Sustainable Approaches to Incorporate Plant-Based Biomaterials in Power Generation
Abstract
1. Introduction

2. Materials and Methods
3. Energy Generation from Mechanical Movement
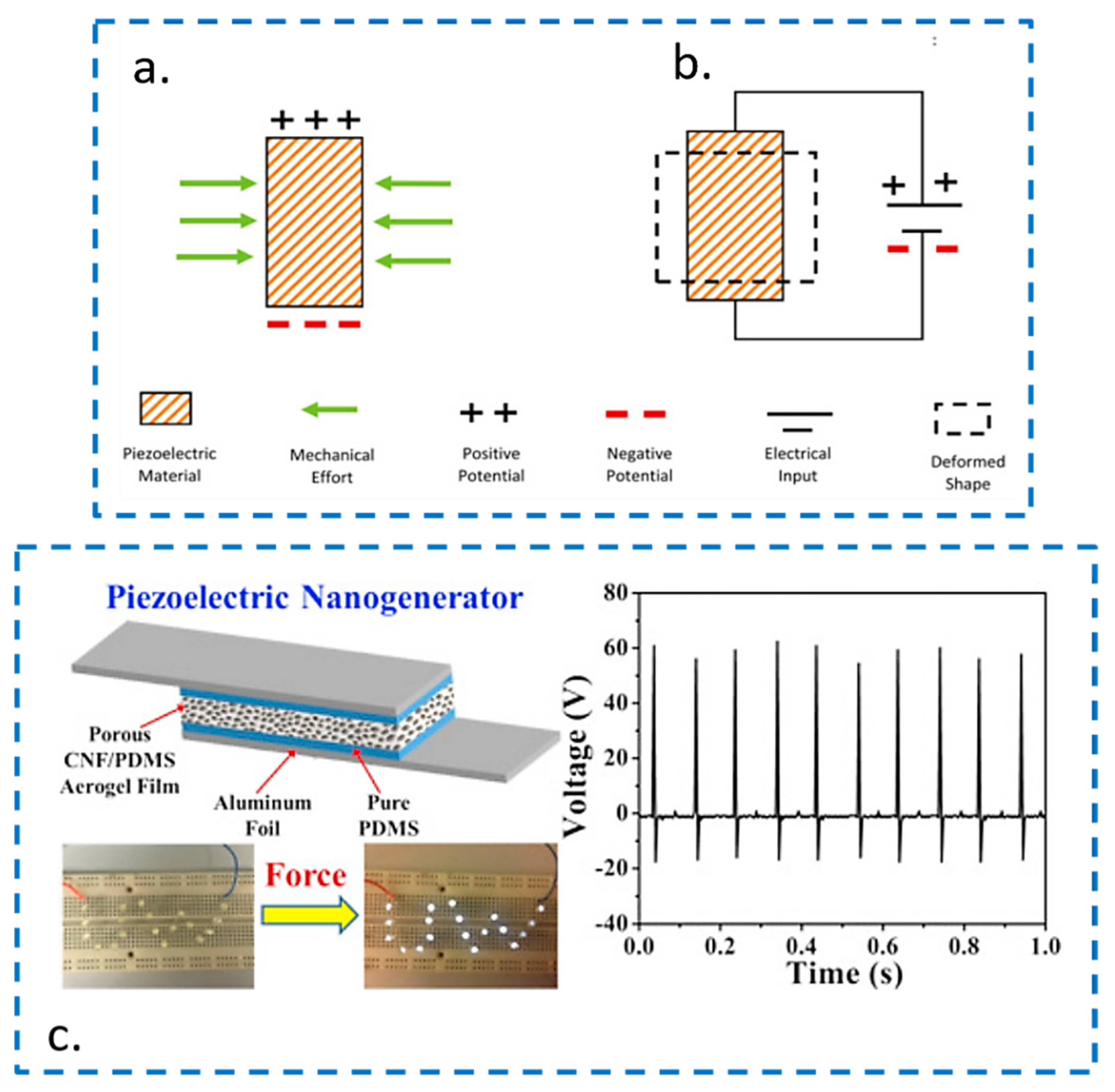
3.1. Piezoelectric Generators
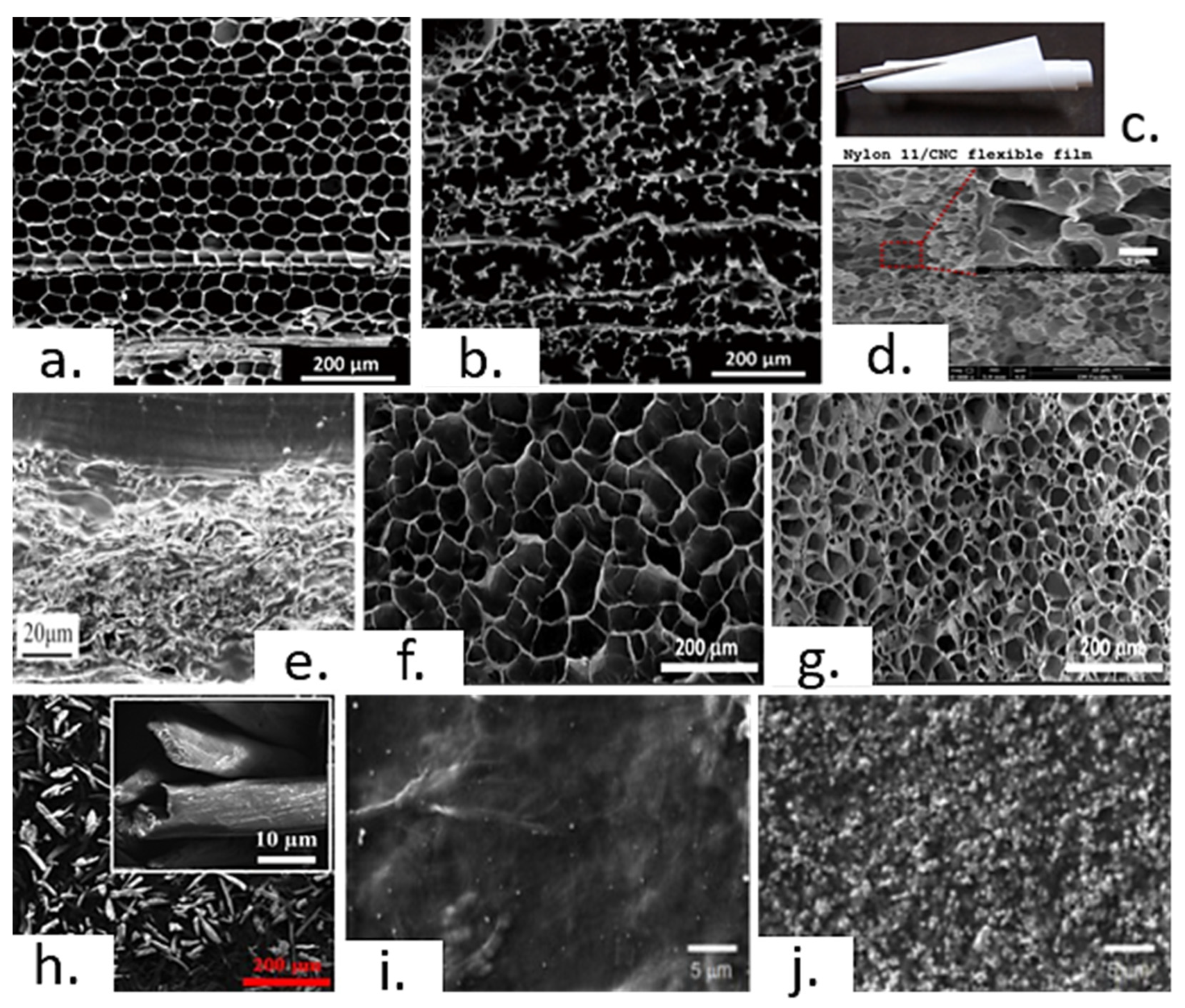
3.2. Triboelectric Nanogenerators

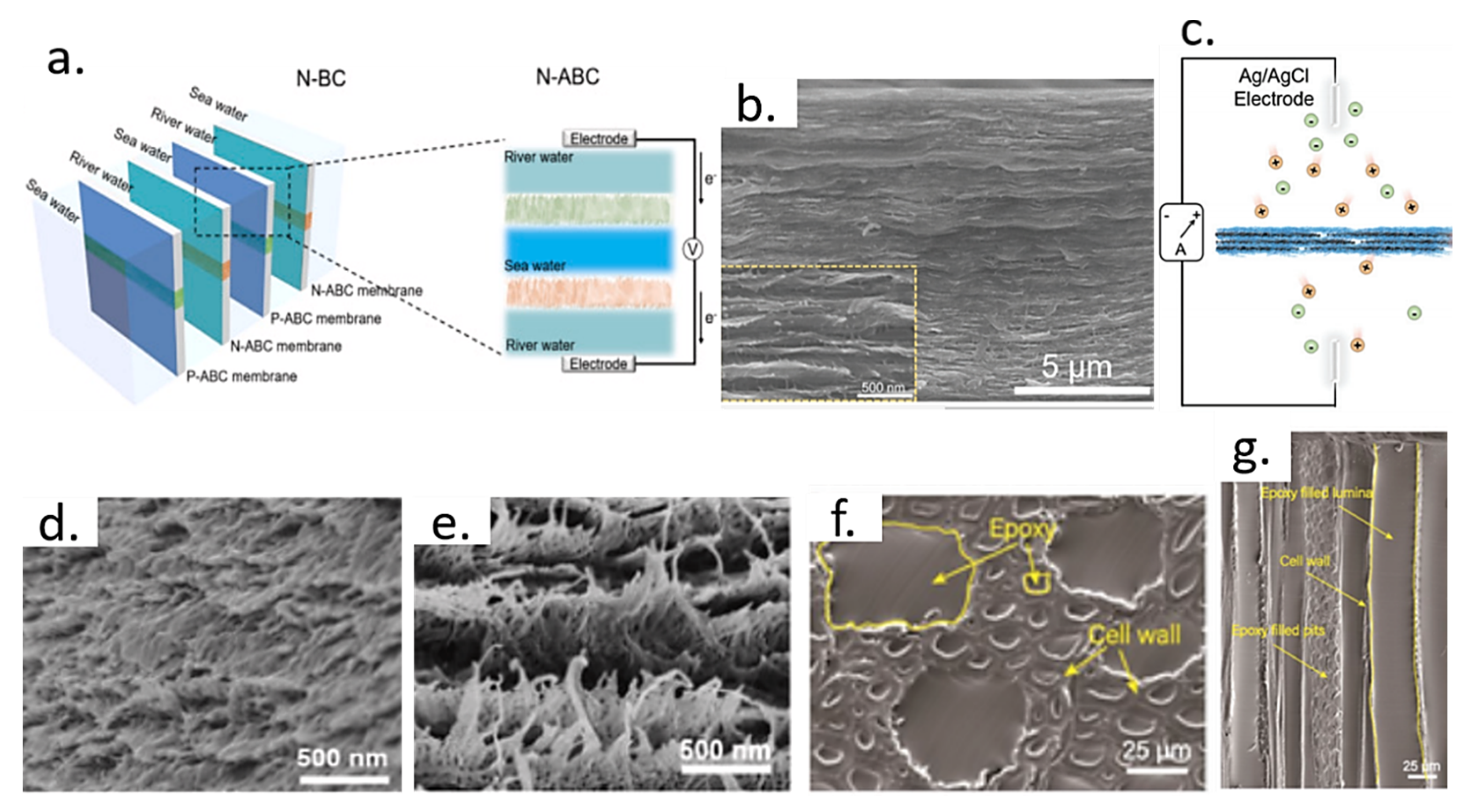
4. Osmotic Energy Harvesting with Cellulose
5. Thermoelectric Energy Harvesting with Cellulose
6. Current Limitations and Opportunities
7. Conclusions and Remarks
Funding
Conflicts of Interest
References
- Booth, M.S. Not carbon neutral: Assessing the net emissions impact of residues burned for bioenergy. Environ. Res. Lett. 2018, 13, 035001. [Google Scholar] [CrossRef]
- Bird, D.N.; Pena, N.; Frieden, D.; Zanchi, G. Zero, one, or in between: Evaluation of alternative national and entity-level accounting for bioenergy. GCB Bioenergy 2012, 4, 576–587. [Google Scholar] [CrossRef]
- Haberl, H.; Sprinz, D.; Bonazountas, M.; Cocco, P.; Desaubies, Y.; Henze, M.; Hertel, O.; Johnson, R.K.; Kastrup, U.; Laconte, P.; et al. Correcting a fundamental error in greenhouse gas accounting related to bioenergy. Energy Policy 2012, 45, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Haberl, H.; Erb, K.H.; Krausmann, F.; Gaube, V.; Bondeau, A.; Plutzar, C.; Gingrich, S.; Lucht, W.; Fischer-Kowalski, M. Quantifying and mapping the human appropriation of net primary production in earth’s terrestrial ecosystems. Proc. Natl. Acad. Sci. USA 2007, 104, 12942–12947. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Huang, X.; Thompson, J.R. Tackle pollution from solar panels. Nature 2014, 509, 563. [Google Scholar] [CrossRef]
- Lourdes Ballinas-Casarrubias, A.C.-D.; Gutierrez-Méndez, N.; Ramos-Sánchez, V.H.; Flores, D.C.; Manjarrez-Nevárez, L.; Zaragoza-Galán, G.; González-Sanchez, G. Biopolymers from Waste Biomass—Extraction, Modification and Ulterior Uses. In Recent Advances in Biopolymers; Perveen, F.K., Ed.; IntechOpen: London, UK, 2015. [Google Scholar]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Dandegaonkar, G.; Ahmed, A.; Sun, L.; Adak, B.; Mukhopadhyay, S. Cellulose based flexible and wearable sensors for health monitoring. Mater. Adv. 2022, 3, 3766–3783. [Google Scholar] [CrossRef]
- Yang, J.; Lu, X.; Liu, X.; Xu, J.; Zhou, Q.; Zhang, S. Rapid and productive extraction of high purity cellulose material via selective depolymerization of the lignin-carbohydrate complex at mild conditions. Green Chem. 2017, 19, 2234–2243. [Google Scholar] [CrossRef]
- Abdul Sisak, M.A.; Daik, R.; Ramli, S. Characterization of cellulose extracted from oil palm empty fruit bunch. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2015; Volume 1678, p. 050016. [Google Scholar]
- Nazir, M.S.; Wahjoedi, B.A.; Yussof, A.W.; Abdullah, M.A. Green extraction and characterization of cellulose fibers from Oil Palm Empty Fruit Bunch. In Proceedings of the 2nd International Conference on Process Engineering and Advance Material (ICPEAM), A Conference of ESTCON, Kuala Lump, Malaysia, 12 June 2012. [Google Scholar]
- Vazquez, A.; Foresti, M.L.; Cerrutti, P.; Galvagno, M. Bacterial Cellulose from Simple and Low Cost Production Media by Gluconacetobacter xylinus. J. Polym. Environ. 2013, 21, 545–554. [Google Scholar] [CrossRef]
- Revin, V.; Liyaskina, E.; Nazarkina, M.; Bogatyreva, A.; Shchankin, M. Cost-effective production of bacterial cellulose using acidic food industry by-products. Braz. J. Microbiol. 2018, 49, 151–159. [Google Scholar] [CrossRef]
- Abol-Fotouh, D.; Hassan, M.A.; Shokry, H.; Roig, A.; Azab, M.S.; Kashyout, A.E.H.B. Bacterial nanocellulose from agro-industrial wastes: Low-cost and enhanced production by Komagataeibacter saccharivorans MD1. Sci. Rep. 2020, 10, 3491. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nano-composites—A review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Himmel, M.E.; Ding, S.-Y. Visualizing chemical functionality in plant cell walls. Biotechnol. Biofuels 2017, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Chupka, É.I.; Rykova, T.M. Electrical properties of lignin. Chem. Nat. Compd. 1983, 19, 78–80. [Google Scholar] [CrossRef]
- Østby, H.; Hansen, L.D.; Horn, S.J.; Eijsink, V.G.; Várnai, A. Enzymatic processing of lignocellulosic biomass: Principles, recent advances and perspectives. J. Ind. Microbiol. Biotechnol. 2020, 47, 623–657. [Google Scholar] [CrossRef]
- Song, Y.; Shi, Z.; Hu, G.-H.; Xiong, C.; Isogai, A.; Yang, Q. Recent advances in cellulose-based piezoelectric and triboelectric nanogenerators for energy harvesting: A review. J. Mater. Chem. A 2021, 9, 1910–1937. [Google Scholar] [CrossRef]
- Dufresne, A.; Belgacem, M.N. Cellulose-reinforced composites: From micro-to nanoscale. Polímeros 2013, 23, 277–286. [Google Scholar] [CrossRef]
- Jawaid, M.; Abdul Khalil, H.P.S. Cellulosic/synthetic fibre reinforced polymer hybrid composites: A review. Carbohydr. Polym. 2011, 86, 1–18. [Google Scholar] [CrossRef]
- Silva, R.R.; Raymundo-Pereira, P.A.; Campos, A.M.; Wilson, D.; Otoni, C.G.; Barud, H.S.; Costa, C.A.R.; Domeneguetti, R.R.; Balogh, D.T.; Ribeiro, S.J.L.; et al. Microbial nanocellulose adherent to human skin used in electrochemical sensors to detect metal ions and biomarkers in sweat. Talanta 2020, 218, 121153. [Google Scholar] [CrossRef]
- Brakat, A.; Zhu, H. Nanocellulose-Graphene Hybrids: Advanced Functional Materials as Multifunctional Sensing Platform. Nano-Micro Lett. 2021, 13, 94. [Google Scholar] [CrossRef]
- Robotti, F.; Sterner, I.; Bottan, S.; Rodríguez, J.M.M.; Pellegrini, G.; Schmidt, T.; Falk, V.; Poulikakos, D.; Ferrari, A.; Starck, C. Microengineered biosynthesized cellulose as anti-fibrotic in vivo protection for cardiac implantable electronic devices. Biomaterials 2020, 229, 119583. [Google Scholar] [CrossRef] [PubMed]
- Petersen, N.; Gatenholm, P. Bacterial cellulose-based materials and medical devices: Current state and perspectives. Appl. Microbiol. Biotechnol. 2011, 91, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Chami Khazraji, A.; Robert, S. Interaction Effects between Cellulose and Water in Nanocrystalline and Amorphous Regions: A Novel Approach Using Molecular Modeling. J. Nanomater. 2013, 2013, 409676. [Google Scholar] [CrossRef]
- Bergander, A.; Brändström, J.; Daniel, G.; Sahnen, L. Fibril angle variability in earlywood of Norway spruce using soft rot cavities and polarization confocal microscopy. J. Wood Sci. 2002, 48, 255–263. [Google Scholar] [CrossRef]
- Tanaka, F.; Koshijima, T.; Okamura, K. Characterization of cellulose in compression and opposite woods of a Pinus densiflora tree grown under the influence of strong wind. Wood Sci. Technol. 1981, 15, 265–273. [Google Scholar] [CrossRef]
- Jakob, H.F.; Fengel, D.; Tschegg, S.E.; Fratzl, P. The Elementary Cellulose Fibril in Picea abies: Comparison of Transmission Electron Microscopy, Small-Angle X-ray Scattering, and Wide-Angle X-ray Scattering Results. Macromolecules 1995, 28, 8782–8787. [Google Scholar] [CrossRef]
- Andersson, S.; Serimaa, R.; Paakkari, T.; Saranpää, P.; Pesonen, E. Crystallinity of wood and the size of cellulose crystallites in Norway spruce (Picea abies). J. Wood Sci. 2003, 49, 531–537. [Google Scholar] [CrossRef]
- Meyers, M.A.; Chen, P.-Y.; Lin, A.Y.-M.; Seki, Y. Biological materials: Structure and mechanical properties. Prog. Mater. Sci. 2008, 53, 1–206. [Google Scholar] [CrossRef]
- Rosenau, T.; Potthast, A.; Hofinger, A.; Bacher, M.; Yoneda, Y.; Mereiter, K.; Nakatsubo, F.; Jäger, C.; French, A.D.; Kajiwara, K. Toward a Better Understanding of Cellulose Swelling, Dissolution, and Regeneration on the Molecular Level, In Cellulose Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 99–125. [Google Scholar]
- Rahmati, S.; Doherty, W.; Dubal, D.; Atanda, L.; Moghaddam, L.; Sonar, P.; Hessel, V.; Ostrikov, K. Pretreatment and fermentation of lignocellulosic biomass: Reaction mechanisms and process engineering. React. Chem. Eng. 2020, 5, 2017–2047. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Kim, H.C.; Kim, H.Y.; Chung, Y.S.; Park, W.H.; Youk, J.H. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef]
- PÉrez, S.; Samain, D. Structure and Engineering of Celluloses. In Advances in Carbohydrate Chemistry and Biochemistry; Horton, D., Ed.; Academic Press: Cambridge, MA, USA, 2010; pp. 25–116. [Google Scholar]
- Rongpipi, S.; Ye, D.; Gomez, E.D.; Gomez, E.W. Progress and Opportunities in the Characterization of Cellulose—An Important Regulator of Cell Wall Growth and Mechanics. Front. Plant Sci. 2019, 9, 1894. [Google Scholar] [CrossRef] [PubMed]
- Oudiani, A.E.; Chaabouni, Y.; Msahli, S.; Sakli, F. Crystal transition from cellulose I to cellulose II in NaOH treated Agave americana L. fibre. Carbohydr. Polym. 2011, 86, 1221–1229. [Google Scholar] [CrossRef]
- Coseri, S.; Biliuta, G.; Simionescu, B.C. Selective oxidation of cellulose, mediated by N-hydroxyphthalimide, under a metal-free environment. Polym. Chem. 2018, 9, 961–967. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. TEMPO-Mediated Oxidation of Native Cellulose. The Effect of Oxidation Conditions on Chemical and Crystal Structures of the Water-Insoluble Fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef]
- Chen, M.; Li, R.-M.; Runge, T.; Feng, J.; Feng, J.; Hu, S.; Shi, Q.-S. Solvent-Free Acetylation of Cellulose by 1-Ethyl-3-methylimidazolium Acetate-Catalyzed Transesterification. ACS Sustain. Chem. Eng. 2019, 7, 16971–16978. [Google Scholar] [CrossRef]
- Yadav, P.; Chacko, S.; Kumar, G.; Ramapanicker, R.; Verma, V. Click chemistry route to covalently link cellulose and clay. Cellulose 2015, 22, 1615–1624. [Google Scholar] [CrossRef]
- Guan, Q.-F.; Yang, H.-B.; Han, Z.-M.; Zhou, L.-C.; Zhu, Y.-B.; Ling, Z.-C.; Jiang, H.-B.; Wang, P.-F.; Ma, T.; Wu, H.-A.; et al. Lightweight, tough, and sustainable cellulose nanofiber-derived bulk structural materials with low thermal expansion coefficient. Sci. Adv. 2020, 6, eaaz1114. [Google Scholar] [CrossRef]
- Antonietti, M. Sustainable Bulk Structural Material Engineered from Cellulose Nanofibers. Matter 2020, 3, 339–340. [Google Scholar] [CrossRef]
- Chen, Y.; Dang, B.; Fu, J.; Wang, C.; Li, C.; Sun, Q.; Li, H. Cellulose-Based Hybrid Structural Material for Radiative Cooling. Nano Lett. 2021, 21, 397–404. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, S.; Li, F.; Shi, J. Cellulose-based sensors for metal ions detection. Cellulose 2020, 27, 5477–5507. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, S.; Li, F.; Yang, Y.; Du, M. Recent advances in cellulose-based membranes for their sensing applications. Cellulose 2020, 27, 9157–9179. [Google Scholar] [CrossRef] [PubMed]
- Ummartyotin, S.; Manuspiya, H. A critical review on cellulose: From fundamental to an approach on sensor technology. Renew. Sustain. Energy Rev. 2015, 41, 402–412. [Google Scholar] [CrossRef]
- Qiu, X.; Hu, S. “Smart” Materials Based on Cellulose: A Review of the Preparations, Properties, and Applications. Materials 2013, 6, 738–781. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, J.; Ma, L.; Zhang, Y.; Shen, L.; Xiong, S.; Li, K.; Qu, M. Multifunctional conductive cellulose fabric with flexibility, superamphiphobicity and flame-retardancy for all-weather wearable smart electronic textiles and high-temperature warning device. Chem. Eng. J. 2020, 390, 124508. [Google Scholar] [CrossRef]
- Sjöström, E. (Ed.) LIGNIN. In Wood Chemistry: Fundamentals and Applications; Academic Press: San Diego, CA, USA, 1993; Chapter 4; pp. 71–89. [Google Scholar]
- Evstigneyev, E.I.; Shevchenko, S.M. Structure, chemical reactivity and solubility of lignin: A fresh look. Wood Sci. Technol. 2019, 53, 7–47. [Google Scholar] [CrossRef]
- Cline, S.P.; Smith, P.M. Opportunities for lignin valorization: An exploratory process. Energy Sustain. Soc. 2017, 7, 26. [Google Scholar] [CrossRef]
- LLP, K.S.I. Lignin Market—Forecasts from 2020 to 2025; Research and Markets: Dublin, Ireland, 2020. [Google Scholar]
- Naomi, R.; Idrus, R.B.H.; Fauzi, M.B. Plant- vs. Bacterial-Derived Cellulose for Wound Healing: A Review. Int. J. Environ. Res. Public Health 2020, 17, 6803. [Google Scholar] [CrossRef]
- Research, G.V. Cellulose Fiber Market Size, Share & Trends Analysis by Product Type (Natural, Synthetic), by Application (Textile, Hygiene, Industrial), by Regions and Segment Forecasts, 2018—2025; Grand View Research: San Francisco, CA, USA, 2016. [Google Scholar]
- Nguyen, N.A.; Meek, K.M.; Bowland, C.C.; Barnes, S.H.; Naskar, A.K. An Acrylonitrile–Butadiene–Lignin Renewable Skin with Programmable and Switchable Electrical Conductivity for Stress/Strain-Sensing Applications. Macromolecules 2018, 51, 115–127. [Google Scholar] [CrossRef]
- Glasser, W.G. About Making Lignin Great Again—Some Lessons from the Past. Front. Chem. 2019, 7, 565. [Google Scholar] [CrossRef]
- Sezer, N.; Koç, M. A comprehensive review on the state-of-the-art of piezoelectric energy harvesting. Nano Energy 2021, 80, 105567. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, H.; Mi, H.; Cai, Z.; Ma, Z.; Gong, S. High-performance flexible piezoelectric nanogenerators consisting of porous cellulose nanofibril (CNF)/poly(dimethylsiloxane) (PDMS) aerogel films. Nano Energy 2016, 26, 504–512. [Google Scholar] [CrossRef]
- Atalla, R.H.; Vanderhart, D.L. Native cellulose: A composite of two distinct crystalline forms. Science 1984, 223, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Yun, G.-Y.; Kim, J.-H.; Kim, J. Dielectric and polarization behaviour of cellulose electro-active paper (EAPap). J. Phys. D Appl. Phys. 2009, 42, 082003. [Google Scholar] [CrossRef]
- Fukada, E. Piezoelectricity of Wood. J. Phys. Soc. Jpn. 1955, 10, 149–154. [Google Scholar] [CrossRef]
- Gong, S.; Zhang, B.; Zhang, J.; Wang, Z.L.; Ren, K. Biocompatible Poly(lactic acid)-Based Hybrid Piezoelectric and Electret Nanogenerator for Electronic Skin Applications. Adv. Funct. Mater. 2020, 30, 1908724. [Google Scholar] [CrossRef]
- Kalimuldina, G.; Turdakyn, N.; Abay, I.; Medeubayev, A.; Nurpeissova, A.; Adair, D.; Bakenov, Z. A Review of Piezoelectric PVDF Film by Electrospinning and Its Applications. Sensors 2020, 20, 5214. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhao, G.; Li, B.; Wang, J. Design optimization of PVDF-based piezoelectric energy harvesters. Heliyon 2017, 3, e00377. [Google Scholar] [CrossRef]
- Wang, J.; Carlos, C.; Zhang, Z.; Li, J.; Long, Y.; Yang, F.; Dong, Y.; Qiu, X.; Qian, Y.; Wang, X. Piezoelectric Nanocellulose Thin Film with Large-Scale Vertical Crystal Alignment. ACS Appl. Mater. Interfaces 2020, 12, 26399–26404. [Google Scholar] [CrossRef]
- Li, J.; Kang, L.; Yu, Y.; Long, Y.; Jeffery, J.J.; Cai, W.; Wang, X. Study of long-term biocompatibility and bio-safety of implantable nanogenerators. Nano Energy 2018, 51, 728–735. [Google Scholar] [CrossRef]
- Mahadeva, S.K.; Walus, K.; Stoeber, B. Piezoelectric Paper Fabricated via Nanostructured Barium Titanate Functionalization of Wood Cellulose Fibers. ACS Appl. Mater. Interfaces 2014, 6, 7547–7553. [Google Scholar] [CrossRef]
- Csoka, L.; Hoeger, I.C.; Rojas, O.J.; Peszlen, I.; Pawlak, J.J.; Peralta, P.N. Piezoelectric Effect of Cellulose Nanocrystals Thin Films. ACS Macro Lett. 2012, 1, 867–870. [Google Scholar] [CrossRef]
- Bairagi, S.; Ghosh, S.; Ali, S.W. A fully sustainable, self-poled, bio-waste based piezoelectric nanogenerator: Electricity generation from pomelo fruit membrane. Sci. Rep. 2020, 10, 12121. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Guo, H.; Schädli, G.N.; Tu, K.; Schär, S.; Schwarze, F.W.; Panzarasa, G.; Ribera, J.; Burgert, I. Enhanced mechanical energy conversion with selectively decayed wood. Sci. Adv. 2021, 7, eabd9138. [Google Scholar] [CrossRef]
- Ram, F.; Radhakrishnan, S.; Ambone, T.; Shanmuganathan, K. Highly Flexible Mechanical Energy Harvester Based on Nylon 11 Ferroelectric Nanocomposites. ACS Appl. Polym. Mater. 2019, 1, 1998–2005. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef]
- Drioli, E.; Brunetti, A.; Di Profio, G.; Barbieri, G. Process intensification strategies and membrane engineering. Green Chem. 2012, 14, 1561–1572. [Google Scholar] [CrossRef]
- Meringolo, C.; Mastropietro, T.F.; Poerio, T.; Fontananova, E.; DE Filpo, G.; Curcio, E.; Di Profio, G. Tailoring PVDF Membranes Surface Topography and Hydrophobicity by a Sustainable Two-Steps Phase Separation Process. ACS Sustain. Chem. Eng. 2018, 6, 10069–10077. [Google Scholar] [CrossRef]
- Oliver-Ortega, H.; Méndez, J.A.; Reixach, R.; Espinach, F.X.; Ardanuy, M.; Mutjé, P. Towards More Sustainable Material Formulations: A Comparative Assessment of PA11-SGW Flexural Performance versus Oil-Based Composites. Polymers 2018, 10, 440. [Google Scholar] [CrossRef]
- Sessini, V.; Haseeb, B.; Boldizar, A.; Re, G.L. Sustainable pathway towards large scale melt processing of the new generation of renewable cellulose–polyamide composites. RSC Adv. 2021, 11, 637–656. [Google Scholar] [CrossRef]
- Alam, M.M.; Mandal, D. Native Cellulose Microfiber-Based Hybrid Piezoelectric Generator for Mechanical Energy Harvesting Utility. ACS Appl. Mater. Interfaces 2016, 8, 1555–1558. [Google Scholar] [CrossRef]
- Choi, H.Y.; Jeong, Y.G. Microstructures and piezoelectric performance of eco-friendly composite films based on nanocellulose and barium titanate nanoparticle. Compos. Part B Eng. 2019, 168, 58–65. [Google Scholar] [CrossRef]
- Shi, K.; Huang, X.; Sun, B.; Wu, Z.; He, J.; Jiang, P. Cellulose/BaTiO3 aerogel paper based flexible piezoelectric nanogenerators and the electric coupling with triboelectricity. Nano Energy 2019, 57, 450–458. [Google Scholar] [CrossRef]
- Ponnamma, D.; Parangusan, H.; Tanvir, A.; AlMa’adeed, M.A.A. Smart and robust electrospun fabrics of piezoelectric polymer nanocomposite for self-powering electronic textiles. Mater. Des. 2019, 184, 108176. [Google Scholar] [CrossRef]
- Li, M.; Jie, Y.; Shao, L.-H.; Guo, Y.; Cao, X.; Wang, N.; Wang, Z.L. All-in-one cellulose based hybrid tribo/piezoelectric nanogenerator. Nano Res. 2019, 12, 1831–1835. [Google Scholar] [CrossRef]
- Pusty, M.; Shirage, P.M. Gold nanoparticle–cellulose/PDMS nanocomposite: A flexible dielectric material for harvesting mechanical energy. RSC Adv. 2020, 10, 10097–10112. [Google Scholar] [CrossRef]
- Toroń, B.; Szperlich, P.; Nowak, M.; Stróż, D.; Rzychoń, T. Novel piezoelectric paper based on SbSI nanowires. Cellulose 2018, 25, 7–15. [Google Scholar] [CrossRef]
- Fan, F.-R.; Tian, Z.-Q.; Wang, Z.L. Flexible triboelectric generator. Nano Energy 2012, 1, 328–334. [Google Scholar] [CrossRef]
- Wang, Z.L. Triboelectric Nanogenerators as New Energy Technology for Self-Powered Systems and as Active Mechanical and Chemical Sensors. ACS Nano 2013, 7, 9533–9557. [Google Scholar] [CrossRef]
- Kim, D.W.; Lee, J.H.; Kim, J.K.; Jeong, U. Material aspects of triboelectric energy generation and sensors. NPG Asia Mater. 2020, 12, 6. [Google Scholar] [CrossRef]
- Song, G.; Kim, Y.; Yu, S.; Kim, M.-O.; Park, S.-H.; Cho, S.M.; Velusamy, D.B.; Cho, S.H.; Kim, K.L.; Kim, J.; et al. Molecularly Engineered Surface Triboelectric Nanogenerator by Self-Assembled Monolayers (METS). Chem. Mater. 2015, 27, 4749–4755. [Google Scholar] [CrossRef]
- Wang, S.; Xie, Y.; Niu, S.; Lin, L.; Liu, C.; Zhou, Y.S.; Wang, Z.L. Maximum Surface Charge Density for Triboelectric Nanogenerators Achieved by Ionized-Air Injection: Methodology and Theoretical Understanding. Adv. Mater. 2014, 26, 6720–6728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.S.; Han, M.D.; Wang, R.X.; Meng, B.; Zhu, F.Y.; Sun, X.M.; Hu, W.; Wang, W.; Li, Z.H.; Zhang, H.X. High-performance triboelectric nanogenerator with enhanced energy density based on single-step fluorocarbon plasma treatment. Nano Energy 2014, 4, 123–131. [Google Scholar] [CrossRef]
- Zhang, R.; Dahlström, C.; Zou, H.; Jonzon, J.; Hummelgård, M.; Örtegren, J.; Blomquist, N.; Yang, Y.; Andersson, H.; Olsen, M.; et al. Cellulose-Based Fully Green Triboelectric Nanogenerators with Output Power Density of 300 W m−2. Adv. Mater. 2020, 32, 2002824. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Hernandez, A.; Yu, Y.; Cai, Z.; Wang, X. Triboelectric nanogenerators and power-boards from cellulose nanofibrils and recycled materials. Nano Energy 2016, 30, 103–108. [Google Scholar] [CrossRef]
- Niu, S.; Wang, Z.L. Theoretical systems of triboelectric nanogenerators. Nano Energy 2015, 14, 161–192. [Google Scholar] [CrossRef]
- Jakmuangpak, S.; Prada, T.; Mongkolthanaruk, W.; Harnchana, V.; Pinitsoontorn, S. Engineering Bacterial Cellulose Films by Nanocomposite Approach and Surface Modification for Biocompatible Triboelectric Nanogenerator. ACS Appl. Electron. Mater. 2020, 2, 2498–2506. [Google Scholar] [CrossRef]
- Shao, Y.; Feng, C.P.; Deng, B.W.; Yin, B.; Yang, M.B. Facile method to enhance output performance of bacterial cellulose nanofiber based triboelectric nanogenerator by controlling micro-nano structure and dielectric constant. Nano Energy 2019, 62, 620–627. [Google Scholar] [CrossRef]
- Nie, S.; Cai, C.; Lin, X.; Zhang, C.; Lu, Y.; Mo, J.; Wang, S. Chemically Functionalized Cellulose Nanofibrils for Improving Triboelectric Charge Density of a Triboelectric Nanogenerator. ACS Sustain. Chem. Eng. 2020, 8, 18678–18685. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, Y.; Wang, Y.; Zhang, S.; Yang, W.; Pan, X.; Wang, Z.L. Cellulose II Aerogel-Based Triboelectric Nanogenerator. Adv. Funct. Mater. 2020, 30, 2001763. [Google Scholar] [CrossRef]
- Bao, Y.; Wang, R.; Lu, Y.; Wu, W. Lignin biopolymer based triboelectric nanogenerators. APL Mater. 2017, 5, 074109. [Google Scholar] [CrossRef]
- Ramage, M.H.; Burridge, H.; Busse-Wicher, M.; Fereday, G.; Reynolds, T.; Shah, D.U.; Wu, G.; Yu, L.; Fleming, P.; Densley-Tingley, D.; et al. The wood from the trees: The use of timber in construction. Renew. Sustain. Energy Rev. 2017, 68, 333–359. [Google Scholar] [CrossRef]
- Zhong, C. Industrial-Scale Production and Applications of Bacterial Cellulose. Front. Bioeng. Biotechnol. 2020, 8, 1425. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Jeon, S.-B.; Kim, J.Y.; Seol, M.-L.; Kim, S.O.; Choi, Y.-K. High-performance nanopattern triboelectric generator by block copolymer lithography. Nano Energy 2015, 12, 331–338. [Google Scholar] [CrossRef]
- Zhang, F.; Li, B.; Zheng, J.; Xu, C. Facile Fabrication of Micro-Nano Structured Triboelectric Nanogenerator with High Electric Output. Nanoscale Res. Lett. 2015, 10, 298. [Google Scholar] [CrossRef]
- Samejima, T.; Soh, Y.; Yano, T. Specific Surface Area and Specific Pore Volume Distribution of Tobacco. Agric. Biol. Chem. 1977, 41, 983–988. [Google Scholar] [CrossRef]
- Zheng, Q.; Fang, L.; Guo, H.; Yang, K.; Cai, Z.; Meador, M.A.; Gong, S. Highly Porous Polymer Aerogel Film-Based Triboelectric Nanogenerators. Adv. Funct. Mater. 2018, 28, 1706365. [Google Scholar] [CrossRef]
- Saadatnia, Z.; Mosanenzadeh, S.G.; Esmailzadeh, E.; Naguib, H.E. A High Performance Triboelectric Nanogenerator Using Porous Polyimide Aerogel Film. Sci. Rep. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Sun, J.-G.; Yang, T.N.; Kuo, I.-S.; Wu, J.-M.; Wang, C.-Y.; Chen, L.-J. A leaf-molded transparent triboelectric nanogenerator for smart multifunctional applications. Nano Energy 2017, 32, 180–186. [Google Scholar] [CrossRef]
- Lee, K.; Mhin, S.; Han, H.; Kwon, O.; Kim, W.B.; Song, T.; Kang, S.; Kim, K.M. A high-performance PDMS-based triboelectric nanogenerator fabricated using surface-modified carbon nanotubes via pulsed laser ablation. J. Mater. Chem. A 2022, 10, 1299–1308. [Google Scholar] [CrossRef]
- Xiao, X.; Lü, C.; Wang, G.; Xu, Y.; Wang, J.; Yang, H. Flexible triboelectric nanogenerator from micro-nano structured polydimethylsiloxane. Chem. Res. Chin. Univ. 2015, 31, 434–438. [Google Scholar] [CrossRef]
- Kim, H.-J.; Yim, E.-C.; Kim, J.-H.; Kim, S.-J.; Park, J.-Y.; Oh, I.-K. Bacterial Nano-Cellulose Triboelectric Nanogenerator. Nano Energy 2017, 33, 130–137. [Google Scholar] [CrossRef]
- Jie, Y.; Jia, X.; Zou, J.; Chen, Y.; Wang, N.; Wang, Z.L.; Cao, X. Natural Leaf Made Triboelectric Nanogenerator for Harvesting Environmental Mechanical Energy. Adv. Energy Mater. 2018, 8, 1703133. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, L.; Zheng, Y.; Wang, D.; Zhou, F.; Liu, W. Leaves based triboelectric nanogenerator (TENG) and TENG tree for wind energy harvesting. Nano Energy 2019, 55, 260–268. [Google Scholar] [CrossRef]
- Xiong, J.; Cui, P.; Chen, X.; Wang, J.; Parida, K.; Lin, M.F.; Lee, P.S. Skin-touch-actuated textile-based triboelectric nanogenerator with black phosphorus for durable biomechanical energy harvesting. Nat. Commun. 2018, 9, 4280. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Y.; Zheng, J.; Xiang, Y.; Zhao, P.; Cui, J.; Zhou, H.; Li, D. Rapidly fabricated triboelectric nanogenerator employing insoluble and infusible biomass materials by fused deposition modeling. Nano Energy 2020, 68, 104382. [Google Scholar] [CrossRef]
- Ramon, G.Z.; Feinberg, B.J.; Hoek, E.M.V. Membrane-based production of salinity-gradient power. Energy Environ. Sci. 2011, 4, 4423–4434. [Google Scholar] [CrossRef]
- Pattle, R.E. Production of Electric Power by mixing Fresh and Salt Water in the Hydroelectric Pile. Nature 1954, 174, 660. [Google Scholar] [CrossRef]
- Liu, X.; He, M.; Calvani, D.; Qi, H.; Gupta, K.B.S.S.; de Groot, H.J.; Sevink, G.A.; Buda, F.; Kaiser, U.; Schneider, G.F. Power generation by reverse electrodialysis in a single-layer nanoporous membrane made from core–rim polycyclic aromatic hydrocarbons. Nat. Nanotechnol. 2020, 15, 307–312. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, C.; Wang, R.; Chen, C.; Gao, J.; Dai, J.; Liu, D.; Lin, Z.; Hu, L. Salinity-Gradient Power Generation with Ionized Wood Membranes. Adv. Energy Mater. 2020, 10, 1902590. [Google Scholar] [CrossRef]
- Moreno, J.; Grasman, S.; Van Engelen, R.; Nijmeijer, K. Upscaling Reverse Electrodialysis. Environ. Sci. Technol. 2018, 52, 10856–10863. [Google Scholar] [CrossRef]
- Siria, A.; Bocquet, M.-L.; Bocquet, L. New avenues for the large-scale harvesting of blue energy. Nat. Rev. Chem. 2017, 1, 0091. [Google Scholar] [CrossRef]
- Długołęcki, P.; Dąbrowska, J.; Nijmeijer, K.; Wessling, M. Ion conductive spacers for increased power generation in reverse electrodialysis. J. Membr. Sci. 2010, 347, 101–107. [Google Scholar] [CrossRef]
- Ding, L.; Xiao, D.; Lu, Z.; Deng, J.; Wei, Y.; Caro, J.; Wang, H. Oppositely Charged Ti3C2Tx MXene Membranes with 2D Nanofluidic Channels for Osmotic Energy Harvesting. Angew. Chem. 2020, 59, 8720–8726. [Google Scholar] [CrossRef]
- Pendse, A.; Cetindag, S.; Rehak, P.; Behura, S.; Gao, H.; Nguyen, N.H.L.; Wang, T.; Berry, V.; Král, P.; Shan, J.; et al. Highly Efficient Osmotic Energy Harvesting in Charged Boron-Nitride-Nanopore Membranes. Adv. Funct. Mater. 2021, 31, 2009586. [Google Scholar] [CrossRef]
- Fu, Y.; Guo, X.; Wang, Y.; Wang, X.; Xue, J. An atomically-thin graphene reverse electrodialysis system for efficient energy harvesting from salinity gradient. Nano Energy 2019, 57, 783–790. [Google Scholar] [CrossRef]
- Wu, Y.; Xin, W.; Kong, X.-Y.; Chen, J.; Qian, Y.; Sun, Y.; Zhao, X.; Chen, W.; Jiang, L.; Wen, L. Enhanced ion transport by graphene oxide/cellulose nanofibers assembled membranes for high-performance osmotic energy harvesting. Mater. Horiz. 2020, 7, 2702–2709. [Google Scholar] [CrossRef]
- Beaumont, M.; Kondor, A.; Plappert, S.; Mitterer, C.; Opietnik, M.; Potthast, A.; Rosenau, T. Surface properties and porosity of highly porous, nanostructured cellulose II particles. Cellulose 2017, 24, 435–440. [Google Scholar] [CrossRef]
- Dahlström, C.; Durán, V.L.; Keene, S.; Salleo, A.; Norgren, M.; Wågberg, L. Ion conductivity through TEMPO-mediated oxidated and periodate oxidated cellulose membranes. Carbohydr. Polym. 2020, 233, 115829. [Google Scholar] [CrossRef] [PubMed]
- Muhmed, S.A.; Nor, N.A.M.; Jaafar, J.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Aziz, F.; Yusof, N. Emerging chitosan and cellulose green materials for ion exchange membrane fuel cell: A review. Energy Ecol. Environ. 2020, 5, 85–107. [Google Scholar] [CrossRef]
- Väisänen, S.; Pönni, R.; Hämäläinen, A.; Vuorinen, T. Quantification of accessible hydroxyl groups in cellulosic pulps by dynamic vapor sorption with deuterium exchange. Cellulose 2018, 25, 6923–6934. [Google Scholar] [CrossRef]
- de Assis Filho, R.B.; de Araújo, C.M.B.; Baptisttella, A.M.S.; Batista, E.B.; Barata, R.A.; Ghislandi, M.G.; da Motta Sobrinho, M.A. Environmentally friendly route for graphene oxide production via electrochemical synthesis focused on the adsorptive removal of dyes from water. Environ. Technol. 2020, 41, 2771–2782. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Wei, Q.; Huang, K.; Cheng, H.-M.; Ren, W. Green synthesis of graphene oxide by seconds timescale water electrolytic oxidation. Nat. Commun. 2018, 9, 145. [Google Scholar] [CrossRef]
- Chufa, B.M.; Gonfa, B.A.; Anshebo, T.Y.; Workneh, G.A. A Novel and Simplest Green Synthesis Method of Reduced Graphene Oxide Using Methanol Extracted Vernonia Amygdalina: Large-Scale Production. Adv. Condens. Matter Phys. 2021, 2021, 6681710. [Google Scholar]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.W.; Voon, C.H. Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Villafaña-López, L.; Reyes-Valadez, D.M.; González-Vargas, O.A.; Suárez-Toriello, V.A.; Jaime-Ferrer, J.S. Custom-Made Ion Exchange Membranes at Laboratory Scale for Reverse Electrodialysis. Membranes 2019, 9, 145. [Google Scholar] [CrossRef]
- Wu, Z.; Ji, P.; Wang, B.; Sheng, N.; Zhang, M.; Chen, S.; Wang, H. Oppositely charged aligned bacterial cellulose biofilm with nanofluidic channels for osmotic energy harvesting. Nano Energy 2021, 80, 105554. [Google Scholar] [CrossRef]
- Sheng, N.; Chen, S.; Zhang, M.; Wu, Z.; Liang, Q.; Ji, P.; Wang, H. TEMPO-Oxidized Bacterial Cellulose Nanofibers/Graphene Oxide Fibers for Osmotic Energy Conversion. ACS Appl. Mater. Interfaces 2021, 13, 22416–22425. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, Y.; Deng, Y.; Gao, H.; Lin, Z.; Zhu, W.; Ye, H. A novel self-powered wireless temperature sensor based on thermoelectric generators. Energy Convers. Manag. 2014, 80, 110–116. [Google Scholar] [CrossRef]
- Mukaida, M.; Kirihara, K.; Horike, S.; Wei, Q. Stable organic thermoelectric devices for self-powered sensor applications. J. Mater. Chem. A 2020, 8, 22544–22556. [Google Scholar] [CrossRef]
- Hewawasam, L.; Jayasena, A.; Afnan, M.; Ranasinghe, R.; Wijewardane, M. Waste heat recovery from thermo-electric generators (TEGs). Energy Rep. 2020, 6, 474–479. [Google Scholar] [CrossRef]
- Hashim, H.T. Energy Harvesting from the Waste Heat of an Electrical Oven via Thermoelectric Generator. J. Phys. Conf. Ser. 2018, 1032, 012024. [Google Scholar] [CrossRef]
- Lin, S.; Li, W.; Chen, Z.; Shen, J.; Ge, B.; Pei, Y. Tellurium as a high-performance elemental thermoelectric. Nat. Commun. 2016, 7, 10287. [Google Scholar] [CrossRef]
- Zheng, Z.-H.; Shi, X.-L.; Ao, D.-W.; Liu, W.-D.; Li, M.; Kou, L.-Z.; Chen, Y.-X.; Li, F.; Wei, M.; Liang, G.-X.; et al. Harvesting waste heat with flexible Bi2Te3 thermoelectric thin film. Nat. Sustain. 2023, 6, 180–191. [Google Scholar] [CrossRef]
- Zhao, X.; Han, W.; Jiang, Y.; Zhao, C.; Ji, X.; Kong, F.; Xu, W.; Zhang, X. A honeycomb-like paper-based thermoelectric generator based on a Bi2Te3/bacterial cellulose nanofiber coating. Nanoscale 2019, 11, 17725–17735. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, C.; Jiang, Y.; Ji, X.; Kong, F.; Lin, T.; Shao, H.; Han, W. Flexible cellulose nanofiber/Bi2Te3 composite film for wearable thermoelectric devices. J. Power Sources 2020, 479, 229044. [Google Scholar] [CrossRef]
- Ying, P.; He, R.; Mao, J.; Zhang, Q.; Reith, H.; Sui, J.; Ren, Z.; Nielsch, K.; Schierning, G. Towards tellurium-free thermoelectric modules for power generation from low-grade heat. Nat. Commun. 2021, 12, 1121. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, Z.; Ren, W.; Cao, G.; Deng, K.; Zhong, Y. Hydrothermal synthesis of single-crystalline Bi2Te3 nanoplates. Mater. Lett. 2008, 62, 4273–4276. [Google Scholar] [CrossRef]
- Kim, J.-J.; Kim, S.-H.; Suh, S.-W.; Choe, D.-U.; Park, B.-K.; Lee, J.-R.; Lee, Y.-S. Hydrothermal synthesis of Bi2Te3 nanowires through the solid-state interdiffusion of Bi and Te atoms on the surface of Te nanowires. J. Cryst. Growth 2010, 312, 3410–3415. [Google Scholar] [CrossRef]
- Fu, J.; Song, S.; Zhang, X.; Cao, F.; Zhou, L.; Li, X.; Zhang, H. Bi2Te3 nanoplates and nanoflowers: Synthesized by hydrothermal process and their enhanced thermoelectric properties. CrystEngComm 2012, 14, 2159–2165. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.; Lacey, S.D.; Mi, R.; Zhao, X.; Jiang, F.; Song, J.; Liu, Z.; Chen, G.; Dai, J.; et al. Cellulose ionic conductors with high differential thermal voltage for low-grade heat harvesting. Nat. Mater. 2019, 18, 608–613. [Google Scholar] [CrossRef]
- Kim, P.; Shi, L.; Majumdar, A.; McEuen, P.L. Thermal Transport Measurements of Individual Multiwalled Nanotubes. Phys. Rev. Lett. 2001, 87, 215502. [Google Scholar] [CrossRef] [PubMed]
- Gnanaseelan, M.; Chen, Y.; Luo, J.; Krause, B.; Pionteck, J.; Pötschke, P.; Qi, H. Cellulose-carbon nanotube composite aerogels as novel thermoelectric materials. Compos. Sci. Technol. 2018, 163, 133–140. [Google Scholar] [CrossRef]
- Abol-Fotouh, D.; Dörling, B.; Zapata-Arteaga, O.; Rodríguez-Martínez, X.; Gómez, A.; Reparaz, J.S.; Laromaine, A.; Roig, A.; Campoy-Quiles, M. Farming thermoelectric paper. Energy Environ. Sci. 2019, 12, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Kumanek, B.; Stando, G.; Wrobel, P.; Krzywiecki, M.; Janas, D. Thermoelectric properties of composite films from multi-walled carbon nanotubes and ethyl cellulose doped with heteroatoms. Synth. Met. 2019, 257, 116190. [Google Scholar] [CrossRef]
- Culebras, M.; Ren, G.; O’Connell, S.; Vilatela, J.J.; Collins, M.N. Lignin Doped Carbon Nanotube Yarns for Improved Thermoelectric Efficiency. Adv. Sustain. Syst. 2020, 4, 2000147. [Google Scholar] [CrossRef]
- Hu, J.; Li, R.; Zhang, K.; Meng, Y.; Wang, M.; Liu, Y. Extract nano cellulose from flax as thermoelectric enhancement material. J. Phys. Conf. Ser. 2021, 1790, 012087. [Google Scholar] [CrossRef]
- Mardi, S.M.; Ambrogioni, R.; Reale, A. Developing printable thermoelectric materials based on graphene nanoplatelet/ethyl cellulose nanocomposites. Mater. Res. Express 2020, 7, 085101. [Google Scholar] [CrossRef]

| Material | VOC | ISC | Force | Frequency | Power Output | Ref. |
|---|---|---|---|---|---|---|
| PVDF | - | - | - | - | 112.8 μW | [65] |
| Pomelo skin | 15 V | 130 μA | 0.05 N | 100 Hz | 12 μW/cm−3 | [70] |
| Wood incubated with G. applanatum | 0.87 V | 13.3 nA | 10 N | - | - | [71] |
| Nylon 11/cellulose nanocrystals | 6.95 V | - | 23 N | - | 500 μW cm−3 | [72] |
| PDMS-coated cellulose nanofibrils | 60.2 V | 10.1 μA | 0.05 MPa | 10 Hz | 6.3 mW/cm−3 | [59] |
| Cellulose microfibre/PDMS | 30 V | 500 nA | - | - | 9.0 μW/cm−3 | [78] |
| BaTiO3 -Wood Cellulose fibres | 2.86 V | 262.35 nA | 1 N | 1 Hz | 8.41 μW/cm−3 | [79] |
| Cellulose/BaTiO3 aerogel | 15.5 V | 3.3 μA | 80 kPa | 3 Hz | 11.8 μW | [80] |
| PVDF-HFP/cellulose nanocrystals | 12 V | 1.9 μA cm−2 | 2.5 N | 45 Hz | 490 μW/cm−3 | [81] |
| Nitrocellulose nanofibril/ BaTiO3/MWCNT | 22 V | 220 nA cm−2 | 2 N cm−2 | 5 Hz | 1.21 μW cm−2 | [82] |
| Au nanoparticle/cellulose/PDMS | 6 V | 700 nA | 3 N | - | 8.34 mW m−2 | [83] |
| Cellulose/SbSI nanowires | 24 mV | - | 90 dB | 175 Hz | 41.5 nW cm−3 | [84] |
| Material | VOC | ISC | Force | Frequency | Power Generation | Ref. |
|---|---|---|---|---|---|---|
| Nanopatterned PDMS | 414.63 V | 40.03 μA | - | - | 7.69 W m−2 | [107] |
| Nitrocellulose nanofibril/ BaTiO3/MWCNT | 37 V | 1.23 μA cm−2 | 2 N cm−2 | 5 Hz | 10.6 μW cm−2 | [82] |
| Regenerated cellulose | 300 V | 2.6 mA | - | 5 Hz | 307 W m−2 | [91] |
| Cellulose nanofibrils | 10–30 V | 10–90 μA | - | 10–60 Hz | - | [92] |
| Bacterial cellulose/ZnO nanoparticles | 57.6 V | 5.78 μA | 2 N | 5 Hz | 42 mW m−2 | [94] |
| Bacterial cellulose/BaTiO3 particles | 181 V | 21 μA | 42 N | 2 Hz | 4.8 W m−2 | [95] |
| Aminosilane-functionalised cellulose nanofibril | 195 V | 13.4 μA | - | - | - | [96] |
| Cellulose II aerogel | 65 V | 1.86 µA | 40 N | 4 Hz | 127 mW m−2 | [97] |
| Lignin/starch | 1.04 V cm−2 | 3.96 nA cm−2 | - | 0.5 Hz | - | [98] |
| Micropatterned PDMS | 56 V | 3.1 µA | - | - | - | [106] |
| Hosta leaf | 230 V | 9.5 µA | - | 2 Hz | 45 mW m−2 | [110] |
| Dry leaf modified with Poly-L-Lysine | 1000 V | 60 µA | - | 5 Hz | - | [111] |
| Black phosphorous encapsulated with hydrophobic cellulose oleoyl ester nanoparticles | 250–880 | 0.48–1.1 µA | 5 N | 4 Hz | 0.52 mW cm−2 | [112] |
| Lignin/PDMS | 308 V | 61.6 µA | 10 N | 30 Hz | 5.93 W m−2 | [113] |
| Material | Power Output | Charge Density (Cation Film) | Charge Density (Anion Film) | Ionic Conductivity (Cation Film) | Ionic Conductivity (Anion Film) | Ref. |
|---|---|---|---|---|---|---|
| Polyethylene/polystyrene | 15 mW | - | - | - | - | [115] |
| Polycyclic aromatic hydrocarbon | 67 W m−2 | - | - | - | - | [116] |
| Nanocellulose | 0.23 W m−2 | 3.13 mC m−2 | −2.66 mC m−2 | 0.42 mS cm−1 | 1.0 mS cm−1 | [136] |
| Cellulose nanofibrils/graphene oxide | 4.19 W m−2 | - | - | - | - | [124] |
| Ionised wood | 5.14 mW m−2 | 2.25 mC m−2 | −3.09 mC m−2 | 0.4 mS cm−1 | 0.2 mS cm−1 | [117] |
| Cellulose oxide/graphene oxide | 0.53 W m–2 | - | −3.00 mC m–2 | - | 0.8 mS cm–1 | [137] |
| Materials | Power Factor (μW m−1 K−2) | Seebeck Coefficient (μV K−1) | Figure of Merit | Ref. |
|---|---|---|---|---|
| Ag-doped Bi2Te3 | 200 | - | 1.2 | [143] |
| Cellulose ionic conductor | 1150 | 24 | - | [150] |
| Bi2Te3/bacterial cellulose | 25.5 | 135 | - | [144] |
| CNF/Bi0·5Sb1·5Te3 | - | 154 | - | [145] |
| CNF/Bi2Se0·3Te2.7 | - | −130 | - | [145] |
| CNT/bacteria cellulose | 20 | 30 | 2 × 10−3 | [153] |
| Boron-doped CNTs/ethyl cellulose | - | ~25 | ~8 × 10−6 | [154] |
| Nitrogen-doped CNTs/ethyl cellulose | - | ~−20 | ~3 × 10−5 | [154] |
| Lignin-doped carbon nanotube yarns | 132.2 | 100 | - | [155] |
| Graphene nanoplatelets/ethyl cellulose | 0.254 | 15–20 | - | [157] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Gonzalez, A.; Wang, M.; Haseloff, J. Sustainable Approaches to Incorporate Plant-Based Biomaterials in Power Generation. Solids 2023, 4, 133-155. https://doi.org/10.3390/solids4020009
Ruiz-Gonzalez A, Wang M, Haseloff J. Sustainable Approaches to Incorporate Plant-Based Biomaterials in Power Generation. Solids. 2023; 4(2):133-155. https://doi.org/10.3390/solids4020009
Chicago/Turabian StyleRuiz-Gonzalez, Antonio, Mingqing Wang, and Jim Haseloff. 2023. "Sustainable Approaches to Incorporate Plant-Based Biomaterials in Power Generation" Solids 4, no. 2: 133-155. https://doi.org/10.3390/solids4020009
APA StyleRuiz-Gonzalez, A., Wang, M., & Haseloff, J. (2023). Sustainable Approaches to Incorporate Plant-Based Biomaterials in Power Generation. Solids, 4(2), 133-155. https://doi.org/10.3390/solids4020009






