Exploring Cranial Growth Patterns from Birth to Adulthood for Forensic Research and Practice
Abstract
1. Introduction
Why Is the Cranial Complex, Complex?
2. Materials and Methods
2.1. Samples
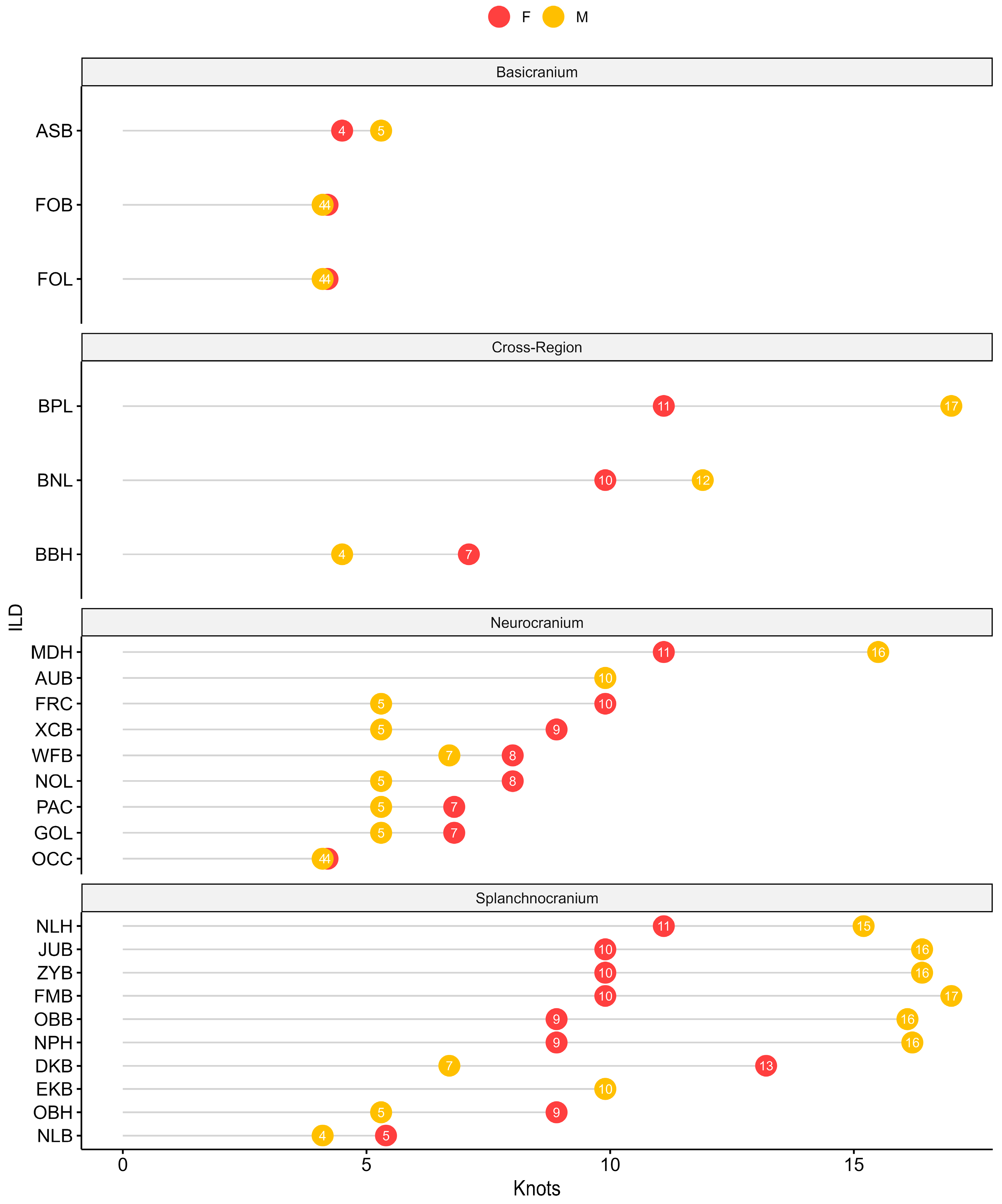
2.2. Interlandmark Distances (ILDs)
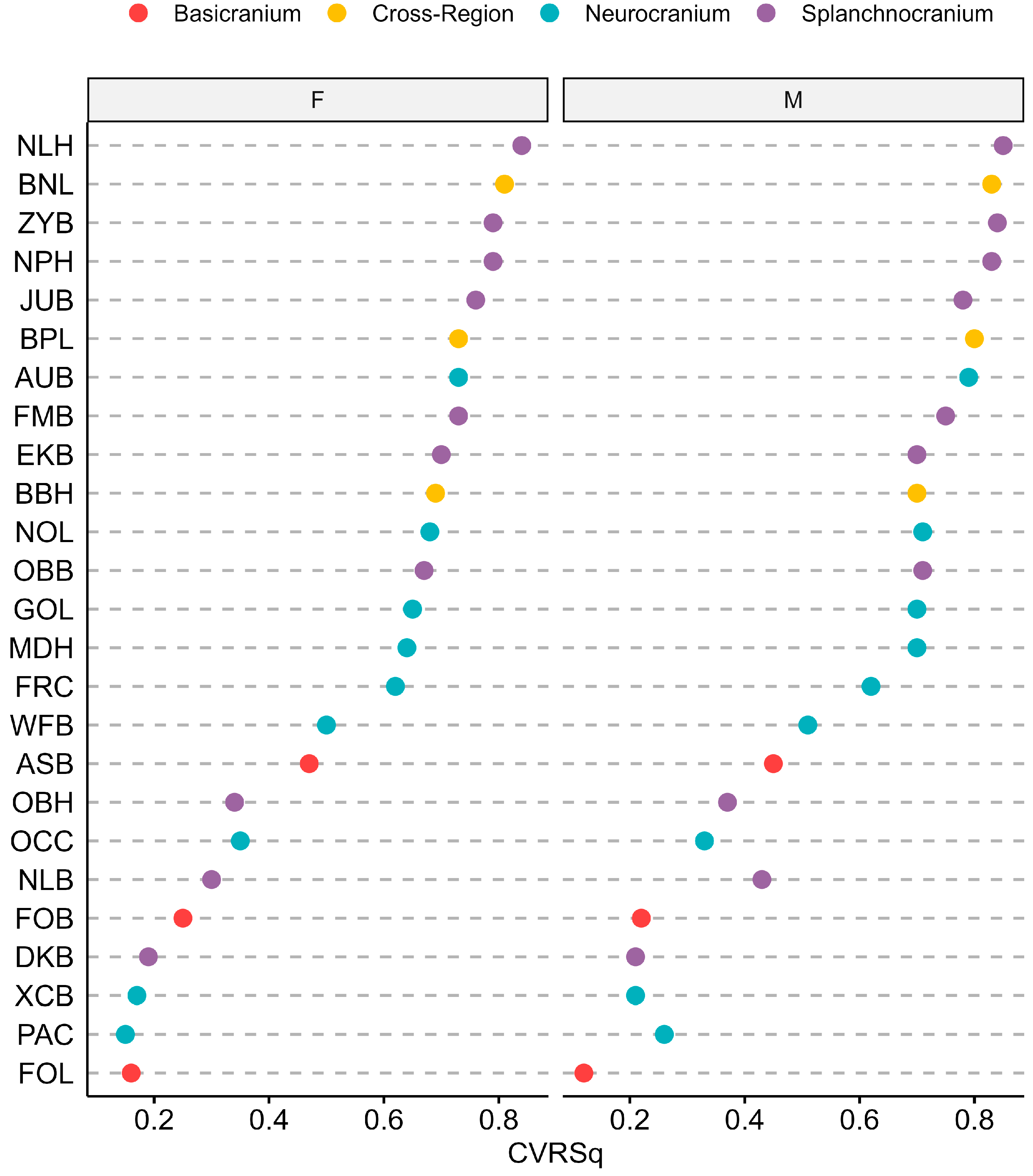
2.3. Statistical Analyses
3. Results
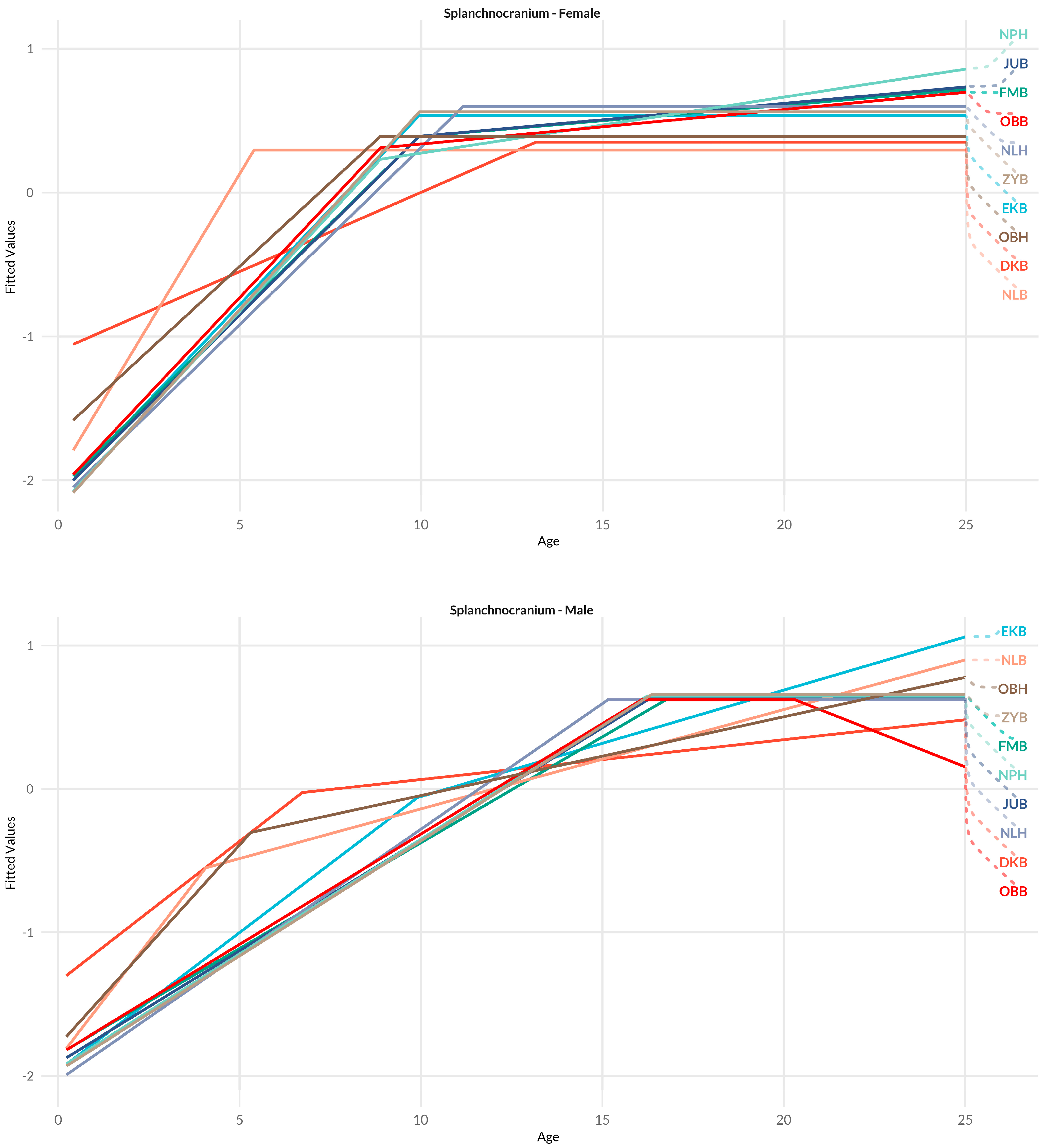
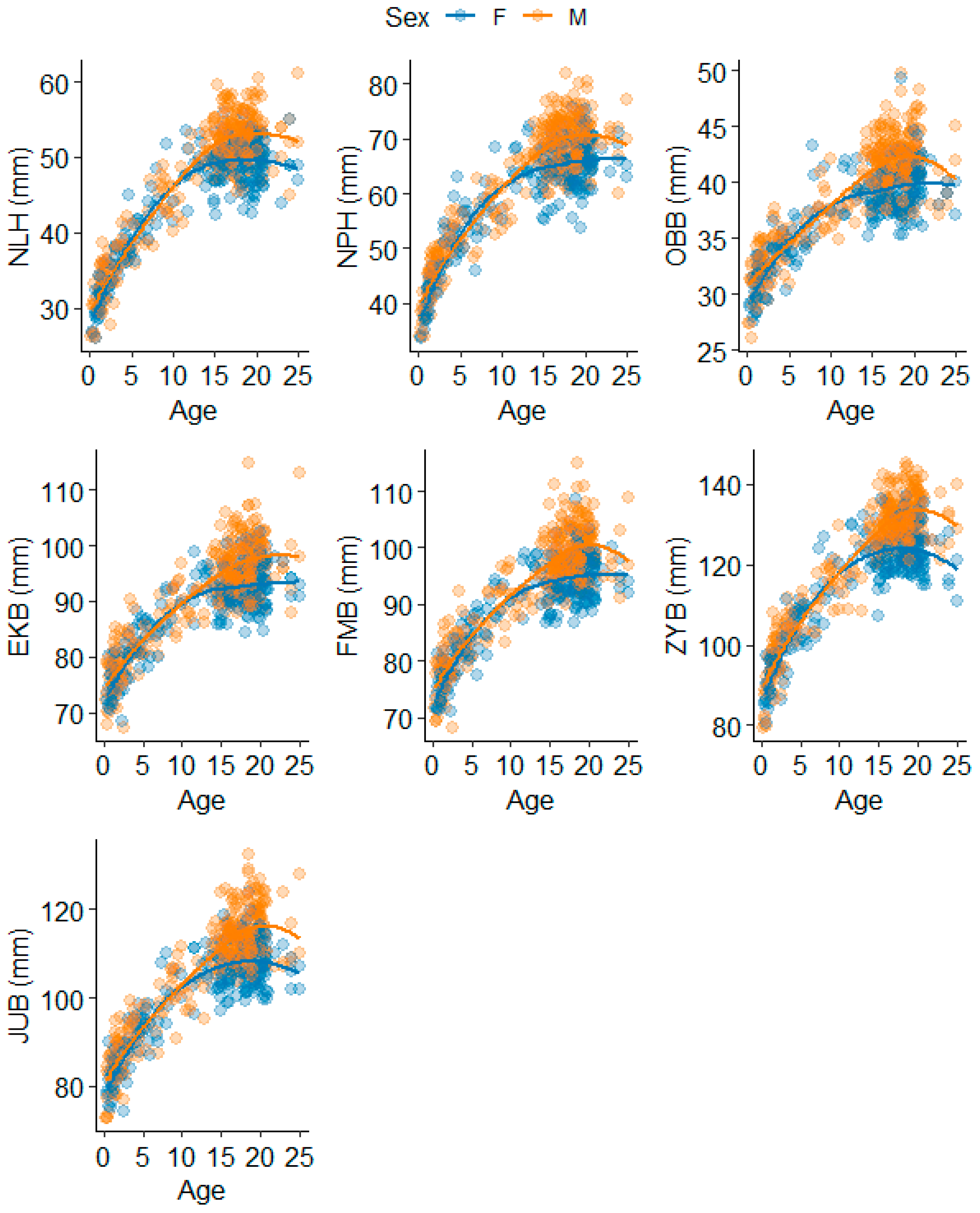
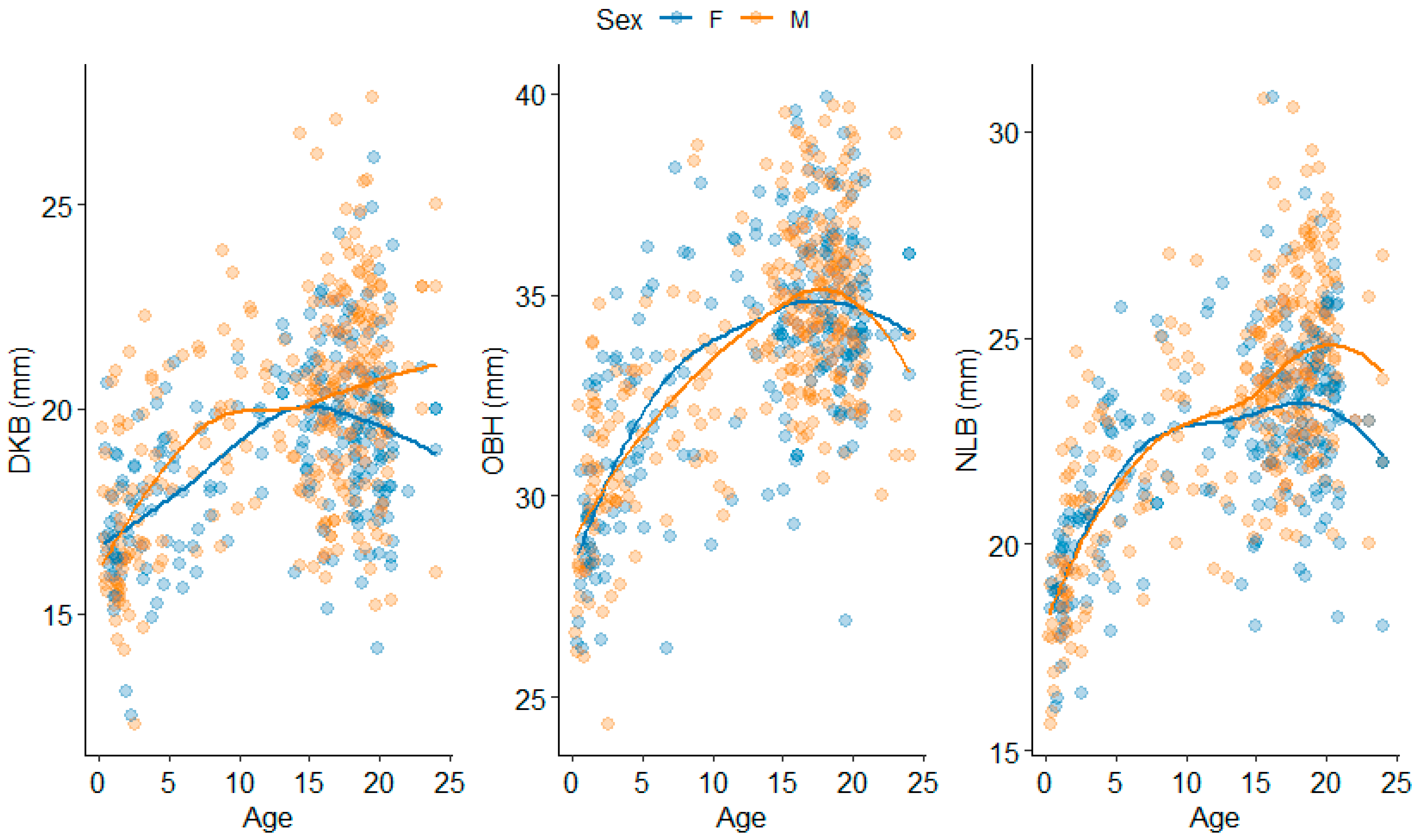
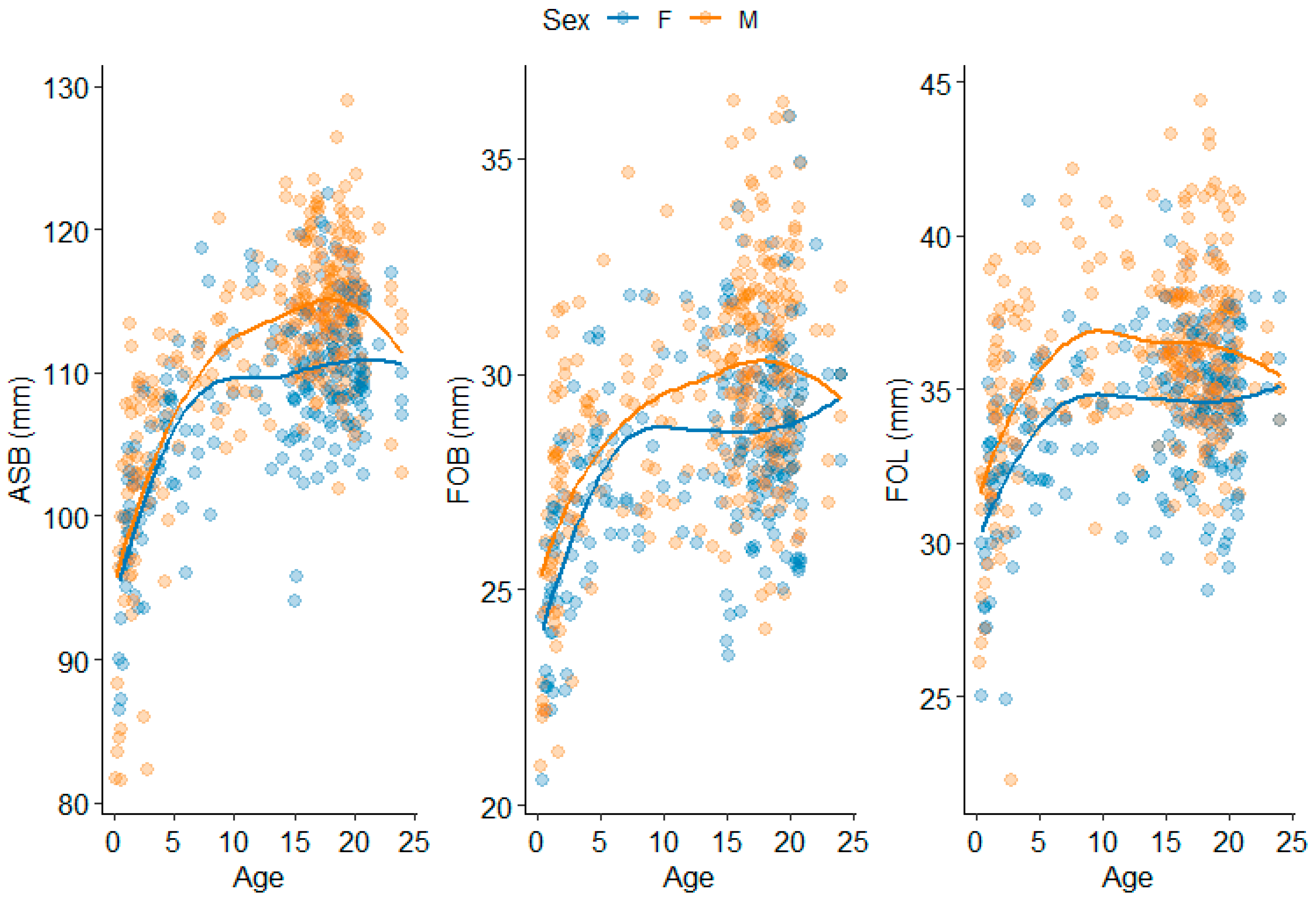
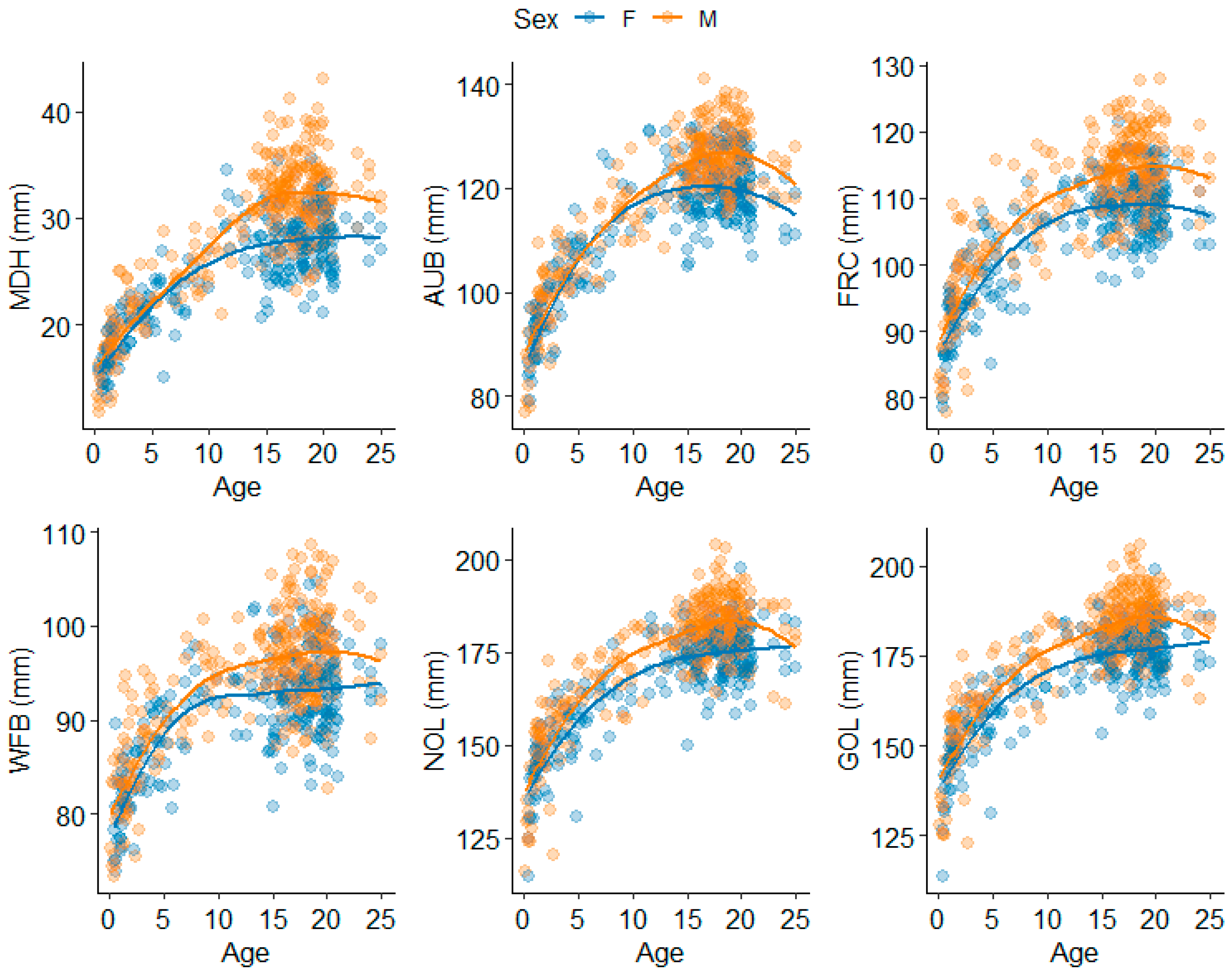
3.1. Splanchnocranium
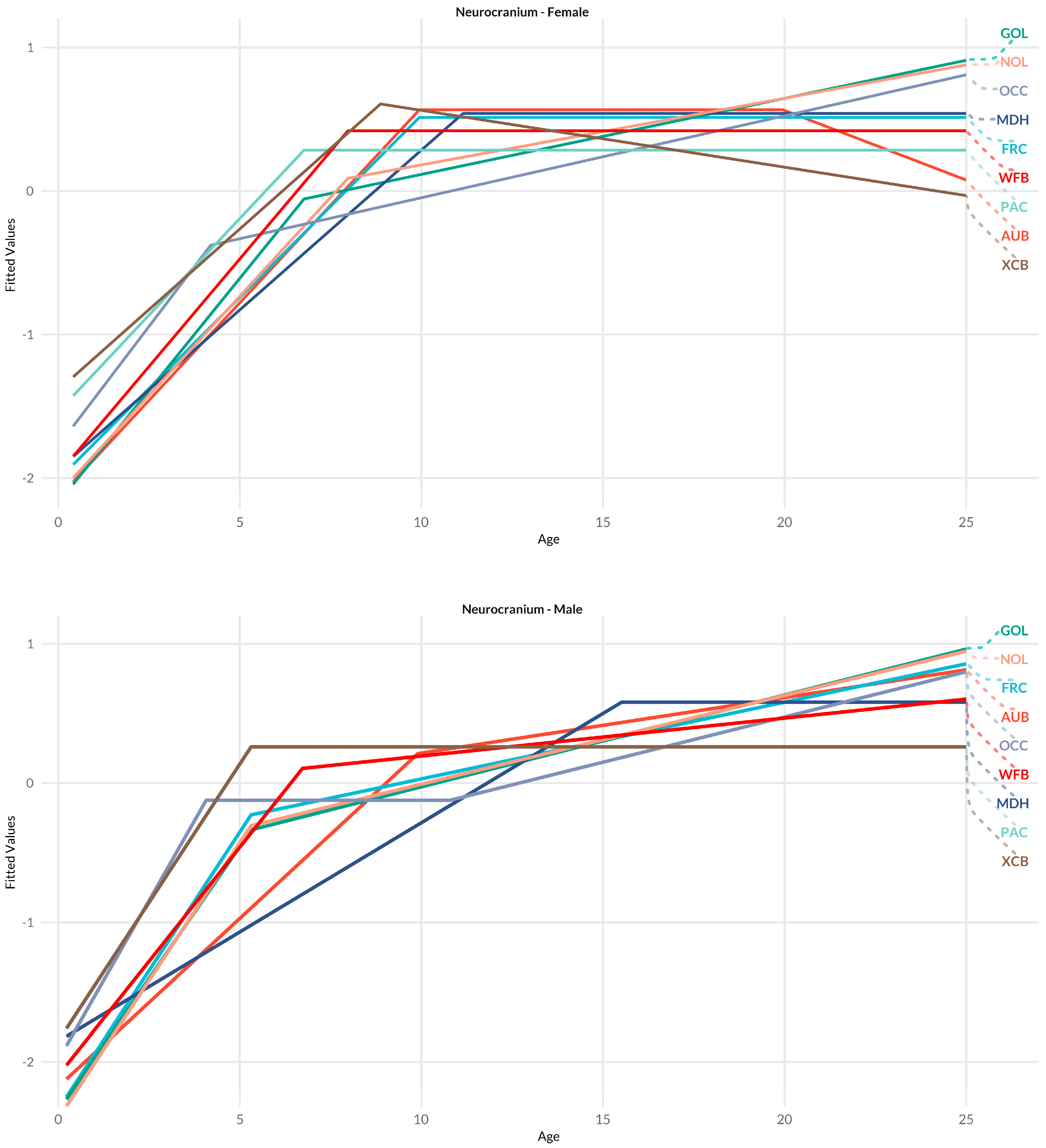
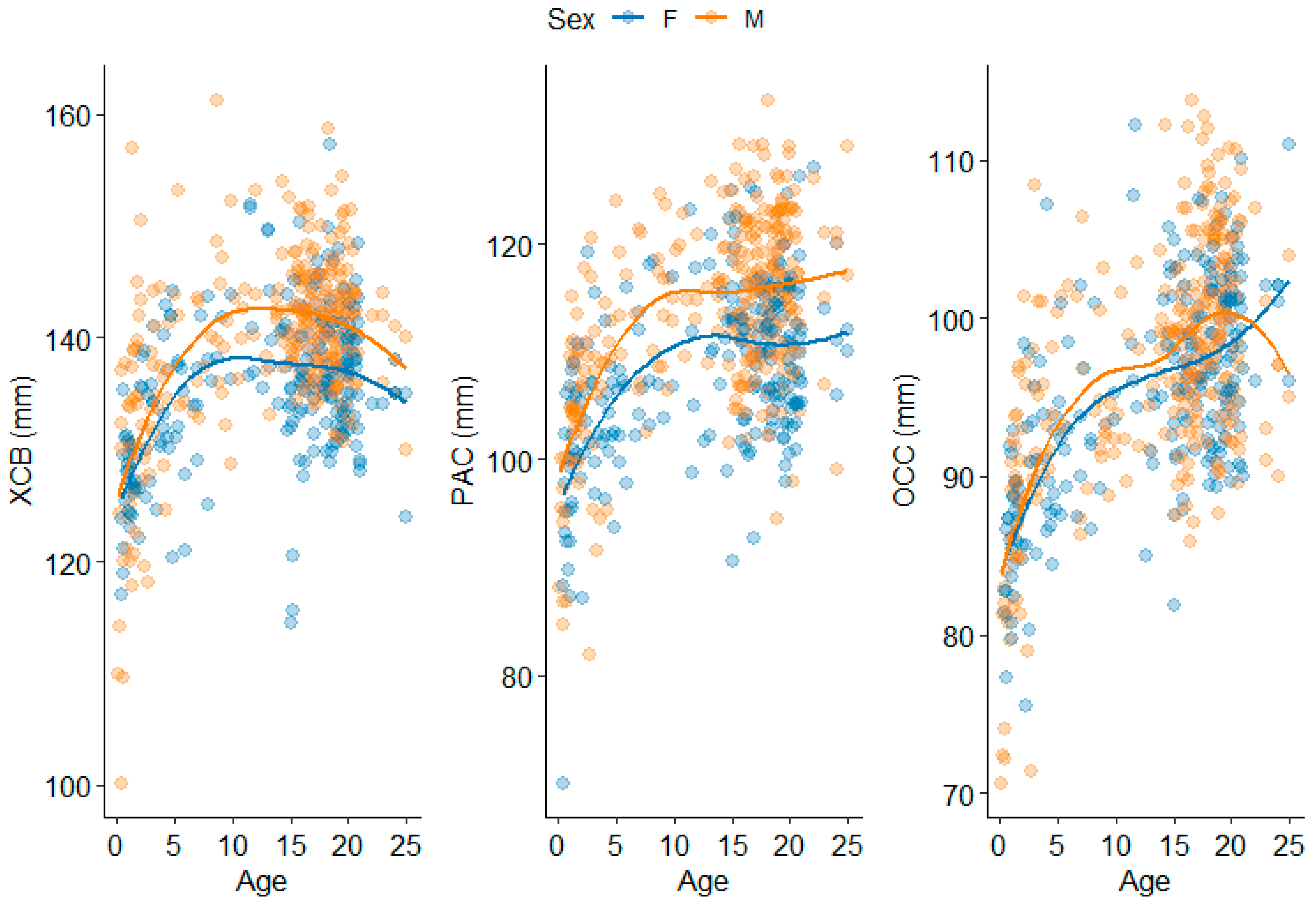
3.2. Neurocranium
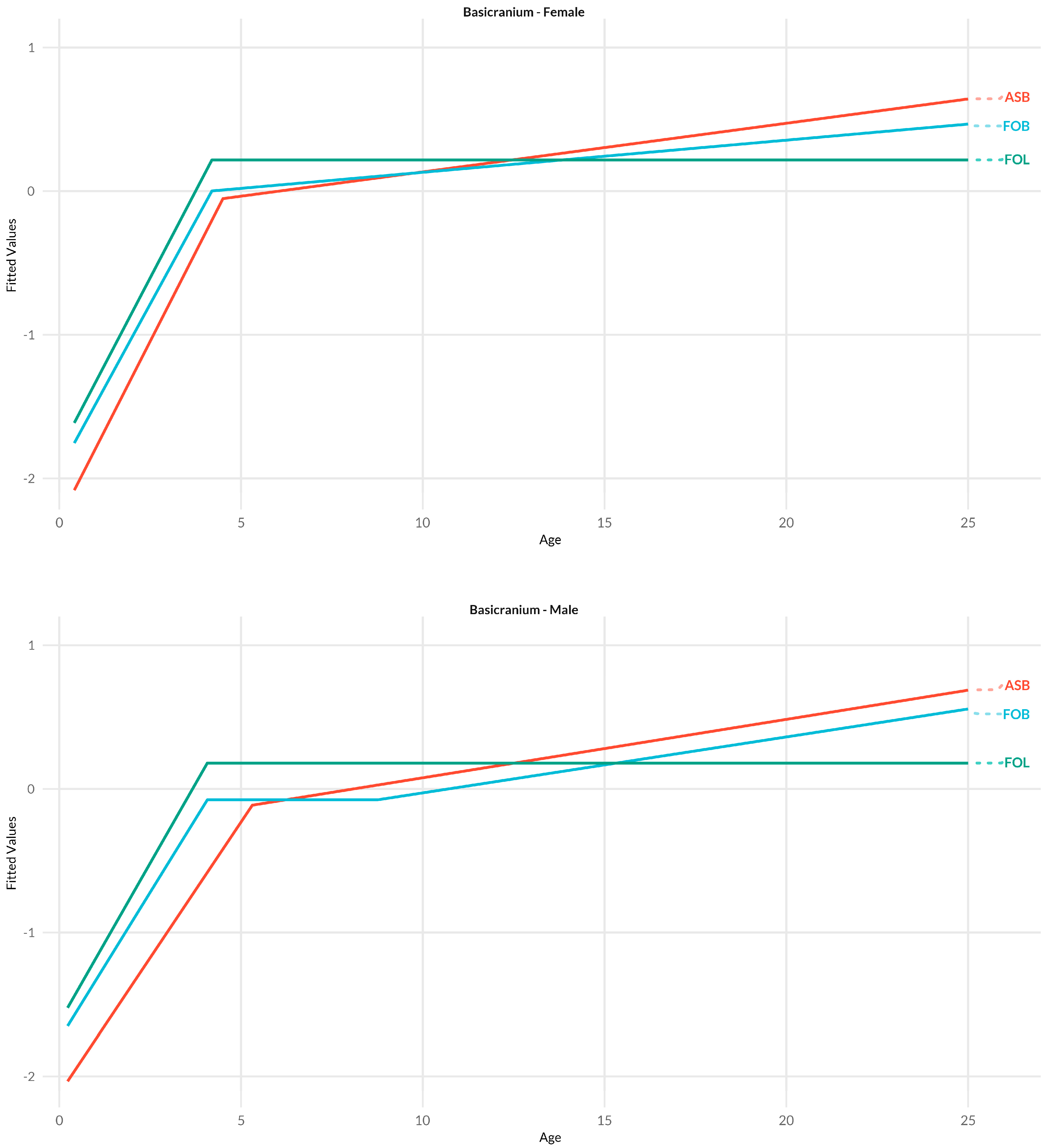
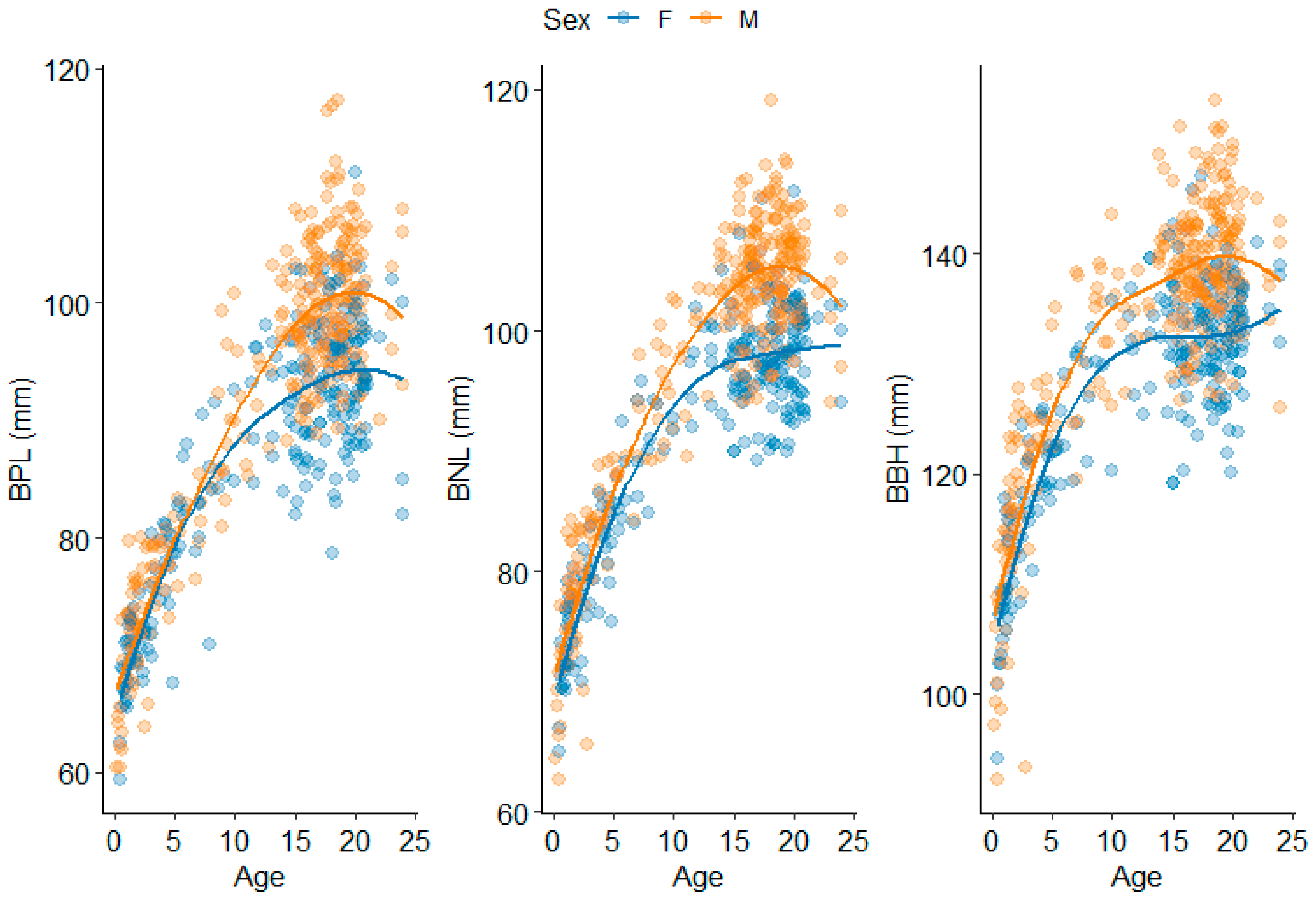
3.3. Basicranium
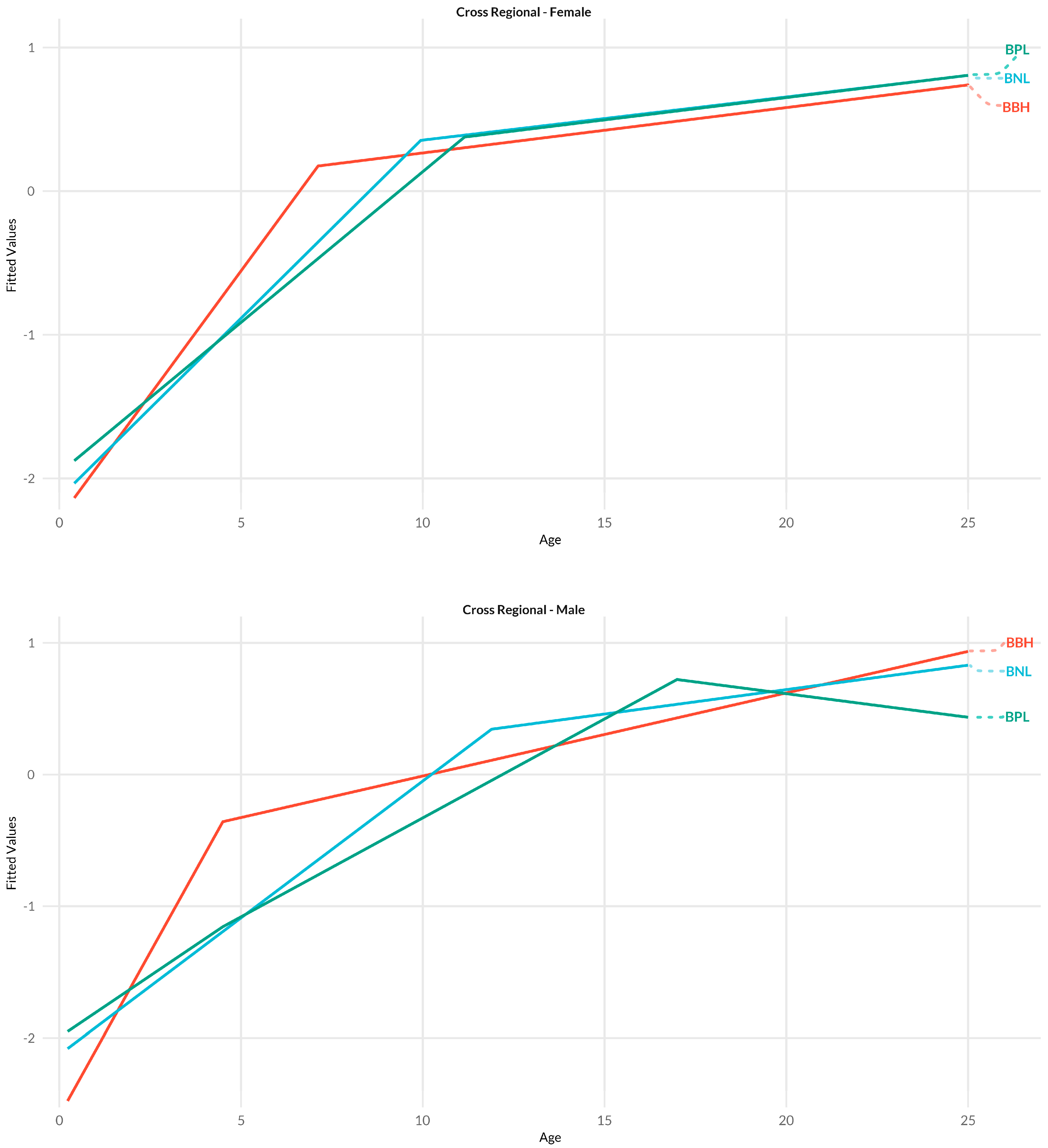
3.4. Cross-Regional
4. Discussion
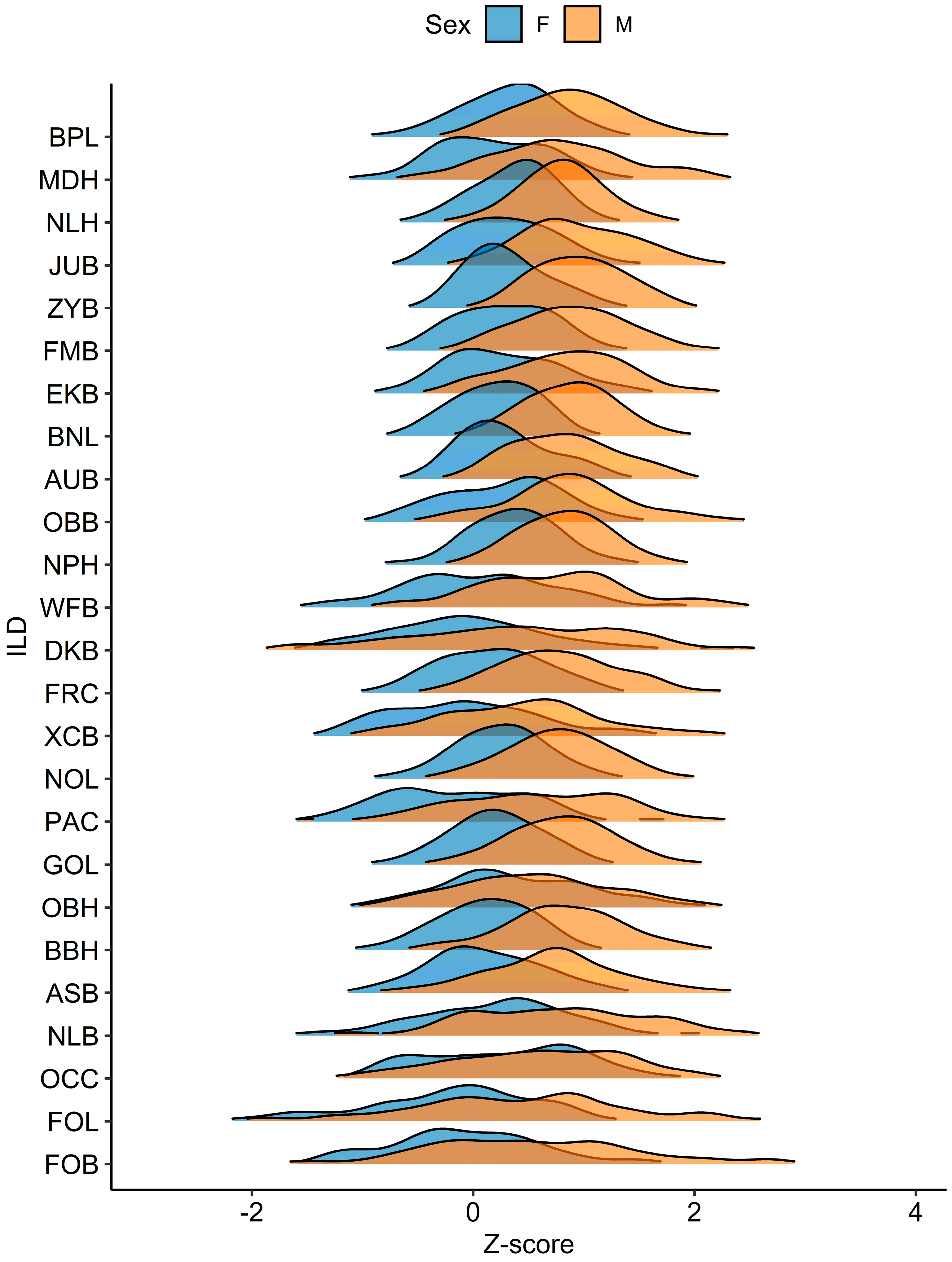
4.1. Age-Patterned ILD Variance and Growth Trajectories
4.1.1. Splanchnocranium
4.1.2. Neurocranium
4.1.3. Basicranium
4.1.4. Cross-Region
4.2. ILD Sexual Dimorphism
4.3. Forensic Implications of Exploring Ontogenetic Variation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stock, M.K. A Preliminary Analysis of the Age of Full Expression of Sexually Dimorphic Cranial Traits. J. Forensic Sci. 2018, 63, 1802–1808. [Google Scholar] [CrossRef]
- Coqueugniot, H.; Weaver, T.D. Brief Communication: Infracranial Maturation in the Skeletal Collection From Coimbra, Portugal: New Aging Standards for Ephiphyseal Union. Am. J. Phys. Anthropol. 2007, 134, 424–437. [Google Scholar] [CrossRef]
- Fazekas, I.G.; Kósa, F. Forensic Fetal Osteology; Akadémiai Kiadó: Budapest, Hungary, 1978; ISBN 978-963-05-1491-0. [Google Scholar]
- Konie, J.C. Comparative Value Of X-Rays Of The Spheno-Occipital Synchondrosis And Of The Wrist For Skeletal Age Assessment. Angle Orthod. 1964, 34, 303–313. [Google Scholar]
- Niel, M.; Adalian, P. New Models to Estimate Fetal and Young Infant Age with the Pars Basilaris Biometry. Forensic Sci. Int. 2023, 342, 111531. [Google Scholar] [CrossRef]
- Powell, T.V.; Brodie, A.G. Closure of the Spheno-occipital Synchondrosis. Anat. Rec. 1963, 147, 15–23. [Google Scholar] [CrossRef]
- Sahni, D.; Jit, I.; Neelam; Suri, S. Time of Fusion of the Basisphenoid with the Basilar Part of the Occipital Bone in Northwest Indian Subjects. Forensic Sci. Int. 1998, 98, 41–45. [Google Scholar] [CrossRef]
- Scheuer, L.; MacLaughlin-Black, S. Age Estimation from the Pars Basilaris of the Fetal and Juvenile Occipital Bone. Int. J. Osteoarchaeol. 1994, 4, 377–380. [Google Scholar] [CrossRef]
- Ubelaker, D.H.; Khosrowshahi, H. Estimation of Age in Forensic Anthropology: Historical Perspective and Recent Methodological Advances. Forensic Sci. Res. 2019, 4, 1–9. [Google Scholar] [CrossRef]
- Wood, C. The Age-Related Emergence of Cranial Morphological Variation. Forensic Sci. Int. 2015, 251, 220.e1–220.e20. [Google Scholar] [CrossRef]
- Cole, S.J. Developing Subadult Sex Estimation Standards Using Adult Morphological Sex Traits and an Ontogenetic Approach. Ph.D. Dissertation, University of Nevada, Reno, Reno, NV, USA, 2022. [Google Scholar]
- Saunders, S.R. Juvenile Skeletons and Growth-Related Studies. In Biological Anthropology of the Human Skeleton; Katzenberg, M.A., Saunders, S.R., Eds.; Wiley: Hoboken, NJ, USA, 2008; pp. 115–147. ISBN 978-0-471-79372-4. [Google Scholar]
- Corron, L.K.; Marchal, F.; Condemi, S.; Adalian, P. A Critical Review of Sub-Adult Age Estimation in Biological Anthropology: Do Methods Comply with Published Recommendations? Forensic Sci. Int. 2018, 288, 328.e1–328.e9. [Google Scholar] [CrossRef]
- Winburn, A.P.; Yim, A.; Stock, M.K. Recentering Forensic Anthropology within a Multifaceted Body of Evolutionary Theory: Strengthening Method by Making Theory Explicit. Am. J. Biol. Anthropol. 2022, 179, 535–551. [Google Scholar] [CrossRef]
- Anzelmo, M.; Barbeito-AndrÉs, J.; Ventrice, F.; Pucciarelli, H.M.; Sardi, M.L. Ontogenetic Patterns of Morphological Variation in the Ectocranial Human Vault. Anat. Rec. 2013, 296, 1008–1015. [Google Scholar] [CrossRef]
- Barbeito-Andrés, J.; Anzelmo, M.; Ventrice, F.; Pucciarelli, H.M.; Sardi, M.L. Morphological Integration of the Orbital Region in a Human Ontogenetic Sample: Integration of Human Orbital Region. Anat. Rec. 2016, 299, 70–80. [Google Scholar] [CrossRef]
- Bastir, M.; Rosas, A.; O’Higgins, P. Craniofacial Levels and the Morphological Maturation of the Human Skull: Spatiotemporal Pattern of Cranial Ontogeny. J. Anat. 2006, 209, 637–654. [Google Scholar] [CrossRef]
- Bulygina, E.; Mitteroecker, P.; Aiello, L. Ontogeny of Facial Dimorphism and Patterns of Individual Development within One Human Population. Am. J. Phys. Anthropol. 2006, 131, 432–443. [Google Scholar] [CrossRef]
- Evteev, A.; Anikin, A.; Satanin, L. Midfacial Growth Patterns in Males from Newborn to 5 Years Old Based on Computed Tomography. Am. J. Hum. Biol. 2018, 30, e23132. [Google Scholar] [CrossRef]
- Freidline, S.E.; Martinez-Maza, C.; Gunz, P.; Hublin, J.-J. Exploring Modern Human Facial Growth at the Micro- and Macroscopic Levels. In Building Bones: Bone Formation and Development in Anthropology; Percival, C.J., Richtsmeier, J.T., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 104–127. ISBN 978-1-316-38890-7. [Google Scholar]
- Hardin, A.M.; Knigge, R.P.; Oh, H.S.; Valiathan, M.; Duren, D.L.; McNulty, K.P.; Middleton, K.M.; Sherwood, R.J. Estimating Craniofacial Growth Cessation: Comparison of Asymptote- and Rate-Based Methods. Cleft Palate Craniofacial J. 2022, 59, 230–238. [Google Scholar] [CrossRef]
- Humphrey, L.T. Growth Patterns in the Modern Human Skeleton. Am. J. Phys. Anthr. 1998, 105, 57–72. [Google Scholar] [CrossRef]
- Jeffery, N.S.; Humphreys, C.; Manson, A. A Human Craniofacial Life-course: Cross-sectional Morphological Covariations during Postnatal Growth, Adolescence, and Aging. Anat. Rec. 2022, 305, 81–99. [Google Scholar] [CrossRef]
- Jeon, S.; Chung, J.H.; Baek, S.-H.; Yang, I.H.; Choi, K.Y.; Seo, H.J.; Shin, J.Y.; Kim, B.J. Characterization of Cranial Growth Patterns Using Craniometric Parameters and Best-Fit Logarithmic Growth Curves. J. Cranio-Maxillofac. Surg. 2024, 52, 30–39. [Google Scholar] [CrossRef]
- Liang, C.; Profico, A.; Buzi, C.; Khonsari, R.H.; Johnson, D.; O’Higgins, P.; Moazen, M. Normal Human Craniofacial Growth and Development from 0 to 4 Years. Sci. Rep. 2023, 13, 9641. [Google Scholar] [CrossRef]
- Lieberman, D.E.; McCarthy, R.C. The Ontogeny of Cranial Base Angulation in Humans and Chimpanzees and Its Implications for Reconstructing Pharyngeal Dimensions. J. Hum. Evol. 1999, 36, 487–517. [Google Scholar] [CrossRef]
- Neubauer, S.; Gunz, P.; Hublin, J. The Pattern of Endocranial Ontogenetic Shape Changes in Humans. J. Anat. 2009, 215, 240–255. [Google Scholar] [CrossRef]
- Niemann, K.; Lazarus, L.; Rennie, C.O. Developmental Changes of the Facial Skeleton from Birth to 18 Years within a South African Cohort (A Computed Tomography Study). J. Forensic Leg. Med. 2021, 83, 102243. [Google Scholar] [CrossRef]
- Noble, J.; Cardini, A.; Flavel, A.; Franklin, D. Geometric Morphometrics on Juvenile Crania: Exploring Age and Sex Variation in an Australian Population. Forensic Sci. Int. 2019, 294, 57–68. [Google Scholar] [CrossRef]
- Patcas, R.; Keller, H.; Markic, G.; Beit, P.; Eliades, T.; Cole, T.J. Craniofacial Growth and SITAR Growth Curve Analysis. Eur. J. Orthod. 2022, 44, 325–331. [Google Scholar] [CrossRef]
- Ross, A.H.; Williams, S.E. Craniofacial Growth, Maturation, and Change: Teens to Midadulthood. J. Craniofac. Surg. 2010, 21, 458–461. [Google Scholar] [CrossRef]
- Sardi, M.L.; Ramírez Rozzi, F.V. A Cross-Sectional Study of Human Craniofacial Growth. Ann. Hum. Biol. 2005, 32, 390–396. [Google Scholar] [CrossRef]
- Syutkina, T.; Anikin, A.; Satanin, L.; Evteev, A. Sexual Dimorphism in Human Midfacial Growth Patterns from Newborn to 5 Years Old Based on Computed Tomography. J. Anat. 2023, 242, 132–145. [Google Scholar] [CrossRef]
- Wellens, H.L.L.; Kuijpers-Jagtman, A.M.; Halazonetis, D.J. Geometric Morphometric Analysis of Craniofacial Variation, Ontogeny and Modularity in a Cross-Sectional Sample of Modern Humans. J. Anat. 2013, 222, 397–409. [Google Scholar] [CrossRef]
- Gkantidis, N.; Halazonetis, D.J. Morphological Integration between the Cranial Base and the Face in Children and Adults: Cranial Base and Face Integration. J. Anat. 2011, 218, 426–438. [Google Scholar] [CrossRef]
- Klingenberg, C.P. Cranial Integration and Modularity: Insights into Evolution and Development from Morphometric Data. Hystrix Ital. J. Mammal. 2013, 24, 43–58. [Google Scholar] [CrossRef]
- Lesciotto, K.M.; Richtsmeier, J.T. Craniofacial Skeletal Response to Encephalization: How Do We Know What We Think We Know? Am. J. Phys. Anthropol. 2019, 168, 27–46. [Google Scholar] [CrossRef]
- Mitteroecker, P.; Stansfield, E. A Model of Developmental Canalization, Applied to Human Cranial Form. PLoS Comput. Biol. 2021, 17, e1008381. [Google Scholar] [CrossRef]
- von Cramon-Taubadel, N. Multivariate Morphometrics, Quantitative Genetics, and Neutral Theory: Developing a “Modern Synthesis” for Primate Evolutionary Morphology. Evol. Anthropol. Issues News Rev. 2019, 28, 21–33. [Google Scholar] [CrossRef]
- Kawasaki, K.; Richtsmeier, J.T. Association of the Chondrocranium and Dermatocranium in Early Skull Formation. In Building Bones: Bone Formation and Development in Anthropology; Percival, C.J., Richtsmeier, J.T., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 52–78. ISBN 978-1-316-38890-7. [Google Scholar]
- Milella, M.; Franklin, D.; Belcastro, M.G.; Cardini, A. Sexual Differences in Human Cranial Morphology: Is One Sex More Variable or One Region More Dimorphic? Anat. Rec. 2021, 304, 2789–2810. [Google Scholar] [CrossRef]
- Mitteroecker, P.; Gunz, P.; Neubauer, S.; Müller, G. How to Explore Morphological Integration in Human Evolution and Development? Evol. Biol. 2012, 39, 536–553. [Google Scholar] [CrossRef]
- West-Eberhard, M.J. Modularity as a Universal Emergent Property of Biological Traits. J. Exp. Zoolog. B Mol. Dev. Evol. 2019, 332, 356–364. [Google Scholar] [CrossRef]
- Cheverud, J.M. Developmental Integration and the Evolution of Pleiotropy. Am. Zool. 1996, 36, 44–50. [Google Scholar] [CrossRef]
- González-José, R.; Van Der Molen, S.; González-Pérez, E.; Hernández, M. Patterns of Phenotypic Covariation and Correlation in Modern Humans as Viewed from Morphological Integration. Am. J. Phys. Anthropol. 2004, 123, 69–77. [Google Scholar] [CrossRef]
- Moss, M.L.; Young, R.W. A Functional Approach to Craniology. Am. J. Phys. Anthropol. 1960, 18, 281–292. [Google Scholar] [CrossRef]
- Carlson, B. Human Embryology and Developmental Biology, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Lieberman, D.E. Ontogeny, Homology, and Phylogeny in the Hominid Craniofacial Skeleton: The Problem of the Browridge. In Development, Growth and Evolution: Implications for the Study of Hominid Skeletal Evolution; O’Higgins, P., Cohn, M., Eds.; Academic Press: London, UK, 2000; pp. 85–122. [Google Scholar]
- Nie, X. Cranial Base in Craniofacial Development: Developmental Features, Influence on Facial Growth, Anomaly, and Molecular Basis. Acta. Odontol. Scand. 2005, 63, 127–135. [Google Scholar] [CrossRef]
- Lieberman, D.E.; Pearson, O.M.; Mowbray, K.M. Basicranial Influence on Overall Cranial Shape. J. Hum. Evol. 2000, 38, 291–315. [Google Scholar] [CrossRef]
- Cunningham, C.; Scheuer, L.; Black, S. Developmental Juvenile Osteology; Elsevier Science: Amsterdam, The Netherlands, 2016; ISBN 978-0-12-382107-2. [Google Scholar]
- Tubbs, R.S.; Bosmia, A.N.; Cohen-Gadol, A.A. The Human Calvaria: A Review of Embryology, Anatomy, Pathology, and Molecular Development. Childs Nerv. Syst. 2012, 28, 23–31. [Google Scholar] [CrossRef]
- von Cramon-Taubadel, N. The Relative Efficacy of Functional and Developmental Cranial Modules for Reconstructing Global Human Population History. Am. J. Phys. Anthropol. 2011, 146, 83–93. [Google Scholar] [CrossRef]
- von Cramon-Taubadel, N. Congruence of Individual Cranial Bone Morphology and Neutral Molecular Affinity Patterns in Modern Humans. Am. J. Phys. Anthropol. 2009, 140, 205–215. [Google Scholar] [CrossRef]
- Barbeito-Andrés, J.; Bonfili, N.; Nogué, J.M.; Bernal, V.; Gonzalez, P.N. Modeling the Effect of Brain Growth on Cranial Bones Using Finite-Element Analysis and Geometric Morphometrics. Surg. Radiol. Anat. 2020, 42, 741–748. [Google Scholar] [CrossRef]
- Frassanito, P.; Bianchi, F.; Pennisi, G.; Massimi, L.; Tamburrini, G.; Caldarelli, M. The Growth of the Neurocranium: Literature Review and Implications in Cranial Repair. Childs Nerv. Syst. 2019, 35, 1459–1465. [Google Scholar] [CrossRef]
- Garvin, H.M.; Ruff, C.B. Sexual Dimorphism in Skeletal Browridge and Chin Morphologies Determined Using a New Quantitative Method. Am. J. Phys. Anthr. 2012, 147, 661–670. [Google Scholar] [CrossRef]
- Kleisner, K.; Tureček, P.; Roberts, S.C.; Havlíček, J.; Valentova, J.V.; Akoko, R.M.; Leongómez, J.D.; Apostol, S.; Varella, M.A.C.; Saribay, S.A. How and Why Patterns of Sexual Dimorphism in Human Faces Vary across the World. Sci. Rep. 2021, 11, 5978. [Google Scholar] [CrossRef]
- Weston, E.M.; Friday, A.E.; Liò, P. Biometric Evidence That Sexual Selection Has Shaped the Hominin Face. PLoS ONE 2007, 2, e710. [Google Scholar] [CrossRef]
- Ousley, S.D.; Jantz, R.L. The Forensic Data Bank: Documenting Skeletal Trends in the United States. In Forensic Osteology: Advances in the Identification of Human Remains; Reichs, K.J., Ed.; Charles C Thomas: Springfield, IL, USA, 1998; pp. 441–458. [Google Scholar]
- Stull, K.E.; Corron, L.K. The Subadult Virtual Anthropology Database (SVAD): An Accessible Repository of Contemporary Subadult Reference Data. Forensic Sci. 2022, 2, 20–36. [Google Scholar] [CrossRef]
- Berry, S.D.; Edgar, H.J. Announcement: The New Mexico Decedent Image Database. Forensic Imaging 2021, 24, 200436. [Google Scholar] [CrossRef]
- Spradley, M.K.; Wolfe, C.A.; Stull, K.E.; Chu, E.Y.; Broehl, K.A.; Vlemincq-Mendieta, T.; Pilloud, M.A.; Scott, G.R.; Corron, L.K. Subadult Virtual Anthropology Database (SVAD) Data Collection Protocol: Cranial Landmarks and Craniometrics (2.0); Zenodo: Geneva, Switzerland, 2021. [Google Scholar] [CrossRef]
- Stull, K.E.; Corron, L.K. Subadult Virtual Anthropology Database (SVAD) Data Collection Protocol: Amira (1.0); Zenodo: Geneva, Switzerland, 2021. [Google Scholar] [CrossRef]
- Stock, M.K.; Garvin, H.M.; Corron, L.K.; Hulse, C.N.; Cirillo, L.E.; Klales, A.R.; Colman, K.L.; Stull, K.E. The Importance of Processing Procedures and Threshold Values in CT Scan Segmentation of Skeletal Elements: An Example Using the Immature Os Coxa. Forensic Sci. Int. 2020, 309, 110232. [Google Scholar] [CrossRef]
- Stock, M.K.; Stull, K.E.; Garvin, H.M.; Klales, A.R. Development of Modern Human Subadult Age and Sex Estimation Standards Using Multi-Slice Computed Tomography Images from Medical Examiner’s Offices. In Proceedings of the Proc. SPIE 9967, Developments in X-Ray Tomography X, San Diego, CA, USA, 29–31 August 2016; Volume 9967, pp. 99670E–99670E-14. [Google Scholar]
- Langley-Shirley, N.; Jantz, R.L. A Bayesian Approach to Age Estimation in Modern Americans from the Clavicle. J. Forensic Sci. 2010, 55, 571–583. [Google Scholar] [CrossRef]
- Chu, E.Y. Explorations into Appendicular Ontogeny Using a Cross-Sectional, Contemporary U.S. Sample. Ph.D. Dissertation, University of Nevada, Reno, Reno, NV, USA, 2022. [Google Scholar]
- Chu, E.Y.; Stull, K.E. An Investigation of the Relationship between Long Bone Measurements and Stature: Implications for Estimating Skeletal Stature in Subadults. Int. J. Legal Med. 2025, 139, 441–453. [Google Scholar] [CrossRef]
- Barbeito-Andrés, J.; Anzelmo, M.; Ventrice, F.; Sardi, M.L. Measurement Error of 3D Cranial Landmarks of an Ontogenetic Sample Using Computed Tomography. J. Oral Biol. Craniofacial Res. 2012, 2, 77–82. [Google Scholar] [CrossRef]
- Corron, L.K.; Marchal, F.; Condemi, S.; Chaumoître, K. Evaluating the Consistency, Repeatability, and Reproducibility of Osteometric Data on Dry Bone Surfaces, Scanned Dry Bone Surfaces, and Scanned Bone Surfaces Obtained from Living Individuals. Bull. Mém. Société Anthropol. Paris 2017, 29, 33. [Google Scholar] [CrossRef]
- Howells, W.W. Cranial Variation in Man: A Study by Multivariate Analysis of Patterns of Difference Among Recent Human Populations; Papers of the Peabody Museum of Archaeology and Ethnolgy; Harvard University Press: Cambridge, MA, USA, 1973; ISBN 0-87365-189-8. [Google Scholar]
- Langley, N.R.; Jantz, L.M.; Ousley, S.D.; Jantz, R.L.; Milner, G.R. Data Collection Procedures for Forensic Skeletal Material 2.0; Department of Anthropology, The University of Tennessee: Knoxville, TN, USA, 2016. [Google Scholar]
- Corron, L.K.; Broehl, K.A.; Chu, E.Y.; Vlemincq-Mendieta, T.; Wolfe, C.A.; Pilloud, M.A.; Scott, G.R.; Spradley, M.K.; Stull, K.E. Agreement and Error Rates Associated with Standardized Data Collection Protocols for Skeletal and Dental Data on 3D Virtual Subadult Crania. Forensic Sci. Int. 2022, 334, 111272. [Google Scholar] [CrossRef]
- Edgar, H.J.H.; Spradley, M.K.; Kamnikar, K.R.; McKeown, A.H. Roadmap to the Future: Calipers to Digitizing to Virtual Osteology. Forensic Anthropol. 2024, 7, 89. [Google Scholar] [CrossRef]
- Milborrow, S. Derived from mda:mars by T. Hastie and R. Tibshirani. Earth: Multivariate Adaptive Regression Spline Models (5.3.4). R Package. 2024. Available online: http://www.milbo.users.sonic.net/earth/ (accessed on 18 May 2025).
- Friedman, J.H. Multivariate Adaptive Regression Splines. Ann. Stat. 1991, 19, 1–67. [Google Scholar] [CrossRef]
- Friedman, J.H.; Roosen, C.B. An Introduction to Multivariate Adaptive Regression Splines. Stat. Methods Med. Res. 1995, 4, 197–217. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction, 2nd ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Corron, L.K.; Marchal, F.; Condemi, S.; Telmon, N.; Chaumoitre, K.; Adalian, P. Integrating Growth Variability of the Ilium, Fifth Lumbar Vertebra, and Clavicle with Multivariate Adaptive Regression Splines Models for Subadult Age Estimation. J. Forensic Sci. 2019, 64, 34–51. [Google Scholar] [CrossRef]
- Corron, L.K.; Marchal, F.; Condemi, S.; Chaumoître, K.; Adalian, P. A New Approach of Juvenile Age Estimation Using Measurements of the Ilium and Multivariate Adaptive Regression Splines (MARS) Models for Better Age Prediction. J. Forensic Sci. 2017, 62, 18–29. [Google Scholar] [CrossRef]
- Stull, K.E.; L’Abbé, E.N.; Ousley, S.D. Using Multivariate Adaptive Regression Splines to Estimate Subadult Age from Diaphyseal Dimensions. Am. J. Phys. Anthr. 2014, 154, 376–386. [Google Scholar] [CrossRef]
- Stull, K.E.; Chu, E.Y.; Corron, L.K.; Price, M.H. Subadult Age Estimation Using the Mixed Cumulative Probit and a Contemporary United States Population. Forensic Sci. 2022, 2, 741–779. [Google Scholar] [CrossRef]
- Milborrow, S. Notes on the Earth Package; R Vignette: 2024; pp. 1–68. Available online: http://www.milbo.org/doc/earth-notes.pdf (accessed on 19 May 2025).
- Middleton, K.M.; Duren, D.L.; McNulty, K.P.; Oh, H.; Valiathan, M.; Sherwood, R.J. Cross-Sectional Data Accurately Model Longitudinal Growth in the Craniofacial Skeleton. Sci. Rep. 2023, 13, 19294. [Google Scholar] [CrossRef]
- Bartholdy, B.P.; Hoogland, M.L.P.; Waters-Rist, A. How Old Are You Now? A New Ageing Method for Nonadults Based on Dental Wear. Int. J. Osteoarchaeol. 2019, 29, 622–633. [Google Scholar] [CrossRef]
- Nikita, E.; Nikitas, P. On the Use of Machine Learning Algorithms in Forensic Anthropology. Leg. Med. 2020, 47, 101771. [Google Scholar] [CrossRef]
- Stull, K.E.; Armelli, K. Combining Variables to Improve Subadult Age Estimation. Forensic Anthropol. 2021, 3, 203. [Google Scholar] [CrossRef]
- Krüger, G.C.; L’Abbé, E.N.; Stull, K.E. Sex Estimation from the Long Bones of Modern South Africans. Int. J. Legal Med. 2017, 131, 275–285. [Google Scholar] [CrossRef]
- Liebenberg, L.; L’Abbé, E.N.; Stull, K.E. Population Differences in the Postcrania of Modern South Africans and the Implications for Ancestry Estimation. Forensic Sci. Int. 2015, 257, 522–529. [Google Scholar] [CrossRef]
- Stull, K.E.; Corron, L.K.; Price, M.H. Subadult Age Estimation Variables: Exploring Their Varying Roles across Ontogeny. In Remodeling Forensic Skeletal Age; Elsevier: Amsterdam, The Netherlands, 2021; pp. 49–73. ISBN 978-0-12-824370-1. [Google Scholar]
- Kochanek, K.; Murphy, S.L.; Xu, J.; Arias, E. Mortality in the United States, 2022; NCHS Data Brief, no 492; National Center for Health Statistics: Hyattsville, MD, USA, 2023. [Google Scholar] [CrossRef]
- Stull, K.E.; Wolfe, C.A.; Corron, L.K.; Heim, K.; Hulse, C.N.; Pilloud, M.A. A Comparison of Subadult Skeletal and Dental Development Based on Living and Deceased Samples. Am. J. Phys. Anthropol. 2021, 175, 36–58. [Google Scholar] [CrossRef]
- Farkas, L.G.; Posnick, J.C.; Hreczko, T.M. Growth Patterns of the Face: A Morphometric Study. Cleft Palate Craniofacial J. 1992, 29, 308–315. [Google Scholar] [CrossRef]
- Snodell, S.F.; Nanda, R.S.; Currier, G.F. A Longitudinal Cephalometric Study of Transverse and Vertical Craniofacial Growth. Am. J. Orthod. Dentofacial Orthop. 1993, 104, 471–483. [Google Scholar] [CrossRef]
- Nanda, R.; Snodell, S.F.; Bollu, P. Transverse Growth of Maxilla and Mandible. Semin. Orthod. 2012, 18, 100–117. [Google Scholar] [CrossRef]
- Waitzman, A.A.; Posnick, J.C.; Armstrong, D.C.; Pron, G.E. Craniofacial Skeletal Measurements Based on Computed Tomography: Part I. Accuracy and Reproducibility. Cleft Palate Craniofac. J. 1992, 29, 112–117. [Google Scholar] [CrossRef]
- Bastir, M.; Megía, I.; Torres-Tamayo, N.; García-Martínez, D.; Piqueras, F.M.; Burgos, M. Three-dimensional Analysis of Sexual Dimorphism in the Soft Tissue Morphology of the Upper Airways in a Human Population. Am. J. Phys. Anthropol. 2020, 171, 65–75. [Google Scholar] [CrossRef]
- Butaric, L.N.; Nicholas, C.L.; Kravchuk, K.; Maddux, S.D. Ontogenetic Variation in Human Nasal Morphology. Anat. Rec. 2022, 305, 1910–1937. [Google Scholar] [CrossRef]
- Maddux, S.D.; Butaric, L.N.; Yokley, T.R.; Franciscus, R.G. Ecogeographic Variation across Morphofunctional Units of the Human Nose. Am. J. Phys. Anthropol. 2017, 162, 103–119. [Google Scholar] [CrossRef]
- Hall, B.K.; Precious, D.S. Cleft Lip, Nose, and Palate: The Nasal Septum as the Pacemaker for Midfacial Growth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, 442–447. [Google Scholar] [CrossRef]
- Holton, N.E.; Alsamawi, A.; Yokley, T.R.; Froehle, A.W. The Ontogeny of Nasal Shape: An Analysis of Sexual Dimorphism in a Longitudinal Sample: The Ontogeny of Nasal Shape. Am. J. Phys. Anthropol. 2016, 160, 52–61. [Google Scholar] [CrossRef]
- Holton, N.E.; Yokley, T.R.; Figueroa, A. Nasal Septal and Craniofacial Form in European- and African-derived Populations. J. Anat. 2012, 221, 263–274. [Google Scholar] [CrossRef]
- Scott, J.H. The Growth of the Human Face. Proc. R. Soc. Med. 1954, 47, 91–100. [Google Scholar] [CrossRef]
- Franciscus, R.G.; Long, J.C. Variation in Human Nasal Height and Breadth. Am. J. Phys. Anthropol. 1991, 85, 419–427. [Google Scholar] [CrossRef]
- Hubbe, M.; Hanihara, T.; Harvati, K. Climate Signatures in the Morphological Differentiation of Worldwide Modern Human Populations. Anat. Rec. 2009, 292, 1720–1733. [Google Scholar] [CrossRef]
- Noback, M.L.; Harvati, K.; Spoor, F. Climate-related Variation of the Human Nasal Cavity. Am. J. Phys. Anthropol. 2011, 145, 599–614. [Google Scholar] [CrossRef]
- Zaidi, A.A.; Mattern, B.C.; Claes, P.; McEvoy, B.; Hughes, C.; Shriver, M.D. Investigating the Case of Human Nose Shape and Climate Adaptation. PLoS Genet. 2018, 14, e1007207. [Google Scholar] [CrossRef]
- Bastir, M.; Godoy, P.; Rosas, A. Common Features of Sexual Dimorphism in the Cranial Airways of Different Human Populations. Am. J. Phys. Anthropol. 2011, 146, 414–422. [Google Scholar] [CrossRef]
- Landi, F.; Barraclough, J.; Evteev, A.; Anikin, A.; Satanin, L.; O’Higgins, P. The Role of the Nasal Region in Craniofacial Growth: An Investigation Using Path Analysis. Anat. Rec. 2022, 305, 1892–1909. [Google Scholar] [CrossRef]
- Ye, F.; Ji, Y.; Chen, Y.; He, F.; Fan, X. Orbital Growth Is Associated with Eyeball Size: A Study Using CT-Based Three-Dimensional Techniques. Curr. Eye Res. 2022, 47, 317–324. [Google Scholar] [CrossRef]
- Barbeito-Andres, J.; Pucciarelli, H.M.; Sardi, M.L. An Ontogenetic Approach to Facial Variation in Three Native American Populations. Homo-J. Comp. Hum. Biol. 2011, 62, 56–67. [Google Scholar] [CrossRef]
- Harvati, K.; Weaver, T.D. Human Cranial Anatomy and the Differential Preservation of Population History and Climate Signatures. Anat. Rec.—Part A Discov. Mol. Cell. Evol. Biol. 2006, 288, 1225–1233. [Google Scholar] [CrossRef]
- Hefner, J.T.; Plemons, A.M.; Kamnikar, K.R.; Ousley, S.D.; Linde, K.C. User Guide for MMS [Macromorphoscopic Traits] (v.1.6.1); Michigan State University: East Lansing, MI, USA, 2018. [Google Scholar]
- Martínez-Abadías, N.; Esparza, M.; Sjøvold, T.; González-José, R.; Santos, M.; Hernández, M. Heritability of Human Cranial Dimensions: Comparing the Evolvability of Different Cranial Regions. J. Anat. 2009, 214, 19–35. [Google Scholar] [CrossRef]
- Plemons, A.M. The Interaction Between Genetics and Climate on Craniofacial Variation: Examining the Causative Forces of Macromorphoscopic Trait Expression. Ph.D. Dissertation, Michigan State University, East Lansing, MI, USA, 2022. [Google Scholar]
- von Cramon-Taubadel, N. Evolutionary Insights into Global Patterns of Human Cranial Diversity: Population History, Climatic and Dietary Effects. J. Anthropol. Sci. 2014, 92, 43–77. [Google Scholar] [CrossRef]
- New, B.T.; Auchter, L.E.; Chu, E.Y.; Corron, L.K.; Go, M.C.; Hefner, J.T.; Spradley, M.K.; Wolfe, C.A.; Stull, K.E. Patterns of Covariation in Cranial Metric and Macromorphoscopic Variables. In Proceedings of the 76th Meeting of the American Academy of Forensic Sciences, Baltimore, MD, USA, 19–24 February 2025. [Google Scholar]
- Stull, K.E.; New, B.T.; Corron, L.; Auchter, L.E.; Spradley, K.; Wolfe, C.A.; Chu, E.Y.; Hefner, J.T. Exploring Mutual and Exclusive Biological Information in Cranial Metric and Morphological Variables. Forensic Anthropol. 2024, 7, 141. [Google Scholar] [CrossRef]
- Hefner, J.T.; Spradley, M.K.; Anderson, B. Ancestry Assessment Using Random Forest Modeling. J. Forensic Sci. 2014, 59, 583–589. [Google Scholar] [CrossRef]
- Liebenberg, L. South African Cranial Variation: A Combined Metric-Macromorphoscopic Method for Ancestry Estimation. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2023. [Google Scholar]
- Navega, D.; Coelho, C.; Vicente, R.; Ferreira, M.T.; Wasterlain, S.; Cunha, E. AncesTrees: Ancestry Estimation with Randomized Decision Trees. Int. J. Legal Med. 2015, 129, 1145–1153. [Google Scholar] [CrossRef]
- Spradley, M.K. Use of Craniometric Data to Facilitate Migrant Identifications at the United States/Mexico Border. Am. J. Phys. Anthropol. 2021, 175, 486–496. [Google Scholar] [CrossRef]
- Hallgrímsson, B.; Jamniczky, H.; Young, N.M.; Rolian, C.; Parsons, T.E.; Boughner, J.C.; Marcucio, R.S. Deciphering the Palimpsest: Studying the Relationship Between Morphological Integration and Phenotypic Covariation. Evol. Biol. 2009, 36, 355–376. [Google Scholar] [CrossRef]
- Friede, H. Normal Development and Growth of the Human Neurocranium and Cranial Base. Scand. J. Plast. Reconstr. Surg. 1981, 15, 163–169. [Google Scholar] [CrossRef]
- Pereira-Pedro, A.S.; Bruner, E. Craniofacial Orientation and Parietal Bone Morphology in Adult Modern Humans. J. Anat. 2022, 240, 330–338. [Google Scholar] [CrossRef]
- Kumar, A.; Vandekar, S.; Schilling, K.G.; Bhatia, A.; Landman, B.A.; Smith, S. Mapping Pediatric Spinal Cord Development with Age. In Proceedings of the Medical Imaging 2022: Image Processing, San Diego, CA, USA, 20–24 February 2022; Išgum, I., Colliot, O., Eds.; SPIE: San Diego, CA, USA, 2022; p. 41. [Google Scholar]
- Parenteau, C.S.; Wang, N.C.; Zhang, P.; Caird, M.S.; Wang, S.C. Quantification of Pediatric and Adult Cervical Vertebra—Anatomical Characteristics by Age and Gender for Automotive Application. Traffic Inj. Prev. 2014, 15, 572–582. [Google Scholar] [CrossRef]
- Corron, L.K.; Wolfe, C.A.; Stull, K.E. A Multifaceted Exploration of Ontogenetic Variation in Vertebral Neural Canal Size across Contemporary Populations. Am. J. Biol. Anthropol. 2022, 180, 328–351. [Google Scholar] [CrossRef]
- Lottering, N.; MacGregor, D.M.; Alston, C.L.; Watson, D.; Gregory, L.S. Introducing Computed Tomography Standards for Age Estimation of Modern Australian Subadults Using Postnatal Ossification Timings of Select Cranial and Cervical Sites. J. Forensic Sci. 2016, 61, S39–S52. [Google Scholar] [CrossRef]
- Miller, C.A.; Hwang, S.J.; Cotter, M.M.; Vorperian, H.K. Cervical Vertebral Body Growth and Emergence of Sexual Dimorphism: A Developmental Study Using Computed Tomography. J. Anat. 2019, 234, 764–777. [Google Scholar] [CrossRef]
- Ford, D.M.; McFadden, K.D.; Bagnall, K.M. Sequence of Ossification in Human Vertebral Neural Arch Centers. Anat. Rec. 1982, 203, 175–178. [Google Scholar] [CrossRef]
- Piatt, J.H.; Grissom, L.E. Developmental Anatomy of the Atlas and Axis in Childhood by Computed Tomography: Clinical Article. J. Neurosurg. Pediatr. 2011, 8, 235–243. [Google Scholar] [CrossRef]
- Smith, O.A.M.; Nashed, Y.S.G.; Duncan, C.; Pears, N.; Profico, A.; O’Higgins, P. 3D Modeling of Craniofacial Ontogeny and Sexual Dimorphism in Children. Anat. Rec. 2021, 304, 1918–1926. [Google Scholar] [CrossRef]
- Bogin, B. Patterns of Human Growth, 3rd ed.; Cambridge University Press: Cambridge, UK, 2020; ISBN 978-1-108-37997-7. [Google Scholar]
- Ellison, P.T. Endocrinology, Energetics, and Human Life History: A Synthetic Model. Horm. Behav. 2017, 91, 97–106. [Google Scholar] [CrossRef]
- Marshall, W.A.; Tanner, J.M. Variations in the Pattern of Pubertal Changes in Boys. Arch. Dis. Child. 1970, 45, 13–23. [Google Scholar] [CrossRef]
- Wheeler, M.D. Physical Changes of Puberty. Endocrinol. Metab. Clin. 1991, 20, 1–14. [Google Scholar] [CrossRef]
- Purkait, R. Growth of Cranial Volume: An Anthropometric Study. J. Plast. Reconstr. Aesthet. Surg. 2011, 64, e115–e117. [Google Scholar] [CrossRef]
- Klales, A.R.; Cole, S.J. MorphoPASSE: The Morphological Pelvis and Skull Sex Estimation Database Manual; Washburn University: Topeka, KS, USA, 2016; Volume 1. [Google Scholar]
- Walker, P.L. Sexing Skulls Using Discriminant Function Analysis of Visually Assessed Traits. Am. J. Phys. Anthropol. 2008, 136, 39–50. [Google Scholar] [CrossRef]
- Garvin, H.M. The Effects of Living Conditions on Human Cranial and Postcranial Sexual Dimorphism. Ph.D. Dissertation, Johns Hopkins University, Baltimore, MD, USA, 2012. [Google Scholar]
- Garvin, H.M.; Sholts, S.B.; Mosca, L.A. Sexual Dimorphism in Human Cranial Trait Scores: Effects of Population, Age, and Body Size. Am. J. Phys. Anthr. 2014, 154, 259–269. [Google Scholar] [CrossRef]
- Del Bove, A.; Menéndez, L.; Manzi, G.; Moggi-Cecchi, J.; Lorenzo, C.; Profico, A. Mapping Sexual Dimorphism Signal in the Human Cranium. Sci. Rep. 2023, 13, 16847. [Google Scholar] [CrossRef]
- Petaros, A.; Sholts, S.B.; Slaus, M.; Bosnar, A.; Wärmländer, S.K.T.S. Evaluating Sexual Dimorphism in the Human Mastoid Process: A Viewpoint on the Methodology. Clin. Anat. 2015, 28, 593–601. [Google Scholar] [CrossRef]
- L’Abbé, E.N.; Kenyhercz, M.; Stull, K.E.; Keough, N.; Nawrocki, S. Application of Fordisc 3.0 to Explore Differences Among Crania of North American and South African Blacks and Whites, J. Forensic Sci. 2013, 58, 1579–1583. [Google Scholar] [CrossRef]
- ANSI/ASB Standard 090; Standard for Sex Estimation in Forensic Anthropology, 1st ed. AAFS Standards Board: Colorado Springs, CO, USA, 2019.
- ANSI/ASB Standard 045; Standard for Stature Estimation in Forensic Anthropology, 1st ed. AAFS Standards Board: Colorado Springs, CO, USA, 2019.
- ANSI/ASB Standard 132; Standard for Population Affinity Estimation in Forensic Anthropology, 1st ed. AAFS Standards Board: Colorado Springs, CO, USA, 2023.
- Corron, L.K.; Santos, F.; Adalian, P.; Chaumoitre, K.; Guyomarc’h, P.; Marchal, F.; Brůžek, J. How Low Can We Go? A Skeletal Maturity Threshold for Probabilistic Visual Sex Estimation from Immature Human Os Coxae. Forensic Sci. Int. 2021, 325, 110854. [Google Scholar] [CrossRef]
- Auchter, L.E.; Stull, K. Development and Validation of a Subadult Sex Estimation Method Using Pelvic Metrics. Forensic Anthropol. 2025, 8, 19. [Google Scholar] [CrossRef]
- Sanchez, J.; Hoppa, R. Is Adulthood Required? Examining the Accuracy of Pelvic Sex Estimation Throughout Pubertal Growth. Bioarchaeology Int. 2022, 7, 173. [Google Scholar] [CrossRef]
- Klales, A.R. Current State of Sex Estimation in Forensic Anthropology. Forensic Anthropol. 2021, 4, 118–133. [Google Scholar] [CrossRef]
- Maier, C. Evaluating Mixed-Methods Models for the Estimation of Ancestry from Skeletal Remains. Forensic Anthropol. 2019, 2, 22–28. [Google Scholar] [CrossRef]
- Stull, K.E.; Wolfe, C.A.; Corron, L.K.; New, B.T.; Spradley, M.K. Growth and Development of the Cranial Complex and Its Implications for Sex Estimation. Forensic Sci. 2025, 5, 43. [Google Scholar] [CrossRef]
- Richtsmeier, J.T.; Lesciotto, K.M. From Phenotype to Genotype And Back Again. Bull. Mém. Société Anthropol. Paris 2020, 32, 8–17. [Google Scholar] [CrossRef]
- Roseman, C.C. Random Genetic Drift, Natural Selection, and Noise in Human Cranial Evolution. Am. J. Phys. Anthropol. 2016, 160, 582–592. [Google Scholar] [CrossRef]
- Carson, E.A. Maximum Likelihood Estimation of Human Craniometric Heritabilities. Am. J. Phys. Anthropol. 2006, 131, 169–180. [Google Scholar] [CrossRef]
- Katz, D.C.; Grote, M.N.; Weaver, T.D. A Mixed Model for the Relationship between Climate and Human Cranial Form. Am. J. Phys. Anthropol. 2016, 160, 593–603. [Google Scholar] [CrossRef]
- Tallman, S.; Kincer, C.; Plemons, E. Centering Transgender Individuals in Forensic Anthropology and Expanding Binary Sex Estimation in Casework and Research. Forensic Anthropol. 2021, 5, 161–180. [Google Scholar] [CrossRef]
- Meloro, R.; Tallman, S.D.; Streed, C.G.; Stowell, J.T.; Delgado, T.A.; Haug, J.D.; Redgrave, A.; Winburn, A.P. A Framework for Incorporating Diverse Gender Identities into Forensic Anthropology Casework and Theory: Recommendations for Inclusive Practices. Curr. Anthropol. 2025, 66, 1–27. [Google Scholar] [CrossRef]
- Márquez-Grant, N.; Baldini, E.; Jeynes, V.; Biehler-Gomez, L.; Aoukhiyad, L.; Passalacqua, N.V.; Giordano, G.; Di Candia, D.; Cattaneo, C. How Do Drugs Affect the Skeleton? Implications for Forensic Anthropology. Biology 2022, 11, 524. [Google Scholar] [CrossRef]
- Boogers, L.S.; Sikma, B.T.; Bouman, M.-B.; Van Trotsenburg, A.S.P.; Den Heijer, M.; Wiepjes, C.M.; Hannema, S.E. Shaping the Skeleton: Impact of GnRH Analogue and Sex Hormone Therapy on Skeletal Dimensions in Transgender Individuals. J. Clin. Endocrinol. Metab. 2025, 110, e1411–e1419. [Google Scholar] [CrossRef]
- Roseman, C.C. Detecting Interregionally Diversifying Natural Selection on Modern Human Cranial Form by Using Matched Molecular and Morphometric Data. Proc. Natl. Acad. Sci. USA 2004, 101, 12824–12829. [Google Scholar] [CrossRef]
- Angel, J.L. A New Measure of Growth Efficiency: Skull Base Height. Am. J. Phys. Anthropol. 1982, 58, 297–305. [Google Scholar] [CrossRef]
- Senator, M.; Kwiatkowska, B.; Gronkiewicz, S. Height of Skull Base as an Indicator of Living Conditions in Historical Native Populations from Europe, Australia and Africa. HOMO 2009, 60, 535–549. [Google Scholar] [CrossRef]
- Wescott, D.J.; Jantz, R.L. Assessing Craniofacial Secular Change in American Blacks and Whites Using Geometric Morphometry. In Modern Morphometrics in Physical Anthropology; Developments in Primatology: Progress and Prospects; Slice, D.E., Ed.; Kluwer Academic Publishers-Plenum Publishers: New York, NY, USA, 2005; pp. 231–245. ISBN 978-0-306-48697-5. [Google Scholar]
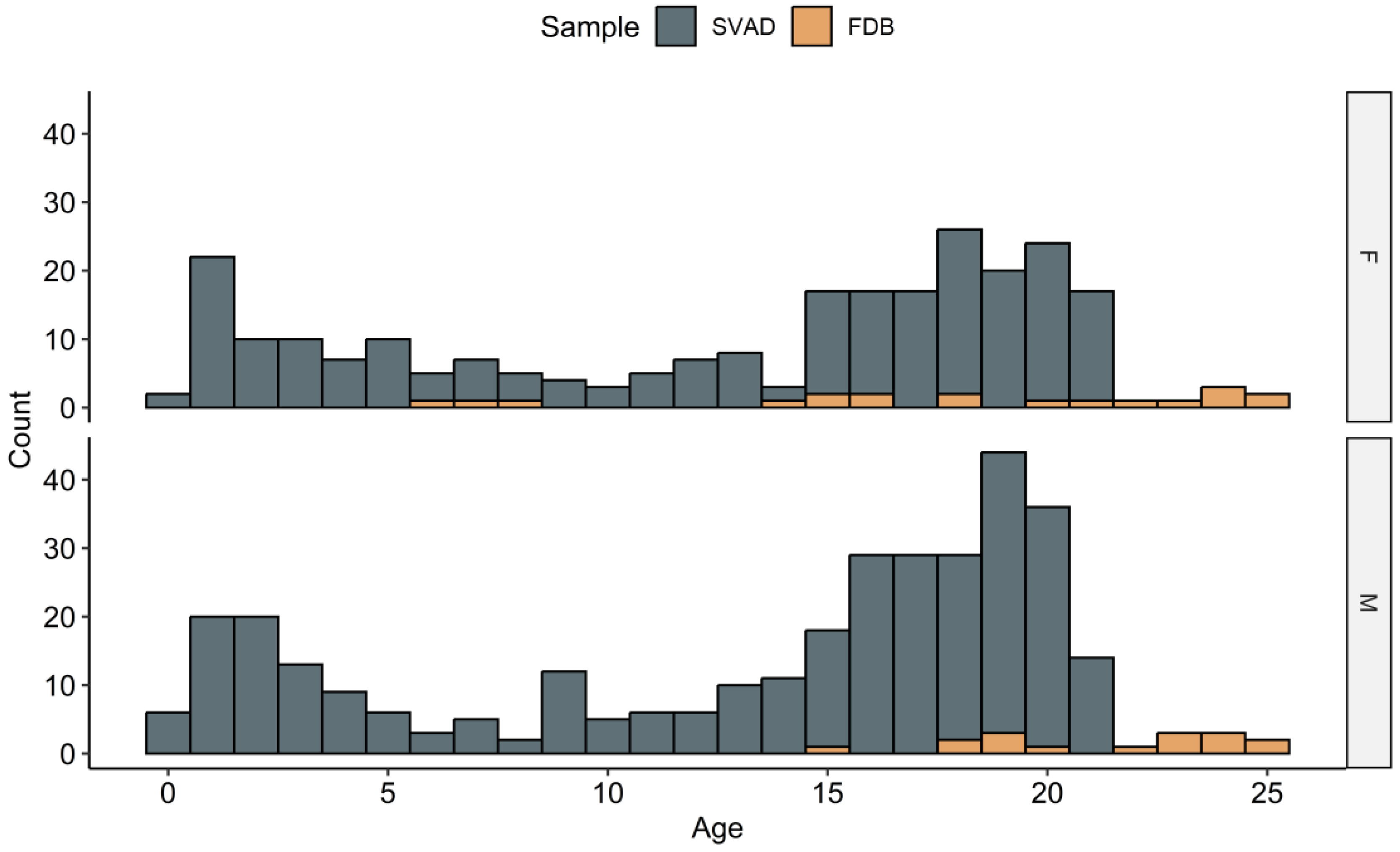
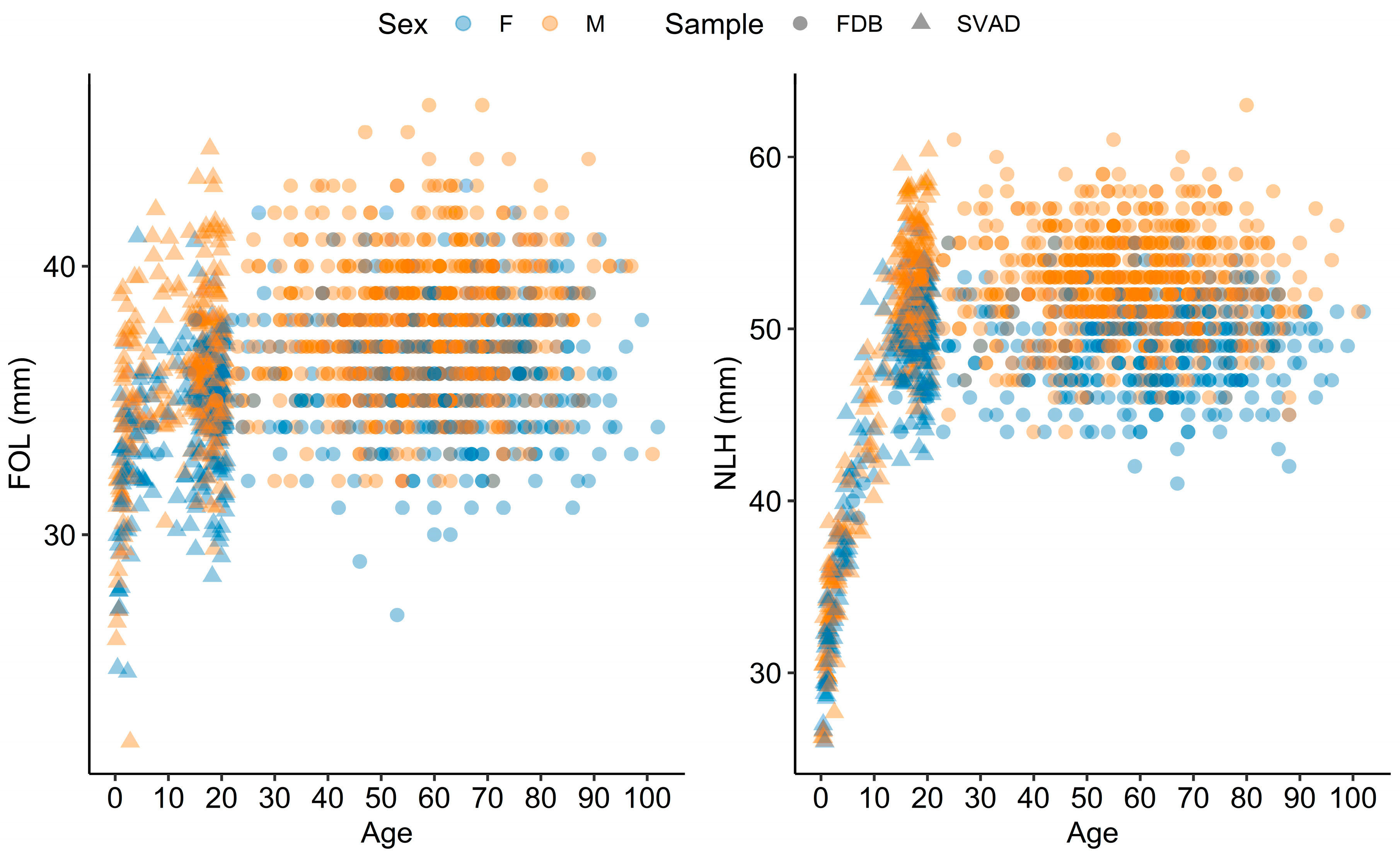
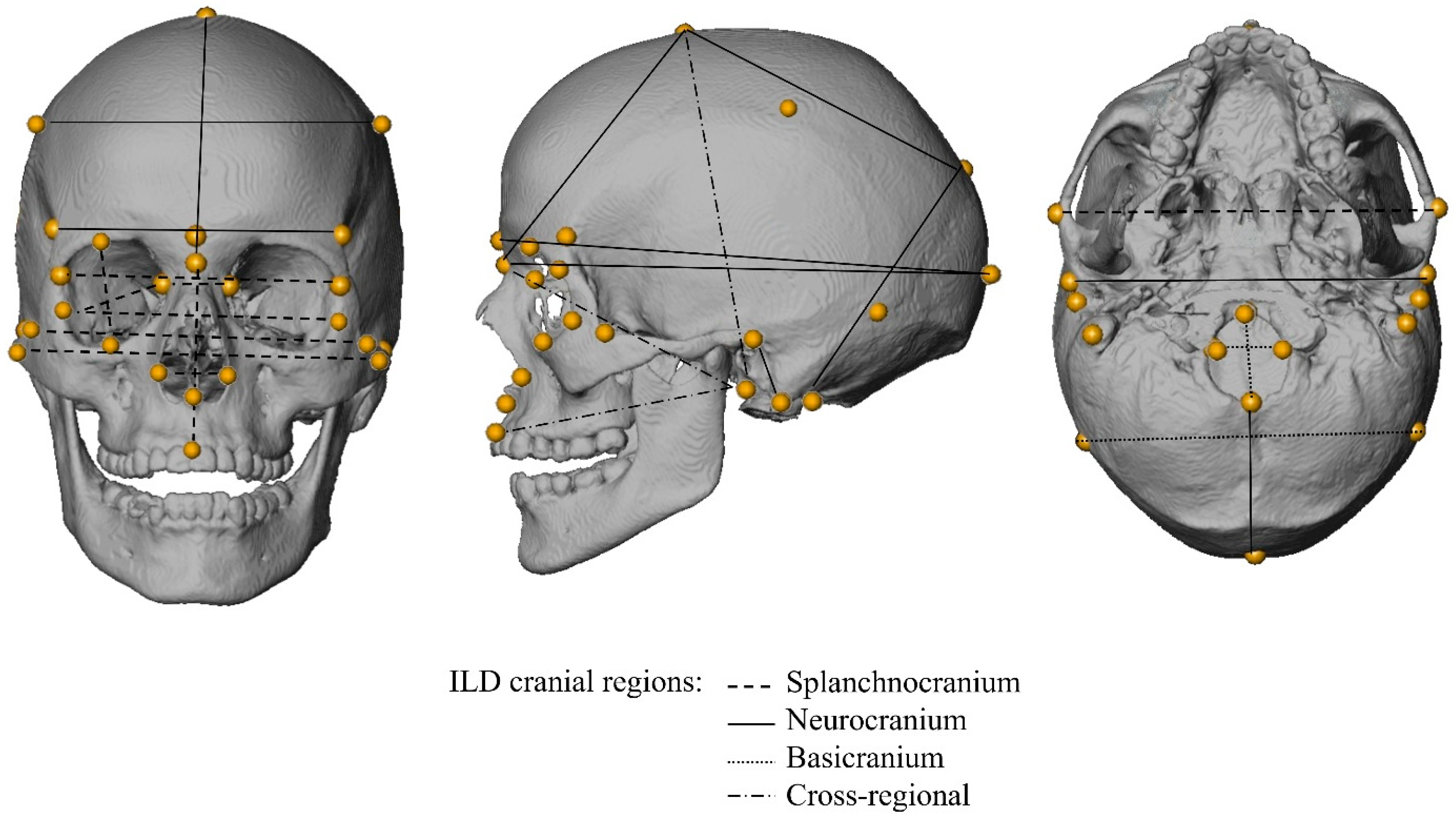
| Region | ILD | Abbreviation | Definitions |
|---|---|---|---|
| Splanchnocranium | Nasal Height | NLH | Nasion to most inferior nasal border (L) |
| Nasion-Prosthion Height | NPH | Nasion to prosthion | |
| Nasal Breadth | NLB | Alare (L) to alare (R) | |
| Orbital Height | OBH | Orbit height inferior (R) to orbit height superior (R) | |
| Orbital Breadth | OBB | Dacryon (R) to ectoconchion (R) | |
| Interorbital Breadth | DKB | Dacryon (L) to dacryon (R) | |
| Biorbital Breadth | EKB | Ectoconchion (L) to ectoconchion (R) | |
| Bifrontal Breadth | FMB | Frontomalare anterior (L) to frontomalare anterior (R) | |
| Bizygomatic Breadth | ZYB | Zygion (L) to zygion (R) | |
| Bijugal Breadth | JUB | Jugale (L) to jugale (R) | |
| Neurocranium | Maximum Cranial Length | GOL | Glabella to opisthocranion |
| Nasio-occipital Length | NOL | Nasion to opisthocranion | |
| Mastoid Height | MDH | Porion (R) to mastoideale (R) | |
| Maximum Cranial Breadth | XCB | Euryon (L) to euryon (R) | |
| Minimum Frontal Breadth | WFB | Frontotemporale (L) to frontotemporale (R) | |
| Frontal Chord | FRC | Nasion to bregma | |
| Parietal Chord | PAC | Bregma to lambda | |
| Occipital Chord | OCC | Lambda to opisthion | |
| Biauricular Breadth | AUB | Radiculare (L) to radiculare (R) | |
| Basicranium | Biasterionic Breadth | ASB | Asterion (L) to asterion (R) |
| Foramen Magnum Length | FOL | Basion to opisthion | |
| Foramen Magnum Breadth | FOB | Foramen magnum breadth (L) to foramen magnum breadth (R) | |
| Cross-Region | Cranial Base Length | BNL | Basion to nasion |
| Basion-Bregma Height | BBH | Basion to bregma | |
| Basion-Prosthion Length | BPL | Basion to prosthion |
| Female | Male | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cranial Region | ILD | Knots h(Age − t) | GCV | GRSq | CVRSq | Knots h(Age − t) | GCV | GRSq | CVRSq |
| Splanchnocranium | NLH | 11.1 | 0.14 | 0.86 | 0.84 | 15.2 | 0.14 | 0.86 | 0.85 |
| NPH | 8.9 | 0.17 | 0.83 | 0.79 | 16.2 | 0.17 | 0.83 | 0.83 | |
| NLB | 5.4 | 0.63 | 0.37 | 0.3 | 4.1 | 0.55 | 0.45 | 0.43 | |
| OBH | 8.9 | 0.58 | 0.43 | 0.34 | 5.3 | 0.58 | 0.42 | 0.37 | |
| OBB | 8.9 | 0.30 | 0.71 | 0.67 | 16.1 | 0.26 | 0.74 | 0.71 | |
| 20.3 | |||||||||
| DKB | 13.2 | 0.76 | 0.25 | 0.19 | 6.7 | 0.77 | 0.23 | 0.21 | |
| EKB | 9.9 | 0.25 | 0.75 | 0.7 | 9.9 | 0.26 | 0.74 | 0.7 | |
| FMB | 9.9 | 0.23 | 0.77 | 0.73 | 17.0 | 0.23 | 0.77 | 0.75 | |
| ZYB | 9.9 | 0.18 | 0.82 | 0.79 | 16.4 | 0.15 | 0.85 | 0.84 | |
| JUB | 9.9 | 0.21 | 0.79 | 0.76 | 16.4 | 0.20 | 0.80 | 0.78 | |
| Neurocranium | GOL | 6.8 | 0.28 | 0.72 | 0.65 | 5.3 | 0.29 | 0.71 | 0.7 |
| NOL | 8.0 | 0.26 | 0.74 | 0.68 | 5.3 | 0.27 | 0.73 | 0.71 | |
| MDH | 11.1 | 0.30 | 0.70 | 0.67 | 15.5 | 0.28 | 0.73 | 0.7 | |
| XCB | 8.9 | 0.76 | 0.24 | 0.17 | 5.3 | 0.70 | 0.30 | 0.21 | |
| WFB | 8.0 | 0.46 | 0.54 | 0.5 | 6.7 | 0.45 | 0.55 | 0.51 | |
| FRC | 9.9 | 0.32 | 0.68 | 0.62 | 5.3 | 0.34 | 0.66 | 0.62 | |
| PAC | 6.8 | 0.73 | 0.28 | 0.15 | 5.3 | 0.70 | 0.30 | 0.26 | |
| OCC | 4.2 | 0.61 | 0.39 | 0.35 | 4.1 | 0.65 | 0.35 | 0.33 | |
| 10.8 | |||||||||
| AUB | 9.9 | 0.23 | 0.77 | 0.73 | 9.9 | 0.20 | 0.80 | 0.79 | |
| 20.0 | |||||||||
| Basicranium | ASB | 4.5 | 0.49 | 0.51 | 0.47 | 5.3 | 0.50 | 0.50 | 0.45 |
| FOL | 4.2 | 0.76 | 0.24 | 0.16 | 4.1 | 0.83 | 0.17 | 0.12 | |
| FOB | 4.2 | 0.69 | 0.32 | 0.25 | 4.1 | 0.75 | 0.25 | 0.22 | |
| 8.6 | |||||||||
| Cross-Region | BNL | 9.9 | 0.16 | 0.84 | 0.81 | 11.9 | 0.15 | 0.85 | 0.83 |
| BBH | 7.1 | 0.27 | 0.73 | 0.69 | 4.5 | 0.27 | 0.73 | 0.7 | |
| BPL | 11.1 | 0.23 | 0.77 | 0.73 | 17.0 | 0.19 | 0.81 | 0.8 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
New, B.T.; Stull, K.E.; Corron, L.K.; Wolfe, C.A. Exploring Cranial Growth Patterns from Birth to Adulthood for Forensic Research and Practice. Forensic Sci. 2025, 5, 32. https://doi.org/10.3390/forensicsci5030032
New BT, Stull KE, Corron LK, Wolfe CA. Exploring Cranial Growth Patterns from Birth to Adulthood for Forensic Research and Practice. Forensic Sciences. 2025; 5(3):32. https://doi.org/10.3390/forensicsci5030032
Chicago/Turabian StyleNew, Briana T., Kyra E. Stull, Louise K. Corron, and Christopher A. Wolfe. 2025. "Exploring Cranial Growth Patterns from Birth to Adulthood for Forensic Research and Practice" Forensic Sciences 5, no. 3: 32. https://doi.org/10.3390/forensicsci5030032
APA StyleNew, B. T., Stull, K. E., Corron, L. K., & Wolfe, C. A. (2025). Exploring Cranial Growth Patterns from Birth to Adulthood for Forensic Research and Practice. Forensic Sciences, 5(3), 32. https://doi.org/10.3390/forensicsci5030032






