1. Introduction
Over a century and a half after the Neotropical genus
Ceratozamia Brongn. was first established [
1], only 12 species were recognized [
2]. However, the pace of species discovery within the genus has accelerated considerably in recent years, with the number of accepted species nearly tripling to 34 since the year 2000 [
3]. This notable burst of species discovery has been achieved through the efforts of researchers surveying previously unexplored areas for cycads as well as the critical examination of herbarium specimens (e.g., [
4,
5,
6,
7,
8]). The increase in species discovery in recent years is consistent with the prediction by Walters et al. [
9], who suggested that the number of species in
Ceratozamia will approximate 40 once the field explorations and the taxonomic determinations of known populations are completed. Identifying the entirety of the constituent species within
Ceratozamia will contribute to a better understanding of its evolutionary history, and this in turn will allow for the identification of evolutionarily significant units [
10] within the genus to aid in future conservation efforts.
Species of the genus
Ceratozamia can be found in a wide range of elevations, from 0 to 2000 m a.s.l., mainly in tropical forests, oak forests and cloud forests in mountainous regions of Mexico, Guatemala, Belize and Honduras [
11]. Most species (26 of 34) occur in the States of Oaxaca, Chiapas and Veracruz, in southeastern Mexico: An area that represents the richest floristic region in the country [
12]. This high biodiversity can be explained by the high neo- and paleoendemism [
13] originated by processes of diversification mainly influenced by climatic fluctuations which occurred between 5 and 20 million years ago [
14,
15]. Indeed, Wendt [
16] identified the mountains of Uxpanapa, Veracruz and the “arc area” (a mountain chain that extends from Uxpanapa to northern Chiapas) as one of several regions that could have served as refugia for warm-tropical communities during the glaciation cycles that took place from 20 million years to 10,000 years ago. Recent studies on cycads have supported this hypothesis [
15,
17,
18]; yet little is known about the influence of these refugia on the evolution of
Ceratozamia.
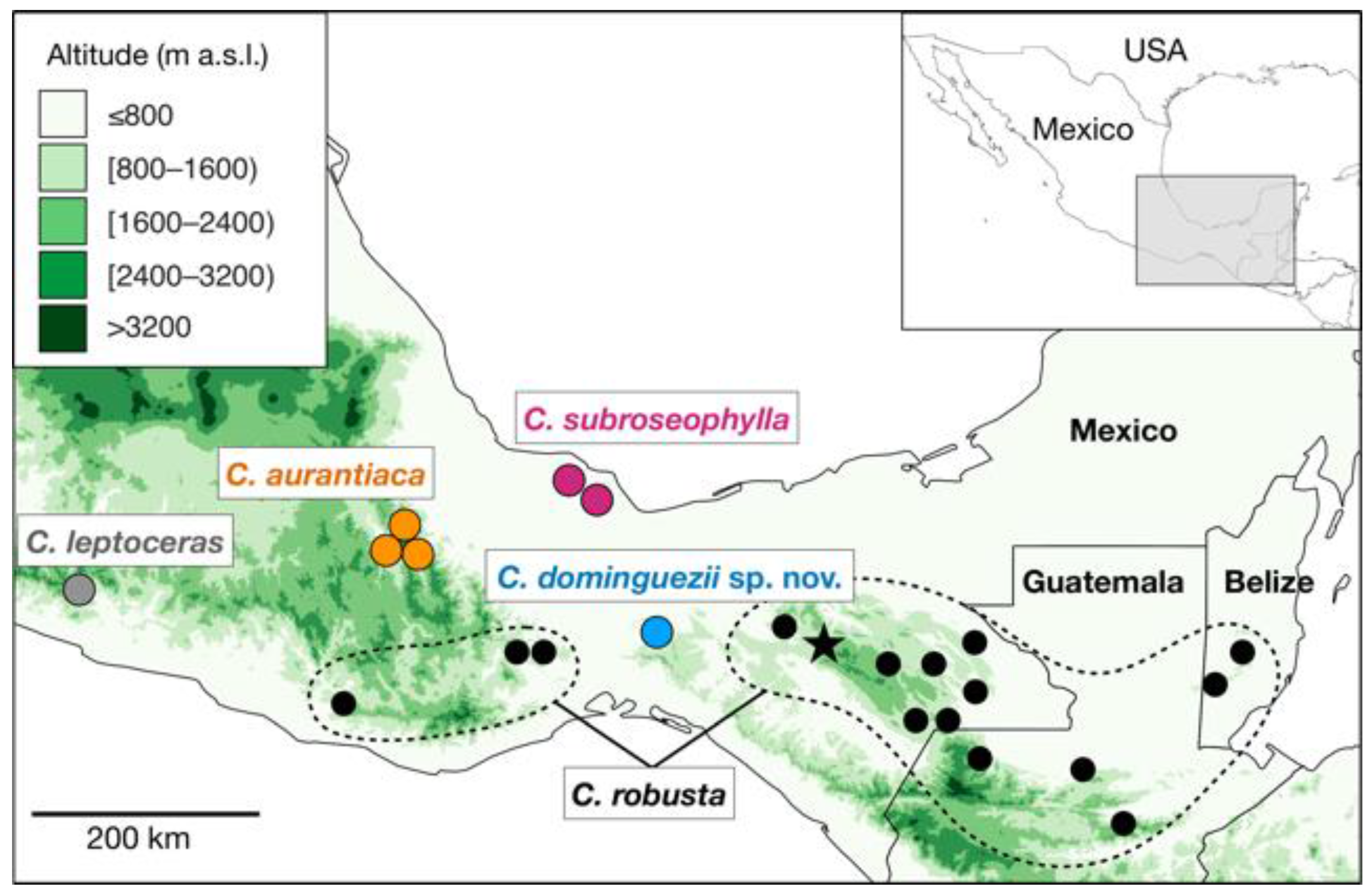
In the year 2000, we started reviewing the taxonomy and distribution of the
Ceratozamia robusta Miq. species complex, a group of closely related species characterized by having robust trunks, large strobili, and long leaves [
19]. The complex occurs in the mountainous areas of Chiapas, Guerrero, Oaxaca and Veracruz, Mexico and ranges into adjacent Guatemala and Belize (
Figure 1). It is currently comprised of
C. robusta, C. subroseophylla Mart.-Domínguez and Nic.-Mor.,
C. aurantiaca Pérez-Farr., Gut. Ortega, J.L. Haynes and Vovides, and
C. leptoceras Mart.-Domínguez, Nic.-Mor., D.W.Stev and Lorea-Hern., the latter occurring in the State of Guerrero [
20], each species distinguished from the others by qualitative and quantitative traits such as the color of emerging leaves, size and color of strobili, or size and shape of leaves and leaflets [
8]. In the process of reviewing specimens of the
C. robusta complex, we located a few specimens deposited at HEM, XAL and MEXU herbaria (acronyms according to [
21]) which were collected in the floristic refuge of Uxpanapa (Veracruz) and required closer scrutiny to ensure its taxonomic status. These plants, previously identified as
C. robusta, were originally discovered and first collected in 1975 by Dr. Mario Vázquez Torres of the Institute of Biological Sciences of Universidad Veracruzana.
In mid-2019, we located populations of this
Ceratozamia in the wild in order to better understand its variation and ecology and collect more specimens. At this time, we observed that the plants from Uxpanapa appeared to differ from the contemporary circumscription of
C. robusta. For example, the leaves of these plants were larger than those of any other known species in the genus (
Figure 2). Additionally, the distal sporophyll faces of mature seed and pollen strobili were a dark-violet color that fades to yellowish-brown at the margin, a distinct difference to the brownish margins found in
C. robusta. These observations led us to consider the possibility that the plants from Uxpanapa could represent a new species. Previous studies of
C. robusta sensu lato have led to the discovery of new species [
7,
8], so we tested whether this was also the case for populations from Uxpanapa, by evaluating their morphological variation and testing its delimitation as a species when compared to other species within the
C. robusta species complex.
3. Results
The putative new species from Uxpanapa can be distinguished from each of the three compared species utilizing different qualitative traits (
Table 2). Most remarkable is the unique caramel color (similar to light-brown) of the emerging leaves, which are orange in
C. aurantiaca, yellowish-brown in
C. subroseophylla, reddish-brown in
C. robusta and green to copper-green in
C. leptoceras. Likewise, the measurements of morphometric traits were informative to understand general differences between the putative new species in respect to three closely related species in the
C. robusta species complex (
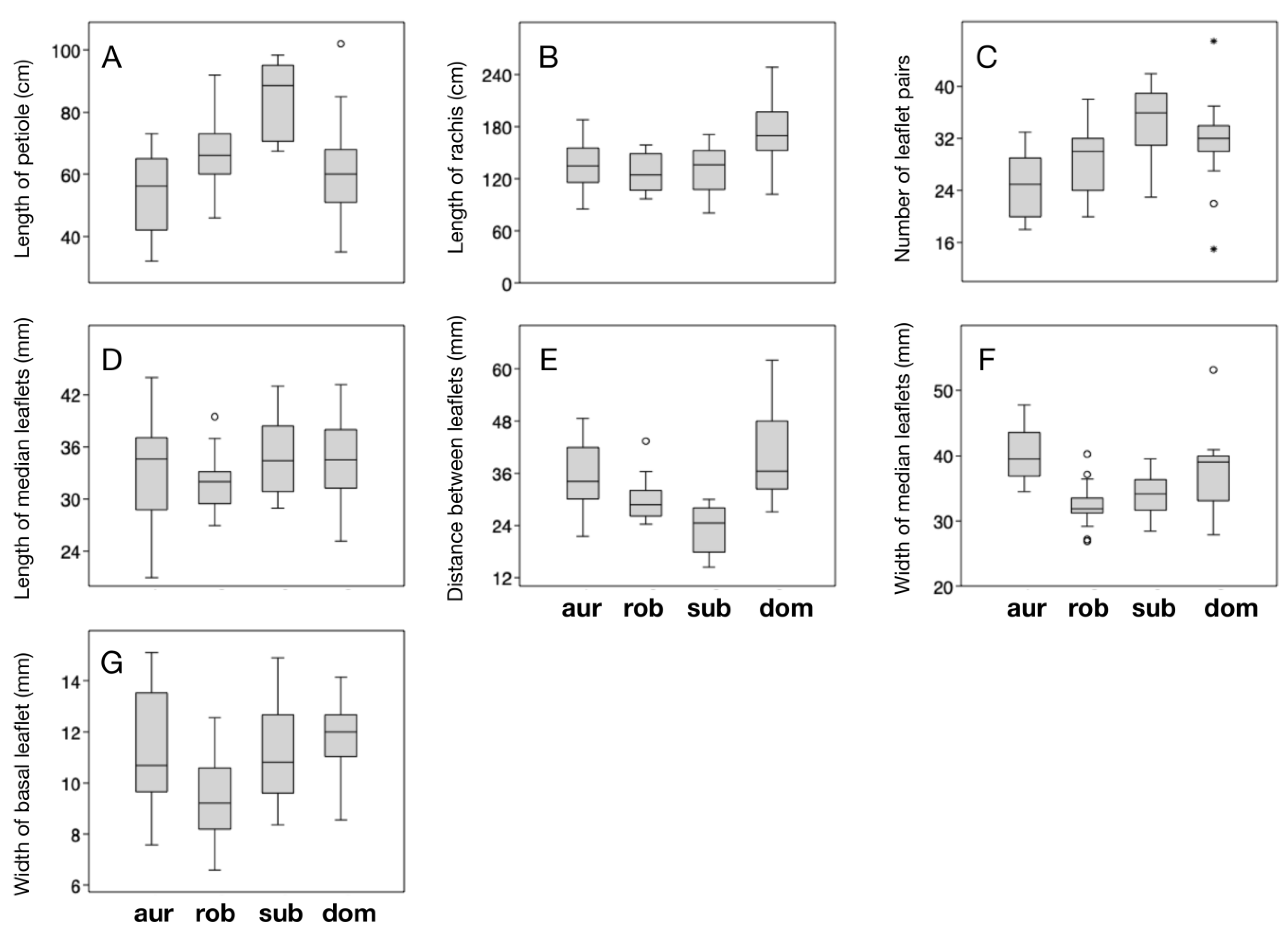
Table 3,
Figure 3). The plants from Uxpanapa have the longest rachides (mean = 172.83 ± 35.30 cm), longer distances between median leaflets (mean = 40.05 ± 10.53 mm), and widest basal leaflets (mean = 11.77 ± 1.47 mm) among the examined species.
C. subroseophylla presents the longest petioles (mean = 84.46 ± 11.93 cm), the most leaflet pairs (34.9 ± 5.3) and the longest median leaflets (mean = 34.70 ± 4.45 cm).
C. aurantiaca has the widest median leaflets (mean = 40.11 ± 3.85 mm). Surprisingly,
C. robusta, although it has been historically considered as the largest sized species (due to its long leaves and robust trunks) in the genus [
23], did not rank first in any of the examined traits.
The ANOVA test demonstrated that six of the seven quantitative traits have significantly different mean values among the four compared species (
Table 4). The length of median leaflets showed no significant differentiation (
p = 0.1908), suggesting that this trait has little diagnostic utility within the
C. robusta species complex. The Tukey’s Q values of pairwise differentiation gave more detailed information on how the examined species can be distinguished (
Table 5). Concordant with our prior observations, the plants from Uxpanapa were found to have significantly longer rachides than the other species (
Figure 2 and
Figure 3B,
Table 5). In addition, the plants from Uxpanapa have significantly longer rachides than
C. aurantiaca (
p < 0.005), significantly longer distance between median leaflets than
C. robusta (
p < 0.005) and
C. subroseophylla (
p < 0.001), and significantly narrower median leaflets (
p < 0.05) and wider basal leaflets than
C. robusta (p < 0.005).
The LDA summarized the total variation among species in three axes, each representing 68.97% (axis 1), 22.35% (axis 2) and 8.683% (axis 3) of the total variation. Axis 1 clearly separates
C. subroseophylla from
C. robusta,
C. aurantiaca and the plants from Uxpanapa (
Figure 4A). The longest biplots B and E suggest that the length of rachis and the distance between median leaflets were the main traits that dispersed the four groups. Axes 2 and 3 do not improve the segregation, showing that a few individuals from Uxpanapa overlap with the convex hulls of
C. aurantiaca (
Figure 4B). The confusion matrix obtained from LDA (
Table 6) confirmed that only two individuals from Uxpanapa may be confused with either
C. aurantiaca (1 individual) or
C. robusta (1 individual). Additionally, one individual of
C. aurantiaca may be confused as the putative species from Uxpanapa. However, the squared Mahalanobis distances were significantly high in all pairwise comparisons, suggesting that the overall morphometric variation is completely sorted among the examined taxa, confirming that the population from Uxpanapa represents a different species (
Table 7).
In summary, the presented evidence suggests that the plants from Uxpanapa, Veracruz, have the longest leaves within the C. robusta species complex, when compared to the type population of each species. Additionally, overall, plants look similar to C aurantiaca but have leaflets with a papyraceous (vs. coriaceous) texture, and emergent leaves that are of a unique caramel color not found in other members of the group. Additionally, the central portion of distal faces of the sporophylls of both the pollen and seed cones of the Uxpanapa plants are dark-violet that fades to yellowish-brown towards the margins, a unique trait within the genus. Because this suite of characters distinguishes these plants from the rest in the genus, we propose that the Ceratozamia populations from Uxpanapa should be recognized as a new species, and we name it as Ceratozamia dominguezii sp. nov.
New species description:
Holotype:—MEXICO. Veracruz, Uxpanapa, 130 m a.s.l. Pérez-Farrera M.A, 29 May 2021, 4013 ♂, (HEM). Isotypes: (XAL; MEXU).
Ceratozamia dominguezii can be distinguished by having long rachides with very separated median leaflets, emergent leaves of caramel color, papyraceous leaflets, and distal faces of mega- and microsporophylls with a dark-violet color that fades to yellowish-brown at the margin.
Additional specimens examined:—MEXICO. Veracruz: Uxpanapa, 16 January 1975, Mario Vázquez 1760 (MEXU, XAL, MO); Jesús Carranza, 12 April 1982, Mario Vázquez et al., 2430 (MEXU); Jesús Carranza, 19 February 2009, David Jimeno DJS1045 (MEXU, UV); Uxpanapa, 8 May 2019, Miguel A. Pérez Farrera, Michael Calonje and Cesar Daniel Coutiño Ovando 3763 (HEM); 4 November 2019, Miguel A. Pérez Farrera and Hector Gómez Domínguez 3800 (HEM); 23 October 2020, Miguel A. Pérez Farrera and Héctor Gómez Domínguez 3868 (HEM); 4 September 2021 Miguel A. Pérez Farrera and Pedro Díaz Jiménez 4064 ♀ (HEM).
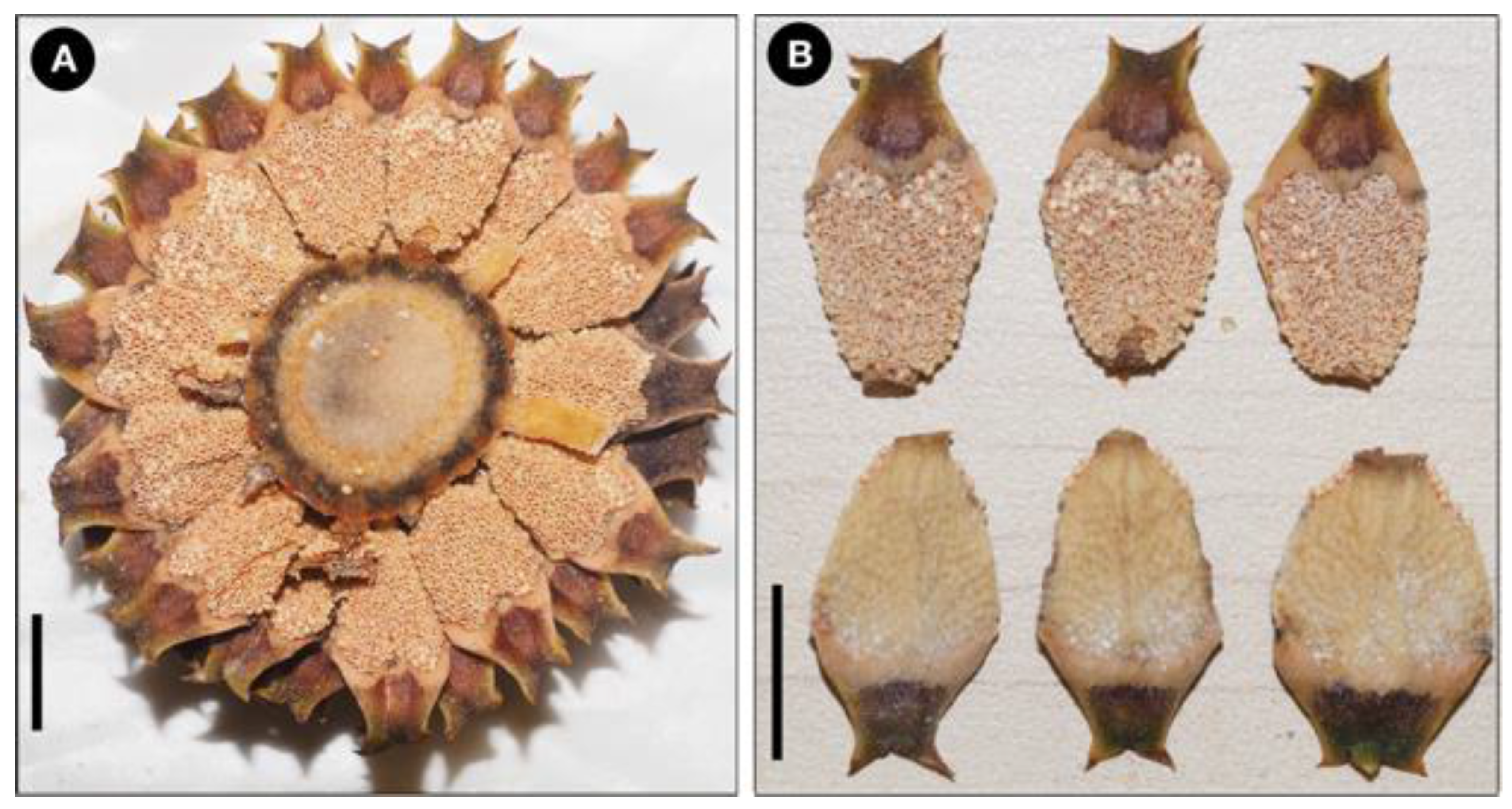
Plant rupicolous, arborescent, unbranching. Stem cylindrical, erect, sometimes decumbent with age, covered with persistent leaf bases, 32–132 cm tall, 20.69–30 cm in diameter. Cataphylls persistent, brown and densely tomentose at emergence, triangular apex acuminate. Leaves pinnate, 22–43 per crown, forming a semi-open crown, erect, ascending, olive green, keeled lightly in the basal part of leaves, 165.5–316.0 cm long, 50.4–68.4 cm wide, caramel color at emergence, turning green at maturity. Petiole terete, 35–103 cm long, densely armed with thick prickles. Rachis green, terete, 102–248 cm long, erect, with sparse prickles diminishing into the distal end of the rachis. Leaflets 15–37 pairs, papyraceous, subopposite to alternate in the basal part of the leaf, opposite to subopposite in the median part, linear, subfalcate to falcate, margin entire, apex acute to acuminate, asymmetric; base broad attenuate, articulation green adaxially, light green abaxially 0.85–1.4 cm wide, veins 30–33, parallel, inconspicuous, slightly translucent; median leaflets 25–43 cm long, 2.7–4.5 cm wide, spaced 2.7–6.2 cm between leaflets. Microstrobilus solitary, conical, erect, orange tomentose when emerging, 34–50 cm long, 5–6 cm diameter, peduncle densely tomentose, 8–9 cm long, 3 cm diameter. Microsporophyll cuneiform, 17.37–22.96 mm long, 8.79–13.38 mm wide, distal face bicornate, with dark-violet color at the central portion, which fades to brown-yellowish towards the margins, horns 1.5–2.7 mm long, separated by 120–135° angle, sporangia zone on abaxial surface 10.22–14.81 mm long, microsporangia 220–240 grouped in 3–4 per sorus. Megastrobilus solitary, cylindrical, erect, 44–48 cm long, 10–12 cm diameter; apex mucronate, light orange pubescent at emergence, peduncle tomentose, 7–9 cm long, 2.2–3.2 cm in diameter. Megasporophylls peltate, bicornate, 4–5.5 cm wide, 2.2–2.5 cm tall, distal face pubescent, when mature, distal face presents a dark-violet color at the central portion, which fades to brown-yellowish towards the margins (including horns). Seed ovoid, sarcotesta cream when immature, sclerotesta beige when mature, 1.5–2.0 cm diameter, 2.5–2.9 cm long, with micropylar ridges.
Habitat description:
Ceratozamia dominguezii grows between 100 and 130 m a.s.l., in karst tropical forest, according to the vegetation classification of Rzedowski [
24]. Associated flora includes
Brosimum alicastrum Sw.,
Pseudobombax ellipticum (Kunth) Dugand,
Swietenia macrophylla King,
Bursera simaruba (L.) Sarg.,
Stenanona sp.,
Coccoloba sp.,
Amphitecna sp.,
Plumeria rubra L.,
Astrocaryum mexicanum Liebm. ex Mart.,
Chamaedorea ernesti-augustii H. Wendl.,
Ch. pinnatifrons H. Wendl.,
Ch. oblongata Mart.,
Ch. elatior Mart.,
Ch. tepejilote Liebm.,
Begonia nelumbonifolia Schltdl. and Cham.,
Anthurium pedatoradiatum Schott,
A. schlechtendalii Kunth,
Monstera deliciosa Liebm., and
Philodendron radiatum Schott.
Etymology: the specific epithet was chosen to honor Héctor Gómez-Domínguez (M.Sc.), retired technician from the Eizi Matuda Herbarium (Chiapas) whose field explorations and specimen collections resulted in the discovery of several new cycad species from southern Mexico.
4. Discussion
Although the
Ceratozamia populations from Uxpanapa, Veracruz, have historically been considered as
C. robusta, our results demonstrate that the set of morphological traits presented by these populations are not consistent with the concept of
C. robusta, as defined by plants occurring at or near its type locality. The neotype of
C. robusta [
25] is from Parque Nacional Cañón del Sumidero, northwest of Tuxtla Gutiérrez, Chiapas (
Table 1), a locality that is relatively close to Uxpanapa (
Figure 1). However, the plants from Uxpanapa (here recognized as
C. dominguezii) have significantly longer rachides (
Figure 3B), wider distances between leaflets along the rachis (
Figure 3E), wider median leaflets (
Figure 3F) and wider basal leaflets (
Figure 3G,
Table 5). This taxon is also distinguishable from
C. robusta and the rest of the species in the complex based on qualitative traits (
Table 2), most notable being the unique caramel color of emerging leaves (
Figure 6), and the dark-violet coloration at the central portion of distal faces of strobili (
Figure 7 and
Figure 8).
The occurrence of
C. dominguezii and the distribution of the other species in the
C. robusta species complex can be informative about the evolutionary history of this group (
Figure 1). The Isthmus of Tehuantepec has been identified as a main geographic barrier causing the divergence of lineages at either side [
17].
Ceratozamia dominguezii and
C. robusta occur at the eastern side of the isthmus, whereas
C. subroseophylla,
C. aurantiaca, and
C. leptoceras occur at the western side. However, there is evidence of historical connectivities between the two sides. Wendt [
16] identified that the regions of Uxpanapa (home of
C. dominguezii), the northern mountains of Chiapas (home of
C. robusta in Chiapas), as well as the Izabal floristic refugium region (home of
C. robusta in Guatemala and Belize, Los Tuxtlas (home of
C. subroseophylla), and the northern mountains of Oaxaca (home of
C. aurantiaca) harbor highly similar karstic, tropical rainforests, although each with their own endemic species. These areas are thought to represent floristic refugia that allowed the persistence of paleoendemic groups such as
Oreomunnea Oerst. and
Ceratozamia [
26] during the climatic fluctuations which occurred since the Miocene to the Late Pleistocene [
16]. Events of vicariance in plant lineages inhabiting throughout these areas may have occurred during these times, since events of vicariance are expected to have influenced cycad divergence leading to speciation [
15,
17,
18,
27]. Future phylogenetic analyses may clarify the times of divergence, as well as its position and delimitation in the phylogeny of the genus.
To date, we have identified four populations of
C. dominguezii in Uxpanapa, Veracruz, Mexico. However, Veracruz is one of the states with the highest deforestation rate in the country [
28], and the forest of Uxpanapa is one of the most severely affected [
29]. Uxpanapa also hosts two other cycad species:
Ceratozamia euryphyllidia Vázq. Torres, Sabato and D.W.Stev., a distantly related species belonging to the
C. miqueliana species complex [
30,
31], and
Zamia purpurea Vovides, J.D.Rees and Vázq. Torres. Both species are listed as Critically Endangered in the Red List of the International Union for the Conservation of Nature [
32]. We propose that
C. dominguezii should be considered under the same status, CR, given the same threat and according to the criteria B2ab(i,ii,iii,iv,v) [
32]. Conservation measures for in-situ protection and representation of this species in ex-situ collections are urgently necessary.