A New Species of Desmoscolex (Nematoda: Desmoscolecidae) from the Northwestern Pacific and Its Implications for Lip-Region Ultrastructure in Species Delimitation †
Abstract
1. Introduction
2. Materials and Methods
2.1. Habitat Characteristics of the Study Area
2.2. Field Sampling and Sample Processing
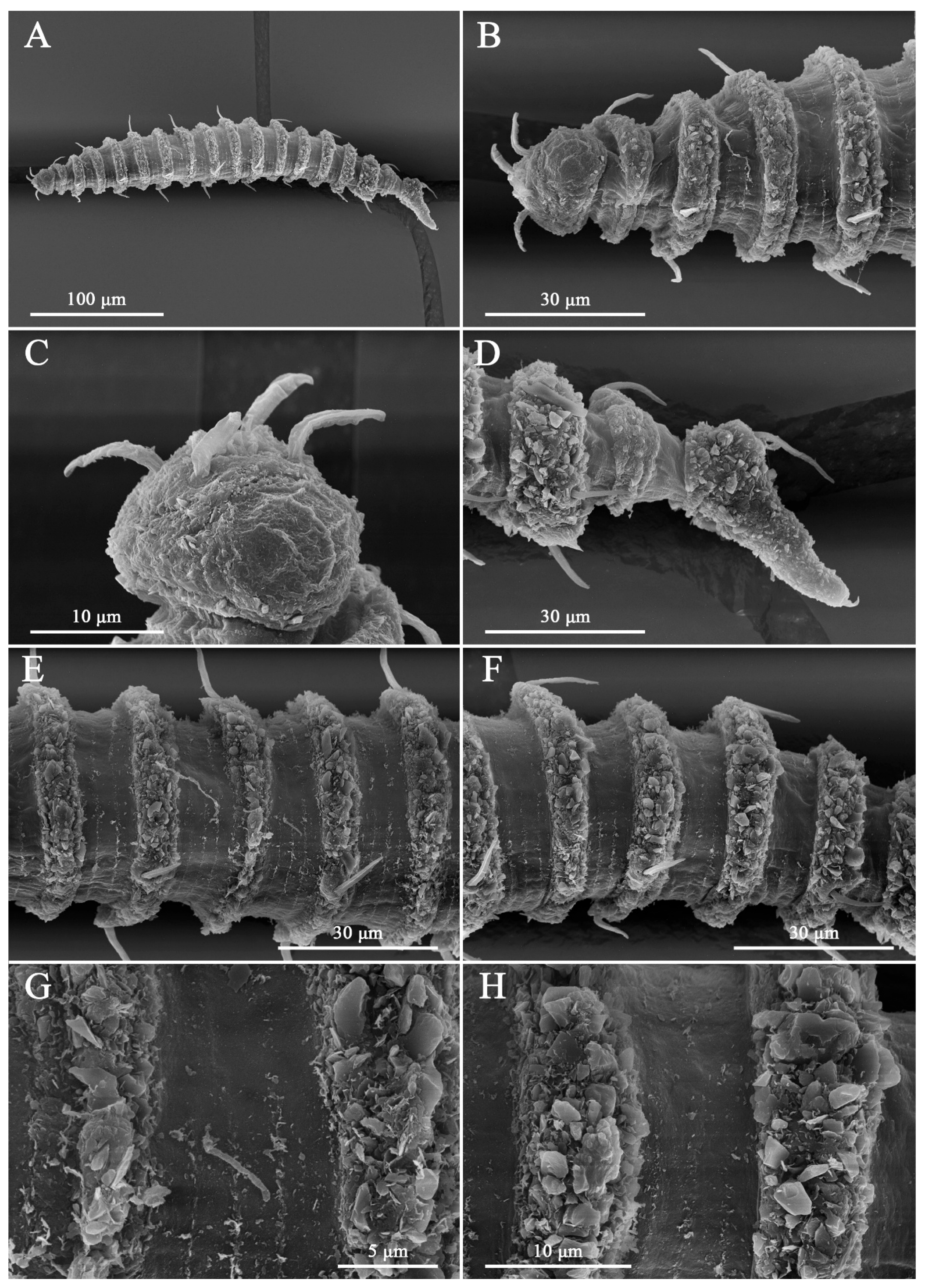
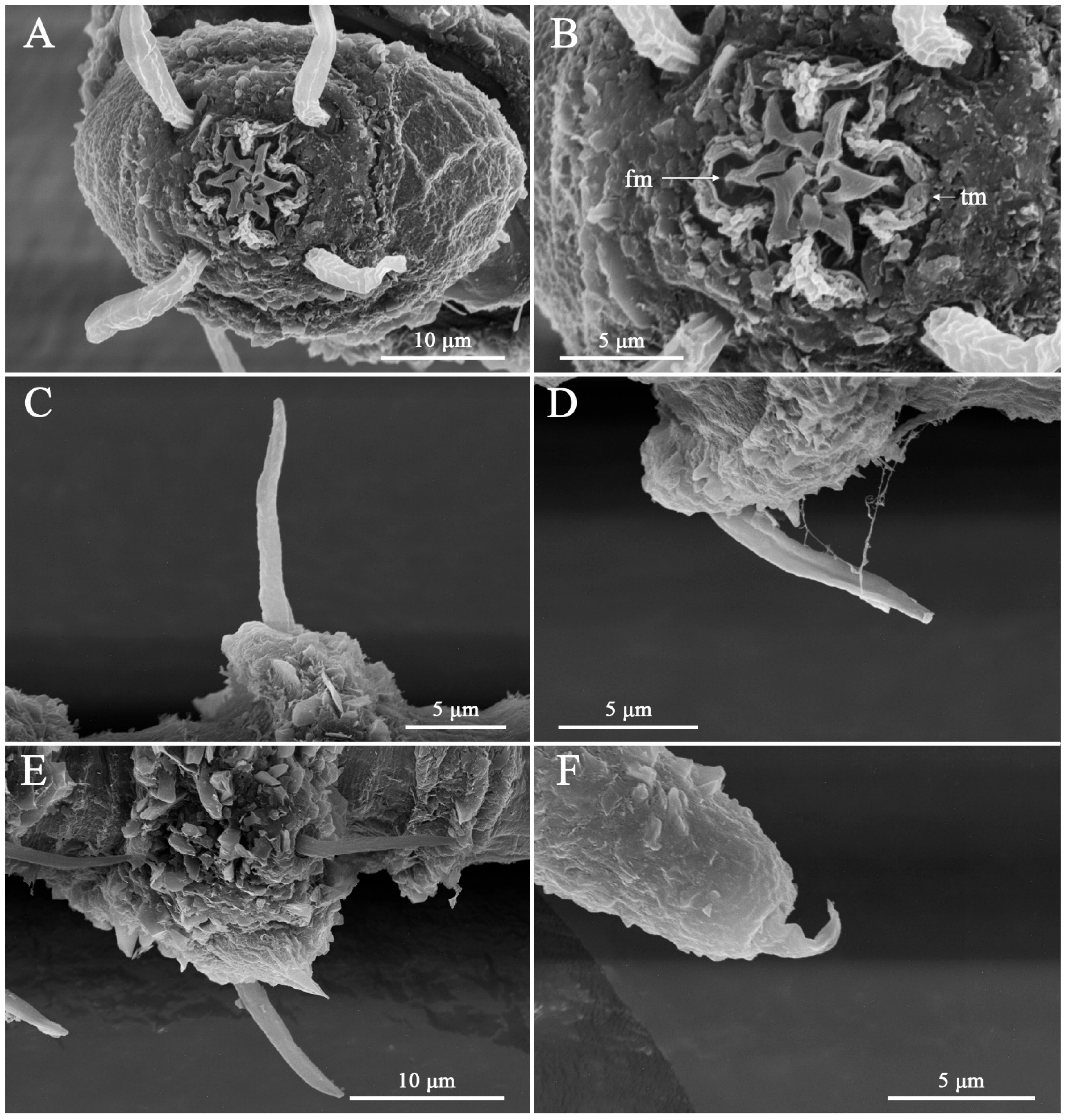
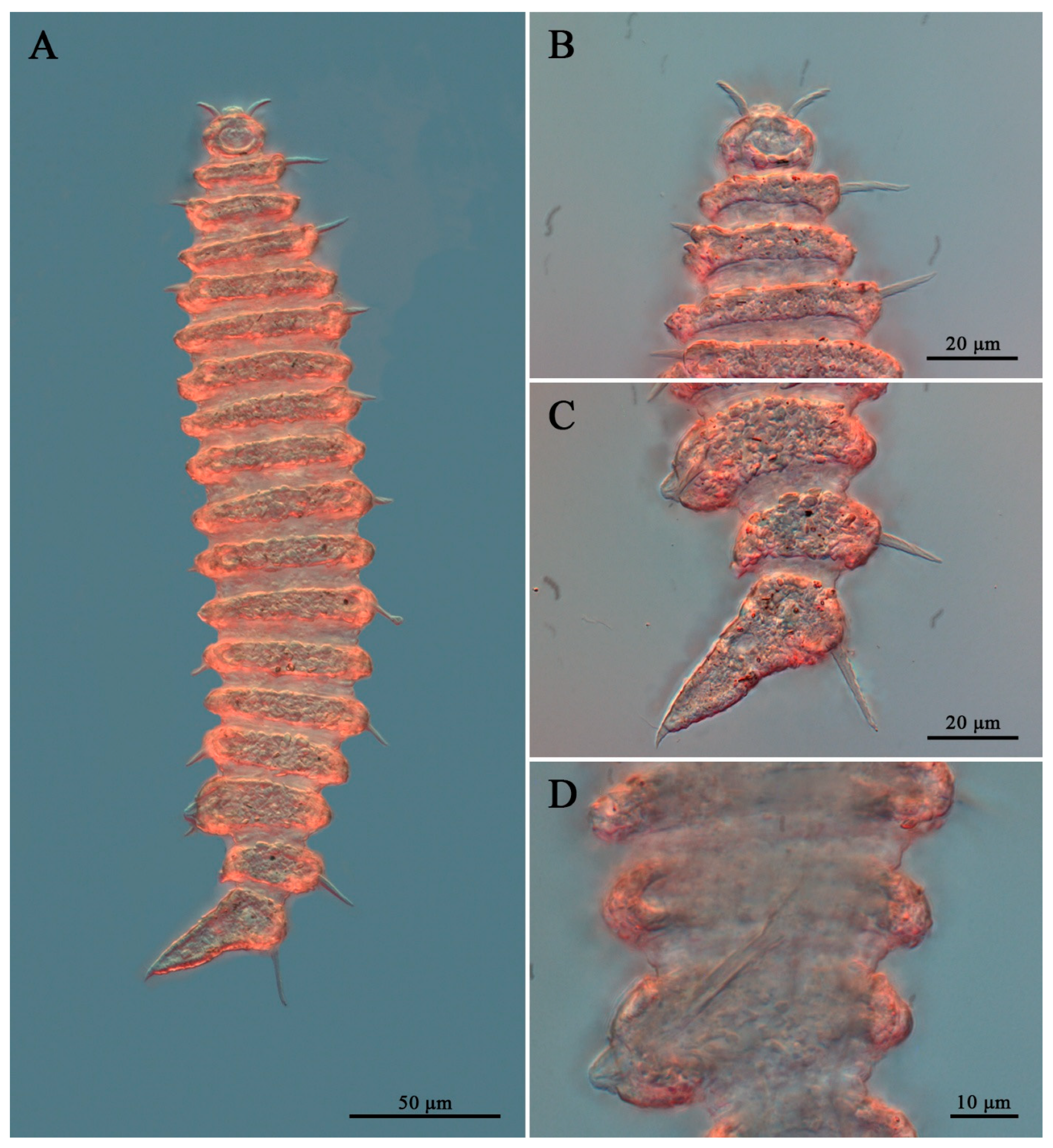
2.3. DIC and SEM–Based Morphological Examination
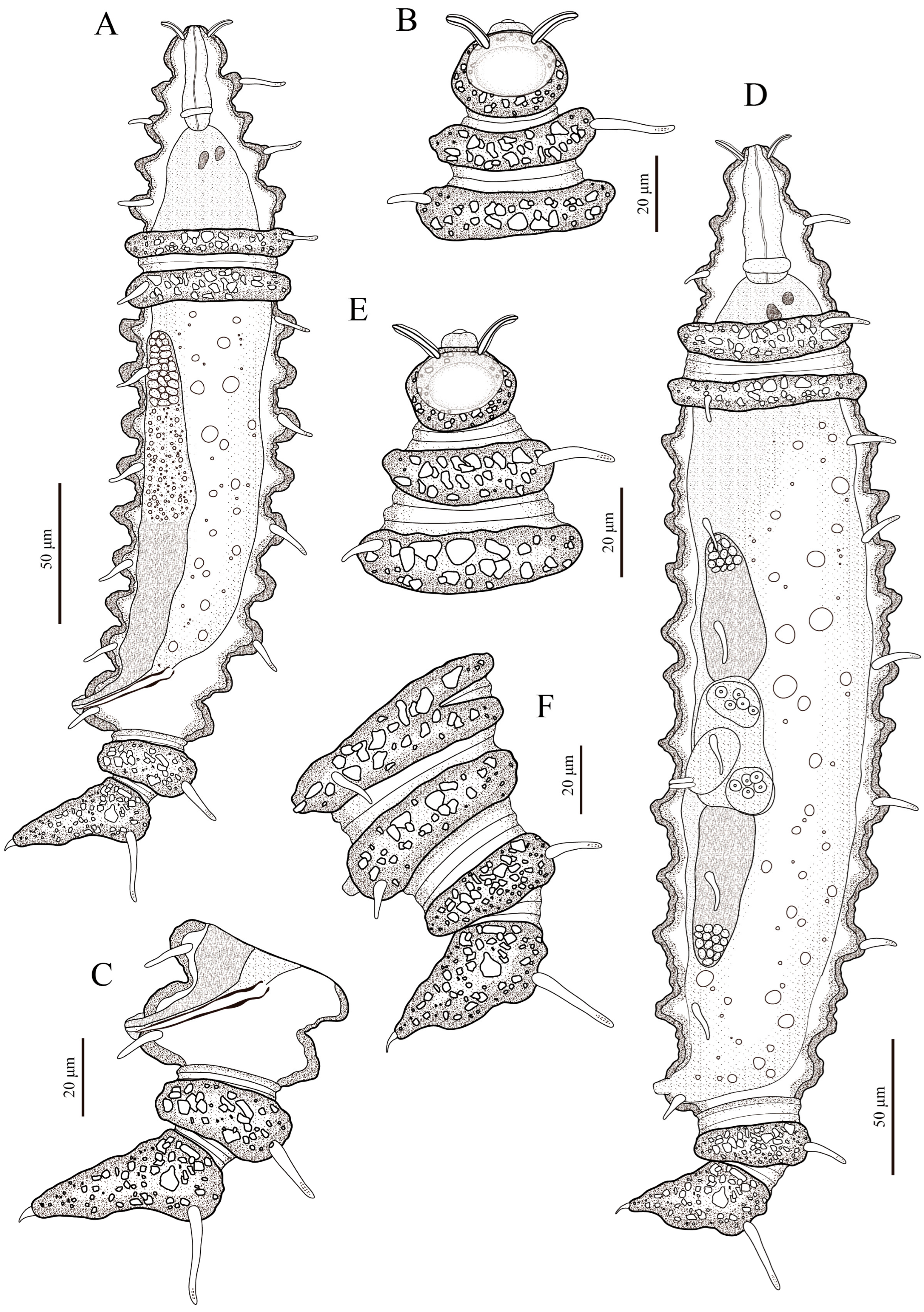
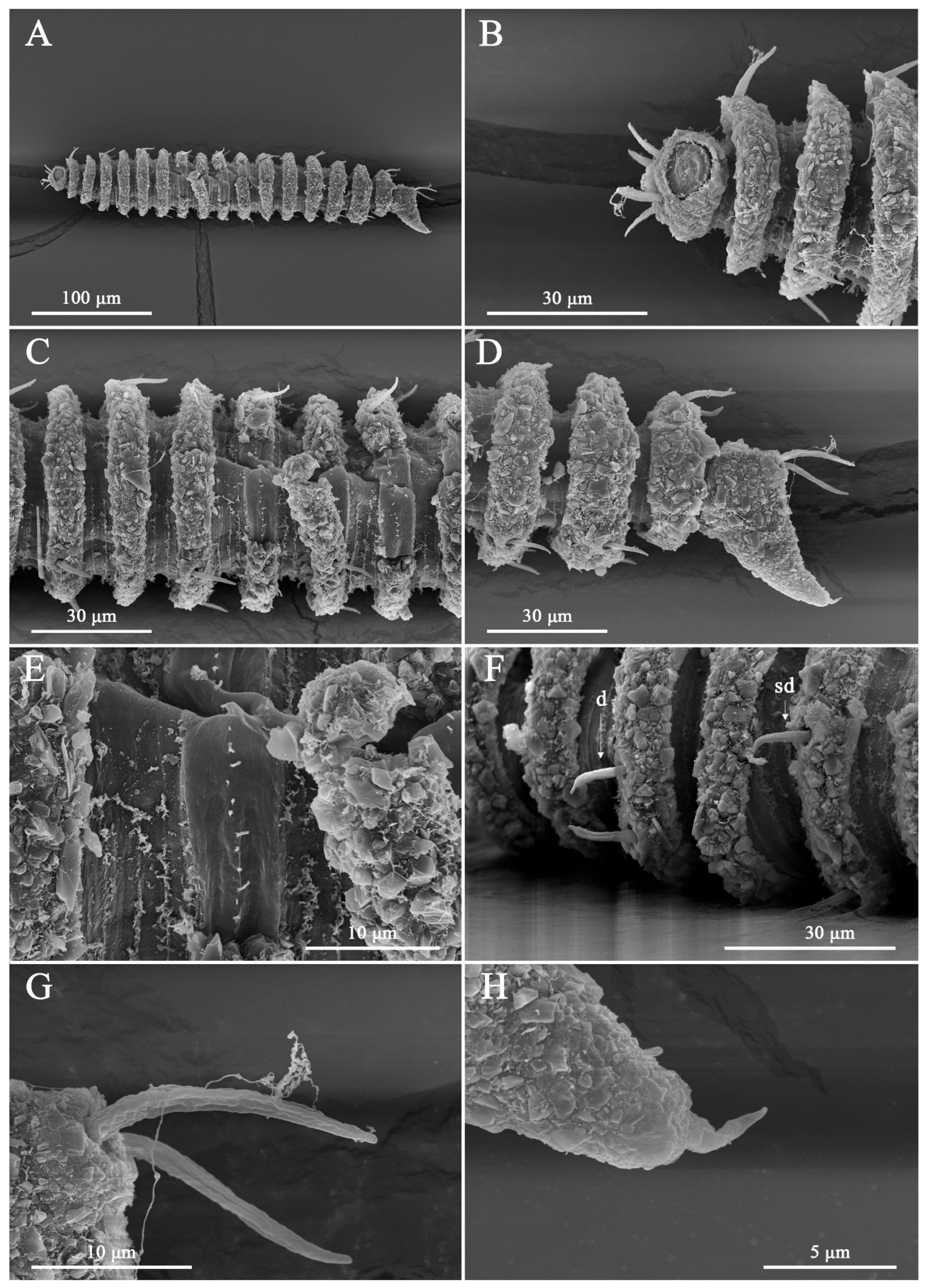
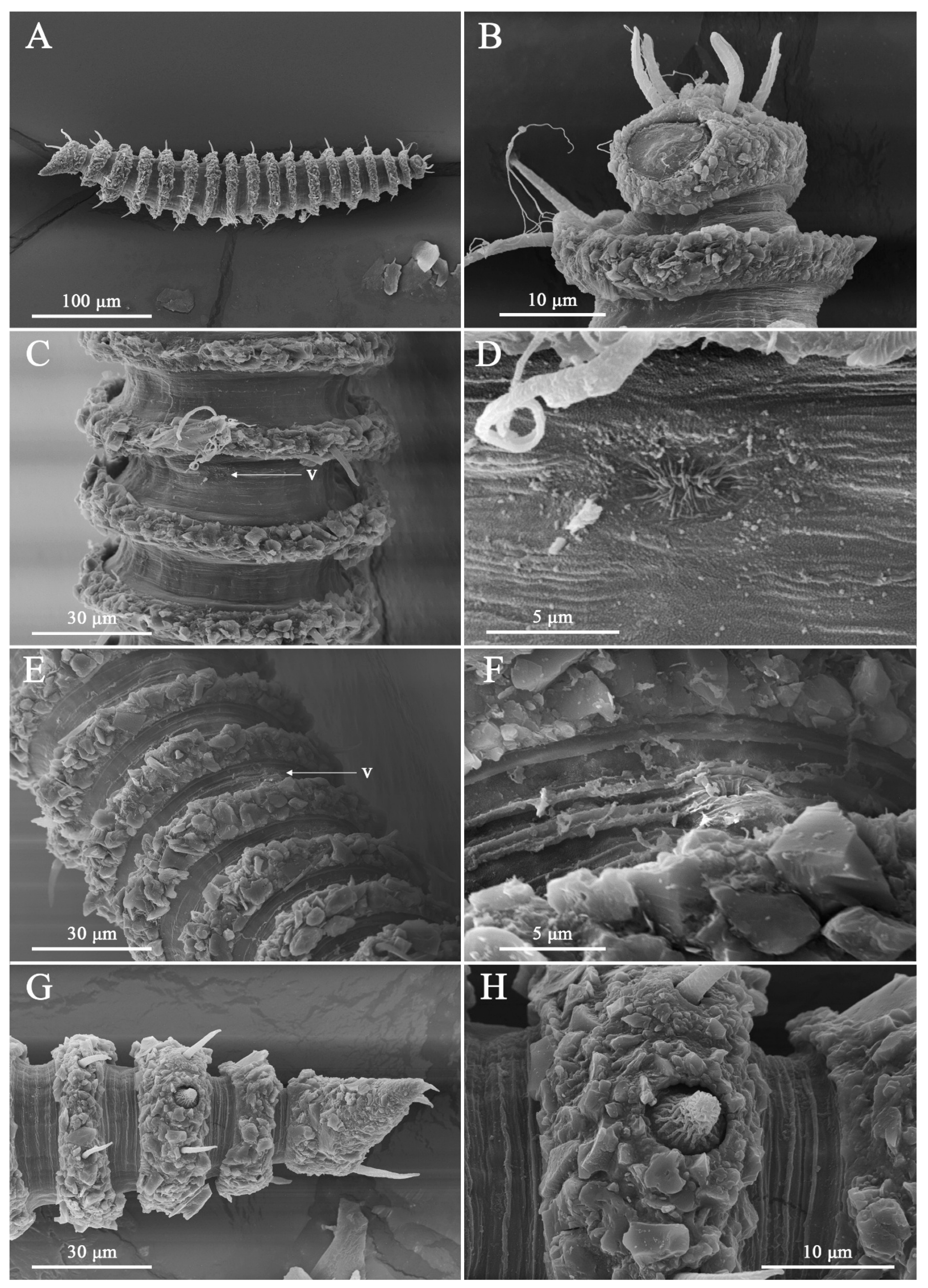
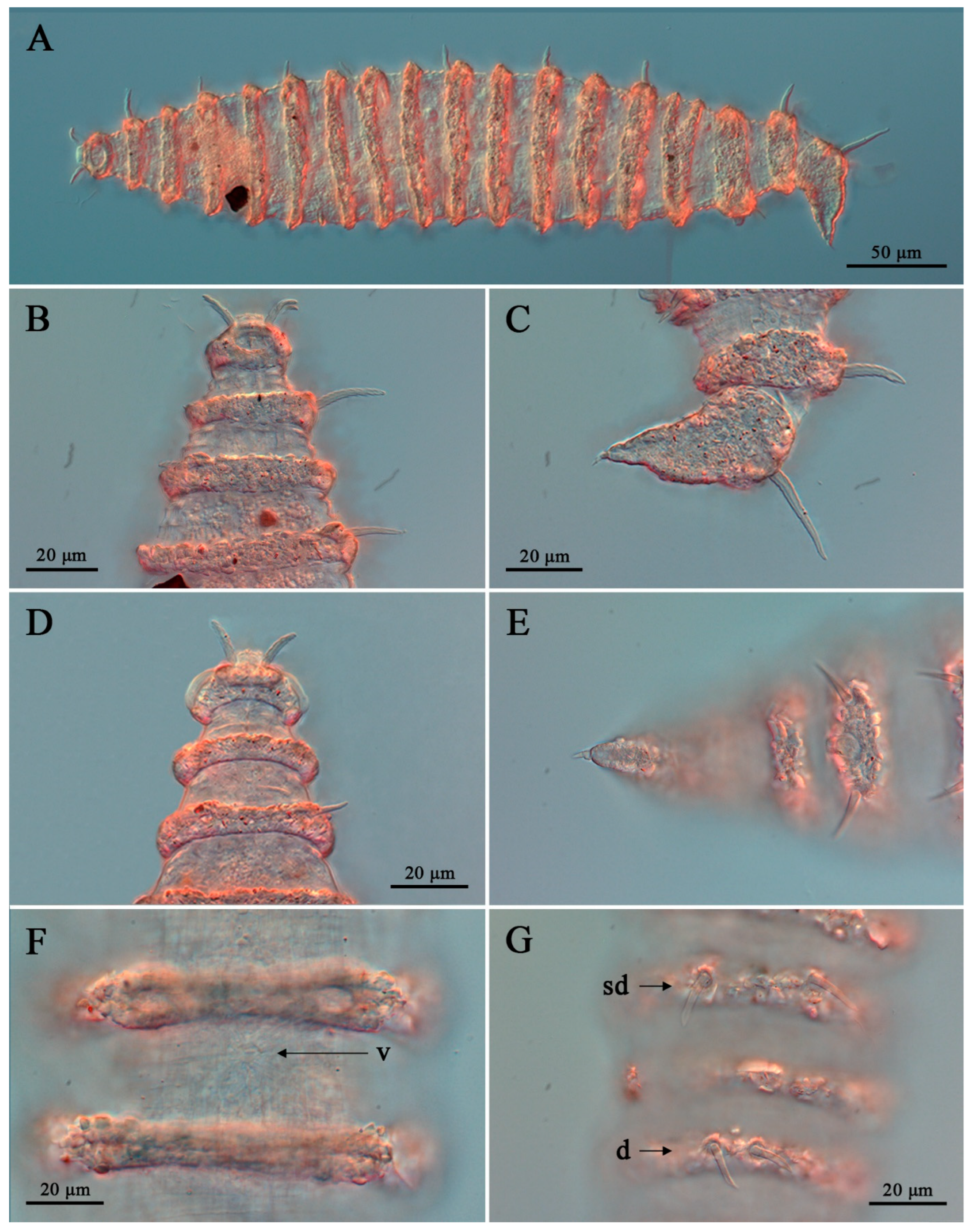
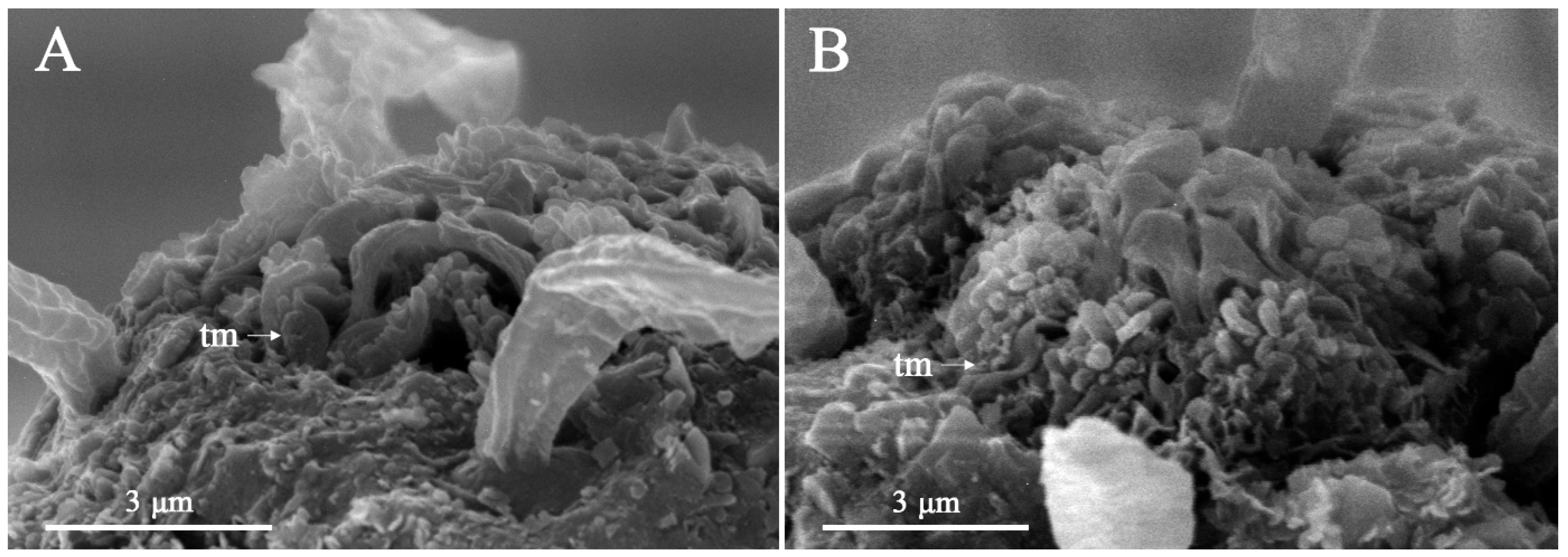
3. Results
3.1. Systematic Accounts
3.2. Type Locality
3.3. Material Examined
3.4. Etymology
3.5. Diagnosis
3.6. Measurements
3.7. Description
- Subdorsal: 1, 3, 5, 7, 9, 11, 13, 16, 17 = 9
- Subventral: 2, 4, 6, 8, 10, 12, 14, 15 = 8
3.8. Differential Diagnosis
3.9. Intraspecific Variation
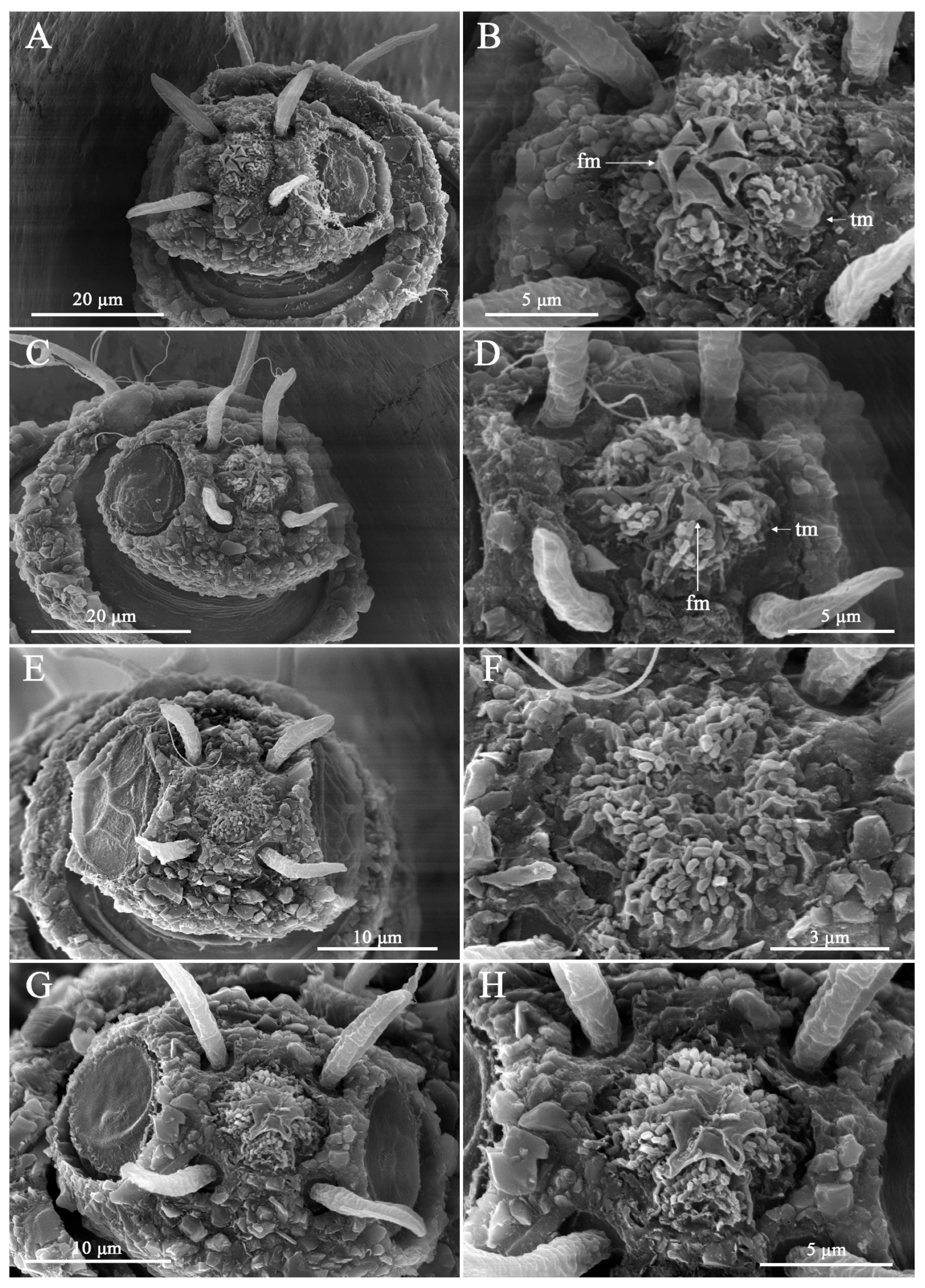
4. Discussion
4.1. Limitations of Cephalic Setae as Diagnostic Characters
4.2. Interpretation of Lip-Region Ultrastructure
4.3. Current Knowledge of Lip-Region Diversity in Desmoscolex
4.4. Taxonomic Implications of Lip-Region Ultrastructure
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| hd | maximum head diameter |
| cs | length of cephalic seta |
| sdn | length of subdorsal setae on main ring n |
| svn | length of subventral setae on main ring n |
| mbd | maximum body diameter |
| (mbd) | maximum body diameter (foreign material not included) |
| oes | length of pharynx |
| spic | length of spicules measured along the median line |
| gub | length of gubernaculum measured along the median line |
| abd | anal body diameter |
| t | tail length |
| tmr | length of terminal ring |
| tmrw | maximum width of terminal ring |
| V (%) | position of the vulva as percentage of the body length |
| a | body length divided by maximum body diameter |
| b | body length divided by pharynx length |
| c | body length divided by tail length |
References
- Lorenzen, S. Desmoscoleciden (eine Gruppe freilebender Meeresnematoden) aus Küstensalzwiesen. Veröff. Inst. Meeresforsch. Bremerhav. 1969, 12, 231–265. [Google Scholar]
- Timm, R.W. A Revision of the Nematode Order Desmoscolecida Filipjev, 1929; University of California Press: Berkeley, CA, USA, 1970; Volume 93, pp. 1–115. [Google Scholar]
- Freudenhammer, I. Desmoscolecida aus der Iberischen Tiefsee, zugleich eine Revision dieser Nematoden-Ordnung. Meteor Forschungsergebnisse Reihe D Biol. 1975, 20, 1–65. [Google Scholar]
- Decraemer, W. Taxonomic problems within the Desmoscolecida (Nematoda). Ann. Soc. R. Zool. Belg. 1979, 108, 13–20. [Google Scholar]
- Decraemer, W.; Rho, H.S. Order Desmoscolecida. In Handbook of Zoology, Volume 2 Nematoda; Schmidt-Rhaesa, A., Ed.; De Gruyter: Berlin, Germany, 2014; pp. 351–372. [Google Scholar] [CrossRef]
- Jung, W.; Rho, H.S. Discovery of two new deep-sea Desmoscolex species (Nematoda: Desmoscolecidae) with wing-like cephalic setae from the Ulleung Basin, the East Sea, Korea. J. Mar. Sci. Eng. 2024, 12, 2257. [Google Scholar] [CrossRef]
- García-Cobo, M.; González-Casarrubios, A.; Sánchez, N. Description of two new species of Desmoscolex Claparède, 1863 (Nematoda: Desmoscolecidae) from the Cassidaigne Canyon, Mediterranean Sea. Eur. J. Taxon. 2025, 1004, 211–236. [Google Scholar] [CrossRef]
- Shirayama, Y.; Hope, W.D. Cephalic tubercles, a new character useful for the taxonomy of Desmoscolecidae (Nematoda). Trans. Am. Microsc. Soc. 1992, 111, 211–222. [Google Scholar] [CrossRef]
- Decraemer, W. New information on the ultrastructure of the lip region in the genus Desmoscolex and description of Desmoscolex (Desmoscolex) parvospiculatus sp. n. (Nemata: Desmoscolecida) from Papua New Guinea. Bull. Inst. R. Sci. Nat. Belg. Biol. 1996, 54, 1–19. [Google Scholar]
- Jung, W.; Kihm, J.-H.; Rho, H.S. Two new species of Desmoscolex (Nematoda: Desmoscolecidae) from subtidal benthic habitats in Korea, with a comparative analysis of cephalic setae variability. J. Mar. Sci. Eng. 2024, 12, 2168. [Google Scholar] [CrossRef]
- Seinhorst, J.W. A rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematologica 1959, 4, 67–69. [Google Scholar] [CrossRef]
- Decraemer, W. The cuticular structure in Desmoscolex with description of D. spinosus sp. n. and redescription of D. michaelseni Steiner, 1916 (Nematoda: Desmoscolecida). Biol. Jaarb. Dodonaea 1976, 44, 123–134. [Google Scholar]
- Decraemer, W.; Sturhan, D. Desmoscolex (Desmolorenzenia) camerunensis n. sp. (Nematoda: Desmoscolecida) from Cameroon. Fundam. Appl. Nematol. 1997, 20, 233–238. [Google Scholar]
- Decraemer, W. Scientific report on the Belgian expedition to the Great Barrier Reef in 1967. Nematodes II: Desmoscolex species (Nematoda: Desmoscolecida) from Yonge Reef, Lizard Island and Nymphe Island. Zool. Scr. 1974, 3, 167–176. [Google Scholar] [CrossRef]
- Kreis, H.A. Weiterer Beitrag zur Kenntnis der freilebenden marinen Nematoden. Arch. Naturg. 1926, 8, 1–29. [Google Scholar]
- Decraemer, W. Scientific report on the Belgian expedition to the Great Barrier Reef in 1967. Nematodes V: Observations on Desmoscolex (Nematoda: Desmoscolecida) with description of three new species. Zool. Scr. 1974, 3, 243–255. [Google Scholar] [CrossRef]
- Decraemer, W. Scientific report on the Belgian expedition to the Great Barrier Reef in 1967. Nematodes I: Desmoscolex-species (Nematoda- Desmoscolecidae) from Yonge Reef, Lizard Island and Nymphe Island with general characteristics of the genus Desmoscolex. Ann. Soc. R. Zool. Belg. 1975, 104, 105–130. [Google Scholar]
- Lim, H.W.; Chang, C.Y. First record of Desmoscolex (Nematoda: Desmoscolecida) from Korea. Integr. Biosci. 2006, 10, 219–225. [Google Scholar] [CrossRef]
- Rho, H.S.; Kim, W.; Chang, C.Y. Description of two new free-living marine nematode species of the subgenus Desmolorenzenia (Desmoscolecida, genus Desmoscolex) from Korea. J. Nat. Hist. 2007, 41, 313–326. [Google Scholar] [CrossRef]
| Characters (μm) | Males | Females | |||
|---|---|---|---|---|---|
| Holotype (n = 1) | Paratypes (n = 3) | Paratypes (n = 13) | |||
| Min–Max | Mean ± SD | Min–Max | Mean ± SD | ||
| body length | 326 | 398–428 | 409.4 ± 16.2 | 341–447 | 400.3 ± 33.7 |
| hd (width) | 22 | 23–26 | 23.0 ± 0.5 | 20–25 | 23.0 ± 1.1 |
| hd (length) | 17 | 16–18 | 16.5 ± 1.1 | 13–17 | 15.5 ± 1.2 |
| amphid (width) | 11 | 10–14 | 12.1 ± 2.0 | 10–14 | 11.8 ± 1.1 |
| amphid (length) | 12 | 9–12 | 9.8 ± 1.8 | 7–10 | 8.5 ± 1.2 |
| cs (width) | 3 | 2–3 | 2.4 ± 0.2 | 2–3 | 2.7 ± 0.4 |
| cs (length) | 12 | 8–11 | 10.0 ± 1.5 | 9–15 | 11.4 ± 1.4 |
| sd1 | 16 | 14–17 | 15.4 ± 1.1 | 13–19 | 16.8 ± 1.9 |
| sd3 | 12 | 11–12 | 11.4 ± 0.5 | 10–17 | 12.6 ± 1.9 |
| sd5 | 10 | 9–14 | 11.9 ± 2.4 | 7–16 | 12.4 ± 2.4 |
| sd7 | 10 | 8–13 | 11.2 ± 3.1 | 8–15 | 12.2 ± 2.2 |
| sd9 | 12 | 11–14 | 12.2 ± 1.6 | 7–16 | 12.5 ± 2.6 |
| sd11 | 12 | 11–12 | 11.4 ± 0.7 | 7–16 | 12.7 ± 2.4 |
| sd13 | 12 | 11–15 | 12.9 ± 2.2 | 11–15 | 12.7 ± 1.2 |
| sd16 | 17 | 15–17 | 15.8 ± 1.4 | 14–25 | 16.4 ± 2.8 |
| sd17 | 24 | 23–25 | 24.2 ± 1.3 | 22–29 | 25.4 ± 2.3 |
| sv2 | 7 | 8–9 | 8.3 ± 0.5 | 6–13 | 9.8 ± 1.8 |
| sv4 | 6 | 6–10 | 8.4 ± 2.1 | 8–13 | 10.4 ± 1.4 |
| sv6 | 6 | 7–12 | 9.2 ± 2.2 | 7–15 | 11.0 ± 1.9 |
| sv8 | 9 | 6–11 | 9.5 ± 2.8 | 8–14 | 10.1 ± 1.8 |
| sv10 | 10 | 7–14 | 11.4 ± 3.7 | 8–14 | 10.9 ± 1.9 |
| sv12 | 11 | 8–13 | 11.3 ± 2.8 | 7–14 | 10.9 ± 2.0 |
| sv14 | 11 | 11–13 | 11.8 ± 0.9 | 10–13 | 11.6 ± 1.1 |
| sv15 | 12 | 9–13 | 11.8 ± 2.1 | 8–16 | 12.4 ± 1.8 |
| mbd | 64 | 52–62 | 57.2 ± 4.9 | 60–88 | 73.5 ± 10.9 |
| (mbd) | 49 | 43–51 | 46.8 ± 4.3 | 44–75 | 60.6 ± 12.2 |
| oes | 53 | 51–52 | 51.2 ± 0.4 | 34–48 | 43.5 ± 3.8 |
| spic | 33 | 32–38 | 34.5 ± 3.2 | – | – |
| V (%) | – | – | – | 49.2–53.8 | 52.5 ± 1.6 |
| abd | 52 | 50–52 | 50.9 ± 1.1 | 44–68 | 57.3 ± 6.7 |
| t | 83 | 79–86 | 82.4 ± 3.7 | 69–98 | 87.3 ± 7.9 |
| tmr | 49 | 44–46 | 45.5 ± 1.5 | 36–58 | 50.2 ± 5.6 |
| tmrw | 26 | 25 | 25.0 ± 0.0 | 24–31 | 28.9 ± 1.9 |
| a | 5.1 | 6.4–7.7 | 7.2 ± 0.7 | 4.3–7.3 | 5.5 ± 0.9 |
| b | 6.2 | 7.8–8.3 | 8.0 ± 0.3 | 7.2–12.6 | 9.3 ± 1.4 |
| c | 3.9 | 4.9–5.1 | 5.0 ± 0.1 | 4.0–15.6 | 5.4 ± 3.1 |
| spinneret | 4 | 4 | 3.8 ± 0.5 | 3–6 | 4.5 ± 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Han, S.; Lee, H.J.; Lee, H.; Rho, H.S. A New Species of Desmoscolex (Nematoda: Desmoscolecidae) from the Northwestern Pacific and Its Implications for Lip-Region Ultrastructure in Species Delimitation. Taxonomy 2026, 6, 5. https://doi.org/10.3390/taxonomy6010005
Han S, Lee HJ, Lee H, Rho HS. A New Species of Desmoscolex (Nematoda: Desmoscolecidae) from the Northwestern Pacific and Its Implications for Lip-Region Ultrastructure in Species Delimitation. Taxonomy. 2026; 6(1):5. https://doi.org/10.3390/taxonomy6010005
Chicago/Turabian StyleHan, Seungyeop, Hyo Jin Lee, Heegab Lee, and Hyun Soo Rho. 2026. "A New Species of Desmoscolex (Nematoda: Desmoscolecidae) from the Northwestern Pacific and Its Implications for Lip-Region Ultrastructure in Species Delimitation" Taxonomy 6, no. 1: 5. https://doi.org/10.3390/taxonomy6010005
APA StyleHan, S., Lee, H. J., Lee, H., & Rho, H. S. (2026). A New Species of Desmoscolex (Nematoda: Desmoscolecidae) from the Northwestern Pacific and Its Implications for Lip-Region Ultrastructure in Species Delimitation. Taxonomy, 6(1), 5. https://doi.org/10.3390/taxonomy6010005






