A New Species of the Genus Ptyctolaemus Peters, 1864 (Squamata, Agamidae) from Yunnan, China †
Abstract
1. Introduction
2. Materials and Methods
3. Results
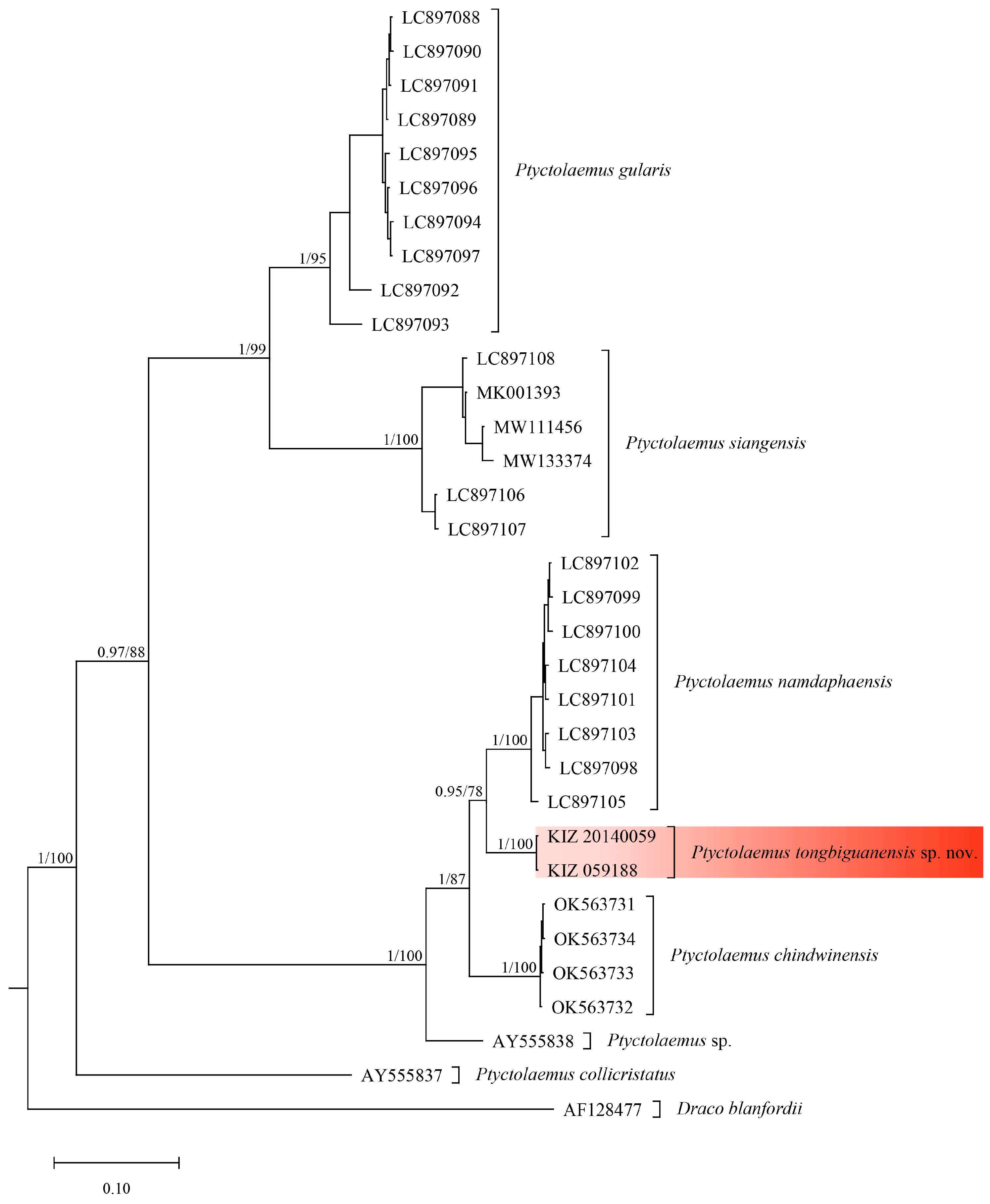
3.1. Phylogenetic Relationship
3.2. Morphological Analysis
3.3. Taxonomic Account
3.4. Etymology
3.5. Diagnosis
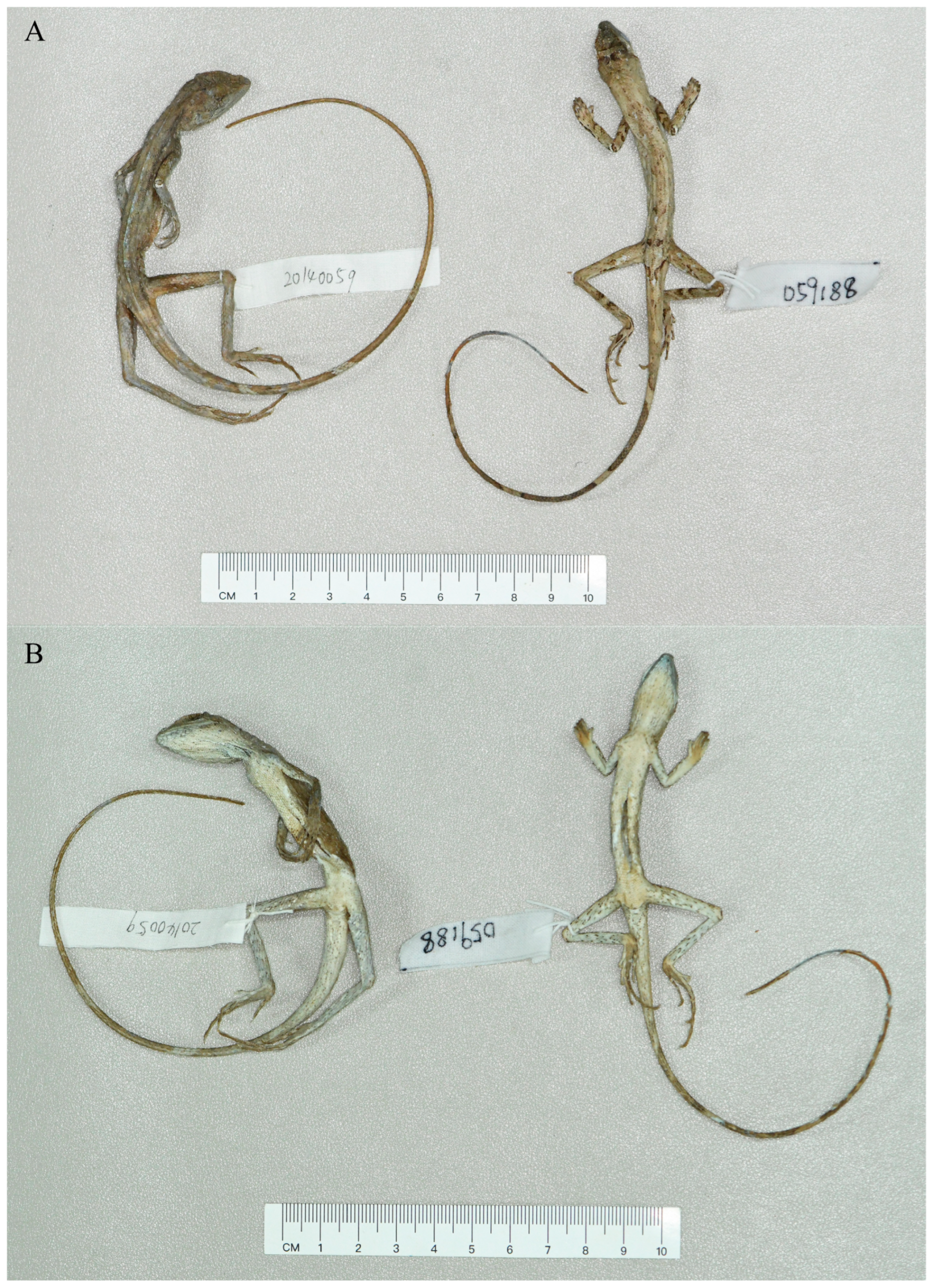
3.6. Description of Holotype
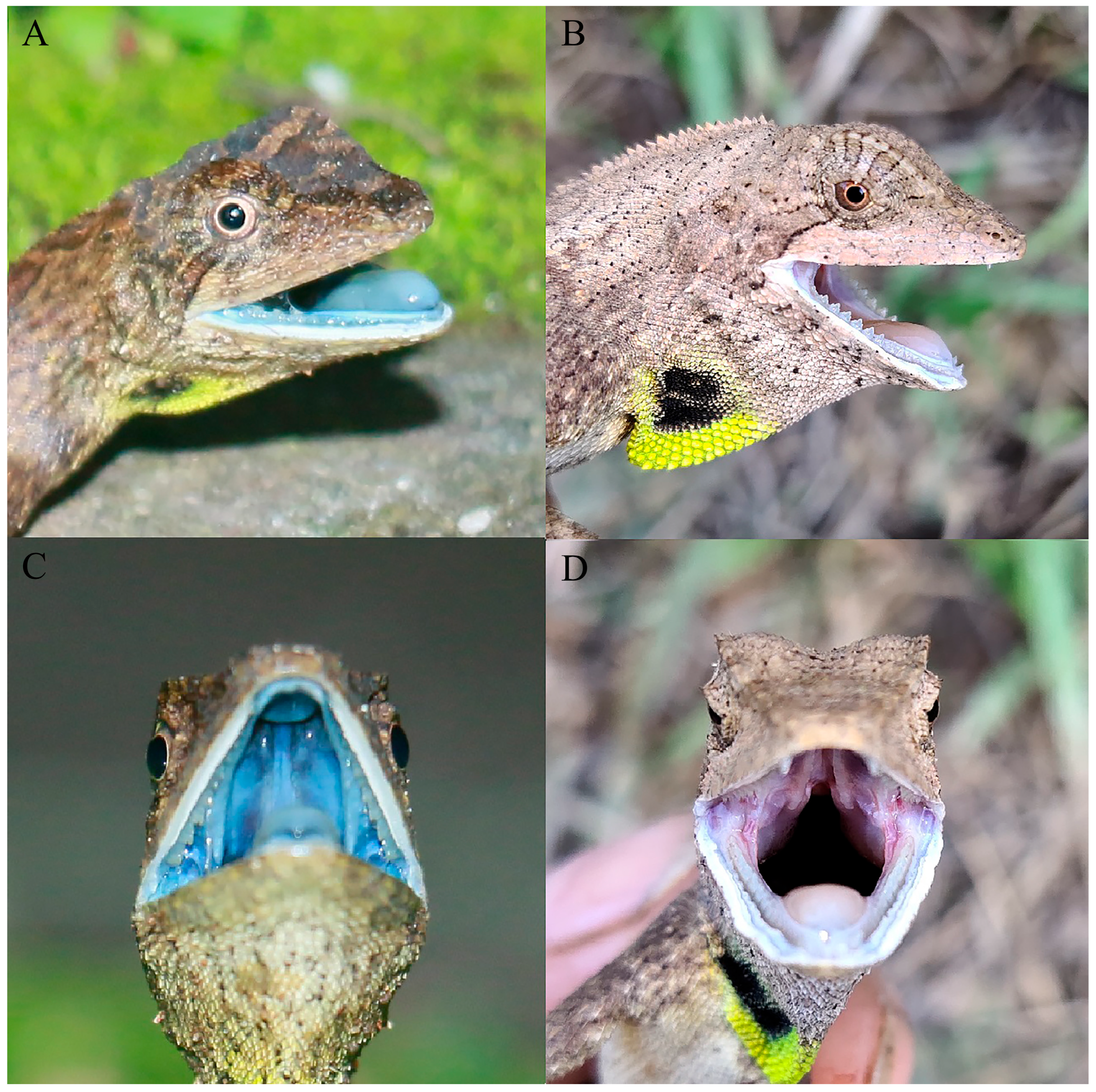
3.7. Coloration of Holotype in Life
3.8. Coloration of Holotype in Preservative
3.9. Variations
3.10. Natural History
3.11. Distribution
3.12. Comparisons
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ananjeva, N.B.; Stuart, B.L. The Agamid Lizard Ptyctolaemus Phuwuanensis Manthey and Nabhitabhata, 1991 from Thailand and Laos Represents a New Genus. Russ. J. Herpetol. 2001, 8, 165–170. [Google Scholar]
- Balan, A.; Das, A.; Boruah, B.; Tillack, T.; Lalronunga, S.; Deepak, V. Description of two new species of Ptyctolaemus (Squamata: Agamidae) from northeast India. Vert. Zool. 2025, 75, 487–516. [Google Scholar] [CrossRef]
- Manthey, U.; Nabhitabhata, J. Eine neue Agame, Ptyctolaemus phuwuanensis sp. n. (Sauria: Agamidae), aus Nordost-Thailand. Sauria 1991, 13, 3–6. [Google Scholar]
- Schulte, J.A., II; Vindum, J.V.; Win, H.; Thin, T.; Lwin, K.S.; Shein, A.K. Phylogenetic relationships of the genus Ptyctolaemus (Squamata: Agamidae), with a description of a new species from Chin Hills of Western Myanmar. Proc. Calif. Acad. Sci. 2004, 55, 222–247. [Google Scholar]
- Liu, S.; Hou, M.; Lwin, Y.H.; Rao, D.Q. A new species of the genus Ptyctolaemus Peters, 1864 (Squamata, Agamidae) from Sagaing, Myanmar. Evol. Sys. 2021, 5, 347–357. [Google Scholar] [CrossRef]
- Uetz, P.; Freed, P.; Hošek, J. The Reptile Database. Available online: http://www.reptiledatabase.org (accessed on 28 November 2025).
- Yang, Y.M.; Du, F. Intergrated Scientific Studies of Yunnan Tongbiguan Nature Reserve; Yunnan Science and Technology Press: Kunming, China, 2006. [Google Scholar]
- Zhao, T.; Yi, X.; Zeng, Z.; Feng, T. MobileNet-Yolo Based Wildlife Detection Model: A Case Study in Yunnan Tongbiguan Nature Reserve, China. J. Intell. Fuzzy Syst. 2021, 41, 2171–2181. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Macey, J.R.; Schulte, J.A.; Larson, A.; Ananjeva, N.B.; Wang, Y.; Pethiyagoda, R.; Rastegar-Pouyani, N.; Papenfuss, T.J. Evaluating trans-tethys migration: An example using acrodont lizard phylogenetics. Syst. Biol. 2000, 49, 233–256. [Google Scholar] [CrossRef]
- Pal, S.; Vijayakumar, S.; Shanker, K.; Jayarajan, A.; Deepak, V. A systematic revision of Calotes Cuvier, 1817 (Squamata: Agamidae) from the Western Ghats adds two genera and reveals two new species. Zootaxa 2018, 4482, 401–450. [Google Scholar] [CrossRef]
- Burland, T.G. DNASTAR’s Lasergene sequence analysis software. Methods Mol. Biol. 2000, 132, 71–91. [Google Scholar]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the Sensitivity of Progressive Multiple Sequence Alignment Through Sequence Weighting, Position-Specific Gap Penalties and Weight Matrix Choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast Model Selection for Accurate Phylogenetic Estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen, H.H.; Ninh, H.T.; Le, L.T.H.; Bui, H.T.; Orlov, N.; Hoang, C.V.; Ziegler, T. Zhangixalus thaoae sp. nov., a new green treefrog species from Vietnam (Anura, Rhacophoridae). ZooKeys 2024, 1197, 93–113. [Google Scholar] [CrossRef]
- Giri, V.B.; Chaitanya, R.; Mahony, S.; Lalrounga, S.; Lalringhhana, C.; Das, A.; Sarkar, V.; Karanth, P.; Deepak, V. On the systematic status of the genus Oriocalotes Günther, 1864 (Squamata: Agamidae: Draconinae) with the description of a new species from Mizoram state, Northeast India. Zootaxa 2019, 4638, 451–484. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org (accessed on 21 November 2022).
- Grismer, L.L.; Quah, E.S.H.; Wood, P.L., Jr.; Anuar, S.; Muin, A.; Davis, H.R.; Murdoch, M.L.; Grismer, J.L.; Cota, M.; Cobos, A.J. Dragons in the mist: Three new species of Pseudocalotes Fitzinger (Squamata: Agamidae) from the sky island archipelago of Peninsular Malaysia. Zootaxa 2016, 4136, 461–490. [Google Scholar] [CrossRef]
- Deepak, V.; Giri, V.B.; Asif, M.; Dutta, S.K.; Vyas, R.; Zambre, A.M.; Bhosale, H.; Karanth, K.P. Systematics and phylogeny of Sitana (Reptilia: Agamidae) of Peninsular India, with the description of one new genus and five new species. Contrib. Zool. 2016, 85, 67–111. [Google Scholar] [CrossRef]
- Ambekar, M.; Murthy, A.; Mirza, Z.A. A new species of fan-throated lizard of the genus Sitana Cuvier, 1829 (Squamata: Agamidae) from northern Karnataka, India. Bonn Zool. Bull. 2020, 69, 157–164. [Google Scholar] [CrossRef]
- Das, S.; Pal, S.; Narayanan, S.; Subin, K.; Palot, M.J.; Rajkumar, K.P.; Deepak, V. Discovery of a new species of kangaroo lizard (Squamata: Agamidae: Agasthyagama) from the southern Western Ghats of India. Vert. Zool. 2024, 74, 151–168. [Google Scholar] [CrossRef]
- Wang, K.; Ren, J.; Wu, J.; Jiang, K.; Jin, J.; Hou, S.; Zheng, P.; Xie, F.; Siler, C.D.; Che, J. Systematic revision of Mountain Dragons (Reptilia: Agamidae: Diploderma) in China, with descriptions of six new species and discussion on their conservation. J. Zool. Syst. Evol. Res. 2021, 59, 222–263. [Google Scholar] [CrossRef]
- Wang, K.; Gao, W.; Wu, J.; Dong, W.; Feng, X.; Shen, W.; Jin, J.; Shi, X.; Qi, Y.; Siler, C.D.; et al. Two new species of Diploderma Hallowell, 1861 (Reptilia: Squamata: Agamidae) from the Hengduan Mountain Region in China and rediscovery of D. brevicaudum (Manthey, Wolfgang, Hou, Wang, 2012). Zootaxa 2021, 4941, 1–32. [Google Scholar] [CrossRef]
- Marín, C.M.; Bocanumenth, D.; Daza, J.M. The colorful giants: Revisiting the systematics of the Anolis latifrons series (Squamata: Anolidae). Vert. Zool. 2025, 75, 441–457. [Google Scholar] [CrossRef]
- Poe, S.; Ryan, M.J. Description of two new species similar to Anolis insignis (Squamata: Iguanidae) and resurrection of Anolis (Diaphoranolis) brooksi. Amph. Rep. Cons. 2017, 11, 1–16. [Google Scholar]
- Ayala-Varela, F.; Valverde, S.; Poe, S.; Narváez, A.E.; Yánez-Muñoz, M.H.; Torres-Carvajal, O. A new giant anole (Squamata: Iguanidae: Dactyloinae) from southwestern Ecuador. Zootaxa 2021, 4991, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Harvey, M.B.; Hamidy, A.; Kurniawan, N.; Shaney, K.; Smith, E.N. Three new species of Pseudocalotes (Squamata: Agamidae) from southern Sumatra, Indonesia. Zootaxa 2014, 3841, 211–238. [Google Scholar] [CrossRef]
- Harvey, M.B.; Shaney, K.; Hamidy, A.; Kurniawan, N.; Smith, E.N. A new species of Pseudocalotes (Squamata: Agamidae) from the Bukit Barisan Range of Sumatra with an Estimation of its phylogeny. Zootaxa 2017, 4276, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wu, J.W.; Jiang, K.; Chen, J.M.; Miao, B.F.; Siler, C.D.; Che, J. A new species of Mountain Dragon (Reptilia: Agamidae: Diploderma) from the D. dymondi complex in southern Sichuan Province, China. Zool. Res. 2019, 40, 456–465. [Google Scholar] [CrossRef]
- Liu, S.; Hou, M.; Mo, M.Z.; Rao, D.Q. A new species of the genus Acanthosaura (Squamata, Agamidae) from Yunnan, China, with comments on its conservation status. ZooKeys 2020, 959, 113–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hou, M.; Rao, D.; Ananjeva, N.B. Three new species of Diploderma Hallowell, 1861 (Squamata, Agamidae) from the Hengduan Mountain Region, south-western China. ZooKeys 2022, 1131, 1–30. [Google Scholar] [CrossRef]
- Xu, Y.; Gong, Y.; Hou, M.; Weng, S.; Liu, S.; Deng, J.; Hu, J.; Peng, L. A New Species of the Genus Pseudocalotes (Squamata: Agamidae) from Southwest Yunnan, China. Animals 2024, 14, 826. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.J.; Wu, Y.Y.; Zhang, J.D.; Yang, G.; Liu, S.; Chen, X.; Chang, J.; Xie, Q.; Cai, B. A new species of Diploderma Hallowell, 1861 (Squamata, Agamidae) discovered in the upper Dadu River valley of the Hengduan Mountains, Sichuan, China. ZooKeys 2025, 1251, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Zug, G.R.; Brown, H.H.; Schulte, J.A.; Vindum, J.V. Systematics of the garden lizards, Calotes versicolor group (Reptilia, Squamata, Agamidae), in Myanmar: Central dry zone populations. Proc. Calif. Acad. Sci. 2006, 57, 35–68. [Google Scholar]





| Species | Locality | Voucher | GenBank |
|---|---|---|---|
| Ptyctolaemus chindwinensis | Htamanthi, Sagaing, Myanmar | SEABRI 2019120076 | OK563731 |
| Ptyctolaemus chindwinensis | Htamanthi, Sagaing, Myanmar | SEABRI 2019120016 | OK563732 |
| Ptyctolaemus chindwinensis | Htamanthi, Sagaing, Myanmar | SEABRI 2019120031 | OK563733 |
| Ptyctolaemus chindwinensis | Htamanthi, Sagaing, Myanmar | SEABRI 2019120046 | OK563734 |
| Ptyctolaemus collicristatus | Min Dat, Chin, Myanmar | USNM 559811 | AY555837 |
| Ptyctolaemus gularis | Changlang, Arunachal Pradesh, India | WII-ADR3292 | LC897088 |
| Ptyctolaemus gularis | Lohit, Arunachal Pradesh, India | WII-ADR3018 | LC897091 |
| Ptyctolaemus gularis | Changlang, Arunachal Pradesh, India | WII-ADR1406 | LC897090 |
| Ptyctolaemus gularis | East Garo Hills, Meghalaya, India | WII-ADR1436 | LC897093 |
| Ptyctolaemus gularis | East Khasi Hills, Meghalaya, India | WII-ADR3180 | LC897092 |
| Ptyctolaemus gularis | East Jaintia Hills, Meghalaya, India | ZSI-R-29477 | LC897094 |
| Ptyctolaemus gularis | Mamit, Mizoram, India | WII-ADR1152 | LC897096 |
| Ptyctolaemus gularis | Lawngtlai, Mizoram, India | WII-ADR1054 | LC897095 |
| Ptyctolaemus gularis | Cachar, Assam, India | WII-ADR3476 | LC897097 |
| Ptyctolaemus gularis | Dibrugarh, Assam, India | WII-ADR3475 | LC897089 |
| Ptyctolaemus namdaphaensis | Changlang, Arunachal Pradesh, India | WII-ADR1403 | LC897099 |
| Ptyctolaemus namdaphaensis | Changlang, Arunachal Pradesh, India | WII-ADR1409 | LC897100 |
| Ptyctolaemus namdaphaensis | Changlang, Arunachal Pradesh, India | WII-ADR1410 | LC897101 |
| Ptyctolaemus namdaphaensis | Changlang, Arunachal Pradesh, India | WII-ADR1411 | LC897102 |
| Ptyctolaemus namdaphaensis | Changlang, Arunachal Pradesh, India | WII-ADR1427 | LC897103 |
| Ptyctolaemus namdaphaensis | Changlang, Arunachal Pradesh, India | WII-ADR3178 | LC897105 |
| Ptyctolaemus namdaphaensis | Changlang, Arunachal Pradesh, India | WII-ADR3283 | LC897104 |
| Ptyctolaemus namdaphaensis | Changlang, Arunachal Pradesh, India | ZSI-R-29481 | LC897098 |
| Ptyctolaemus siangensis | East Siang, Arunachal Pradesh, India | WII-ADR1176 | LC897107 |
| Ptyctolaemus siangensis | Upper Siang, Arunachal Pradesh, India | WII-ADR1210 | LC897108 |
| Ptyctolaemus siangensis | Lower Subansiri, Arunachal Pradesh, India | WII-ADR465 | LC897106 |
| Ptyctolaemus siangensis | Medog, Xizang, China | KIZ 016452 | MK001393 |
| Ptyctolaemus siangensis | Medog, Xizang, China | KIZ 06654 | MW111456 |
| Ptyctolaemus siangensis | Medog, Xizang, China | KIZ 09947 | MW133374 |
| Ptyctolaemus sp. | Putao, Kachin, Myanmar | CAS 221515 | AY555838 |
| Ptyctolaemus tongbiguanensis sp. nov. | Yingjiang, Yunnan, China | KIZ 20140059 | PX724370 |
| Ptyctolaemus tongbiguanensis sp. nov. | Yingjiang, Yunnan, China | KIZ 059188 | PX724371 |
| Draco blanfordii | An Khe, Gia Lai, Vietnam | MVZ 222156 | AF128477 |
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| 1 Ptyctolaemus chindwinensis | ||||||
| 2 Ptyctolaemus collicristatus | 26.3 | |||||
| 3 Ptyctolaemus gularis | 23.6 | 22.1 | ||||
| 4 Ptyctolaemus namdaphaensis | 9.2 | 26.2 | 23.7 | |||
| 5 Ptyctolaemus siangensis | 25.9 | 25.0 | 15.2 | 25.3 | ||
| 6 Ptyctolaemus sp. | 10.3 | 26.0 | 22.2 | 9.5 | 24.9 | |
| 7 Ptyctolaemus tongbiguanensis sp. nov. | 8.5 | 26.3 | 24.1 | 7.7 | 25.3 | 11.2 |
| KIZ 20140059 Holotype Male | KIZ 059188 Paratype Female | |
|---|---|---|
| SVL | 78.5 | 68.4 |
| TAL | 192.5 | 178.0 |
| TAL/SVL | 2.45 | 2.60 |
| HL | 22.0 | 18.8 |
| HW | 12.2 | 11.2 |
| HW/HL | 0.55 | 0.60 |
| HL/SVL | 0.28 | 0.27 |
| SEL | 8.6 | 7.0 |
| OD | 6.5 | 6.1 |
| IOD | 7.7 | 6.8 |
| FLL | 36.0 | 32.9 |
| HLL | 71.1 | 63.8 |
| FLL/SVL | 0.46 | 0.48 |
| HLL/SVL | 0.91 | 0.93 |
| T4L | 16.9 | 15.4 |
| T4L/SVL | 0.22 | 0.23 |
| T4L/HLL | 0.24 | 0.24 |
| TRL | 37.4 | 33.6 |
| SupL | 8/8 | 8/7 |
| InfL | 8/8 | 8/9 |
| T4S | 36/37 | 37/37 |
| NC | 21 | 12 |
| MB | 86 | 88 |
| VSR | 68 | 72 |
| VT | 90 | 93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Liu, S.; Zhu, X.; Hou, M.; Duan, Z.; Zuo, C.; Yin, F.; Rao, D. A New Species of the Genus Ptyctolaemus Peters, 1864 (Squamata, Agamidae) from Yunnan, China. Taxonomy 2026, 6, 4. https://doi.org/10.3390/taxonomy6010004
Liu S, Zhu X, Hou M, Duan Z, Zuo C, Yin F, Rao D. A New Species of the Genus Ptyctolaemus Peters, 1864 (Squamata, Agamidae) from Yunnan, China. Taxonomy. 2026; 6(1):4. https://doi.org/10.3390/taxonomy6010004
Chicago/Turabian StyleLiu, Shuo, Xiaoyu Zhu, Mian Hou, Zhengpan Duan, Changsheng Zuo, Fawang Yin, and Dingqi Rao. 2026. "A New Species of the Genus Ptyctolaemus Peters, 1864 (Squamata, Agamidae) from Yunnan, China" Taxonomy 6, no. 1: 4. https://doi.org/10.3390/taxonomy6010004
APA StyleLiu, S., Zhu, X., Hou, M., Duan, Z., Zuo, C., Yin, F., & Rao, D. (2026). A New Species of the Genus Ptyctolaemus Peters, 1864 (Squamata, Agamidae) from Yunnan, China. Taxonomy, 6(1), 4. https://doi.org/10.3390/taxonomy6010004






