Enhancing the Structural Stability and Electrochemical Performance of δ-MnO2 Cathodes via Fe3+ Doping for Aqueous Zinc-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Characterization
2.3. Electrochemical Measurements
3. Results
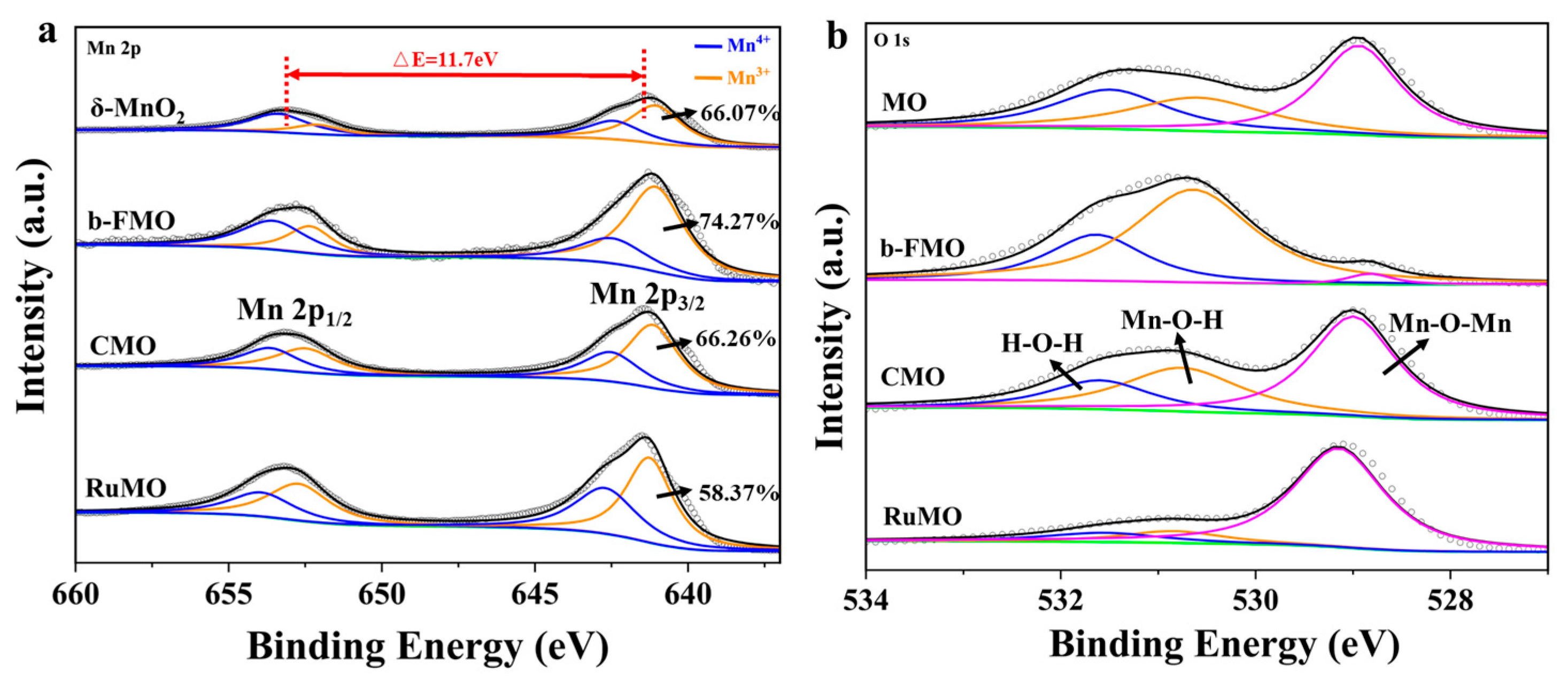
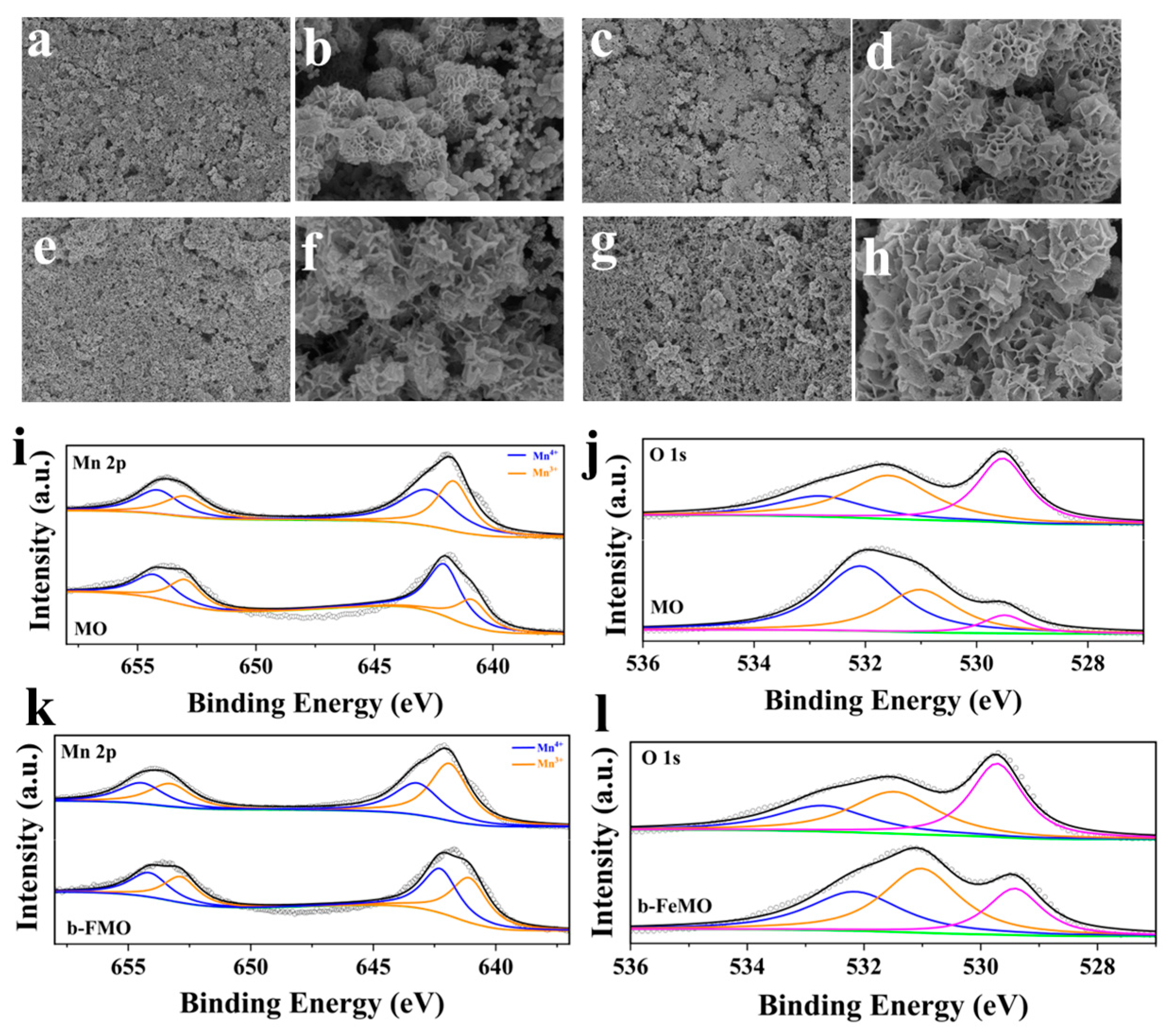
3.1. Structural and Morphological Characterization
3.2. Electrochemical Performance
3.3. Post-Cycling SEM and XPS Analysis:
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, Y.; Chua, R.; Srinivasan, M. Anode Materials for Rechargeable Aqueous Al-Ion Batteries: Progress and Prospects. Chemnanomat 2022, 8, 1–17. [Google Scholar] [CrossRef]
- Guo, A.; Wang, Z.; Chen, L.; Liu, W.; Zhang, K.; Cao, L.; Liang, B.; Luo, D. A Comprehensive Review of the Mechanism and Modification Strategies of V2O5 Cathodes for Aqueous Zinc-Ion Batteries. ACS Nano 2024, 18, 27261–27286. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, J.; Batmunkh, M.; Li, W.; Fu, H.; Wang, L.; Al-Mamun, M.; Qi, D.; Liu, P.; Zhang, S.; et al. Scalable Spray Drying Production of Amorphous V2O5-EGO 2D Hetero-structured Xerogels for High-Rate and High-Capacity Aqueous Zinc Ion Batteries. Small 2022, 18, 2105761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liao, Y.; Ye, M.; Cai, W.; Xiao, M.; Hu, C.; Zhong, B.; Wan, F.; Guo, X. Regeneration of Spent Lithium Manganate Batteries into Al-Doped MnO2 Cathodes toward Aqueous Zn Batteries. ACS Appl. Mater. Interfaces 2023, 15, 59475–59481. [Google Scholar] [CrossRef]
- Qin, L.; Li, S.; Li, L.; Fang, G.; Cheng, H.; Zhu, Q.; Gao, H.; Chen, S. N/Br co-doped C coating Zn2VO4 as excellent electrochemical performance cathode material for aqueous zinc ion batteries. Mater. Lett. 2022, 315, 131949. [Google Scholar] [CrossRef]
- Wang, R.; Yao, M.; Huang, S.; Tian, J.; Niu, Z. An anti-freezing and anti-drying multifunctional gel electrolyte for flexible aqueous zinc-ion batteries. Sci. China Mater. 2022, 65, 2189–2196. [Google Scholar] [CrossRef]
- Ma, D.; Li, F.; Ouyang, K.; Chen, Q.; Zhao, J.; Chen, M.; Yang, M.; Wang, Y.; Chen, J.; Mi, H.; et al. An electrochemically driven hybrid interphase enabling stable versatile zinc metal electrodes for aqueous zinc batteries. Nat. Commun. 2025, 16, 4817. [Google Scholar] [CrossRef]
- Kim, A.; Park, Y.; Choi, J.; Yu, S.-H.; Nam, K.W. A Comprehensive Review of Cathode Materials for Advanced Aqueous Zinc-Ion Batteries. ACS Appl. Energy Mater. 2025, 8, 6806–6828. [Google Scholar] [CrossRef]
- Zhong, W.; Tan, C.; Li, L.; Zhang, S.; Wang, X.; Cheng, H.; Lu, Y. Regulation of aqueous electrolyte interface via electrolyte strategies for uniform zinc deposition. Nano Res. 2024, 17, 8678–8693. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, H.; Zhang, X.; Liu, Y.; Zhang, Y. Kinetics-Driven MnO2 Nanoflowers Supported by Interconnected Porous Hollow Carbon Spheres for Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2023, 15, 14388–14398. [Google Scholar] [CrossRef]
- Minakshi, M.; Aughterson, R.; Sharma, P.; Sunda, A.P.; Ariga, K.; Shrestha, L.K. Micelle-Assisted Electrodeposition of γ-MnO2 on Lead Anodes: Structural and Electrochemical Insights. ChemNanoMat 2025, 1–11. [Google Scholar] [CrossRef]
- Zhang, N.; Cheng, F.; Liu, J.; Wang, L.; Long, X.; Liu, X.; Li, F.; Chen, J. Rechargeable aqueous zinc-manganese dioxide batteries with high energy and power densities. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Liu, X.J.; Liu, X.; Bi, Y.F.; Ke, J. Construction of Lithium-Rich Manganese-Based Cathodes Based on Activated Manganese Dioxide for Enhanced Energy Storage Performances. Energy Fuels 2022, 36, 13238–13245. [Google Scholar] [CrossRef]
- Zhong, S.; Xin, Y.; Mo, L.; He, B.; Zhang, F.; Zhao, C.; Hu, L.; Tian, H. Intercalation and Interface Engineering of Layered MnO2 Cathodes toward High-Performance Aqueous Zinc-Ion Batteries. J. Phys. Chem. C 2025, 129, 6684–6696. [Google Scholar] [CrossRef]
- Khamsanga, S.; Pornprasertsuk, R.; Yonezawa, T.; Mohamad, A.A.; Kheawhom, S. δ-MnO2 nanoflower/graphite cathode for rechargeable aqueous zinc ion batteries. Sci. Rep. 2019, 9, 8441. [Google Scholar] [CrossRef]
- Zhang, B.; Dong, P.; Yuan, S.; Zhang, Y.; Zhang, Y.; Wang, Y. Manganese-Based Oxide Cathode Materials for Aqueous Zinc-Ion Batteries: Materials, Mechanism, Challenges, and Strategies. Chem Bio Eng. 2024, 1, 113–132. [Google Scholar] [CrossRef]
- Zhai, X.; Yu, Y.; Hu, Y. Engineering Aqueous Zn-MnO2 Microbatteries Using a Synergistic Reaction Mechanism. ACS Appl. Energy Mater. 2023, 6, 6171–6182. [Google Scholar] [CrossRef]
- Li, W.; Qin, L.; Liu, Z.; Li, L.; Li, W.; Fang, G. Potassium-Ion-Doped Manganese Oxides and Kaolinite Electrolyte Additives for Aqueous Zinc-Ion Batteries. ACS Appl. Nano Mater. 2024, 7, 9720–9729. [Google Scholar] [CrossRef]
- Kumari, P.; Kundu, R. Zinc-Ion Batteries: Promise and Challenges for Exploring the Post-Lithium Battery Materials. ACS Appl. Energy Mater. 2024, 7, 9634–9669. [Google Scholar] [CrossRef]
- Jiang, S.; Tian, S.; Zhang, S.; Fang, L.; Wang, Z.; Nie, P.; Han, W.; Xue, X.; Zhao, C.; Lu, M.; et al. Iron-Doped Nanorods of MnO2 for Applications in Zinc-Ion Batteries. ACS Appl. Nano Mater. 2024, 7, 27648–27655. [Google Scholar] [CrossRef]
- Ding, X.; Wen, Y.; Qing, C.; Wei, Y.; Wang, P.; Liu, J.; Peng, Z.; Song, Y.; Chen, H.; Rong, Q. Cr-induced enhancement of structural stability in δ-MnO2 for aqueous Zn-ion batteries. J. Alloys Compd. 2024, 986, 174041. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Zhang, Y.; Liu, X.; Yan, D.; Huang, J.; Peng, S. Selection of Cu2+ intercalation from electronegativity perspective: Improving cycle stability and rate performance of δ-MnO2 cathode material for aqueous zinc-ion batteries. Sci. China Mater. 2022, 66, 531–540. [Google Scholar] [CrossRef]
- Zheng, J.; Xia, R.; Yaqoob, N.; Kaghazchi, P.; Elshof, J.E.T.; Huijben, M. Simultaneous Enhancement of Lithium Transfer Kinetics and Structural Stability in Dual-Phase TiO2 Elec trodes by Ruthenium Doping. ACS Appl. Mater. 2024, 16, 8616–8626. [Google Scholar] [CrossRef]
- Hashemzadeh, F.; Kashani Motlagh, M.M.; Maghsoudipour, A. A Comparative Study of Hydrothermal and Sol-Gel Methods in the Synthesis of MnO2 Nanostructures. J. Sol-Gel Sci. Technol. 2009, 51, 169–174. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X.; Zhao, H.; Xu, L.; Xia, J.; Luo, M.; Yang, Y.; Du, Y. Superior-Performance Aqueous Zinc Ion Battery Based on Structural Transformation of MnO2 by Rare Earth Doping. J. Phys. Chem. C 2019, 123, 22735–22741. [Google Scholar] [CrossRef]
- Gu, H.; Yang, X.; Chen, S.; Zhang, W.; Yang, H.Y.; Li, Z. Oxygen Vacancies Boosted Proton Intercalation Kinetics for Aqueous Aluminum–Manganese Batteries. Nano Lett. 2023, 23, 11842–11849. [Google Scholar] [CrossRef]
- Wang, R.; Chen, W.; Zhang, C.; Zhao, R.; Wang, X. Electrochemically Active Mn2+ Enabling High-Performance Aqueous Zinc Ion Batteries. Energy Fuels 2024, 38, 13436–13443. [Google Scholar] [CrossRef]
- Fang, Y.; Xie, X.; Zhang, B.; Chai, Y.; Lu, B.; Liu, M.; Zhou, J.; Liang, S. Regulating Zinc Deposition Behaviors by the Conditioner of PAN Separator for Zinc-Ion Batteries. Adv. Funct. Mater. 2021, 32, 2109671. [Google Scholar] [CrossRef]
- Kamenskii, M.A.; Popov, A.Y.; Eliseeva, S.N.; Kondratiev, V.V. The Effect of the Synthesis Method of the Layered Manganese Dioxide on the Properties of Cathode Materials for Aqueous Zinc-Ion Batteries. Russ. J. Electrochem. 2023, 59, 1092–1101. [Google Scholar] [CrossRef]
- Feng, Y.P.; Zhang, Y.F.; Zhang, Y.C. In situ growth of the δ-manganese dioxide on carbon cloth by different concen-trations of reactants for eco-friendly battery applications. J. Solid State Electrochem. 2023, 27, 2691–2700. [Google Scholar] [CrossRef]
- Ni, Z.; Liang, X.; Zhao, L.; Zhao, H.; Ge, B.; Li, W. Tin doping manganese dioxide cathode materials with the improved stability for aqueous zinc-ion batteries. Mater. Chem. Phys. 2022, 287, 126238. [Google Scholar] [CrossRef]
- Xu, J.; Hu, X.; Alam, A.; Muhammad, G.; Lv, Y.; Wang, M.; Zhu, C.; Xiong, W. Al-doped α-MnO2 coated by lignin for high-performance rechargeable aqueous zinc-ion batteries. RSC Adv. 2021, 11, 35280–35286. [Google Scholar] [CrossRef] [PubMed]




| Material | MO | b-FeMO | CuMO | RuMO |
|---|---|---|---|---|
| Initial discharge specific capacity (mAh/g) | 85.15 | 116.24 | 146.53 | 210.57 |
| Discharge specific capacity after 200 cycles (mAh/g) | 16.95 | 48.47 | 51.67 | 44.58 |
| Capacity retention rate (%) | 19.9 | 41.70 | 35.23 | 21.17 |
| Average Coulombic efficiency (%) | 99.72 | 100 | 99.59 | 99.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Yu, H.; Zou, C.; Wu, Y.; Chen, Z. Enhancing the Structural Stability and Electrochemical Performance of δ-MnO2 Cathodes via Fe3+ Doping for Aqueous Zinc-Ion Batteries. Solids 2025, 6, 45. https://doi.org/10.3390/solids6030045
Wang P, Yu H, Zou C, Wu Y, Chen Z. Enhancing the Structural Stability and Electrochemical Performance of δ-MnO2 Cathodes via Fe3+ Doping for Aqueous Zinc-Ion Batteries. Solids. 2025; 6(3):45. https://doi.org/10.3390/solids6030045
Chicago/Turabian StyleWang, Pengfei, Haiyang Yu, Chengyan Zou, Yuxue Wu, and Zhengfei Chen. 2025. "Enhancing the Structural Stability and Electrochemical Performance of δ-MnO2 Cathodes via Fe3+ Doping for Aqueous Zinc-Ion Batteries" Solids 6, no. 3: 45. https://doi.org/10.3390/solids6030045
APA StyleWang, P., Yu, H., Zou, C., Wu, Y., & Chen, Z. (2025). Enhancing the Structural Stability and Electrochemical Performance of δ-MnO2 Cathodes via Fe3+ Doping for Aqueous Zinc-Ion Batteries. Solids, 6(3), 45. https://doi.org/10.3390/solids6030045





