Abstract
The concept of urban mining refers to the recovery and valorization of valuable resources from urban and industrial waste, contributing to circular economy principles. Within this framework, the present study provides a critical review of alkali-activated binders incorporating bivalve mollusk shells as alternative calcium sources. Shells from oysters, scallops, mussels, clams, cockles, and periwinkles were examined, either in their natural or calcined forms, for use as calcium sources, alkaline activators, or fillers in low-carbon binders. The review evaluates key processing parameters, including precursor composition, type and concentration of alkaline activators, curing conditions, and calcination temperatures, and compares the resulting mechanical, chemical, and microstructural properties. In addition, several studies report applications of these binders in soil stabilization and heavy metal immobilization, demonstrating performances comparable to Portland cement. The findings confirm the technical potential of mollusk shell residues and their contribution to the circular economy by diverting aquaculture waste from landfills and marine environments. Nonetheless, significant knowledge gaps persist, including the limited investigation of non-oyster species, the absence of field-scale studies, and the lack of resource mapping, life cycle, or economic assessments. This synthesis highlights preliminary insights, such as optimal calcination temperatures between 700 and 900 °C and effective combinations with silica and alumina-rich residues. Overall, it outlines a pathway toward transforming an underutilized waste stream into sustainable and technically viable construction materials.
1. Introduction
Cement remains one of the most widely used construction materials in modern society, serving as a fundamental binder in concrete and enabling the development of large-scale civil infrastructure. However, the production of commercial (Portland) cement is associated with significant environmental costs, particularly in terms of carbon dioxide emissions. Approximately 8% of total global CO2 emissions are attributed to this process, primarily due to the calcination and grinding of limestone. The growing demand for cement has raised concerns about the sustainability of its production, the durability of the resulting structures, and the depletion of natural resources. These concerns underscore the need for alternative, more environmentally sustainable production routes [1].
The development of alternative materials derived from industrial waste has gained increasing attention, especially in the construction sector. This trend is driven by the scarcity of natural raw materials, the high carbon footprint of traditional cement, and the environmental impact associated with conventional building materials [2]. In this context, alkali activation has emerged as a viable pathway to convert various industrial by-products into sustainable binders, mortars, and concretes, offering reduced energy demand and environmental impact [2,3]. Recent discoveries have also allowed the creation of ultra-high strength alkali-activated geopolymers (UHS-EGC), reaching surprising unconfined compressive strengths (UCSs) (above 150 MPa) [4]. Alkali-activated binders are synthesized through a chemical reaction between an aluminosilicate precursor and an alkaline activator. The incorporation of calcium sources into the formulation reduces the amount of activator required and improves curing efficiency under ambient conditions [5].
A wide range of raw materials has been investigated for the production of alkali-activated binders, including rice husk ash [6,7], sugarcane bagasse ash [8], and iron ore tailings [5,9]. In parallel, various calcium-rich residues have been assessed, such as eggshells [8], carbide lime [6] and by-products from the fishing industry, including shells of oysters [10], mussels [11], scallops [12], and periwinkles [13], all of which are classified as bivalve mollusks.
In 2022, the global production of bivalve mollusks reached 18.9 million tonnes, with China accounting for approximately 16 million tonnes of this total. In the same year, international trade in bivalve mollusks generated an estimated six billion US dollars, representing about three percent of all aquaculture-related animal exports [14]. A critical environmental challenge associated with this industry lies in the large quantities of shell waste generated annually. Most of this material lacks appropriate disposal routes, leading to significant environmental concerns [15].
Urban mining is an activity that aims to transform waste into resources that can be economically reused, reducing the need for virgin raw materials. This allows the circulation of unused materials and the transformation of materials that would otherwise be discarded into products with economic value and social function [16]. Framed within the concept of urban mining, this study interprets shell waste not only as a calcium-rich by-product but as a strategic secondary mineral resource integrated into urban and peri-urban waste flows. Shell waste can thus be regarded as a “distributed limestone reserve” concentrated in coastal cities and urban areas with seafood markets, restaurants, and processing facilities. Urban mining aims to recover materials from anthropogenic stocks to reduce dependence on primary extraction and to close resource loops in a circular economy [17,18]. In this context, the recovery of shells for alkali activation is positioned as a direct replacement strategy for quarried limestone, with the potential to reduce CO2 emissions, energy consumption, and environmental degradation, while also integrating into existing urban waste management networks. Practical routes for this recovery have already been documented, including the recollection of oyster and clam shells from restaurants for calcium carbonate extraction and reuse [19].
Shells of oysters, scallops, mussels, cockles, and periwinkles typically contain more than 90 percent calcium carbonate, often above 95 percent, which can be thermally converted into calcium oxide through calcination. This enables their application as alternative lime sources in sustainable binders and mortars, reducing reliance on conventional raw materials [15,20]. Beyond simple substitution, recent studies show technical feasibility to replace limestone itself in cementitious systems, including limestone calcined clay cement formulations and alkali-activated matrices, with favorable effects on strength and microstructure when process conditions are properly optimized [21,22,23].
Accordingly, the aim of this study is to provide the first semi-systematic literature review that synthesizes the technical performance, processing variables, and sustainability potential of bivalve shell waste in alkali-activated binders under the explicit framework of urban mining. This approach not only consolidates scattered scientific evidence but also identifies critical knowledge gaps, including the absence of field-scale tests, resource mapping, and life cycle assessments, thereby defining a clear research and implementation agenda for replacing natural limestone in a circular and low-carbon construction industry
2. Review Methodology
This study presents a semi-systematic review of the current literature concerning the reuse of bivalve mollusk shell waste from the aquaculture industry in the context of alkali activation, with a particular focus on oyster, mussel, scallop, periwinkle, cockle, and clam shells.
The review involved the analysis and categorization of existing scientific studies on the topic, identifying both commonalities and divergences among them through a detailed examination of the parameters adopted by the authors, such as types of alkaline activators, raw materials, curing times, testing procedures used to evaluate the binders, calcination temperatures, and material preparation methods.
The literature survey was based on scientific articles retrieved from the Scopus and Web of Science databases, with the search conducted up to 16 June 2025. The search terms employed are listed in Table 1. The bibliometric data was collected exclusively from the Scopus and Web of Science databases. These two sources were chosen because they are internationally recognized as the most authoritative and comprehensive repositories of peer-reviewed scientific literature, maintaining rigorous indexing standards that ensure consistency, reliability, and quality of data. Several methodological studies have highlighted that the combined use of Scopus and Web of Science effectively captures the vast majority of relevant scientific output, thus providing a robust foundation for bibliometric analysis. Although other databases, such as Google Scholar or Dimensions, may offer broader coverage, they frequently present issues such as record duplication, inclusion of non-peer-reviewed materials, and lack of metadata standardization, which may compromise reproducibility. Therefore, the combination of Scopus and Web of Science was considered sufficient and appropriate for the objectives of this review.

Table 1.
Results of mollusk shell article searches in Scopus and Web of Science up to 16 June 2025.
The total number of articles found in the databases was 62 before any separation. After that, duplicate articles were removed using the statistical software R version 4.3.3. Bibliometric data, were analyzed using the “bibliometrix 4.3.4” package and its interactive interface “biblioshiny”, both available in the R environment. The resulting data were adapted by the authors into graphs, visual schematics, and maps, which are discussed in the following section.
It is important to emphasize that, despite the use of the keywords listed in Table 1, some retrieved publications did not correspond to the specific topic of alkali activation focused on mollusk shells, as the term was mentioned without being central to the research. Additionally, the term “geopolymers” is broader in scope and often used in the literature, but it allows for ambiguity and may or may not refer specifically to alkali-activated cements [24].
Accordingly, a set of exclusion criteria was defined and applied in two stages. In the first stage, duplicates from different databases were removed. Subsequently, articles that mentioned mollusk shells but did not actually employ them in the experimental investigation were excluded. Review papers were also excluded, since the present study is grounded in experimental research with reported results. Furthermore, conference abstracts were disregarded due to the limited methodological detail they provide, which is insufficient for the level of analysis required in this review.
Following the initial screening, an additional criterion was adopted to further narrow the scope of the selection: verification of whether the core subject of the studies truly involved alkali activation. As previously discussed, the term “geopolymers” is often employed in a generic sense and may refer to acid-based activation processes or to alternative binders incorporating industrial residues, without necessarily involving alkali activation. These cases were considered outside the scope of this research. Therefore, only studies that met all predefined criteria and clearly addressed the intended topic were included in the final analysis. The flowchart illustrating the article exclusion criteria can be seen in Figure 1.
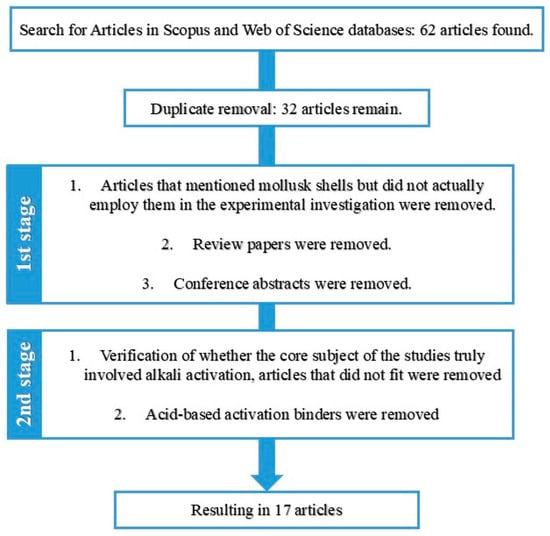
Figure 1.
Flowchart with the exclusion process and selection of the articles.
3. Literature Overview and Discussion
A total of 62 articles were initially identified across the Scopus and Web of Science databases. However, after applying the exclusion criteria described in the “Review Methodology” section, this number was reduced to 32 studies, which form the basis of the analysis presented in this section. The following discussion addresses the results obtained through the use of the R software, in combination with the bibliometrix package and its biblioshiny interface. The outcomes were adapted and presented in the form of graphs, visual schematics, and maps, accompanied by their respective interpretations.
3.1. Annual Scientific Production and Country Scientific Production
Figure 2 illustrates the annual scientific output of publications related to geopolymers produced using mollusk shells. The evolution in the number of publications between 2010 and 2025 demonstrates a clear upward trend in scientific production over time. Between 2010 and 2015, there was a preliminary phase of low productivity, with at most one publication per year. This period may be interpreted as an academic maturation phase, potentially associated with the early stages of research, academic training, or the establishment of dedicated research lines.
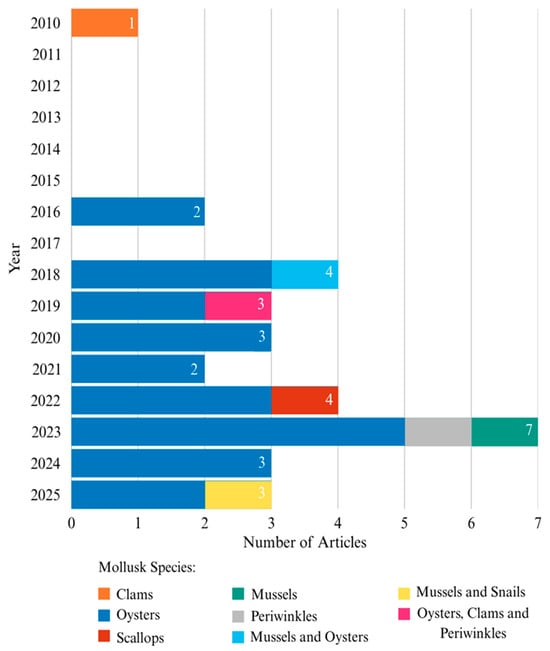
Figure 2.
Annual scientific production of articles on geopolymers produced using mollusk shells based on initial selection. Note: In the year 2025, articles are only considered up until the month of June.
In 2016, a gradual increase in the number of publications was observed, with two articles published that year, indicating the beginning of a more active research phase. In 2018 and 2019, this upward trend became more pronounced, with four and three publications, respectively, suggesting a period of consolidation in scientific output. The year 2020 maintains this elevated level, with three publications, indicating continuity in research activity even in the face of potential external challenges, such as the COVID-19 pandemic.
In 2021, a slight decline occurred, with two articles published; however, this drop is promptly reversed in 2022, which again records four publications. The peak of the time series is reached in 2023, with a total of seven articles published, representing the highest number across the entire period analyzed. This peak likely reflects the conclusion of medium-term research projects, increased participation in research networks, or the maturation of previously established collaborations.
In the subsequent years, 2024 and 2025, the number of publications remains elevated, with three articles in each year. This suggests a stabilization of research productivity at a level higher than that observed during the initial phases. Such consistency indicates that the scientific and academic development process has reached a stage of maturity, in which article publication has become a regular and sustained activity over time.
Overall, the data reveal a consistent and positive trajectory in scientific production, with progressive and consolidated growth in recent years. This trend reflects the strengthening of academic engagement and the maintenance of an active research agenda.
Oyster shells are by far the most widely studied among the mollusk shell types investigated in alkali-activated binder systems. This predominance is attributed to the significantly higher global production of oysters compared to the other mollusks considered in this review [13], which results in a larger quantity of available waste material. Among the 32 articles selected after applying the exclusion criteria, 27 involve oyster shells, 3 involve mussel shells, 2 address periwinkle shells, 2 clam shells, and 1 study utilizes scallop shells.
Despite the relatively greater number of studies involving oyster shells, substantial research gaps remain. There is a broad range of materials that could still be investigated as precursors in combination with these shells. The number of studies focused on scallop, Periwinkle, clam, and mussel shells remains limited, and no articles were found that specifically explore cockle shells, although these have previously been used as aggregates in cementitious materials and are identified as promising candidates for future research.
Figure 3 presents the percentage distribution and geographic location of global scientific production on geopolymers incorporating mollusk residues, based on the country of affiliation or nationality of the authors of the analyzed studies. For this reason, the total count exceeds the number of articles themselves. The data reveal a significant concentration of research in Asian countries, with China standing out as the leading contributor, responsible for 25.8% of total publications, corresponding to 34 articles over the period analyzed. This figure reinforces China’s central role as the primary knowledge producer on this topic, likely driven by the country’s extensive availability of marine-based waste. China has been the world’s leading producer of aquaculture-derived animals since 2002, an activity practiced for over two thousand years. With the country’s technological advancement, aquaculture has reached an industrial scale, including the development of improved species [25].
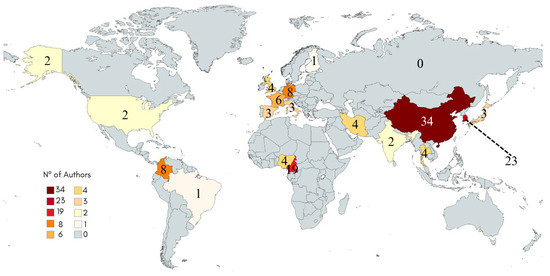
Figure 3.
Country scientific production by number of authors after initial exclusion of articles.
China’s prominent role in aquaculture has resulted in the generation of significant quantities of marine animal waste, including bivalve mollusk shells, which require proper management. This scenario may have encouraged investment in sustainable technologies. Combined with the growing interest in alternative materials to Portland cement, this has likely contributed to the increasing number of studies on alkali activation involving bivalve shells.
South Korea ranks second, with 23 publications (17.4%), followed by Cameroon with 19 publications (14.4%). South Korea is currently the seventh-largest aquaculture producer globally. Similar to China, the country benefits from substantial investment in research and development. In addition, the Asian continent is responsible for approximately 90% of global bivalve mollusk production [13]. The considerable number of studies from Cameroon is particularly noteworthy, given that it is an African country not typically recognized as a major aquaculture producer. This highlights a growing regional interest in reusing organic waste for the development of sustainable cementitious materials. Unlike China and South Korea, Cameroon’s position in the global aquaculture industry is modest, making its high level of scientific output in this field a notable indication of the globalization of research on alkali-activated materials, even in countries where aquaculture is not a dominant economic sector.
Other countries with relevant contributions include Germany and Colombia, each with six publications (6.1%), and France, with four publications (4.5%). In Latin America, Colombia emerges as the main contributor. The global map (Figure 3) provides a clear visualization of the geographic distribution of these studies, highlighting the principal centers of scientific activity. Although the majority of publications are concentrated in Asia, research efforts are also observed across other continents, including North and South America, Europe, and Africa.
In addition to the countries already mentioned, further contributions were identified from Japan, Iran, Nigeria, Spain, the United Kingdom, Thailand, Italy, and the United States, with publication counts ranging from one to four. It is worth noting that virtually all countries engaged in this line of research have access to coastal regions. This suggests a strong correlation between the availability of mollusk waste and the development of scientific studies focused on its application in cementitious systems. Proximity to coastal environments facilitates both access to raw materials and local interest in sustainable waste reuse strategies.
Overall, the data presented in Figure 3 indicate that the use of mollusk residues in alkali-activated systems is gaining global relevance, with particular prominence in Asia and select regions of Europe, Africa, and Latin America. This scenario underscores the growing importance of sustainable solutions in construction and highlights the strategic role of locally available resources in advancing low-carbon cement technologies.
In total, authors from 17 different countries were identified in the articles retrieved from the Scopus and Web of Science databases. International collaborations among researchers from different nations were also observed. These findings suggest that the topic remains relatively new and has considerable potential for growth within the research community. Given that the United Nations has 193 member states, the involvement of researchers from only 17 countries in studies on alkali activation using mollusk residues demonstrates that this field is still emerging.
3.2. Word Cloud and Network Frame
In addition to the discussions based on annual scientific production and the scientific output of each country by number of authors, valuable insights can also be derived from analyzing the words that most frequently appear in the titles of these articles, as well as from mapping and network analyses that illustrate the interconnections among these terms. Figure 4 presents the word cloud generated from the analysis of titles, abstracts, and keywords of the selected articles related to alkali activation with mollusk residues. This visual tool enables rapid and intuitive identification of the most frequently used terms in the scientific literature and, by extension, the main research trends within the field.
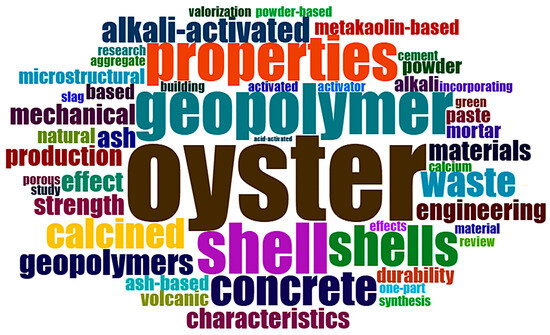
Figure 4.
Word Cloud of article titles on alkali activation using mollusks after initial exclusion of articles.
The most prominent term is “oyster”, indicating that oyster shells have been the most extensively investigated type of mollusk residue used as a raw material for the formulation of alternative binders. The terms “shell” and “shells” also appear with high frequency, reflecting a general interest in the utilization of marine-derived calcareous waste in cementitious systems.
The keywords “geopolymers” and “alkali-activated” demonstrate that the majority of studies focus on the production of alkali-activated binders, with particular emphasis on geopolymer technology. This focus is frequently associated with the pursuit of more sustainable materials with lower environmental impact compared to conventional Portland cement.
Additionally, terms such as “properties”, “mechanical”, “strength”, “microstructural”, “durability”, and “characteristics” indicate that many studies are primarily concerned with evaluating the physical, mechanical, and structural performance of the developed materials. Words such as “concrete”, “mortar”, and “paste” point to the practical application of these binders in cementitious formulations, further emphasizing the technological orientation of the investigations.
The presence of terms such as “calcined”, “slag”, “ash-based”, “metakaolin-based”, “volcanic”, and “natural” suggests that mollusk residues are often combined with other precursor materials, both industrial and natural in origin. Finally, terms such as “waste”, “production”, and “valorization” (even when implicit) reflect the environmental concerns that underpin these studies, particularly those aimed at waste valorization and the reduction in carbon footprint in the construction sector.
Therefore, the word cloud confirms that research on alkali activation involving mollusk residues has predominantly focused on oyster shells as alternative materials for geopolymer production, with an emphasis on characterizing their properties and assessing their potential application as sustainable binders.
Figure 5 presents the mapping of keyword co-occurrences from the analyzed articles, represented as a term network. In this visualization, the nodes correspond to the most frequent words in the literature, while the connecting lines indicate the frequency with which these terms appear together within a given study. The size of the nodes reflects the frequency of each term, and the thickness of the connections represents the strength of the relationship between terms.
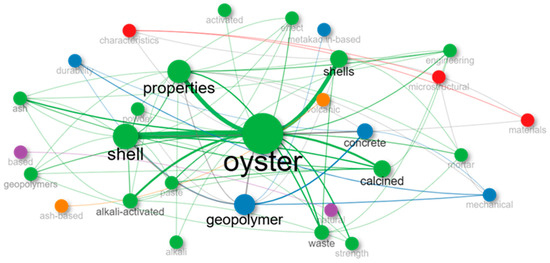
Figure 5.
Network Frame of article titles on alkali activation using mollusks after initial exclusion of articles.
The term “oyster” stands out as the central node within the network, reaffirming that oyster shells represent the primary mollusk residue studied in the production of alkali-activated materials. Surrounding this core are terms such as “shell”, “shells”, “geopolymer”, “properties”, “concrete”, “calcined”, and “alkali-activated”, forming a highly interconnected cluster. This indicates that the reviewed studies frequently associate the use of oyster shells with geopolymer production and the investigation of their physical and mechanical properties, particularly when used in concrete or mortar formulations.
The presence of technical terms such as “microstructural”, “durability”, “mechanical”, “strength”, “mortar”, and “waste” reflects the multidisciplinary nature of the research, which combines aspects of performance characterization, sustainability, and waste reuse. Furthermore, terms such as “calcined”, “metakaolin-based”, “ash-based”, “volcanic”, and “slag” refer to the various precursors or additives that are commonly combined with mollusk residues in the studies.
The network also reveals the presence of term clusters that highlight specific subtopics within the broader research field, such as durability and sustainability (in green), mechanical performance and microstructure (in red and blue), and types of activated materials (in purple). The dense interconnections among nodes indicate that the studies frequently address material properties, types of waste, and practical applications in an integrated manner.
This network analysis complements the information provided by the word cloud (Figure 4), allowing for the identification of not only the most frequent terms but also the relationships among them within the field of alkali activation involving mollusk residues.
The comparison between the word cloud and the co-occurrence network reinforces the relevance of the central terms within this field of study while offering different levels of analytical insight. Whereas the word cloud highlights the isolated frequency of keywords, enabling a quick overview of the most recurrent terms, the co-occurrence network deepens this understanding by illustrating how these concepts are interrelated. In this way, both visualizations are complementary, providing an integrated view of both the presence and the thematic connections found in studies focused on the use of mollusk residues in alkali-activated materials.
4. Cement Industry and Its Associated Environmental Impacts
The production of construction materials is responsible for approximately 25 percent of all greenhouse gas emissions worldwide. Concrete is the most widely used material in the construction of urban environments. It is primarily composed of cement and steel, and its manufacturing process involves high energy consumption and significant CO2 emissions. As a result, cement production alone is estimated to account for about 8 percent of total global emissions [1,26].
Concrete is the most consumed man-made material in the world, and its primary binder is Portland cement. In addition to its high CO2 emissions, the massive demand for cement leads to the excessive extraction of raw materials, the generation of large volumes of industrial waste, and substantial water consumption. Consequently, the construction industry is considered the largest consumer of freshwater resources globally [27].
Cement is mainly composed of calcined lime, which provides calcium oxide, and clay, which is rich in silica, alumina, and iron oxide. These raw materials are obtained through quarry blasting and rock drilling using explosives, contributing further to the exploitation of natural resources. The manufacturing process involves pyroprocessing, during which lime and clay are subjected to high-temperature treatments to induce the chemical reactions required to produce usable cement. These thermal processes rely on fossil fuels or other combustible materials, resulting in the emission of large quantities of pollutants, including not only CO2 but also CO, NOX, SOX, and numerous other substances harmful to human health and the environment [28]. In addition, the handling, loading, and transportation of cement clinker generate particulate emissions, contributing further to air pollution [29].
In 2024, global production of Portland cement reached 4.1 billion tonnes. China remains by far the largest consumer market, with a production volume of 1.9 billion tonnes, followed by India (420 million tonnes), Vietnam (110 million tonnes), the United States (86 million tonnes), Turkey (81 million tonnes), Iran (71 million tonnes), and both Indonesia and Brazil (67 million tonnes each) [30].
Given these considerations, and in light of the projected 23 percent increase in global Portland cement demand by 2050, it becomes imperative to develop solutions that reduce pollution and environmental degradation associated with the construction industry. One such strategy involves the introduction of alternative binders capable of partially (supplementary cementitious materials) or fully (alkali-activated cements) replacing Portland cement [27,31].
4.1. Alkali-Activated Binders
Alkali-activated binders remain a relatively recent research topic. Despite this, they are extremely versatile materials, used primarily in the production of cements and mortars, and have even been reported as inorganic adhesives capable of replacing organic polymers in the encapsulation of DUV LEDs [24,32]. As such, numerous studies are currently being conducted to address existing knowledge gaps and explore questions related to environmental cost–benefit, economic feasibility, and performance quality of these binders [33]. The precursors commonly used in the production of these materials include industrial residues derived from the metallurgy, energy, agriculture, food, and mining sectors. When combined with alkaline activators, these residues can form binders with favorable mechanical strength and durability properties [34]. The alkaline activators are typically applied in aqueous solution, most often based on hydroxides, silicates, carbonates, or sulfates of alkali metals [35].
Alkali-activated cements can generally be classified into three categories: high-calcium systems, low-calcium systems, and hybrid systems. High-calcium alkali-activated cements are also rich in silica, with the combined content of SiO2 and CaO exceeding 70% by weight, and contain a lower proportion of alumina. These systems typically lead to the formation of calcium silicate hydrate (C-A-S-H) gels under moderate alkaline conditions. Ground granulated blast furnace slag is a common example of this category [3].
Alkali-activated binders can be produced using two different mixing strategies. The first is the one-part method, in which dry powders are mixed with water only. The second is the two-part method, where a solid precursor is combined with a liquid alkaline activator [24].
Alkali-activated binders are still considered an emerging technology, with many ongoing studies aimed at understanding their behavior and overcoming current limitations. This technology relies on aluminosilicate-rich waste materials such as rice husk ash, ground granulated blast furnace slag, fly ash, corn cob ash, metakaolin, volcanic ash, and glass powder, all of which are combined with an alkaline medium [27]. To replace commercial lime, bivalve mollusk shells have shown significant potential due to their high calcium carbonate content. These shells can be used for the production of hydrated and quicklime, as well as in construction blocks, bricks, asphalt, ceramics, and polyethylene foam [36].
4.2. Alkali Activation Using Bivalve Mollusk Shells
The rapid expansion of the aquaculture sector has intensified the generation of waste from these practices. Focusing specifically on bivalve mollusc shells, production in 2022 reached nearly 19 million tons. This volume highlights the magnitude of shell waste produced annually, a considerable proportion of which is subject to inappropriate disposal practices [14]. Mollusk shells represent between 40 and 70 percent of the animal’s total weight, and following their culinary use, they are frequently discarded into sewage systems, oceans, vacant lots, or landfills in an irregular manner. Such practices cause environmental imbalances in ecosystems, unpleasant odors, and the proliferation of insects [15,20,23,37].
Researchers have investigated the use of residues obtained from shells, such as oyster shells, in various applications, including the production of construction materials, water treatment for pollution control, plastics manufacturing, and even in medicine for dietary supplements, agricultural fungicides, and fire-retardant agents [38]. Other types of shells, such as those from eggs, snails, cockles, mussels, and scallops, have also been studied and applied across different industries with the objective of reusing these by-products, which have historically lacked adequate utilization [39].
Alkaline materials can be synthesized from a wide variety of aluminosilicate precursors, which differ in terms of reactivity, availability, cost, and value across different regions of the world. This diversity underscores the versatility and potential for local adaptation of these materials [24]. Shells from marine mollusks can participate in alkaline activation reactions as a calcium source (in high-calcium systems) when combined with other residues rich in silica and alumina. The product of this reaction is the formation of calcium-aluminosilicate hydrate (C-A-S-H) gels, which are chemically similar to those formed during the hydration of Portland cement, where calcium carbonate derived from limestone and aluminosilicates from clay are the main reactive components [3,40].
Brazil is the second-largest producer of bivalve mollusks in Latin America, and shellfish farming represents the country’s most prominent aquaculture activity [36]. In 2023, Brazil’s production of oysters, scallops, and mussels totaled 8,729,164 units [41], with the state of Santa Catarina being the largest domestic producer due to favorable geographic and water quality conditions [36]. Given that oysters and mussels are the most widely cultivated mollusks in Brazil [42] and that they generate millions of tonnes of poorly managed waste annually, coupled with their potential reuse as raw materials in construction, there is clear justification for further research into alternative uses for these residues. Such efforts could contribute significantly to reducing the environmental impact associated with discarded shell waste [43].
Table 2 aims to synthesize the data extracted from the articles selected for this review, providing a clearer understanding of how these studies relate to one another through a categorization based on the type of bivalve mollusk shells used, the additional materials investigated, the alkaline activators employed, the analyses performed, the strengths achieved, and their respective references.

Table 2.
Description of the articles related to alkali activation using mollusk shells.
From the aspects presented in Table 2, specific features of each approach adopted in the studies selected for this review can be observed. By examining the chemical, physical, and microstructural analyses performed by each author, it is possible to understand the main focus of each work. Furthermore, the table allows the identification of which activators, mollusk shell types, and additional materials have been used in combination, thereby facilitating the recognition of existing gaps that still require further investigation. In addition to discussing the elements summarized in Table 2, this section also presents relevant interpretations and recurring patterns reported in the reviewed studies, providing a comprehensive overview of the current literature on alkali activation using mollusk shells.
Studies available in the literature on alkali activation using oyster shells have been conducted by authors such as Zhang, Lin, and Wang [45], Zhang et al. [1], Djobo et al. [47], Nasaeng et al. [10], Le et al. [46], Monneron-Gyurits et al. [11], Yang and Jang [22], Shao et al. [51], Okoro and Oyebisi [44], Angdiarto, Chen, and Chang [49], Deng et al. [52], Yanting et al. [53], and Choi et al. [50]. Oyster shells are unquestionably the most studied bivalve mollusk waste in the field of alkali activation. This trend can be explained primarily by availability, as oysters are the most widely produced bivalve mollusks on a global scale [14]. Consequently, oyster shell residues are far more abundant, and their disposal represents a greater environmental concern compared to other mollusk shells that are generated in smaller quantities.
In contrast, studies focused on scallop, periwinkle, and mussel shells have been conducted by Hasnaoui et al. [12], Oladele et al. [13], Monneron-Gyurits et al. [11] and López et al. [54], respectively. These materials remain underexplored in the context of alkali activation. All of the aforementioned studies will be analyzed and discussed in this section, with particular emphasis on their methodologies, materials, alkaline activators, and specific experimental approaches.
Notably, studies by Angdiarto, Chen, and Chang [49], Choi et al. [50], and Yang and Jang [22] utilized mollusk shells themselves as the alkaline activator, offering an innovative perspective on alkaline activation systems. In the study by Choi et al. [50], calcined oyster shells were compared with other more conventional commercial alkaline activators. The best unconfined compressive strength (UCS) results obtained by Choi et al. [50] for activators based on calcined oyster shells, calcium hydroxide, sodium hydroxide, and potassium hydroxide were recorded at 7 days of curing using the highest slag-to-sand ratio (16%), resulting in strengths of 1.2 MPa, 1.5 MPa, 2.9 MPa, and 3 MPa, respectively.
Although the calcined oyster shells yielded lower strength values compared to other activators, the authors emphasized their chemical similarity to calcium hydroxide. The UCS results for oyster shell-based systems were only slightly lower at 7 days. However, when compared to sodium or potassium hydroxide, the results remained considerably inferior. Nonetheless, oyster shells were deemed suitable for extended curing periods, during which their reactivity improved and led to enhanced performance [50].
Bivalve mollusk shells are rich in calcium carbonate (CaCO3), which is abundantly present in their composition. Upon calcination, CaCO3 decomposes into calcium oxide (CaO), enabling the reuse of these residues as substitutes for conventional materials in sectors such as construction [15]. Through calcination, mollusk shells can reach CaO contents exceeding 90%, as confirmed by characterization studies on individual shell types: oysters [22], scallops [12], mussels [11], cockles [55], clams [56], and periwinkles [13], which reported CaCO3 contents of 98.0%, 96.6%, 90.71%, 98.9%, 96.8%, and 94.7%, respectively.
Calcination temperatures varied among studies, ranging from 500 °C [46,47,48] to 1000 °C [22,44]. The longest calcination time was reported by Le et al. [46], who conducted calcination at 500 °C for three days. The shortest was 30 min at 800 °C, reported by Nasaeng et al. [10]. Therefore, calcination temperatures for bivalve mollusk shells remain unstandardized, both in terms of duration and temperature. This lack of standardization poses a significant challenge for large-scale industrial applications, highlighting the need for the development of specific guidelines and regulations based on future advances in research to enable effective implementation in industrial processes.
It is important to note that in studies such as those by Shao et al. [51], Yanting et al. [53], Zhang et al. [1], Zhang, Lin, and Wang [45], Deng et al. [52], Hasnaoui et al. [12] and Yanting et al. [53], oyster shells were only ground and not subjected to calcination. In these cases, the shells primarily acted as fillers or fine aggregates in the alkali-activated matrix. Even so, these studies reported improvements in the physical and mechanical properties of the binders, demonstrating the potential of uncalcined oyster shells when used in conjunction with alkali-activated systems.
Cements produced from mollusk shells demonstrated satisfactory unconfined compressive strength (UCS) values in the studies analyzed in this review. However, significant variations in strength were observed among the studies, mainly due to differences in the precursors used in combination with the shell-based materials. The data presented in the table illustrate these variations, showing UCS values of up to 80 MPa at 28 days, which exceeds the typical strength range required for conventional Portland cement, generally between 25 and 40 MPa at 28 days [57].
Monneron-Gyurits et al. [11] compared the performance of calcined and uncalcined oyster and mussel shells. Their conclusions indicated that when uncalcined shells were used, the reaction occurred predominantly via physical interaction, serving mainly as filler in sodium silicate-based systems. However, a small portion of the shells could still react depending on the reactivity of the other materials present in the system. Zhang et al. [1] reported that the incorporation of uncalcined oyster shells nonetheless promoted the formation of additional C-A-S-H gels.
Calcined shells participate much more actively in alkaline reactions. In such systems, two competing reactions occur: accelerated polycondensation and subsequent hydration. The formation of portlandite and C-A-S-H gels confirms that the hydration reaction is predominant. This behavior is attributed to the high reactivity of lime produced during calcination, which actively contributes to gel formation [11]. It is worth noting, however, that compressive strength was not evaluated in that study, as the authors focused primarily on chemical characterization of the binder and the reactions associated with either calcined or uncalcined shells.
Bivalve mollusk shells can contribute to pore filling within the cement matrix, thereby enhancing the overall density of the cementitious structure. This effect occurs independently of calcination, as the shells promote the precipitation of calcite through the carbonation of portlandite, which further contributes to matrix densification. However, this incorporation should be carefully controlled, since excessive amounts of oyster shell material in geopolymer formulations may negatively affect structural integrity. Most studies recommend additions of up to approximately 30 percent to achieve the desired balance between densification and mechanical performance [58,59,60].
Curing times for the specimens varied depending on the experimental approach adopted in each study. The shortest curing durations were one day, as seen in the works of Djobo et al. [48] and Deng et al. [52]. The longest durations were 180 days, as reported by Zhang et al. [1] and Djobo et al. [48]. The most commonly used activator was NaOH, reported in 12 studies, often in combination with commercial sodium silicate. This aspect is explained by the fact that NaOH has a more accessible commercial value, good solubility in aqueous media, and easy availability, which provides a more favorable cost benefit [61].
Overall, studies indicated that curing times of 28 days or more yielded significantly improved strength outcomes when optimal binder and activator proportions were used. Exceptions include studies that focused on specific parameters under fixed curing durations, such as Zhang et al. [1], which investigated high-temperature performance at 180 days; Shao et al. [51], which examined alkaline solution influence, binder proportions, and foaming agents at 7 days; and Monneron-Gyurits et al. [11], which compared oyster and mussel shells at 15 days. Therefore, these studies are not directly comparable within this context.
The reason for increased strength at extended curing times is related to the delayed participation of mollusk shells in the reaction. After one day of curing, the quantity of C-A-S-H gels formed is not significantly influenced by the proportion of shell powder. However, as curing time increases, calcium ions are gradually released, promoting gel formation. The binder matrix becomes increasingly interconnected, forming a denser, less porous, and more uniform three-dimensional C-A-S-H network, thereby improving both compressive and tensile strength [45].
Regarding the precursors used in the selected studies, ground granulated blast furnace slag was the most common, appearing in 9 out of 17 studies. However, it was often combined with other materials, such as ceramic waste powder [1,45], nanosilica [49], fly ash [52], and spent coffee grounds [46]. Other unconventional materials were also employed, including volcanic ash [47,48], pumice [10], and cocoa pod ash [13]. Granulated blast furnace slag has been widely used in alkali activation studies due to its uniform and optimized pore distribution, which results in a dense and durable cementitious matrix. In addition, it provides good resistance to high temperatures, leading to alkali-activated cements with favorable physical and mechanical properties. This explains its frequent use in alkali activation research involving different precursors [62]. The combination of non-calcined oyster shells with granulated blast furnace slag has been shown to enhance the physical properties of alkali-activated cements, promoting matrix densification and reducing porosity during extended curing periods [53]. When calcined, oyster shells further improved compressive strength by 14.52 percent and 31.55 percent with substitutions of 10 and 20 percent, respectively, in combination with 3 percent nanosilica [49].
Oyster shells were the most commonly used type, featured in 14 of the 17 studies on alkali activation using bivalve mollusk residues. Investigations involving scallop, periwinkle, and mussel shells remain scarce, with only Monneron-Gyurits et al. [11], Hasnaoui et al. [12], and Oladele et al. [13] addressing their application. Cockle and clam shells have not yet been explored in the context of alkali-activated binder production.
Nevertheless, cockle and clam shells exhibit similar properties to the shells already studied and thus present considerable potential for future research. Although they have not been studied for alkaline binder production, they have been investigated as aggregates for improving the strength of cement and mortar formulations and characterized in studies by Lertwattanaruk, Makul, and Siripattarapravat [56], Olivia, Oktaviani, and Ismeddiyanto [63], Oyejobi et al. [64], Chung et al. [55], and Khankhaje et al. [65].
An additional noteworthy application of calcined and alkali-activated mollusk shells is in the stabilization of clayey soils. When used as a soil stabilizer, the material has been shown to improve unconfined compressive strength (UCS), often yielding results comparable to or even surpassing those obtained with traditional Portland cement. For instance, a mixture containing 11% mollusk shells and a 1 M alkaline activator produced a UCS of 3756 kPa at 7 days, compared to 3453 kPa achieved with Portland cement. At 28 days, the UCS for the Portland cement mixture was 6150 kPa, while the mixture with mollusk shells reached 4605 kPa. Additionally, mollusk shells have been shown to raise soil pH and promote the formation of C-S-H and C-A-S-H gels, which contribute to the immobilization of heavy metals in contaminated soils, as reported by López et al. [54].
Based on the findings discussed in this section, it is evident that the selected studies adopt a wide range of approaches. This methodological diversity should be viewed as a positive aspect, as it enhances the understanding of material behavior across different variables and highlights the versatility of mollusk shells. These studies collectively demonstrate the critical role that mollusk shells can play in alkali activation research and underscore their potential as a valuable resource in the development of sustainable cementitious materials.
The results reported by the selected authors show that when mollusk shells are used in combination with aluminosilicate-rich precursors in alkaline activation systems, they can improve the physical, chemical, and microstructural properties of the resulting binders. Due to their high calcium content and chemical similarity to limestone used in conventional cement, these shells represent a viable and promising alternative. Alkali activation using mollusk residues is founded on the principle of minimizing environmental degradation and holds the potential to significantly reshape cement production practices. This alternative should be further disseminated and explored, as it can provide considerable benefits to both society and the environment.
4.3. Scallops, Mussels and Periwinkle Shells
As reported in the previous section, mussel, scallop, and periwinkle shells have received limited attention in alkali activation research. This is primarily due to their lower global production compared to oyster shells, which results in a smaller quantity of waste available for reuse. Therefore, this section focuses on studies that have incorporated mussel, scallop, and periwinkle shells, discussing their findings and offering guidance for future research on the use of these materials. In general, as previously mentioned, the chemical composition of scallop, mussel, and periwinkle shells is extremely similar to that of oysters, leading to comparable results. Unfortunately, the lack of studies involving clam and cockle shells prevents further discussion of their performance under alkaline conditions.
The use of scallop shells has shown strength gains and reduced water demand for moderate replacement levels, typically between 20 and 30 percent. Studies involving mussel shells indicate a replacement limit of up to 40 percent or an addition of up to 30 percent before the physical characteristics of alkali-activated cements begin to deteriorate. Both studies reported improvements in the physical and mechanical properties of alkali-activated cements with the inclusion of scallop and mussel shells. However, it should be noted that only mussel shells underwent calcination, conducted at 850 °C. Unconfined compressive strength (UCS) for mussel-based materials was evaluated mainly in soil applications, with calcination temperatures between 700 and 900 °C, reaching 4.6 MPa at 28 days at the optimal dosage. For scallop shells, the maximum strength was achieved at 90 days, with an average of 60 MPa [11,12,54].
The addition of periwinkle shells resulted in faster setting of mortars, reducing the required curing time for binders based on cocoa pod ash, kaolin clay, and quarry dust. Calcination was performed at 700 °C, yielding a peak UCS of approximately 35 MPa at 28 days. However, the replacement levels were low, with the best results obtained at 2 to 3 percent addition, and a maximum of 5 percent [13].
From a mineralogical perspective, a slight difference exists between mussel and oyster shells. Mussel shells contain a mixture of calcite and aragonite, whereas oyster shells are composed predominantly of calcite. This difference may influence the optimal calcination temperature [11].
Overall, although oyster, mussel, scallop, and periwinkle shells have shown promising results, the limited number of available studies—only four to date—prevents the establishment of a consistent behavioral trend. Further research is needed to standardize procedures and to improve the understanding of the advantages and limitations of using these shells in comparison with oyster shells for alkali activation applications.
4.4. Urban Mining as a Framework for Bivalve Shell Valorization
The generation of bivalve mollusk shell waste occurs in predictable locations such as aquaculture processing plants, seafood markets, and coastal restaurants. This spatial concentration allows bivalve shells to be integrated into a strategic resource flow within the framework of urban mining. In contrast, agroindustrial residues such as rice husks and sugarcane bagasse are distributed across more dispersed and geographically variable sources, making their collection and transportation less predictable and often more costly. Therefore, when bivalve shells are incorporated into targeted collection and recycling systems, they can provide a consistent and localized supply of calcium-rich material suitable for cement formulations, thereby effectively operationalizing the concept of urban mining [17,18].
From a resource management perspective, urban mining shifts the perception of shell waste from an environmental liability to an accessible “secondary limestone reserve” embedded within urban metabolism. This reframing is essential for closing material loops in the construction sector, as it enables the partial substitution of quarried limestone without expanding mining frontiers. Such substitution not only mitigates landscape degradation and biodiversity loss associated with quarrying but also reduces transportation distances when shells are sourced from local coastal cities rather than remote mineral extraction sites [19,21].
The reviewed studies show that shell-derived calcium carbonate, whether in calcined or uncalcined form, can perform competitively in alkali-activated binder systems, meeting both strength and durability requirements [22,52]. Embedding these findings in an urban mining strategy creates a pathway for integrating construction material manufacturing into local circular economies, where waste recovery, processing, and end-use occur within the same regional supply chain. This can foster decentralized production models that are more resilient to disruptions in global raw material markets.
Moreover, urban mining of shells offers co-benefits beyond material substitution. It supports waste diversion from landfills and marine environments, contributes to local job creation in collection and processing, and strengthens community engagement in sustainable waste management. The challenge lies in scaling up from pilot initiatives to region-wide, coordinated recovery systems, supported by policy incentives, technical standards, and partnerships between the aquaculture and construction industries.
4.5. Study Limitations
One of the main limitations of this review lies in the fact that alkali activation using mollusk shells is still an emerging research topic, with a relatively small number of studies published to date. This limited exploration makes it difficult to establish consistent patterns regarding the behavior of the binders evaluated. Several knowledge gaps and research limitations remain to be addressed in the field, including the behavior of mollusk shells when combined with other unstudied residues; the lack of studies involving shells of scallops, mussels, cockles, clams, and periwinkles, as research efforts have largely focused on oyster shells; and the evaluation of these binders under extreme climatic conditions, since most studies have been conducted under controlled laboratory environments without accounting for real-world weathering scenarios. Furthermore, detailed cost analyses and life cycle assessments remain scarce, despite their importance for determining the industrial and environmental feasibility of large-scale implementation.
An additional limitation, highlighted by the urban mining framework adopted in this work, is the absence of systematic studies that quantify the spatial distribution, seasonal variability, and collection logistics of bivalve shell waste in urban and peri-urban contexts. While isolated reports document recovery from restaurants and seafood markets [19], there is no comprehensive mapping of these anthropogenic deposits to support reliable feedstock supply models. Similarly, research rarely addresses the integration of shell recovery into existing municipal waste management systems, which is crucial for scaling up from pilot projects to regional or national programs.
Another limitation of this review is its reliance on only two databases, Scopus and Web of Science. Nevertheless, these databases are widely recognized within the academic community and include many of the most relevant peer-reviewed journals. Moreover, the authors took care to examine whether additional relevant studies cited within the selected literature were not indexed in the aforementioned databases, ensuring that no key references were overlooked in the context of this review.
5. Conclusion Remarks
Based on the analysis and discussion of studies addressing the use of bivalve mollusk shells in alkali activation, considering different precursors, activators, analytical methods, curing times, calcination temperatures, and material preparation techniques, several specific conclusions can be drawn. This review is, to the best of our knowledge, the first to synthesize the available literature explicitly under the urban mining perspective, reframing shell waste as a secondary limestone resource embedded in urban and peri-urban environments. While the individual technical, chemical, and mechanical aspects of shell-based alkali-activated binders have been previously reported, no prior work has integrated these findings into a holistic framework that connects material performance with the recovery of valuable materials from urban waste streams, circular economy strategies, and the displacement of natural limestone extraction.
The current body of research remains limited in scope, with the majority of studies focusing on laboratory-scale assessments of oyster shells. There is a complete absence of field-based evaluations that demonstrate the feasibility of implementing urban mining supply chains for shell recovery, processing, and use in binder production. Likewise, there is no systematic mapping of the spatial distribution, seasonal availability, or collection logistics of shell waste, all of which are essential for ensuring reliable feedstock supply in large-scale industrial applications.
From a technical perspective, the reviewed literature confirms that both calcined and uncalcined bivalve shells can be effectively used in high-calcium alkali-activated systems, achieving mechanical strength, durability, and microstructural properties comparable to or exceeding those of conventional binders. However, significant knowledge gaps persist regarding the compatibility of shell-derived calcium carbonate with a broader range of precursors beyond those already studied, the influence of regional and species-specific compositional variability on reactivity and long-term performance, and the durability of shell-based binders under real-world exposure conditions, including aggressive climates and cyclic loading.
In terms of economic and environmental feasibility, no comprehensive techno-economic analyses or life cycle assessments have been conducted within an urban mining framework for shell waste utilization in alkali-activated binders. This absence of quantitative sustainability metrics limits the ability to verify potential reductions in carbon footprint, energy use, and cost when compared to conventional limestone-based systems. Moreover, there is insufficient evidence on the integration of shell recovery into existing municipal or regional waste management systems, which is a key operational challenge for realizing the full circular economy potential of urban mining strategies.
Considering the high global production of bivalve mollusks and the volumes of shell waste that remain improperly disposed of, the application of an urban mining framework to alkali-activated binder production presents both a technical opportunity and an environmental imperative. While laboratory-scale studies confirm technical feasibility, industrial adoption will depend on addressing the identified research gaps, developing urban resource mapping and recovery networks, and validating environmental and economic performance through field trials and robust life cycle assessment studies.
In addition to identifying these gaps, the comparative analysis of the reviewed studies provides preliminary process guidelines. The most successful results were obtained when shells were calcined at temperatures between approximately 700 °C and 900 °C, which is sufficient to ensure the decomposition of CaCO3 into reactive CaO while avoiding excessive energy consumption and sintering effects. Combinations with blast furnace slag, fly ash, and certain agricultural ashes appear particularly promising, as they optimize the Ca/Si ratio and promote the formation of C-A-S-H and N-A-S-H gels under ambient curing conditions. Lower calcination temperatures combined with mechanical activation and fine grinding can also enhance reactivity in applications where complete conversion to CaO is not required. Other notable findings include the use of calcined bivalve mollusk shells as alkaline activators themselves, as soil stabilizing agents, and in combination with various silica and alumina-rich precursors, demonstrating the versatility of this material. Although these processing strategies still require validation at larger scales, they provide a solid foundation for future field trials aimed at demonstrating both technical performance and economic feasibility within the operational framework of urban mining systems.
Furthermore, based on the trends identified in the results and the knowledge gaps outlined in the discussion, other calcium-rich residues such as carbide lime, eggshells, and demolition-derived concrete fines, as well as silica- and alumina-rich wastes such as rice husk ash, sugarcane bagasse ash, and mine tailings, represent promising candidates for co-utilization with bivalve shells. Such combinations could enhance the chemical balance of alkali-activated systems, improve mechanical performance, and increase the robustness of supply chains, while also addressing multiple waste streams simultaneously through an integrated urban mining approach.
By consolidating the scattered scientific evidence into a unified framework that bridges materials science, waste management, and sustainability assessment, this study not only identifies the untapped potential of bivalve shells as an urban mineral resource but also sets the research and implementation agenda required to transform this abundant waste into a strategic raw material. Emphasizing the urban mining perspective provides a clear pathway for coupling technical feasibility with resource recovery logistics, offering a viable, circular, and locally sourced alternative to traditional limestone exploitation in the construction industry.
Author Contributions
Conceptualization, A.P.C., G.J.B. and E.P.K.; methodology, A.P.C., G.J.B. and E.P.K.; validation, A.P.C., G.J.B. and E.P.K.; formal analysis, A.P.C.; investigation, A.P.C. and G.J.B.; resources, A.P.C., G.J.B. and E.P.K.; data curation, A.P.C., G.J.B. and E.P.K.; writing—original draft preparation, A.P.C. and G.J.B.; writing—review and editing, E.P.K.; visualization, A.P.C., G.J.B. and E.P.K.; supervision, G.J.B. and E.P.K.; project administration, G.J.B. and E.P.K.; funding acquisition, G.J.B. and E.P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Conselho Nacional de Desenvolvimento Científico Tecnológico (Master’s fellow-ship and Research Productivity fellowship—grant number 305910/2023–0).
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CO2 | Carbon Dioxide |
| NOX | Nitrogen Oxides |
| SOX | Sulfur Oxides |
| C-A-S-H | calcium-aluminosilicate hydrate |
| UCS | unconfined compressive strength |
| XRF | X-ray fluorescence |
| XRD | X-ray diffraction |
| SEM | Scanning Electron Microscope |
| EDS | Energy-Dispersive X-ray Spectroscopy |
| NaOH | Sodium Hydroxide |
| SiO2 | Silicon Dioxide |
| Na2O | Sodium Oxide |
| TGA | Thermogravimetric analysis |
| FT-IR | Infrared spectroscopy |
| UPV | Ultrasonic pulse velocity |
| Na2SiO3 | Sodium Metasilicate |
| MIP | Modified Impact Penetration test |
| H2O | Water |
| ITC | Isothermal titration calorimetry |
| TCLP | Toxicity Characteristic Leaching Procedure |
| RCP | Rapid Chloride Permeability |
| RCM | Chloride migration coefficient |
| EIS | Electrochemical impedance spectroscopy |
| DTG | Derivative Thermogravimetry |
| WSL | Waste seashell |
References
- Zhang, G.Y.; Lee, S.; Han, Y.; Wang, X.Y. The Effect of Oyster Shell Powder on the High-Temperature-Properties of Slag-Ceramic Powder-Based Geopolymer. Materials 2023, 16, 3706. [Google Scholar] [CrossRef]
- Mo, K.H.; Alengaram, U.J.; Jumaat, M.Z.; Lee, S.C.; Goh, W.I.; Yuen, C.W. Recycling of Seashell Waste in Concrete: A Review. Constr. Build. Mater. 2018, 162, 751–764. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Palomo, A. Low Environmental Impact Hybrid Cements: Reducing Clinker Content. Alconpat 2015, 5, 141–151. [Google Scholar] [CrossRef]
- Huang, B.-T.; Xi, H.; Ma, R.-Y.; Zhang, Z.-L.; Lao, J.-C.; Zhang, H.; Shen, Y.-N.; Shi, D.-D.; Xu, L.-Y. Ultra-High-Strength Engineered Geopolymer Composites (UHS-EGC) with Mineral Processing Waste: Probabilistic Modeling of Cracking Behavior. Theor. Appl. Fract. Mech. 2025, 140, 105138. [Google Scholar] [CrossRef]
- Mateus, W.; Levandoski, K.; Mota, J.D.; Menegolla, C.; Ferrazzo, S.T.; Bruschi, G.J.; Korf, E.P. Mechanical Strength, Mineralogical Characteristics and Leaching Behavior of Iron Ore Tailings Stabilized with Alkali-Activated Rice Husk Ash and Eggshell Lime Binder. Minerals 2025, 15, 567. [Google Scholar] [CrossRef]
- Pelisser, G.; Ferrazzo, S.T.; Mota, J.D.; dos Santos, C.P.; Pelisser, C.; Rosa, F.D.; Korf, E.P. Rice Husk Ash-Carbide Lime as an Alternative Binder for Waste Foundry Sand Stabilization. Environ. Sci. Pollut. Res. 2023, 30, 42176–42191. [Google Scholar] [CrossRef] [PubMed]
- Pompermaier, C.L.; Ferrazzo, S.T.; Levandoski, W.M.K.; Bruschi, G.J.; Prietto, P.D.M.; Korf, E.P. Stabilization of Waste Foundry Sand with Alkali-Activated Binder: Mechanical Behavior, Microstructure and Leaching. Constr. Build. Mater. 2024, 444, 137772. [Google Scholar] [CrossRef]
- Ferrazzo, S.T.; de Araújo, M.T.; Bruschi, G.J.; Chaves, H.M.; Korf, E.P.; Consoli, N.C. Mechanical and Environmental Behavior of Waste Foundry Sand Stabilized with Alkali-Activated Sugar Cane Bagasse Ash-Eggshell Lime Binder. Constr. Build. Mater. 2023, 383, 131313. [Google Scholar] [CrossRef]
- da Veiga, F.P.; Levandoski, W.M.K.; Bruschi, G.J.; Krogel, M.; Piovesan, M.A.; Pelissaro, D.T.; Prietto, P.D.M.; Korf, E.P. Utilizing Iron Ore Tailings for the Development of a Sustainable Alkali-Activated Binder. Mining 2025, 5, 26. [Google Scholar] [CrossRef]
- Nasaeng, P.; Wongsa, A.; Cheerarot, R.; Sata, V.; Chindaprasirt, P. Strength Enhancement of Pumice-Based Geopolymer Paste by Incorporating Recycled Concrete and Calcined Oyster Shell Powders. Case Stud. Constr. Mater. 2022, 17, e01307. [Google Scholar] [CrossRef]
- Monneron-Gyurits, M.; Joussein, E.; Soubrand, M.; Fondanèche, P.; Rossignol, S. Valorization of Mussel and Oyster Shells toward Metakaolin-Based Alkaline Activated Material. Appl. Clay Sci. 2018, 162, 15–26. [Google Scholar] [CrossRef]
- Hasnaoui, A.; Bourguiba, A.; Sebaibi, N.; Boutouil, M. Valorization of Queen Scallop Shells in the Preparation of Metakaolin-Based Geopolymer Mortars. J. Build. Eng. 2022, 53, 104578. [Google Scholar] [CrossRef]
- Oladele, O.L.; Adesanya, E.D.; Arbe, A.; Iturrospe, A.; Ogundiran, M.B. Mitigation of Efflorescence, Drying Shrinkage and Water Demand of Calcined Clay-Based Geopolymers with Biological Waste Ashes as Activator and Hardener. Appl. Clay Sci. 2023, 243, 107050. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2024—Blue Transformation in Action; FAO: Rome, Italy, 2024; Volume 35. [Google Scholar] [CrossRef]
- Yao, Z.; Xia, M.; Li, H.; Chen, T.; Ye, Y.; Zheng, H. Bivalve Shell: Not an Abundant Useless Waste but a Functional and Versatile Biomaterial. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2502–2530. [Google Scholar] [CrossRef]
- Moita Neto, J.M.; Leal, R.C.; Araújo, N.L.d.S.; da Silva, E.A. Challenges and Opportunities for the Development of Urban Mining in Brazil. Minerals 2025, 15, 593. [Google Scholar] [CrossRef]
- Cossu, R.; Williams, I.D. Urban mining: Concepts, terminology, challenges. Waste Manag. 2015, 45, 1–3. [Google Scholar] [CrossRef]
- Xavier, L.H.; Ottoni, M.; Abreu, L.P.P. A comprehensive review of urban mining and the value recovery from e waste materials. Resour. Conserv. Recycl. 2023, 190, 106840. [Google Scholar] [CrossRef]
- Águila-Almanza, E.; Hernández-Cocoletzi, H.; Rubio-Rosas, E.; Calleja-González, M.; Lim, H.R.; Khoo, K.S.; Singh, V.; Maldonado-Montiel, J.C.; Show, P.L. Recuperation and Characterization of Calcium Carbonate from Residual Oyster and Clamshells and Their Incorporation into a Residential Finish. Chemosphere 2022, 288, 132550. [Google Scholar] [CrossRef]
- Bellei, P.; Torres, I.; Solstad, R.; Flores-Colen, I. Potential Use of Oyster Shell Waste in the Composition of Construction Composites: A Review. Buildings 2023, 13, 1546. [Google Scholar] [CrossRef]
- Her, S.; Im, S.; Liu, J.; Suh, H.; Kim, G.; Sim, S.; Wi, K.; Park, D.; Bae, S. Exploring the Potential of Pulverized Oyster Shell as a Limestone Substitute in Limestone Calcined Clay Cement (LC3) and Its Implications for Performance. Constr. Build. Mater. 2024, 425, 135918. [Google Scholar] [CrossRef]
- Yang, B.; Jang, J.G. Environmentally Benign Production of One-Part Alkali-Activated Slag with Calcined Oyster Shell as an Activator. Constr. Build. Mater. 2020, 257, 119552. [Google Scholar] [CrossRef]
- Her, S.; Park, T.; Zalnezhad, E.; Bae, S. Synthesis and Characterization of Cement Clinker Using Recycled Pulverized Oyster and Scallop Shell as Limestone Substitutes. J. Clean. Prod. 2021, 278, 123987. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-Activated Materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Yue, G.H.; Tay, Y.X.; Wong, J.; Shen, Y.; Xia, J. Aquaculture Species Diversification in China. Aquac. Fish. 2024, 9, 206–217. [Google Scholar] [CrossRef]
- Belaïd, F. How Does Concrete and Cement Industry Transformation Contribute to Mitigating Climate Change Challenges? Resour. Conserv. Recycl. Adv. 2022, 15, 200084. [Google Scholar] [CrossRef]
- Adesina, A. Recent Advances in the Concrete Industry to Reduce Its Carbon Dioxide Emissions. Environ. Challenges 2020, 1, 100004. [Google Scholar] [CrossRef]
- Dunuweera, S.P.; Rajapakse, R.M.G. Cement Types, Composition, Uses and Advantages of Nanocement, Environmental Impact on Cement Production, and Possible Solutions. Adv. Mater. Sci. Eng. 2018, 2018, 4158682. [Google Scholar] [CrossRef]
- Mohamad, N.; Muthusamy, K.; Embong, R.; Kusbiantoro, A.; Hashim, M.H. Environmental Impact of Cement Production and Solutions: A Review. Mater. Today Proc. 2021, 48, 741–746. [Google Scholar] [CrossRef]
- USGS. U.S. Geological Survey, Mineral Commodity Summaries. January 2025. Available online: https://www.usgs.gov/ (accessed on 30 May 2025).
- CSI-ECRA. CSI/ECRA—Technology Papers 2017: Development of State of the Art Techniques in Cement Manufacturing: Trying to Look Ahead; European Cement Research Academy: Düsseldorf, Germany, 2017. Available online: https://ecra-online.org (accessed on 27 May 2025).
- Sun, Q.; Cui, C.; Li, J.; Liu, F.; Chen, B.; Liu, Y.; Tu, K.N.; Jiang, H. Geopolymer Adhesives for DUV LED Packaging: Synthesis and Bonding Mechanism. J. Alloys Compd. 2025, 1036, 181903. [Google Scholar] [CrossRef]
- Gökçe, H.S. Durability of Slag-Based Alkali-Activated Materials: A Critical Review. J. Aust. Ceram. Soc. 2024, 60, 885–903. [Google Scholar] [CrossRef]
- Nasir, M.; Mahmood, A.H.; Bahraq, A.A. History, Recent Progress, and Future Challenges of Alkali-Activated Binders—An Overview. Constr. Build. Mater. 2024, 426, 136141. [Google Scholar] [CrossRef]
- Provis, J.L. Geopolymers and Other Alkali Activated Materials: Why, How, and What? Mater. Struct. Constr. 2014, 47, 11–25. [Google Scholar] [CrossRef]
- Santos, M.E.M.; de Oliveira Moura, E.M. Potencialidades e Impactos Ambientais Dos Resíduos Oriundos Da Malacocultura. Acta Fish. Aquat. Resour. 2017, 5, 147–152. [Google Scholar] [CrossRef]
- Chierighini, D.; Bridi, R.; Rocha, A.A.; Lapa, K.R. Possibilidades do Uso das Conchas de Moluscos. In Proceedings of the 3rd International Workshop Advances in Cleaner Production, São Paulo, Brazil, 18–20 May 2011; Available online: http://www.advancesincleanerproduction.net/third/files/sessoes/6A/6/Chierighini_D%20-%20Paper%20-%206A6.pdf (accessed on 4 April 2025).
- Silva, T.H.; Mesquita-Guimarães, J.; Henriques, B.; Silva, F.S.; Fredel, M.C. The Potential Use of Oyster Shell Waste in New Value-Added by-Product. Resources 2019, 8, 13. [Google Scholar] [CrossRef]
- Aimikhe, V.J.; Lekia, G.B. An Overview of the Applications of Periwinkle (Tympanotonus fuscatus) Shells. Curr. J. Appl. Sci. Technol. 2021, 40, 31–58. [Google Scholar] [CrossRef]
- de Castro, V.G. Cimento Portland. In Compósitos Madeira-Cimento: Um Produto Sustentável Para o Futuro; EdUFERSA: Mossoró, Brazil, 2021; pp. 13–21. [Google Scholar] [CrossRef]
- IBGE—Instituto Brasileiro de Geografia e Estatística. Pesquisa da Pecuária Municipal 2023 (PPM); IBGE: Rio de Janeiro, Brazil, 2023. Available online: https://www.ibge.gov.br/estatisticas/economicas/agricultura-e-pecuaria/9107-producao-da-pecuaria-municipal.html?=&t=resultados (accessed on 8 April 2025).
- Igarashi, M.A. Aspectos Do Desenvolvimento Tecnológico Do Cultivo de Ostra No Sul Do Brasil Aspects of the Technological Development of Oyster Culture in Southern Brazil. Semiárido Visu 2018, 8, 28–44. [Google Scholar] [CrossRef]
- Pessoa, K.d.A.R.; Salgado, R.; Da Mata, A.M.A.T.; Quintella, C.M. Oyster Shell: Recent Trends in Environmental Uses for Circular Economy. Cad. Prospecção 2019, 12, 849–864. [Google Scholar] [CrossRef]
- Okoro, W.; Oyebisi, S. Mechanical and Durability Assessments of Steel Slag-Seashell Powder-Based Geopolymer Concrete. Heliyon 2023, 9, e13188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Y.; Lin, R.S.; Wang, X.Y. Effect of Waste Oyster Shell Powder on the Properties of Alkali-Activated Slag-Waste Ceramic Geopolymers. J. Mater. Res. Technol. 2023, 22, 1768–1780. [Google Scholar] [CrossRef]
- Le, T.T.; Park, S.S.; Lee, J.C.; Lee, D.E. Strength Characteristics of Spent Coffee Grounds and Oyster Shells Cemented with GGBS-Based Alkaline-Activated Materials. Constr. Build. Mater. 2021, 267, 120986. [Google Scholar] [CrossRef]
- Djobo, Y.J.N.; Elimbi, A.; Dika Manga, J.; Djon Li Ndjock, I.B. Partial Replacement of Volcanic Ash by Bauxite and Calcined Oyster Shell in the Synthesis of Volcanic Ash-Based Geopolymers. Constr. Build. Mater. 2016, 113, 673–681. [Google Scholar] [CrossRef]
- Djobo, J.N.Y.; Tchakouté, H.K.; Ranjbar, N.; Elimbi, A.; Tchadjié, L.N.; Njopwouo, D. Gel Composition and Strength Properties of Alkali-Activated Oyster Shell-Volcanic Ash: Effect of Synthesis Conditions. J. Am. Ceram. Soc. 2016, 99, 3159–3166. [Google Scholar] [CrossRef]
- Angdiarto, S.P.; Chen, C.T.; Chang, T.P. Engineering Characteristics of One-Part Alkali-Activated Slag-Based Paste Incorporating Nano-Silica and Calcined Oyster Shells Ash. J. Chinese Inst. Eng. Trans. Chinese Inst. Eng. A 2024, 48, 1–14. [Google Scholar] [CrossRef]
- Choi, S.-G.; Park, S.-S.; Wang, K. Early-Age Strength of Alkali-Activated Slag Mortar Based on Burned Oyster Shell and Other Chemical Activators. J. Mater. Civ. Eng. 2019, 31, 04019186. [Google Scholar] [CrossRef]
- Shao, W.C.; Lu, C.L.; Dong, Y.W.; Chen, J.W.; Chiang, Y.T. Research on Innovative Green Building Materials from Waste Oyster Shells into Foamed Heat-Insulating Bricks. Clean. Mater. 2024, 11, 100222. [Google Scholar] [CrossRef]
- Deng, Z.; Yang, Z.; Bian, J.; Pan, X.; Wu, G.; Guo, F.; Fu, R.; Yan, H.; Deng, Z.; Chen, S. Engineering Properties of PVA Fibre-Reinforced Geopolymer Mortar Containing Waste Oyster Shells. Materials 2022, 15, 7013. [Google Scholar] [CrossRef]
- Yanting, M.; Bo, D.; Xiaotong, Y.; Da, C.; Yingdi, L. Study on Mechanical Properties and Durability of Geopolymer Concrete with Oyster Shell Aggregate. Constr. Build. Mater. 2025, 472, 140926. [Google Scholar] [CrossRef]
- López, L.C.S.; Ramos, J.C.L.; de la Rosa, Y.E.N.; Bruschi, G.J.; Baldovino, J.D.J.A. Stabilization of Clay Soils Using a Lime Derived from Seashell. Materials 2025, 18, 2723. [Google Scholar] [CrossRef]
- Chung, S.Y.; Oh, S.E.; Jo, S.S.; Lehmann, C.; Won, J.; Elrahman, M.A. Microstructural Investigation of Mortars Incorporating Cockle Shell and Waste Fishing Net. Case Stud. Constr. Mater. 2023, 18, e01719. [Google Scholar] [CrossRef]
- Lertwattanaruk, P.; Makul, N.; Siripattarapravat, C. Utilization of Ground Waste Seashells in Cement Mortars for Masonry and Plastering. J. Environ. Manag. 2012, 111, 133–141. [Google Scholar] [CrossRef]
- NBR 16697:2018; Cimento Portland—Requisitos. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 2018.
- Kim, M.O.; Lee, M.K. Strength and Microstructural Changes in Cementitious Composites Containing Waste Oyster Shell Powder. Buildings 2023, 13, 3078. [Google Scholar] [CrossRef]
- Seo, J.H.; Park, S.M.; Yang, B.J.; Jang, J.G. Pó de concha de ostra calcinada como aditivo expansivo em argamassa de cimento. Materiais 2019, 12, 1322. [Google Scholar] [CrossRef]
- Cha, I.; Kim, J.; Lee, H. Aumento da resistência à compressão em compósitos cimentícios por meio do uso eficaz de conchas de ostras desperdiçadas e aditivos. Buildings 2023, 13, 2787. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S.J. Geopolymers: Structure, Processing, Properties and Industrial Applications; Woodhead Publishing: Great Abington, UK, 2009. [Google Scholar] [CrossRef]
- Paswan, R.K.; Kumar, P.; Kumar, V.; Sembeta, R.Y. Mechanical Properties of Alkali Activated Slag Binder Based Concrete at Elevated Temperatures. Discov. Sustain. 2025, 6, 744. [Google Scholar] [CrossRef]
- Olivia, M.; Oktaviani, R. Ismeddiyanto Properties of Concrete Containing Ground Waste Cockle and Clam Seashells. Procedia Eng. 2017, 171, 658–663. [Google Scholar] [CrossRef]
- Oyejobi, D.O.; Raji, S.A.; Aina, S.T.; Siva, A. Physio-Chemical and Microstructural Characteristics of Selected Pozzolanic Materials for Cement and Concrete Production. Niger. J. Technol. Dev. 2019, 16, 111–119. [Google Scholar] [CrossRef]
- Khankhaje, E.; Salim, M.R.; Mirza, J.; Salmiati; Hussin, M.W.; Khan, R.; Rafieizonooz, M. Properties of Quiet Pervious Concrete Containing Oil Palm Kernel Shell and Cockleshell. Appl. Acoust. 2017, 122, 113–120. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).