The Diagnosis, Treatment, Surveillance and Control of Cystic Echinococcosis in the Province of Rio Negro: The “One-Health” Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Work Area
2.2. Primary Prevention
2.3. Secondary Prevention
2.4. Tertiary Prevention
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larrieu, E.; Gavidia, C.M.; Lightowlers, M.W. Control of cystic echinococcosis: Background and prospects. Zoonoses Public Health 2019, 66, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Budke, C.M.; Deplazes, P.; Torgerson, P.R. Global socioeconomic impact of cystic echinococcosis. Emerg. Infect. Dis. 2006, 2, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Eckert, J.; Gemmel, M.A.; Meslin, F.X.; Pawlowski, Z.S. WHO/OIE Manual on Echinococcosis in Humans and Animals: A Public Health Problem of Global Concern; WHO/FAO: Paris, France, 2001; p. 265. [Google Scholar]
- Craig, P.S.; Hegglin, D.; Lightowlers, M.W.; Torgerson, P.R.; Wang, Q. Echinococcosis: Control and Prevention. Adv. Parasitol. 2017, 96, 55–158. [Google Scholar]
- Pavletic, C.F.; Larrieu, E.; Guarnera, E.A.; Casas, N.; Irabedra, P.; Ferreira, C.; Sayes, J.; Gavidia, C.M.; Caldas, E.; Zini Lise, M.; et al. Cystic echinococcosis in America: A call for action. Rev. Panam. Salud Publica 2017, 21, 41–42. [Google Scholar]
- Uchiumi, L.; Mujica, G.; Araya, D.; Salvitti, J.C.; Sobrino, M.; Moguillansky, S.; Solari, A.; Blanco, P.; Barrera, F.; Lamunier, J.; et al. Prevalence of human cystic echinococcosis in the towns of Ñorquinco and Ramos Mexia in Rio Negro Province, Argentina, and direct risk factors for infection. Parasit. Vectors 2021, 19, 262. [Google Scholar] [CrossRef]
- Marcos, E. The One Health Concept as Integrator of the Interface Human-Animal-Environmental, Against Diseases Emerging, Reemerging and Cross-Border. Epidemiol. Salud. 2013, 1, 16–20. [Google Scholar]
- FAO; OIE; OMS. A Tripartite Guide to Addressing Zoonotic Diseases in Countries. 2019. Available online: https://www.oie.int/fileadmin/Home/eng/Media_Center/docs/EN_TripartiteZoonosesGuide_webversion.pdf (accessed on 18 July 2021).
- Laing, G.; Vigilato, M.A.N.; Cleaveland, S.; Thumbi, S.M.; Blumberg, L.; Salahuddin, N.; Abdela-Ridder, B.; Harrison, W. One Health for neglected tropical diseases. Trans. R. Soc. Trop. Med. Hyg. 2021, 28, 182–184. [Google Scholar] [CrossRef]
- Larrieu, E.; Costa, M.T.; Cantoni, G.; Labanchi, J.L.; Bigatti, R.; Pérez, A.; Araya, D.; Mancini, S.; Herrero, M.E.; Talmon, G.; et al. Control program of hydatid disease in the province of Río Negro Argentina 1980–1997. Bol. Chil. Parasitol. 2000, 55, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Larrieu, E.; Uchiumi, L.; Salvitti, J.C.; Sobrino, M.; Panomarenko, O.; Tissot, H.; Mercapide, C.H.; Sustercic, J.; Arezo, M.; Mujica, G.; et al. Epidemiology, diagnosis, treatment and follow up of cystic echinococcosis in asymptomatic carriers. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Arezo, M.; Mujica, G.; Uchiumi, L.; Santillán, G.; Herrero, E.; Labanchi, J.L.; Araya, D.; Salvitti, J.C.; Cabrera, M.; Grizmado, C.; et al. Identification of potential ′hot spots′ of cystic echinococcosis transmission in the province of Río Negro, Argentina. Acta Trop. 2020, 204, 105341. [Google Scholar] [CrossRef]
- Vignolo, J.; Vacarezza, M.; Alvarez, C.; Sosa, A. Levels of care, prevention and primary health care. Arch. Med. 2011, 33, 1. [Google Scholar]
- Leavell, H.R.; Clark, E.G. Preventive Medicine for the Doctor in His Community; McGraw-Hill Book Company: New York, NY, USA, 1965. [Google Scholar]
- Larrieu, E.; Mujica, G.; Araya, D.; Labanchi, J.L.; Arezo, M.; Herrero, E.; Santillán, G.; Vizcaychipi, K.; Uchiumi, L.; Salvitti, J.C.; et al. Pilot field trial of the EG95 vaccine against ovine cystic echinococcosis in Rio Negro, Argentina: 8 years of work. Acta Trop. 2019, 191, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.; Costa, M.T.; Cantoni, G.; Mancini, S.; Mercapide, C.; Herrero, E.; Volpe, M.; Araya, D.; Talmon, G.; Chiosso, C.; et al. Epidemiological surveillance of cystic echinococcosis in dogs, sheep farms and humans in the Rio Negro Province. Medicina 2006, 66, 193–200. [Google Scholar] [PubMed]
- Salvitti, J.C.; Sobrino, M.; Del Carpio, M.; Mercapide, C.; Uchiumi, L.; Moguilensky, J.; Moguilansky, S.; Frider, B.; Larrieu, E. Hydatidosis: Ultrasonographyc screening in the Río Negro Province 25 years after the first screening. Acta Gastroenterol. Latamer. 2014, 44, 311–315. [Google Scholar]
- Larrieu, E.; Del Carpio, M.; Mercapide, C.H.; Salvitti, J.C.; Sustercic, J.; Moguilensky, J.; Panomarenko, H.; Uchiumi, L.; Herrero, E.; Talmon, G.; et al. Programme for ultrasound diagnoses and treatment with albendazole of cystic echinococcosis in asymptomatic carriers: 10 years of follow-up of cases. Acta Trop. 2011, 117, 1–5. [Google Scholar] [CrossRef]
- Del Carpio, M.; Mercapide, C.H.; Salvitti, J.C.; Uchiumi, L.; Sustercic, J.; Panomarenko, H.; Moguilensky, J.; Herrero, E.; Talmon, G.; Volpe, M.; et al. Early diagnosis, treatment and follow-up of cystic echinococcosis in remote rural areas in Patagonia: Impact of ultrasound training of non-specialists with a focused approach on CE. PLoS. Neg. Trop. Dis. 2011, 6, e1444. [Google Scholar] [CrossRef]
- Mercapide, C.H.; Pereyra, R.; Giménez, R.; Pérez, C.; Michelena, F. Tránsitos hidatídicos abdomino torácicos. Prensa Med. Argent. 1993, 80, 300–306. [Google Scholar]
- Mercapide, C.H.; Gimenez, R.; Pereyra, R.; Perez, C.; Michelena, F. Tratamiento de la hidatidosis hepática. Prensa Med. Argent. 1994, 81, 275–281. [Google Scholar]
- Brunett, E.; Garcia, H.H.; Junghanss, T. Cystic echinococcosis: Chronic, complex, and still neglected. PLoS. Negl. Trop. Dis. 2011, 5, e1146. [Google Scholar] [CrossRef] [Green Version]
- Larrieu, E.; Frider, B.; del Carpio, M.; Salvitti, J.C.; Mercapide, C.; Pereyra, R.; Costa, M.; Odriozola, M.; Pérez, A.; Cantoni, G.; et al. Asymptomatic carriers of hydatidosis: Epidemiology, diagnosis, and treatment. Rev. Panam. Salud Publ. 2000, 8, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Larrieu, E.; Costa, M.T.; Cantoni, G.; Alvarez, J.; Pérez, A.; Giménez, N.; Giménez, R.; Odriozzola, M. Control of hydatidosis in the Province of Rio Negro, Argentina: Epidemiology. Bol. Chil. Parasitol. 1991, 46, 3–7. [Google Scholar]
- Bingham, G.M.; Larrieu, E.; Uchiumi, L.; Mercapide, C.H.; Mujica, G.; Del Carpio, M.; Hererro, E.; Salvitti, J.C.; Norby, B.; Budke, C.M. The Economic Impact of Cystic Echinococcosis in Rio Negro Province, Argentina. Am. J. Trop. Med. Hyg. 2016, 94, 615–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canali, M.; Aragrande, M.; Angheben, A.M.; Capelli, G.; Drigo, M.; Gobbi, F.; Tamarozzi, F.; Cassini, R. Epidemiologic-economic models and the One Health paradigm: Echinococcosis and leishmaniasis, case studies in Veneto region, Northeastern Italy. One Health 2019, 27, 100115. [Google Scholar] [CrossRef] [PubMed]
- Abdulhameed, M.F.; Robertson, I.D.; Al-Azizz, S.A.; Habib, I. Neglected Zoonoses and the Missing Opportunities for One Health Education: The Case of Cystic Echinococcosis among Surgically Operated Patients in Basrah, Southern Iraq. Diseases 2019, 7, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larrieu, E.; Zanini, F. Critical analysis of the strategies to control cystic echinococcosis and the use of praziquantel in South America: 1980–2009. Rev. Panam. Salud Publica 2012, 31, 73–81. [Google Scholar]

| Levels of Prevention | Actor | Action | Community Participation |
|---|---|---|---|
| Primary prevention (avoid agent entry into the body) | Teacher | Health education in schools | Proactive attitude in responsible dog |
| Sanitary agent | Health education and deworming of dogs in homes | ownership and proper disposal of viscera. | |
| Veterinarian | Dog deworming, sheep vaccination, infection surveillance in dogs and sheep, rural education | Willingness to actively participate in the Control Program (facilitation of the deworming of dogs and the vaccination of sheep) | |
| Physician First level of care | Health education in the community | ||
| Laboratory | Diagnosis of infection in dogs, sheep and the environment | ||
| Secondary prevention (early diagnosis to avoid complications generated by the agent) | Teacher | Organization of students for screening operations (abdominal ultrasound surveys) in schools | |
| Veterinarian | Organization of screening operations, surveillance in people | ||
| Physician First level of care | Organization of screening operations, execution of ultrasound surveys | Cooperation, so that children can be studied by ultrasonography | |
| Medical specialist | Confirmation of cases detected in screening | ||
| Tertiary prevention (elimination of the agent, and recovery of health) | Physician First level of care | Antiparasitic treatment of cases | Cooperation to ensure antiparasitic treatments in the place of origin |
| Surgeon | Surgery |
| Year | Events |
|---|---|
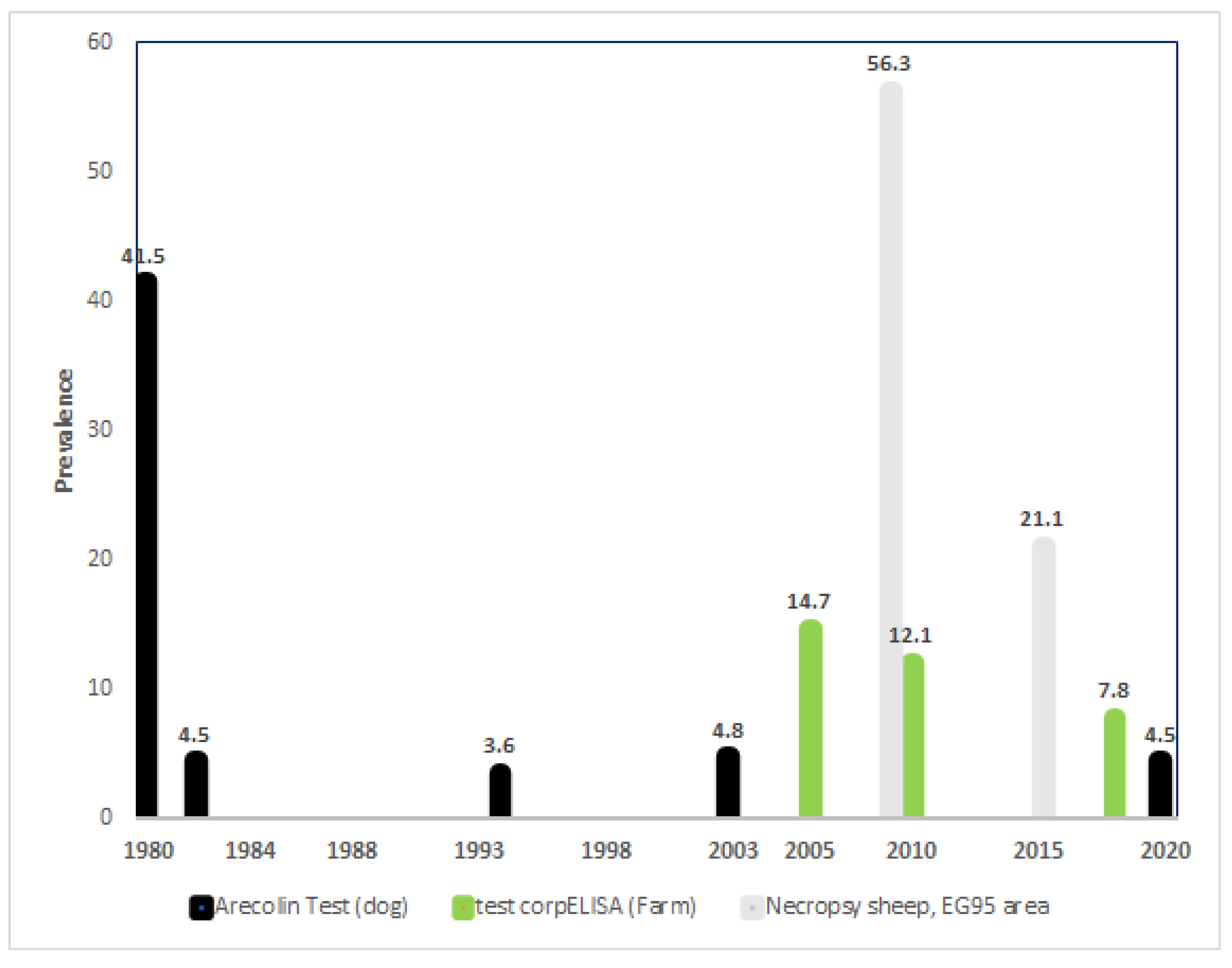
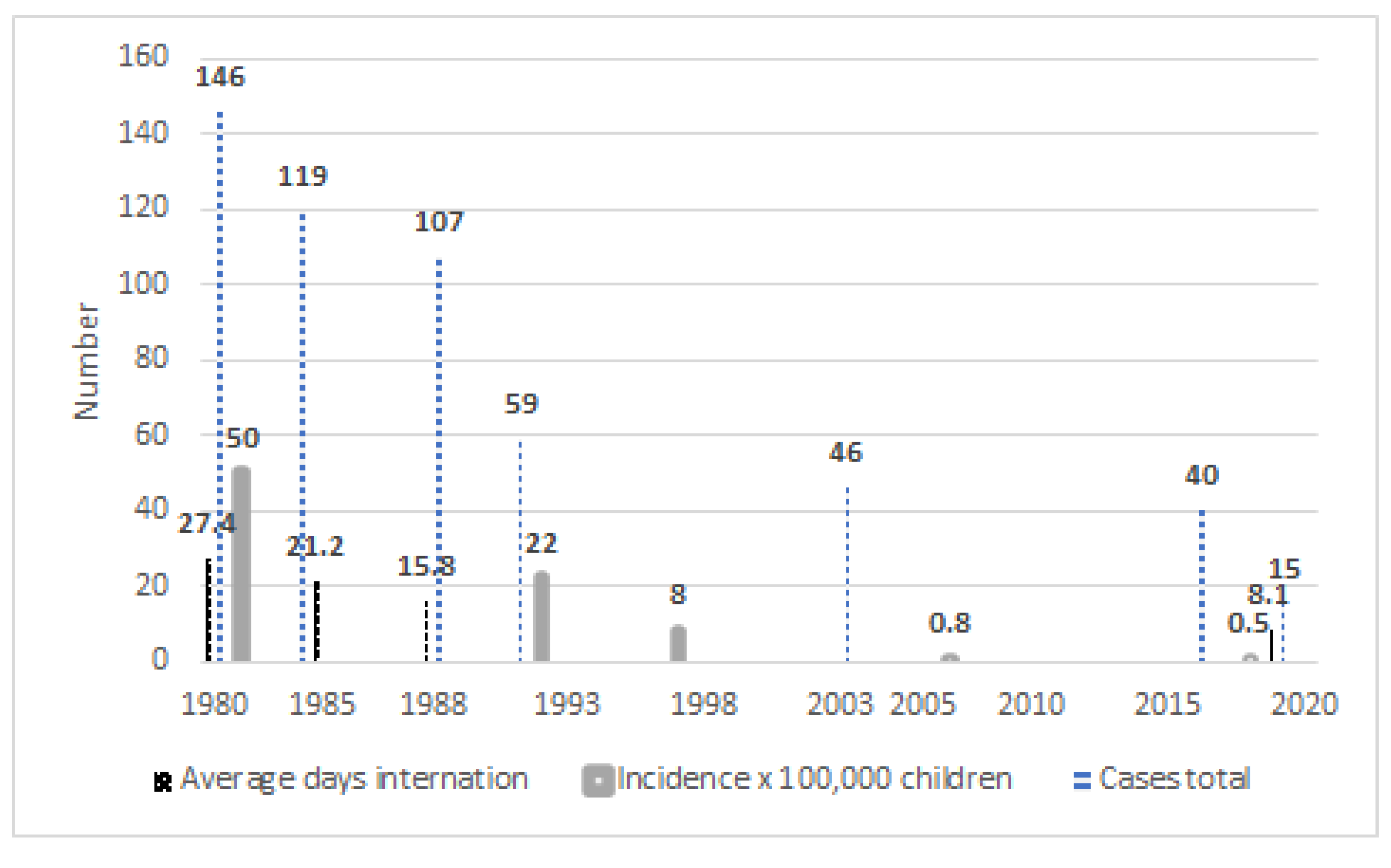
| 1979 | Beginning of arecolina tests on dogs |
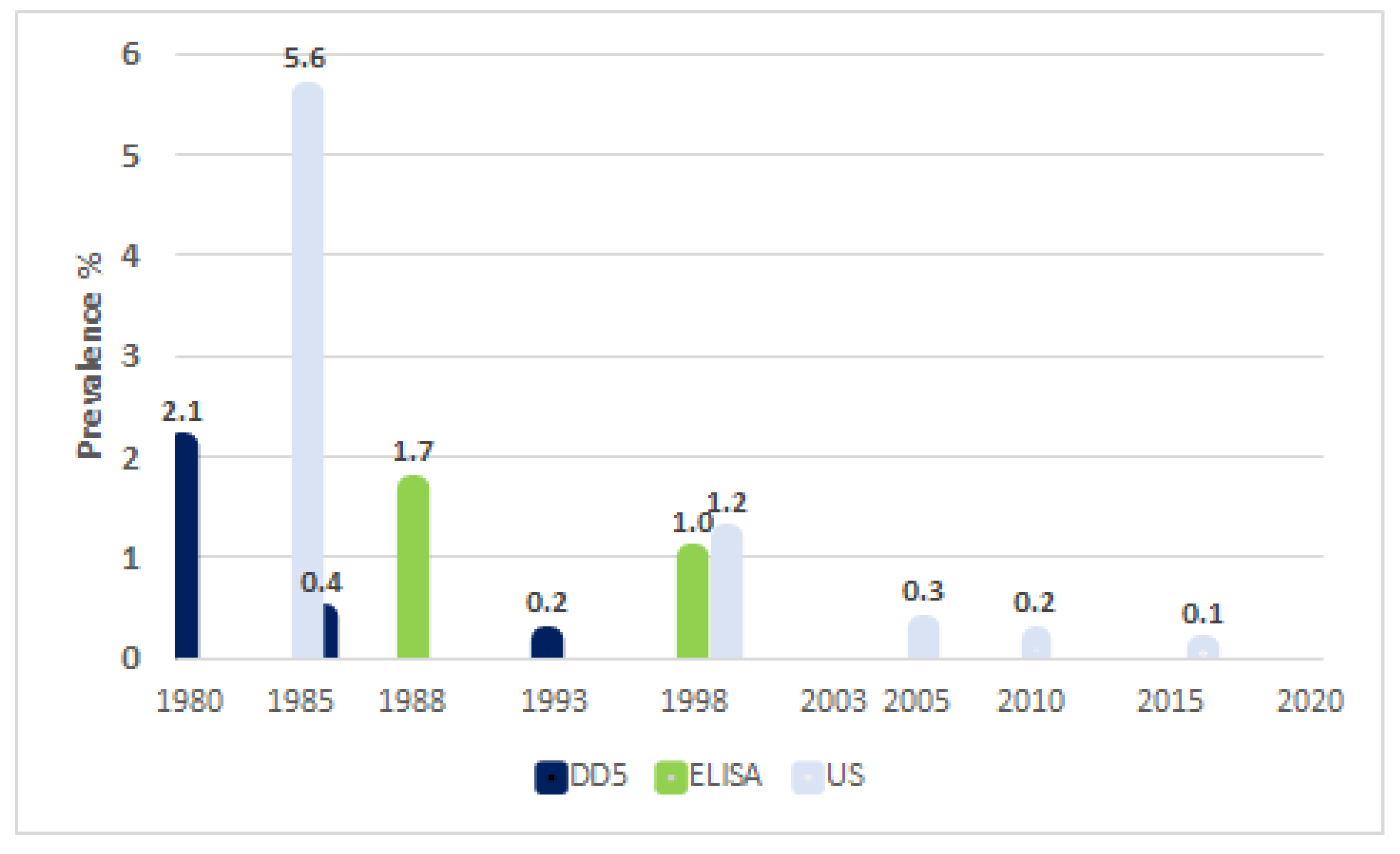
| 1980 | Beginning of canine deworming with PZQ Beginning of screening with DD5 in Children |
| 1984 | First screening with US |
| 1988 | Beginning of screening with ELISA for children |
| 1992 | First use of ABZ |
| 1997 | Last use of seroepidemiology Beginning of US screening for children |
| 2000 | Introduction of FASE courses |
| 2005 | Last use of arecoline tests on dogs Beginning of copro ELISA tests on farms |
| 2006 | Beginning of vaccinations with EG95 in lambs |
| 2020 | Continuation of copro ELISA screening Continuation of deworming and vaccination Continuation of US screening and FASE courses |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mujica, G.; Uchiumi, L.; Araya, D.; Salvitti, J.C.; Labanchi, J.L.; Sobrino, M.; Herrero, E.; Panomarenko, O.; Blanco, P.; Talmon, G.; et al. The Diagnosis, Treatment, Surveillance and Control of Cystic Echinococcosis in the Province of Rio Negro: The “One-Health” Model. Parasitologia 2021, 1, 177-187. https://doi.org/10.3390/parasitologia1040019
Mujica G, Uchiumi L, Araya D, Salvitti JC, Labanchi JL, Sobrino M, Herrero E, Panomarenko O, Blanco P, Talmon G, et al. The Diagnosis, Treatment, Surveillance and Control of Cystic Echinococcosis in the Province of Rio Negro: The “One-Health” Model. Parasitologia. 2021; 1(4):177-187. https://doi.org/10.3390/parasitologia1040019
Chicago/Turabian StyleMujica, Guillermo, Leonardo Uchiumi, Daniel Araya, Juan Carlos Salvitti, Jose Luis Labanchi, Mariano Sobrino, Eduardo Herrero, Oscar Panomarenko, Patricia Blanco, Gabriel Talmon, and et al. 2021. "The Diagnosis, Treatment, Surveillance and Control of Cystic Echinococcosis in the Province of Rio Negro: The “One-Health” Model" Parasitologia 1, no. 4: 177-187. https://doi.org/10.3390/parasitologia1040019
APA StyleMujica, G., Uchiumi, L., Araya, D., Salvitti, J. C., Labanchi, J. L., Sobrino, M., Herrero, E., Panomarenko, O., Blanco, P., Talmon, G., Tissot, H., Grizmado, C., Arezo, M., Seleiman, M., Mercapide, C. H., & Larrieu, E. (2021). The Diagnosis, Treatment, Surveillance and Control of Cystic Echinococcosis in the Province of Rio Negro: The “One-Health” Model. Parasitologia, 1(4), 177-187. https://doi.org/10.3390/parasitologia1040019






