Data on Demodex Ectoparasite Infestation in Patients Attending an Outpatient Clinic in Greece
Abstract
1. Introduction
2. Results
3. Materials and Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Desch, C.; Nutting, B.W. Demodex folliculorum (Simon) and D. brevis Akbulatova of Man: Redescription and Reevaluation. J. Parasitol. 1972, 58, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Forton, F. Demodex and perifollicular inflammation in man: Review and report of 69 biopsies. Ann. Dermatol. Venereol. 1986, 113, 1047–1058. [Google Scholar] [PubMed]
- Panopoulou, A.D.; Ieronymaki, A.; Chryssou, S.E. Human Demodex mites (Demodex folliculorum and Demodex brevis) and Demodicosis. Acta Microbilogica Hell. 2017, 62, 7–16. [Google Scholar]
- Wesolowska, M.; Knysz, B.; Reich, A.; Blazejewska, D.; Czarnecki, M.; Gladysz, A.; Pozowski, A.; Misiuk-Hojlo, M. Prevalence of Demodex spp. in eyelash follicles in different populations. Arch. Med. Sci. 2014, 12, 319–324. [Google Scholar] [CrossRef]
- Bonnar, E.; Eustace, P.; Powell, F.C. The Demodex mite population in rosacea. J. Am. Acad. Dermatol. 1993, 28, 443–448. [Google Scholar] [CrossRef]
- Forton, F.; Seys, B. Density of Demodex folliculorum in rosacea: A case-control study using standardized skin surface biopsy. Br. J. Dermatol. 1993, 128, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Forton, F.M. Papulopustular rosacea, skin immunity and Demodex: Pityriasis folliculorum as a missing link. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 19–28. [Google Scholar] [CrossRef]
- Erbağci, Z.; Ozgöztaşi, O. The significance of Demodex folliculorum density in rosacea. Int. J. Dermatol. 1998, 37, 421–425. [Google Scholar] [CrossRef]
- Dolenc- Voljc, M.; Pohar, M.; Lunder, T. Density of Demodex folliculorum in perioral dermatitis. Acta Derm. Venereol. 2005, 85, 211–215. [Google Scholar] [CrossRef]
- Smith, G.; Manzano Marín, A.; Reyes-Prieto, M.; Ribeiro Antunes, C.S.; Ashworth, V.; Goselle, O.N.; Jan, A.A.A.; Moya, A.; Latorre, A.; Perotti, M.A.; et al. Human follicular mites: Ectoparasites becoming symbionts. Mol. Biol. Evol. 2022, 21, 39. [Google Scholar] [CrossRef]
- Plewig, G.; Kligman, A.M. Acne and Rosacea, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 507–508. [Google Scholar]
- Przydatek-Tyrajska, R.; Sędzikowska, A.; Bartosik, K. Primary facial demodicosis as a health problem and aesthetic challenge: A case report. J. Cosmet. Dermatol. 2021, 20, 420–424. [Google Scholar] [CrossRef]
- Baima, B.; Sticherling, M. Demodicidosis revisited. Acta Derm. Venereol. 2002, 82, 3–6. [Google Scholar] [CrossRef]
- Ricardo-Gonzalez, R.R.; Kotas, M.E.; O’Leary, C.E.; Singh, K.; Damsky, W.; Liao, C.; Arouge, E.; Tenvooren, I.; Marquez, D.M.; Schroeder, A.W.; et al. Innate type 2 immunity controls hair follicle commensalism by Demodex mites. Immunity 2022, 11, 1891–1908. [Google Scholar] [CrossRef]
- Lacey, N.; Kavanagh, K.; Tseng, S.C. Under the lash: Demodex mites in human diseases. Biochemist 2009, 1, 2–6. [Google Scholar] [CrossRef]
- Karabay, A.E.; Çerman, A.A. Demodex folliculorum infestations in common facial dermatoses: Acne vulgaris, rosacea, seborrheic dermatitis. An. Bras. Dermatol. 2020, 95, 187–193. [Google Scholar] [CrossRef]
- Erdal, B.; Albayrak, H. Investigation of the Prevalence of Demodex spp. in Dermatological Diseases. Turk. Parazitol. Derg. 2022, 1, 54–59. [Google Scholar] [CrossRef]
- Yasak-Guner, R.; Tosun, M.; Akyol, M.; Hayta, S.B. Demodex infestation as a cause of sensitive skin in a dermatology outpatient clinic. J. Cosmet. Dermatol. 2022, 21, 1610–1615. [Google Scholar] [CrossRef]
- Zeytun, E.; Yazıcı, M. Human Demodex Mites (Acari: Demodicidae) as a Possible Etiological Factor in Rosacea—A Cross-Sectional Study from Turkey. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Aşkin, U.; Seçkin, D. Comparison of the two techniques for measurement of the density of Demodex folliculorum: Standardized skin surface biopsy and direct microscopic examination. Br. J. Dermatol. 2010, 162, 1124–1126. [Google Scholar] [CrossRef]
- Forton, F.; Germaux, M.A.; Brasseur, T.; De Liever, A.; Laporte, M.; Mathys, C.; Sass, U.; Stene, J.J.; Thibaut, S.; Tytgat, M.; et al. Demodicosis and rosacea: Epidemiology and significance in daily dermatologic practice. J. Am. Acad. Dermatol. 2005, 52, 74–87. [Google Scholar] [CrossRef]
- Eser, A.; Erpolat, S.; Kaygusuz, I.; Balci, H.; Kosus, A. Investigation of Demodex folliculorum frequency in patients with polycystic ovary syndrome. An. Bras. Dermatol. 2017, 92, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Karapsias, S.; Patelis, A.; Sgourou, A. Demodex Outbreak Causing Palpebra Skin Lesions. J. Pigment. Disord. 2017, 4, 1–2. [Google Scholar]
- Georgala, S.; Katoulis, A.C.; Kylafis, G.D.; Koumantaki-Mathioudaki, E.; Georgala, C.; Aroni, K. Increased density of Demodex folliculorum and evidence of delayed hypersensitivity reaction in subjects with papulopustular rosacea. J. Eur. Acad. Dermatol. Venereol. 2001, 15, 441–444. [Google Scholar] [CrossRef]
- Lazaridou, E.; Apalla, Z.; Sotiraki, S.; Ziakas, N.G.; Fotiadou, C.; Ioannides, D. Clinical and laboratory study of rosacea in northern Greece. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 410–414. [Google Scholar] [CrossRef]
- Aylesworth, R.; Vance, C. Demodex folliculorum and Demodex brevis in cutaneous biopsies. J. Am. Acad. Dermatol. 1982, 7, 583–589. [Google Scholar] [CrossRef]
- Paichitrojjana, A.; Chalermchai, T. The Association Between Acne Vulgaris, Acne Vulgaris with Nonspecific Facial Dermatitis, and Demodex Mite Presence. Clin. Cosmet. Investig. Dermatol. 2024, 22, 137–146. [Google Scholar] [CrossRef]
- Sędzikowska, A.; Bartosik, K.; Przydatek-Tyrajska, R.; Dybicz, M. Shared Makeup Cosmetics as a Route of Demodex folliculorum Infections. Acta Parasitol. 2021, 66, 631–637. [Google Scholar] [CrossRef]
- Van Atteveld, J.; Graaf, M.; Grotel, M.; Van den Heuvel-Eibrink, M.M. Demodicosis in Pediatric Cancer. J. Pediatr. Hematol. Oncol. 2017, 39, 402–406. [Google Scholar] [CrossRef]
- Álvarez-Salafranca, M.; Vicente, A.; Prat Torres, C.; Combalia, A.; Monsonís, M.; Celis-Passini, V.P.; González-Enseñat, M.A. Demodicosis in two patients with a previous history of Langerhans cell histiocytosis. Pediatr. Dermatol. 2017, 34, e299–e301. [Google Scholar] [CrossRef]
- Douglas, A.; Zaenglein, A.L. A case series of demodicosis in children. Pediatr. Dermatol. 2019, 36, 651–654. [Google Scholar] [CrossRef]
- Sarac, G.; Cankaya, C.; Ozcan, K.N.; Cenk, H.; Kapicioglu, Y.K. Increased frequency of Demodex blepharitis in rosacea and facial demodicosis patients. J. Cosmet. Dermatol. 2020, 19, 1260–1265. [Google Scholar] [CrossRef]
- Zhang, A.C.; Muntz, A.; Wang, M.T.M.; Craig, J.P.; Downie, L.E. Ocular Demodex: A systematic review of the clinical literature. Ophthalmic Physiol. Opt. 2020, 40, 389–432. [Google Scholar] [CrossRef]
- Cheng, A.M.; Galor, A.; Banoub, R.; Gupta, S.K. The impact of ocular demodicosis on the eyes. Eye 2023, 37, 3061–3062. [Google Scholar] [CrossRef]
- Chudzicka-Strugała, I.; Gołębiewska, I.; Brudecki, G.; Elamin, W.; Zwoździak, B. Demodicosis in Different Age Groups and Alternative Treatment Options—A Review. J. Clin. Med. 2023, 19, 1649. [Google Scholar] [CrossRef]


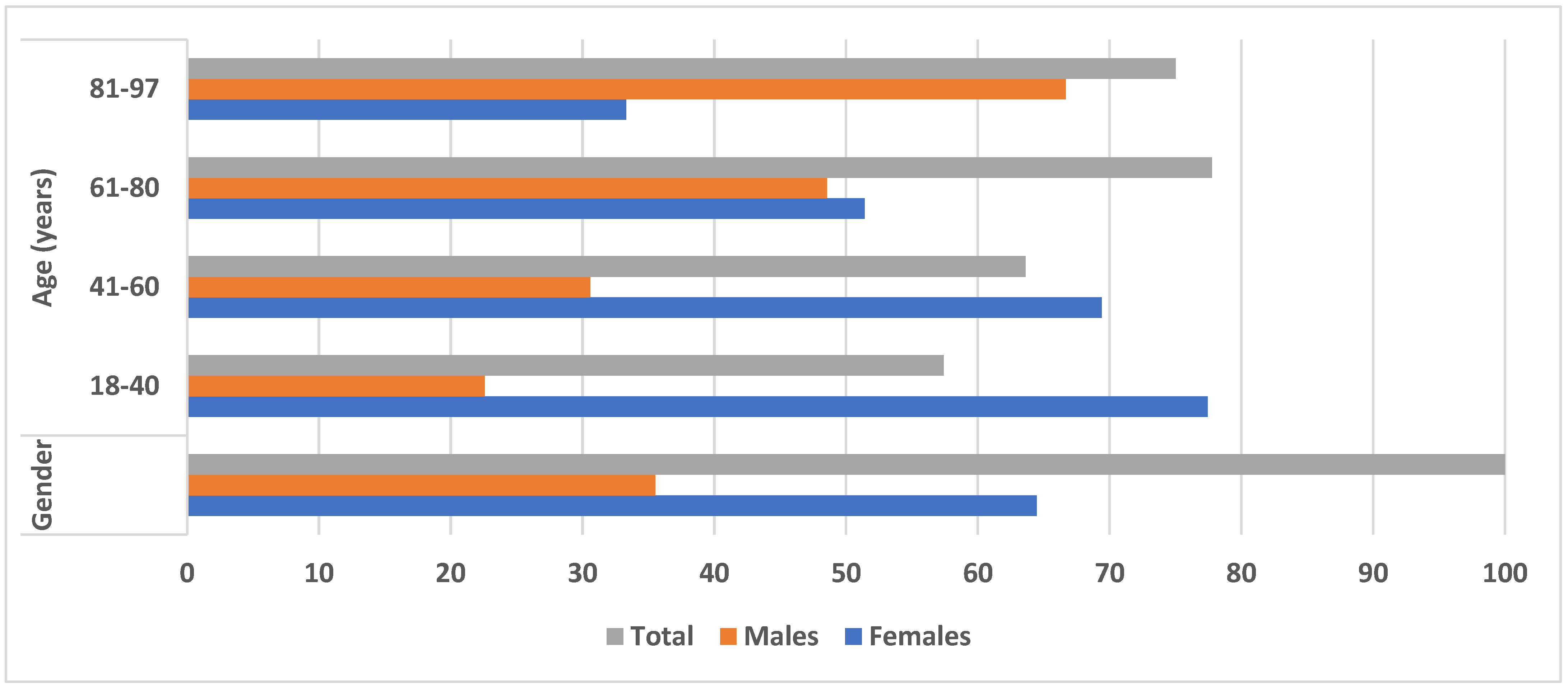
| Prevalence of Demodex spp. | Parameters | ||||
|---|---|---|---|---|---|
| Gender Number/Percentage | Age (Years) | ||||
| 18–40 | 41–60 | 61–80 | 81–97 | ||
| Female | 78 (64.46%) | 24 (77.42%) | 34 (69.4%) | 18 (51.42%) | 2 (33.33%) |
| Male | 43 (35.54%) | 7 (22.58%) | 15 (30.6%) | 17 (48.57%) | 4 (66.67%) |
| Total | 121 (100%) | 31 (57.41%) | 49 (63.63%) | 35 (77.77%) | 6 (75%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kargadouri, A.; Beleri, S.; Patsoula, E. Data on Demodex Ectoparasite Infestation in Patients Attending an Outpatient Clinic in Greece. Parasitologia 2024, 4, 129-136. https://doi.org/10.3390/parasitologia4020011
Kargadouri A, Beleri S, Patsoula E. Data on Demodex Ectoparasite Infestation in Patients Attending an Outpatient Clinic in Greece. Parasitologia. 2024; 4(2):129-136. https://doi.org/10.3390/parasitologia4020011
Chicago/Turabian StyleKargadouri, Anastasia, Stavroula Beleri, and Eleni Patsoula. 2024. "Data on Demodex Ectoparasite Infestation in Patients Attending an Outpatient Clinic in Greece" Parasitologia 4, no. 2: 129-136. https://doi.org/10.3390/parasitologia4020011
APA StyleKargadouri, A., Beleri, S., & Patsoula, E. (2024). Data on Demodex Ectoparasite Infestation in Patients Attending an Outpatient Clinic in Greece. Parasitologia, 4(2), 129-136. https://doi.org/10.3390/parasitologia4020011






