Abstract
The presence of the Varroa destructor mite requires the use of acaricide treatments for honeybee colonies. Amitraz is one of the most common acaricide-active ingredients used by beekeepers. Certain Varroa mite populations have developed resistance to amitraz, thereby leading to a loss in the efficacy of amitraz-based treatments. Two products, Apivar and Supatraz, were applied in the same apiary in France to evaluate their efficacy. Both treatments are amitraz-based but have different galenics. Thanks to field data, a dynamic model was used to simulate the actions of Apivar and Supatraz on the mite population. We considered two parameters to compare the products as follows: the daily mortality rate and the treatment duration. In the field, the percentage of the efficacy of the two products was not significantly different, but Supatraz kills mites faster and decreases 90% of the mite infestation in 28.4 days compared with 50.9 days when using Apivar. Through modeling, we showed the daily impact of the two different products on mite population. Supatraz has a higher daily mortality rate during the first two weeks than Apivar. Supatraz requires a lower efficacy (% of varroa mites killed during all the treatment) to stabilize the varroa mite population due to its faster release of active ingredients than Apivar, thereby needing a shorter period to achieve the same result. Depending on the model, Supatraz conserves effective efficacy when used against moderately resistant mites (with mite mortality being 40–70% at the LC90) but not against highly resistant mites (with mite mortality being <40% at the LC90). These results show that the comparison of the efficacy of the two products with different characteristics (duration of treatment and daily mortality rate) should be analyzed with caution.
1. Introduction
Varroa destructor is an ectoparasitic mite of honeybees responsible for varoosis [1]. It is also responsible for the spread of viruses in honeybee colonies [2,3]. Today, beekeepers must integrate varroa management to limit colony collapses. The control methods include the acaricide (chemical) and biotechnical methods [4]. Treatments should be sufficiently effective to significantly reduce the varroa mite population every year. However, a result of the lack of rotations of synthetic acaricides is that of the development of resistance to the acaricide substance by varroa mites [5]. Resistance to tau-fluvalinate, flumethrin, coumaphos and amitraz was detected in the laboratory [6,7,8,9,10,11] and in the field [11,12,13]. For the moment, synthetic acaricides are the most prone to resistance. Currently, no resistance to acaricides that are used in organic beekeeping has been demonstrated [5,14,15]. Resistance development can come from different mechanisms as follows: physiological, metabolic or target mutations. In relation to resistance to tau-fluvalinate, protein changes have been demonstrated through increases in detoxifying enzymes [16]. More recently, molecular resistance, such as target mutation, is also believed to be involved in the development of resistance to tau-fluvalinate [17,18]. The detection of amitraz resistance was demonstrated in the laboratory and in the field [6,8,9,13]. Recently, target mutation resistance has been described in correlation with varroa mite resistance to amitraz [19]. Mutations were different between populations from France (N87S) and United States (Y215H). This discovery demonstrates the ability of the varroa mite to adapt according to its geographical area.
In this context, beekeepers must put in place control strategies to limit the development of resistance and maintain high treatment efficacy [5]. Among these strategies, that of alternating control (alternation of the acaricide substance over several years) has been identified. Unfortunately, the alternation of acaricides is generally not performed in France, and beekeepers mainly use Apivar treatment every year [20]. In addition, because of the loss of efficacy, beekeepers are advised by vets to leave the treatment in the beehives for a longer period [21]. Unfortunately, long-term treatments increase the risk of developing resistance [22].
More generally, for amitraz, several pharmaceutical treatments are available all around the world [4]. Each treatment has different characteristics that influence the varroa population and its resistance to the acaricide substance as follows: duration of treatment, active ingredient concentration and galenic formulation. The release of the active ingredient also varies according to the galenic presentation. This variation in release could explain the differences in efficacy observed for the varroa population between treatments. The choice of products in addition to the choice of the active ingredient may influence the efficacy and the development of resistance.
A recent modeling study shows the influence of resistance on the loss of efficacy with Apivar® (Vetopharma, Palaiseau, France) treatment in France [23]. This model explains how the level-resistant mites could be the explanation for the Apivar efficacy observed in the field. However, the model must be adapted to each medicine to fit with the observed field efficacy. A mechanistic model could allow us to understand how treatments kill mites and the impact of the efficacy loss on each type of treatment.
Our goal in this study is to assess the different amitraz-based products that are used for varroa mite management through a mechanistic model. Two points have been studied as follows: (1) the duration of treatment and (2) the rate of falling dead mites. Two products will be compared in the same environment as follows: Apivar® and Supatraz®. Modeling the action of the treatments may help explain the differences between these two medicines that have been observed for the efficacy parameters, such as those of the optimal treatment duration and residual mites.
2. Materials and Methods
2.1. Mathematical Modeling
The model of natural mite population dynamics is derived from the model developed by Calis et al. [24]. The action of Apivar® treatment was added according to the model developed by Almecija [23]. The input and output variables are presented in Table 1. R software (version 3.6.2) was used for simulations and statistical analysis.

Table 1.
Input and output variables of the model.
The natural population dynamics of varroa mites is defined by Equation (1) [23]. The number of phoretic mites is represented by P(t). The variable I(t) corresponds to the number of mites infesting the worker brood, and E(t) corresponds to the number of mites emerging with young, mature females. The number of individual mites that died from natural mortality is given by M(t). The treatment’s effect on the honeybee population dynamics is represented by an additional daily mortality. MT(t) is the global number of deaths (dying from natural mortality and from mortality induced through the treatment). The treatment starts on 7 August for all simulations.
The action of the treatment depends on 3 parameters as follows: duration of treatment, dT, daily mortality, kT, and the level of initial infestations in the colony, Pi. The action of Apivar® treatment on the population can be simulated through a daily mortality rate, kT, and a constant for the duration of treatment, kTAPIVAR [23]. The Apivar® treatment duration is 10 weeks (70 days) [25]. The model had to be readjusted for the Supatraz® (amitraz) treatment, which has different pharmacokinetic characteristics from those of Apivar®. Supatraz® is an 8-week treatment (56 days).
2.2. Efficacy Determination
2.2.1. In the Field
The efficacy monitoring method is that of the standard European method [26]. Efficacy monitoring was carried out in 2019 and 2020 on Apivar® and Supatraz® in an apiary of 30 beehives located in Nieul-sur-mer, France. Before treatment, the mite infestation level was monitored to homogenize groups (level of phoretic mites by alcohol wash between 4 and 5% and 3 ± 0.5 frames of brood per colony at the beginning of the experiment). In 2020, the efficacies of Apivar® and Supatraz® were followed, respectively, on 7 and 5 beehives. The colonies used were 6-frame nucs, so only one strip per colony was used. The strips were introduced into the colonies on 26 August and 8 July, respectively, for the years 2019 and 2020. The characteristics of each treatment are presented in Table 2. During treatment, the number of dead mites, which drop to the floor of the beehive, is counted with a sticky board every three days (Vtreatment).

Table 2.
Characteristics of Apivar® and Supatraz®.
At the end of the treatment, a control treatment is applied (Apistan® (Tau-fluvalinate)) killing the remaining mites (Vremaining). From these data, the percentage of field treatment efficacy, EffFIELD, is evaluated according to Equation (2) as follows:
2.2.2. With the Model
Our model replicates the protocol that is applied in the field to determine treatment efficacy (including control treatment). The model estimates the number of cumulative dead mites, McumMODEL(t), during treatment and control treatment (Equation (3)). The treatment starts at day pT. The model simulates the efficacy of the treatment using the same protocol as that in the field (Equation (4)). The number of remaining mites according to the model, VremainingMODEL, is evaluated through a simulation of a control treatment (with a daily efficiency of 90%). Efffield MODEL represents a simulation of the efficacy reproducing the protocol used in the field.
2.3. Validation of Supatraz® Modeling
The Apivar® model was validated using previous data from efficacy monitoring in 2020 [23]. The model showed that Apivar® presents the constant daily mortality rate, kT, during the treatment duration. Here, the model had to be adjusted to match the action of the Supatraz® treatment. Indeed, Supatraz® presents a daily mortality rate, kTSUPATRAZ, of 0.342 for the first week of treatment and a variable value between 0.03 and 1 after one week of treatment.
The validation of the Supatraz modeling is based on the efficacy monitoring conducted in 2019 and 2020 in Nieul-sur-mer (France). Two parameters were set as follows: start of treatment (pT = August 7) and duration of treatment (dT = 8 weeks). The initial infestation, Pi, is adjusted according to the number of dead mites during the treatment (McumMODEL). The model is validated by comparing the slope of the cumulative number of dead mites over the total duration of treatment between the field and the model. The slopes of cumulative dead mites during treatment are defined in the field as well as through the model. They are evaluated over a duration of treatment of 70 days for Apivar® and one of 56 days for Supatraz®.
2.4. Time to Kill 90% of the Mite Population: L90
A lethal time of 90% (LT90) represents the time required to kill 90% of the total varroa population. This period is evaluated from data from the field. It is evaluated through the ECOTOX package and the simulation of a GLM model tracing varroa mortality during treatment. LT90s are evaluated for Apivar® and Supatraz® in 2019 and 2020.
2.5. Influence of the Proportion of Resistant Mites on the Treatment Efficacy
Resistance in the mite population is modeled through the decrease in the daily mortality rate, kT. The decrease in kT corresponds to the proportion of resistant mites that are insensitive to treatment. In our model, the proportion of resistant mites hypothetically remains stable and does not increase during the treatment. The percentage of efficacy is evaluated according to whether there are percentages of 0%, 20%, 40%, 60% and 80% of resistant mites in the population observed for both treatments.
Amitraz resistance has different impacts depending on the treatments of either Apivar® or Supatraz®. Apivar® kills mites with a similar kT during all the treatment. For Supatraz®, the resistance simulation is more complicated because of the inconstant kT. The reduction of kT to simulate resistance is not enough. The influence of resistance on the efficacy of Supatraz® differs depending on the two hypotheses as follows: (1) the resistant mites are killed the first week with stable kT, 0.394; (2) the resistant mites are not killed in the first week of treatment due to a high resistance ratio. In this second hypothesis, the proportion of kT decreases in the same proportion as that of resistant mites.
3. Results
3.1. Field Efficacy
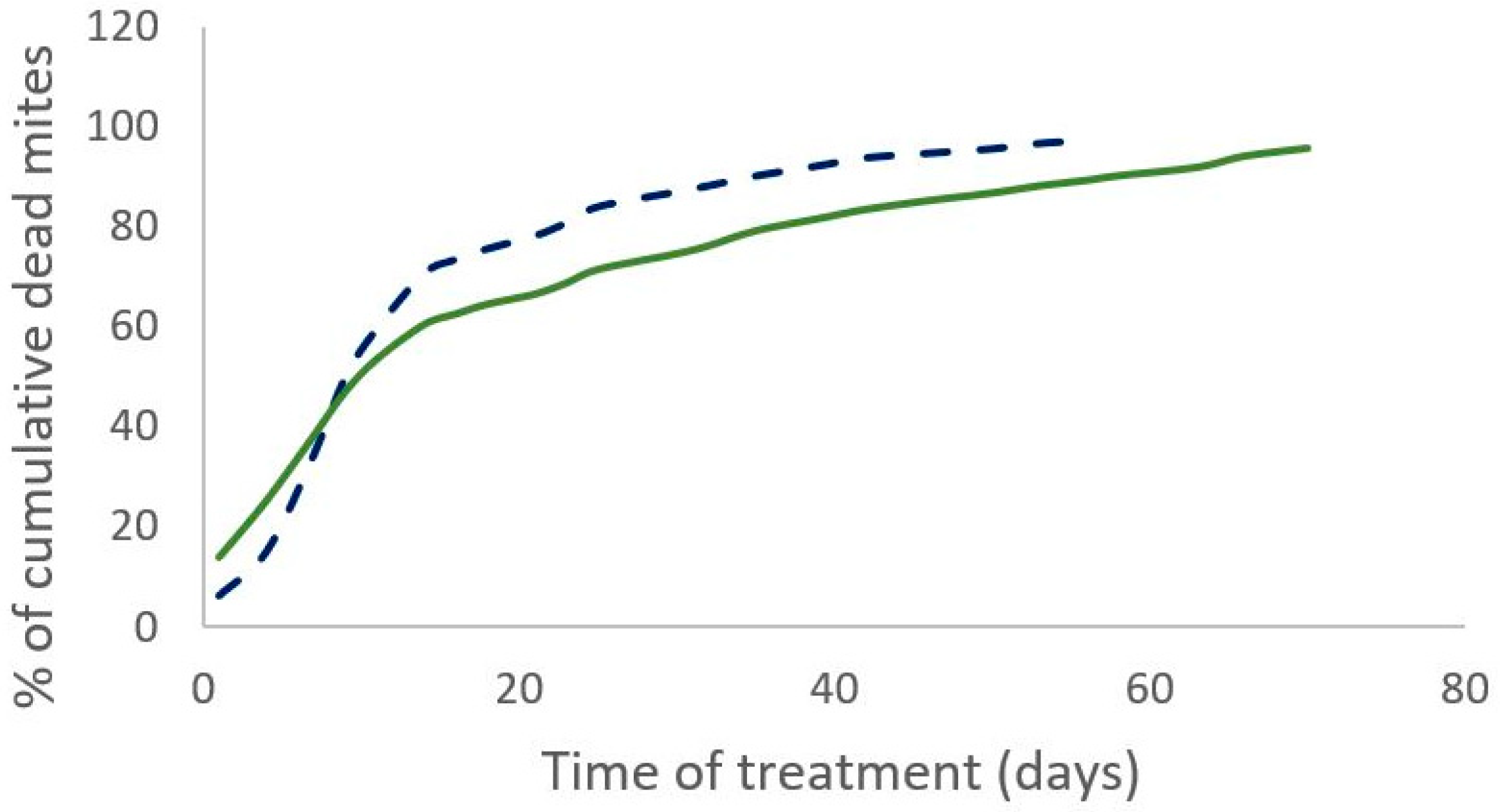
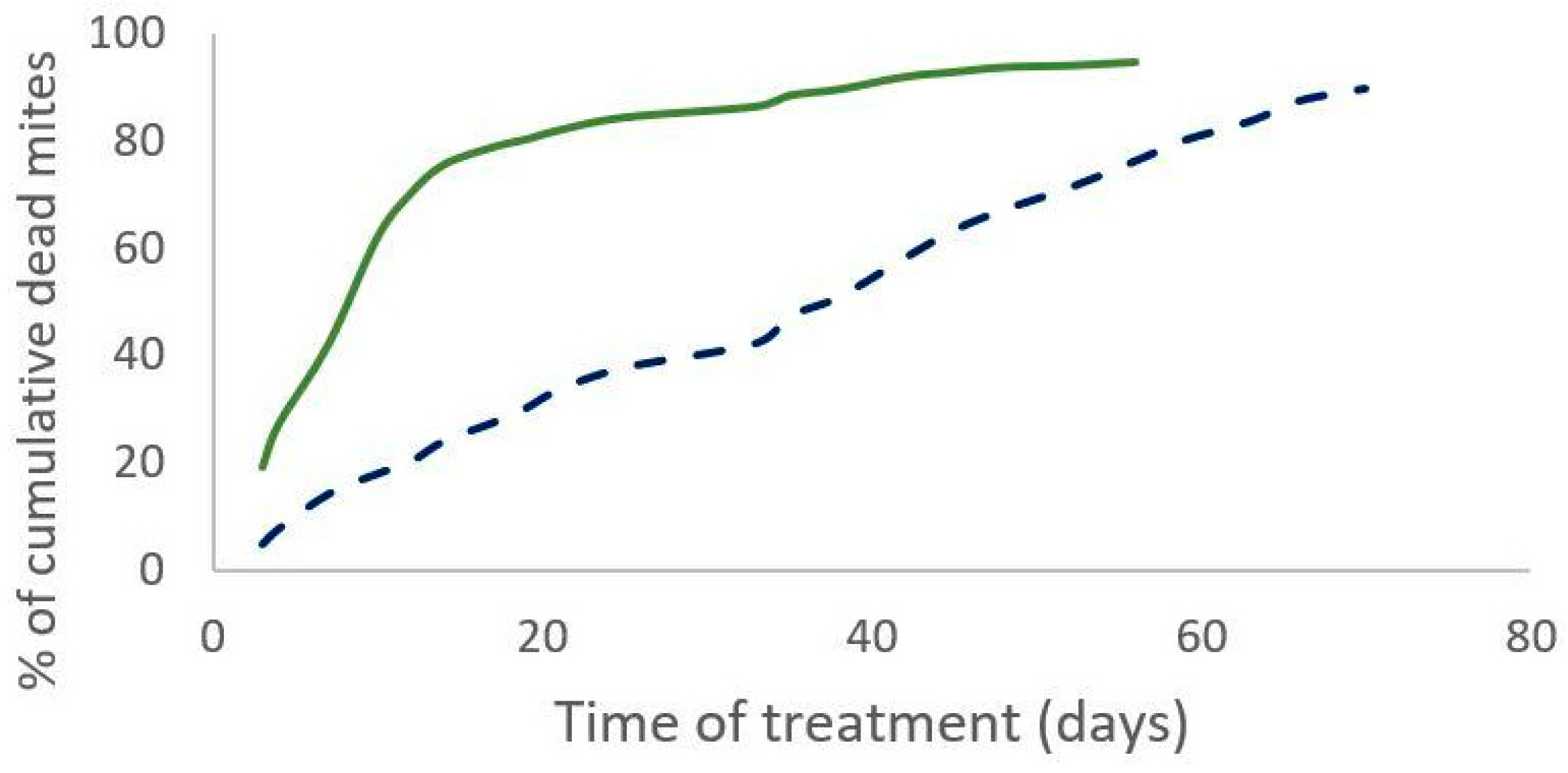
The percentage of the field efficacy is not significantly different between both treatments (Mann–Whitney test, W = 19, p = 0.3 (2019) and W = 12, p = 0.43 (2020)). In 2019, the cumulative number of dead mites during treatment was not significantly different between the two treatments (Mann–Whitney test, W = 35, n = 11, p = 0.35). The time required to kill 90% of the varroa population was significantly shorter for the Supatraz® treatment than for the Apivar® one in 2019 (Wilcoxon test, W = 79, p = 7 × 10−4) and in 2020 (Wilcoxon test, W = 35, p = 2.5 × 10−3) (Table 3). Supatraz® acts faster than Apivar® (Figure 1 and Figure 2).

Table 3.
Comparison of results obtained in the field in 2019 and 2020 between Apivar® and Supatraz®. Column followed by different letters indicate that they are significantly different (p < 0.05).
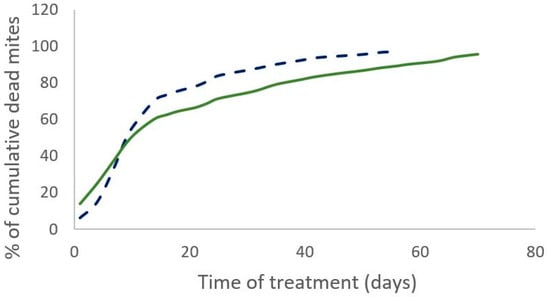
Figure 1.
Percentage of cumulative dead mites during treatment observed for Apivar® (solid line) and Supatraz® (dotted line) in 2019.
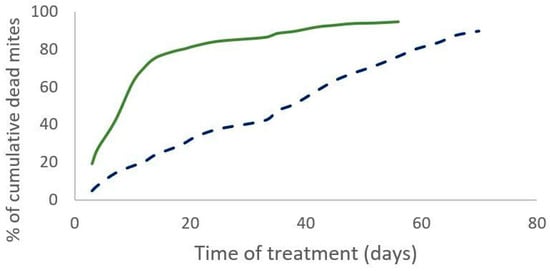
Figure 2.
Percentage of cumulative dead mites during treatment observed for Apivar® (solid line) and Supatraz® (dotted line) in 2020.
Residual varroa counts are significantly lower for Supatraz®-treated colonies (Mann–Whitney test, W = 21, n = 11, p = 0.04). The same observations are made for the year 2020 (Table 3). The percentage of efficacy alone is not enough to explain the real field efficacy of the treatment.
3.2. Modeling Efficacy
3.2.1. Validation of Apivar® and Supatraz®
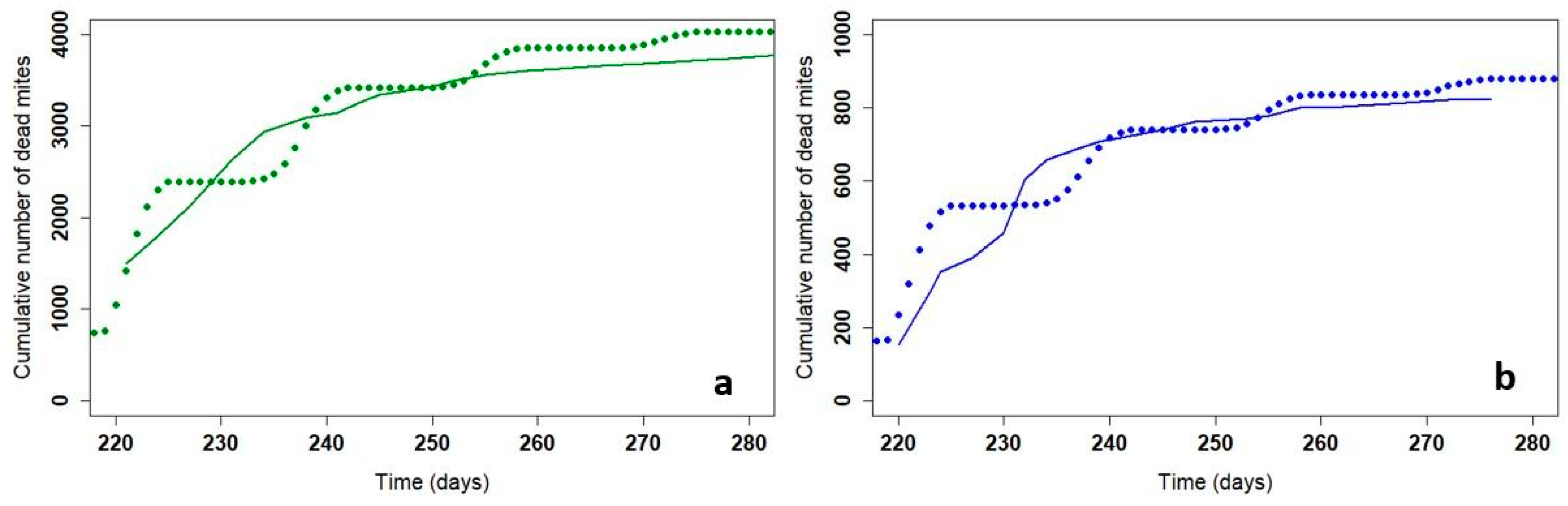
The model must represent the kinetics of the mite drop during treatment to match the reality. The action of Apivar® treatment was modeled in 2019 and 2020 on beehives followed by the ADA AURA (France) and APINOV in Nieul-sur-Mer (France). Maintaining the same parameter values, we represent the curve of the cumulative dead mites from the apiary in Nieul-sur-Mer (Figure 3).
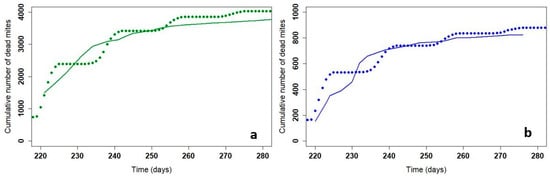
Figure 3.
Cumulative dead mites in the field (solid line) and with the model (dotted line) for Apivar (a) and for Supatraz (b). Field data come from one beehive in Nieul-sur-Mer in the 2019 trial.
Figure 3 shows the number of mites that fell during treatment with Supatraz® in the field and with the model. The cumulative dead mites from the model correspond to the mite count in the field. Moreover, the 56-day slope is not significantly different between the field and model data (Wilcoxon test, p = 0.37).
3.2.2. Stabilization of the Mite Population with Supatraz® and Apivar®
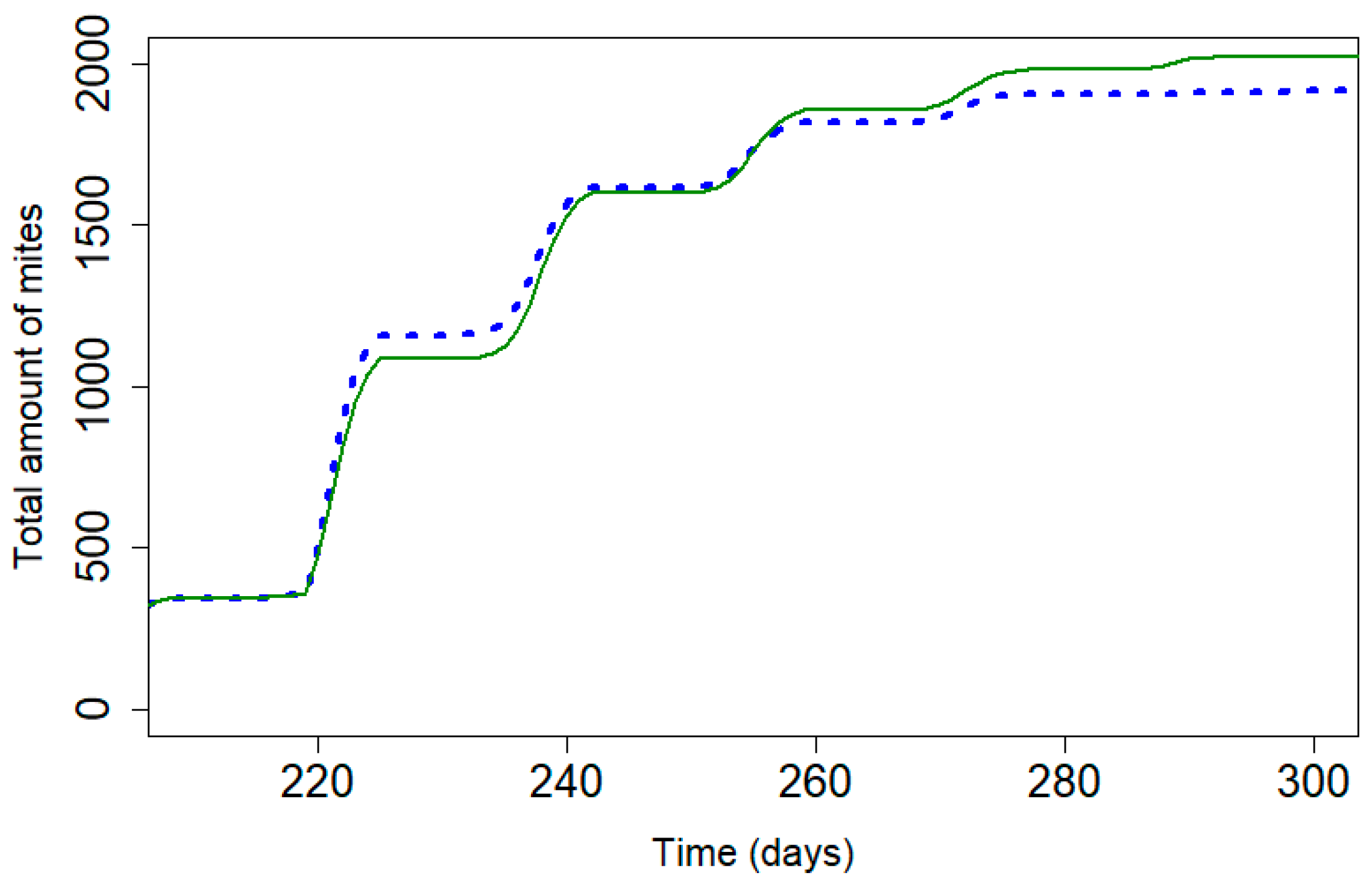
Table 4 presents the different parameters used to stabilize the varroa mite population for both products. The initial daily mortality rate, kT, is higher for Supatraz® than for Apivar®. According to the model, a percentage of 97.0% for Supatraz® is enough to stabilize the varroa population from year to year, while Apivar® must have an efficacy rate of 98.77% [23]. The model shows that the number of dead mites during treatment would be lower for Supatraz® and the number of residual varroa mites would be higher. The model, which is free of all environmental factors, shows less of a difference in the cumulative dead mites between those observed for Apivar® and Supatraz® than the mortalities observed in the field (Figure 1, Figure 2 and Figure 4). In this condition, both treatments present identical results for the mite population as follows: efficacy, LT90 and number of dead mites.

Table 4.
Modeling values needed to stabilize the varroa population for Apivar® (10 weeks of treatment) and Supatraz® (8 weeks of treatment) (Pi = 50).
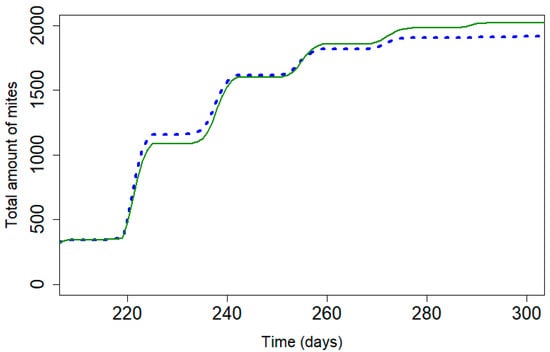
Figure 4.
Cumulative mite mortality with the model for Apivar® (solid line) and Supatraz® (dotted line) (Pi = 50) for stabilized the mite population year to year.
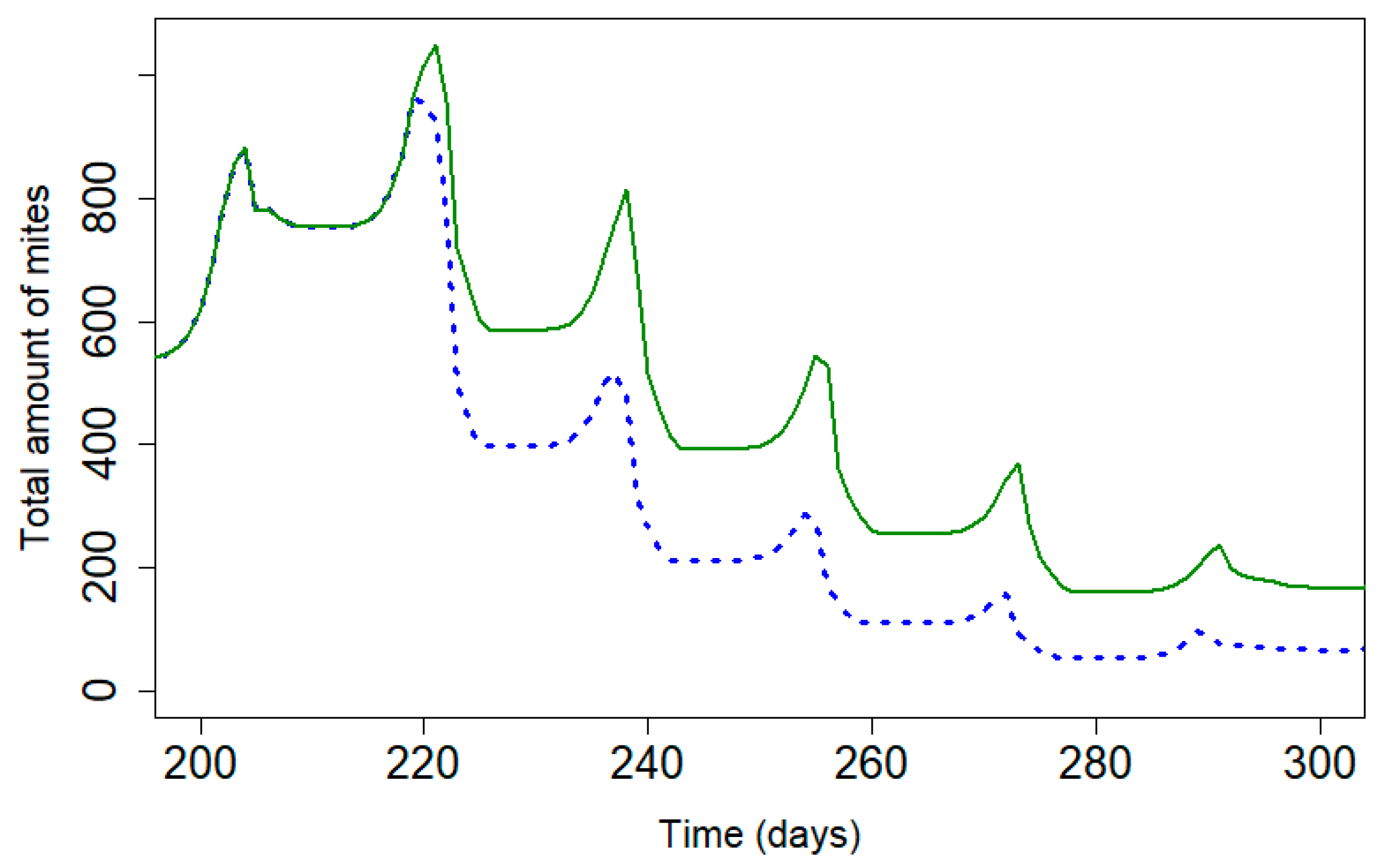
3.3. Influence of Decreasing Efficacy on Mite Population
Figure 5 presents the mite population curve during treatment applications of Apivar® and Supatraz® for a theoretical efficacy that is equal to 95% for both treatments. Supatraz® has a faster rate of action on the varroa population than Apivar® treatment, thereby allowing for a reduction in the reproduction rate of varroa mites early in treatment. The mite population decreases faster with Supatraz® than Apivar®, and the number of remaining mites is lower with Supatraz®.
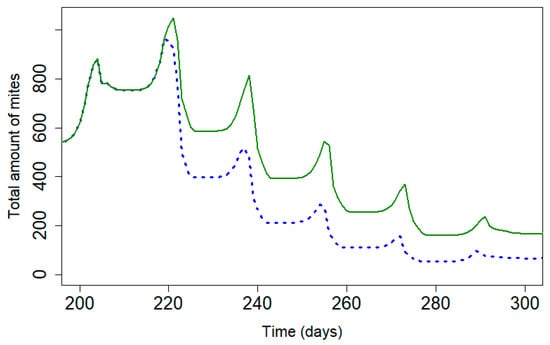
Figure 5.
Mite population during treatment with Apivar® (solid line) and Supatraz® (dotted line) for 95% efficacy across both products (Pi = 50). (kTAPIVAR = 0.178 and kTSUPATRAZ = 0.24).
Table 5 presents the influence of both products on mite population depending on the percentage of efficacy. The LT90 is identical to the values observed in the field (Table 2). For the same efficacy, the model shows that Supatraz® has fewer remaining mites; moreover, the LT90s are shorter for Supatraz®.

Table 5.
Number of cumulative dead mites and remaining mites with Apivar® and Supatraz® at different efficacies (with Pi = 50).
3.4. Influence of Resistant Mites on Treatment Efficacy
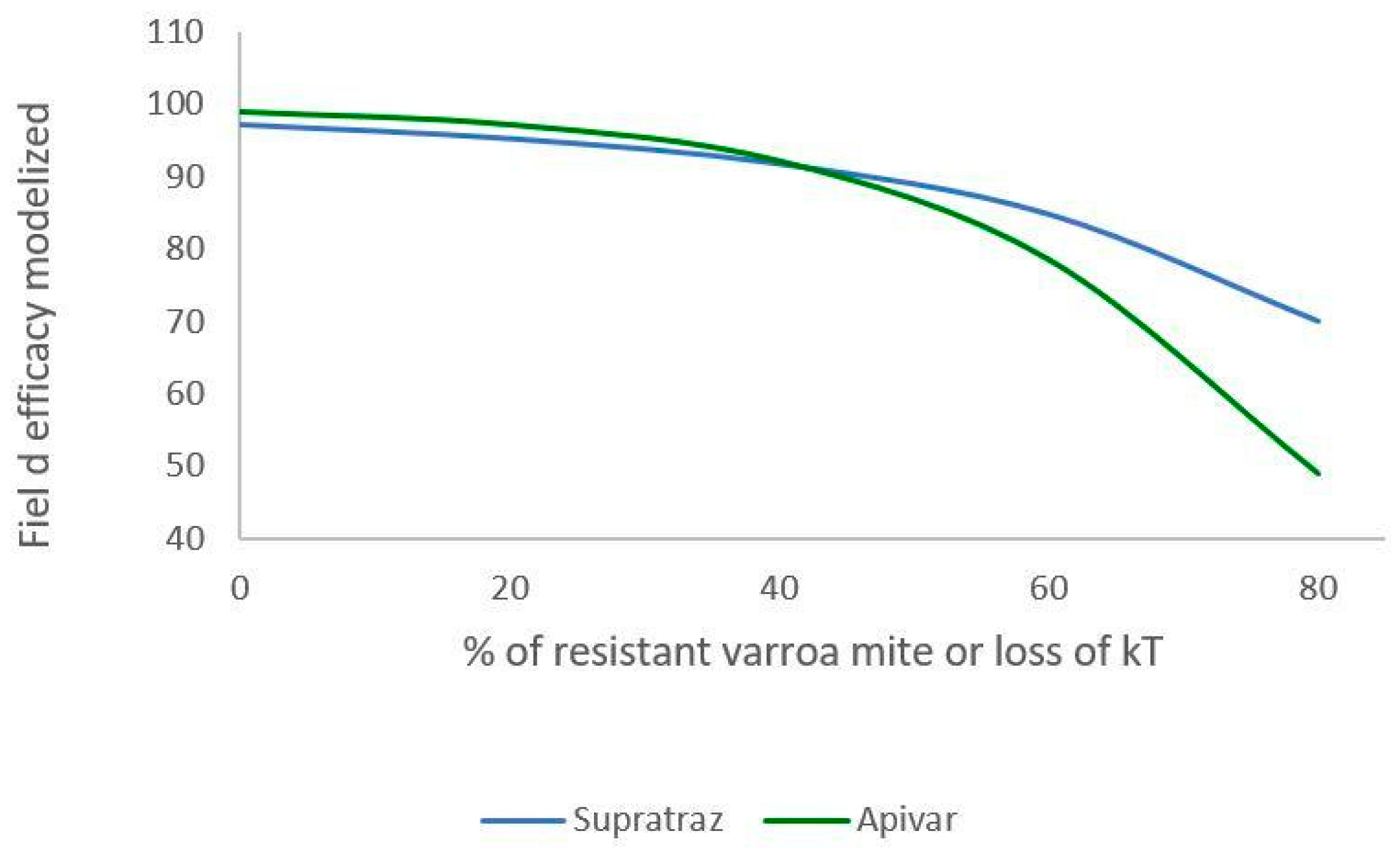
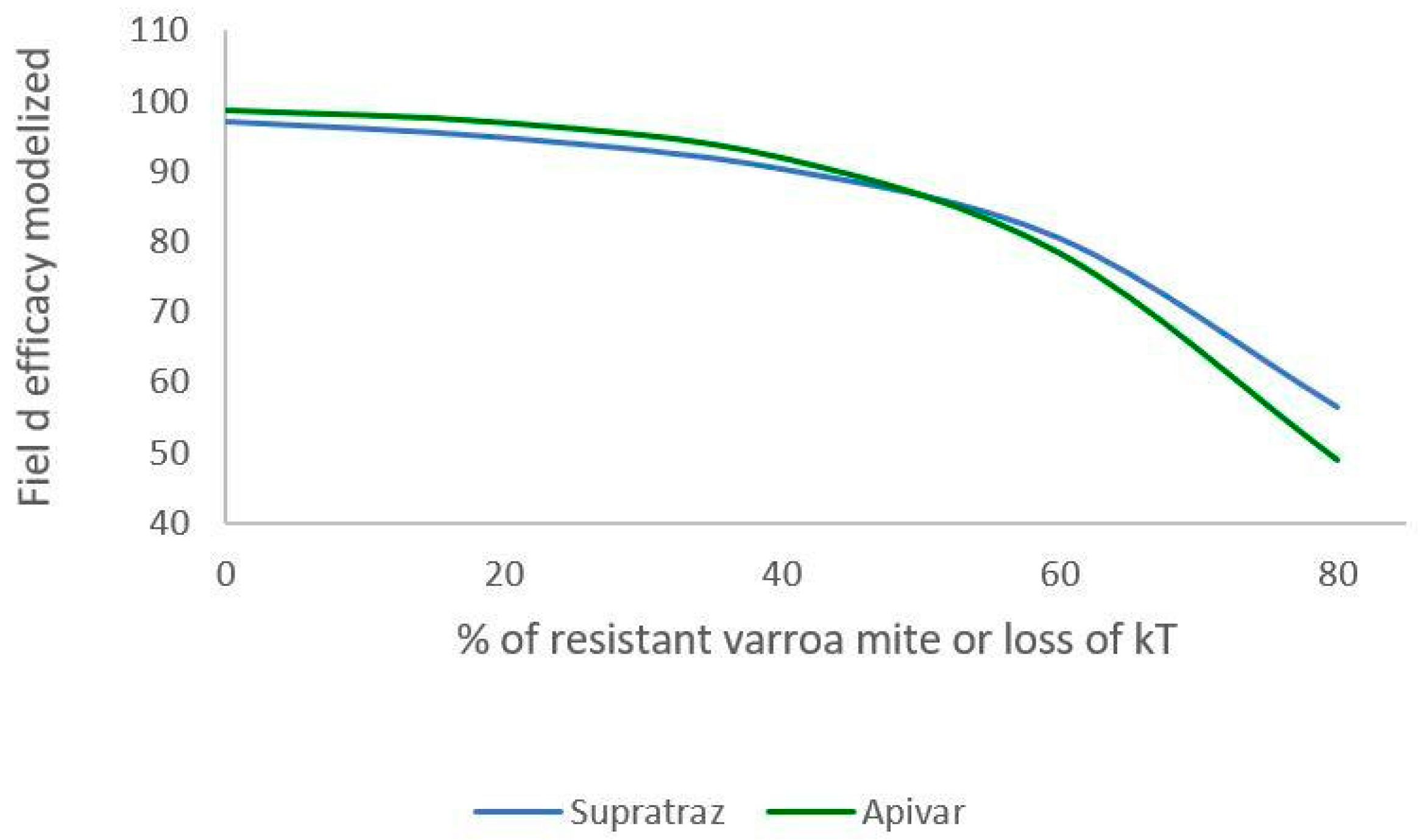
Figure 6 and Figure 7 present the decrease in the efficacy when resistance increases in the mite population. With Apivar®, efficacy decreases from 98.77% to 48.9% (Figure 6). With Supatraz® and hypothesis 1, the efficacy decreases from 97.17 to 70.13% (Figure 7). With hypothesis 2, the efficacy decreases from 97.17% to 56.4%. In both cases, the efficacy is low when the resistance is high (>40%).
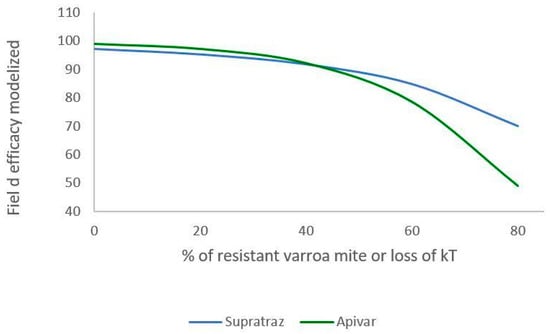
Figure 6.
Modeling of efficacy loss depending on the percentage of resistance (Apivar: Pi = 50, dT = 10; Supatraz: Pi = 50, dT = 8). For Supatraz, hypothesis 1 was applied for this simulation. The proportion of kT decreases in the same proportion as that of resistant mites.
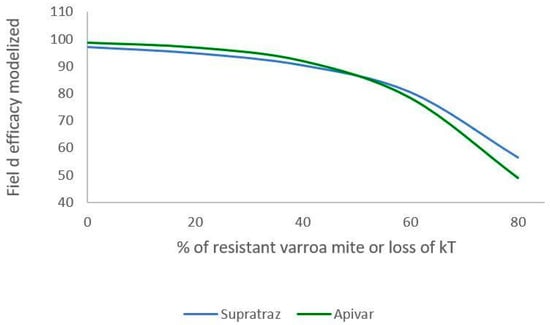
Figure 7.
Modeling of efficacy loss depending on the percentage of resistance (Apivar: Pi = 50, dT = 10; Supatraz: Pi = 50, dT = 8). For Supatraz, hypothesis 2 was applied for this simulation. The varroa mites were sufficiently resistant to be safe during the first week of treatment.
When the proportion of resistant mites is under 40%, the efficacy decreases but is higher than 90% for both Apivar® and Supatraz®. Thus, the mite population is not stabilized but is sufficiently decreased for beekeepers. However, when the proportion of resistant mites is higher than 40%, the efficacy for all the cases decreases quickly, thereby leading to honeybee colony collapse.
4. Discussion
In the field, Supatraz® has a higher efficacy than Apivar®. The number of cumulative dead mites was lower with Supatraz® than Apivar®, thereby indicating faster action with Supatraz® [23]. Each medicine has different characteristics that affect the impact on the varroa population. The amount of the active ingredient as well as galenic changes the amount or speed of the active ingredient that is released. This could be consistent with the Supatraz® galenic due to the support matrix, which contains oil, and the higher amount of the active ingredient (Figure 5). The product’s galenic and formulation play an important role in the action of the treatment. The same observation was evaluated with different thymol-based treatments [27].
A mechanistic model was used to understand the action of both treatments on the varroa population. The action model depends on several parameters. The amitraz release in the beehive is not well understood and we cannot model it; however, the model can show us how each medicine influences the mite population reduction. Without dissociating the origin of this variation in the amitraz released (galenic and amount of the active compound), the modeling of the daily mortality rate of Apivar® and Supatraz® (kTAPIVAR and kTSUPATRAZ) allows for analysis of the performance of the medicines.
The population stabilization point between the treatments shows that the comparison of the efficacy between the treatments is subtle. To stabilize the mite population, the model shows that Supatraz® requires lower efficacy (Table 3). Moreover, in the case of a percentage of efficacy that was theoretically the same, Supatraz® presents fewer remaining mites and a shorter LT90 due to faster action (Table 4). Supatraz® could be useful to quickly reduce infestation, as shown in reference [28], which is probably due to both a higher amitraz concentration and formulation.
Our model also explained how the resistance level influences the efficacy of both treatments, especially for Supatraz®, with a higher daily mortality rate (kT) in the first week. In both cases, the resistance of varroa mites has a high impact on the varroa population when more than 40% of the mites are considered to be resistant to amitraz. Even if the medicines present a different mode of action on the varroa mite population, the impact of the resistance is quite similar.
Depending on the treatment action, the risk of resistance development may vary, as was shown with other mites [29]. In fact, resistance development in mites and insects depends on many parameters, such as dose, pattern of application and timing and sequence of insecticide, are used [30,31]. Sensitive mites do not need high doses of the active ingredient; however, low doses can rapidly cause resistance. A treatment duration that is too long increases the risk of developing resistance. But, either a too high or too low concentration of the active ingredient can also increase the risk of developing resistance [22]. To prevent resistance, the treatment duration must be as short as possible and the amitraz concentration adapted. At present, no information is available on resistance trigger thresholds for the amitraz concentration and the treatment duration for varroa mites. The amitraz application could influence resistance development. However, more information is needed on the resistance in order to model the impact on the efficacy. It should be noted that our model has the same limitations that are described in the model developed for Apivar® [23] as follows: a population dynamic of bees with a total and long winter breakup and no reinfestation. Any changes in bee dynamics would alter these observations as they would affect the varroa population dynamics [28]. In addition, the virus quantity was not included in the model.
5. Conclusions
In the field, Supatraz® presentation is faster acting than that of Apivar® in reducing the mite population. By comparing the action of the Apivar® and Supatraz® treatments, we have shown that pharmacokinetic characteristics influence the global efficacy of the treatment. The model showed that comparing the efficacy of different products with different treatment times is not reliable. Moreover, low resistance probably affects both products in the same way, even if fast action can lead to an increase in efficacy and reduce the treatment duration. The trade-off between the treatment duration and the quantity of the active ingredient could allow the medications to decrease the risk of resistance development in the mite population.
Author Contributions
Conceptualization, G.A.; data curation, G.A.; analysis, G.A. and C.S.; writing—original draft preparation, G.A.; writing—review and editing, G.A., B.P., C.S., P.M. and M.W.; supervision, B.P. and C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This manuscript is funded by the National Agency of Technology Research (ANRT) (n°2018/0060), Vita beehealth Europe and the Research and Training center, APINOV.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available by email from the corresponding author.
Acknowledgments
The authors thank Robin Azemar, Elise Poisson, Precillia Cochard for his help in the apiary and for the counting of dead mites. The authors also would like to thank the reviewers for their advice and their participation in the improvement of the clarity of this article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study, analyses, interpretation of data or in the decision to publish the results.
References
- Anderson, D.; Trueman, J. Varroa jacobsoni (Acari: Varroidae) is more than one species. Exp. Appl. Acarol. 2000, 24, 165–189. [Google Scholar] [CrossRef]
- Beaurepaire, A.; Piot, N.; Doublet, V.; Antunez, K.; Campbell, E.; Chantawannakul, P.; Chejanovsky, N.; Gajda, A.; Heerman, M.; Panziera, D.; et al. Diversity and Global Distribution of Viruses of the Western Honey Bee, Apis mellifera. Insects 2020, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Traynor, K.S.; Mondet, F.; de Miranda, J.R.; Techer, M.; Kowallik, V.; Oddie, M.A.; Chantawannakul, P.; McAfee, A. Varroa destructor: A Complex Parasite, Crippling Honey Bees Worldwide. Trends Parasitol. 2020, 36, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.A.; Wilson, J.M.; Tignor, K.R.; Gross, A.D. Biology and Management of Varroa destructor (Mesostigmata: Varroidae) in Apis mellifera (Hymenoptera: Apidae) Colonies. J. Integr. Pest Manag. 2020, 11, 1. [Google Scholar] [CrossRef]
- Almecija, G.; Poirot, B.; Cochard, P.; Suppo, C. Inventory of Varroa destructor susceptibility to amitraz and tau-fluvalinate in France. Exp. Appl. Acarol. 2020, 82, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Elzen, P.J.; Baxter, J.R.; Spivak, M.; Wilson, W.T. Control of Varroa jacobsoni Oud. resistant to fluvalinate and amitraz using coumaphos. Apidologie 2000, 31, 437–441. [Google Scholar] [CrossRef]
- Kamler, M.; Nesvorna, M.; Stara, J.; Erban, T.; Hubert, J. Comparison of tau-fluvalinate, acrinathrin, and amitraz effects on susceptible and resistant populations of Varroa destructor in a vial test. Exp. Appl. Acarol. 2016, 69, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Maggi, M.D.; Ruffinengo, S.R.; Gende, L.B.; Eguaras, M.J.; Sardella, N.H. LC50 baseline levels of amitraz, coumaphos, fluvalinate and flumethrin in populations of Varroa destructor from Buenos Aires Province, Argentina. J. Apic. Res. 2008, 47, 292–295. [Google Scholar] [CrossRef]
- Milani, N. The resistance of Varroa jacobsoni Oud to pyrethroids: A laboratory assay. Apidologie 1995, 26, 415–429. [Google Scholar] [CrossRef]
- Trouiller, J. Monitoring Varroa jacobsoni resistance to pyrethroids in western Europe. Apidologie 1998, 29, 537–546. [Google Scholar] [CrossRef]
- Adjlane, N. Evaluation of the resistance of the mite Varroa destructor to the amitraz in colonies of the honey bees (Apis mellifera) in Algeria. Uludağ Arıcılık Derg. 2017, 17, 1–6. [Google Scholar] [CrossRef][Green Version]
- Rinkevich, F.D. Detection of amitraz resistance and reduced treatment efficacy in the Varroa Mite, Varroa destructor, within commercial beekeeping operations. PLoS ONE 2020, 15, e0227264. [Google Scholar] [CrossRef]
- A Berry, J.; Bartlett, L.J.; Bruckner, S.; Baker, C.; Braman, S.K.; Delaplane, K.S.; Williams, G.R. Assessing Repeated Oxalic Acid Vaporization in Honey Bee (Hymenoptera: Apidae) Colonies for Control of the Ectoparasitic Mite Varroa destructor. J. Insect Sci. 2022, 22, 15. [Google Scholar] [CrossRef] [PubMed]
- Bava, R.; Castagna, F.; Palma, E.; Marrelli, M.; Conforti, F.; Musolino, V.; Carresi, C.; Lupia, C.; Ceniti, C.; Tilocca, B.; et al. Essential Oils for a Sustainable Control of Honeybee Varroosis. Vet. Sci. 2023, 10, 308. [Google Scholar] [CrossRef] [PubMed]
- Hillesheim, E.; Ritter, W.; Bassand, D. First data on resistance mechanisms of Varroa jacobsoni (OUD.) against tau-fluvalinate. Exp. Appl. Acarol. 1996, 20, 283–296. [Google Scholar] [CrossRef]
- González-Cabrera, J.; Rodríguez-Vargas, S.; Davies, T.G.E.; Field, L.M.; Schmehl, D.; Ellis, J.D.; Krieger, K.; Williamson, M.S. Novel Mutations in the Voltage-Gated Sodium Channel of Pyrethroid-Resistant Varroa destructor Populations from the Southeastern USA. PLoS ONE 2016, 11, e0155332. [Google Scholar] [CrossRef] [PubMed]
- Millán-Leiva, A.; Marín, Ó.; De la Rúa, P.; Muñoz, I.; Tsagkarakou, A.; Eversol, H.; Christmon, K.; Vanengelsdorp, D.; González-Cabrera, J. Mutations associated with pyrethroid resistance in the honey bee parasite Varroa destructor evolved as a series of parallel and sequential events. J. Pest Sci. 2021, 94, 1505–1517. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, C.S.; Moreno-Martí, S.; Almecija, G.; Christmon, K.; Johnson, J.D.; Ventelon, M.; Vanengelsdorp, D.; Cook, S.C.; González-Cabrera, J. Resistance to amitraz in the parasitic honey bee mite Varroa destructor is associated with mutations in the β-adrenergic-like octopamine receptor. J. Pest Sci. 2022, 95, 1179–1195. [Google Scholar] [CrossRef]
- Brodschneider, R.; Schlagbauer, J.; Arakelyan, I.; Ballis, A.; Brus, J.; Brusbardis, V.; Cadahía, L.; Charrière, J.-D.; Chlebo, R.; Coffey, M.F.; et al. Spatial clusters of Varroa destructor control strategies in Europe. J. Pest Sci. 2023, 96, 759–783. [Google Scholar] [CrossRef]
- Apivar—Sachet de 10 Lanières—VETO PHARMA|France Véto. Available online: https://france-veto.com/produit/apivar-sachet-de-10-lanieres-veto-pharma/ (accessed on 26 January 2024).
- Code International de Conduite Pour la Distribution et l’utilisation des Pesticides (Version Révisée): (Version Adoptée lors de la Cent Vingt-troisième Session du Conseil de la FAO en Novembre 2002); FAO: Rome, Italy, 2003.
- Almecija, G.; Poirot, B.; Ventelon, M.; Suppo, C. Modelling the impact of Apivar treatment on a Varroa mite population and the influence of resistance. Pest Manag. Sci. 2021, 78, ps.6698. [Google Scholar] [CrossRef] [PubMed]
- Calis, J.N.; Fries, I.; Ryrie, S.C. Population modelling of Varroa jacobsoni Oud. Apidologie 1999, 30, 111–124. [Google Scholar] [CrossRef]
- RCP. Available online: https://www.ircp.anmv.anses.fr/rcp.aspx?NomMedicament=APIVAR+LANIERES+POUR+RUCHES+A+500+MG+D%27AMITRAZ (accessed on 24 January 2024).
- Veterinary Medicinal Products Controlling Varroa Destructor Parasitosis in Bees—Scientific Guideline|European Medicines Agency. [En Ligne]. Available online: https://www.ema.europa.eu/en/veterinary-medicinal-products-controlling-varroa-destructor-parasitosis-bees-scientific-guideline (accessed on 26 January 2024).
- Gracia, M.J.; Moreno, C.; Ferrer, M.; Sanz, A.; Peribáñez, M.; Estrada, R. Field efficacy of acaricides against Varroa destructor. PLoS ONE 2017, 12, e0171633. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.J. The role of Varroa and viral pathogens in the collapse of honeybee colonies: A modelling approach. J. Appl. Ecol. 2001, 38, 1082–1093. [Google Scholar] [CrossRef]
- Bianchi, M.; Barré, N.; Messad, S. Factors related to cattle infestation level and resistance to acaricides in Boophilus microplus tick populations in New Caledonia. Veter-Parasitol. 2003, 112, 75–89. [Google Scholar] [CrossRef]
- Brattsten, L.B.; Holyoke, C.W.; Leeper, J.R.; Raffa, K.F. Insecticide resistance: Challenge to pest management and basic research. Science 1986, 231, 1255–1260. [Google Scholar] [CrossRef]
- Rinkevich, F.D.; Moreno-Martí, S.; Hernández-Rodríguez, C.S.; González-Cabrera, J. Confirmation of the Y215H mutation in the β2-octopamine receptor in Varroa destructor is associated with contemporary cases of amitraz resistance in the United States. Pest Manag. Sci. 2023, 79, 2840–2845. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).