3. Results
3.1. Taxonomy
Superfamily—Lysianassoidea Dana, 1849.
Family—Cyphocarididae Lowry & Stoddart, 1997.
Genus—Cyphocaris Boeck, 1871.
Type species—Cyphocaris anonyx Boeck, 1871.
Composition of the genus—the genus comprises 18 species, including the new species described here, as follows: Cyphocaris ananke Hughes & Lowry, 2015; C. anonyx Boeck, 1871; C. bellona Lowry & Stoddart, 1994; C. boecki sp. nov.; C. bouvieri Chevreux, 1916; C. challengeri Stebbing, 1888; C. cornuta Ledoyer, 1978; C. faurei K. H. Barnard, 1916; C. geyserensis Ledoyer, 1986; C. johnsoni Shoemaker, 1934; C. latirama Hendrycks & Conlan, 2003; C. nesoi Hughes & Lowry, 2015; C. ohtsukai Tomikawa, 2009; C. pedroi Sorrentino Alves, Johnsson & Senna, 2016; C. polaris Gurjanova, 1951; C. richardi Chevreux, 1905; C. tartaros Hughes & Lowry, 2015; and C. tunicola Lowry & Stoddart, 1997.
Cyphocaris boecki sp. nov.
Figures 1–7.
3.2. Material Examined
Holotype—female, ovigerous, 33.3 mm in length, in 70% ethanol, dissected, and illustrated, obtained off the Rocas Atoll (initial trawl coordinates: 3°58′ S 34°03′ W; final trawl coordinates: 3°57′ S 34°04′ W), Rio Grande do Norte state, Brazil, at 525 m depth, 6 October 2015 (MOUFPE 20068); paratype—juvenile, 17 mm length, in 70% ethanol, same sampling data as for holotype (MOUFPE 20069).
3.3. Type Locality
Off the Rocas Atoll, Rio Grande do Norte state, Brazil (initial trawl coordinates: 3°58′ S 34°03′ W; final trawl coordinates: 3°57′ S 34°04′ W).
3.4. Etymology
The species name is dedicated to Dr. Axel Boeck, who established the genus Cyphocaris, for his great contributions to the early study of amphipods.
3.5. Diagnosis
Head with eyes present; lateral cephalic lobe rounded. Pereonite 1 dorsally smooth, anterodorsal corner not overlapping head. Antenna 1 flagellum article 1 elongated, ventral margin with two fields of well-developed callynophores. Antenna 2 very elongated, almost reaching body length, peduncle article 4 ventral margin expanded; flagellum elongated. Mandible palp article 2 ventral margin with a medial strong lobe; left lacinia mobilis broad and apically blunt; right lacinia mobilis absent. Gnathopod 1 weakly subchelate; palm extremely acute, with a robust seta defining palm; dactylus inner margin serrate. Gnathopod 2 carpus and propodus anterofacially setulose; palm acute; dactylus bifid. Pereopod 4 coxa posterior margin with a truncate projection. Pereopod 5 basis posterior margin with a long and strongly tapered acute spur. Pereopods 6–7 basis posterior margin weakly serrate. Pereopod 7 basis posterodistal lobe weakly produced. Epimeral plates 1–2 with facial oblique keel, forming a subacute posteroventral projection. Epimeral plate 3 posteroventral corner sharply pointed. Uropod 3 rami with plumose setae; outer ramus article 1 broadened in the middle. Telson deeply cleft, with dorsal scales, without apical nails.
3.6. Description
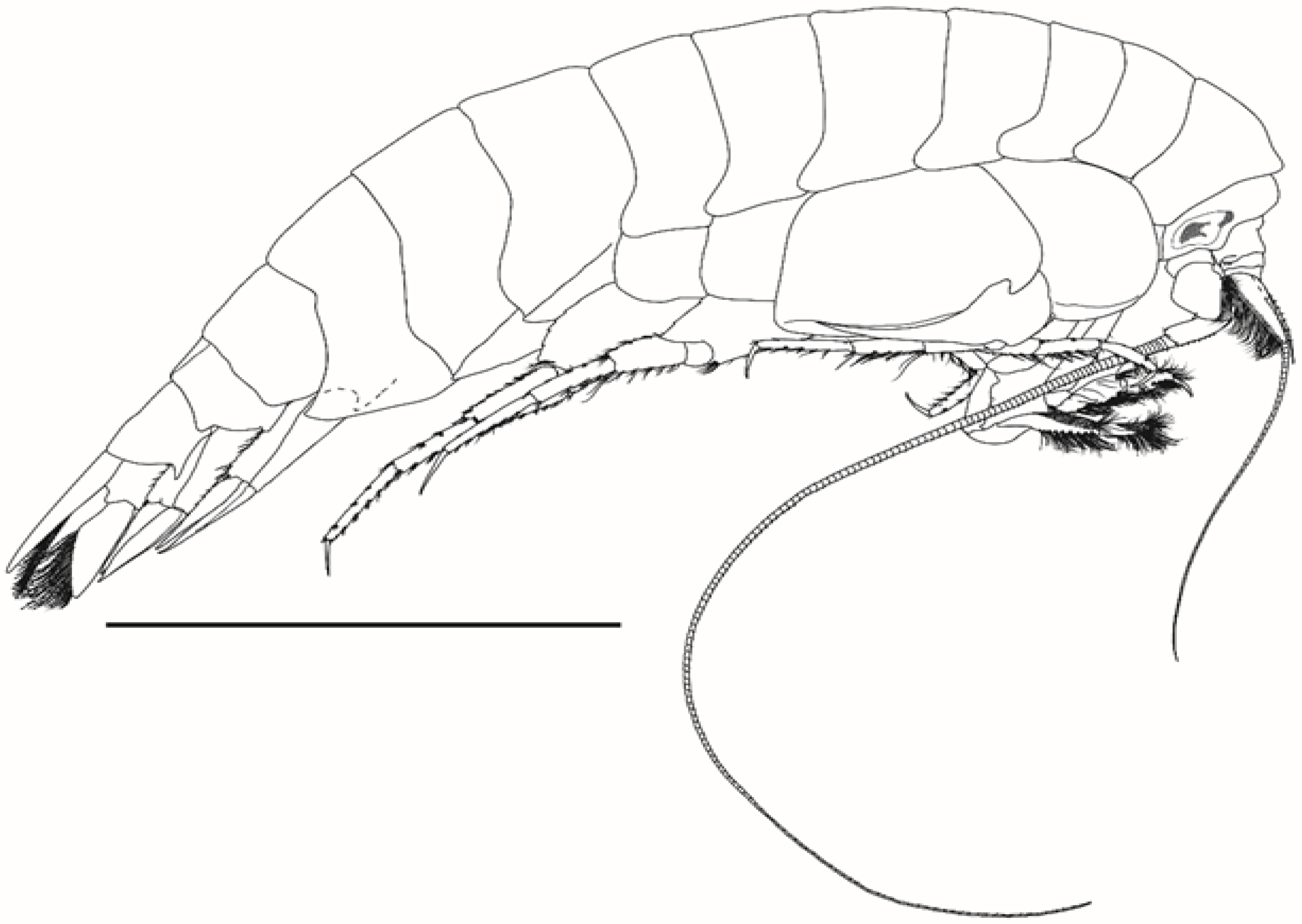
Body as in
Figure 1. Head (
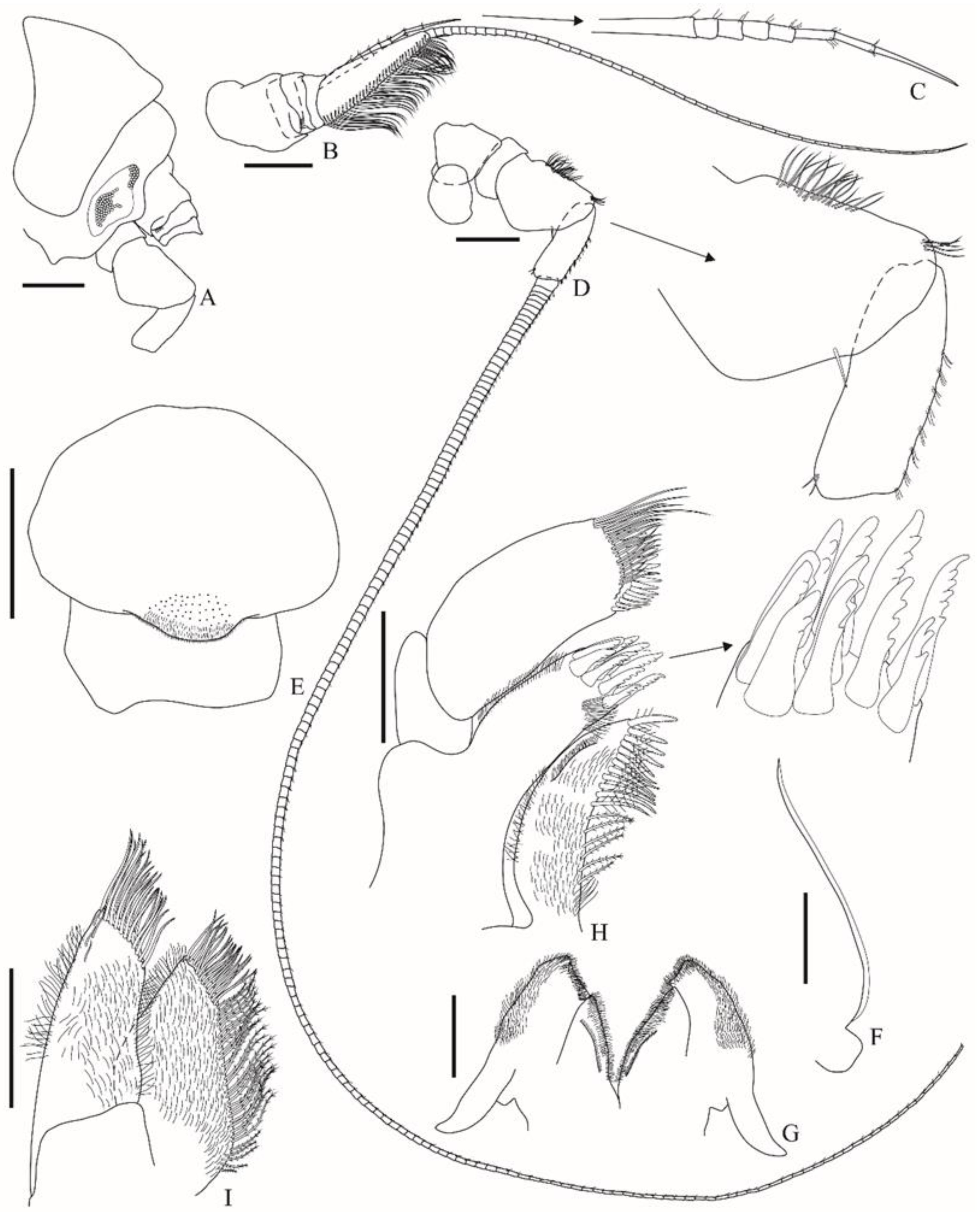
Figure 2A) has well-defined eyes, which are pear-shaped, and bear few ommatidia. Antenna 1 (
Figure 2B) peduncle article 1 about 1.6 × longer than articles 2–3 combined, has a ventral margin with an acute distal projection, bearing a distoventral row of six small setae, articles 2–3 dorsal margin with an acute distal projection; primary flagellum 48-articulate, article 1 elongated, slightly longer than the length of peduncle articles 1–3 combined, ventral margin with two fields of well-developed callynophores; accessory flagellum (
Figure 2C) 8-articulate. Antenna 2 (
Figure 2D) peduncle article 4 dorsal margin has few medial and distal setae, the ventral margin is produced; article 5 dorsal margin have tufts of setules; flagellum is elongated, 167-articulate. Upper lip (
Figure 2E) is rounded, and apex is slightly produced, bearing setules. Epistome (
Figure 2F) is without setae, with a dorsal keel, and the anterior margin is slightly sinuous and descending with a small projection above the articulation with upper lip. Lower lip (
Figure 2G) is setulose. Right and left mandibles (
Figure 3B,C) left incisor has three teeth, right incisor has two teeth, molar is well-developed and triturative, left lacinia mobilis is broad and apically blunt, right lacinia mobilis is absent, and accessory setal row has six robust multicuspidate setae; palp 3-articulate: article 1 is short, article 2 is about 1.5× longer than article 3, mesial margin has a strong medial rounded lobe and a row of 20–28 setae extending to the facial margin, article 3 facial margin is setulose, ventral margin is strongly setose, and apex has four to five pectinate setae. Maxilla 1 (
Figure 2H) inner plate is subequal to outer, facially setulose, mesial margin has six plumose setae, and a row of 12 papposerrate setae extending to apex; outer plate setal teeth produce a distal corona in a modified 6/5 arrangement; STA and STB 2-cuspidate, STC 3-cuspidate, STD 2-cuspidate, ST1 2-cuspidate, ST2 1-cuspidate, ST3, ST4, and ST5 2-cuspidate, ST6 4-cuspidate, and ST7 5-cuspidate; palp 2-articulate, article 1 is short, apicolateral apex is rounded, article 2 is broad, apex has 16 robust setae and a subapical row with slender setae. Maxilla 2 (
Figure 2I) has an inner plate about 0.8× the length of the outer, is facially setulose, has mesial margin with plumose setae, an apex bearing an apical and a subapical row of pectinate setae, lateral margin with a row of setules; outer plate facially setulose, mesial and lateral margins have a row of setules, apex bears an apical and a subapical row of pectinate setae. Maxilliped (
Figure 3A) inner plate with two nodular apical robust setae, bearing a subapical row with four plumose and six papposerrate setae, mesial margin has plumose setae; outer plate apicolateral margin has nine setae, mesial margin proximal third has plumose setae, distal two-thirds bear a row of short robust setae with minute accessory setae and a submarginal row of sinuous setae, last third of the plate has acute projections between the robust setae, apex has pappose setae, facially setulose subapically; palp 4-articulate, article 1 ovate, is about 2.2× longer than wide, mesial margin is moderately setose, apicolateral corner has five setae, article 2 mesial margin has a dense row of plumose setae as well as a submarginal row, lateral margin has a distal row of five setae, article 3 is facially setulose, mesial margin has plumose setae, lateral margin with three rows of setae, article 4 facially setulose, about 1.2× longer than article 3, margins have plumose setae.b
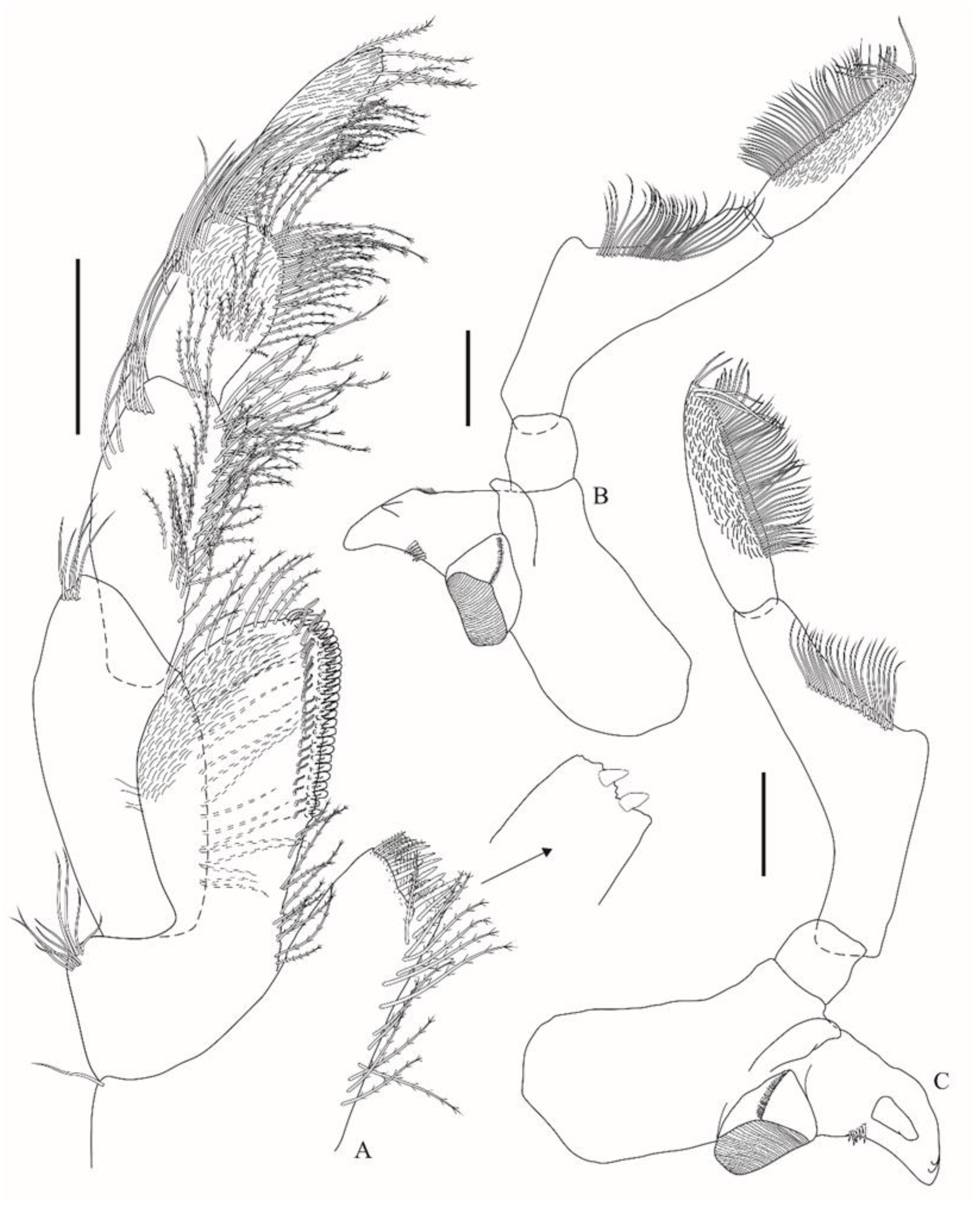
Gnathopod 1 (
Figure 4A) coxa is subtrapezoidal, ventral margin is convex; basis is 7.2× longer than wide, posterodistal corner has three slender setae; ischium posterodistal corner has a row of nine setae; merus has a distal blunt projection facially, posterior margin has a row of setae; carpus is 1.8× longer than wide, with two facial rows of setae, ventral margin setulose; propodus is about 0.8× the length of the carpus, with three facial rows of setae, posterior margin is serrated; palm is extremely acute, with a robust seta defining palm; dactylus outer margin with a proximal seta, inner margin serrate. Gnathopod 2 (
Figure 4B) coxa is subtriangular and is 1.7× deeper than broad; basis is 6.1 × longer than wide, elongate and curved backwards; ischium is 3.6× longer than wide, anterior margin has seven setae; merus is about 0.7× the length of the ischium; carpus is 4.2× longer than wide, anterofacially setulose, anterior margin has a distal row of 11 setae, posterior margin with nine rows of setae; propodus is about half the length of the carpus, anterofacially setulose, anterior margin with a distal dense tuft of setae, and posterior margin has proximal setules and eight rows of setae; palm is acute, defined by the sudden change in angle to the posterior margin; dactylus is broad, bifid, outer margin has one proximal and one distal seta, and inner margin is distally setulose.
Pereopod 3 (
Figure 5A) coxa is subtrapezoidal; basis is 5.9× longer than wide; merus is 3.6× longer than wide, anterior margin has seven distal setae, posterior margin has a few setae; carpus is slightly shorter than the merus, anterior margin has a medial and a distal row of setae, posterior margin has 11 robust setae and a few slender setae; propodus anterior and posterior margins have few slender and robust setae; dactylus is about half the length of propodus, outer margin has one proximal and one distal seta. Pereopod 4 (
Figure 5B) coxa is broad, anterior margin is convex, posterior margin has a subdistal, small, subtriangular, and apically truncate projection; basis is 7.4× longer than wide; remaining articles are very similar to pereopod 3, differing only in setal arrangement. Pereopod 5 (
Figure 5C) coxa has well-developed anterodistal lobe, which is curved backwards, posterior margin is strongly expanded, ventrally with a facial submarginal ridge that fits the spur of the basis; the basis anterior margin has four setae, posterior margin is smooth, with a long acute spur, reaching about 82% of the facial submarginal ridge of the coxa and about two-thirds of the propodus; the merus has an anterior margin with six robust and four slender setae; carpus is slightly longer than merus, anterior margin has four sets of robust and slender setae; propodus is 1.3× longer than carpus, anterior margin has five sets of robust and slender setae; dactylus is about 40% the length of the propodus. Pereopod 6 (
Figure 5D) coxa is subquadrate; basis anterior margin is sinuous and weakly setose, anterodistal corner has a row of six setae, posterior margin is weakly serrate, posteroventral lobe acutely produced, reaching about a quarter of the length of merus; ischium is short and subrectangular; merus, carpus and propodus anterior and posterior margins with sets of simple and robust setae; dactylus inner margin is minutely combed, about half the length of propodus. Pereopod 7 (
Figure 5E) coxa is subquadrate; basis posterior margin weakly serrate, posterodistal lobe is weakly produced; ischium is short; merus anterior and posterior margins have simple and robust setae, bearing a distal facial row of 11 setae; carpus and propodus anterior and posterior margins have sets of simple and robust setae; dactylus inner margin is minutely combed, and is about 40% the length of propodus.
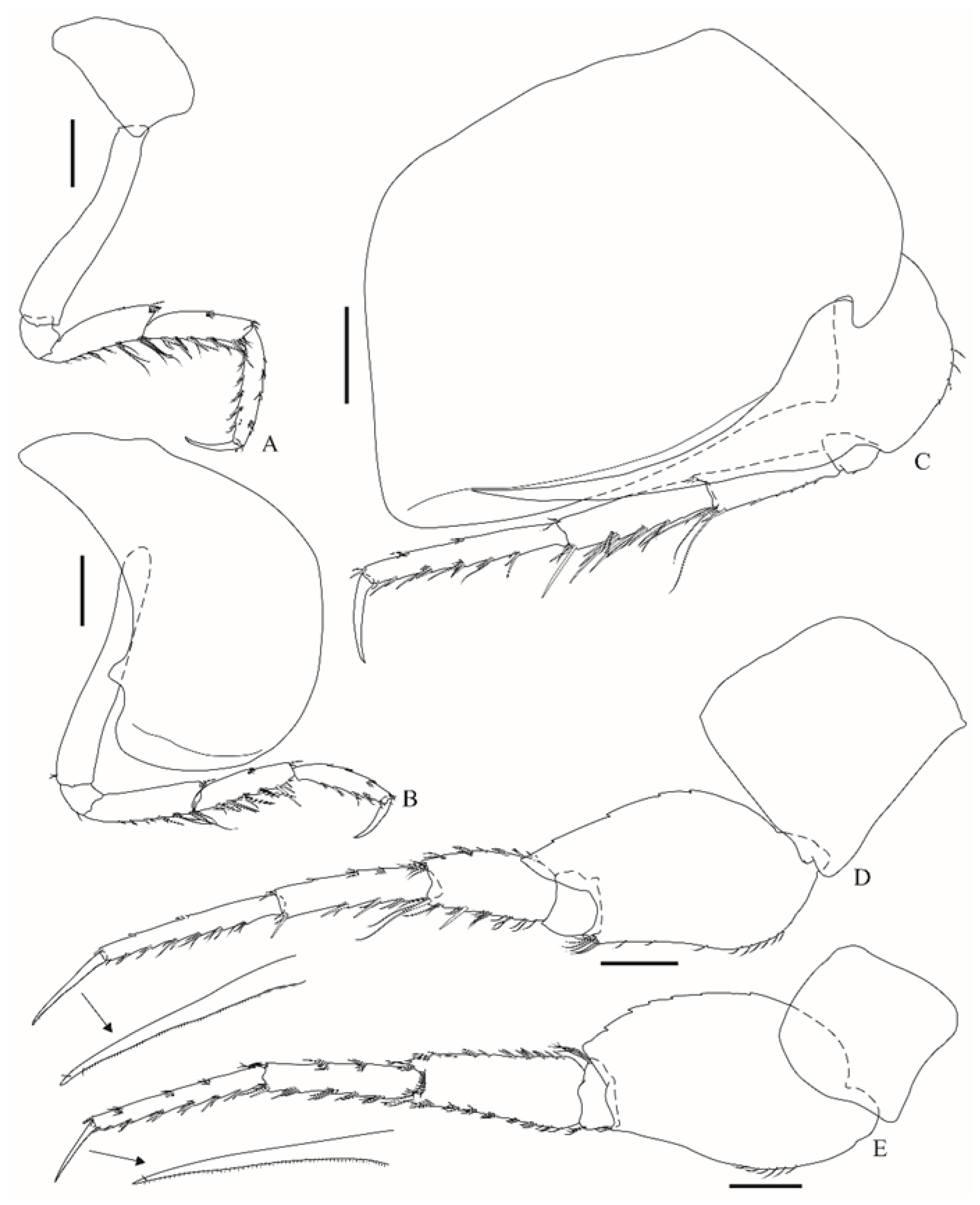
Epimeral plate 1 (
Figure 6A) anterior margin is concave, anteroventral corner is anteriorly produced and blunt, ventral margin is almost straight, oblique facial keel forms a subacute posteroventral projection, posterior margin is convex. Epimeral plate 2 (
Figure 6B) anterior margin is sinuous, oblique facial keel forms a subacute posteroventral projection, posterior margin is convex. Epimeral plate 3 (
Figure 6C) anterior margin is concave, ventral margin is slightly convex, posteroventral corner is sharply pointed, posterior margin is slightly concave. Uropod 1 (
Figure 6D) peduncle is about 1.3× longer than rami, dorsomesial margin has 11 setae; rami are subequal in length, dorsal margin is serrated; inner ramus dorsal margin has five robust setae. Uropod 2 (
Figure 6E) peduncle is about 1.2× longer than rami, dorsomesial margin has 10 setae; rami are subequal in length, dorsal margin is serrated; inner ramus dorsal margin has three robust setae. Uropod 3 (
Figure 6F) peduncle is about 1.3× longer than wide, dorsomesial margin has a row of plumose setae; rami are subequal in length; outer ramus is 2-articulate, article 1 dorsal margin is covered by plumose setae on its distal two thirds, article 2 is missing; inner ramus reaches the apex of telson, dorsal margin is covered by plumose setae. Telson (
Figure 6G) 2× longer than wide, about 70% cleft, tapering distally, and finely scaly.
The juvenile paratype is quite similar to the holotype, presenting small differences, as follows: antenna 1 accessory flagellum (
Figure 7A) is 3-articulate; propodus of gnathopod 1 (
Figure 7B) and gnathopod 2 (
Figure 7C) have fewer setal rows; and epimeral plate 1 (
Figure 7D) anterior margin is weakly concave, anteroventral corner is not produced. Although there is a substantial difference in length between the juvenile and the adult female, pereopod 5 (
Figure 7E) is almost indistinct, with a long basis spur reaching about two-thirds of the propodus. In addition, uropod 3 of the juvenile has an entire outer ramus (
Figure 7F) with 2 articles, article 2 is about 10% the length of article 1, and has a ventral margin with a row of minute setules.
4. Discussion
Cyphocaris boecki sp. nov. is among the species of the genus that possess eyes (with clearly visible or indistinct ommatidia), as follows: C. ananke, C. anonyx, C. bellona, C. cornuta, C. faurei, C. johnsoni, C. latirama, C. nesoi, C. ohtsukai, C. polaris, C. richardi, C. tartaros, and C. tunicola. The new species is easily differentiated from all these species (except for C. faurei and C. latirama) by its dorsally smooth pereonite 1, which has no projection. Nevertheless, Cyphocaris boecki sp. nov. can be distinguished from C. latirama, recorded from the north Pacific, by the following character states (the character states of C. latirama are given within parentheses): head with a rounded lateral cephalic lobe (vs. slightly concave); maxilliped inner plate with two nodular apical robust setae (vs. three); pereopod 5 basis posterior margin is smooth, with a long acute spur (vs. posterior margin is strongly serrated, without a spur); pereopod 6 basis posterior margin is weakly serrated (vs. strongly serrated); pereopod 7 basis posterior margin is weakly serrated and slightly longer than pereopod 6 basis (vs. strongly serrated, and about 1.2× longer than pereopod 6 basis); posterodistal lobe is weakly produced (vs. strongly produced); telson lobes are without setae or spines (vs. lobes with five dorsal and one subapical spine); about 70% cleft (vs. about 84% cleft).
The new species also differs from
C. faurei, which is considered a pan-oceanic species with a very wide bathymetric range [
13,
14].
Cyphocaris faurei was described by K.H. Barnard based on a 30 mm long male from Cape Point, South Africa, Southeastern Atlantic [
15]; since then, it has been recorded by different authors from several locations. A record by Schellenberg from Cape Agulhas, South Africa [
16], was based on a 23 mm long female and an 8 mm juvenile. Birstein, Vinogradov, and Gurjanova briefly commented on
C. faurei, reporting from the Pacific Ocean [
17,
18]. Ledoyer presented a redescription and illustrations of
C. faurei from Madagascar in the Indian Ocean [
19], based on a 15 mm long male. Later, Andres recorded a 33 mm female from Antarctic waters [
20], and provided minor remarks based on Schellenberg’s work.
Cyphocaris faurei was also reported from the Magellan Strait by Costanzo and Crescenti [
21], who analyzed six female individuals from 18.8 to 27 mm in length, and provided comments, illustrated by plates and scanning electron microscopy images. The most recently described or illustrated record of the species was by Hughes and Lowry, who based it on a 25 mm male from the Tasman Sea [
9].
As cited above, reports of C. faurei in the literature are extensive and comprise accounts of many morphological variations, which may reflect intraspecific differences related to the stage of maturity, geographic distribution, or even an overlooked species complex. Nevertheless, C. boecki sp. nov. can be distinguished from C. faurei (described in Barnard’s work) by the following character states: epistome descending with a projection above the upper lip (vs. not projecting above the upper lip); pereonite 1 is shorter than the length of pereonites 2 and 3 together (vs. longer); anterodorsal corner is not swollen and not overlapping the head (vs. slightly swollen, slightly overlapping the head); maxilliped inner plate has two nodular apical robust setae (vs. three); gnathopod 1 basis is 1.2× longer than the length from ischium to dactylus (vs. equal in length); coxa 3 is about 1.7× deeper than long (vs. as deep as long); coxa 4 posterior margin is weakly concave, with a subdistal apically truncate projection (vs. strongly concave, with a subdistal subacute projection); pereopod 5 basis spur reaches about two-thirds of propodus (vs. reaching the end of carpus); coxa 6 is subquadrate (vs. subrectangular); coxa 7 is subquadrate (vs. semicircular); and telson is about 0.6× the length of urosomites 1–3 together (vs. equal in length).
The new species also differs from Schellenberg’s description of C. faurei in the following character states: eyes present (vs. absent); antenna 1 flagellum without calceoli (vs. with calceoli); pereonite 1 anterodorsal corner is not produced and not overlapping the head (vs. slightly produced, slightly overlapping the head); pereopod 5 basis spur reaches about two-thirds of propodus (vs. reaching the end of carpus); pereopods 6 and 7 basis posterior margins are weakly serrated, with small spines in the juvenile (vs. strongly serrated, with large spines in the juvenile). We found an inconsistency between Schellenberg’s description and the illustration of pereopod 5 of C. faurei. The description states that it reaches the end of the carpus, whereas in the illustration, it appears to reach a part of the propodus.
Birstein and Vinogradov’s comments are brief, and mostly highlight the differences between C. faurei and C. challengeri. They claim that there is a “hood” formed by pereonite 1, slightly extending over the head of C. faurei; however; this feature is not present in the new species. Gurjanova provides comments and a figure based on Schellenberg’s work, which is discussed above.
Ledoyer provides a description and illustrations of appendages. Our species can be distinguished from his report of C. faurei by the following character states: head lower corner is not extended (vs. extended); antenna 1 accessory flagellum is 8-articulate (vs. 4-articulate); epistome slightly sinuous (vs. not produced); mandible incisor has two or three teeth (vs. one); palp article 2 mesial margin has a strong rounded medial lobe (vs. weak lobe); maxilla 1 palp article 2 are not broadened distally, and are about 2.1× longer than wide (vs. strongly broadened, about 1.3× longer than wide); maxilla 2 plates are facially setulose (vs. facially naked); inner plate is apically subtriangular (vs. rounded); maxilliped inner plate has two nodular apical robust setae (vs. three); coxa 3 is about 1.7× deeper than long (vs. as deep as long); pereopods 6 and 7 basis have smaller spines on posterior margin (vs. larger spines); posterodistal lobe of pereopod 7 is weakly produced and not exceeding ischium (vs. exceeding ischium); uropod 3 outer ramus article 1 is very broadened in the middle and is about 2.2× longer than wide (vs. weakly broadened and about 2.8× longer than wide); and the telson has dorsal scales (vs. smooth).
Costanzo and Crescenti’s short description of C. faurei compares it with Schellenberg’s description using the shape of pereopod 5 basis as a definitive character to confirm species identity. Here, we show that C. boecki sp. nov. presents features different from those of C. faurei: pereonite 1 is shorter than the length of pereonites 2 and 3 together (vs. slightly longer); pereopod 7 coxa is subquadrate (vs. subrectangular), basis posterior margin has smaller spines (vs. larger spines), posterodistal lobe weakly produced, not exceeding ischium (vs. exceeding ischium); and telson dorsally scaly (vs. smooth).
Finally, Hughes and Lowry provided a description and figures for C. faurei. Our species can be differentiated from it by the following character states: antenna 1 accessory flagellum is 8-articulate (vs. 4-articulate); mandible palp article 3 is facially setulose (vs. facially naked); maxilla 1 palp article 2 is not broadened distally and is about 2.1× longer than wide (vs. weakly broadened and about 1.6× longer than wide); maxilla 2 plates are facially setulose (vs. facially naked); maxilliped inner plate has two nodular apical robust setae (vs. three); outer plate almost reaches the apex of palp article 1 (vs. reaching the middle of palp article 2); gnathopod 2 is without a robust seta on palmar corner (vs. with one robust seta); pereopod 6 coxa is subquadrate, without a ventral lobe (vs. subrectangular, with a ventral lobe); pereopod 7 basis has a weakly produced posterodistal lobe, not exceeding the apex of the ischium (vs. moderately produced and exceeding the apex of the ischium); and the telson has dorsal scales (vs. smooth).
There is distributional proximity between C. faurei and the new species, since the former is known from the eastern Atlantic but is not yet recorded from its southwestern portion. However, given all the morphological variations reported for C. faurei, the new species can be considered distinct from it, based on the following: pereonite 1 is shorter than the length of pereonites 2 and 3 together; the anterodorsal corner is not produced and not overlapping any part of the head; maxilla 1 palp article 2 is not broadened distally; maxilla 2 plates are facially setulose; maxilliped inner plate has two nodular apical robust setae; pereopod 6 and 7 coxa are subquadrate, basis posterior margin is weakly serrated; pereopod 7 posterodistal lobe is weakly produced and not exceeding ischium; and the telson has dorsal scales.
Although the presence of eyes in our species is a significant difference from C. pedroi, described from Brazil, other character states also make it recognizable (the character states of C. pedroi are given within parentheses), as follows: head with rounded lateral cephalic lobe (vs. subacute); antenna 2 is about 0.9× the length of body (vs. antenna 2 about 0.2× the length of body); epistome anterior margin is slightly sinuous (vs. straight); maxilliped inner plate has two nodular apical robust setae (vs. three); gnathopod 1 merus posterodistal corner is regular, without any additional structures (vs. bearing a subtrapezoidal serrated structure); pereopod 5 coxa anterodistal lobe is without a robust seta (vs. with a robust plumose seta); basis spur is apically acute, reaching about two-thirds of propodus (vs. spur apically rounded, reaching the apex or carpus); telson is without apical nails and dorsal margin is entirely scaly (vs. with apical nail on each lobe and dorsal margin scaly only on the distal half).
Further thorough research on Cyphocaris is required, including morphological analyses of type and non-type material available in collections, as well as molecular studies. This may elucidate geographical patterns of widely distributed species such as C. faurei and comprehend the actual diversity of the genus.