Abstract
Acanthamoeba keratitis (AK) is a rare cornea disease caused by species of the Acanthamoeba genus. The antifungal voriconazole blocks the ergosterol synthesis in the protozoan membrane and is active against the cysts and trophozoites of Acanthamoeba spp. Due to the low stability of voriconazole, its options for eye drops are scarce. This study aimed to investigate the stability of the biological activity of voriconazole against two strains of Acanthamoeba castellanii and one clinical isolate from a patient with AK. To evaluate the stability of the biological activity of voriconazole, strains of A. castellanii (ATCC 50492) were exposed to different periods and voriconazole concentrations stored at 4 °C for 7, 15, and 30 days. The cytotoxicity assays were performed using SIRC (ATCC CCL-60™) cell line. The results indicated the amoebicidal effect of voriconazole against Acanthamoeba spp. within 24 h and 48 h of exposure, and the voriconazole solution was stable and retained antiamoebic activity when stored at 4 °C for up to 30 days. In the cytotoxicity test, the result demonstrated low cytotoxicity of the drug to the corneal rabbit cell line. However, there is a need to carry out further synergistic effects with other antiamoebic drugs and then in vivo experiments in the AK animal model.
1. Introduction
Free-living amoeba of the genus Acanthamoeba are the etiological agents of Acanthamoeba keratitis (AK), an inflammation of the cornea []. Described as an ocular pathogen in the 1970s, these protozoan presents two forms in its life cycle: the metabolically active trophozoite form, capable of reproducing, feeding, and invading host cells, and the cyst form, known to be highly resistant and efficient in resisting extreme adverse conditions [,]. AK affects individuals with previous corneal injury and is characterized by gradual vision loss, usually affecting one eye. Approximately 90% of AK cases relate to using contact lenses (CL) and the improper cleaning and storage of the lenses and their cases []. The symptoms are related to corneal invasion and degradation, generating photophobia, tearing, opacity, redness, and swelling of the eyelids. Almost 85% of AK patients experience impairment of vision, followed by ocular pain, redness, excessive tearing, and a white spot on the cornea []. A ring infiltrate is the most typical sign observed in AK cases []. AK initiates through the adhesion of Acanthamoeba spp. trophozoites on the surface of the corneal tissue, followed by the production of hydrolytic enzymes degrading the extracellular matrix with subsequent cell death [,]. Protocols for treating AK are based on the combination of antimicrobials, which can provide synergistic effects []. Currently, polyhexamethylene biguanide (PHMB) 0.02–0.08% and chlorhexidine digluconate (CLX) 0.02–0.06% represent the first line of treatment, especially against trophozoites; hexamidine (Desomedine®) 0.1%, propamidine isethionate (Brolene®) 0.1% are active against Acanthamoeba spp. trophozoites and cysts [,]. Other drugs are also used in combination: antibiotics (aminoglycosides, neomycin) and antifungals (azoles, itraconazole, voriconazole, amphotericin B) [,,]. The resistance of Acanthamoeba cysts to antimicrobials is the most difficult in treating AK, as they can remain encysted and return to the trophozoite form after using therapies [,,,,,,,]. In addition, the prolonged treatment time causes side effects triggered by drug toxicity, making it difficult to regenerate corneal tissue, causing complications such as cataracts, iris atrophy, and corneal ulceration []. Treatment may last several months to prevent reinfection and is usually given topically every hour for 3 to 4 weeks []. If the treatment fails, the infection may progress to corneal ulceration and permanent blindness, and keratoplasty may be recommended []. Azole derivatives are widely used by the topical ocular route to treat infectious keratitis [,]. Like other triazole antifungals, voriconazole inhibits the demethylation of 14-alpha-lanosterol, impairing the synthesis of ergosterol, leading to abnormalities in the cell wall of fungi and protozoa such as Acanthamoeba spp. []. The voriconazole demonstrated amoebicidal activity against Acanthamoeba strains, reaching adequate concentrations for ocular use []. The scarcity of therapeutic ocular formulations of voriconazole leads to the reformulations of the intravenous preparations available, using a lyophilized powder for injection diluted in biocompatible ocular vehicles []. Due to the few options of standardized specific therapies that act against both life forms of Acanthamoeba spp. and the promising data in the literature for using voriconazole to treat AK, this study aimed to evaluate the in vitro amoebicidal stability of free voriconazole against a strain of Acanthamoeba castellanii to the application in the treatment of amoebic keratitis.
2. Results
2.1. Activity of Voriconazole against Trophozoites of Acanthamoeba spp.
To evaluate the biological activity of voriconazole, two strains of A. castellanii and a regional clinical isolate were tested against different concentrations of the antifungal. In macroscopic analysis 6 and 12 h after the treatment with voriconazole, it was possible to observe rounding of the trophozoitic forms, increasing its vacuolization and granularity. The 24 h period was selected to count viable cells, as changes in the membrane, presence of cell debris, and cell lysis were observed.
The viability of trophozoites after 24 h of incubation at 0.7 μM was 86.1 ± 8.6% for A. castellanii Neff strains (ATCC30010), 82.8 ± 7.7% for the A. castellanii T4 (ATCC 50492), and 72.7 ± 5.2% for the clinical isolate AM. At a concentration of 35.8 μM of voriconazole, the viabilities were 58.9 ± 13.2%, 44.2 ± 9.4%, and 48.6 ± 14.1%, respectively. Figure 1A demonstrates the amoebic viabilities after 24 h. The IC50 value found for A. castellanii T4 (ATCC50492) was 13.5 µM. For the A. castellanii Neff strain (ATCC30010) and the clinical isolate AM T4, it was not possible to calculate the IC50 within 24 h of exposure to the drug.
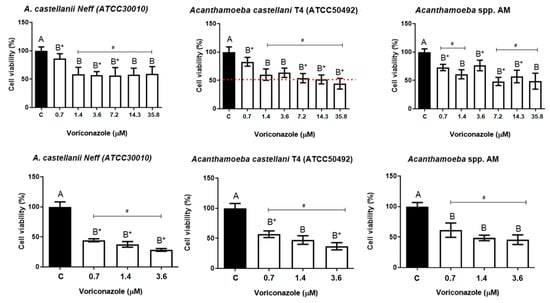
Figure 1.
Amoebicidal activity of voriconazole against Acanthamoeba spp. (A) Viability assays for the trophozoite strain A. castellanii Neff (ATCC 30010), A. castellanii T4 (ATCC 50492), and the regional clinical isolate AM T4 after 24-h incubation with voriconazole; (B) Viability assays of trophozoite strain A. castellanii Neff (ATCC 30010), A. castellanii T4 (ATCC 50492), and the regional clinical isolate AM, after 48 h of incubation with voriconazole. The results correspond to the mean ± SEM of three independent experiments in triplicate. # Value of p < 0.05 compared to the negative control (DMSO 0.125%) and test concentrations of voriconazole with * p < 0.05 compared to each other (Kruskall-Wallis post-test). The dashed line indicates the IC50 calculated through linear regression.
The amoebic viability was evaluated in 48 h at three concentrations (0.7 µM, 1.4 µM, and 3.6 μM). At the lowest concentration tested, the trophozoites viability were 44.4 ± 2.6%, 56.8 ± 5.7%, and 61.2 ± 9.3% for the A. castellanii Neff, A. castellanii T4, and AM regional clinical isolate, respectively. At 3.6 μM, it was observed a decrease in viability with an average of 28.4 ± 2.3%, 36.8 ± 5.7%, and 45.7 ± 8.0% for the A. castellanii Neff, A. castellanii T4, and AM regional clinical isolate, respectively. Figure 1B shows the viability results after 48 h of incubation with voriconazole. Again, rounded and hyper-vacuolized trophozoites were observed in the analyzed period without forming cysts.
2.2. Evaluation of the Stability of Biological Activity of Voriconazole against Trophozoites of Acanthamoeba castellanii
The results demonstrated a significant decrease in the viability of trophozoites after treatment in all storage times. Figure 2A shows the viability percentage of A. castellanii T4 trophozoites (ATCC 50492) after 48 h of exposure to 1.4 μM, 3.6 μM, 7.2 μM, 14.3 μM, and 35.8 μM of voriconazole stored for 7, 15, and 30 days after reconstitution of SE1. After 7 days of storage and with 48 h of incubation, the viability values varied between 22.5 ± 4.5% and 29.8 ± 2.8%, while in 15 days, the viability was from 33.7 ± 4.5% to 40 ± 4.5%, and in 30 days the values were between 46.8 ± 2.8% and 52.4 ± 4.3%. Figure 3A presents the viability percentage of A. castellanii T4 trophozoites (ATCC 50492) within 72 h of incubation. The results demonstrate a decrease in the biological activity of voriconazole with trophozoite viability values ranging from 4.2 ± 1.7 and 6.5 ± 1.2% observed 7 days after preparing SE1, a decrease of 6.8 ± 1.5–11.2 ± 1.3% in 15 days after preparing the SE1, and 17.8 ± 1.6–25.0 ± 2.2% after 30 days. Micrographs demonstrate the decrease in trophozoite viability at different voriconazole concentrations (Figure 2B and Figure 3B).
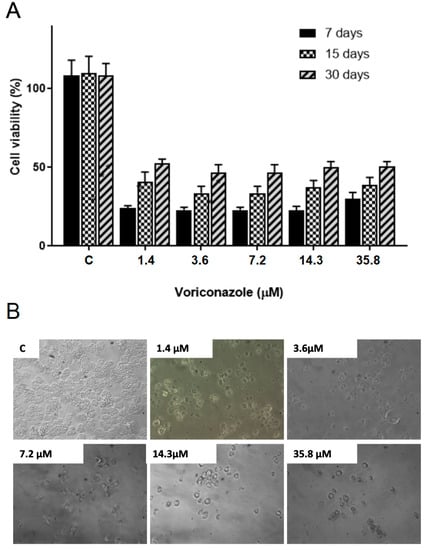
Figure 2.
Evaluation of the stability of the biological activity of voriconazole against Acanthamoeba castellanii T4 trophozoites. (A) Viability for the A. castellanii T4 trophozoite strain (ATCC 50492) after incubation for 48 h with free voriconazole stored for 7, 15, and 30 days. The results correspond to the mean ± SEM of the mean of three independent experiments. * p-value < 0.05 compared to the negative control (Kruskall-Wallis post-test). (B) Micrographs demonstrate the decrease in viability at different voriconazole concentrations. Images at 200× magnification.
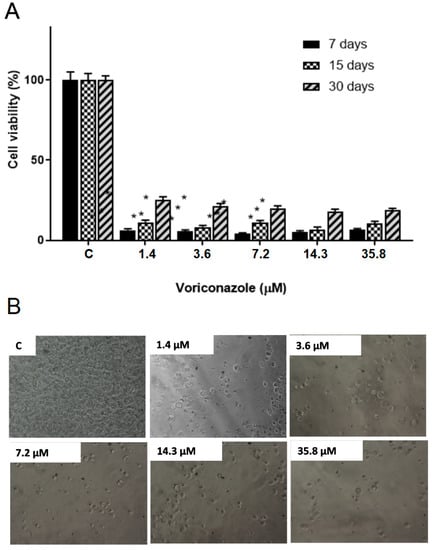
Figure 3.
Evaluation of the stability of the biological activity of voriconazole against Acanthamoeba castellanii T4 trophozoites. (A) Viability for the A. castellanii T4 trophozoite strain (ATCC 50492) after incubation for 72 h with free voriconazole stored for 7, 15, and 30 days. * p-value < 0.05 compared to the negative control (Kruskall-Wallis post-test). The results correspond to the mean ± SEM of the mean of three independent experiments. (B) Micrographs demonstrate the decrease in viability at different voriconazole concentrations. Images at 200× magnification.
2.3. Activity of Voriconazole against Cysts of Acanthamoeba castellanii
The cysticidal activity of voriconazole was evaluated against cysts of the clinical isolate AM T4 at concentrations of 0.7–35.8 µM in a 24 h period. Figure 4 presents the percentage of cyst viability compared to the negative control (DMSO 0.125%). The mean viability obtained demonstrates the performance of voriconazole against the cysts of the regional clinical isolate AM concerning the negative control in all test concentrations with values of 57.3% ± 11.3%, 62.2% ± 9.4%, 55.6% ± 10.1%, 55.8 5 ± 8.2%, 60.7% ± 8.8% and 64.3% ± 9.2% respectively, demonstrating a plateau in drug activity (p ≤ 0.05).
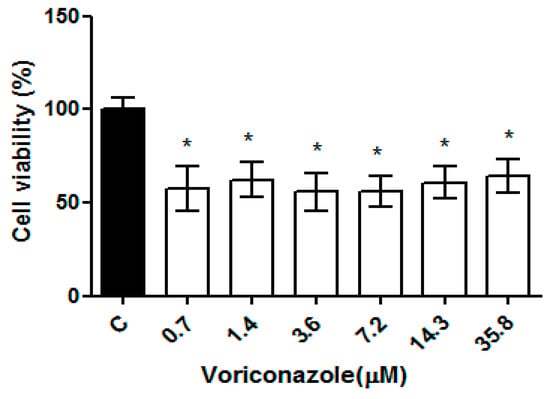
Figure 4.
Evaluation of the cysticidal activity of voriconazole against Acanthamoeba spp. Viability of cysts of the regional clinical isolate AM T4 of Acanthamoeba spp. after 24 h of incubation with voriconazole. The results correspond to the mean ± standard error of the mean of three independent experiments in triplicate. * p-value < 0.05 compared to the negative control (Kruskall-Wallis post-test).
2.4. Evaluation of Voriconazole Cytotoxicity against SIRC Cells
The cytotoxicity of voriconazole was evaluated against the SIRC cell line (Statens Seruminstitut Rabbit Cornea) (ATCC®–CCL 60) after 24 h of exposure in concentrations ranging from 0.7 to 35.8 µM, as demonstrated in Figure 5. The average cell viability was 74%. At the lowest tested concentration (0.7 µM), 85.35 ± 3.07, while at 35.8 µM, the average viability (%) was 74.33 ± 1.41. The negative control of 0.125% DMSO proved a little toxic to SIRC cells, with a viability (%) of 90.69 ± 0.60. The biguanides used as positive controls, 0.02% chlorhexidine and 0.02% PHMB, promoted 100% cell death.
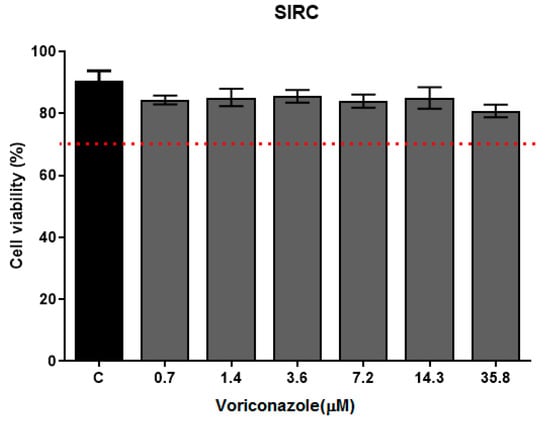
Figure 5.
Cell viability of SIRC after voriconazole treatment. Cytotoxicity assay with SIRC cells (Statens Seruminstitut Rabbit Cornea) (ATCC®–CCL 60) in exposure to voriconazole for 24 h followed by the addition of MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide) 0.5 mg/mL. The results correspond to the mean ± standard error of the mean of three independent experiments in triplicate. * p-value < 0.05, compared to the negative control—DMSO 0.125% (Kruskall-Wallis post-test). The dashed line indicates 70% viability.
3. Discussion
Drug repositioning is vital in the search for a safe and efficient treatment for AK. Especially due to the lack of specific therapies associated with high resistance of the cystic life form of Acanthamoeba spp. and the high toxicity of drugs used in the therapy of amoebic keratitis [,,,,]. The triazole antifungal voriconazole acts in the ergosterol synthesis, causing cell damage []. Several studies have reported the voriconazole efficiency in causing cell death in Acanthamoeba spp. [,,]. Martín-Navarro’s (2015) study demonstrated that voriconazole induced cell death in A. castellanii by non-necrotic mechanisms, making this a promising drug to fight AK []. Commercially, voriconazole is available for oral and intravenous administration (IV) []. To our knowledge, only one formulation for ocular use contains voriconazole as a drug. Vozole® (Aurolab) is sold as a lyophilized powder at a concentration of 0.1% w/v solution and is indicated to be used as an intravitreal injection. Still, preparations using the lyophilized powder of voriconazole diluted in water for injection, or lacrimal solution, are widely used in ophthalmologic clinics [].
The present work investigated the biological activity of voriconazole with ≥98% purity against Acanthamoeba spp. to evaluate the in vitro amoebicidal stability against strains of A. castellanii and clinical isolate T4 genotype Acanthamoeba spp., to the application in the treatment of amoebic keratitis. The results indicate a time-dependent action of the drug, as it was observed that the amoebic viability varied between 24 h and 48 h of incubation, with a greater decrease in viability with a longer period of incubation time. Likewise, other studies have presented similar results. Cabello-Vílchez et al. (2014) evaluated the amoebicidal activity of voriconazole [], and in 96 h of treatment, the IC50 calculated was approximately 7.7 μM. A minimum inhibitory concentration (MIC) of 1 to 2 mg/L after 48 h of incubation of the strains of Acanthamoeba polyphaga and A. castellanii confirmed the effective inhibition of trophozoite proliferation.
In the viability tests of voriconazole against trophozoites, it was observed that voriconazole did not induce encystment. This is an essential feature for the treatment of AK. The encystment process in Acanthamoeba enables the protozoan to survive disinfection by heat, chlorination, and treatment with different drugs [,,]. For the patient, encystment may signify a recrudescence of the infection. The amoeba may encyst in ocular tissue, resist drug therapies, and lead to recurrent keratitis [].
To evaluate whether the solution stored at a temperature of ±4 °C maintained its activity constant, tests were conducted to analyze amoeba viability after 7, 15, and 30 days of storage. The previous drug freezing did not result in a loss of pharmacological activity since the voriconazole remained active for up to 30 days. Some studies with voriconazole demonstrate data on its stability [,,,]. Isla Tejera et al. demonstrated that voriconazole was solubilized in water for injection and kept at 2–8 °C and 22–24 °C maintained its physicochemical characteristics for 30 days of storage []. However, the solution lost its biological activity after 3 weeks of preparation. Conversely, Amóros-Reboreo et al. (2005) reported that even after 90 days stored at −20 °C, a solution containing 1% voriconazole was stable and retained antifungal activity [].
Tests to assess anti-cystic activity using Acanthamoeba spp. of the clinical isolate AM T4 were performed in the present study. Despite little incubation time and the low concentrations tested, there was a significant reduction in the number of cysts, but with the limited cystic activity of voriconazole. The result observed is due to the low metabolic activity of the cyst, which is in a latent state, as voriconazole has no direct action on membranes and cell wall of the protozoan but acts by inhibiting the synthesis of ergosterol in the active phase of formation of the trophozoite membranes and the cyst wall [].
SIRC cells have been used to assess the cytotoxicity of chemical substances that induce lesions in eyes that do not cause irritation, according to the Eye Irritation or Serious Eye Damage test (Oecd Guidelines for The Testing Of Chemicals, Section 4, 27 June 2018. In the cytotoxicity test, despite the 24-h exposure to the treatment, the result demonstrated cell viability greater than 70%, confirming the low toxicity of the drug to the corneal tissue, bringing greater safety to continue with research. Studies have already reported the amoebicidal activity of voriconazole, demonstrating that the therapy is well tolerated and capable of treating infectious agents that cause keratitis with a low potential risk of adverse effects [], being a promising alternative for the topical ocular use for the treatment of AK.
4. Materials and Methods
4.1. Preparation of Voriconazole
Voriconazole (≥98%, HPLC: Sigma-Aldrich) was reconstituted in dimethylsulfoxide (DMSO—P.A Neon, Brazil) at a final concentration of 2 mg/mL, called stock solution 1 (SE1). SE1 was stored in 100 µL aliquots at −80 °C, according to instructions described by the manufacturer. Samples were stored at 4 °C and protected from light to perform stability tests. One batch was analyzed immediately after preparation, and other batches were stored at −20 °C until 30 days to test activity antiamoebic stability.
4.2. Amoebicidal Activity Experiments
4.2.1. Culture of Acanthamoeba spp.
The amoebicidal activity tests were performed with A. castellanii Neff (ATCC 30010), A. castellanii (ATCC 50492), and the regional clinical isolate AM T4 genotype characterized at LADIPE—Research Laboratory Applied to Emerging Protozoa []. Trophozoites were cultivated axenically in PYG medium [0.75% (w/v) protease peptone, 0.75% (w/v) yeast extract, and 1.5% (w/v) glucose with penicillin-streptomycin antibiotics 400 UI/mL (each)] and incubated at 30 °C. To obtain the cysts, 5 × 105 trophozoites/mL of the clinical isolate AM were incubated for 72 h in an encystment medium (95 mM NaCl, 5 mM KCl, 8 mM MgSO4, 0.4 mM CaCl2, 1 mM NaHCO3, 20 mM Tris-HCl, pH 9.0) []. Afterward, the encystment medium was removed, and a 0.5% SDS solution was added for 5 min for the lysis of immature cysts and trophozoites still present.
4.2.2. Activity of Voriconazole against Trophozoites of Acanthamoeba spp.
To evaluate the biological activity of voriconazole against Acanthamoeba spp., 8 × 104 trophozoites/mL in PYG medium of A. castellanii Neff (ATCC 30010), A. castellanii T4 (ATCC 50492), and the clinical isolate AM were aliquot in 96-well plates. Voriconazole were tested at concentrations of 0.7 µM, 1.4 µM, 3.6 µM, 7.2 µM, 14.3 µM, and 35.8 µM. The plates were incubated at 30 °C for 24 and 48 h, with an observation of the growth kinetics at 6, 12, 24, and 48 h of incubation. As a negative control, 0.125% DMSO solution in PYG was used. Viable cells were quantified using the Fuchs–Rosenthal counting chamber, and the cell viability was analyzed using 0.4% trypan blue. The experiments were performed in independent triplicates. The results obtained were expressed as a percentage of viable trophozoites.
4.2.3. Activity of Voriconazole against Trophozoites of Acanthamoeba castellanii after Storage of Voriconazole
The stability of biological activity of voriconazole after 7, 15, and 30 days of preparation of SE1 were evaluated by aliquoting 8 × 104 trophozoites/mL of A. castellanii T4 (ATCC 50492) in 96-well plates. Concentrations of 0.7 µM, 1.4 µM, 3.6 µM, 7.2 µM, 14.3 µM, and 35.8 µM of voriconazole were tested within 48 and 72 h of incubation at 30 °C. As a negative control, a 0.125% DMSO solution in PYG was evaluated. Viable cells were quantified using the Fuchs–Rosenthal counting chamber, and the cell viability was analyzed using 0.4% trypan blue. The experiments were performed in independent triplicates. The results obtained were expressed as a percentage of viable trophozoites.
4.2.4. Activity of Voriconazole against Cysts of Acanthamoeba castellanii
The biological activity of voriconazole was tested against the clinical isolate AM, T4 genotype. To obtain the cysts, 5 × 105 trophozoites/mL of the isolate AM were incubated in a Tris-HCl encystment medium. After the encysting medium was removed, a 0.5% SDS solution was added to lyse the immature cysts and trophozoites still present. The cysts were suspended in PBS and inoculated into 96-well plates in 8 × 104 cysts/mL density. Concentrations of 1.4 µM, 7.2 µM, 14.3 µM, and 35.8 µM of voriconazole were tested within 24 h of incubation at 30 °C. As a negative control, PBS with 0.125% DMSO was evaluated, with 0.02% of CLX and 0.02% of PHMB as the positive control. Cyst viability was determined by hemocytometer count using trypan blue viability dye.
4.3. Evaluation of Voriconazole Cytotoxicity against SIRC Cells
To assess ocular toxicity, SIRC cells (SIRC; Statens Seruminstitut Rabbit Cornea, ATCC®CCL-60™) were cultivated in DMEM medium supplemented in 10% with fetal bovine serum (FBS) and incubated in an atmosphere of 5% CO2 at 37 °C. The 5 × 105 cells/well concentration was cultivated in 96-well plates and incubated for 24 h. After, the medium was removed, and the treatments were added. The concentrations of voriconazole tested were 0.7 µM, 1.4 µM, 3.6 µM, 7.2 µM, 14.3 µM, and 35.8 µM. The following solutions were used as controls: DMSO 0.125% as the negative control. All treatments were in contact with the cells for 24 h, followed by adding 0.5 mg/mL MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide/Sigma-Aldrich) for 2 h. After the MTT was removed and DMSO was added for the solubilization of the MTT reduction product to be revealed. The reading was performed in an ELX800 spectrophotometer (BIOTEK®, USA) at a wavelength of 540 nm. The percentage of cell viability was calculated to the negative control (100% viability). The experiments were performed in triplicate.
4.4. Statistical Analysis
Statistical analyses were performed using Graph Pad Prism (La Jolla, CA, USA) for analysis of variance (ANOVA) with a comparison of means, followed by the Kruskal-Wallis post-test. The results obtained in the antiamoebic and cytotoxicity tests were expressed as percent viability, mean ± standard error of the mean (SEM) for at least 3 replicates, and p-values less than 0.05 were considered significant.
5. Conclusions
The voriconazole solution prepared was stable and retained antiamoebic activity when stored at 4 °C for 30 days, and well tolerated due to the low cytotoxic potential to corneal cells, concluding that the voriconazole can be used for topical ocular formulation therapy and with a low potential risk of causing adverse effects. Voriconazole has the potential for use in formulations composed with potent antiamoebics, improving tolerance and for use in cases of polymicrobial infections by Acanthamoeba spp. and fungi.
Author Contributions
Conceptualization, K.S.C., B.C.R. and F.B.F.-M.; methodology, B.C.R. and C.d.J.d.C.; resources, F.B.F.-M. and K.S.C.; data curation, B.C.R., C.d.J.d.C. and M.L.C.B.; writing—original draft preparation, B.C.R. and M.L.C.B.; writing—review and editing, M.L.C.B., F.B.F.-M. and K.S.C.; supervision, K.S.C.; project administration, K.S.C.; funding acquisition, K.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The National Council for Scientific and Technological Development (CNPq)-Universal MCTI/CNPq No. 432566/2016-3.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES) and The National Council for Scientific and Technological Development (CNPq) (Universal MCTI/CNPq No. 432566/2016-3). The authors thank the Marcos José Machado for assistance in the statistical analysis of the data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lorenzo-Morales, J.; Khan, N.A.; Walochnik, J. An update on Acanthamoeba keratitis: Diagnosis, pathogenesis and treatment. Parasite 2015, 22, 10. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.K.; Sharma, P.; Shyam, K.; Tejan, N.; Ghoshal, U. Acanthamoeba and its pathogenic role in granulomatous amebic encephalitis. Exp. Parasitol. 2020, 208, 107788. [Google Scholar] [CrossRef] [PubMed]
- Marciano-Cabral, F.; Cabral, G. Acanthamoeba spp. as Agents of Disease in Humans. Clin. Microbiol. Rev. 2003, 16, 273–307. [Google Scholar] [CrossRef]
- Buchele, M.L.C.; Wopereis, D.B.; Casara, F.; de Macedo, J.P.; Rott, M.B.; Monteiro, F.B.F.; Bazzo, M.L.; Spada, F.D.R.; dos Santos, J.I.; Caumo, K.S. Contact lens-related polymicrobial keratitis: Acanthamoeba spp. genotype T4 and Candida albicans. Parasitol. Res. 2018, 117, 3431–3436. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, J.; Lalitha, P.; Prajna, N.V.; Srinivasan, M.; Das, M.; D’Silva, S.S.; Oldenburg, C.E.; Borkar, D.S.; Esterberg, E.J.; Lietman, T.M. Acanthamoeba, Fungal, and Bacterial Keratitis: A comparison of risk factors and clinical features. Am. J. Ophthalmol. 2014, 157, 56–62. [Google Scholar] [CrossRef]
- Varacalli, G.; di Zazzo, A.; Mori, T.; Dohlman, T.H.; Spelta, S.; Coassin, M.; Bonini, S. Challenges in Acanthamoeba Keratitis: A review. J. Clin. Med. 2021, 10, 942. [Google Scholar] [CrossRef]
- De Lacerda, A.G.; Lira, M. Acanthamoeba keratitis: A review of biology, pathophysiology and epidemiology. Ophthalmic Physiol. Opt. 2021, 41, 116–135. [Google Scholar] [CrossRef]
- Fanselow, N.; Sirajuddin, N.; Yin, X.-T.; Huang, A.; Stuart, P. Keratitis, Pathology, Diagnosis and Treatment. Pathogens 2021, 10, 323. [Google Scholar] [CrossRef]
- Elsheikha, H.M.; Siddiqui, R.; Khan, N.A. Drug Discovery against. Pathogens 2020, 9, 405. [Google Scholar] [CrossRef]
- Trabelsi, H.; Dendana, F.; Sellami, A.; Cheikhrouhou, F.; Neji, S.; Makni, F.; Ayadi, A. Pathogenic free-living amoebae: Epidemiology and clinical review. Pathol. Biol. 2012, 60, 399–405. [Google Scholar] [CrossRef]
- Thomson, S.; Rice, C.A.; Zhang, T.; Edrada-Ebel, R.; Henriquez, F.L.; Roberts, C.W. Characterisation of sterol biosynthesis and validation of 14α-demethylase as a drug target in Acanthamoeba. Sci. Rep. 2017, 7, 8247. [Google Scholar] [CrossRef] [PubMed]
- Gueudry, J.; Le Goff, L.; Compagnon, P.; Lefèvre, S.; Colasse, E.; Aknine, C.; Duval, F.; François, A.; Razakandrainibe, R.; Ballet, J.J.; et al. Evaluation of voriconazole anti-Acanthamoeba polyphaga in vitro activity, rat cornea penetration and efficacy against experimental rat Acanthamoeba keratitis. J. Antimicrob. Chemother. 2018, 73, 1895–1898. [Google Scholar] [CrossRef] [PubMed]
- Rice, C.A.; Colon, B.L.; Chen, E.; Hull, M.V.; Kyle, D.E. Discovery of repurposing drug candidates for the treatment of diseases caused by pathogenic free-living amoebae. PLoS Negl. Trop. Dis. 2020, 14, e0008353. [Google Scholar] [CrossRef]
- Maycock, N.J.R.; Jayaswal, R. Update on Acanthamoeba Keratitis: Diagnosis, Treatment, and Outcomes. Cornea 2016, 35, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Carrijo-Carvalho, L.C.; Sant’Ana, V.P.; Foronda, A.S.; De Freitas, D.; de Souza Carvalho, F.R. Therapeutic agents and biocides for ocular infections by free-living amoebae of Acanthamoeba genus. Surv. Ophthalmol. 2017, 62, 203–218. [Google Scholar] [CrossRef]
- Saeed, A.; Arcy, F.D.; Stack, J.; Collum, L.M.; Power, W.; Beatty, S. Risk factors, microbiological findings, and clinical outcomes in cases of microbial keratitis admitted to a tertiary referral center in ireland. Cornea 2009, 28, 285–292. [Google Scholar] [CrossRef]
- Nakaminami, H.; Tanuma, K.; Enomoto, K.; Yoshimura, Y.; Onuki, T.; Nihonyanagi, S.; Hamada, Y.; Noguchi, N. Evaluation of In Vitro Antiamoebic Activity of Antimicrobial Agents Against Clinical Acanthamoeba Isolates. J. Ocul. Pharmacol. Ther. 2017, 33, 629–634. [Google Scholar] [CrossRef]
- Díaz-Tomé, V.; García-Otero, X.; Varela-Fernández, R.; Martín-Pastor, M.; Conde-Penedo, A.; Aguiar, P.; González-Barcia, M.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. In situ forming and mucoadhesive ophthalmic voriconazole/HPβCD hydrogels for the treatment of fungal keratitis. Int. J. Pharm. 2021, 597, 120318. [Google Scholar] [CrossRef]
- Martín-Navarro, C.M.; López-Arencibia, A.; Sifaoui, I.; Reyes-Batlle, M.; Valladares, B.; Martínez-Carretero, E.; Piñero, J.E.; Maciver, S.K.; Lorenzo-Morales, J. Statins and voriconazole induce programmed cell death in Acanthamoeba castellanii. Antimicrob. Agents Chemother. 2015, 59, 2817–2824. [Google Scholar] [CrossRef]
- Mm, H.; Badami, A.; Vokuda, H.; Venkatachalam, K. An Update on Voriconazole in Ophthalmology. Off. Sci. J. Delhi Ophthalmol. Soc. 2016, 27, 9–15. [Google Scholar] [CrossRef]
- Cabello-Vílchez, A.M.; Martín-Navarro, C.M.; López-Arencibia, A.; Reyes-Batlle, M.; Sifaoui, I.; Valladares, B.; Piñero, J.E.; Lorenzo-Morales, J. Voriconazole as a first-line treatment against potentially pathogenic Acanthamoeba strains from Peru. Parasitol. Res. 2014, 113, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Iovieno, A.; Miller, D.; Ledee, D.R.; Alfonso, E.C. Cysticidal activity of antifungals against different genotypes of Acanthamoeba. Antimicrob. Agents Chemother. 2014, 58, 5626–5628. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Martínez, D.; Reyes-Batlle, M.; Castelan-Ramírez, I.; Hernández-Olmos, P.; Vanzzini-Zago, V.; Ramírez-Flores, E.; Sifaoui, I.; Piñero, J.E.; Lorenzo-Morales, J.; Omaña-Molina, M. Evaluation of the sensitivity to chlorhexidine, voriconazole and itraconazole of T4 genotype Acanthamoeba isolated from Mexico. Exp. Parasitol. 2019, 197, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, C.J.; Lynch, S.C.; Rah, M.J.; Millard, K.A.; Morris, T.W. Acanthamoeba encystment: Multifactorial effects of buffers, biocides, and demulcents present in contact lens care solutions. Clin. Ophthalmol. 2015, 9, 1905–1913. [Google Scholar] [CrossRef]
- Shing, B.; Balen, M.; McKerrow, J.H.; Debnath, A. Acanthamoeba Keratitis: An update on amebicidal and cysticidal drug screening methodologies and potential treatment with azole drugs. Expert Rev. Anti-Infect. Ther. 2021, 19, 1427–1441. [Google Scholar] [CrossRef]
- Tejera, B.I.; de Almagro, C.G.M.; Aranzana, M.C.; Rodrigo, I.P.; Rubio, M.A.; Sánchez, R.G. Estabilidad y actividad in vitro de voriconazol en colirio a una concentración de 3 μg/mL. Farm. Hosp. 2005, 29, 331–334. [Google Scholar] [CrossRef]
- Bardin, C.; Astier, A.; Vulto, A.; Sewell, G.; Vigneron, J.; Trittler, R.; Daouphars, M.; Paul, M.; Trojniak, M.; Pinguet, F. Guidelines for the practical stability studies of anticancer drugs: A European consensus conference. Ann. Pharm. Fr. 2011, 69, 221–231. [Google Scholar] [CrossRef]
- Amorós-Reboredo, P.; Bastida-Fernandez, C.; Guerrero-Molina, L.; Soy-Muner, D.; López-Cabezas, C. Stability of frozen 1% voriconazole ophthalmic solution. Am. J. Health Syst. Pharm. 2015, 72, 479–482. [Google Scholar] [CrossRef]
- Curti, C.; Lamy, E.; Primas, N.; Fersing, C.; Jean, C.; Bertault-Peres, P.; Vanelle, P. Stability studies of five anti-infectious eye drops under exhaustive storage conditions. Pharmazie 2017, 72, 741–746. [Google Scholar] [CrossRef]
- Lau, D.; Fedinands, M.; Leung, L.; Fullinfaw, R.; Kong, D.; Davies, G.; Daniell, M. Penetration of voriconazole, 1%, eyedrops into human aqueous humor: A prospective open-label study. Arch. Ophthalmol. 2008, 126, 343–346. [Google Scholar] [CrossRef]
- Song, S.-M.; Han, B.-I.; Moon, E.-K.; Lee, Y.-R.; Yu, H.S.; Jha, B.K.; Danne, D.-B.S.; Kong, H.-H.; Chung, D.-I.; Hong, Y. Autophagy protein 16-mediated autophagy is required for the encystation of Acanthamoeba castellanii. Mol. Biochem. Parasitol. 2012, 183, 158–165. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).