An Extraordinary Rosette and Resurrection New Spikemoss, Selaginella iridescens (Selaginellaceae) from Hainan Island, China †
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphological Observations
2.2. Taxon Sampling and Sequencing
2.3. Phylogenetic Analysis
2.4. Character Evolution Analysis
3. Results
3.1. Taxonomy Treatment
3.2. Phylogenetic Analysis
3.3. Ancestral Reconstruction of Rosette Character
4. Discussion
4.1. Morphological Comparison with Three Related Rosette Species
4.2. How Many Rosette Species in Selaginella?
4.3. Key to S. iridescens, S. pseudotamariscina, S. pulvinata, and S. tamariscina
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jermy, A.C. Subgeneric names in Selaginella. Fern Gaz. 1986, 13, 117–118. [Google Scholar]
- Zhang, X.C.; Nooteboom, H.P.; Kato, M. Selaginellaceae. In Flora of China; Wu, Z.Y., Raven, P.H., Hong, D.Y., Eds.; Science Press: Beijing, China, 2013; Volumes 2–3, pp. 37–66. [Google Scholar]
- Weststrand, S.; Korall, P. Phylogeny of Selaginellaceae: There is value in morphology after all! Am. J. Bot. 2016, 103, 2136–2159. [Google Scholar] [CrossRef]
- Weststrand, S.; Korall, P. A subgeneric classification of Selaginella (Selaginellaceae). Am. J. Bot. 2016, 103, 2160–2169. [Google Scholar] [CrossRef] [PubMed]
- Korall, P.; Kenrick, P. Phylogenetic relationships in Selaginellaceae based on rbcL sequences. Am. J. Bot. 2002, 89, 506–517. [Google Scholar] [CrossRef]
- Zhou, X.M.; Zhang, L.B. A classification of Selaginella (Selaginellaceae) based on molecular (chloroplast and nuclear), macromorphological, and spore features. Taxon 2015, 64, 1117–1140. [Google Scholar] [CrossRef]
- Zhang, H.R.; Wei, R.; Xiang, Q.P.; Zhang, X.C. Plastome-based phylogenomics resolves the placement of the sanguinolenta group in the spikemoss of lycophyte (Selaginellaceae). Mol. Phylogenet. Evol. 2020, 147, 106788. [Google Scholar] [CrossRef] [PubMed]
- Jermy, A.C. Selaginellaceae. In The Families and Genera of Vascular Plants, Pteridophytes and Gymnosperms; Kramer, K.U., Green, P.S., Eds.; Springer: Berlin, Germany, 1990; Volume 1, pp. 39–45. [Google Scholar]
- Zhang, X.C. Selaginellaceae. In Flora Reipublica Popularis Sinicae; Zhang, X.C., Ed.; Science Press: Beijing, China, 2004; Volume 6, pp. 86–219. [Google Scholar]
- Zhou, X.M.; Rothfels, C.J.; Zhang, L.; He, Z.R.; Le Péchon, T.; He, H.; Lu, N.T.; Knapp, R.; Lorence, D.; He, X.J.; et al. A large-scale phylogeny of the lycophyte genus Selaginella (Selaginellaceae: Lycopodiopsida) based on plastid and nuclear loci. Cladistics 2015, 32, 360–389. [Google Scholar] [CrossRef]
- Zhang, X.C.; Xiang, Q.P.; Nooteboom, H.P. A new species of Selaginella from Hainan Island, China, with a key to the Hainan species. Bot. J. Linnean Soc. 2005, 148, 323–327. [Google Scholar] [CrossRef][Green Version]
- Zhang, M.H.; Yang, J.; Luu, H.T.; Tran, G.; Zhang, X. Selaginella pseudotamariscina (Selaginellaceae), an overlooked rosette-forming resurrection spikemoss from Vietnam. Guihaia 2021. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 491–587. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree, Version 1.4.2. Computer Program and Documentation Distributed by the Author. 2014. Available online: https://tree.bio.ed.ac.uk/software/figtree/ (accessed on 10 September 2021).
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis. Version 3.61. 2019. Available online: https://www.mesquiteproject.org (accessed on 14 September 2021).
- Lewis, P.O. A likelihood approach to estimating phylogeny from discrete morphological character data. Syst. Biol. 2001, 50, 913–925. [Google Scholar] [CrossRef] [PubMed]
- IUCN. Guidelines for Using the IUCN Red List Categories and Criteria, Version 14. Prepared by the Standards and Petitions Committee. 2019. Available online: https://www.iucnredlist.org/documents/RedListGuidelines.pdf (accessed on 24 December 2020).
- Smith, A.R.; Reeves, T. Selaginella gypsophila (Selaginellaceae), yet another new edaphic endemic from northern Mexico. SIDA Contrib. Bot. 1984, 10, 211–215. [Google Scholar]
- Mickel, J.T.; Smith, A.R.; Valdespino, I.A. Selaginella. In The Pteridophytes of Mexico; Mickel, J.T., Smith, A.R., Eds.; Parts I and II Memoirs of the New York Botanical Garden; New York Botanical Garden Press: New York, NY, USA, 2004; pp. 88, 550–602, 968–989. [Google Scholar]
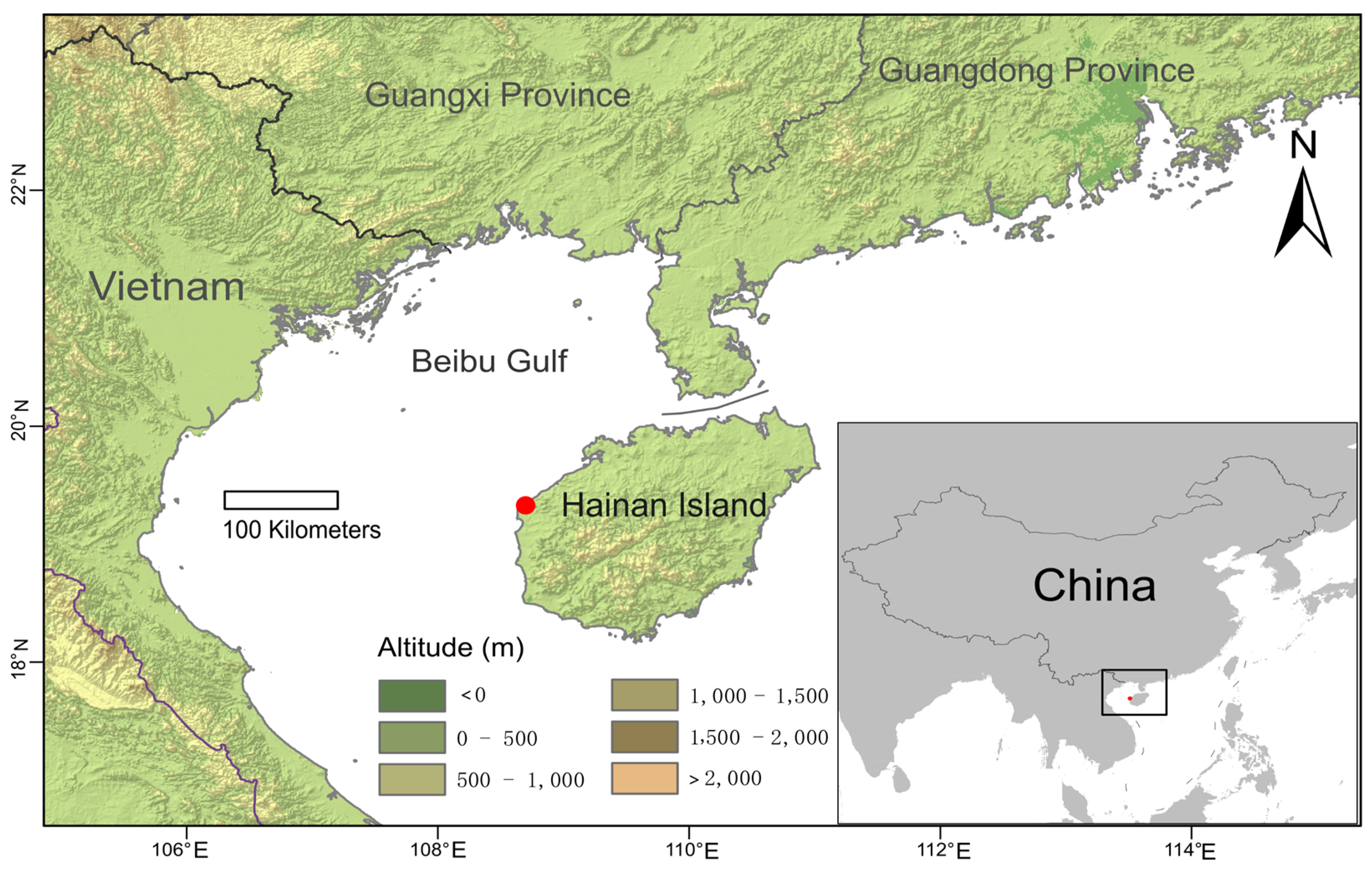
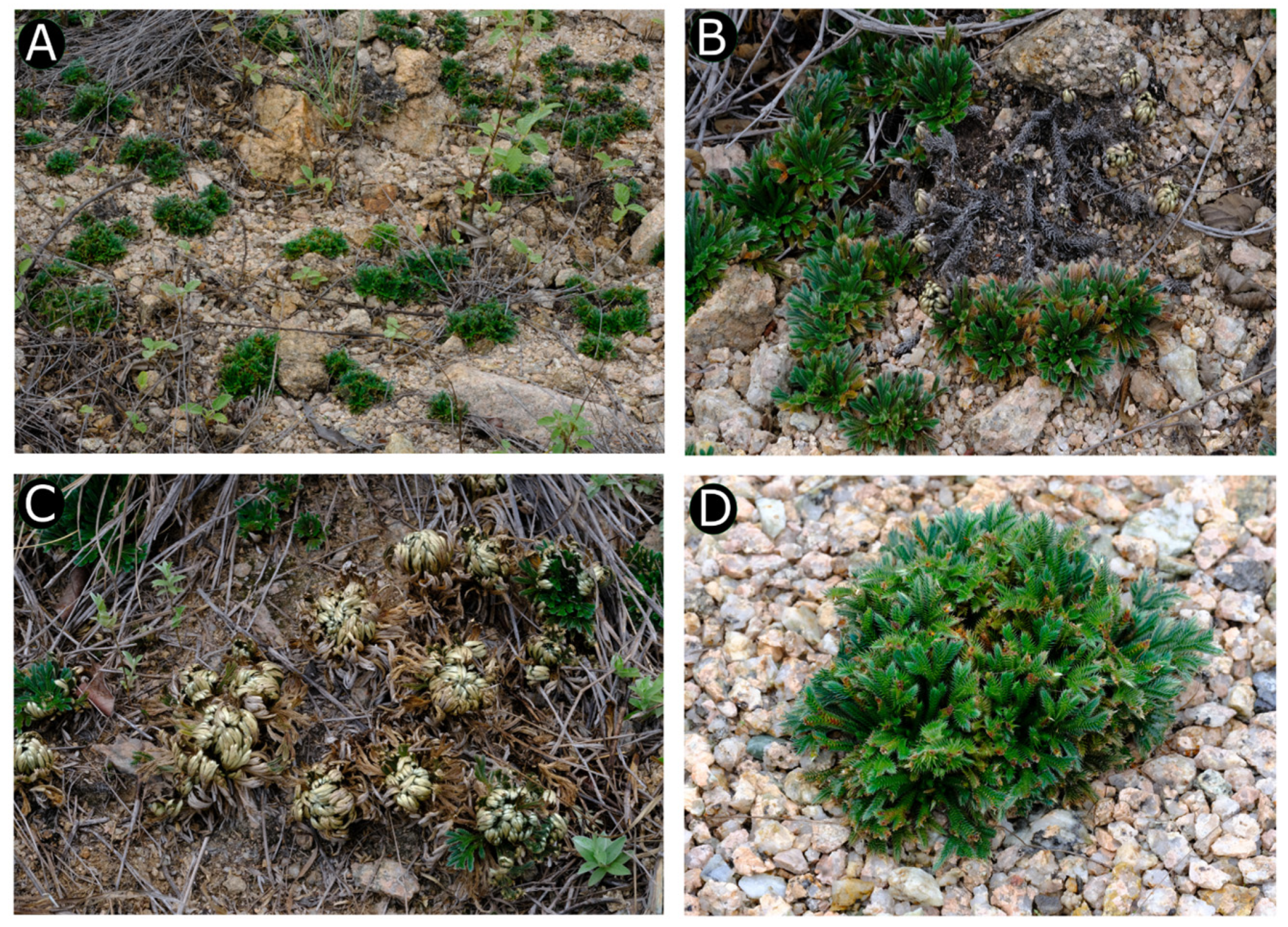


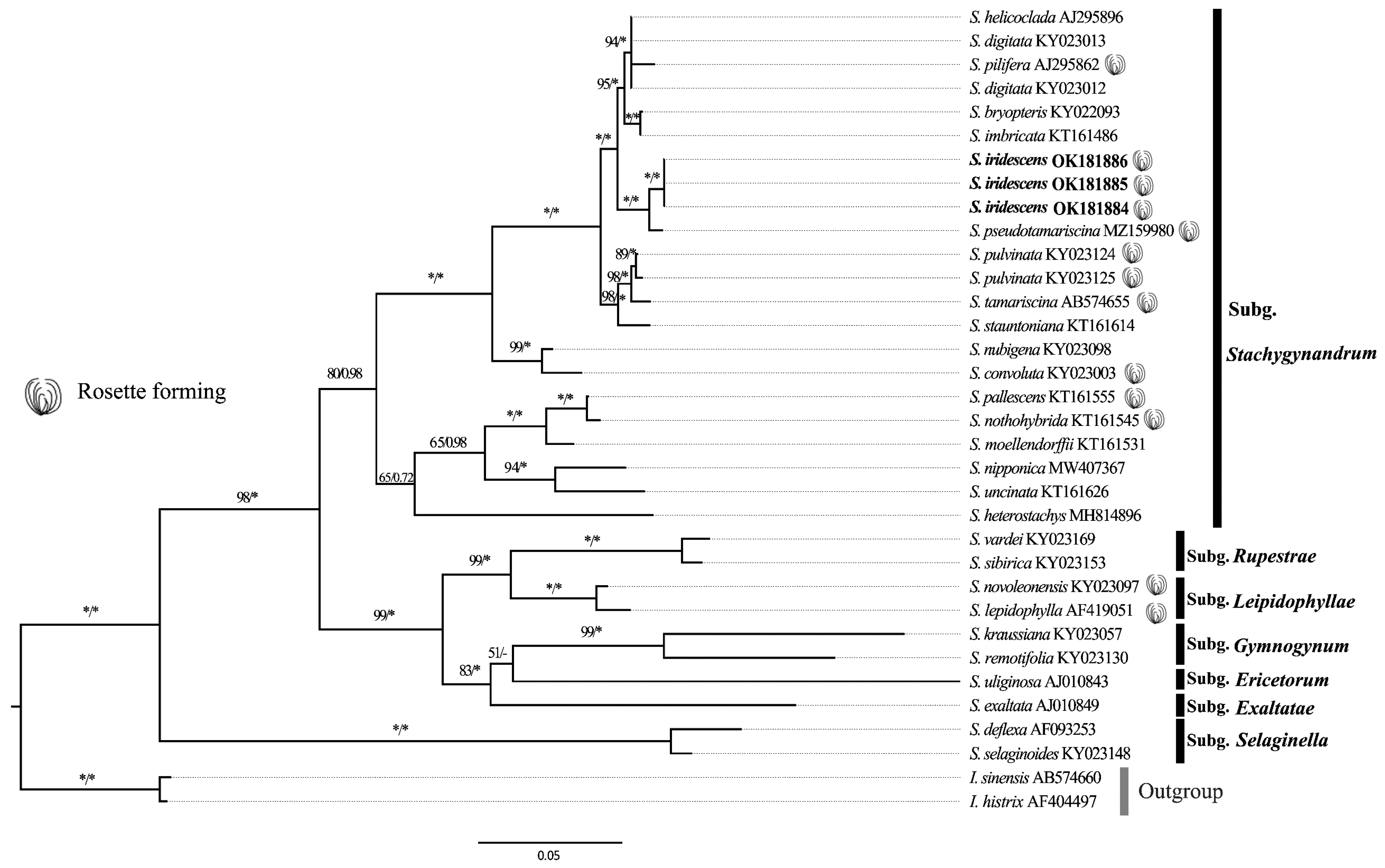
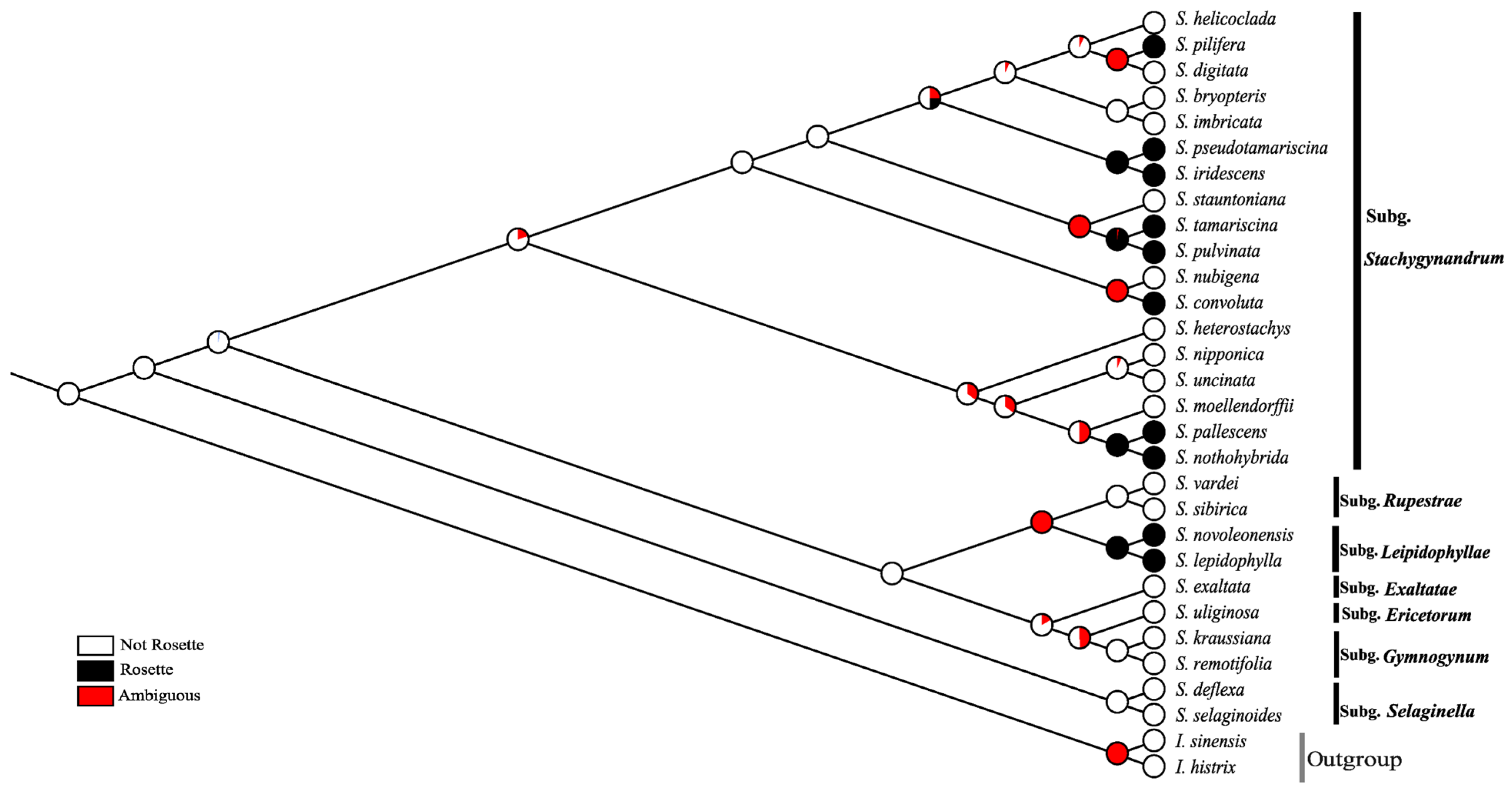
| No. | Species | Collector and Voucher No. | Locality | Herbarium | Accession No. |
|---|---|---|---|---|---|
| 1 | Selaginella bryopteris (L.) Baker | C. R. Fraser-Jenkins 4370 | Nepal | L | KY022983 |
| 2 | S. convoluta (Arn.) Spring | R. M. Harley 16181 | Brazil: Bahia | U | KY023003 |
| 3 | S. deflexa Brack. | D. Pamer 2651 | USA: Hawaii | KANU | AF093253 |
| 4 | S. digitata Spring | N. Wikström et al. 110319-2 | Madagascar | S | KY023013 |
| 5 | S. digitata Spring | P. Phillipson 1826 | Madagascar | L | KY023012 |
| 6 | S. exaltata (Kunze) Spring | Korall 1996-1 | Sweden | S | AJ010849 |
| 7 | S. helicoclada Alston | Rakotondrainibe 3262 | Madagascar | P | AJ295896 |
| 8 | S. heterostachys Baker | X. C. Zhang 7088 | China: Guizhou | PE | MH814896 |
| 9 | S. imbricata (Forssk.) Spring ex Decne. | Rothfels et al. 4275 | Oman | DUKE | KT161486 |
| 10 | S. iridescens X. C. Zhang and Y. R. Wang | Y. R. Wang and L. X. Yuan wyr20200110-1 | China: Hainan | PE | OK181884 |
| 11 | S. iridescens X. C. Zhang and Y. R. Wang | Y. R. Wang and L. X. Yuan wyr20200110-2 | China: Hainan | PE | OK181885 |
| 12 | S. iridescens X. C. Zhang and Y. R. Wang | Y. R. Wang and L. X. Yuan wyr20200110-3 | China: Hainan | PE | OK181886 |
| 13 | S. kraussiana A. Braun | M. Mokoso 3098 | Democratic Republic of the Congo | BR | KY023057 |
| 14 | S. lepidophylla (Hook. and Grev.) Spring | Worthington s.n. | USA: Texas | US | AF419051 |
| 15 | S. moellendorffii Hieron. | Ju and Deng HGX12295 | China: Sichuan | CDBI | KT161531 |
| 16 | S. nipponica Franch. and Sav. | X. C. Zhang et al. 7066 | China: Guizhou | PE | MW407367 |
| 17 | S. nothohybrida Valdespino | Rothfels et al. 3069 | Mexico: San Luis Potosí | DUKE | KT161545 |
| 18 | S. novoleonensis Hieron. | F. Drouet and D. Richards 3942 | Mexico: Sonora | S | KY023097 |
| 19 | S. nubigena J.P.Roux | A. Larsson AL810 | South Africa | UPS | KY023098 |
| 20 | S. pallescens (C. Presl) Spring | Beck 1120 | Mexico: Hidalgo | DUKE | KT161555 |
| 21 | S. pilifera A. Braun | Pringle 13959 | N/A 1 | S | AJ295862 |
| 22 | S. pseudotamariscina X.C. Zhang and C.W. Chen | C.W. Chen Wade 5314 | Vietnam: Khanh Hoa Province | PE | MZ159980 |
| 23 | S. pulvinata (Hook. et Grev.) Maxim | D. E. Boufford et al. 37879 | China: Sichuan | A | KY023124 |
| 24 | S. pulvinata | D. E. Boufford et al. 35254 | China: Yunnan | A | KY023125 |
| 25 | S. remotifolia Spring | Gaoligong Shan Biodiversity Survey; 21081 | China: Yunnan | GH | KY023130 |
| 26 | S. selaginoides (L.) P. Beauv. ex Mart. and Schrank | S. Weststrand 104 | Sweden | UPS | KY023148 |
| 27 | S. sibirica (Milde) Hieron. | L. A. Viereck and K. Jones 5667 | USA: Alaska | S | KY023153 |
| 28 | S. stauntoniana Spring | Zhao 169 | China: Beijing | CDBI | KT161614 |
| 29 | S. tamariscina (P.Beauv.) Spring | N/A TNS759348 | Japan: Okinawa | TNS | AB574655 |
| 30 | S. uliginosa Spring | Holmgren and Wanntorp 253 | Sweden | S | AJ010843 |
| 31 | S. uncinata (Desv. ex Poir.) Spring | Zhang and Zhou DJY04101 | China: Sichuan | CDBI | KT161626 |
| 32 | S. vardei H. Lév. | D. E. Boufford et al. 32425 | China: Tibet | A | KY023169 |
| 33 | Isoetes sinensis Palmer | N/A 743727 | Japan | TNS | AB574660 |
| 34 | I. histrix Bory and Durand | Wanntorp N/A | Sweden | S | AF404497 |
| Characters/Species | S. iridescens | S. pseudotamariscina | S. pulvinata | S. tamariscina |
|---|---|---|---|---|
| Distribution | Hainan of China | Khanh Hoa and Ninh Thuan of Vietnam | Southwestern and central China, India, Thailand, Vietnam | Eastern and Southeastern China (including Hainan), Japan, Korean Peninsula, Philippines, Thailand |
| Habit | Stems and roots entangled forming slender, dorsiventrally prostrate trunk | Stems and roots entangled forming thick, radial erect treelike trunk | Stems and roots entangled not forming treelike trunk | Stems and roots entangled forming treelike trunk |
| Leaves | Iridescent | Not iridescent | Not iridescent | Not iridescent |
| Axillary leaves | Ovate-oblong, margin membranaceous (about 2/3 of the entire leaf), lacerate or subentire, base ciliolate or denticulate | Lanceate, or ovate-lanceate, margin membranaceous (about 1/2 of the entire leaf), acerate-ciliolate | Ovate to triangular, margin membranaceous (about 1/2 of the entire leaf), lacerate-ciliolate, base ciliolate or lacerate | Ovate, ovate-triangular, or elliptic, margin membranaceous (about 1/3 of the entire leaf), denticulate-lacerate, base ciliolate or denticulate |
| Dorsal leaves | Symmetrical, lanceate, adaxially sulcate extends to the top, apex with long arista (ca. 0.76 mm long), margin ciliolate or denticulate | Symmetrical, lanceate, adaxially sulcate extends to half of the leaves, apex with short arista (ca. 0.2 mm long), margin ciliolate or denticulate | Asymmetrical, obliquely ovate or triangular, adaxially not sulcate, margin lacerate or entire | Asymmetrical, elliptic, adaxially not sulcate, margin denticulate (shortly ciliolate at base) |
| Ventral leaves | Ovate-lanceate to ovate-triangular, adaxially sulcate, apex with long arista (ca. 0.35–1.20 mm long) | Ovate-lanceate to ovate-triangular, adaxially sulcate, apex with short arista (0.02–0.40 mm long) | Oblong, adaxially not sulcate; apex with long arista (ca. 0.35–1.20 mm long) | Ovate to triangular or oblong-ovate, adaxially not sulcate; apex arista |
| Strobili | Slightly dorsoventrallycomplanate | Slightly dorsoventrally complanate | Tetragonal | Tetragonal |
| Sporophylls | Slightly anisophyllous; sporangia borne only on the ventral sporophylls | Slightly anisophyllous; sporangia borne only on the ventral sporophylls | Isophyllous; sporangia borne on both dorsal and ventral sporophylls | Isophyllous; sporangia borne on both dorsal and ventral sporophylls |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Zhang, M.-H.; Wang, Y.-R.; Yuan, L.-X.; Zhang, X.-C. An Extraordinary Rosette and Resurrection New Spikemoss, Selaginella iridescens (Selaginellaceae) from Hainan Island, China. Taxonomy 2021, 1, 302-312. https://doi.org/10.3390/taxonomy1040024
Yang J, Zhang M-H, Wang Y-R, Yuan L-X, Zhang X-C. An Extraordinary Rosette and Resurrection New Spikemoss, Selaginella iridescens (Selaginellaceae) from Hainan Island, China. Taxonomy. 2021; 1(4):302-312. https://doi.org/10.3390/taxonomy1040024
Chicago/Turabian StyleYang, Jie, Meng-Hua Zhang, Ya-Rong Wang, Lang-Xing Yuan, and Xian-Chun Zhang. 2021. "An Extraordinary Rosette and Resurrection New Spikemoss, Selaginella iridescens (Selaginellaceae) from Hainan Island, China" Taxonomy 1, no. 4: 302-312. https://doi.org/10.3390/taxonomy1040024
APA StyleYang, J., Zhang, M.-H., Wang, Y.-R., Yuan, L.-X., & Zhang, X.-C. (2021). An Extraordinary Rosette and Resurrection New Spikemoss, Selaginella iridescens (Selaginellaceae) from Hainan Island, China. Taxonomy, 1(4), 302-312. https://doi.org/10.3390/taxonomy1040024







