Comparative Morphology, Phylogeny, Classification and Evolution of Interstitial Habits in Microcambevine Catfishes (Siluriformes: Trichomycteridae) †
Abstract
1. Introduction
History of Microcambevine Taxonomy
2. Materials and Methods
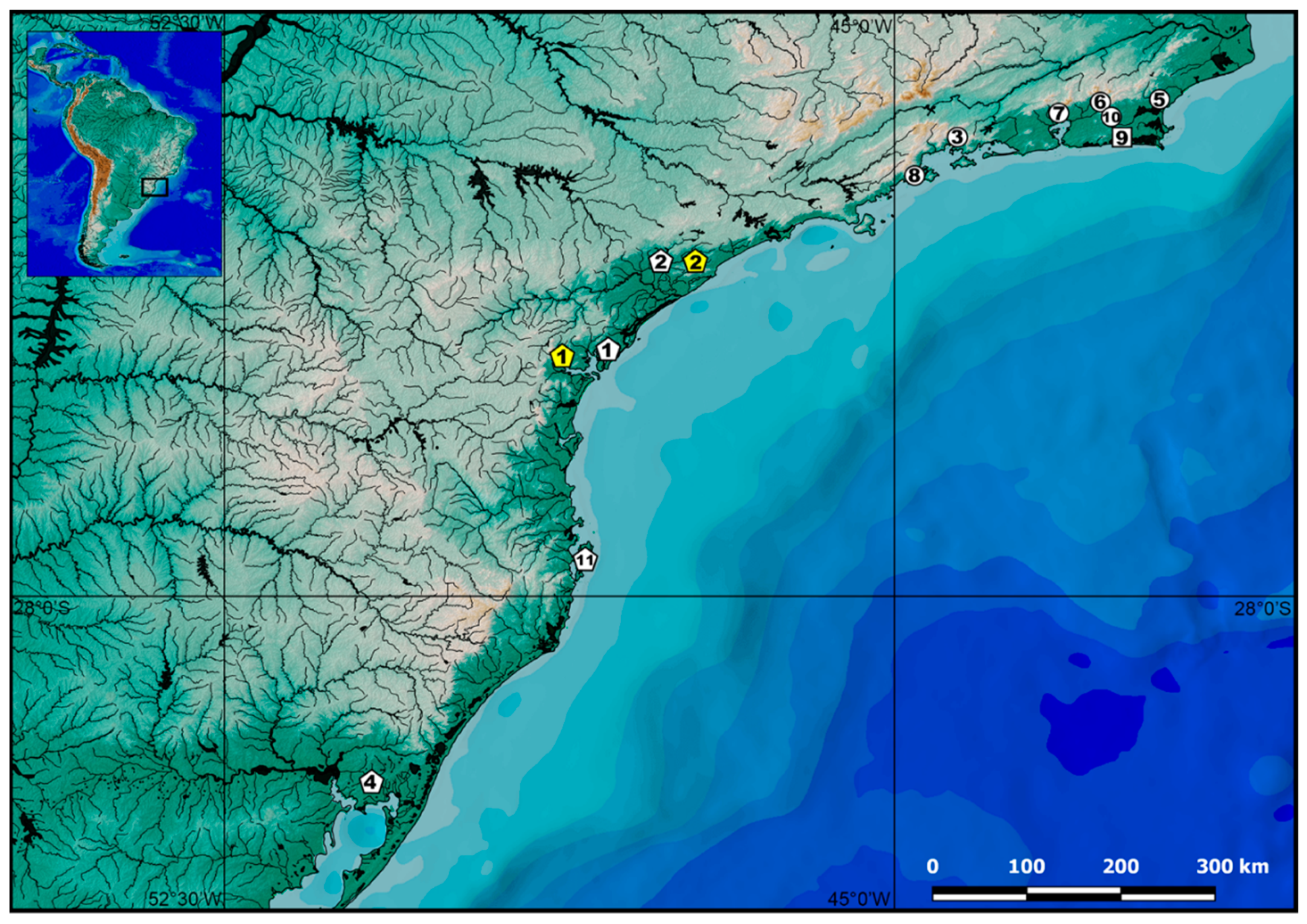
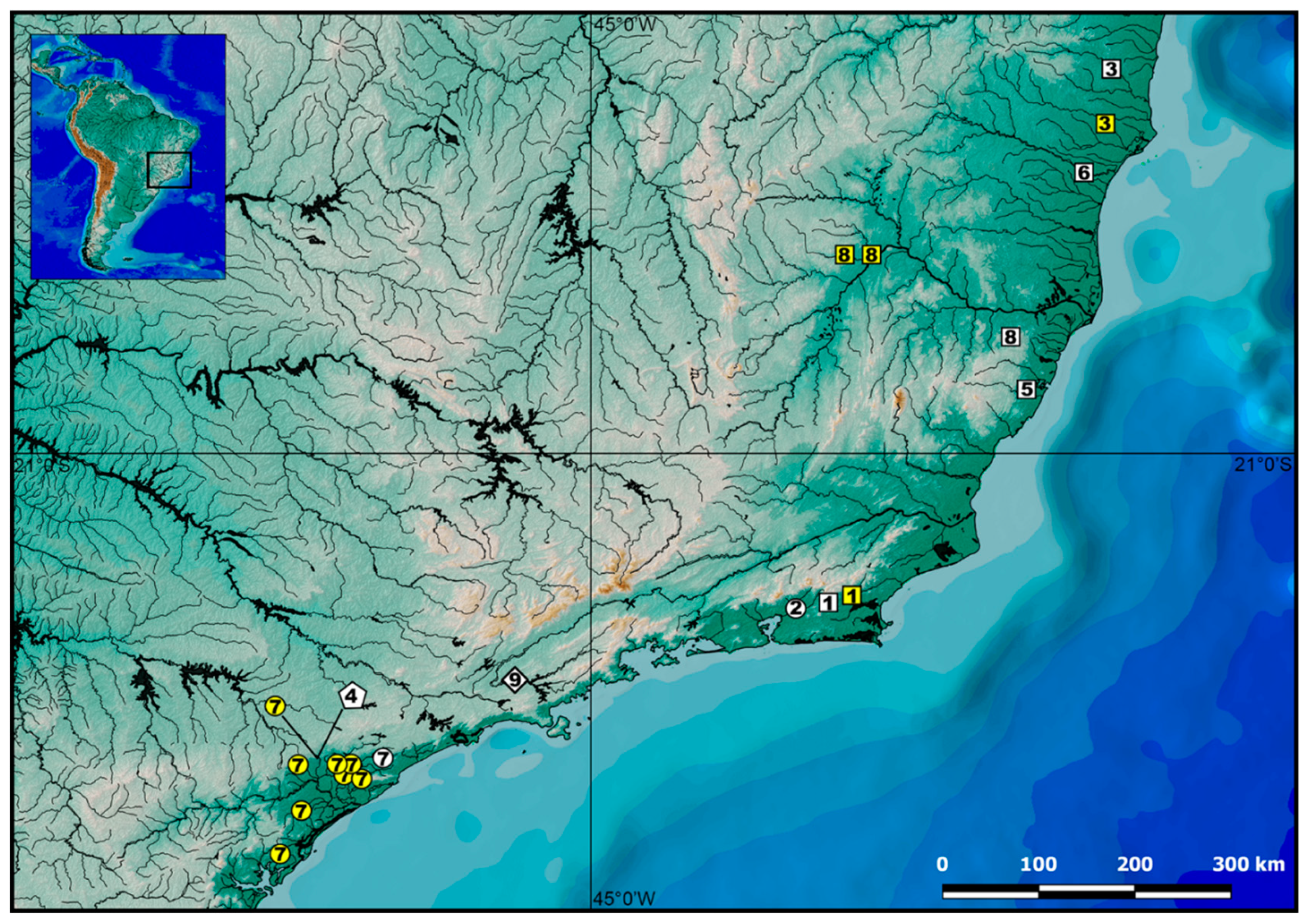
2.1. Specimens
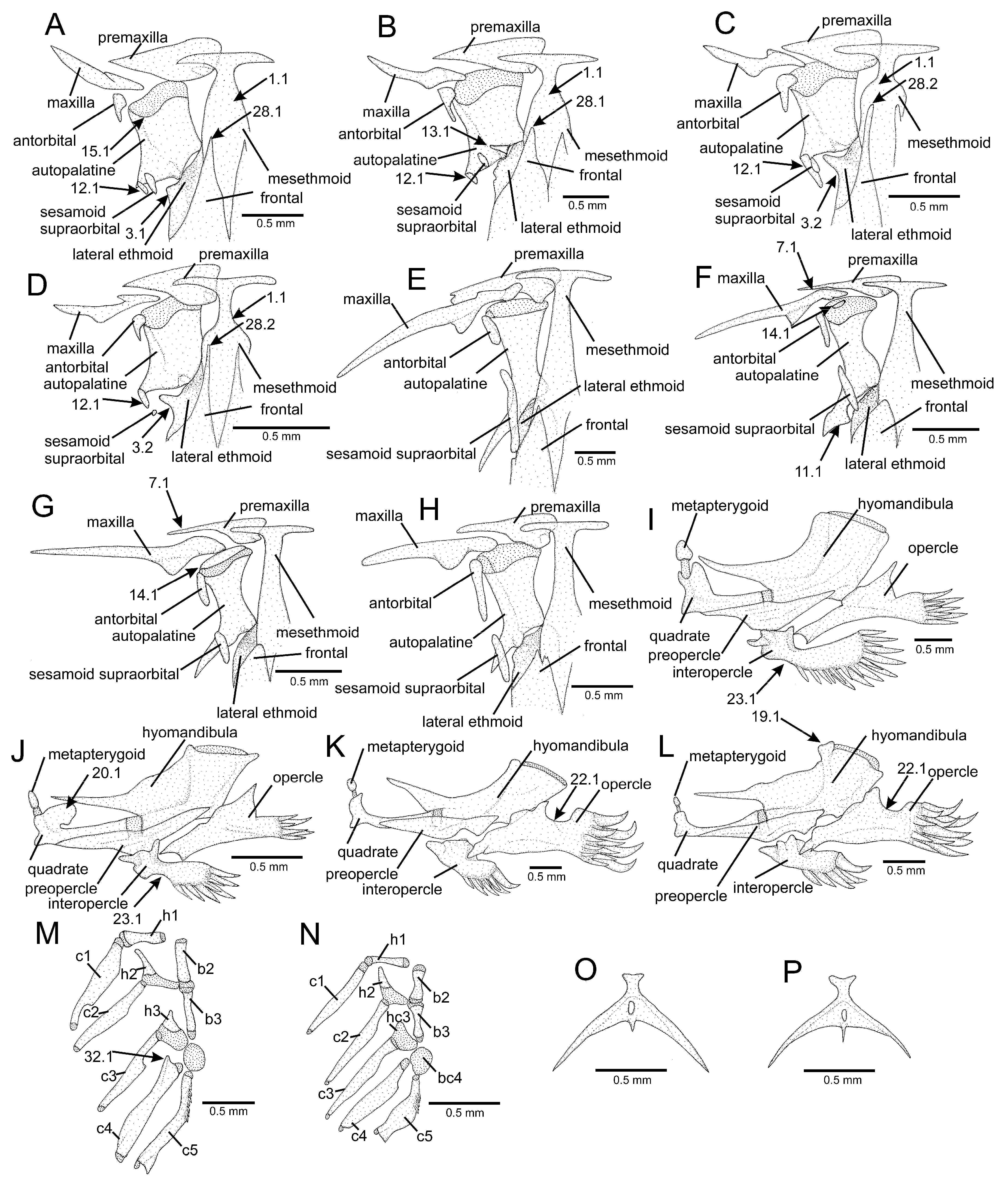
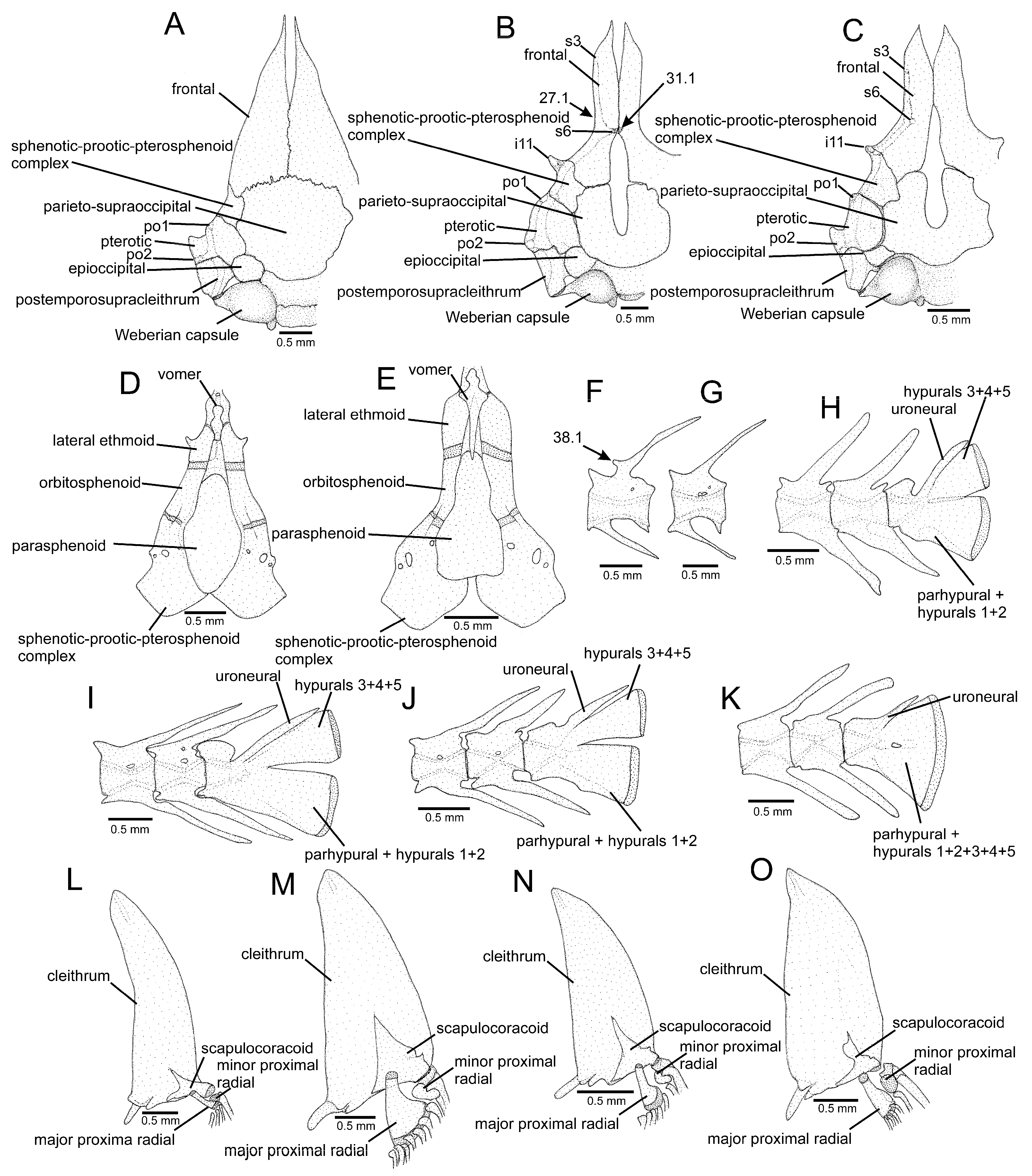
2.2. Morphological Data
2.3. DNA Sequences
2.4. Phylogenetic Analyses
2.5. Taxonomical Accounts
3. Results
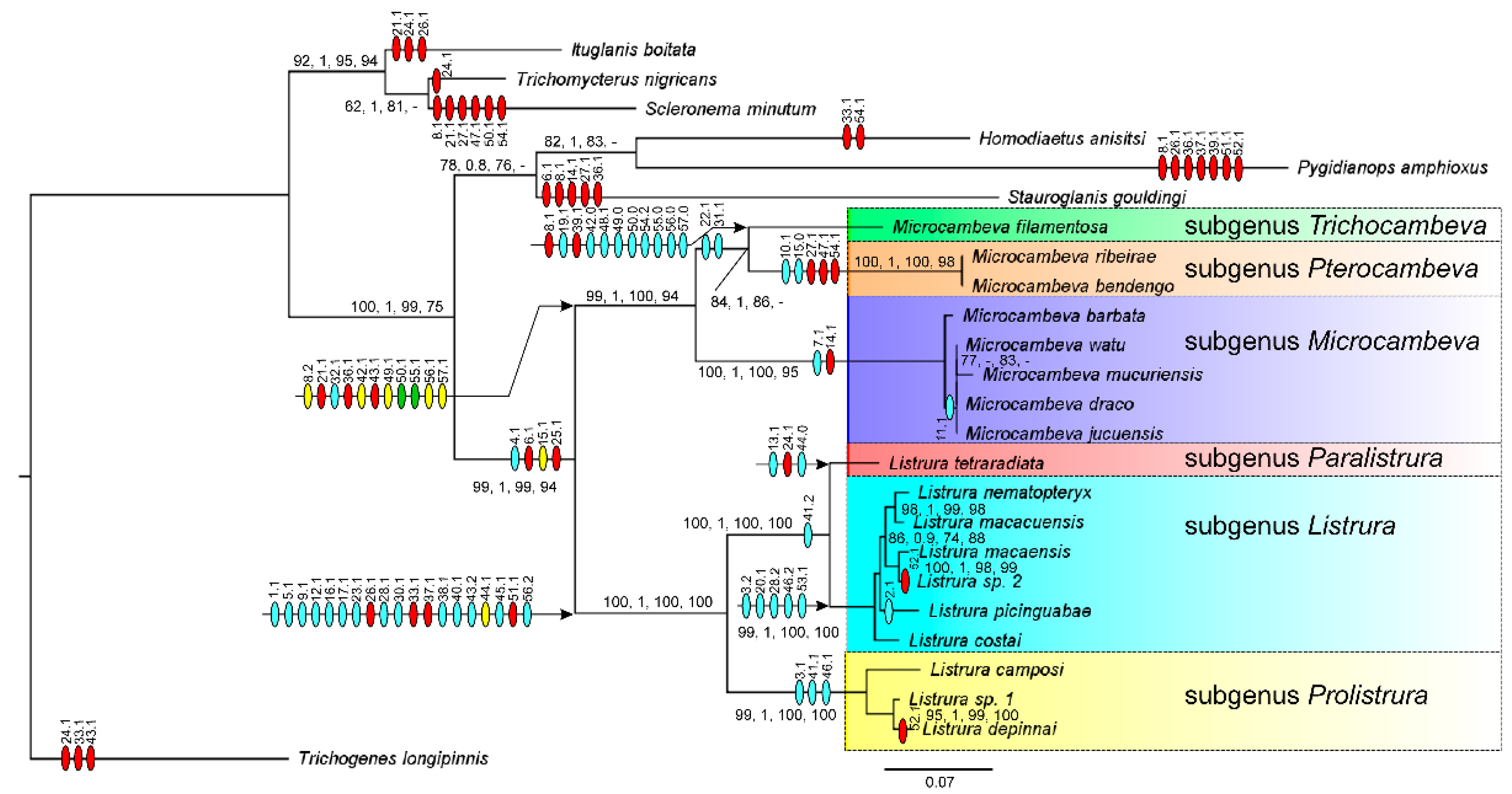
3.1. Phylogenetic Relationships
3.2. Taxonomic Accounts
3.2.1. Listrura de Pinna, 1988

Subgenus Listrura de Pinna, 1988
Listrura macaensis sp. nov.
Listrura macacuensis sp. nov.
Paralistrura, New Subgenus
Prolistrura, New Subgenus
3.2.2. Microcambeva Costa & Bockmann, 1994
Subgenus Microcambeva
Pterocambeva, New Subgenus
Trichocambeva, New Subgenus
3.3. Key for Identification of Genera and Subgenera of Microcambeviinae
4. Discussion
4.1. Phylogenetic Relationships and Morphological Support
4.2. Morphological Diversity and Evolution of Fossorial Lifestyle in Listrura
4.3. Morphological Diversity and Evolution of Psammophily in Microcambeva
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- Listrura camposi: UFRJ 11404, 5 (2 C&S).
- Listrura costai: MNRJ 31917, H; MNRJ 31535, 3 P; MNRJ 31918, 5 P; MNRJ 39620, 4 P; UFRJ 7214, 3 P; UFRJ 6577, 3 P; UFRJ 7215, 4; UFRJ 7214, 6; UFRJ 11224, 4.
- Listrura depinnai: UFRJ 12552, 5; UFRJ 10689, 2 (1 C&S).
- Listrura nematopteryx: UFRJ 707, 20; UFRJ 8623, 1; UFRJ 5952, 5 (C&S).
- Listrura picinguabae: UFRJ 6111, H; MCP 38921, 2 P; UFRJ 5948, 1 P; UFRJ 5949, 2 P; UFRJ 5950, 15 P; UFRJ 5951, 4 P (C&S); UFRJ 5991, 2 P; UFRJ 6138, 5 P (C&S).
- Listrura tetraradiata: MZUSP 52572, H; UFRJ 4586, 17P; UFRJ 4588, 6 P (C&S); UFRJ 4587, 17 P; UFRJ 4590, 7 P; MZUSP 50164, 3 P; UFRJ 11458, 8; UFRJ 11457, 9.
- Listrura sp. 1: UFRJ 9601, 11; UFRJ 1278, 1; UFRJ 9692, 3 (C&S); UFRJ 11522, 2 (C&S); UFRJ 9498, 11; UFRJ 1279, 1 (C&S); UFRJ 9994, 1 (C&S).
- Microcambeva barbata: MZUSP 43678, H; MZUSP 43679, 1 P; UFRJ 684, 2 P (C&S); UFRJ 12129, 1; UFRJ 7000, 1 (C&S).
- Microcambeva draco: MCP 17796, H; MCP 17796, 1P (C&S).
- Microcambeva filamentosa: UFRJ 12187, P; UFRJ 12180, 2 P; UFRJ 12188, 1 P; UFRJ 12345, 2 P (C&S); UFRJ 12551, 9 P.
- Microcambeva jucuensis: UFRJ 12124, H; UFRJ 11083, 18 P; UFRJ 11011, 4 P; UFRJ 11840, 4 P (C&S); CICCAA 03524, 2 P.
- Microcambeva mucuriensis: UFRJ 12123, H; UFRJ 11091, 22 P; UFRJ 11028, 4 P; UFRJ 11841, 5P (C&S); CICCAA 03525, 2 P;
- Microcambeva ribeirae: MZUSP 84301, H; MZUSP 78617, 5 P; MZUSP 68169, 3 P (C&S); MZUSP 74669, 10 P; MZUSP 79953, 20 P; MNRJ 12314, 1 P; MNRJ 14304, 4 P (1 C&S); MZUSP 49463, 1 P (C&S); UFRJ 12189, 2; UFRJ 6999, 1 (C&S); UFRJ 12549, 3.
Appendix B
| COI | CYTB | RAG2 | |
| Trichogenes longipinnis | MK123682 | MK123704 | MF431117 |
| Trichomycterus nigricans | MN813005 | MK123723 | MK123765 |
| Ituglanis boitata | MK123684 | MK123706 | MK123758 |
| Scleronema minutum | MK123685 | MK123707 | MK123759 |
| Stauroglanis gouldingi | MN385800 | KY858033 | ––––– |
| Pygidianops amphioxus | MN385801 | ––––– | MN385823 |
| Homodiateus anistisi | MN385798 | ––––– | MN385821 |
| Microcambeva filamentosa | MN385808 | OK334289 | MN385831 |
| Microcambeva ribeirae | MN385807 | OK334290 | MN385832 |
| Microcambeva barbata | MN385804 | ––––– | MN385829 |
| Microcambeva jucuensis | MN385805 | ––––– | MN385830 |
| Microcambeva mucuriensis | MN385806 | ––––– | MN385828 |
| Listrura tetraradiata | JQ231083 | JQ231088 | MN385826 |
| Listrura nematopteryx | HM245417 | HM245425 | ––––– |
| Listrura macacuensis | MN385802 | OK143231 | MN385825 |
| Listrura macaensis | OK144133 | OK143232 | ––––– |
| Listrura costai | HM245414 | HM245421 | ––––– |
| Listrura picinguabae | HM245416 | HM245424 | ––––– |
| Listrura camposi | MK123703 | MK123732 | MN385824 |
| Listrura depinnai | ––––– | OK143234 | ––––– |
| Listrura sp. 1 | MN385803 | OK143233 | MN385827 |
| Listrura sp. 2 | JN830897 | JN830896 | ––––– |
Appendix C
- Mesethmoid, main axis, area posterior to cornu basis, abrupt lateral widening: (0) absent; (1) present.
- Mesethmoid, cornu, shape: (0) narrow and cylindrical; (1) broad and flat.
- Lateral ethmoid, lateral margin, surface; (0) smooth, without projections; (1) with flattened, subtriangular projection; (2) with pronounced subcylindrical process.
- Vomer, lateral constriction: (0) absent; (1) present.
- Vomer, posterior process: (0) present; (1) absent.
- Premaxilla, postero-medial region, dorsal surface, small projection: (0) absent; (1) present.
- Premaxilla, lateral extremity, distinctive process: (0) absent; (1) present.
- Maxilla, length relative to distance between mesethmoid cornua: (0) equal or slightly longer; (1) about one time and half; (2) about twice or slightly more.
- Autopalatine, general shape: (0) slender, with well-developed postero-lateral process; (1) broad, postero-lateral process absent.
- Autopalatine, posterior region, length relative to autopalatine length: (0) short, about half length or slightly less; (1) long, about two thirds.
- Autopalatine, postero-lateral process, posterior portion, expansion: (0) absent; (1) present.
- Autopalatine, postero-lateral tip, narrow and flat ossification postero-medially directed: (0) absent; (1) present.
- Autopalatine, postero-medial margin, latero-dorsally directed process: (0) absent; (1) present.
- Autopalatine, anterior cartilage head, anterior margin, ossification: (0) absent; (1) present.
- Autopalatine, osseous portion, anterior margin, shape: (0) slightly curved to about straight; (1) strongly sinuous.
- Antorbital, shape: (0) rod-shaped; (1) comma-shaped.
- Supraorbital, development: (0) well-developed; (1) rudimentary.
- Metapterygoid, development: (0) well-developed; (1) rudimentary.
- Hyomandibula, dorsal margin, area just anterior to cartilage articulating hyomandibula and neurocranium, prominent expansion: (0) absent; (1) present.
- Quadrate, dorsal process, posterior margin, laminar extension: (0) absent; (1) present.
- Opercle, odontode patch, width relative to length of cartilage articulating hyomandibula and neurocranium: (0) smaller; (1) larger.
- Opercle, dorsal surface, area just anterior to odontode patch: (0) straight to slightly concave; (1) with prominent concavity.
- Interopercle, area between dorsal process and odontode patch, shape: (0) not constricted; (1) strongly constricted.
- Interopercle, odontode patch, length relative to width of opercular odontode patch: (0) equal or smaller; (1) about twice larger.
- Interopercle, lateral surface, projection: (0) absent; (1) present.
- Neurocranium, roof, dorsal surface; aperture: (0) with open fontanel (1) roof solid, without apertures.
- Neurocranium, antero-frontal, lateral constriction: (0) gradual and weak; (1) abrupt and strong.
- Neurocranium, frontal, anterior extent relative to autopalatine structures: (0) posterior to articular facet for lateral ethmoid; (1) near postero-medial tip overlapping vomer; (2) about mid-length between postero-medial tip and limit between osseus portion and anterior cartilage.
- Neurocranium, anterior supraorbital canal (between pores s1–s3): (0) present; (1) absent.
- Neurocranium, posterior supraorbital canal (between pores s3–po1): (0) present; (1) absent.
- Neurocranium, supraorbital pore s6: (0) paired; (1) single, median.
- Ceratobranchial 4, sub-proximal region, process: (0) present; (1) absent.
- Hypobranchial 3, ossification: (0) with well-developed anterior ossification; (1) ossification rudimentary, usually absent.
- Parurohyal, lateral process, shape: (0) short and broad; (1) long and narrow.
- Ribs, number: (0) six or more; (1) four or fewer (taken from Costa & Bockmann [6]).
- Trunk, pigmentation: (0) well pigmented, making body wall opaque; (1) hyaline, with a few scattered chromatophores.
- Trunk, shape: (0) fusiform; (1) eel-like.
- Trunk, precaudal vertebrae, neural spines, anterior process: (0) present; (1) absent.
- Trunk, dorsal surface, pre-dorsal region, adipose fold: (0) absent; (1) present.
- Caudal peduncle, procurrent caudal-fin rays, anterior extent: (0) limited to area close to caudal fin; (1) anteriorly extending to near dorsal and anal fins.
- Caudal skeleton, dorsal and ventral hypural plates, fusion: (0) unfused, separated by broad gap; (1) partially fused, at least with posterior gap separating plates; (2) fused, often resting small antero-middle notch.
- Caudal skeleton, lower hypurals, shape: (0) subtrapezoidal; (1) subrectangular as a result of an anteroventral expansion. Remarks: In taxa with the character state 1, the ventral lobe of the caudal fin is slightly expanded posteriorly and there is a reduced number (4–7) of ventral procurrent caudal-fin rays, apomorphic conditions here considered as possibly interdependent, thus not analysed separately.
- Caudal fin, general morphology: (0) subtruncate; (1) rounded, almost indistinct between caudal peduncle procurrent rays; (2) bilobed.
- Caudal fin, principal caudal-fin rays, number: (0) 17; (1) 13; (2) 11 or 12.
- Pectoral girdle, main components (cleithrum and proximal radials), development: (0) robust and thick; (1) narrow and thin.
- Pectoral fin, rays, development: (0) first and remaining rays well developed; (1) first rays developed, followed by one to three rudimentary rays; (2) single barbel-like rayed.
- Pectoral fin, first ray, length relative to subsequent rays: (0) slightly longer; (1) shorter. Remarks: the taxa having a relatively short and robust first pectoral-fin ray (character state 1), also have a hypertrophied major proximal radial, considered to be dependent conditions of a single event.
- Pectoral fin, first ray, tip, filament, length relative to pectoral fin length: (0) short, 20% or less; (1) long, about 70–80%.
- Pectoral fin, minor proximal radial, ossification: (0) cartilaginous; (1) ossified.
- Pelvic fin, medial interspace: (0) in close proximity; (1) broader than pelvic-fin base; (?) taxa without pelvic fin.
- Pelvic fin: (0) present; (1) absent.
- Dorsal fin: (0) present; (1) absent.
- Anal fin, origin, position relative to principal dorsal-fin rays: (0) posteriorly placed, in vertical through or posterior to them; (1) anteriorly placed, in vertical anterior to them.
- Nasal barbel, development: (0) well-developed, reaching between opercular odontode patch and pectoral fin base; (1) rudimentary, barely reaching posterior nostril.
- Eye, position in head: (0) anterior; (1) posterior.
- Eye, size expressed as percentage of head length: (0) medium sized (about 12–16%); (1) hypertrophied (about 18–22%); (2) minute (about 7–10%).
- Head, ventral surface, branchiostegal region, paired barbel-like short projection: (0) absent; (1) present.
Appendix D
| Trichogenes longipinnis | 0000000000000000000000010000000010000000001000000000?0000 |
| Ituglanis boitata | 000000000000000000001001010000000000000000010000000000000 |
| Trichomycterus nigricans | 000000000000000000000001000000000000000000010000000000000 |
| Scleronema minutum | 000000010000000000001000001000?00000000000010010010001000 |
| Homodiaetus anisitsi | 0?00000000000000?1000000000010?01111000000010000000001000 |
| Stauroglanis gouldingi | 000001010000010001000000001010000111000000010000000000100 |
| Pygidianops amphioxus | ??00000100000000?100000?01001??001111010????0???0?11?0??0 |
| Microcambeva filamentosa | 000101010000001001101100100010110111001000110001000002000 |
| Microcambeva ribeirae | 000101020100000001001100101010110111000001110010110001111 |
| Microcambeva bendego | 000101020100000001001100101010110111000001110010110001111 |
| Microcambeva barbata | 000101120000011001001000100010010111000001110000110000111 |
| Microcambeva watu | 000101120010011001001000?000100101110?0001110000110000111 |
| Microcambeva jucuensis | 000101120010011001001000100010010111000001110000110000111 |
| Microcambeva mucuriensis | 000101120010011001001000100010010111000001110000110000111 |
| Microcambeva draco | 000101120010011001001000100010010111000001110000110000111 |
| Listrura tetraradiata | 100111001001101111000011110111?011101101202110000?1000020 |
| Listrura nematoptetyx | 102111001001001111010010110211?011101101202212000?1010020 |
| Listrura macacuensis | 102111001001001111010010110211?0111011012022120?0?1010020 |
| Listrura sp. 2 | ???????????????????????????2?????????????????2????11????? |
| Listrura macaensis | 102111001001001111010010110211?0111011012022120?0?1010020 |
| Listrura costai | 102111001001001111010010110211?0111011012022120?0?1010020 |
| Listrura picinguabae | 112111001001001111010010110211?0111011012022120?0?1010020 |
| Listrura camposi | 101111001001001111000010110111?0111011011022110?0?1000020 |
| Listrura sp. 1 | 101111001001001111000010110111?0111011011022110?0?1000020 |
| Listrura depinnai | 101111001001001111000010110111?0111011011022110?0?11?0020 |
Appendix E
| Partition | Base Pairs | Evolutive Model |
| COI 1st | 219 | TNe+G4 |
| COI 2nd | 218 | F81+F+I |
| COI 3rd | 219 | TIM+F+I+G4 |
| CYTB 1st | 361 | TNe+G4 |
| CYTB 2nd | 362 | HKY+F |
| CYTB 3rd | 361 | TN+F+G4 |
| RAG2 1st | 274 | TIM2e+I |
| RAG2 2nd | 275 | K2P+I |
| RAG2 3rd | 275 | HKY+F+I |
| Morphology | 57 | JC+I+G4 |
References
- McConnell, R.; Lowe-McConnell, R.H. Ecological Studies in Tropical Fish Communities; Cambridge University Press: Cambridge, UK, 1987. [Google Scholar]
- Myers, N.; Mittermeir, R.A.; Mittermeir, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Costa, W.J.E.M.; Henschel, E.; Katz, A.M. Multigene phylogeny reveals convergent evolution in small interstitial catfishes from the Amazon and Atlantic forests (Siluriformes: Trichomycteridae). Zool. Scr. 2020, 49, 159–173. [Google Scholar] [CrossRef]
- De Pinna, M.C.C. A new genus of trichomycterid catfish (Siluroidei, Glanapteryginae), with comments on its phylogenetic relationships. Rev. Suisse de Zool. 1988, 95, 113–128. [Google Scholar] [CrossRef]
- Costa, W.J.E.M.; Bockmann, F.A. A new genus and species of Sarcoglanidinae (Siluriformes: Trichomycteridae) from southeastern Brazil, with a re-examination of subfamilial phylogeny. J. Nat. Hist. 1994, 28, 715–730. [Google Scholar] [CrossRef]
- Costa, W.J.E.M.; Bockmann, F.A. Un nouveau genre néotropical de la famille des Trichomycteridae (Siluriformes: Loricarioidei). Rev. Fr. d’Aquariologie Herpétologie 1993, 20, 43–46. [Google Scholar]
- Villa-Verde, L.; Costa, W.J.E.M. A new glanapterygine catfish of the genus Listrura (Siluriformes: Trichomycteridae) from the southeastern Brazilian coastal plains. Zootaxa 2006, 1142, 43–50. [Google Scholar] [CrossRef]
- Landim, M.I.; Costa, W.J.E.M. Listrura tetraradiata (Siluriformes: Trichomycteridae): A new glanapterygine catfish from the southeastern Brazilian coastal plains. Copeia 2002, 2002, 152–156. [Google Scholar] [CrossRef]
- De Pinna, M.C.C.; Wosiacki, W. A new interstitial catfish of the genus Listrura from southern Brazil (Siluriformes, Trichomycteridae, Glanapteryginae). Proc. Biol. Soc. Wash. 2002, 115, 720–726. [Google Scholar]
- Villa-Verde, L.; Lazzarotto, H.; Lima, S.Q.M. A new glanapterygine catfish of the genus Listrura (Siluriformes: Tricho-mycteridae) from southeastern Brazil, corroborated by morphological and molecular data. Neotrop. Ichthyol. 2012, 10, 527–538. [Google Scholar] [CrossRef]
- Villa-Verde, L.; Ferrer, J.; Malabarba, L.R. A New Species of Listrura from Laguna dos Patos System, Brazil: The Southernmost Record of the Glanapteryginae (Siluriformes: Trichomycteridae). Copeia 2013, 2013, 641–646. [Google Scholar] [CrossRef]
- Costa, W.J.E.M.; Lima, S.M.Q.; Bizerril, R.S. Microcambeva ribeirae sp. n. (Teleostei: Siluriformes: Trichomycteridae): A New Sarcoglanidine Catfish from the Rio Ribeira do Iguape Basin, Southeastern Brazil. Zootaxa 2004, 563, 1–10. [Google Scholar] [CrossRef][Green Version]
- Mattos, J.L.; Lima, S.M.Q. Microcambeva draco, a new species from north-eastern Brazil (Siluriformes: Trichomycteridae). Ichthyol. Explor. Freshw. 2010, 21, 233–238. [Google Scholar]
- Costa, W.J.E.M.; Katz, A.M.; Mattos, J.L.O.; Rangel-Pereira, F.S. Two new species of miniature psammophilic sarcoglanidine catfishes of the genus Microcambeva from the Atlantic Forest of eastern Brazil (Siluriformes: Trichomycteridae). J. Nat. Hist. 2019, 53, 1837–1851. [Google Scholar] [CrossRef]
- De Medeiros, L.S.; Moreira, C.R.; de Pinna, M.C.C.; Lima, S.M.Q. A new catfish species of Microcambeva Costa & Bockmann 1994 (Siluriformes: Trichomycteridae) from a coastal basin in Rio de Janeiro State, southeastern Brazil. Zootaxa 2020, 4895, 111–123. [Google Scholar]
- De Medeiros, L.S.; Sarmento-Soares, L.M.; Lima, S.M.Q. A new psammophilous catfish of the genus Microcambeva (Teleostei: Trichomycteridae) from the Rio Doce basin, southeastern Brazil. Ichthyol. Explor. Freshw. 2021, 1–13. [Google Scholar] [CrossRef]
- Costa, W.J.; Vilardo, P.; Katz, A.M. Sympatric sister species with divergent morphological features of psammophilic catfishes of the southeastern Brazilian genus Microcambeva (Siluriformes: Trichomycteridae). Zool. Anz. 2020, 285, 12–17. [Google Scholar] [CrossRef]
- Costa, W. Comparative Osteology, Phylogeny and Classification of the Eastern South American Catfish Genus Trichomycterus (Siluriformes: Trichomycteridae). Taxonomy 2021, 1, 160–191. [Google Scholar] [CrossRef]
- Costa, W.J.E.M. Description de huit nouvelles espèces du genre Trichomycterus (Siluriformes: Trichomycteridae), du Brésil oriental. Rev. Fr. d’Aquariologie Herpetol. 1992, 18, 101–110. [Google Scholar]
- Costa, W.J.E.M.; Katz, A.M.; Mattos, J.L.O.; Amorim, P.F.; Mesquita, B.O.; Vilardo, P.J.; Barbosa, M.A. Historical review and redescription of three poorly known species of the catfish genus Trichomycterus from south-eastern Brazil (Siluriformes: Trichomycteridae). J. Nat. Hist. 2020, 53, 2905–2928. [Google Scholar] [CrossRef]
- Taylor, W.R.; Van Dyke, G.C. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium 1985, 9, 107–119. [Google Scholar]
- Costa, W.J.E.M.; Katz, A.M. Integrative taxonomy supports high species diversity of south-eastern Brazilian mountain catfishes of the T. reinhardti group (Siluriformes: Trichomycteridae). Syst. Biodivers. 2021, 19, 601–621. [Google Scholar] [CrossRef]
- Arratia, G.; Huaquin, L. Morphology of the lateral line system and of the skin of diplomystic and certain primitive loricarioid catfishes and systematic and ecological considerations. Bonn Zool. Monogr. 1995, 36, 1–110. [Google Scholar]
- Bockmann, F.A.; Casatti, L.; de Pinna, M.C.C. A new species of trichomycterid catfish from the Rio Paranapanema, southeastern Brazil (Teleostei; Siluriformes), with comments on the phylogeny of the family. Ichthyol. Explor. Freshw. 2004, 15, 225–242. [Google Scholar]
- Hardman, M.; Page, L.M. Phylogenetic relationships among bullhead catfishes of the genus Ameiurus (Siluriformes: Ictaluridae). Copeia 2003, 2003, 20–33. [Google Scholar] [CrossRef]
- Ward, R.D.; Zemlak, T.S.; Innes, B.H.; Last, P.R.; Hebert, P. DNA barcoding Australia’s fish species. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Chenna, R. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003, 31, 3497–3500. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Sereno, P.C. Logical basis for morphological characters in phylogenetics. Cladistics 2007, 23, 565–587. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Chernomor, O.; Von Haeseler, A.; Minh, B.Q. Terrace Aware Data Structure for Phylogenomic Inference from Supermatrices. Syst. Biol. 2016, 65, 997–1008. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Anisimova, M.; Gil, M.; Dufayard, J.F.; Dessimoz, C.; Gascuel, O. Survey of branch support methods demonstrates accuracy, power, and robustness of fast likelihood-based approximation schemes. Syst. Biol. 2011, 60, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Nguyen, M.A.T.; Von Haeseler, A. Ultrafast Approximation for Phylogenetic Bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; Von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2017, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Goloboff, P.A.; Farris, J.S.; Nixon, K.C. TNT, a free program for phylogenetic analysis. Cladistics 2008, 24, 774–786. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Anoop, V.; Britz, R.; Arjun, C.; Dahanukar, N.; Raghavan, R. Pangio bhujia, a new, peculiar species of miniature subterranean eel loach lacking dorsal and pelvic fins from India (Teleostei: Cobitidae). Zootaxa 2019, 4683, 144–150. [Google Scholar] [CrossRef]
- Britz, R.; Doherty-Bone, T.M.; Kouete, M.T.; Sykes, D.; Gower, D.J. Monopterus luticolus, a new species of swamp eel from Cameroon (Teleostei: Synbranchidae). Ichthyol. Explor. Freshw. 2016, 27, 309–323. [Google Scholar]
- De Pinna, M.; Zuanon, J.; Py-Daniel, L.R.; Petry, P. A new family of neotropical freshwater fishes from deep fossorial Amazonian habitat, with a reappraisal of morphological characiform phylogeny (Teleostei: Ostariophysi). Zool. J. Linn. Soc. 2018, 182, 76–106. [Google Scholar] [CrossRef]
- Yamada, T.; Sugiyama, T.; Tamaki, N.; Kawakita, A.; Kato, M. Adaptive radiation of gobies in the interstitial habitats of gravel beaches accompanied by body elongation and excessive vertebral segmentation. BMC Evol. Biol. 2009, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Adriaens, D.; Baskin, J.N.; Coppens, H. Evolutionary morphology of trichomycterid catfishes: About hanging on and digging in. In Origin and Phylogenetic Interrelationships of Teleosts; Nelson, J.S., Schultze, H.P., Wilson, M.V.H., Eds.; Verlag Dr. Friedrich Pfeil: München, Germany, 2010; pp. 337–362. [Google Scholar]
- Wagner, M.; Bračun, S.; Skofitsch, G.; Kovačić, M.; Zogaris, S.; Iglésias, S.; Sefc, K.M.; Koblmüller, S. Diversification in gravel beaches: A radiation of interstitial clingfish (Gouania, Gobiesocidae) in the Mediterranean Sea. Mol. Phylogenetics Evol. 2019, 139, 106525. [Google Scholar] [CrossRef]
- Villa-Verde, L.; Britto, M.R.; Abilhoa, V. Novos exemplares de Listrura boticario de Pinna & Wosiacki (Siluriformes: Trichomycteridae). Bol. Soc. Bras. de Ictiol. 2008, 91, 5–6. [Google Scholar]
- Villa-Verde, L.; Lima, S.M.Q.; Carvalho, P.H.; de Pinna, M.C.C. Rediscovery, taxonomic status and conservation of the threatened catfish Listrura camposi (Miranda-Ribeiro) (Siluriformes: Trichomycteridae). Neotrop. Ichthyol. 2013, 11, 55–64. [Google Scholar] [CrossRef][Green Version]
- Nico, L.G.; de Pinna, M.C.C. Confirmation of Glanapteryx anguilla (Siluriformes, Trichomycteridae) in the Orinoco River basin, with notes on the distribution and habitats of the Glanapteryginae. Ichthyol. Explor. Freshw. 1996, 7, 27–32. [Google Scholar]
- De Almeida-Val, V.M.F.; Fé, L.M.L.; de Campos, D.F. Evolutionary aspects on the comparative biology of lungfishes: Emphasis on South-American Lungfish, Lepidosiren paradoxa. In Phylogeny, Anatomy and Physiology of Ancient Fishes; Zaccone, G., Dabrowski, K., Hedrick, M.S., Fernandes, J.M.O., Icardo, J.M., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 38–56. [Google Scholar]
- Zuanon, J.; Bockmann, F.A.; Sazima, I. A remarkable sand-dwelling fish assemblage from central Amazonia, with comments on the evolution of psammophily in South American freshwater fishes. Neotrop. Ichthyol. 2006, 4, 107–118. [Google Scholar] [CrossRef]
- Zuanon, J.; Sazima, I. Natural history of Stauroglanis gouldingi (Siluriformes: Trichomycteridae), a miniature sand-dwelling candiru from central Amazonia streamlets. Ichthyol. Explor. Freshw. 2004, 15, 201–208. [Google Scholar]
- Baskin, J.N. Structure and Relationships of the Trichomycteridae; City University of New York: New York, NY, USA, 1973. Supplementary material of de Pinna. Neotrop. Ichthyol. 2016, 14, S1–S62. [Google Scholar]
- De Pinna, M.C.C. A new sarcoglandine catfish, phylogeny of its subfamily, and an appraisal of the phyletic status of the Trichomycterinae (Teleostei, Trichomycteridae). Am. Mus. Novit. 1989, 2950, 1–39. [Google Scholar]
- Myers, G.S.; Weitzman, S.H. Two remarkable new trichomycterid catfishes from the Amazon basin in Brazil and Colombia. J. Zool. 1966, 149, 277–287. [Google Scholar] [CrossRef]
- Costa, W.J.E.M. A new genus and species of Sarcoglanidinae (Siluriformes: Trichomycteridae) from the Araguaia basin, central Brazil, with notes on subfamilial phylogeny. Ichthyol. Explor. Freshw. 1994, 5, 207–216. [Google Scholar]
- Henschel, E.; Katz, A.M.; Costa, W.J.E.M. A new candiru of the genus Paracanthopoma (Siluriformes: Trichomycteridae) from the Araguaia River basin, Central Brazil. J. Fish Biol. 2021, in press. [Google Scholar] [CrossRef]
- Schaefer, S.A.; Provenzano, F.; de Pinna, M.; Baskin, J.N. New and noteworthy Venezuelan glanapterygine catfishes (Siluriformes, Trichomycteridae), with discussion of their biogeography and psammophily. Am. Mus. Novit. 2005, 3496, 1–27. [Google Scholar] [CrossRef]
- Near, T.J.; Dornburg, A.; Eytan, R.I.; Keck, B.P.; Smith, W.L.; Kuhn, K.L.; Moore, J.A.; Price, S.A.; Burbrink, F.T.; Friedman, M.; et al. Phylogeny and tempo of diversification in the superradiation of spiny-rayed fishes. Proc. Natl. Acad. Sci. USA 2013, 110, 12738–12743. [Google Scholar] [CrossRef]
- Betancur-R, R.; Ortí, G.; Pyron, R.A. Fossil-based comparative analyses reveal ancient marine ancestry erased by extinction in ray-finned fishes. Ecol. Lett. 2015, 18, 441–450. [Google Scholar] [CrossRef] [PubMed]
- De Pinna, M.C.C. Phylogenetic relationships of neotropical Siluriformes (Teleostei: Ostariophysi): Historical overview and synthesis of hypotheses. In Phylogeny and Classification of Neotropical Fishes; Malabarba, L.R., Reis, R.E., Vari, R.P., Lucena, Z.M.S., Lucena, C.A.S., Eds.; Edipucrs: Porto Alegre, Brazil, 1998; pp. 279–330. [Google Scholar]
- Sullivan, J.P.; Lundberg, J.G.; Hardman, M. A phylogenetic analysis of the major groups of catfishes (Teleostei: Siluriformes) using rag1 and rag2 nuclear gene sequences. Mol. Phylogenetics Evol. 2006, 41, 636–662. [Google Scholar] [CrossRef] [PubMed]



| Holotype | Paratypes (n = 5) | |
|---|---|---|
| Standard length (SL) | 42.7 | 25.5–31.7 |
| Percentage of standard length | ||
| Body depth | 8.0 | 7.0–8.9 |
| Caudal peduncle depth | 7.5 | 6.8–9.2 |
| Body width | 4.0 | 3.2–4.5 |
| Caudal peduncle width | 2.1 | 1.4–2.2 |
| Dorsal-fin base length | 4.4 | 3.5–4.9 |
| Anal-fin base length | 5.0 | 5.1–6.7 |
| Pectoral-fin length | 7.5 | 8.5–10.6 |
| Pre-dorsal length | 73.6 | 70.1–76.7 |
| Pre-anal length | 71.0 | 66.0–71.9 |
| Head length | 9.7 | 9.9–11.4 |
| Percentage of head length | ||
| Head depth | 50.6 | 46.4–49.1 |
| Head width | 79.0 | 77.2–83.0 |
| Preorbital length | 33.7 | 36.4–43.9 |
| Eye diameter | 8.9 | 8.3–14.5 |
| Interorbital width | 28.9 | 25.6–35.1 |
| Holotype | Paratypes (n = 12) | |
|---|---|---|
| Standard length (SL) | 29.8 | 32.0–40.2 |
| Percentage of standard length | ||
| Body depth | 8.7 | 7.7–9.8 |
| Caudal peduncle depth | 9.3 | 8.0–10.0 |
| Body width | 5.4 | 3.7–5.3 |
| Caudal peduncle width | 2.1 | 1.7–2.9 |
| Dorsal-fin base length | 3.5 | 3.1–4.1 |
| Anal-fin base length | 4.7 | 4.1–5.4 |
| Pectoral-fin length | 8.7 | 6.9–8.7 |
| Pre-dorsal length | 69.1 | 67.7–80.1 |
| Pre-anal length | 67.1 | 64.8–77.4 |
| Head length | 9.7 | 7.7–9.7 |
| Percentage of head length | ||
| Head depth | 51.2 | 50.3–63.3 |
| Head width | 93.0 | 88.5–105.0 |
| Pre-orbital length | 36.5 | 25.3–41.9 |
| Eye diameter | 5.1 | 4.6–6.3 |
| Interorbital width | 22.2 | 22.2–30.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, W.J.E.M.; Katz, A.M. Comparative Morphology, Phylogeny, Classification and Evolution of Interstitial Habits in Microcambevine Catfishes (Siluriformes: Trichomycteridae). Taxonomy 2021, 1, 313-344. https://doi.org/10.3390/taxonomy1040025
Costa WJEM, Katz AM. Comparative Morphology, Phylogeny, Classification and Evolution of Interstitial Habits in Microcambevine Catfishes (Siluriformes: Trichomycteridae). Taxonomy. 2021; 1(4):313-344. https://doi.org/10.3390/taxonomy1040025
Chicago/Turabian StyleCosta, Wilson J. E. M., and Axel M. Katz. 2021. "Comparative Morphology, Phylogeny, Classification and Evolution of Interstitial Habits in Microcambevine Catfishes (Siluriformes: Trichomycteridae)" Taxonomy 1, no. 4: 313-344. https://doi.org/10.3390/taxonomy1040025
APA StyleCosta, W. J. E. M., & Katz, A. M. (2021). Comparative Morphology, Phylogeny, Classification and Evolution of Interstitial Habits in Microcambevine Catfishes (Siluriformes: Trichomycteridae). Taxonomy, 1(4), 313-344. https://doi.org/10.3390/taxonomy1040025






