Effect of Heat Treatment on the Microstructure and Mechanical Properties of Vanadis 60 Steel: A Statistical Design Approach
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
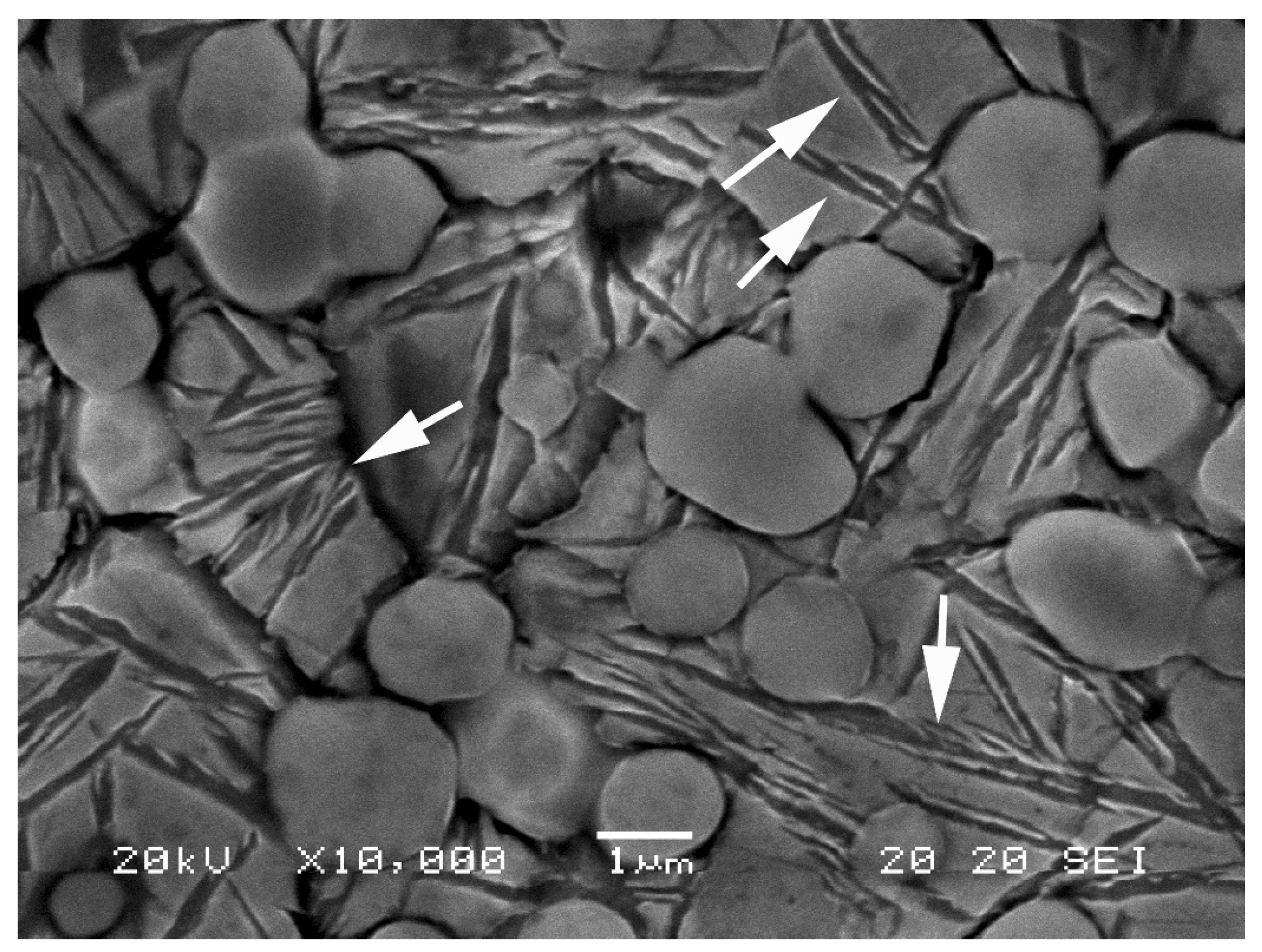
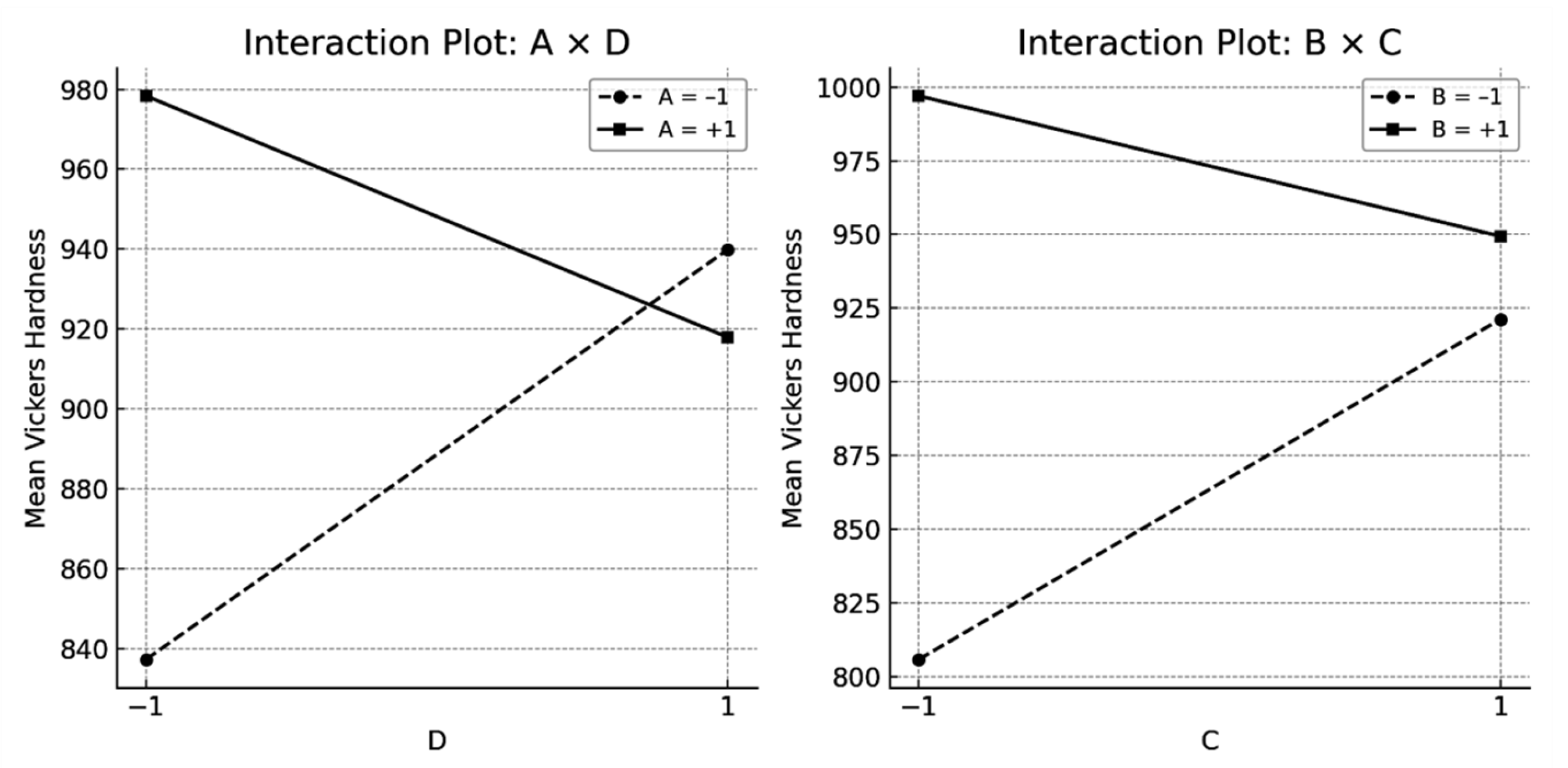
- Vickers hardness increased significantly when austenitisation was carried out at 1180 °C, followed by oil quenching and tempering at 560 °C. This thermal sequence promotes the dissolution of carbon and alloying elements into austenite, enhancing the tetragonality of martensite and favouring secondary hardening during tempering. It also reduces the amount of retained austenite.
- A statistically significant interaction was identified between the austenitisation temperature and the number of tempering cycles. Three temperings were sufficient to compensate for the reduced hardness associated with lower austenitisation temperatures.
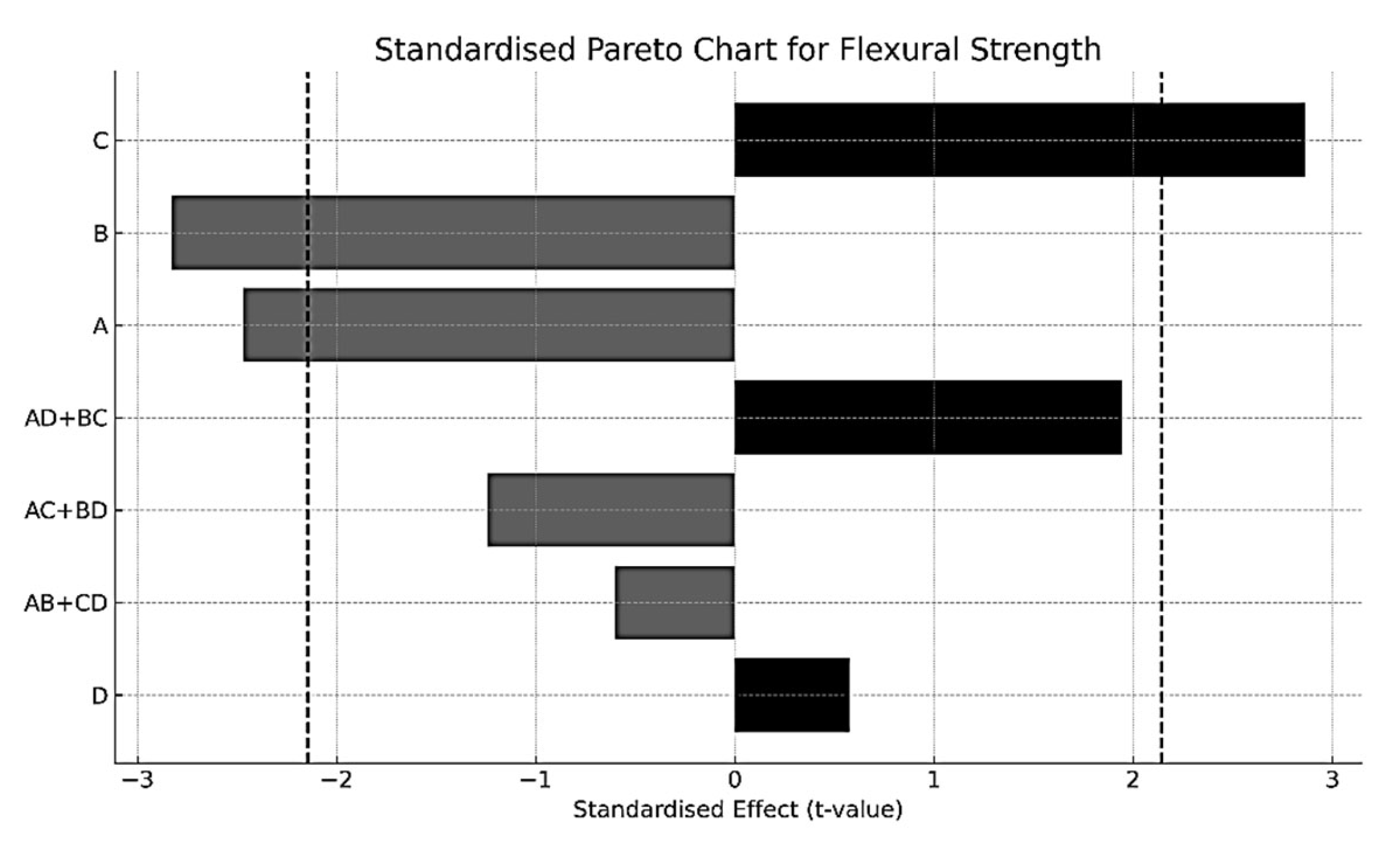
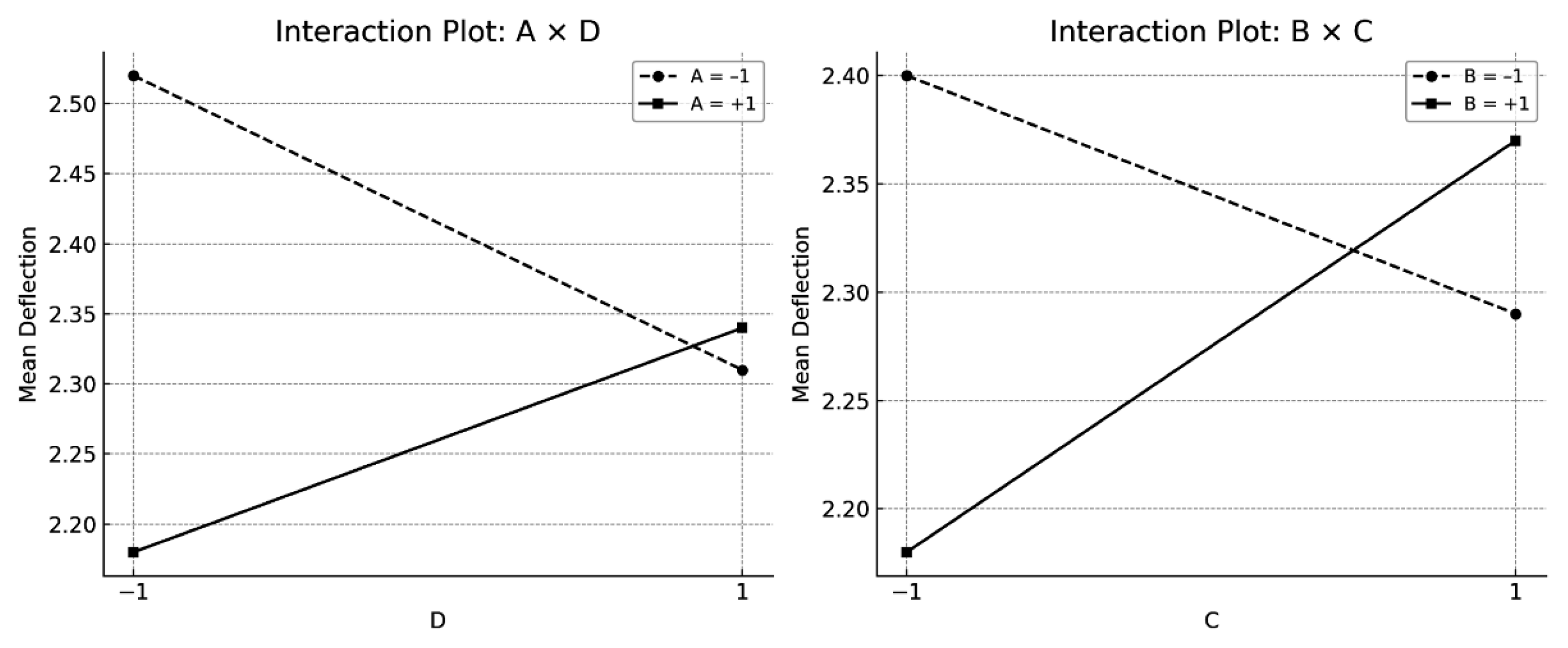
- Flexural strength increased after austenitisation at 1020 °C, followed by air cooling, and tempering at 560 °C. This condition promotes the development of a bainitic microstructure and a lower tetragonality of martensite. A marked reduction in the retained austenite content was also observed as a result of the tempering temperature. Ductility, assessed via the maximum deflection, was significantly enhanced under bainitic microstructures. Moreover, tempering at 560 °C proved effective in mitigating the embrittling effect of oil quenching, contributing to a balanced mechanical behaviour.
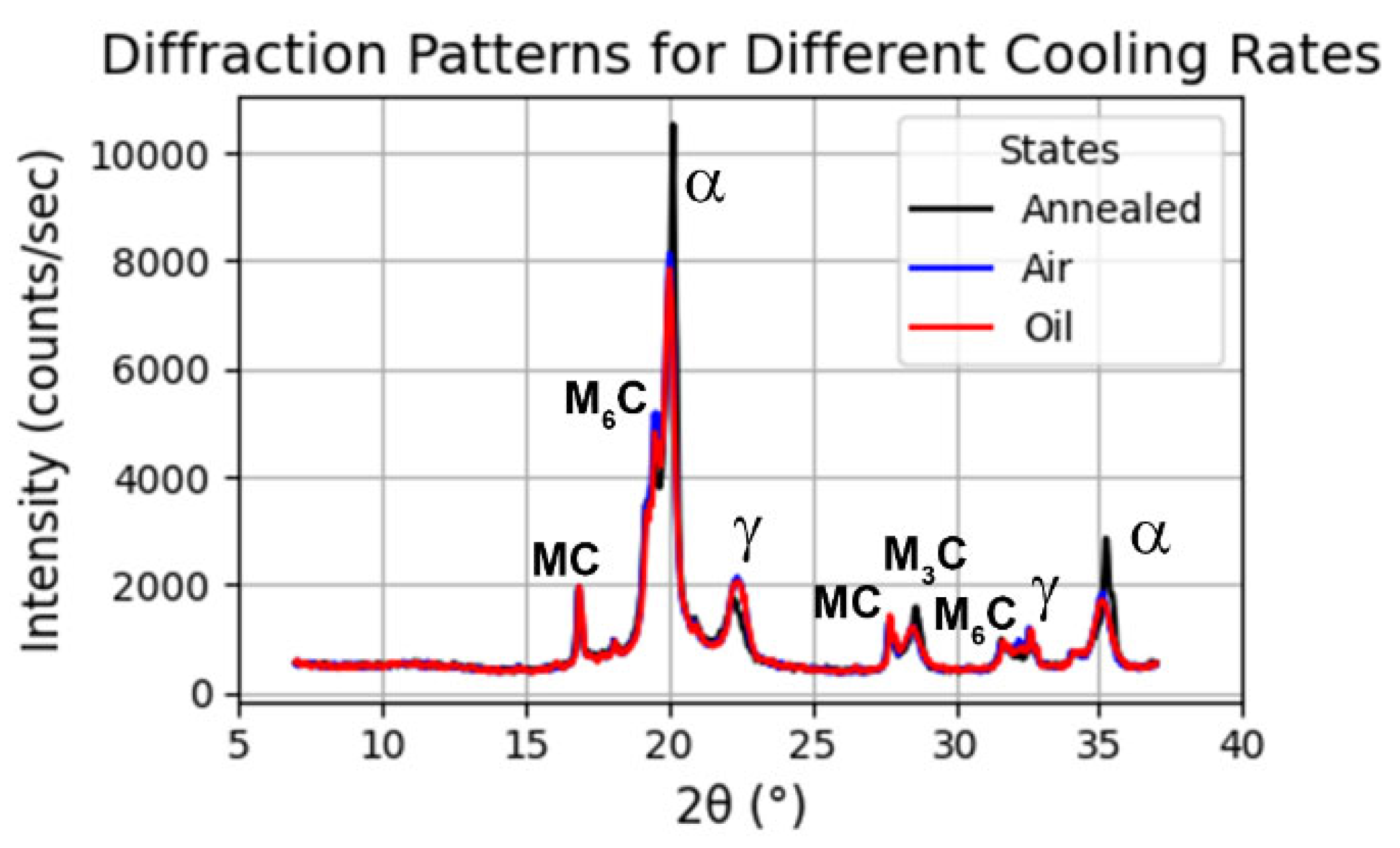
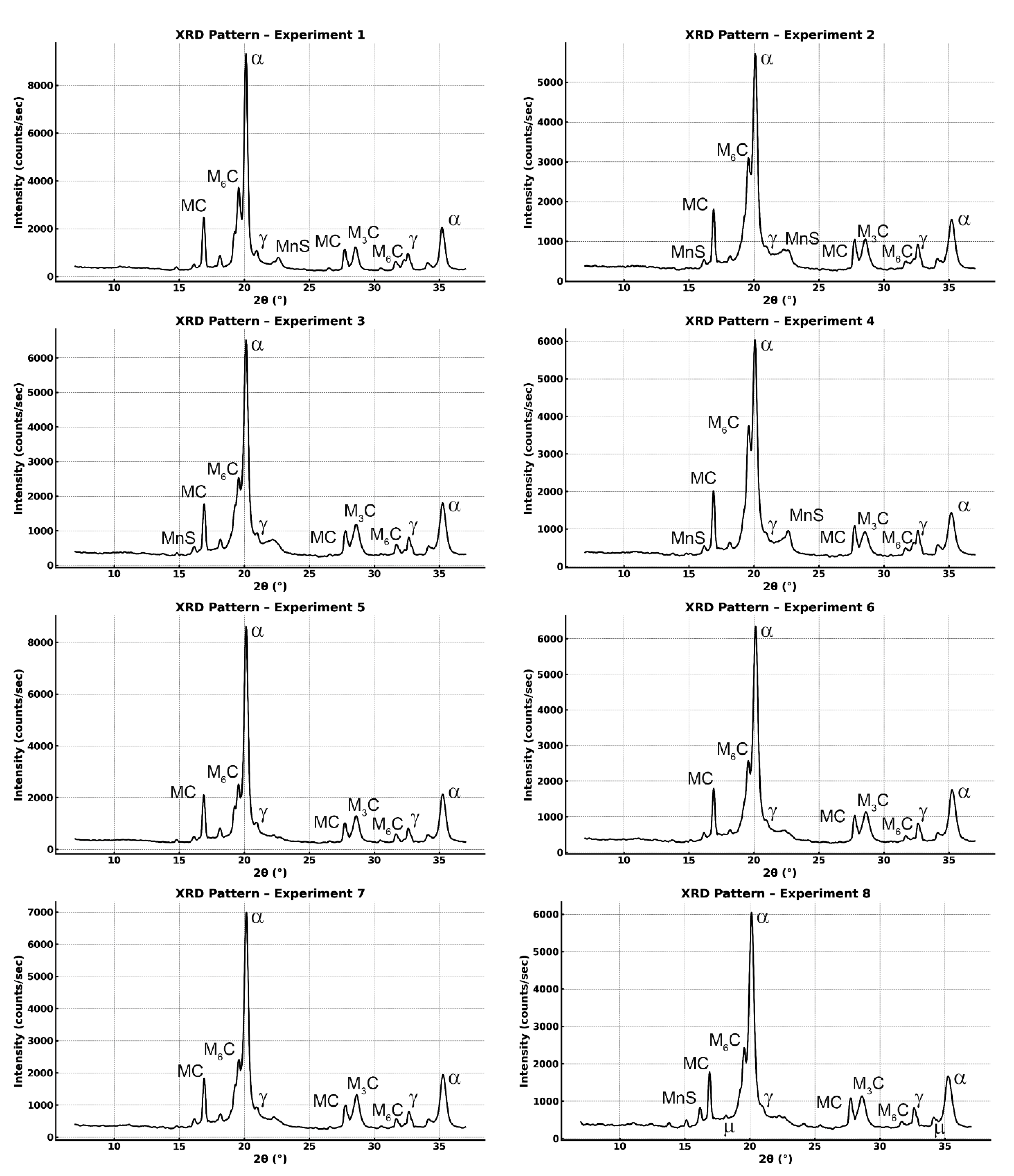
- XRD analysis confirmed that tempering at 560 °C reduced the retained austenite and promoted martensite stabilisation, in line with the observed increase in hardness. The analysis of lattice parameters showed that austenitisation at 1180 °C increased the unit cell parameter of tempered martensite, due to greater dissolution of carbon and alloying elements and the higher cooling rate.
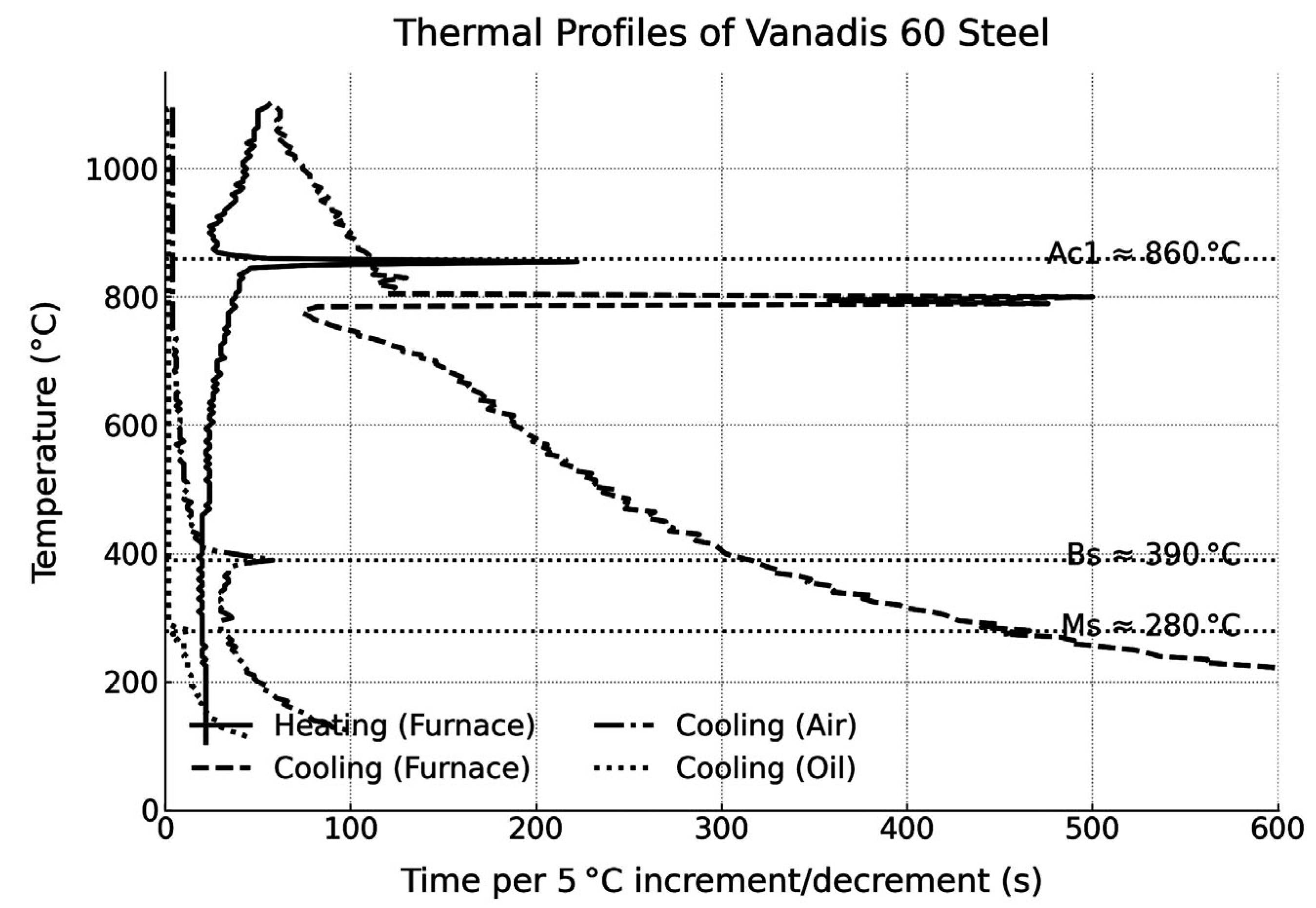
- Thermal analysis allowed the identification of the characteristic transformation temperatures for Vanadis 60 steel: austenitisation at 860 °C, formation of upper bainite at 390 °C by air cooling, and the onset of martensitic transformation at 280−300 °C.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chang, S.-H.; Chen, C.-Y.; Huang, K.-T. Microstructure, retained austenite, and mechanical properties evaluation of Vanadis 60-HfC-Ta0.6Nb0.4C steel matrix composite by vacuum sintering, sub-zero, and heat treatments. Vacuum 2023, 210, 111885. [Google Scholar] [CrossRef]
- Lemecha, M.; Napiorkowski, J.; Ligier, K.; Tarasiuk, W.; Sztukowski, K. Analysis of Wear Properties of Powder Metallurgy Steel in Abrasive Soil Mass. Materials 2022, 15, 6888. [Google Scholar] [CrossRef]
- Huang, K.-T.; Chang, S.-H.; Chang, C.-W. Microstructure, Strengthening Mechanism and Mechanical Properties of Vanadis 60-Ta0.5Nb0.5C-B4C High-speed Steel Composite via Vacuum Sintering, Sub-zero, and Heat Treatments. ISIJ Int. 2024, 64, 154–164. [Google Scholar] [CrossRef]
- Alvarez-Antolin, F.; Gonzalez-Pociño, A.; Cofiño-Villar, A.; Alvarez-Perez, C.H. Optimisation of Thermal Processes with Plasma Nitriding on Vanadis 4 High Speed Steel. Materials 2022, 15, 906. [Google Scholar] [CrossRef]
- Halfa, H.; Seikh, A.H.; Abdo, H.S.; Alnaser, I.A.; Soliman, M.S.; Ragab, S.M. Study on the Microstructure of Vanadium-Modified Tungsten High-Speed Steel-Coded SAE-AISI T1 Steel. Adv. Mater. Sci. Eng. 2022, 2022, 1–18. [Google Scholar] [CrossRef]
- Scholl, L.M.; Bezold, A.; Broeckmann, C. Influences of Manufacturing-Related Microstructural Variations on Fatigue in Carbide-Rich Tool Steels. Steel Res. Int. 2023, 94, 2200578. [Google Scholar] [CrossRef]
- Akhmetov, A.S.; Eremeeva, Z.V. Investigation of the Structure of Sintered Blanks from Powder Mixture of R6M5K5 High-Speed Steel Containing Diffusion-Alloyed Powder. Metallurgist 2022, 66, 299–303. [Google Scholar] [CrossRef]
- Bombač, D.; Terčelj, M.; Fazarinc, M.; Kugler, G. On the Increase of Intrinsic Workability and Hot Working Temperature Range of M42 Ledeburitic Super High Steel in As-Cast and Wrought States. Mater. Sci. Eng. A 2017, 703, 438–450. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, J.; Wang, H.; Lu, L.; Cui, H.; Zhang, J. Microstructures and Mechanical Properties of Spray Formed H13 Tool Steel. Acta Metall. Sin. 2014, 50, 787–794. [Google Scholar]
- Pavlícková, M.; Vojtech, D.; Novák, P.; Gemperlová, J.; Gemperle, A.; Zárubová, N.; Lejcek, P.; Jurci, P.; Stolar, P. Thermal Treatment of PM-Tool Steel Alloyed with Niobium. Mater. Sci. Eng. A 2003, 356, 200–207. [Google Scholar] [CrossRef]
- Pan, Y.; Pi, Z.; Liu, B.; Xu, W.; Zhang, C.; Qu, X.; Lu, X. Influence of Heat Treatment on the Microstructural Evolution and Mechanical Properties of W6Mo5Cr4V2Co5Nb (825 K) High Speed Steel. Mater. Sci. Eng. A 2020, 787, 139480. [Google Scholar] [CrossRef]
- Liu, J.; Chi, H.; Wu, H.; Ma, D.; Zhou, J. Discussion of the Segregation and Low Hardness of Large-Diameter M3 High-Speed Steel Produced by Spray Forming. Materials 2023, 16, 482. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, P.; Wang, K.; Li, H.; Gong, M.; Tong, W. Microstructure and Mechanical Properties of a Mo Alloyed High Chromium Cast Iron after Different Heat Treatments. Vacuum 2018, 156, 59–67. [Google Scholar] [CrossRef]
- Chang, S.-H.; Chang, C.-H.; Huang, K.-T. In Situ TEM Observation of the Microstructure Characteristics of the Vacuum Sintering, Sub-Zero and Heat Treatments of Vanadis 23 High-Speed Steel by Adding Cr3C2-TaC-TiC Powders. Powder Metall. 2023, 66, 151–163. [Google Scholar] [CrossRef]
- Wang, F.; Xu, L. Microstructure and Erosion Wear Characterization of a New Cast High-Vanadium-Chromium Alloy (HVCA). Int. J. Met. 2023, 17, 466–480. [Google Scholar] [CrossRef]
- Zinchenko, S.A. Thermocyclic treatment (TCT)–as a method to decrease carbide segregation of hypereutectoid steels. Eur. Phys. J.-Spec. Top. 2020, 229, 459–465. [Google Scholar] [CrossRef]
- Chen, K.-J.; Hung, F.-Y.; Lui, T.-S.; Shih, Y.-R. Wear Inducing Phase Transformation of Plasma Transfer Arc Coated Tools during Friction Stir Welding with Al Alloy. J. Eng. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, D.; Zhu, W.; Fang, F.; Tui, Y.; Jiang, J. Morphology, microstructure and decomposition behavior of M2C carbides in high speed steel. J. Iron Steel Res. Int. 2017, 24, 43–49. [Google Scholar] [CrossRef]
- Jin, J.; Gao, R.; Peng, H.; Guo, H.; Gong, S.; Chen, B. Rapid Solidification Microstructure and Carbide Precipitation Behavior in Electron Beam Melted High-Speed Steel. Metall. Mater. Trans. A 2020, 51, 2411–2429. [Google Scholar] [CrossRef]
- Guo, J.; Liu, L.; Feng, Y.; Liu, S.; Ren, X.; Yang, Q. Crystallographic Characterizations of Eutectic and Secondary Carbides in a Fe-12Cr-2.5Mo-1.5W-3V-1.25C Alloy. Met. Mater.-Int. 2017, 23, 313–319. [Google Scholar] [CrossRef]
- Jaworski, J.; Kluz, R.; Trzepiecinski, T. Influence of Heat Treatment on Content of the Carbide Phases in the Microstructure of High-Speed Steel. Arch. Foundry Eng. 2017, 17, 59–62. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Y.; Sun, C.; Chen, L.; Fang, F.; Jiang, J. Optimization on mechanical properties of transition metal carbides: A combined experimental and theoretical study. Mater. Chem. Phys. 2022, 282, 125955. [Google Scholar] [CrossRef]
- Michalcova, A.; Pecinka, V.; Kacenka, Z.; Serak, J.; Kubasek, J.; Novak, P.; Vojtech, D. Microstructure, Mechanical Properties, and Thermal Stability of Carbon-Free High Speed Tool Steel Strengthened by Intermetallics Compared to Vanadis 60 Steel Strengthened by Carbides. Metals 2021, 11, 1901. [Google Scholar] [CrossRef]
- Rakhadilov, B.; Kurbanbekov, S.; Skakov, M.; Wieleba, W.; Zhurerova, L. Effect plasma beam irradiation on the microstructure and phase composition of high-speed steel R6M5. Mater. Test. 2020, 62, 1138–1142. [Google Scholar] [CrossRef]
- Shen, W.; Yu, L.; Li, Z.; He, Y.; Zhang, Q.; Zhang, H.; Jiang, Y.; Lin, N. In situ synthesis and strengthening of powder metallurgy high speed steel in addition of LaB6. Met. Mater.-Int. 2017, 23, 1150–1157. [Google Scholar] [CrossRef]
- Serna, M.M.; Rossi, J.L. MC complex carbide in AISI M2 high-speed steel. Mater. Lett. 2009, 63, 691–693. [Google Scholar] [CrossRef]
- Gonzalez-Pocino, A.; Asensio-Lozano, J.; Alvarez-Antolin, F.; Garcia-Diez, A. Improvement of Impact Toughness and Abrasion Resistance of a 3C-25Cr-0.5Mo Alloy Using a Design of Experiment Statistical Technique: Microstructural Correlations after Heat Treatments. Metals 2021, 11, 595. [Google Scholar] [CrossRef]
- Burja, J.; Nagode, A.; Medved, J.; Balasko, T.; Grabnar, K. Effect of microalloying on tempering of Mo-W high thermal conductivity steel. Metall. Ital. 2023, 114, 22–27. [Google Scholar]
- Zhang, C.; Li, J.; Zhang, Y.; Sun, Z.; Ren, S.; Lv, D.; Nian, B.; Zhao, Y.; Song, Y. Understanding of the Microstructure Evolution and Wear Resistance of Cr12MoV Die Steel during Deep Cryogenic Treatment. J. Mater. Eng. Perform. 2024, 34, 3064–3075. [Google Scholar] [CrossRef]
- Hofinger, M.; Seisenbacher, B.; Landefeld, A.; Ognianov, M.; Turk, C.; Leitner, H.; Schnitzer, R. Influence of thermomechanical fatigue loading conditions on the nanostructure of secondary hardening steels. Mater. Sci. Eng. A 2021, 802, 140672. [Google Scholar] [CrossRef]
- Amirabdollahian, S.; Deirmina, F.; Pellizzari, M.; Bosetti, P.; Molinari, A. Tempering behavior of a direct laser deposited hot work tool steel: Influence of quenching on secondary hardening and microstructure. Mater. Sci. Eng. A 2021, 814, 141126. [Google Scholar] [CrossRef]
- Liu, F.; Kang, C.; Qian, R.; Jiang, Z.; Geng, X.; Li, H. Effect of Tempering Temperature on Microstructure and Properties of a New Type of Nitrogen-Containing Hot-Work Die Steel 3Cr7Mo2NiSiVN. Steel Res. Int. 2022, 93, 2200013. [Google Scholar] [CrossRef]
- Bae, K.; Moon, H.-S.; Park, Y.; Jo, I.; Lee, J. Influence of Tempering Temperature and Time on Microstructure and Mechanical Properties of Additively Manufactured H13 Tool Steel. Materials 2022, 15, 8329. [Google Scholar] [CrossRef]
- Ozer, M. Influence of heat treatments on microstructure and wear behavior of AISI H13 tool steel. Kov. Mater.-Met. Mater. 2022, 60, 387–396. [Google Scholar] [CrossRef]
- Mochtar, M.A.; Putra, W.N.; Abram, M. Effect of tempering temperature and subzero treatment on microstructures, retained austenite, and hardness of AISI D2 tool steel. Mater. Res. Express 2023, 10, 056511. [Google Scholar] [CrossRef]
- Miyauchi, H.; Matsumoto, H.; Yokota, K. Development of a Periodic Laminate Structure in H13 Steel Using Laser Powder Bed Fusion: Effects of Tempering on Hardness Evolution. Steel Res. Int. 2023, 94, 00622. [Google Scholar] [CrossRef]
- Deirmina, F.; Quarzago, L.; Butcher, D.; Bettini, E.; Mehraban, S.; Hann, J.; Pettersson, N.H.; Lavery, N.; Rottger, A.; Pellizzari, M. General investigations on the heat treatment and thermal fatigue behavior of an experimental hot work tool steel tailored for laser powder bed fusion. Mater. Sci. Eng. A 2024, 901, 146554. [Google Scholar] [CrossRef]
- Wang, Y.; Chu, S.; Mao, B.; Xing, H.; Zhang, J.; Sun, B. Microstructure, residual stress, and mechanical property evolution of a spray-formed vanadium-modified high-speed steel processed by post-heat treatment. J. Mater. Res. Technol. 2022, 18, 1521–1533. [Google Scholar] [CrossRef]
- Jiao, W.-C.; Li, H.-B.; Feng, H.; Wang, H.-J.; Zhu, H.-C.; Zhang, S.-C.; Jiang, Z.-H.; Wu, W. Exploring the Influence Mechanisms of Tempering Temperature and N-alloying on Mechanical Properties of AISI M42 High-Speed Steel. Steel Res. Int. 2023, 94, 0824. [Google Scholar] [CrossRef]
- Zhou, T.; Spartacus, G.; Dahlstrom, A.; Babu, R.P.; Davydok, A.; Hedstrom, P. Computational thermodynamics and kinetics-guided re-engineering of a high-performance tool steel. Scr. Mater. 2023, 232, 115496. [Google Scholar] [CrossRef]
- Kumar, A.; Agarwal, G.; Petrov, R.; Goto, S.; Sietsma, J.; Herbig, M. Microstructural evolution of white and brown etching layers in pearlitic rail steels. Acta Mater. 2019, 171, 48–64. [Google Scholar] [CrossRef]
- Bergmueller, S.; Kaserer, L.; Fuchs, L.; Braun, J.; Weinberger, N.; Letofsky-Papst, I.; Leichtfried, G. Crack-free in situ heat-treated high-alloy tool steel processed via laser powder bed fusion: Microstructure and mechanical properties. Heliyon 2022, 8, e10171. [Google Scholar] [CrossRef]
- Firouzi, A.; Yazdani, S.; Tavangar, R.; Shakerifard, B.; Khan, F. Fracture Toughness Evaluation of Powder Metallurgical ASP2030 High-Speed Steels Using Flexural Specimens and Finite Element Method. Strength Mater. 2022, 54, 1064–1081. [Google Scholar] [CrossRef]
- Zhikai, Y.; Jianlin, B.; Xinyue, Z. Effect of heat treatment on microstructure and mechanical property of selective laser melted high speed steel. Cailiao Gongcheng 2022, 50, 135–142. [Google Scholar] [CrossRef]
- Jurkovic, K.; Cajner, H.; Mrvar, P.; Bauer, B. Analysis of Factor Effects in Process of Vertical Centrifugal Casting. Mater. Manuf. Process. 2023, 39, 386–397. [Google Scholar] [CrossRef]
- Fukuda, I.M.; Pinto, C.F.F.; Moreira, C.S.; Saviano, A.M.; Lourenço, F.R. Design of Experiments (DoE) applied to Pharmaceutical and Analytical Quality by Design (QbD). Braz. J. Pharm. Sci. 2018, 54, e01006. [Google Scholar] [CrossRef]
- Marco, L.; Tort-Martorell, X.; Cuadrado, J.A.; Pozueta, L. Optimization of a Car Brake Prototype as a Consequence of Successful DOE Training. Qual. Reliab. Eng. Int. 2004, 20, 469–480. [Google Scholar] [CrossRef]
- Monikandan, V.V.; Mandal, A. Application of the Statistical Method to Analyze the High-Temperature Tribological Properties of Aluminum Composites. Trans. Indian Inst. Met. 2022, 76, 2383–2389. [Google Scholar] [CrossRef]
- Beg, S.; Raza, K. Full Factorial and Fractional Factorial Design Applications in Pharmaceutical Product Development. In Design of Experiments for Pharmaceutical Product Development: Volume I: Basics and Fundamental Principles; Beg, S., Ed.; Springer: Singapore, 2021; pp. 43–53. [Google Scholar] [CrossRef]
- Weng, L.-C.; Elsawah, A.M.; Fang, K.-T. Cross-Entropy Loss for Recommending Efficient Fold-Over Technique. J. Syst. Sci. Complex 2021, 34, 402–439. [Google Scholar] [CrossRef]
- Barrionuevo, G.O.; Ramos-Grez, J.A.; Sánchez-Sánchez, X.; Zapata-Hidalgo, D.; Mullo, J.L.; Puma-Araujo, S.D. Influence of the Processing Parameters on the Microstructure and Mechanical Properties of 316L Stainless Steel Fabricated by Laser Powder Bed Fusion. J. Manuf. Mater. Process. 2024, 8, 35. [Google Scholar] [CrossRef]
- García-López, E.; Siller, H.R.; Rodríguez, C.A. Development of AISI 316L stainless steel coronary stent. In Proceedings of the Laser-Based Micro- and Nanoprocessing XII, Spie Lase, San Francisco, CA, USA, 27 January–1 February 2018; pp. 147–153. [Google Scholar] [CrossRef]
- Yang, Y.J.; Draper, N.R. Two-Level Factorial and Fractional Factorial Designs in Blocks of Size Two. J. Qual. Technol. 2003, 35, 294–305. [Google Scholar] [CrossRef]
- Srinivasan, P.; Dharmakkan, N.; Vishnu, S.; Prasath, H.; Gogul, R. Thermal conductivity analysis of Al2O3/water-ethylene glycol nanofluid by using factorial design of experiments in a natural convection heat transfer apparatus. Hem. Ind. 2021, 75, 341–352. [Google Scholar] [CrossRef]
- Mendonça, C.; Capellato, P.; Bayraktar, E.; Gatamorta, F.; Gomes, J.; Oliveira, A.; Sachs, D.; Melo, M.; Silva, G. Recycling Chips of Stainless Steel Using a Full Factorial Design. Metals 2019, 9, 842. [Google Scholar] [CrossRef]
- Mazen, A.; McClanahan, B.; Weaver, J.M. Factors affecting ultimate tensile strength and impact toughness of 3D printed parts using fractional factorial design. Int. J. Adv. Manuf. Technol. 2022, 119, 2639–2651. [Google Scholar] [CrossRef]
- Sahoo, A.K.; Panda, A.; Kumar, R.; Das, R.K.; Das, D. Investigation on machinability characteristics during turning Al6063 alloy using uncoated carbide insert. Mater. Today Proc. 2018, 5, 18120–18128. [Google Scholar] [CrossRef]
- Lauka, D.; Blumberga, D. Electrolysis Process Analysis by Using Low Carbon Content Additives: A Batch Test Study. Energy Procedia 2015, 72, 196–201. [Google Scholar] [CrossRef]
- Reddy, M.S.; Vinoth Kumar, M. Friction stir welding parameters optimization of naval grade AA5083 alloy: RSM. Int. J. Interact. Des. Manuf. 2023, 19, 25–36. [Google Scholar] [CrossRef]
- Ayele, M.; Abay, A.G. Analyzing the Effect of Various Sizing Machine Settings on Abrasion Resistance and Size Pick-Up of Polyester/Cotton Blend Sized Yarn Using Box-Behnken Design. J. Nat. Fibers 2023, 20, 2165591. [Google Scholar] [CrossRef]
- Desai, B.; Mokashi, P.; Anand, R.L.; Burli, S.B.; Khandal, S.V. Effect of Additives on Green Sand Molding Properties Using Design of Experiments and Taguchi’s Quality Loss Function—An Experimental Study. IOP Conf. Ser. Mater. Sci. Eng. 2016, 149, 012006. [Google Scholar] [CrossRef]
- Hosseinzadeh, M.; Hosseini, M.R. Investigation and Optimization of Influencing Parameters on the Copper Extraction from a Low-Grade Oxide Deposit by Acid Leaching. Metall. Res. Technol. 2019, 116, 305. [Google Scholar] [CrossRef]
- González-Pociño, A.; García-García, M.A.; Alvarez-Antolín, F.; Segurado-Frutos, E. Effect of Shot Peening and Nitriding on Toughness and Abrasive Wear Resistance of Powder Metallurgic Steels Highly Alloyed with Vanadium. Metals 2024, 14, 22. [Google Scholar] [CrossRef]
- Nassif, N.; Zeiada, W.; Al-Khateeb, G.; Haridy, S.; Altoubat, S. Assessment of Punching Shear Strength of Fiber-Reinforced Concrete Flat Slabs Using Factorial Design of Experiments. Jordan J. Civ. Eng. 2022, 16, 139–154. [Google Scholar]
- Nurulhuda, A.; Hafizzal, Y.; Izzuddin, M.; Sulawati, M.; Rafidah, A.; Suhaila, Y.; Fauziah, A. Analysis on Flexural Strength of A36 Mild Steel by Design of Experiment (DOE). IOP Conf. Ser. Mater. Sci. Eng. 2017, 226, 012153. [Google Scholar] [CrossRef]
- Gasan, H.; Erturk, F. Effects of a Destabilization Heat Treatment on the Microstructure and Abrasive Wear Behavior of High-Chromium White Cast Iron Investigated Using Different Characterization Techniques. Metall. Mater. Trans. A 2013, 44, 4993–5005. [Google Scholar] [CrossRef]
- Mamidi, S.; Gundeboina, R.; Kurra, S.; Velchuri, R.; Muga, V. Aurivillius Family of Layered Perovskites, BiREWO6 (RE = La, Pr, Gd, and Dy): Synthesis, Characterization, and Photocatalytic Studies. Comptes Rendus Chim. 2018, 21, 547–552. [Google Scholar] [CrossRef]
- Guje, R.; Ravi, G.; Palla, S.; Rao, K.N.; Vithal, M. Synthesis, Characterization, Photocatalytic Conductivity Studies of Defect Pyrochlore KM0.33Te1.67O6 (M = Al, Cr and Fe). Mater. Sci. Eng. B 2015, 198, 1–9. [Google Scholar] [CrossRef]
- Ravi, G.; Palla, S.; Veldurthi, N.K.; Reddy, J.R.; Padmasri, H.A.; Vithal, M. Solar Water-Splitting with the Defect Pyrochlore Type of Oxides KFe0.33W1.67O6 and Sn0.5Fe0.33W1.67O6·Xh2O. Int. J. Hydrogen Energy 2014, 39, 15352–15361. [Google Scholar] [CrossRef]
- Carbajal, L.; Sainz, M.A.; Serena, S.; Caballero, A.C.; Caballero, A. Solid-State Compatibility in Two Regions of the System ZnO–CaO–P2O5. J. Am. Ceram. Soc. 2011, 94, 2213–2219. [Google Scholar] [CrossRef]
- Stephens, P.W. Dealing with Anisotropic Peak Broadening in Rietveld Refinements. Acta Crystallogr. A 1999, 55, 90. [Google Scholar]
- Arıcı, M.; Demirtaş, M.; Yılmaz, R. Effect of Ni and Co Additions on Microstructure and Mechanical Properties of Fe–Mn–Al–C Steels. Eng. Sci. Technol. Int. J. 2021, 24, 479–489. [Google Scholar] [CrossRef]
- Pero-Sanz Elorz, J.A. Aceros: Metalúrgica Física, Selección y Diseño; CIE Inversiones Editoriales Dossat 2000: Madrid, Spain, 2004; pp. 539–551. [Google Scholar]
- Li, Z.; Li, P.; Luo, Y.; Zhou, X.; Qi, L.; Li, S.; Wang, Z. Effect of Austenitizing Temperature and Prior Martensite on Ultra-Fine Bainite Transformation Kinetics. Metals 2019, 9, 1309. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Zhang, Z.; Li, Y.; Lv, B.; Zheng, C.; Zhang, P.; Zhang, F. A Review of Heat Treatment Processes for Bainitic Steel. J. Mater. Res. Technol. 2025, 37, 279–307. [Google Scholar] [CrossRef]
- Liu, B.; Qin, T.; Xu, W.; Jia, C.; Wu, Q.; Chen, M.; Liu, Z. Effect of Tempering Conditions on Secondary Hardening of Carbides and Retained Austenite in Spray-Formed M42 High-Speed Steel. Materials 2019, 12, 3714. [Google Scholar] [CrossRef]
- Pegues, J.W.; Melia, M.A.; Rodriguez, M.A.; Babuska, T.F.; Gould, B.; Argibay, N.; Greco, A.; Kustas, A.B. In Situ Synchrotron X-Ray Imaging and Mechanical Properties Characterization of Additively Manufactured High-Entropy Alloy Composites. J. Alloys Compd. 2021, 876, 159505. [Google Scholar] [CrossRef]
- Ding, J.; Zhu, W.; Ma, Y.; Liu, W.; Huang, Y.; Liang, C. Evaluation of Phase Relationship in the W–Fe–C Ternary System through Symmetry Principles and First-Principles Calculation. Mater. Des. 2022, 224, 111376. [Google Scholar] [CrossRef]




| C | Cr | Mo | W | V | Co | Fe |
|---|---|---|---|---|---|---|
| 2.3 | 4.2 | 7.0 | 6.5 | 6.5 | 10.5 | resto |
| Code | Factors | Level −1 | Level +1 |
|---|---|---|---|
| A | Austenitising temperature | 1020 °C (15 min) | 1180 °C (15 min) |
| B | Quenching medium | Air | Oil |
| C | Tempering temperature | 500 °C (1 h) | 560 °C (1 h) |
| D | Number of tempering cycles | 2 | 3 |
| Experiment | A | B | C | D | Effects |
|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | −1 | A B C D AB + CD AC + BD AD + BC |
| 2 | +1 | −1 | −1 | +1 | |
| 3 | −1 | +1 | −1 | +1 | |
| 4 | +1 | −1 | −1 | −1 | |
| 5 | −1 | −1 | +1 | +1 | |
| 6 | +1 | −1 | +1 | −1 | |
| 7 | −1 | +1 | +1 | −1 | |
| 8 | +1 | +1 | +1 | +1 |
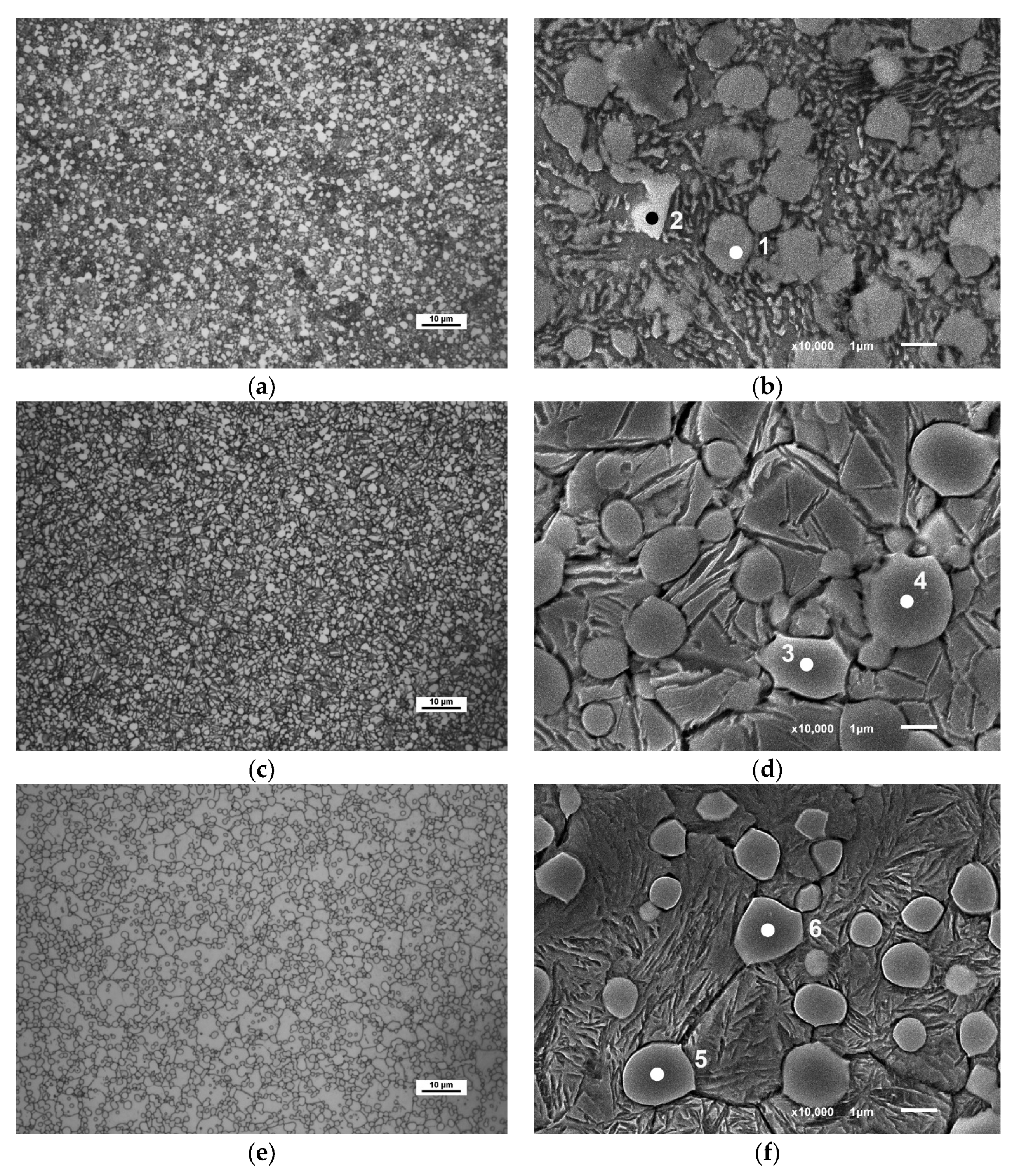
| Spectrum | C | V | Cr | Fe | Co | Mo | W | Carbide Most Likely |
|---|---|---|---|---|---|---|---|---|
| 1 | Rem. | 2.8 | 2.2 | 41.4 | 8.5 | 2.6 | 1.6 | M3C |
| 2 | Rem. | 11.2 | 2.8 | 9.32 | 1.47 | 6.1 | 3.25 | MC |
| 3 | Rem. | 2.3 | 3.1 | 23.3 | 4.9 | 27.2 | 23.5 | M6C |
| 4 | Rem. | 3.0 | 3.1 | 21.5 | 4.7 | 26.0 | 23.4 | M6C |
| 5 | Rem. | 16.3 | 2.7 | 2.3 | 0.8 | 6.1 | 2.3 | MC |
| 6 | Rem. | 16.4 | 2.6 | 2.4 | 0.4 | 6.3 | 2.3 | MC |
| State | Rietveld Fitting | Phases | wt.% | Error (wt.%) | Lattice Parameter (Å) | |
|---|---|---|---|---|---|---|
| Annealed | Rwp = 10.7 Rexp = 5.23 Chi2 = 4.16 | Ferrite | 47.66 | ±1.45 | 2.86747 | ±0.00037 |
| Austenite | 31.36 | ±1.71 | ||||
| M3C carbide | 8.34 | ±1.52 | ||||
| M6C carbide | 1.39 | ±0.16 | ||||
| MC carbide | 11.26 | ±0.69 | ||||
| Air | Rwp = 9.66 Rexp = 5.35 Chi2 = 3.27 | Ferrite | 41.26 | ±4.21 | 2.88274 | ±0.00063 |
| Austenite | 41.15 | ±2.98 | ||||
| M3C carbide | 4.73 | ±1.28 | ||||
| M6C carbide | 1.26 | ±0.17 | ||||
| MC carbide | 11.59 | ±0.95 | ||||
| Oil | Rwp = 9.16 Rexp = 5.43 Chi2 = 3.47 | Ferrite | 42.06 | ±3.99 | 2.88697 | ±0.00066 |
| Austenite | 40.02 | ±2.79 | ||||
| M3C carbide | 5.08 | ±1.33 | ||||
| M6C carbide | 1.35 | ±0.18 | ||||
| MC carbide | 11.49 | ±0.90 | ||||
| Experiment | Replicate 1 | Replicate 2 | Replicate 3 | Mean |
|---|---|---|---|---|
| 1 | 755.13 | 766.22 | 768.41 | 763.26 |
| 2 | 855.97 | 837.87 | 851.20 | 848.35 |
| 3 | 981.14 | 975.74 | 996.21 | 984.36 |
| 4 | 1023.06 | 1005.98 | 999.91 | 1009.65 |
| 5 | 888.89 | 892.40 | 904.67 | 895.32 |
| 6 | 954.17 | 933.73 | 953.31 | 947.07 |
| 7 | 910.69 | 910.29 | 912.71 | 911.23 |
| 8 | 986.12 | 977.09 | 999.45 | 987.55 |
| Effect | Estimated | Upper IC (95%) | Lower IC (95%) | Standardised t-Value |
|---|---|---|---|---|
| A | 59.625 | 66.924 | 52.326 | 7.15 |
| B | 109.692 | 116.991 | 102.393 | 13.16 |
| C | 33.891 | 41.190 | 26.593 | 4.07 |
| D | 21.091 | 28.390 | 13.793 | 2.53 |
| AB + CD | −8.808 | −1.509 | −16.107 | −1.06 |
| AC + BD | 4.392 | 11.690 | −2.907 | 0.53 |
| AD + BC | −81.508 | −74.209 | −88.807 | −9.78 |
| Source | Sum of Squares | df | Mean Square | F-Ratio | p-Value |
|---|---|---|---|---|---|
| A | 21,330.8 | 1 | 21,330.8 | 306.99 | 0.0000 |
| B | 72,193.6 | 1 | 72,193.6 | 1038.99 | 0.0000 |
| C | 6891.87 | 1 | 6891.87 | 99.19 | 0.0000 |
| D | 2669.15 | 1 | 2669.15 | 38.41 | 0.0000 |
| AB + CD | 465.52 | 1 | 465.52 | 6.7 | 0.0215 |
| AC + BD | 115.72 | 1 | 115.72 | 1.67 | 0.2178 |
| AD + BC | 39,861.7 | 1 | 39,861.7 | 573.68 | 0.0000 |
| Blocks | 480.976 | 2 | 240.488 | 3.46 | 0.0601 |
| Total error | 972.777 | 14 | 69.484 |
| Experiment | Replicate 1 | Replicate 2 | Replicate 3 | Mean |
|---|---|---|---|---|
| 1 | 1690.6 | 1671 | 1775.8 | 1712.5 |
| 2 | 1659.5 | 1669.2 | 1797 | 1708.6 |
| 3 | 1315.1 | 1430.7 | 1483.4 | 1409.7 |
| 4 | 1208.2 | 1204.5 | 1211.1 | 1207.9 |
| 5 | 1908.3 | 1991.4 | 1927.9 | 1942.5 |
| 6 | 1647.3 | 1719.7 | 1531 | 1632.7 |
| 7 | 1775.7 | 1806 | 2030.2 | 1870.6 |
| 8 | 1560.3 | 1528 | 1580.5 | 1556.3 |
| Effect | Estimated | Upper IC (95%) | Lower IC (95%) | Standardised t-Value |
|---|---|---|---|---|
| A | −207.483 | −143.756 | −271.210 | −2.849 |
| B | −237.917 | −174.190 | −301.644 | −3.268 |
| C | 240.850 | 304.577 | 177.123 | 3.307 |
| D | 48.350 | 112.077 | −15.377 | 0.664 |
| AB + CD | −50.600 | 13.127 | −114.327 | −0.693 |
| AC + BD | −104.633 | −40.906 | −168.360 | −1.437 |
| AD + BC | 163.767 | 227.494 | 100.040 | 2.248 |
| Source | Sum of Squares | df | Mean Square | F-Ratio | p-Value |
|---|---|---|---|---|---|
| A | 258,296 | 1 | 258,296 | 48.69 | 0.0000 |
| B | 339,626 | 1 | 339,626 | 64.03 | 0.0000 |
| C | 348,052 | 1 | 348,052 | 65.61 | 0.0000 |
| D | 14,026.3 | 1 | 14,026.3 | 2.64 | 0.1262 |
| AB + CD | 15,362.2 | 1 | 15,362.2 | 2.90 | 0.1109 |
| AC + BD | 65,688.8 | 1 | 65,688.8 | 12.38 | 0.0034 |
| AD + BC | 160,917 | 1 | 160,917 | 30.34 | 0.0001 |
| Blocks | 20,519.1 | 2 | 10,259.6 | 1.93 | 0.1813 |
| Total error | 74,264.0 | 14 | 5304.57 |
| Experiment | Replicate 1 | Replicate 2 | Replicate 3 | Mean |
|---|---|---|---|---|
| 1 | 2.84 | 2.81 | 2.85 | 2.83 |
| 2 | 2.68 | 2.64 | 2.90 | 2.74 |
| 3 | 1.93 | 2.23 | 2.16 | 2.11 |
| 4 | 1.89 | 1.87 | 1.93 | 1.90 |
| 5 | 2.64 | 2.73 | 2.70 | 2.69 |
| 6 | 2.33 | 2.51 | 2.18 | 2.34 |
| 7 | 2.52 | 2.58 | 2.85 | 2.65 |
| 8 | 1.95 | 2.22 | 2.23 | 2.13 |
| Effect | Estimated | Upper IC (95%) | Lower IC (95%) | Standardised t-Value |
|---|---|---|---|---|
| A | −0.2925 | −0.3516 | −0.2334 | −2.513 |
| B | −0.4542 | −0.5132 | −0.3950 | −3.901 |
| C | 0.0592 | 0.0000 | 0.1182 | 0.508 |
| D | −0.0125 | −0.0716 | 0.0466 | −0.107 |
| AB + CD | −0.0708 | −0.1299 | −0.0117 | −0.608 |
| AC + BD | −0.1408 | −0.1999 | −0.0817 | −1.210 |
| AD + BC | 0.3308 | 0.2717 | 0.3899 | 2.842 |
| Source | Sum of Squares | df | Mean Square | F-Ratio | p-Value |
|---|---|---|---|---|---|
| A | 0.513338 | 1 | 0.513338 | 37.88 | 0.0000 |
| B | 1.2376 | 1 | 1.2376 | 91.32 | 0.0000 |
| C | 0.0210042 | 1 | 0.0210042 | 1.55 | 0.2336 |
| D | 0.0009375 | 1 | 0.0009375 | 0.07 | 0.7964 |
| AB + CD | 0.0301042 | 1 | 0.0301042 | 2.22 | 0.1583 |
| AC + BD | 0.119004 | 1 | 0.119004 | 8.78 | 0.0103 |
| AD + BC | 0.656704 | 1 | 0.656704 | 48.45 | 0.0000 |
| Blockss | 0.072525 | 2 | 0.0362625 | 2.68 | 0.1037 |
| Error | 0.189742 | 14 | 0.013553 |
| No. | Rietveld Fitting | Phases | wt.% | Lattice Parameter (Å) | ||
|---|---|---|---|---|---|---|
| 1 Austenitised at 1020 °C Air cooled Tempered at 500 °C (2 cycles) | Rwp = 12.8 Rexp = 6.48 Chi2 = 3.89 | Ferrite | 57.57 | ±4.35 | 2.87541 | ±0.00036 |
| Austenite | 13.31 | ±1.20 | ||||
| MC carbide | 25.62 | ±1.43 | ||||
| M6C carbide | 3.5 | ±0.28 | ||||
| M3C carbide | -- | -- | ||||
| 2 Austenitised at 1180 °C Air cooled Tempered at 500 °C (3 cycles) | Rwp = 11.1 Rexp = 7.06 Chi2 = 2.46 | Ferrite | 60.37 | ±4.85 | 2.88140 | ±0.00056 |
| Austenite | 6.79 | ±0.96 | ||||
| MC carbide | 16.45 | ±1.09 | ||||
| M6C carbide | 1.50 | ±0.19 | ||||
| M3C carbide | 14.89 | ±1.72 | ||||
| 3 Austenitised at 1020 °C Oil quenched Tempered at 500 °C (3 cycles) | Rwp = 11.8 Rexp = 7.02 Chi2 = 2.85 | Ferrite | 66.85 | ±5.07 | 2.87863 | ±0.00047 |
| Austenite | -- | -- | ||||
| MC carbide | 16.32 | ±1.07 | ||||
| M6C carbide | 2.25 | ±0.22 | ||||
| M3C carbide | 14.57 | ±1.60 | ||||
| 4 Austenitised at 1180 °C Oil quenched Tempered at 500 °C (2 cycles) | Rwp = 10.6 Rexp = 6.88 Chi2 = 2.36 | Ferrite | 57.04 | ±4.89 | 2.88219 | ±0.00055 |
| Austenite | 11.43 | ±1.25 | ||||
| MC carbide | 19.29 | ±1.26 | ||||
| M6C carbide | 1.43 | ±0.19 | ||||
| M3C carbide | 10.81 | ±1.66 | ||||
| 5 Austenitised at 1020 °C Air cooled Tempered at 560 °C (3 cycles) | Rwp = 10.5 Rexp = 6.68 Chi2 = 2.48 | Ferrite | 71.34 | ±5.20 | 2.87461 | ±0.00041 |
| Austenite | -- | -- | ||||
| MC carbide | 20.27 | ±1.24 | ||||
| M6C carbide | 2.70 | ±0.24 | ||||
| M3C carbide | 5.68 | ±1.34 | ||||
| 6 Austenitised at 1180 °C Air cooled Tempered at 560 °C (2 cycles) | Rwp = 10.8 Rexp = 7.00 Chi2 = 2.39 | Ferrite | 69.86 | ±5.26 | 2.87798 | ±0.00054 |
| Austenite | -- | -- | ||||
| MC carbide | 17.29 | ±1.12 | ||||
| M6C carbide | 1.44 | ±0.18 | ||||
| M3C carbide | 11.41 | ±1.49 | ||||
| 7 Austenitised at 1020 °C Oil quenched Tempered at 560 °C (2 cycles) | Rwp = 11.1 Rexp = 6.96 Chi2 = 2.54 | Ferrite | 70.62 | ±5.12 | 2.87628 | ±0.00045 |
| Austenite | -- | -- | ||||
| MC carbide | 16.94 | ±1.07 | ||||
| M6C carbide | 2.22 | ±0.21 | ||||
| M3C carbide | 10.21 | ±1.41 | ||||
| 8 Austenitised at 1180 °C Oil quenched Tempered at 560 °C (3 cycles) | Rwp = 12.2 Rexp = 7.06 Chi2 = 2.99 | Ferrite | 69.50 | ±5.91 | 2.87638 | ±0.00055 |
| Austenite | -- | -- | ||||
| MC carbide | 16.29 | ±1.16 | ||||
| M6C carbide | 1.26 | ±0.19 | ||||
| M3C carbide | 10.98 | ±1.54 | ||||
| μ (Fe7W6) | 1.97 | ±0.57 | ||||
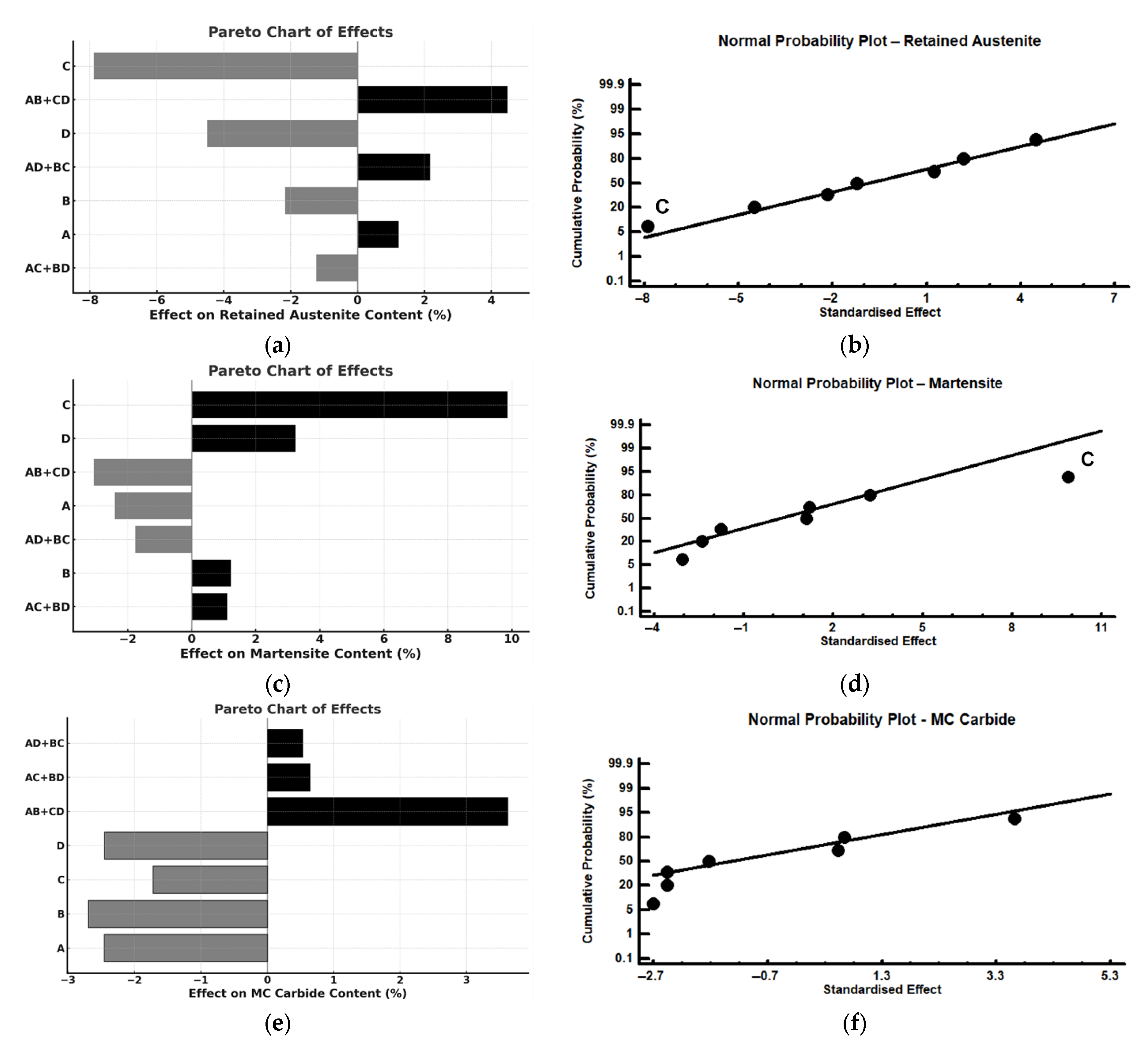
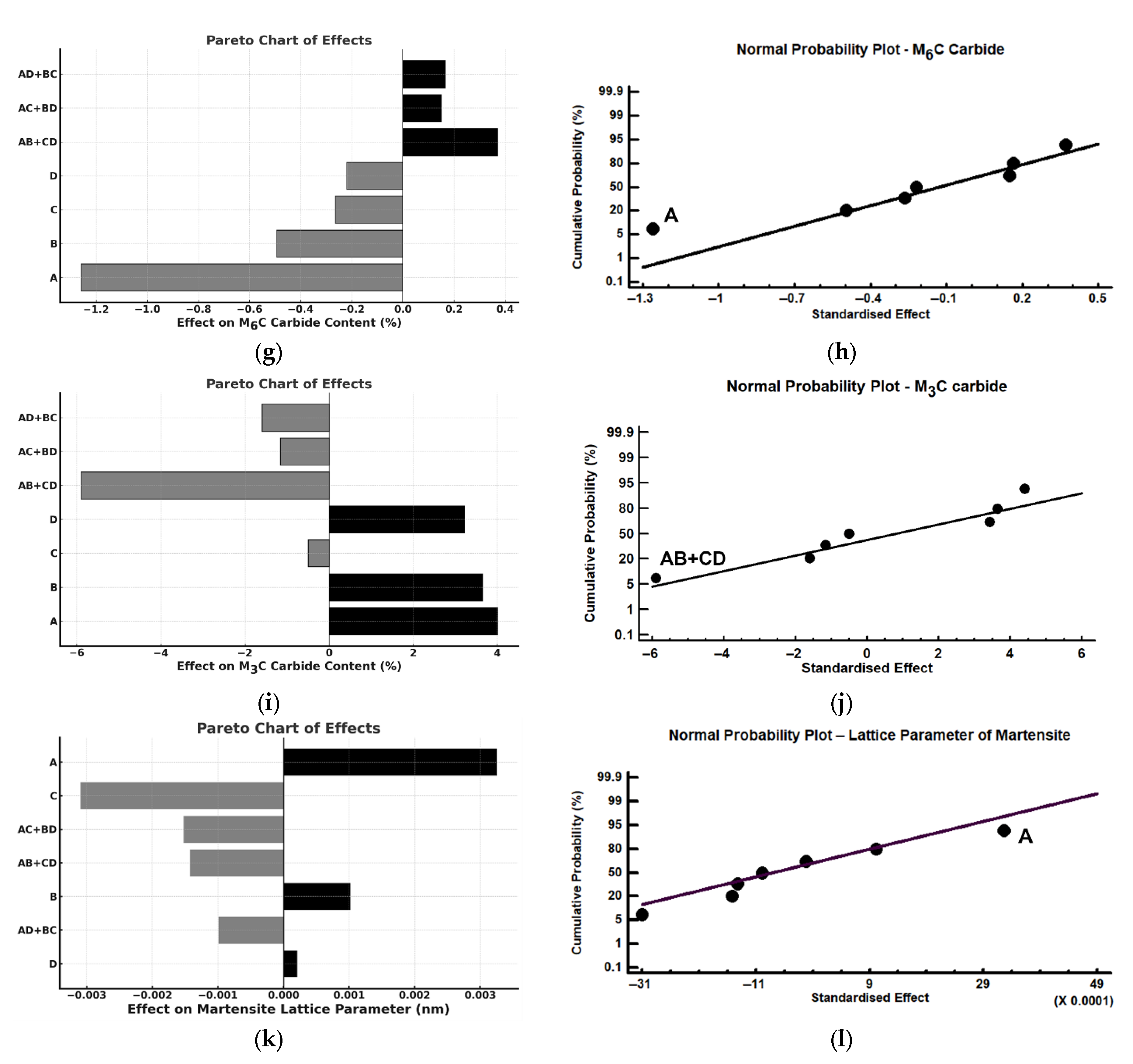
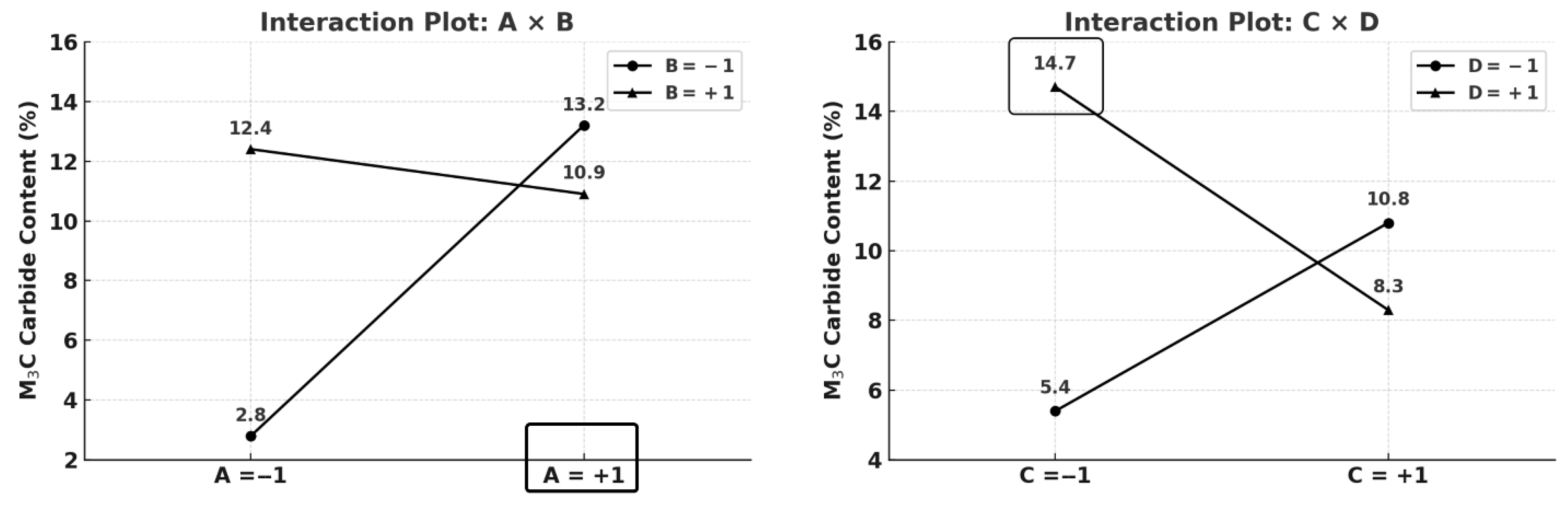
| Effect | Austenite (%) | Martensite (%) | MC (%) | M6C (%) | M3C (%) | a (Å) |
|---|---|---|---|---|---|---|
| Mean | 3.94 | 65.39 | 18.56 | 2.03 | 9.82 | 2.878 |
| A | 1.23 | −2.40 | −2.45 | −1.26 | 4.41 | 0.003 |
| B | −2.17 | 1.21 | −2.69 | −0.49 | 3.64 | 0.001 |
| C | −7.88 | 9.87 | −1.72 | −0.26 | −0.50 | −0.003 |
| D | −4.48 | 3.24 | −2.45 | −0.22 | 3.42 | −0.000 |
| AB + CD | 4.48 | −3.06 | 3.61 | 0.37 | −5.90 | −0.001 |
| AC + BD | −1.23 | 1.10 | 0.64 | 0.15 | −1.16 | −0.001 |
| AD + BC | 2.16 | −1.75 | 0.53 | 0.16 | −1.59 | −0.001 |
| Spectrum | C | V | Cr | Fe | Co | Mo | W | S | Mn | Most Likely Phase |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Rem. | -- | -- | 7.8 | -- | 4.7 | 3.0 | -- | -- | M3C |
| 2 | Rem. | -- | 2.7 | 32.0 | 5.9 | 5.3 | 3.2 | -- | -- | M3C |
| 3 | Rem. | 12.1 | 2.0 | 2.6 | -- | 4.3 | 2.1 | -- | -- | MC |
| 4 | Rem. | -- | -- | 24.8 | -- | -- | -- | 39.0 | 36.2 | MnS |
| 5 | Rem. | -- | -- | 13.8 | 2.6 | 6.4 | 4.2 | -- | -- | M6C |
| 6 | Rem. | 2.8 | -- | 26.1 | 4.2 | -- | -- | -- | -- | M3C |
| 7 | Rem. | -- | -- | 11.7 | 2.6 | 6.6 | 4.1 | -- | -- | M6C |
| 8 | Rem. | 14.4 | -- | 3.3 | -- | 4.8 | 2.1 | -- | -- | MC |
| 9 | Rem. | -- | 2.1 | 14.6 | 2.5 | 9.2 | 5.7 | -- | -- | M6C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez-Antolin, F.; González-Pociño, A. Effect of Heat Treatment on the Microstructure and Mechanical Properties of Vanadis 60 Steel: A Statistical Design Approach. Solids 2025, 6, 46. https://doi.org/10.3390/solids6030046
Alvarez-Antolin F, González-Pociño A. Effect of Heat Treatment on the Microstructure and Mechanical Properties of Vanadis 60 Steel: A Statistical Design Approach. Solids. 2025; 6(3):46. https://doi.org/10.3390/solids6030046
Chicago/Turabian StyleAlvarez-Antolin, Florentino, and Alejandro González-Pociño. 2025. "Effect of Heat Treatment on the Microstructure and Mechanical Properties of Vanadis 60 Steel: A Statistical Design Approach" Solids 6, no. 3: 46. https://doi.org/10.3390/solids6030046
APA StyleAlvarez-Antolin, F., & González-Pociño, A. (2025). Effect of Heat Treatment on the Microstructure and Mechanical Properties of Vanadis 60 Steel: A Statistical Design Approach. Solids, 6(3), 46. https://doi.org/10.3390/solids6030046







