Effect of Neonicotinoid Exposure on the Life History Traits and Susceptibility to Plasmodium Infection on the Major Avian Malaria Vector Culex pipiens (Diptera: Culicidae)
Abstract
:1. Introduction
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Malaria Parasites and Bird Infection
5.2. Mosquito Rearing and Imidacloprid Exposure
5.3. Statistical Analysis
5.4. Ethical Statements
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cohen, J.E. Human Population: The Next Half Century. Science 2003, 302, 1172–1175. [Google Scholar] [CrossRef]
- Crist, E.; Mora, C.; Engelman, R. The Interaction of Human Population, Food Production, and Biodiversity Protection. Science 2017, 356, 260–264. [Google Scholar] [CrossRef]
- Ecobichon, D.J. Pesticide Use in Developing Countries. Toxicology 2001, 160, 27–33. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of Pesticides Use in Agriculture: Their Benefits and Hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Zeiss, M.R.; Geng, S. Agricultural Pesticide Use and Food Safety: California’s Model. J. Integr. Agric. 2015, 14, 2340–2357. [Google Scholar] [CrossRef]
- Hayes, T.B.; Case, P.; Chui, S.; Chung, D.; Haeffele, C.; Haston, K.; Lee, M.; Mai, V.P.; Marjuoa, Y.; Parker, J.; et al. Pesticide Mixtures, Endocrine Disruption, and Amphibian Declines: Are We Underestimating the Impact? Environ. Health Perspect. 2006, 114, 40–50. [Google Scholar] [CrossRef]
- Benachour, N.; Séralini, G.-E. Glyphosate Formulations Induce Apoptosis and Necrosis in Human Umbilical, Embryonic, and Placental Cells. Chem. Res. Toxicol. 2009, 22, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D. Ecology: Pesticides Linked to Bird Declines. Nature 2014, 511, 295–296. [Google Scholar] [CrossRef]
- Kudsk, P.; Mathiassen, S.K. Pesticide Regulation in the European Union and the Glyphosate Controversy. Weed Sci. 2020, 68, 214–222. [Google Scholar] [CrossRef]
- Pisa, L.W.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Downs, C.A.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; McField, M.; et al. Effects of Neonicotinoids and Fipronil on Non-Target Invertebrates. Environ. Sci. Pollut. Res. 2015, 22, 68–102. [Google Scholar] [CrossRef] [Green Version]
- Kumar, U.; Berliner, J.; Adak, T.; Rath, P.C.; Dey, A.; Pokhare, S.S.; Jambhulkar, N.N.; Panneerselvam, P.; Kumar, A.; Mohapatra, S.D. Non-Target Effect of Continuous Application of Chlorpyrifos on Soil Microbes, Nematodes and Its Persistence under Sub-Humid Tropical Rice-Rice Cropping System. Ecotoxicol. Environ. Saf. 2017, 135, 225–235. [Google Scholar] [CrossRef]
- Zaller, J.G.; Brühl, C.A. Non-Target Effects of Pesticides on Organisms Inhabiting Agroecosystems. Front. Environ. Sci. 2019, 7, 75. [Google Scholar] [CrossRef] [Green Version]
- Chagnon, M.; Kreutzweiser, D.; Mitchell, E.A.D.; Morrissey, C.A.; Noome, D.A.; van der Sluijs, J.P. Risks of Large-Scale Use of Systemic Insecticides to Ecosystem Functioning and Services. Environ. Sci. Pollut. Res. 2015, 22, 119–134. [Google Scholar] [CrossRef] [Green Version]
- Stanley, D.A.; Garratt, M.P.D.; Wickens, J.B.; Wickens, V.J.; Potts, S.G.; Raine, N.E. Neonicotinoid Pesticide Exposure Impairs Crop Pollination Services Provided by Bumblebees. Nature 2015, 528, 548–550. [Google Scholar] [CrossRef] [Green Version]
- Stuligross, C.; Williams, N.M. Pesticide and Resource Stressors Additively Impair Wild Bee Reproduction. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201390. [Google Scholar] [CrossRef] [PubMed]
- Fogel, M.N.; Schneider, M.I.; Desneux, N.; González, B.; Ronco, A.E. Impact of the Neonicotinoid Acetamiprid on Immature Stages of the Predator Eriopis Connexa (Coleoptera: Coccinellidae). Ecotoxicology 2013, 22, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, Z.; Yu, X.; Ma, D.; Yu, C.; Liu, F.; Mu, W. Influence of Lethal and Sublethal Exposure to Clothianidin on the Seven-Spotted Lady Beetle, Coccinella Septempunctata L. (Coleoptera: Coccinellidae). Ecotoxicol. Environ. Saf. 2018, 161, 208–213. [Google Scholar] [CrossRef]
- Calvo-Agudo, M.; González-Cabrera, J.; Picó, Y.; Calatayud-Vernich, P.; Urbaneja, A.; Dicke, M.; Tena, A. Neonicotinoids in Excretion Product of Phloem-Feeding Insects Kill Beneficial Insects. Proc. Natl. Acad. Sci. USA 2019, 116, 16817–16822. [Google Scholar] [CrossRef] [Green Version]
- Pereira, J.L.; Antunes, S.C.; Castro, B.B.; Marques, C.R.; Gonçalves, A.M.M.; Gonçalves, F.; Pereira, R. Toxicity Evaluation of Three Pesticides on Non-Target Aquatic and Soil Organisms: Commercial Formulation versus Active Ingredient. Ecotoxicology 2009, 18, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Rumschlag, S.L.; Mahon, M.B.; Hoverman, J.T.; Raffel, T.R.; Carrick, H.J.; Hudson, P.J.; Rohr, J.R. Consistent Effects of Pesticides on Community Structure and Ecosystem Function in Freshwater Systems. Nat. Commun. 2020, 11, 6333. [Google Scholar] [CrossRef] [PubMed]
- Gilliom, R.J. Pesticides in U.S. Streams and Groundwater. Environ. Sci. Technol. 2007, 41, 3408–3414. [Google Scholar] [CrossRef] [Green Version]
- Morrissey, C.A.; Mineau, P.; Devries, J.H.; Sanchez-Bayo, F.; Liess, M.; Cavallaro, M.C.; Liber, K. Neonicotinoid Contamination of Global Surface Waters and Associated Risk to Aquatic Invertebrates: A Review. Environ. Int. 2015, 74, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Lawler, S.P.; Lanzaro, G.C. Managing Mosquitoes on the Farm; UCANR Publications: Davis, CA, USA, 2005. [Google Scholar] [CrossRef] [Green Version]
- Rey, J.R.; Walton, W.E.; Wolfe, R.J.; Connelly, C.R.; O’Connell, S.M.; Berg, J.; Sakolsky-Hoopes, G.E.; Laderman, A.D. North American Wetlands and Mosquito Control. Int. J. Environ. Res. Public Health 2012, 9, 4537–4605. [Google Scholar] [CrossRef]
- Kibuthu, T.W.; Njenga, S.M.; Mbugua, A.K.; Muturi, E.J. Agricultural Chemicals: Life Changer for Mosquito Vectors in Agricultural Landscapes? Parasites Vectors 2016, 9, 500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, K.M.; Gonzaga, W.G.; Pascini, T.V.; Miranda, F.R.; Tomé, H.V.V.; Serrão, J.E.; Martins, G.F. Imidacloprid Impairs the Post-Embryonic Development of the Midgut in the Yellow Fever Mosquito Stegomyia aegypti (=Aedes aegypti). Med. Vet. Entomol. 2015, 29, 245–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czerwinski, M.A.; Sadd, B.M. Detrimental Interactions of Neonicotinoid Pesticide Exposure and Bumblebee Immunity. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2017, 327, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Hohnheiser, B.; Sgolastra, F.; Bosch, J.; Meixner, M.D.; Büchler, R. Immunosuppression Response to the Neonicotinoid Insecticide Thiacloprid in Females and Males of the Red Mason Bee Osmia bicornis L. Sci. Rep. 2020, 10, 4670. [Google Scholar] [CrossRef]
- Stara, A.; Pagano, M.; Capillo, G.; Fabrello, J.; Sandova, M.; Vazzana, I.; Zuskova, E.; Velisek, J.; Matozzo, V.; Faggio, C. Assessing the Effects of Neonicotinoid Insecticide on the Bivalve Mollusc Mytilus galloprovincialis. Sci. Total Environ. 2020, 700, 134914. [Google Scholar] [CrossRef]
- Riaz, M.A.; Chandor-Proust, A.; Dauphin-Villemant, C.; Poupardin, R.; Jones, C.M.; Strode, C.; Régent-Kloeckner, M.; David, J.-P.; Reynaud, S. Molecular Mechanisms Associated with Increased Tolerance to the Neonicotinoid Insecticide Imidacloprid in the Dengue Vector Aedes aegypti. Aquat. Toxicol. 2013, 126, 326–337. [Google Scholar] [CrossRef]
- Mouhamadou, C.S.; de Souza, S.S.; Fodjo, B.K.; Zoh, M.G.; Bli, N.K.; Koudou, B.G. Evidence of Insecticide Resistance Selection in Wild Anopheles Coluzzii Mosquitoes Due to Agricultural Pesticide Use. Infect. Dis. Poverty 2019, 8, 64. [Google Scholar] [CrossRef]
- Fouet, C.; Ashu, A.F.; Ambadiang, M.M.; Tchapga, W.; Wondji, C.S.; Kamdem, C. Resistance of Anopheles gambiae to the New Insecticide Clothianidin Associated with Unrestricted Use of Agricultural Neonicotinoids in Yaoundé, Cameroon. bioRxiv 2020. [Google Scholar] [CrossRef]
- Foster, W.A. Mosquito Sugar Feeding and Reproductive Energetics. Annu. Rev. Entomol. 1995, 40, 443–474. [Google Scholar] [CrossRef] [PubMed]
- Barredo, E.; DeGennaro, M. Not Just from Blood: Mosquito Nutrient Acquisition from Nectar Sources. Trends Parasitol. 2020, 36, 473–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mach, B.M.; Bondarenko, S.; Potter, D.A. Uptake and Dissipation of Neonicotinoid Residues in Nectar and Foliage of Systemically Treated Woody Landscape Plants. Environ. Toxicol. Chem. 2018, 37, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Wintermantel, D.; Odoux, J.-F.; Decourtye, A.; Henry, M.; Allier, F.; Bretagnolle, V. Neonicotinoid-Induced Mortality Risk for Bees Foraging on Oilseed Rape Nectar Persists despite EU Moratorium. Sci. Total Environ. 2020, 704, 135400. [Google Scholar] [CrossRef]
- Di Prisco, G.; Cavaliere, V.; Annoscia, D.; Varricchio, P.; Caprio, E.; Nazzi, F.; Gargiulo, G.; Pennacchio, F. Neonicotinoid Clothianidin Adversely Affects Insect Immunity and Promotes Replication of a Viral Pathogen in Honey Bees. Proc. Natl. Acad. Sci. USA 2013, 110, 18466–18471. [Google Scholar] [CrossRef] [Green Version]
- Brandt, A.; Gorenflo, A.; Siede, R.; Meixner, M.; Büchler, R. The Neonicotinoids Thiacloprid, Imidacloprid, and Clothianidin Affect the Immunocompetence of Honey Bees (Apis mellifera L.). J. Insect Physiol. 2016, 86, 40–47. [Google Scholar] [CrossRef]
- Tosi, S.; Nieh, J.C.; Sgolastra, F.; Cabbri, R.; Medrzycki, P. Neonicotinoid Pesticides and Nutritional Stress Synergistically Reduce Survival in Honey Bees. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171711. [Google Scholar] [CrossRef] [Green Version]
- Whitehorn, P.R.; O’Connor, S.; Wackers, F.L.; Goulson, D. Neonicotinoid Pesticide Reduces Bumble Bee Colony Growth and Queen Production. Science 2012, 336, 351–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Food Safety Authority. Statement on the Findings in Recent Studies Investigating Sub-Lethal Effects in Bees of Some Neonicotinoids in Consideration of the Uses Currently Authorised in Europe. EFSA J. 2012, 10, 2752. [Google Scholar] [CrossRef]
- Glaizot, O.; Fumagalli, L.; Iritano, K.; Lalubin, F.; van Rooyen, J.; Christe, P. High Prevalence and Lineage Diversity of Avian Malaria in Wild Populations of Great Tits (Parus major) and Mosquitoes (Culex pipiens). PLoS ONE 2012, 7, e34964. [Google Scholar] [CrossRef] [PubMed]
- Farajollahi, A.; Fonseca, D.M.; Kramer, L.D.; Marm Kilpatrick, A. “Bird Biting” Mosquitoes and Human Disease: A Review of the Role of Culex pipiens Complex Mosquitoes in Epidemiology. Infect. Genet. Evol. 2011, 11, 1577–1585. [Google Scholar] [CrossRef] [Green Version]
- Lalubin, F.; Delédevant, A.; Glaizot, O.; Christe, P. Temporal Changes in Mosquito Abundance (Culex pipiens), Avian Malaria Prevalence and Lineage Composition. Parasites Vectors 2013, 6, 307. [Google Scholar] [CrossRef] [Green Version]
- Beketov, M.A.; Liess, M. Predation Risk Perception and Food Scarcity Induce Alterations of Life-Cycle Traits of the Mosquito Culex pipiens. Ecol. Entomol. 2007, 32, 405–410. [Google Scholar] [CrossRef]
- Muturi, E.J.; Kim, C.-H.; Alto, B.W.; Berenbaum, M.R.; Schuler, M.A. Larval Environmental Stress Alters Aedes aegypti Competence for Sindbis Virus. Trop. Med. Int. Health 2011, 16, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Tripet, F.; Aboagye-Antwi, F.; Hurd, H. Ecological Immunology of Mosquito—Malaria Interactions. Trends Parasitol. 2008, 24, 219–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bataillard, D.; Christe, P.; Pigeault, R. Impact of Field-Realistic Doses of Glyphosate and Nutritional Stress on Mosquito Life History Traits and Susceptibility to Malaria Parasite Infection. Ecol. Evol. 2020, 10, 5079–5088. [Google Scholar] [CrossRef] [PubMed]
- Delhaye, J.; Aletti, C.; Glaizot, O.; Christe, P. Exposure of the Mosquito Vector Culex Pipiens to the Malaria Parasite Plasmodium relictum: Effect of Infected Blood Intake on Immune and Antioxidant Defences, Fecundity and Survival. Parasites Vectors 2016, 9, 616. [Google Scholar] [CrossRef] [Green Version]
- Starner, K.; Goh, K.S. Detections of the Neonicotinoid Insecticide Imidacloprid in Surface Waters of Three Agricultural Regions of California, USA, 2010–2011. Bull. Environ. Contam. Toxicol. 2012, 88, 316–321. [Google Scholar] [CrossRef]
- Main, A.R.; Headley, J.V.; Peru, K.M.; Michel, N.L.; Cessna, A.J.; Morrissey, C.A. Widespread Use and Frequent Detection of Neonicotinoid Insecticides in Wetlands of Canada’s Prairie Pothole Region. PLoS ONE 2014, 9, e92821. [Google Scholar] [CrossRef] [PubMed]
- Zioga, E.; Kelly, R.; White, B.; Stout, J.C. Plant Protection Product Residues in Plant Pollen and Nectar: A Review of Current Knowledge. Environ. Res. 2020, 189, 109873. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bayo, F.; Goka, K.; Hayasaka, D. Contamination of the Aquatic Environment with Neonicotinoids and Its Implication for Ecosystems. Front. Environ. Sci. 2016, 4, 71. [Google Scholar] [CrossRef]
- Pietrzak, D.; Kania, J.; Malina, G.; Kmiecik, E.; Wątor, K. Pesticides from the EU First and Second Watch Lists in the Water Environment. Clean Soil Air Water 2019, 47, 1800376. [Google Scholar] [CrossRef]
- Bishop, C.A.; Woundneh, M.B.; Maisonneuve, F.; Common, J.; Elliott, J.E.; Moran, A.J. Determination of Neonicotinoids and Butenolide Residues in Avian and Insect Pollinators and Their Ambient Environment in Western Canada (2017, 2018). Sci. Total Environ. 2020, 737, 139386. [Google Scholar] [CrossRef]
- Song, M.Y.; Stark, J.D.; Brown, J.J. Comparative Toxicity of Four Insecticides, Including Imidacloprid and Tebufenozide, to Four Aquatic Arthropods. Environ. Toxicol. Chem. 1997, 16, 2494–2500. [Google Scholar] [CrossRef]
- Antonio-Arreola, G.E.; López-Bello, R.; Romero-Moreno, D.K.; Sánchez, D. Laboratory and Field Evaluation of the Effects of the Neonicotinoid Imidacloprid on the Oviposition Response of Aedes (Stegomyia) aegypti Linnaeus (Diptera: Culicidae). Mem. Inst. Oswaldo Cruz 2011, 106, 997–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomé, H.V.; Pascini, T.V.; Dângelo, R.A.; Guedes, R.N.; Martins, G.F. Survival and Swimming Behavior of Insecticide-Exposed Larvae and Pupae of the Yellow Fever Mosquito Aedes aegypti. Parasites Vectors 2014, 7, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naggar, Y.A.; Giesy, J.P.; Kholy, S.E. Sublethal Effects of Chronic Exposure to Chlorpyrifos or Imidacloprid Insecticides or Their Binary Mixtures on Culex pipiens Mosquitoes. Physiol. Entomol. 2019, 44, 123–132. [Google Scholar] [CrossRef]
- Raby, M.; Zhao, X.; Hao, C.; Poirier, D.G.; Sibley, P.K. Chronic Effects of an Environmentally-Relevant, Short-Term Neonicotinoid Insecticide Pulse on Four Aquatic Invertebrates. Sci. Total Environ. 2018, 639, 1543–1552. [Google Scholar] [CrossRef]
- Kreutzweiser, D.P.; Good, K.P.; Chartrand, D.T.; Scarr, T.A.; Thompson, D.G. Toxicity of the Systemic Insecticide, Imidacloprid, to Forest Stream Insects and Microbial Communities. Bull. Environ. Contam. Toxicol. 2008, 80, 211–214. [Google Scholar] [CrossRef]
- Alexander, A.C.; Culp, J.M.; Liber, K.; Cessna, A.J. Effects of Insecticide Exposure on Feeding Inhibition in Mayflies and Oligochaetes. Environ. Toxicol. Chem. 2007, 26, 1726–1732. [Google Scholar] [CrossRef]
- Chandran, N.N.; Fojtova, D.; Blahova, L.; Rozmankova, E.; Blaha, L. Acute and (Sub)Chronic Toxicity of the Neonicotinoid Imidacloprid on Chironomus riparius. Chemosphere 2018, 209, 568–577. [Google Scholar] [CrossRef]
- Farahat, N.M.; Zyaan, O.H.; Khaled, A.S.; Hussein, M.A. Toxic and Biochemical Effects of Imidacloprid and Tannic Acid on the Culex pipiens larvae (Diptera: Culicidae). Int. J. Mosq. Res. 2018, 5, 111–115. [Google Scholar]
- Li, H.; Zhang, Q.; Su, H.; You, J.; Wang, W.-X. High Tolerance and Delayed Responses of Daphnia Magna to Neonicotinoid Insecticide Imidacloprid: Toxicokinetic and Toxicodynamic Modeling. Environ. Sci. Technol. 2020, 55, 458–467. [Google Scholar] [CrossRef]
- Rauch, N.; Nauen, R. Identification of Biochemical Markers Linked to Neonicotinoid Cross Resistance in Bemisia Tabaci (Hemiptera: Aleyrodidae). Arch. Insect Biochem. Physiol. 2003, 54, 165–176. [Google Scholar] [CrossRef]
- Zewen, L.; Zhaojun, H.; Yinchang, W.; Lingchun, Z.; Hongwei, Z.; Chengjun, L. Selection for Imidacloprid Resistance in Nilaparvata Lugens: Cross-Resistance Patterns and Possible Mechanisms. Pest Manag. Sci. 2003, 59, 1355–1359. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; He, J.; Zhao, X.; Chaimanee, V.; Huang, W.-F.; Nie, H.; Zhao, Y.; Su, S. Differential Physiological Effects of Neonicotinoid Insecticides on Honey Bees: A Comparison between Apis mellifera and Apis cerana. Pestic. Biochem. Physiol. 2017, 140, 1–8. [Google Scholar] [CrossRef]
- Walderdorff, L.; Laval-Gilly, P.; Bonnefoy, A.; Falla-Angel, J. Imidacloprid Intensifies Its Impact on Honeybee and Bumblebee Cellular Immune Response When Challenged with LPS (Lippopolysacharide) of Escherichia Coli. J. Insect Physiol. 2018, 108, 17–24. [Google Scholar] [CrossRef]
- Ewere, E.E.; Reichelt-Brushett, A.; Benkendorff, K. The Neonicotinoid Insecticide Imidacloprid, but Not Salinity, Impacts the Immune System of Sydney Rock Oyster, Saccostrea glomerata. Sci. Total Environ. 2020, 742, 140538. [Google Scholar] [CrossRef] [PubMed]
- Rouzé, R.; Moné, A.; Delbac, F.; Belzunces, L.; Blot, N. The Honeybee Gut Microbiota Is Altered after Chronic Exposure to Different Families of Insecticides and Infection by Nosema ceranae. Microbes Environ. 2019, 34, 226–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Manfredini, F.; Dimopoulos, G. Implication of the Mosquito Midgut Microbiota in the Defense against Malaria Parasites. PLoS Pathog. 2009, 5, e1000423. [Google Scholar] [CrossRef] [Green Version]
- Cirimotich, C.M.; Ramirez, J.L.; Dimopoulos, G. Native Microbiota Shape Insect Vector Competence for Human Pathogens. Cell Host Microbe 2011, 10, 307–310. [Google Scholar] [CrossRef] [Green Version]
- Cirimotich, C.M.; Dong, Y.; Garver, L.S.; Sim, S.; Dimopoulos, G. Mosquito Immune Defenses against Plasmodium Infection. Dev. Comp. Immunol. 2010, 34, 387–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boissière, A.; Tchioffo, M.T.; Bachar, D.; Abate, L.; Marie, A.; Nsango, S.E.; Shahbazkia, H.R.; Awono-Ambene, P.H.; Levashina, E.A.; Christen, R.; et al. Midgut Microbiota of the Malaria Mosquito Vector Anopheles gambiae and Interactions with Plasmodium falciparum Infection. PLoS Pathog. 2012, 8, e1002742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, H.; Cui, C.; Wang, L.; Jacobs-Lorena, M.; Wang, S. Mosquito Microbiota and Implications for Disease Control. Trends Parasitol. 2020, 36, 98–111. [Google Scholar] [CrossRef]
- Ravaiano, S.V.; Barbosa, W.F.; Tomé, H.V.V.; Campos, L.A.D.O.; Martins, G.F. Acute and Oral Exposure to Imidacloprid Does Not Affect the Number of Circulating Hemocytes in the Stingless Bee Melipona quadrifasciata Post Immune Challenge. Pestic. Biochem. Physiol. 2018, 152, 24–28. [Google Scholar] [CrossRef]
- Brevik, K.; Lindström, L.; McKay, S.D.; Chen, Y.H. Transgenerational Effects of Insecticides—Implications for Rapid Pest Evolution in Agroecosystems. Curr. Opin. Insect Sci. 2018, 26, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Xin, J.; Yu, W.; Yi, X.; Gao, J.; Gao, X.; Zeng, X. Sublethal Effects of Sulfoxaflor on the Fitness of Two Species of Wheat Aphids, Sitobion Avenae (F.) and Rhopalosiphum Padi (L.). J. Integr. Agric. 2019, 18, 1613–1623. [Google Scholar] [CrossRef]
- Xiao, D.; Zhao, J.; Guo, X.; Chen, H.; Qu, M.; Zhai, W.; Desneux, N.; Biondi, A.; Zhang, F.; Wang, S. Sublethal Effects of Imidacloprid on the Predatory Seven-Spot Ladybird Beetle Coccinella septempunctata. Ecotoxicology 2016, 25, 1782–1793. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; He, D.; Men, X.; Zheng, L.; Cheng, S.; Tao, L.; Yu, C. Sublethal and Transgenerational Effects of Acetamiprid and Imidacloprid on the Predatory Bug Orius Sauteri (Poppius) (Hemiptera: Anthocoridae). Chemosphere 2020, 255, 126778. [Google Scholar] [CrossRef] [PubMed]
- Lalubin, F.; Delédevant, A.; Glaizot, O.; Christe, P. Natural Malaria Infection Reduces Starvation Resistance of Nutritionally Stressed Mosquitoes. J. Anim. Ecol. 2014, 83, 850–857. [Google Scholar] [CrossRef]
- Valkiunas, G. Avian Malaria Parasites and Other Haemosporidia; CRC Press: Boca Raton, FL, USA, 2004; ISBN 978-0-203-64379-2. [Google Scholar]
- Hellgren, O.; Waldenström, J.; Bensch, S. A New Pcr Assay for Simultaneous Studies of Leucocytozoon, Plasmodium, and Haemoproteus from Avian Blood. J. Parasitol. 2004, 90, 797–802. [Google Scholar] [CrossRef]
- van Rooyen, J.; Lalubin, F.; Glaizot, O.; Christe, P. Avian Haemosporidian Persistence and Co-Infection in Great Tits at the Individual Level. Malar. J. 2013, 12, 40. [Google Scholar] [CrossRef] [Green Version]
- Bensch, S.; Hellgren, O.; Pérez-Tris, J. MalAvi: A Public Database of Malaria Parasites and Related Haemosporidians in Avian Hosts Based on Mitochondrial Cytochrome b Lineages. Mol. Ecol. Resour. 2009, 9, 1353–1358. [Google Scholar] [CrossRef]
- Pigeault, R.; Vézilier, J.; Cornet, S.; Zélé, F.; Nicot, A.; Perret, P.; Gandon, S.; Rivero, A. Avian Malaria: A New Lease of Life for an Old Experimental Model to Study the Evolutionary Ecology of Plasmodium. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carreck, N.L.; Ratnieks, F.L.W. The Dose Makes the Poison: Have “Field Realistic” Rates of Exposure of Bees to Neonicotinoid Insecticides Been Overestimated in Laboratory Studies? J. Apic. Res. 2014, 53, 607–614. [Google Scholar] [CrossRef] [Green Version]
- Cornet, S.; Nicot, A.; Rivero, A.; Gandon, S. Malaria Infection Increases Bird Attractiveness to Uninfected Mosquitoes. Ecol. Lett. 2013, 16, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Vézilier, J.; Nicot, A.; Gandon, S.; Rivero, A. Plasmodium Infection Decreases Fecundity and Increases Survival of Mosquitoes. Proc. R. Soc. B Boil. Sci. 2012, 279, 4033–4041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Handel, E.; Day, J.F. Correlation between Wing Length and Protein Content of Mosquitoes. J. Am. Mosq. Control Assoc. 1989, 5, 180–182. [Google Scholar] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. arXiv 2015, arXiv:1406.5823. [Google Scholar]
- Crawley, M.J. The R Book; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 978-1-118-44896-0. [Google Scholar]

| Resp. Variable | Expl. Variable | Mean ± SE or IC | Test | p | Model Nb. | |
|---|---|---|---|---|---|---|
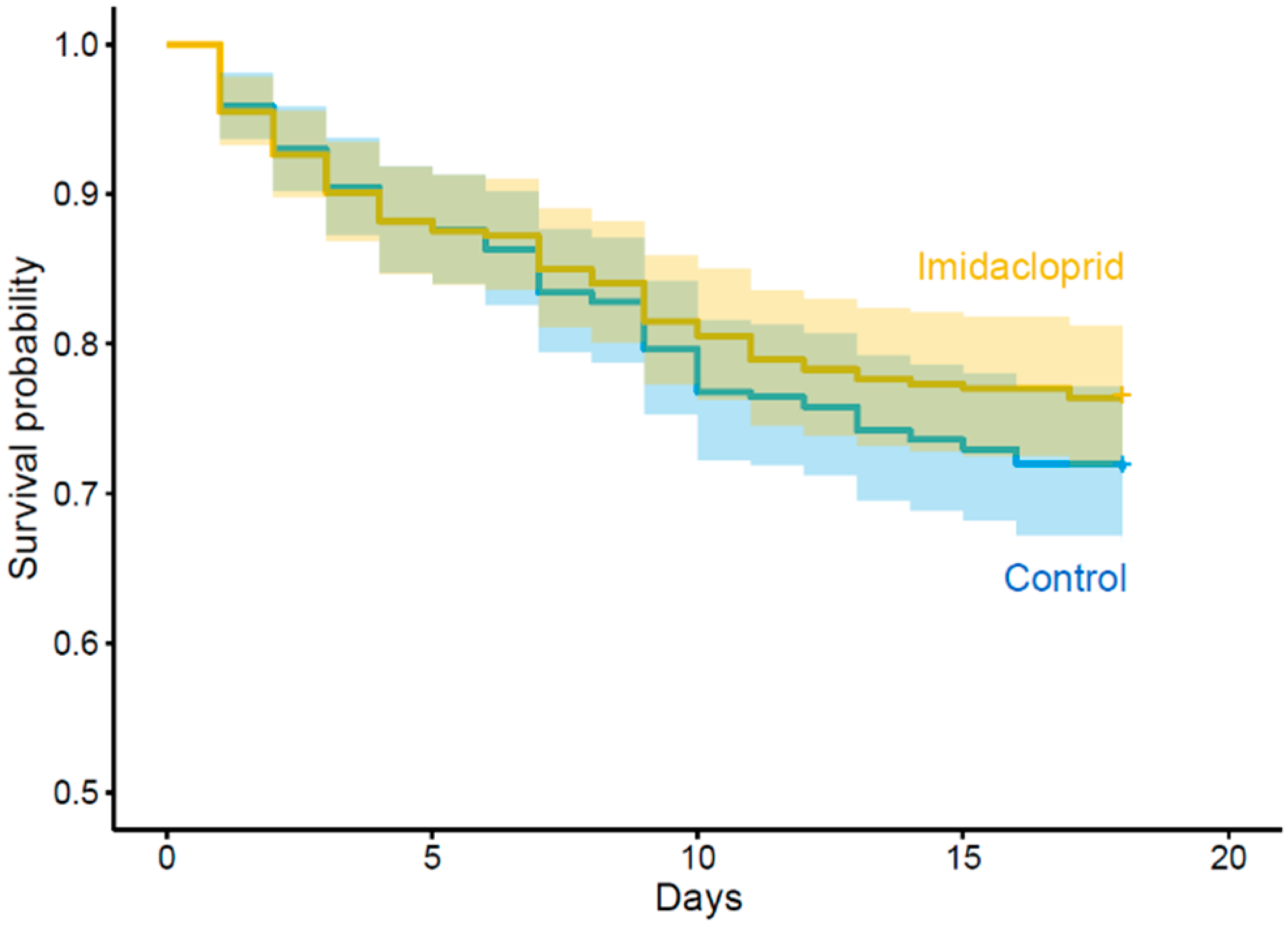
| Survival rate | Larvae exposure status | Unexposed | 0.71, IC: 0.66–0.76 | χ²1 = 1.54 | 0.215 | 1 |
| Exposed | 0.76, IC: 0.71–0.80 | |||||
| Development time | Larvae exposure status | Unexposed | 10.17 days ± 0.11 | F = 0.160 | 0.688 | 2 |
| Exposed | 10.76 days ± 0.10 | |||||
| Sex | Female | 11.33 days ± 0.07 | F = 461.4 | <0.001 | ||
| Male | 9.06 days ± 0.07 | |||||
| Exposure status: Sex | F = 1.230 | 0.268 | ||||
| Wing size | Larvae exposure status | Unexposed | 0.302 cm ± 0.02 | F= 0.050 | 0.817 | 3 |
| Exposed | 0.310 cm ± 0.03 | |||||
| Sex | Female | 0.337 cm ± 0.02 | F = 591.8 | <0.001 | ||
| Male | 0.280 cm ± 0.01 | |||||
| Exposure status: Sex | F = 3.020 | 0.083 | ||||
| Blood meal rate | Larvae exposure status | Unexposed | 0.89, IC: 0.82–0.96 | χ²1 = 0.13 | 0.719 | 4 |
| Exposed | 0.91, IC: 0.84–0.98 | |||||
| Adult exposure status | Unexposed | 0.88, IC: 0.81–0.95 | χ²1 = 0.63 | 0.427 | ||
| Exposed | 0.92, IC: 0.86–0.98 | |||||
| Larvae: Adult exposure status | χ²1 = 1.89 | 0.169 | ||||
| Larvae and Adult unexp. | 0.84, IC: 0.72–0.96 | |||||
| Larvae and Adult exp. | 0.89, IC: 0.79–0.99 | |||||
| Larvae exp. and Adult unexp. | 0.92, IC: 0.83–1.00 | |||||
| Larvae un-exp. And Adult exp. | 0.94, IC: 0.86–1.00 | |||||
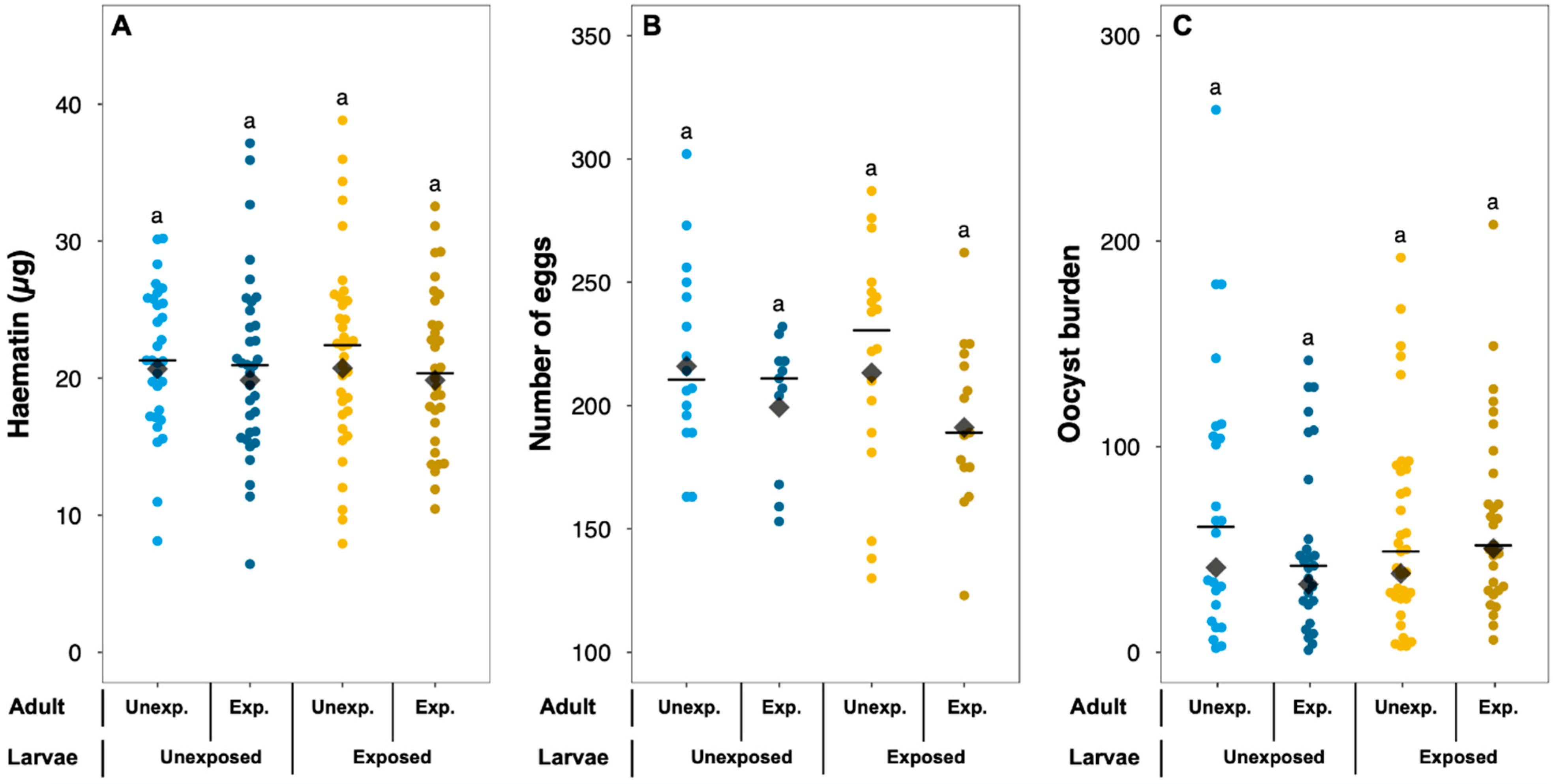
| Blood meal size (haematin, µg) | Larvae exposure status | Unexposed | 21.49 ± 1.06 | χ²1 = 0.11 | 0.742 | 5 |
| Exposed | 21.57 ± 0.96 | |||||
| Adult exposure status | Unexposed | 21.86 ± 1.01 | χ²1 = 0.54 | 0.463 | ||
| Exposed | 21.17 ± 1.00 | |||||
| Larvae: Adult exposure status | χ²1 = 0.29 | 0.169 | ||||
| Larvae and Adult unexp. | 21.41 ± 1.34 | |||||
| Larvae and Adult exp. | 20.86 ± 1.22 | |||||
| Larvae exp. and Adult unexp. | 22.16 ± 1.43 | |||||
| Larvae un-exp. and Adult exp. | 21.58 ± 1.71 | |||||
| Fecundity (nb. of eggs) | Larvae exposure status | Unexposed | 211.74 ± 6.76 | χ²1 = 0.01 | 0.975 | 6 |
| Exposed | 207.39 ± 7.41 | |||||
| Adult exposure status | Unexposed | 218.76 ± 7.26 | χ²1 = 2.65 | 0.104 | ||
| Exposed | 197.04 ± 6.11 | |||||
| Larvae: Adult exposure status | χ²1 = 0.01 | 0.950 | ||||
| Larvae and Adult unexp. | 219.00 ± 9.62 | |||||
| Larvae and Adult exp. | 194.00 ± 8.76 | |||||
| Larvae exp. and Adult unexp. | 218.55 ± 11 | |||||
| Larvae un-exp. and Adult exp. | 201.18 ± 8.41 | |||||
| Parasite burden (nb. of oocysts) | Larvae exposure status | Unexposed | 61.98 ± 7.84 | χ²1 = 0.29 | 0.587 | 7 |
| Exposed | 62.55 ± 5.98 | |||||
| Adult exposure status | Unexposed | 65.34 ± 7.44 | χ²1 = 0.30 | 0.589 | ||
| Exposed | 59.04 ± 5.99 | |||||
| Larvae: Adult exposure status | χ²1 = 2.23 | 0.135 | ||||
| Larvae and Adult unexp. | 73.2 ± 13.73 | |||||
| Larvae and Adult exp. | 65.58 ± 8.64 | |||||
| Larvae exp. and Adult unexp. | 60 ± 8.35 | |||||
| Larvae un-exp. and Adult exp. | 52 ± 8.19 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pigeault, R.; Bataillard, D.; Glaizot, O.; Christe, P. Effect of Neonicotinoid Exposure on the Life History Traits and Susceptibility to Plasmodium Infection on the Major Avian Malaria Vector Culex pipiens (Diptera: Culicidae). Parasitologia 2021, 1, 20-33. https://doi.org/10.3390/parasitologia1010003
Pigeault R, Bataillard D, Glaizot O, Christe P. Effect of Neonicotinoid Exposure on the Life History Traits and Susceptibility to Plasmodium Infection on the Major Avian Malaria Vector Culex pipiens (Diptera: Culicidae). Parasitologia. 2021; 1(1):20-33. https://doi.org/10.3390/parasitologia1010003
Chicago/Turabian StylePigeault, Romain, Danaé Bataillard, Olivier Glaizot, and Philippe Christe. 2021. "Effect of Neonicotinoid Exposure on the Life History Traits and Susceptibility to Plasmodium Infection on the Major Avian Malaria Vector Culex pipiens (Diptera: Culicidae)" Parasitologia 1, no. 1: 20-33. https://doi.org/10.3390/parasitologia1010003
APA StylePigeault, R., Bataillard, D., Glaizot, O., & Christe, P. (2021). Effect of Neonicotinoid Exposure on the Life History Traits and Susceptibility to Plasmodium Infection on the Major Avian Malaria Vector Culex pipiens (Diptera: Culicidae). Parasitologia, 1(1), 20-33. https://doi.org/10.3390/parasitologia1010003







