CaSrxCu3−xTi4O12 Ceramic Oxide Modified with Graphene Oxide and Reduced Graphene Oxide for Supercapacitor Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of CaCu3−xSrxTi4O12 (0 ≤ x ≤ 3.0)
2.2. Synthesis of Graphene Oxide
2.3. Synthesis of Reduced Graphene Oxide
2.4. Preparation of the Films and Electrodes
2.5. Characterization
3. Results and Discussion
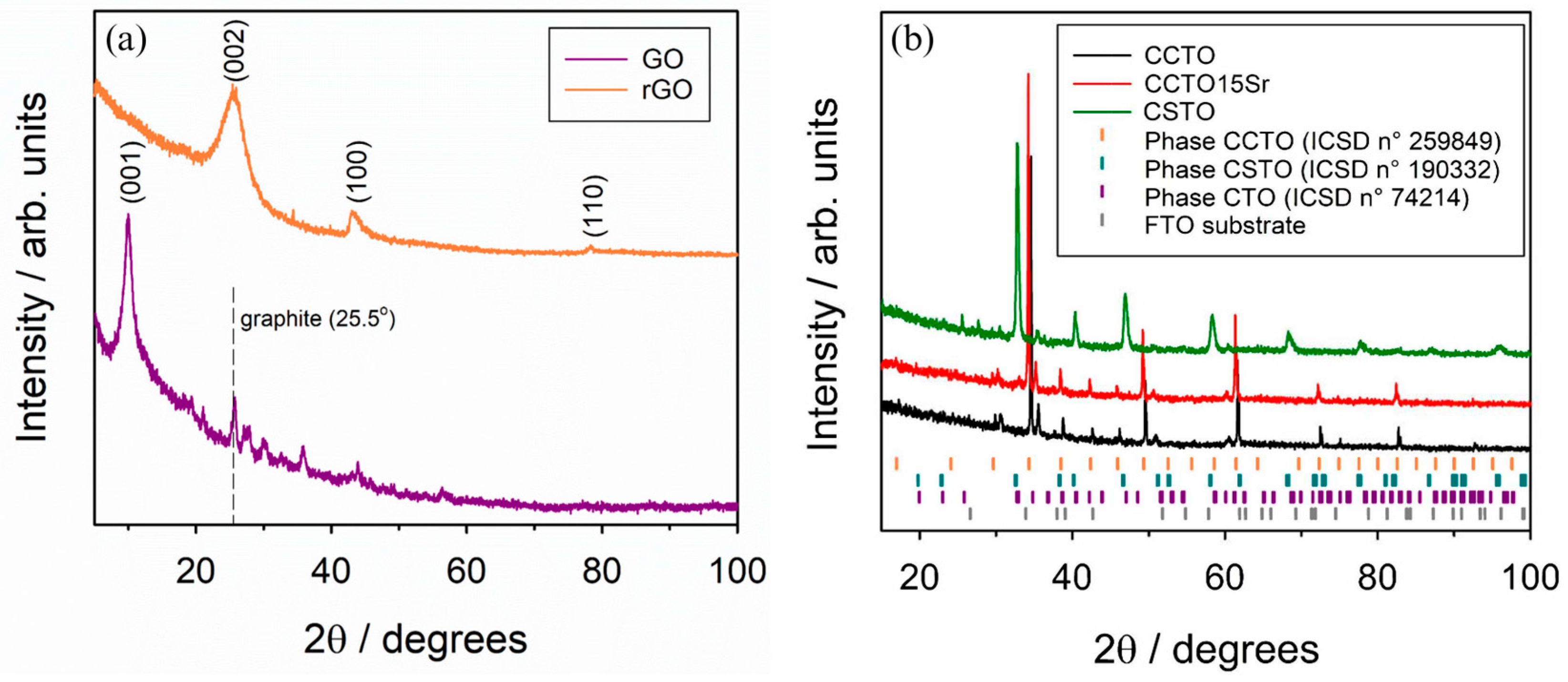
3.1. Structural and Morphological Analysis
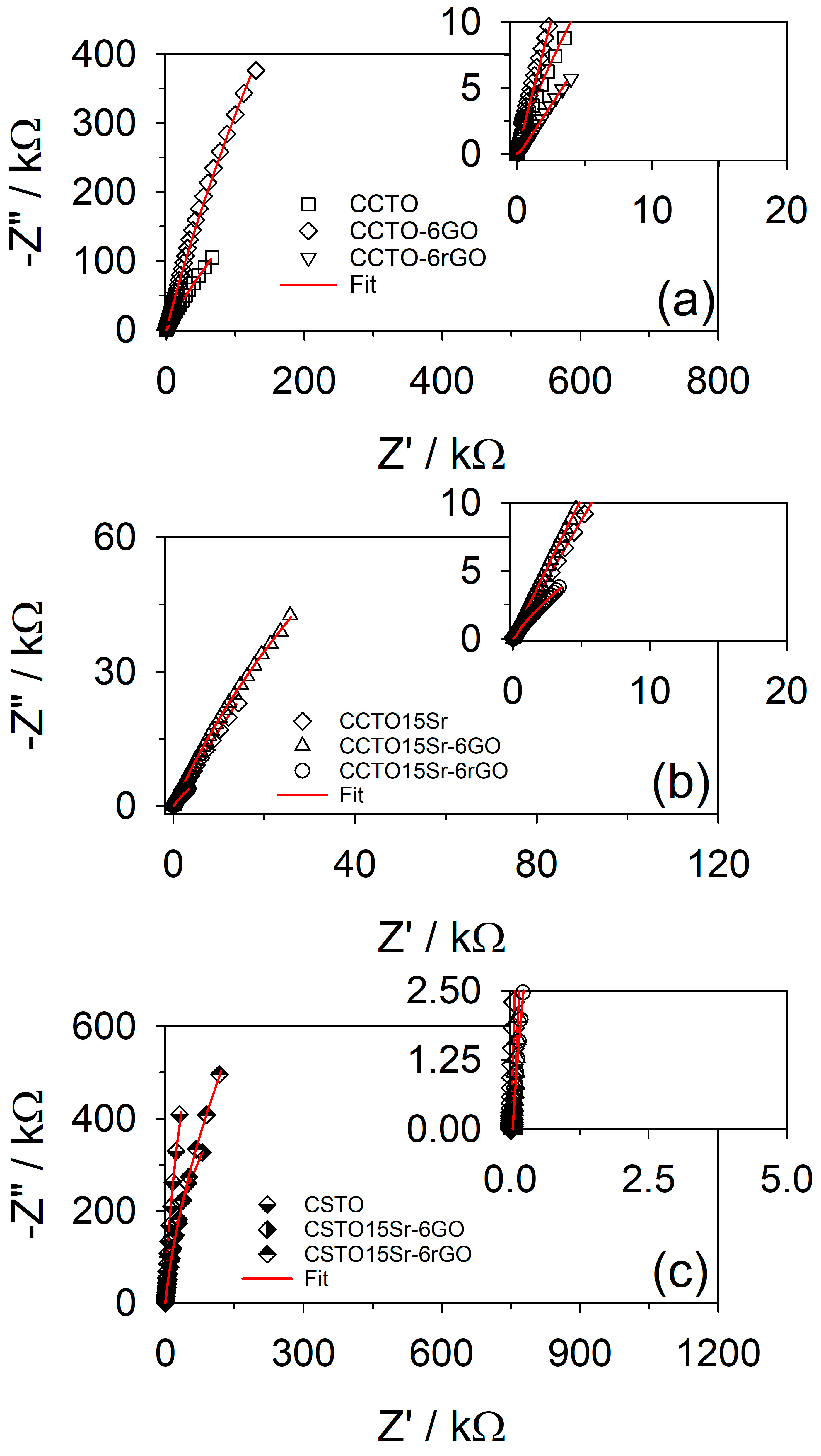
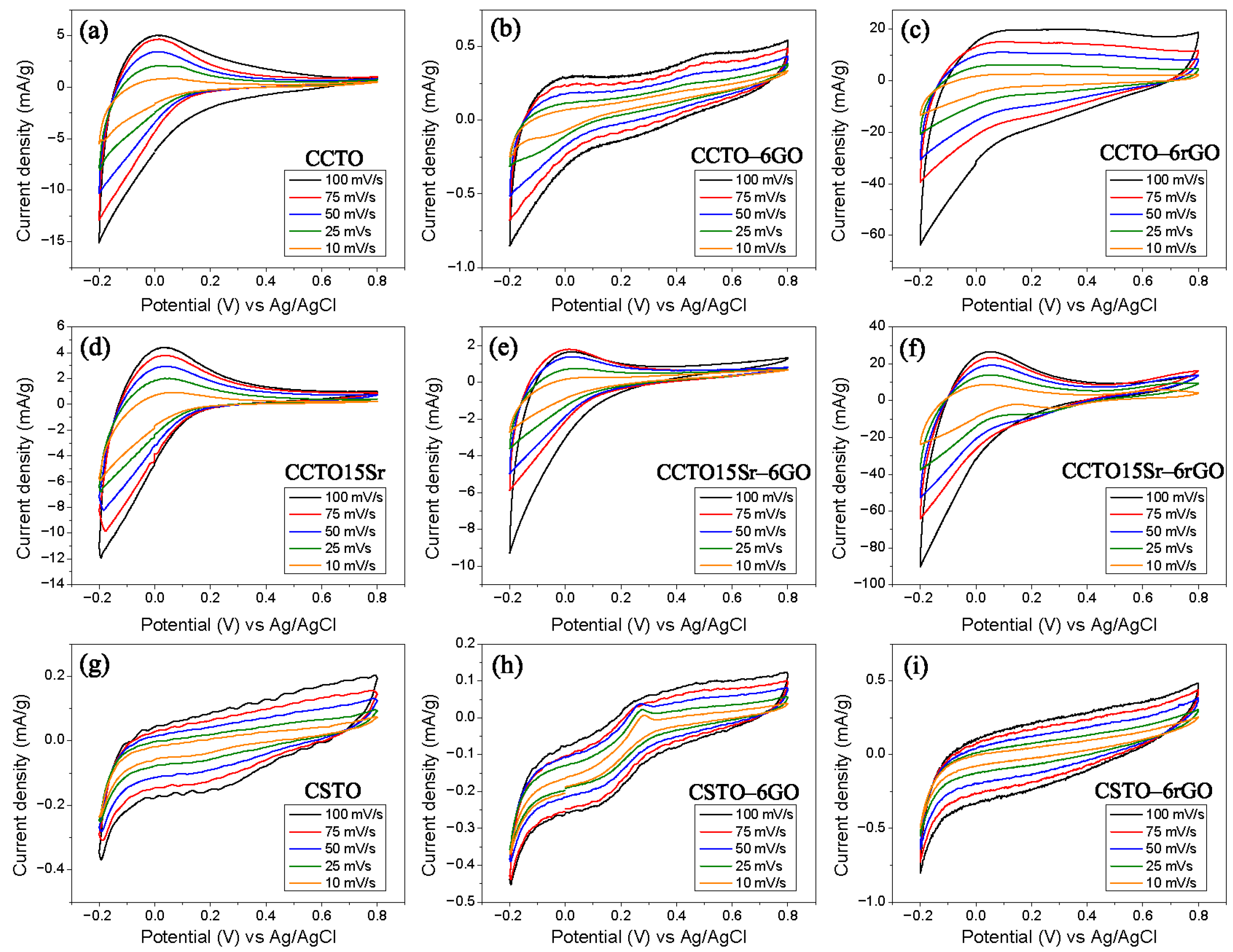
3.2. Electrochemical Response
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCSTO | CaCu3−xSrₓTi4O12 |
| CCTO | CaCu3Ti4O12 |
| CCTO15Sr | CaCu0.85Sr0.15Ti4O12 |
| CCTO15Sr-6GO | 6 wt% GO to CCTO15Sr |
| CCTO15Sr-6rGO | 6 wt% rGO to CCTO15Sr |
| CCTO-6GO | 6 wt% GO to CCTO |
| CCTO-6rGO | 6 wt% rGO to CCTO |
| CV | Cyclic Voltammetry |
| EDLCs | Electric Double-Layer Capacitors |
| GO | graphene oxide |
| ICSD | Inorganic Crystal Structure Database |
| ITO | Indium Tin Oxide |
| rGO | reduced graphene oxide |
| SCs | supercapacitors |
| CSTO | CaSr3T4O12 |
| CSTO-6GO | 6 wt% GO to CSTO |
| CSTO-6rGO | 6 wt% rGO to CSTO |
| SEM | Scanning Electron Microscopy |
| XRD | X-ray Diffraction |
References
- Wang, H.; Wang, H.; Ruan, F.; Feng, Q.; Wei, Y.; Fang, J. High-Porosity Carbon Nanofibers Prepared from Polyacrylonitrile Blended with Amylose Starch for Application in Supercapacitors. Mater. Chem. Phys. 2023, 293, 126896. [Google Scholar]
- Vita, V.; Zafiropoulos, E.P.; Gonos, I.F.; Mladenov, V.M.; Chobanov, V. Power System Studies in the Clean Energy Era: From Capacity to Flexibility Adequacy Through Research and Innovation. In Proceedings of the 21st International Symposium on High Voltage Engineering, Budapest, Hungary, 26–30 August 2019. [Google Scholar]
- Kumar, R.; Lee, D.; Ağbulut, Ü. Different Energy Storage Techniques: Recent Advancements, Applications, Limitations, and Efficient Utilization of Sustainable Energy. J. Therm. Anal. Calorim. 2024, 149, 1895–1933. [Google Scholar] [CrossRef]
- Khalid, M. Smart Grids and Renewable Energy Systems: Perspectives and Grid Integration Challenges. Energy Strategy Rev. 2024, 51, 101299. [Google Scholar] [CrossRef]
- Jeloo, Z.A.G.; Ghasemzadeh, S.; Hosseini-Monfared, H.; Javanbakht, M.; Naji, L.; Najaflo, M.; Hamidi, S. From Barley Straw Biomass to N/S Co-Doped as Electrode Material for High-Performance Supercapacitor Applications. Mater. Chem. Phys. 2024, 323, 129653. [Google Scholar]
- Gogotsi, Y.; Penner, R.M. Energy Storage in Nanomaterials—Capacitive, Pseudocapacitive, or Battery-Like? ACS Nano 2018, 12, 2081–2083. [Google Scholar] [CrossRef]
- Liu, S.; Wei, L.; Wang, H. Review on Reliability of Supercapacitors in Energy Storage Applications. Appl. Energy 2020, 278, 115436. [Google Scholar] [CrossRef]
- Benavides, D.; Arévalo, P.; Aguado, J.A.; Jurado, F. Experimental Validation of a Novel Power Smoothing Method for On-Grid Photovoltaic Systems Using Supercapacitors. Int. J. Electr. Power Energy Syst. 2023, 149, 109050. [Google Scholar] [CrossRef]
- Mindil, A.; Hassan, H.; Iqbal, M.W.; Afzal, A.M.; Amri, N.; Hadia, N.M.A. Enhancing Energy Storage Capability of Advanced Redox-Based Supercapacitors through PANI Incorporation into NiMnS Matrix. Mater. Chem. Phys. 2023, 306, 128077. [Google Scholar]
- Khan, H.A.; Tawalbeh, M.; Aljawrneh, B.; Abuwatfa, W.; Al-Othman, A.; Sadeghifar, H.; Olabi, A.G. A Comprehensive Review on Supercapacitors: Their Promise to Flexibility, High Temperature, Materials, Design, and Challenges. Energy 2024, 295, 131043. [Google Scholar] [CrossRef]
- Lin, Z.; Goikolea, E.; Balducci, A.; Naoi, K.; Taberna, P.L.; Salanne, M.; Yushin, G.; Simon, P. Materials for Supercapacitors: When Li-Ion Battery Power Is Not Enough. Mater. Today 2018, 21, 419–436. [Google Scholar] [CrossRef]
- Liu, C.F.; Liu, Y.C.; Yi, T.Y.; Hu, C.C. Carbon Materials for High-Voltage Supercapacitors. Carbon 2019, 145, 529–548. [Google Scholar] [CrossRef]
- Gaikwad, P.; Tiwari, N.; Kamat, R.; Mane, S.M.; Kulkarni, S.B. A Comprehensive Review on the Progress of Transition Metal Oxides Materials as a Supercapacitor Electrode. Mater. Sci. Eng. B 2024, 307, 117544. [Google Scholar] [CrossRef]
- Azzou, K.A.K.; Terbouche, A.; Ramdane-Terbouche, C.A.; Bataille, T.; Hauchard, D.; Mezaoui, D. Supercapacitor Electrode Based on Ternary Activated Carbon/CuCoO2 Hybrid Material. Mater. Chem. Phys. 2024, 322, 129521. [Google Scholar] [CrossRef]
- Ahangari, M.; Mostafaei, J.; Sayyah, A.; Mahmoudi, E.; Asghari, E.; Coruh, A.; Delibas, N.; Niaei, A. Investigation of Structural and Electrochemical Properties of SrFexCo1-XO3-δ Perovskite Oxides as a Supercapacitor Electrode Material. J. Energy Storage 2023, 63, 107034. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, J.; Li, P.; Deng, T.; Nan, Y.; Lei, Z.; Li, Y.; Li, L. Microstructure and Dielectric Properties of Na and Ni Co-Substituted CaCu3Ti4O12 Ceramics with High Dielectric Constant and Low Loss. Mater. Chem. Phys. 2024, 315, 128973. [Google Scholar] [CrossRef]
- Xue, R.; Zhao, L.; Liu, X.; Wang, H.; Zhu, X.; Ren, Y.; Xiao, Y.; Yuan, C.; Cao, B. Modification of the Dual-Function Varistor-Capacitor Properties of Bi2/3Cu3Ti4O12 Ceramic by Doping with Sr0.99La0.01TiO3. Mater. Today Commun. 2024, 39, 108823. [Google Scholar] [CrossRef]
- Moura, F.; Simões, A.Z.; Deus, R.C.; Silva, M.R.; Varela, J.A.; Longo, E. Intense Photoluminescence Emission at Room Temperature in Calcium Copper Titanate Powders. Ceram. Int. 2013, 39, 3499–3506. [Google Scholar] [CrossRef]
- Kim, I.D.; Rothschild, A.; Hyodo, T.; Tuller, H.L. Microsphere Templating as Means of Enhancing Surface Activity and Gas Sensitivity of CaCu3Ti4O12 Thin Films. Nano Lett. 2006, 6, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Tsyganov, A.; Morozova, N.; Vikulova, M.; Asmolova, A.; Artyukhov, D.; Zotov, I.; Gorokhovsky, A.; Gorshkov, N. The Effect of Lithium Doping on the Dielectric Properties of Solid Solutions LixCa(1−x)Cu3Ti4O12 (x = 0.01–0.1). J. Compos. Sci. 2023, 7, 282. [Google Scholar] [CrossRef]
- Ji, J.H.; Lee, J.W.; Chung, H.; Koh, J.H. (Na,K)NbO3–CaCu3Ti4O12 Perovskite Composites for Supercapacitor Based Piezoelectric Devices. Ceram. Int. 2016, 42, 4978–4983. [Google Scholar] [CrossRef]
- Cortés, J.A.; Moreno, H.; Orrego, S.; Bezzon, V.D.N.; Ramírez, M.A. Dielectric and Non-Ohmic Analysis of Sr2+ Influences on CaCu3Ti4O12-Based Ceramic Composites. Mater. Res. Bull. 2021, 134, 111071. [Google Scholar] [CrossRef]
- Zhang, J.; Li, P.; Deng, T.; Nan, Y.; Lv, Y.; Lei, Z.; Li, Y.; Li, L. Mechanism of the Effect of Al and Sr Substitution on the Electrical Resistance in CaCu3Ti4O12 Ceramics. Phys. B Condens. Matter 2024, 674, 415577. [Google Scholar] [CrossRef]
- Rhouma, S.; Megriche, A.; El Amrani, M.; Said, S.; Roger, S.; Cile Autret-Lambert, C. Effect of Sr/Mg Co-Doping on the Structural, Dielectric, and Electrical Properties of CaCu3Ti4O12 Ceramics. J. Mater. Sci. Mater. Electron. 2022, 33, 4535–4549. [Google Scholar] [CrossRef]
- Kumar, S.; Saeed, G.; Zhu, L.; Hui, K.N.; Kim, N.H.; Lee, J.H. 0D to 3D Carbon-Based Networks Combined with Pseudocapacitive Electrode Material for High Energy Density Supercapacitor: A Review. Chem. Eng. J. 2021, 403, 126352. [Google Scholar] [CrossRef]
- Shah, S.S.; Aziz, M.A. Properties of Electrode Materials and Electrolytes in Supercapacitor Technology. J. Chem. Environ. 2024, 3, 10–56946. [Google Scholar] [CrossRef]
- Qi, W.; Li, L.; Han, R.; Hou, Y.; Zhou, Z.; Chen, G.X.; Li, Q. Enhancing Dielectric Properties of (CaCu3Ti4O12 NWs–Graphene)/PVDF Ternary Oriented Composites by Hot Stretching. ACS Omega 2024, 9, 13298–13305. [Google Scholar] [CrossRef]
- Qu, Y.; Du, Y.; Fan, G.; Xin, J.; Liu, Y.; Xie, P.; You, S.; Zhang, Z.; Sun, K.; Fan, R. Low-Temperature Sintering Graphene/CaCu3Ti4O12 Nanocomposites with Tunable Negative Permittivity. J. Alloys Compd. 2019, 771, 699–710. [Google Scholar] [CrossRef]
- Ahmadipour, M.; Ardani, M.R.; Sarafbidabad, M.; Missaoui, N.; Satgunam, M.; Singh, R.; Kahri, H.; Pal, U.; Pang, A.L.; Iqbal, M.S.; et al. Ultrasonic-Assisted Synthesis of CaCu3Ti4O12/Reduced Graphene Oxide Composites for Enhanced Photocatalytic Degradation of Pharmaceutical Products: Ibuprofen and Ciprofloxacin. Environ. Sci. Pollut. Res. Int. 2024, 31, 27770–27788. [Google Scholar] [CrossRef]
- Praxedes, F.M.; Moreno, H.; Simões, A.Z.; Teixeira, V.C.; Nunes, R.S.; Amoresi, R.A.C.; Ramirez, M.A. Interface Matters: Design of an Efficient CaCu3Ti4O12-RGO Photocatalyst. Powder Technol. 2022, 404, 117478. [Google Scholar] [CrossRef]
- Orrego, S.; Cortés, J.A.; Amoresi, R.A.C.; Simões, A.Z.; Ramírez, M.A. Photoluminescence Behavior on Sr2+ Modified CaCu3Ti4O12 Based Ceramics. Ceram. Int. 2018, 44, 10781–10789. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Padmini, M.; Elumalai, P.; Thomas, P. Symmetric Supercapacitor Performances of CaCu3Ti4O12 Decorated Polyaniline Nanocomposite. Electrochim. Acta 2018, 292, 558–567. [Google Scholar]
- Bard, J.A.; Faulkner, R.L.; White, S.H. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2022. [Google Scholar]
- Venugopal, G.; Krishnamoorthy, K.; Mohan, R.; Kim, S.J. An Investigation of the Electrical Transport Properties of Graphene-Oxide Thin Films. Mater. Chem. Phys. 2012, 132, 29–33. [Google Scholar] [CrossRef]
- Bumberger, A.E.; Nenning, A.; Fleig, J. Transmission Line Revisited—The Impedance of Mixed Ionic and Electronic Conductors. Phys. Chem. Chem. Phys. 2024, 26, 15068–15089. [Google Scholar] [CrossRef]
- Riedl, C.; Siebenhofer, M.; Nenning, A.; Schmid, A.; Weiss, M.; Rameshan, C.; Limbeck, A.; Kubicek, M.; Opitz, A.K.; Fleig, J. In Situ Techniques Reveal the True Capabilities of SOFC Cathode Materials and Their Sudden Degradation Due to Omnipresent Sulfur Trace Impurities. J. Mater. Chem. A Mater. 2022, 10, 14838–14848. [Google Scholar] [CrossRef]
- Moreira, T.F.M.; Pinzón, E.F.; dos Santos, A.; Lopes, L.C.; Bueno, P.R. The Quantum Mechanical Origin of the Supercapacitance Phenomenon in Reduced Graphene Oxide Structures. Carbon 2025, 232, 11. [Google Scholar] [CrossRef]
- Jero, D.; Caussé, N.; Dantras, E.; Roggero, A.; Buffeteau, T.; Pébère, N. Analysis of a Film-Forming Amine Response in Impedance Spectra. Electrochim. Acta 2024, 498, 144690. [Google Scholar] [CrossRef]
- Góes, M.S.; Rahman, H.; Ryall, J.; Davis, J.J.; Bueno, P.R. A Dielectric Model of Self-Assembled Monolayer Interfaces by Capacitive Spectroscopy. Langmuir 2012, 28, 9689–9699. [Google Scholar] [CrossRef]
- Yu, K.; Lou, L.-L.; Liu, S.; Zhou, W. Asymmetric Oxygen Vacancies: The Intrinsic Redox Active Sites in Metal Oxide Catalysts. Adv. Sci. 2020, 7, 1901970. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Speranza, G. Tuning the Oxygen Content of Reduced Graphene Oxide and Effects on Its Properties. ACS Omega 2021, 6, 6195–6205. [Google Scholar] [CrossRef]
- Chatterjee, D.P.; Nandi, A.K. A Review on the Recent Advances in Hybrid Supercapacitors. J. Mater. Chem. A Mater. 2021, 9, 15880–15918. [Google Scholar] [CrossRef]
- Elmouwahidi, A.; Zapata-Benabithe, Z.; Carrasco-Marín, F.; Moreno-Castilla, C. Activated Carbons from KOH-Activation of Argan (Argania Spinosa) Seed Shells as Supercapacitor Electrodes. Bioresour. Technol. 2012, 111, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Veerapandian, M.; Yun, K.; Kim, S.-J. The Chemical and Structural Analysis of Graphene Oxide with Different Degrees of Oxidation. Carbon 2013, 53, 38–49. [Google Scholar] [CrossRef]
- Qureshi, T.S.; Panesar, D.K. Impact of Graphene Oxide and Highly Reduced Graphene Oxide on Cement Based Composites. Constr. Build. Mater. 2019, 206, 71–83. [Google Scholar] [CrossRef]
- Maity, S.; Samanta, M.; Sen, A.; Chattopadhyay, K.K. Investigation of Electrochemical Performances of Ceramic Oxide CaCu3Ti4O12 Nanostructures. J. Solid. State Chem. 2019, 269, 600–607. [Google Scholar] [CrossRef]


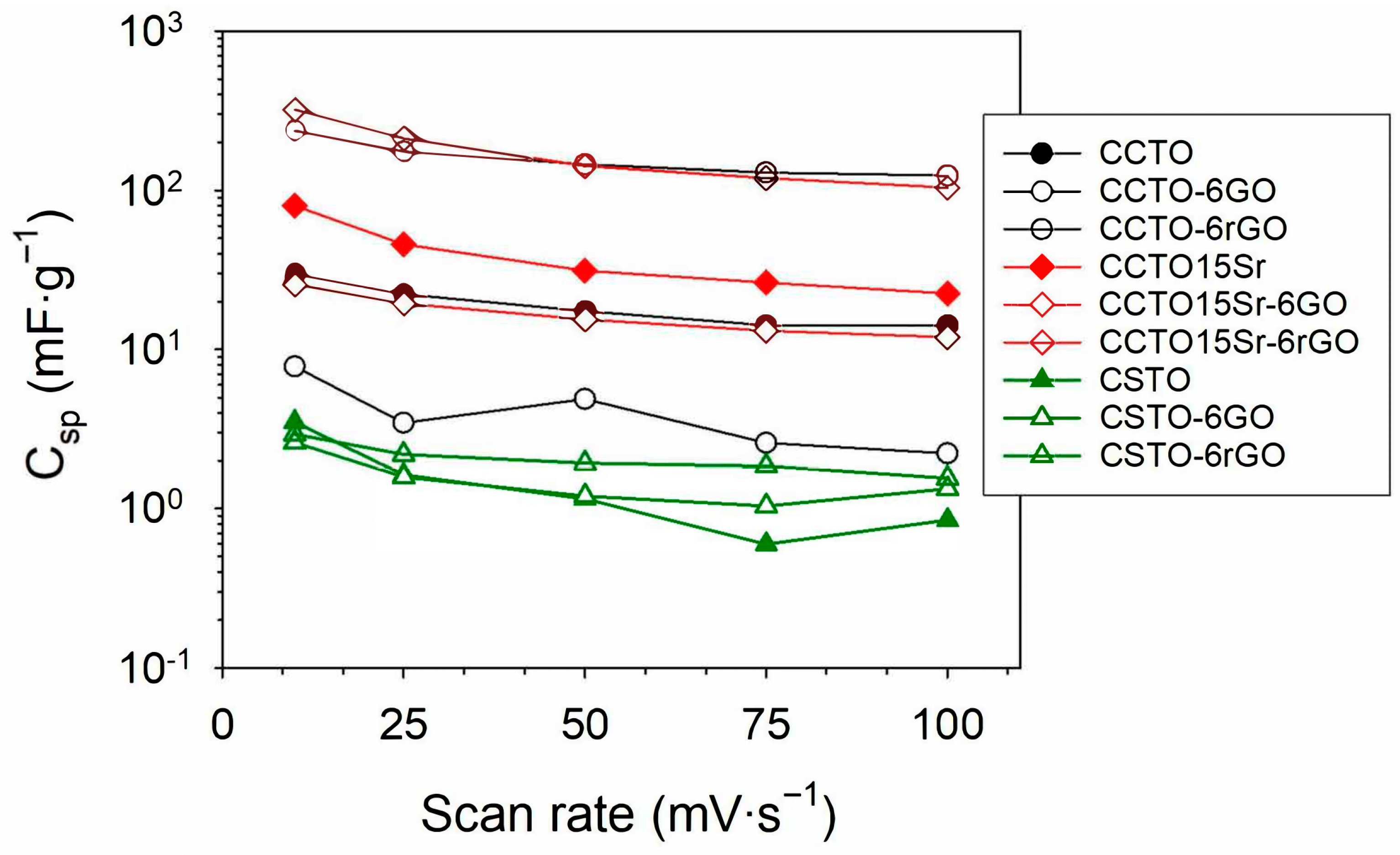
| Scan Rate (mV·s−1) | |||||
|---|---|---|---|---|---|
| Samples | 10 | 25 | 50 | 75 | 100 |
| CCTO | 29.86 | 22.32 | 17.43 | 14.24 | 14.21 |
| CCTO-6GO | 7.83 | 3.46 | 4.89 | 2.59 | 2.22 |
| CCTO-6rGO | 237.76 | 175.63 | 145.35 | 129.28 | 124.20 |
| CCTO15Sr | 80.19 | 45.79 | 31.33 | 26.33 | 22.48 |
| CCTO15Sr-6GO | 25.73 | 19.51 | 15.48 | 13.16 | 10.64 |
| CCTO15Sr-6rGO | 321.63 | 212.89 | 141.90 | 119.69 | 104.25 |
| CSTO | 3.51 | 1.63 | 1.15 | 0.60 | 0.85 |
| CSTO-6GO | 2.60 | 1.58 | 1.20 | 1.04 | 1.33 |
| CSTO-6rGO | 2.93 | 2.19 | 1.93 | 1.85 | 1.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moussa, H.A.K.; Suárez, J.A.C.; La Porta, F.d.A.; Góes, M.S. CaSrxCu3−xTi4O12 Ceramic Oxide Modified with Graphene Oxide and Reduced Graphene Oxide for Supercapacitor Applications. Solids 2025, 6, 58. https://doi.org/10.3390/solids6040058
Moussa HAK, Suárez JAC, La Porta FdA, Góes MS. CaSrxCu3−xTi4O12 Ceramic Oxide Modified with Graphene Oxide and Reduced Graphene Oxide for Supercapacitor Applications. Solids. 2025; 6(4):58. https://doi.org/10.3390/solids6040058
Chicago/Turabian StyleMoussa, Hussein Abdul Karin, Johan Alexander Cortés Suárez, Felipe de Almeida La Porta, and Márcio Sousa Góes. 2025. "CaSrxCu3−xTi4O12 Ceramic Oxide Modified with Graphene Oxide and Reduced Graphene Oxide for Supercapacitor Applications" Solids 6, no. 4: 58. https://doi.org/10.3390/solids6040058
APA StyleMoussa, H. A. K., Suárez, J. A. C., La Porta, F. d. A., & Góes, M. S. (2025). CaSrxCu3−xTi4O12 Ceramic Oxide Modified with Graphene Oxide and Reduced Graphene Oxide for Supercapacitor Applications. Solids, 6(4), 58. https://doi.org/10.3390/solids6040058







