Direct Synthesis of CuP2 and Cu3P and Their Performance as Electrocatalysts for Hydrogen Evolution, Oxygen Evolution, and Oxygen Reduction Reactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of CuP2
2.3. Synthesis of Cu3P
(Reflux at 320 °C, 48 h)
(Reflux at 250 °C, 48 h)
2.4. Characterization
2.5. Electrochemical Performance Study
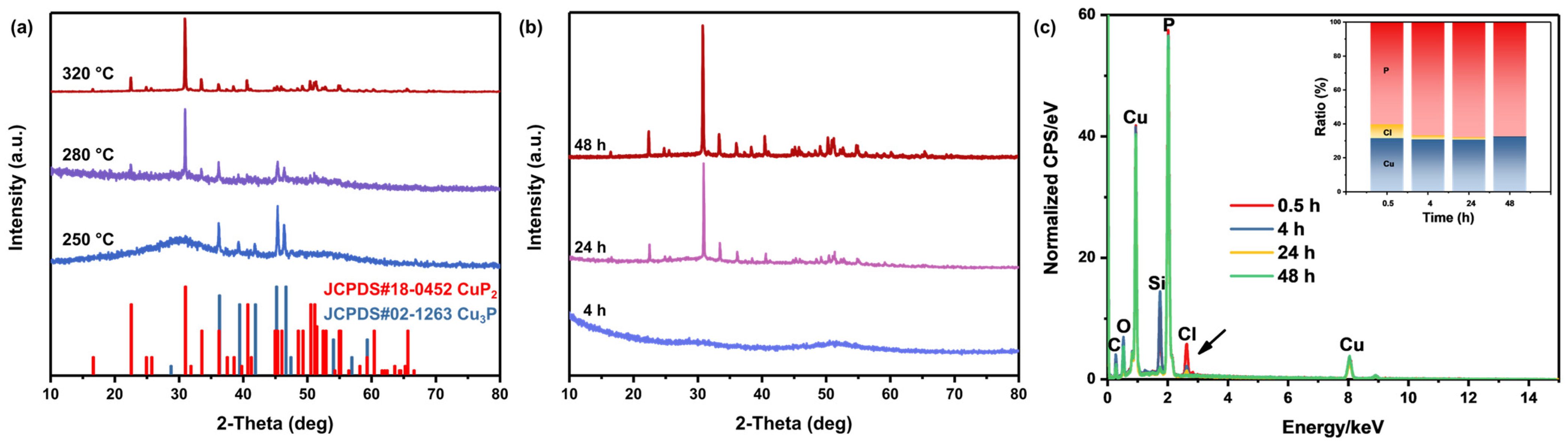
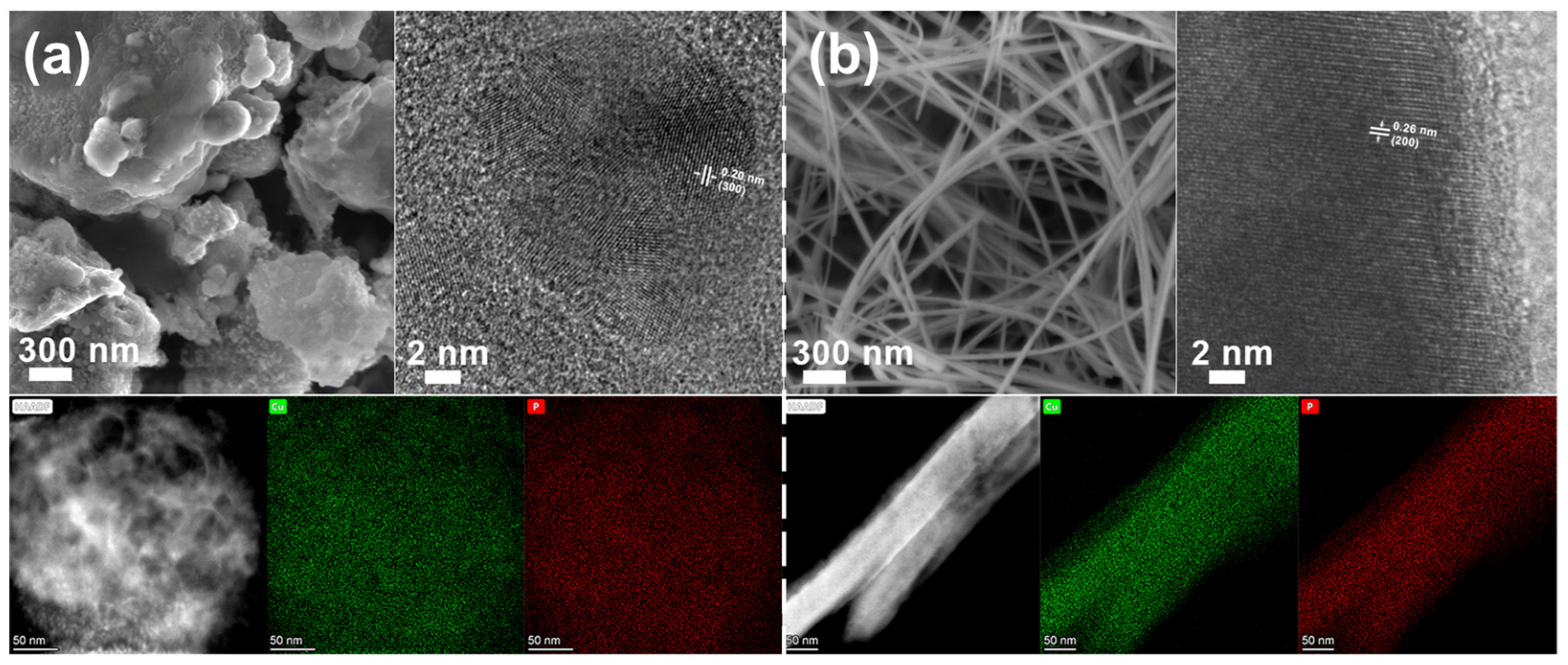
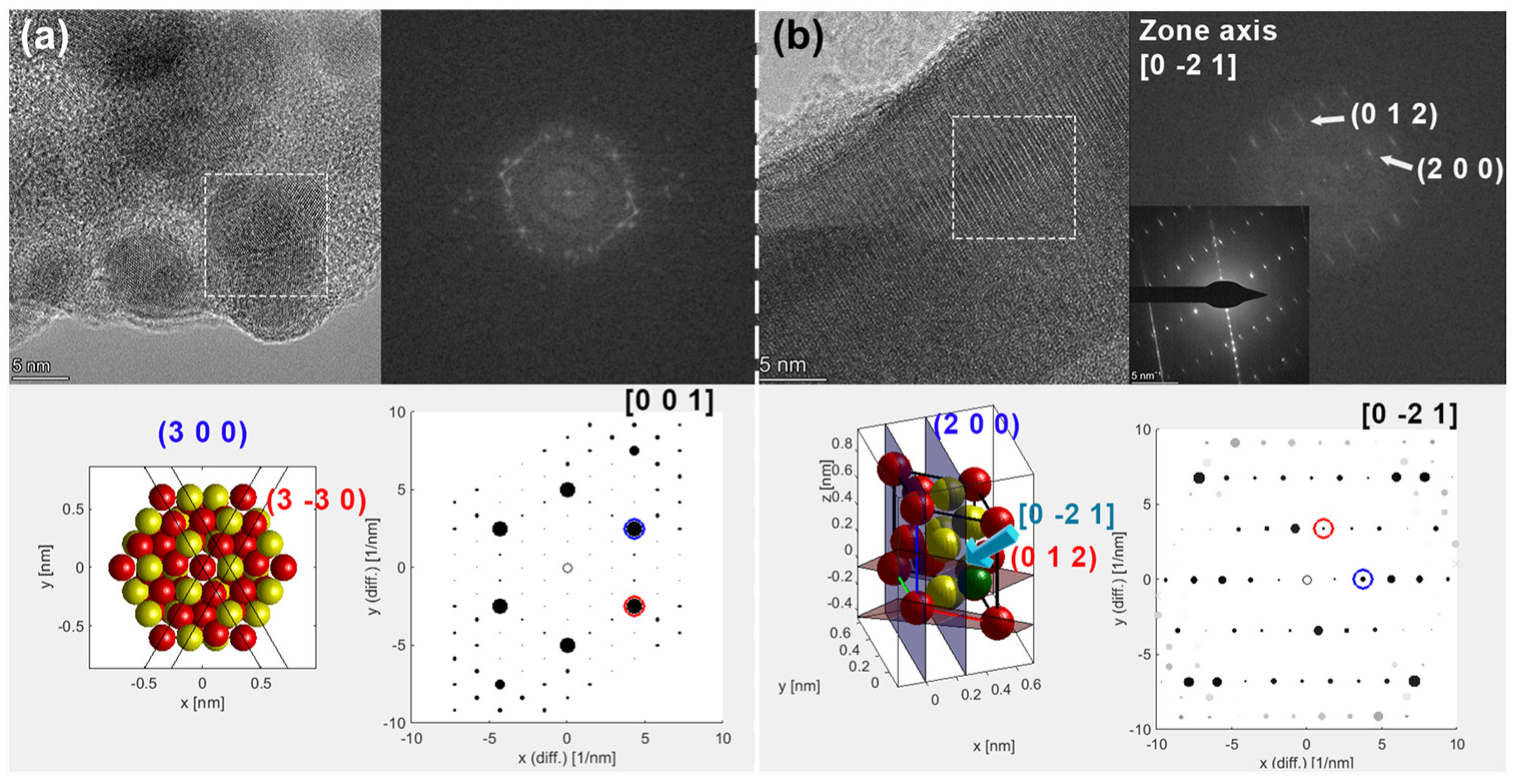
3. Results
3.1. Material Synthesis and Characterization
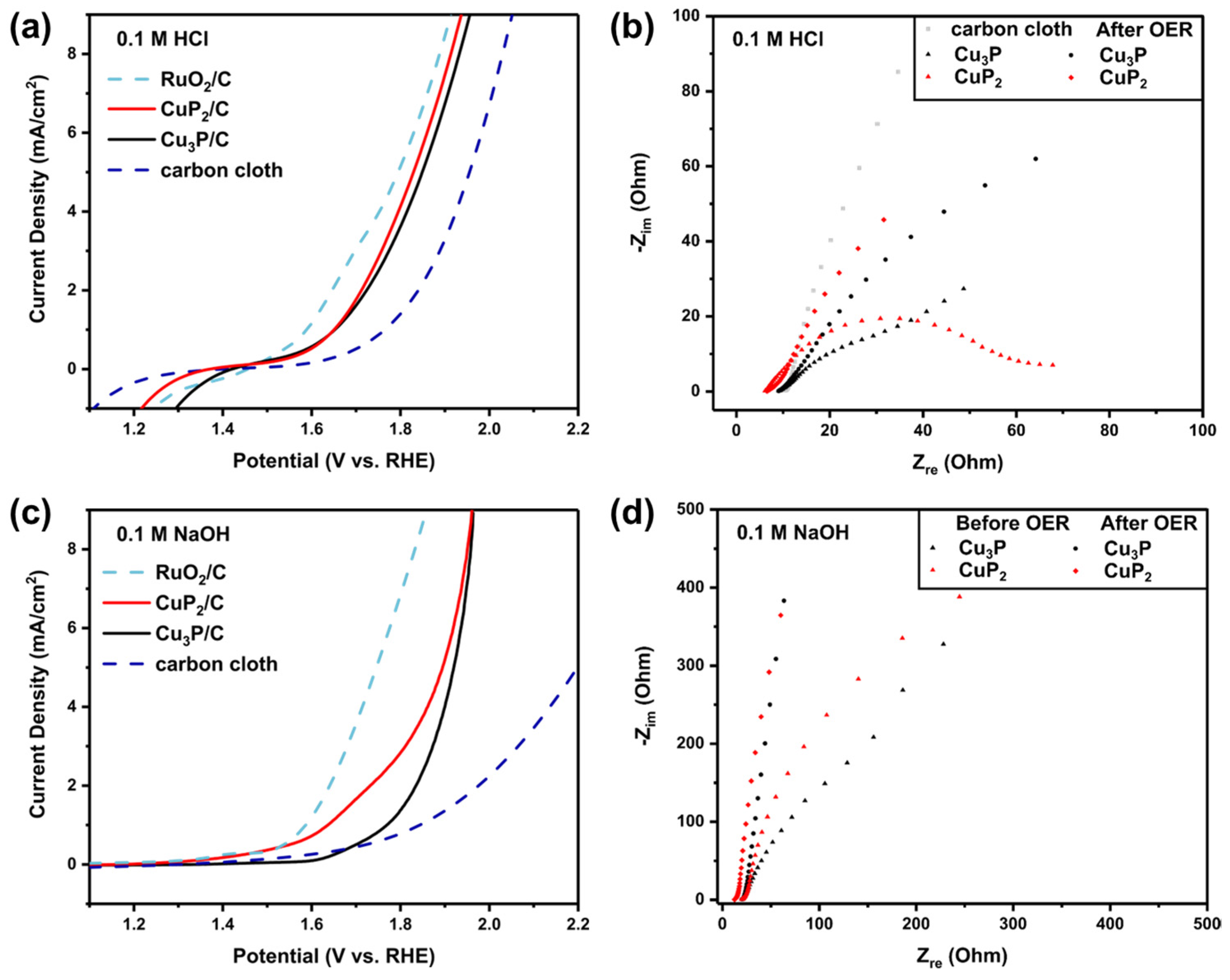
3.2. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of Electrocatalysts for Oxygen- and Hydrogen-Involving Energy Conversion Reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Strmcnik, D.; Lopes, P.P.; Markovic, N.M. Energy and Fuels from Electrochemical Interfaces. Nat. Mater. 2017, 16, 57–69. [Google Scholar] [CrossRef]
- Chen, C.; Kang, Y.; Huo, Z.; Zhu, Z.; Huang, W.; Xin, H.L.; Snyder, J.D.; Li, D.; Herron, J.A.; Mavrikakis, M.; et al. Highly Crystalline Multimetallic Nanoframes with Three-Dimensional Electrocatalytic Surfaces. Science 2014, 343, 1339–1343. [Google Scholar] [CrossRef]
- Liu, J.; Jiao, M.; Lu, L.; Barkholtz, H.M.; Li, Y.; Wang, Y.; Jiang, L.; Wu, Z.; Liu, D.; Zhuang, L.; et al. High Performance Platinum Single Atom Electrocatalyst for Oxygen Reduction Reaction. Nat. Commun. 2017, 8, 15938. [Google Scholar] [CrossRef]
- Zeng, R.; Yang, Y.; Feng, X.; Li, H.; Gibbs, L.M.; DiSalvo, F.J.; Abruña, H.D. Nonprecious Transition Metal Nitrides as Efficient Oxygen Reduction Electrocatalysts for Alkaline Fuel Cells. Sci. Adv. 2022, 8, eabj1584. [Google Scholar] [CrossRef]
- Ren, X.; Wang, H.; Chen, J.; Xu, W.; He, Q.; Wang, H.; Zhan, F.; Chen, S.; Chen, L. Emerging 2D Copper-Based Materials for Energy Storage and Conversion: A Review and Perspective. Small 2023, 19, 2204121. [Google Scholar] [CrossRef]
- Jiang, P.; Liu, Q.; Liang, Y.; Tian, J.; Asiri, A.M.; Sun, X. A Cost-Effective 3D Hydrogen Evolution Cathode with High Catalytic Activity: FeP Nanowire Array as the Active Phase. Angew. Chem. Int. Ed. 2014, 53, 12855–12859. [Google Scholar] [CrossRef]
- Lu, X.F.; Yu, L.; Lou, X.W. Highly Crystalline Ni-Doped FeP/Carbon Hollow Nanorods as All-pH Efficient and Durable Hydrogen Evolving Electrocatalysts. Sci. Adv. 2019, 5, eaav6009. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Li, Y.; Wang, H.; Liang, Y.; Wu, J.Z.; Zhou, J.; Wang, J.; Regier, T.; Wei, F.; Dai, H. An Advanced Ni–Fe Layered Double Hydroxide Electrocatalyst for Water Oxidation. J. Am. Chem. Soc. 2013, 135, 8452–8455. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Onoda, A.; Okuoka, S.; Kitano, T.; Matsumoto, K.; Sakata, T.; Yasuda, H.; Hayashi, T. Nonprecious-Metal Fe/N/C Catalysts Prepared from π-Expanded Fe Salen Precursors toward an Efficient Oxygen Reduction Reaction. ChemCatChem 2018, 10, 743–750. [Google Scholar] [CrossRef]
- Hu, X.; Chen, S.; Chen, L.; Tian, Y.; Yao, S.; Lu, Z.; Zhang, X.; Zhou, Z. What Is the Real Origin of the Activity of Fe–N–C Electrocatalysts in the O2 Reduction Reaction? Critical Roles of Coordinating Pyrrolic N and Axially Adsorbing Species. J. Am. Chem. Soc. 2022, 144, 18144–18152. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, X.; Li, Q.; Zhao, Y.; Pang, H. Copper-Based Materials as Highly Active Electrocatalysts for the Oxygen Evolution Reaction. Mater. Today Chem. 2019, 11, 169–196. [Google Scholar] [CrossRef]
- Cracknell, J.A.; Vincent, K.A.; Armstrong, F.A. Enzymes as Working or Inspirational Electrocatalysts for Fuel Cells and Electrolysis. Chem. Rev. 2008, 108, 2439–2461. [Google Scholar] [CrossRef]
- Thorseth, M.A.; Tornow, C.E.; Tse, E.C.M.; Gewirth, A.A. Cu Complexes That Catalyze the Oxygen Reduction Reaction. Coord. Chem. Rev. 2013, 257, 130–139. [Google Scholar] [CrossRef]
- Parra-Puerto, A.; Ng, K.L.; Fahy, K.; Goode, A.E.; Ryan, M.P.; Kucernak, A. Supported Transition Metal Phosphides: Activity Survey for HER, ORR, OER, and Corrosion Resistance in Acid and Alkaline Electrolytes. ACS Catal. 2019, 9, 11515–11529. [Google Scholar] [CrossRef]
- Pu, Z.; Liu, T.; Amiinu, I.S.; Cheng, R.; Wang, P.; Zhang, C.; Ji, P.; Hu, W.; Liu, J.; Mu, S. Transition-Metal Phosphides: Activity Origin, Energy-Related Electrocatalysis Applications, and Synthetic Strategies. Adv. Funct. Mater. 2020, 30, 2004009. [Google Scholar] [CrossRef]
- Harper, A.F.; Evans, M.L.; Morris, A.J. Computational Investigation of Copper Phosphides as Conversion Anodes for Lithium-Ion Batteries. Chem. Mater. 2020, 32, 6629–6639. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Chen, H.; Liu, H.; Yin, L.; Zhang, Q.; Yu, S.; Liu, P.; Zhong, G.; Lu, C.-Z.; Yang, Y. Quantifying the Reaction Mechanisms of a High-Capacity CuP2/C Composite Anode for Potassium Ion Batteries. J. Mater. Chem. A 2021, 9, 6274–6283. [Google Scholar] [CrossRef]
- Crovetto, A.; Kojda, D.; Yi, F.; Heinselman, K.N.; LaVan, D.A.; Habicht, K.; Unold, T.; Zakutayev, A. Crystallize It before It Diffuses: Kinetic Stabilization of Thin-Film Phosphorus-Rich Semiconductor CuP2. J. Am. Chem. Soc. 2022, 144, 13334–13343. [Google Scholar] [CrossRef] [PubMed]
- Rachkov, A.G.; Schimpf, A.M. Colloidal Synthesis of Tunable Copper Phosphide Nanocrystals. Chem. Mater. 2021, 33, 1394–1406. [Google Scholar] [CrossRef]
- Kim, S.-O.; Manthiram, A. The Facile Synthesis and Enhanced Sodium-Storage Performance of a Chemically Bonded CuP2/C Hybrid Anode. Chem. Commun. 2016, 52, 4337–4340. [Google Scholar] [CrossRef]
- Muruganantham, R.; Chiang, P.-C.; Liu, W.-R. Copper-Diphosphide Composites: A Key Factor Evaluation and Capacity Enhancement Route for High-Energy Lithium-Ion Storage. ACS Appl. Energy Mater. 2018, 1, 3674–3683. [Google Scholar] [CrossRef]
- Li, G.-A.; Wang, C.-Y.; Chang, W.-C.; Tuan, H.-Y. Phosphorus-Rich Copper Phosphide Nanowires for Field-Effect Transistors and Lithium-Ion Batteries. ACS Nano 2016, 10, 8632–8644. [Google Scholar] [CrossRef] [PubMed]
- Barry, B.M. Synthesis of Transition-Metal Polyphosphides. Ph.D. Thesis, University of Iowa, Iowa City, IA, USA, 2010. [Google Scholar] [CrossRef]
- Barry, B.M.; Gillan, E.G. A General and Flexible Synthesis of Transition-Metal Polyphosphides via PCl3 Elimination. Chem. Mater. 2009, 21, 4454–4461. [Google Scholar] [CrossRef]
- Coleman, N., Jr.; Lovander, M.D.; Leddy, J.; Gillan, E.G. Phosphorus-Rich Metal Phosphides: Direct and Tin Flux-Assisted Synthesis and Evaluation as Hydrogen Evolution Electrocatalysts. Inorg. Chem. 2019, 58, 5013–5024. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.; Doert, T.; Hunger, J.; Kaiser, M.; Pallmann, J.; Reinhold, R.; Yogendra, S.; Giebeler, L.; Sichelschmidt, J.; Schnelle, W.; et al. Low-Temperature Tailoring of Copper-Deficient Cu3–xP—Electric Properties, Phase Transitions, and Performance in Lithium-Ion Batteries. Chem. Mater. 2018, 30, 7111–7123. [Google Scholar] [CrossRef]
- Downes, C.A.; Libretto, N.J.; Harman-Ware, A.E.; Happs, R.M.; Ruddy, D.A.; Baddour, F.G.; Ferrell III, J.R.; Habas, S.E.; Schaidle, J.A. Electrocatalytic CO2 Reduction over Cu3P Nanoparticles Generated via a Molecular Precursor Route. ACS Appl. Energy Mater. 2020, 3, 10435–10446. [Google Scholar] [CrossRef]
- Barry, B.M.; Gillan, E.G. Low-Temperature Solvothermal Synthesis of Phosphorus-Rich Transition-Metal Phosphides. Chem. Mater. 2008, 20, 2618–2620. [Google Scholar] [CrossRef]
- Penn, R.L.; Banfield, J.F. Morphology Development and Crystal Growth in Nanocrystalline Aggregates under Hydrothermal Conditions: Insights from Titania. Geochim. Cosmochim. Acta 1999, 63, 1549–1557. [Google Scholar] [CrossRef]
- Dolgin, B.P. Kinetics of the Formation of an Amorphous Layer During a Solid State Reaction. Ph.D. Thesis, California Institute of Technology, Pasadena, CA, USA, 1985. [Google Scholar] [CrossRef]
- Klinger, M.; Jäger, A. Crystallographic Tool Box (CrysTBox): Automated Tools for Transmission Electron Microscopists and Crystallographers. J. Appl. Crystallogr. 2015, 48, 2012–2018. [Google Scholar] [CrossRef]
- Mp-14012: Cu3P (Trigonal, P-3c1, 165). Available online: https://next-gen.materialsproject.org/materials/mp-14012?chemsys=Cu-P (accessed on 4 September 2023).
- mp-927: CuP2 (Monoclinic, P2_1/c, 14). Materials Project. Available online: https://next-gen.materialsproject.org/materials/mp-927?_sort_fields=energy_above_hull&chemsys=Cu-P (accessed on 4 September 2023).
- Yi, Y.; Weinberg, G.; Prenzel, M.; Greiner, M.; Heumann, S.; Becker, S.; Schlögl, R. Electrochemical Corrosion of a Glassy Carbon Electrode. Catal. Today 2017, 295, 32–40. [Google Scholar] [CrossRef]
- Higgins, D.; Wette, M.; Gibbons, B.M.; Siahrostami, S.; Hahn, C.; Escudero-Escribano, M.; García-Melchor, M.; Ulissi, Z.; Davis, R.C.; Mehta, A.; et al. Copper Silver Thin Films with Metastable Miscibility for Oxygen Reduction Electrocatalysis in Alkaline Electrolytes. ACS Appl. Energy Mater. 2018, 1, 1990–1999. [Google Scholar] [CrossRef]
- Choi, M.; Bong, S.; Kim, J.W.; Lee, J. Formation of 1-Butanol from CO2 without *CO Dimerization on a Phosphorus-Rich Copper Cathode. ACS Energy Lett. 2021, 6, 2090–2095. [Google Scholar] [CrossRef]
- Kim, S.-O.; Manthiram, A. Phosphorus-Rich CuP2 Embedded in Carbon Matrix as a High-Performance Anode for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 16221–16227. [Google Scholar] [CrossRef] [PubMed]




| Chemical Composition | Cu3P * | CuP2 |
|---|---|---|
| Space Group | c1 | P21/c |
| Unit cell | 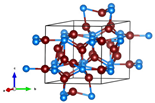 |  |
| a b c | a = 6.91 Å b = 6.91 Å c = 7.13 Å | a = 5.77 Å b =4.78 Å c = 7.50 Å |
| α β γ | α = 90.00° β = 90.00° γ = 120.00° | α = 90° β = 112.63° γ = 90.00° |
| # JCPDS | 02-1263 | 18-0452 |
| ICSD code | 26775 | 35282 |
| # Materials Project | mp-14012 | mp-927 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Huang, X.; Lachgar, A. Direct Synthesis of CuP2 and Cu3P and Their Performance as Electrocatalysts for Hydrogen Evolution, Oxygen Evolution, and Oxygen Reduction Reactions. Solids 2024, 5, 140-150. https://doi.org/10.3390/solids5010010
Ma X, Huang X, Lachgar A. Direct Synthesis of CuP2 and Cu3P and Their Performance as Electrocatalysts for Hydrogen Evolution, Oxygen Evolution, and Oxygen Reduction Reactions. Solids. 2024; 5(1):140-150. https://doi.org/10.3390/solids5010010
Chicago/Turabian StyleMa, Xiao, Xueni Huang, and Abdessadek Lachgar. 2024. "Direct Synthesis of CuP2 and Cu3P and Their Performance as Electrocatalysts for Hydrogen Evolution, Oxygen Evolution, and Oxygen Reduction Reactions" Solids 5, no. 1: 140-150. https://doi.org/10.3390/solids5010010
APA StyleMa, X., Huang, X., & Lachgar, A. (2024). Direct Synthesis of CuP2 and Cu3P and Their Performance as Electrocatalysts for Hydrogen Evolution, Oxygen Evolution, and Oxygen Reduction Reactions. Solids, 5(1), 140-150. https://doi.org/10.3390/solids5010010







