Perspectives for Photochemical Leaching Processes of Chalcopyrite: A Solar Radical-Leaching Process
Abstract
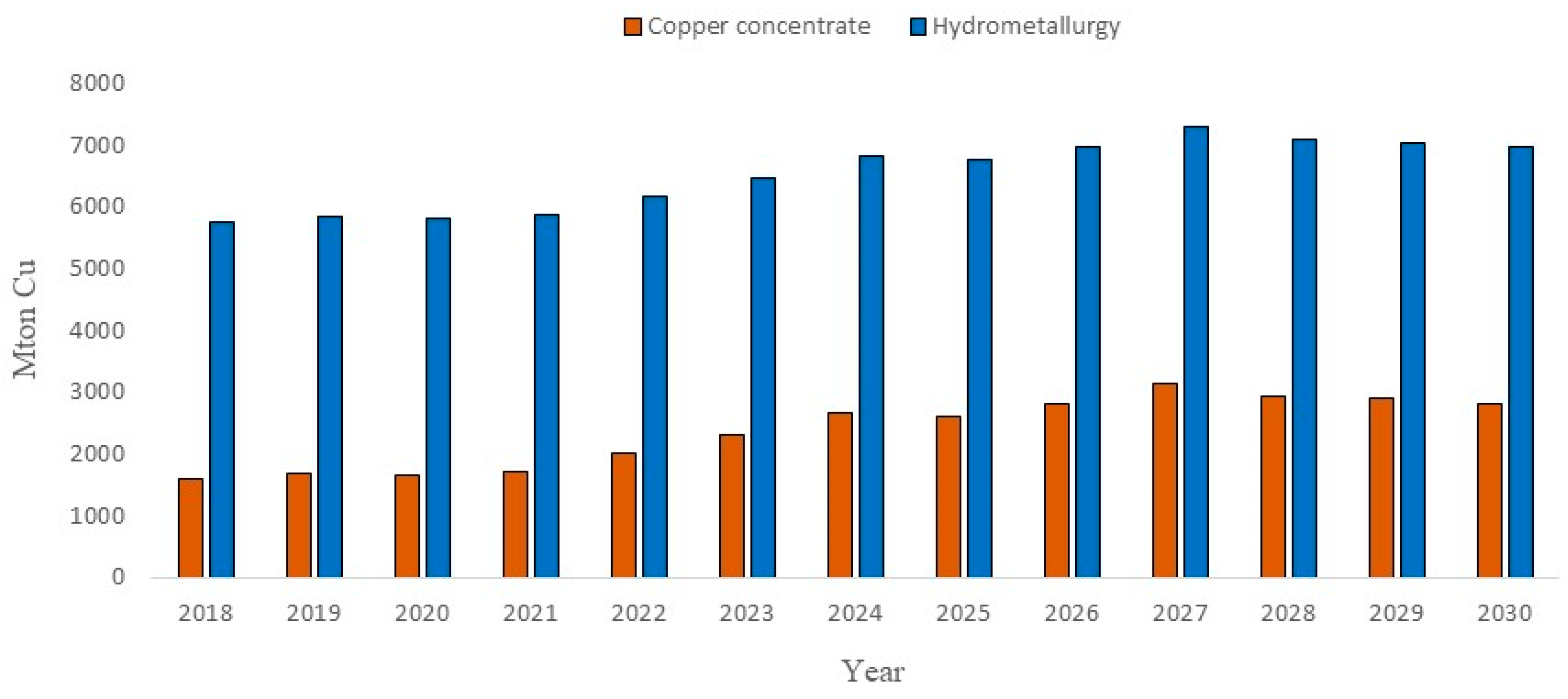
1. Introduction to Metallurgy of Copper Sulfides
2. Materials and Methods
3. Hydrometallurgy of Chalcopyrite
3.1. Acidic Chalcopyrite Leaching
3.2. Ferric Ions (Fe3+) on Chalcopyrite Leaching
3.3. Chalcopyrite Bioleaching
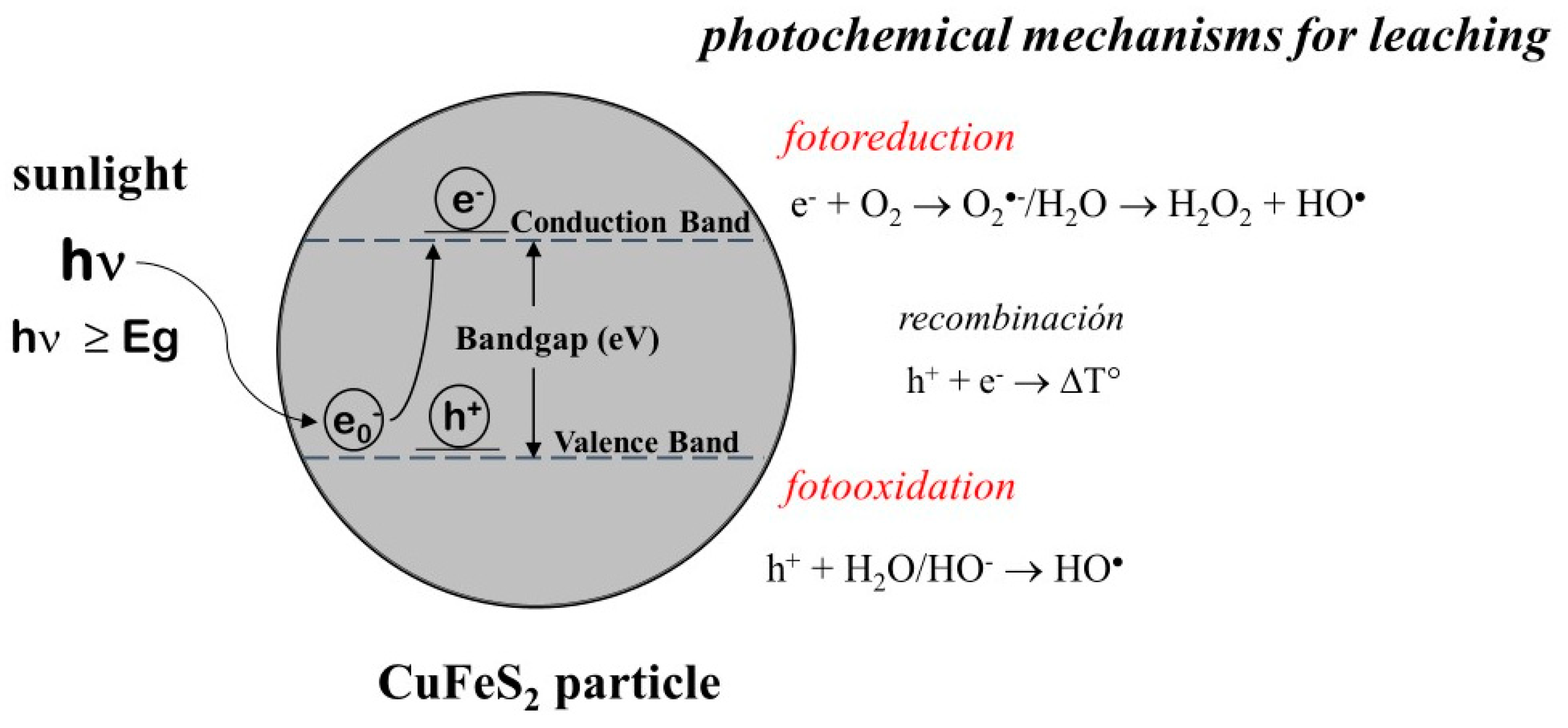
4. Photoleaching: The Use of the Sunlight (UV-Vis) in Chalcopyrite Leaching
4.1. Comparison between Photoleaching and Conventional Acid Leaching of CuFeS2
4.2. Improving Acid Leaching through the Synergistic Action of H2O2
4.3. The Role of H2O2 in Oxidative Acid Leaching v/s in Photo-Assisted Leaching Processes
4.4. Acid Mine Drainage and Its Relationship to Oxidative Dissolution Processes Mediated by Free Radicals
4.5. Photochemical Properties of Passivating Layers Compound
4.6. The Effect of Radiation in the Iron Cycling Fe3+/Fe2+
4.7. The Effect of UV-Vis Radiation on Bioleaching
5. Radical-Leaching Based on Sulfate Radical (SO4•−) in Chalcopyrite Leaching
6. Perspectives and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Sun, X.; Deng, J.; Li, G.; Li, Z.; Jiang, J.; Duan, J. Emission characteristics of heavy metals from a typical copper smelting plant. J. Hazard. Mater. 2022, 424, 127311. [Google Scholar] [CrossRef]
- COCHILCO. Proyección de la Producción de Cobre en Chile 2019–2030; COCHILCO: Santiago, Chile, 2019. [Google Scholar]
- Watling, H.R. e bioleaching of sulphide minerals with emphasis on copper sulphides—A review. Hydrometallurgy 2006, 84, 81–108. [Google Scholar] [CrossRef]
- Mokmeli, M. Pre feasibility study in hydrometallurgical treatment of low-grade chalcopyrite ores from Sarcheshmeh copper mine. Hydrometallurgy 2020, 191, 105215. [Google Scholar] [CrossRef]
- Mokmeli, M.; Parizi, M.T. Low-grade chalcopyrite ore, heap leaching or smelting recovery route? Hydrometallurgy 2022, 211, 105885. [Google Scholar] [CrossRef]
- COCHILCO. Proyección de la Producción de Cobre en Chile 2022–2030; COCHILCO: Santiago, Chile, 2022. [Google Scholar]
- Yepsen, O.; Araneda, E.; Yepsen, R.; Estay, H. The Role of Solar Energy (UV-VIS-NIR) as an Assistant for Sulfide Minerals Leaching and Its Potential Application for Metal Extraction. Minerals 2021, 11, 828. [Google Scholar] [CrossRef]
- Morenjo-Leiva, S.; Haas, J.; Junne, T.; Valencia, F.; Godin, H.; Kracht, W.; Nowak, W.; Eltrop, L. Renewable energy in copper production: A review on systems design and methodological approaches. J. Clean. Prod. 2020, 246, 118978. [Google Scholar] [CrossRef]
- Crundwell, F. The impact of light on understanding the mechanism of dissolution and leaching of sphalerite (ZnS), pyrite (FeS2) and chalcopyrite (CuFeS2). Miner. Eng. 2020, 161, 106728. [Google Scholar] [CrossRef]
- Yepsen, O.; Yanez, J.; Mansilla, H.D. Photocorrosion of copper sulfides: Toward a solar mining industry. Sol. Energy 2018, 171, 106–111. [Google Scholar] [CrossRef]
- Yañez, J.; Torres, S.; Sbarbaro, D.; Parra, R.; Saavedra, C. Analitycal instrumentation for copper pyrometallurgy: Challenges and oportunities. IFAC-PapeersOnLine 2018, 51, 251–256. [Google Scholar] [CrossRef]
- Alpers, C.N.; Blowes, D.W.; Nordstrom, D.K.; Jambor, J.L. Secondary Minerals and Acid Mine-Water Chemistry. In The Environmental Geochemistry of Sulfide Mine-Wastes; MAC: Nepean, Canada, 1994; Chapter 9; pp. 247–270. [Google Scholar]
- Meissner, D.; Memming, R.; Kastening, B. Photoelectrochemistry of cadmium sulfide. 1. Reanalysis of photocorrosion and flat-band potential. J. Phys. Chem. 1988, 92, 3476–3483. [Google Scholar] [CrossRef]
- Ghahremaninezhad, A.; Asselin, E.; Dixon, D.G. Electrochemical evaluation of the surface of chalcopyrite during dissolution in sulfuric acid. Electrochim. Acta 2010, 55, 5041–5056. [Google Scholar] [CrossRef]
- Cordoba, E.M.; Muñoz, J.A.; Bazquez, M.l.; Gonzalez, F.; Ballester, A. Leaching of chalcopyrite with ferric ion. Part II: Effect of redox potential. Hydrometallurgy 2008, 93, 88–96. [Google Scholar] [CrossRef]
- Yang, B.; Lin, M.; Fang, J.; Zhang, R.; Lou, R.; Wang, X.; Liao, R.; Wu, B.; Wang, J.; Gan, M.; et al. Combined effects of jarosite and visible light on chalcopyrite dissolution mediated by Acidithiobacillus ferrooxidans. Sci. Total Environ. 2020, 698, 134175. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.P.; Gerson, A.R. The mechanisms of pyrite oxidation and leaching: A fundamental perspective. Surf. Sci. Rep. 2010, 65, 293–315. [Google Scholar] [CrossRef]
- Antonijevic, M.M.; Jankovic, Z.D.; Dimitrijevic, M.D. Kinetics of chalcopyrite dissolution by hydrogen peroxide in sulphuric acid. Hydrometallurgy 2004, 71, 329–334. [Google Scholar] [CrossRef]
- Li, Y.; Kawashima, N.; Li, J.; Chandra, A.P.; Gerson, A.R. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv. Colloid Interface Sci. 2013, 197–198, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, Z.; Qian, G.; Li, J.; Gerson, A.R. Kinetics and Mechanisms of Chalcopyrite Dissolution at controlled redox potential of 750 mV in sulfuric acid solution. Minerals 2016, 6, 83. [Google Scholar] [CrossRef]
- Tian, Z.; Li, H.; Wei, Q.; Qin, W.; Yang, C. Effects of redox potential on chalcopyrite leaching: An overview. Miner. Eng. 2021, 172, 107135. [Google Scholar] [CrossRef]
- Hiroyoshi, N.; Kuriowa, S.; Miki, H.; Tsunekawa, M.; Hirajima, T. Effects of coexisting metal ions on the redox potential dependence of chalcopyrite leaching in sulfuric acid solutions. Hydrometallurgy 2007, 87, 1–10. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Y.; Zhang, X.; Qian, L.; Sun, M.; Yang, Y.; Zhang, Y.; Wang, J.; Kim, H.; Qiu, G. The dissolution and passivation mechanism of chalcopyrite in bioleaching: An overview. Miner. Eng. 2019, 136, 140–154. [Google Scholar] [CrossRef]
- Vera, M.; Schippers, A.; Hedrich, S.; Sand, W. Progress in bioleaching: Fundamentals and mechanisms of microbial metal sulfide oxidation-part A. Appl. Microbiol. Biotechnol. 2022, 106, 6933–6952. [Google Scholar] [CrossRef] [PubMed]
- Raubach, C.W.; De Santana, Y.V.; Ferrer, M.; Buzolin, P.; Sambrano, J.; Longo, E. Photocatalytic activity of semiconductor sulfide heterostructures. Dalton Trans. 2013, 42, 11111–11116. [Google Scholar] [CrossRef] [PubMed]
- Barton, I.; Hiskey, B. Chemical, crystallographic, and electromagnetic variability in natural chalcopyrite and implications for leaching. Miner. Eng. 2022, 189, 107867. [Google Scholar] [CrossRef]
- Strizh, I.G.; Lysenko, G.; Neverov, K. Photoreduction of Molecular Oxygen in preparations of photosystem II under photoinhibitory conditions. Russ. J. Plant Physiol. 2005, 52, 717–723. [Google Scholar] [CrossRef]
- Brillas, E.; Sires, I.; Oturam, M.A. Electro-Fenton process and related electrochemical technologies based on fenton’s reaction chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef] [PubMed]
- Rozas, O.; Contreras, D.; Mondaca, A.M.; Perez-Moya, M.; Mansilla, H. Experimental design of Fenton and photo-Fenton reactions for the treatment of ampicillin solutions. J. Hazard. Mater. 2010, 177, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Nicol, M. Does galvanic coupling with pyrite increase the rate of dissolution of chalcopyrite under ambient conditions? An electrochemical study. Hydrometallurgy 2022, 208, 105824. [Google Scholar] [CrossRef]
- Nooshabadi, J.; Rao, H. Formation of hydrogen peroxide by chalcopyrite and its influence on flotation. Min. Metall. Explor. 2013, 30, 212–219. [Google Scholar] [CrossRef]
- Petrovic, S.; Bogdanovic, G.; Antonijevic, M. Leaching of chalcopyrite with hydrogen peroxide in hydrochloric acid solution. Trans. Nonferrous Met. Soc. China 2018, 28, 1444–1455. [Google Scholar] [CrossRef]
- Sokic, M.; Markovic, B.; Stankovic, S.; Kamberovic, Z.; Strbac, N.; Manojlovic, V.; Petronijevic, N. Kinetics of Chalcopyrite Leaching by Hydrogen Peroxide in Sulfuric Acid. Metals 2019, 11, 1173. [Google Scholar] [CrossRef]
- Henriquez, A.; Salgado, P.; Albornoz, M.; Melin, V.; Mansilla, H.; Cornejo-Ponce, L.; Contreras, D. Determination of equilibrium constants of iron(iii)-1,2-dihydroxybenzene complexes and the relationship between calculated iron speciation and degradation of rhodamine B. New J. Chem. 2021, 45, 15912–15919. [Google Scholar] [CrossRef]
- Seeger, B. Constantes de Reacciones en Solución Acuosa; El Sur Impresores: Hualpen, Chile, 2007. [Google Scholar]
- Maezono, T.; Tokomura, M.; Sekine, M.; Kawase, Y. Hydroxyl radical concentration profile in photo-Fenton oxidation process: Generation and consumption of hydroxyl radicals during the discoloration of azo-dye Orange II. Chemosphere 2011, 82, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Gil-Lozano, C.; Davila, A.F.; Losa-Adams, E.; Fairen, A.G.; Gago-Duport, L. Quantifying Fenton reaction pathways driven by self-generated H2O2 on pyrite surfacces. J. Azardous Mater. 2017, 7, 122844. [Google Scholar]
- Pignatello, J.; Oliveros, E.; Mackay, A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Cordoba, E.M.; Muñoz, J.A.; Blazquez, M.L.; Gonzalez, F.; Ballester, A. Leaching of Chalcopyrite with Ferric Ion. Part I: General Aspects. Hydrometallurgy 2008, 93, 81–87. [Google Scholar] [CrossRef]
- Thao, N.T.; Tsuji, S.; Jeon, S.; Park, I.; Tabelin, C.B.; Ito, M.; Hiroyoshi, N. Redox potential-dependent chalcopyrite leaching in acidic ferric chloride solutions: Leaching experiments. Hydrometallurgy 2020, 194, 105299. [Google Scholar] [CrossRef]
- Ahmed, H.S.; Mohammed, R.Y. The Effect of Deposition Parameters on Morphological and Optical Properties of Cu2S Thin Films Grown by Chemical Bath Deposition Technique. Photonic 2022, 9, 162. [Google Scholar] [CrossRef]
- Litter, M.; Slodowicz, M. An overview on heterogeneous Fenton and photoFenton reactions using zerovalent iron materials. J. Adv. Oxid. Technol. 2017, 20, 20160164. [Google Scholar] [CrossRef]
- Tokumura, M.; Morito, R.; Hatayama, R.; Kawase, Y. Iron redox cycling in hidroxyl radical generation during the photo-Fenton oxidative degradation: Dynamic change of hydroxyl radical concentration. Appl. Catal. B Environ. 2011, 106, 565–576. [Google Scholar] [CrossRef]
- Nikoloski, A.; O’Malley, G.; Bagas, S.J. The effect of silver on the acidic ferric sulfate leaching of primary copper sulfides under recycle solution conditions observed in heap leaching. Part 1: Kinetics and reaction mechanisms. Hydrometallurgy 2017, 173, 258–270. [Google Scholar] [CrossRef]
- Rawling, D.E. Characteristics and adaptability of iron- and sulfur-oxidizing microorganisms used for the recovery of metals from minerals and their concentrates. Microb. Cell Factories 2005, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Gan, M.; Zhu, J.; Li, Q.; Jie, S.; Yang, B.; Liu, X. Catalytic effect of light illumination on bioleaching of chalcopyrite. Bioresour. Technol. 2015, 182, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.A.; Huie, R.E.; Lymar, S.; Koppenol, W.H.; Merényi, G.; Neta, P.; Stanbury, D.M.; Steenken, S.; Wardman, P. Standard electrode potentials involving radicals in aqueous solution: Inorganic radicals. Bioinorg. React. Mech. 2013, 9, 59–61. [Google Scholar] [CrossRef]
- Yang, Q.; Ma, Y.; Chen, F.; Yao, F.; Sun, J.; Wang, S.; Kaixin, Y.; Lihua, H.; Xiaoming, L.; Wang, D. Recent advances in photo-activated sulfate radical-advanced oxidation process (SR-AOP) for refractory organic pollutants removal in water. Chem. Eng. J. 2019, 378, 122149. [Google Scholar] [CrossRef]
- Giannakis, S.; Lin, A.K.-Y.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]


| Metallic Sulfide | Dissolution Reaction | Kps/pKps |
|---|---|---|
| CuFeS2 | CuFeS2 + 2H+ → Cu2+ + Fe2+ + 2HS− | −35.27 (pKps) |
| Cu2S | Cu2S → 2Cu+ + S2− | 3.16 × 10−49 |
| CuS | CuS → Cu2+ + S2− | 7.9 × 10−37 |
| FeS | FeS → Fe2+ + S2− | 7.9 × 10−19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yepsen, O.; Cornejo-Ponce, L.; Yepsen, R. Perspectives for Photochemical Leaching Processes of Chalcopyrite: A Solar Radical-Leaching Process. Mining 2024, 4, 352-366. https://doi.org/10.3390/mining4020020
Yepsen O, Cornejo-Ponce L, Yepsen R. Perspectives for Photochemical Leaching Processes of Chalcopyrite: A Solar Radical-Leaching Process. Mining. 2024; 4(2):352-366. https://doi.org/10.3390/mining4020020
Chicago/Turabian StyleYepsen, Orlando, Lorena Cornejo-Ponce, and Rodrigo Yepsen. 2024. "Perspectives for Photochemical Leaching Processes of Chalcopyrite: A Solar Radical-Leaching Process" Mining 4, no. 2: 352-366. https://doi.org/10.3390/mining4020020
APA StyleYepsen, O., Cornejo-Ponce, L., & Yepsen, R. (2024). Perspectives for Photochemical Leaching Processes of Chalcopyrite: A Solar Radical-Leaching Process. Mining, 4(2), 352-366. https://doi.org/10.3390/mining4020020







