Abstract
Metabolic engineering of the shikimate pathway offers a promising strategy for enhancing the production of aromatic compounds in microbial hosts. However, feedback inhibition of key enzymes, such as the 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase (DAHP synthase), often limits the yield of target products. In this study, we focused on the DAHP synthase (AroF-I) from Pseudomonas putida. Through computational modeling and experimental validation, we identified specific amino-acid residues responsible for tyrosine-mediated feedback inhibition. By targeted mutagenesis, we engineered DAHP synthase variants that exhibit reduced sensitivity to feedback inhibition. The introduction of these engineered enzymes into a metabolically engineered Pseudomonas putida strain resulted in significantly increased production of p-coumaric acid. Our findings provide valuable insights into the regulation of the shikimate pathway and demonstrate the potential of protein engineering to improve microbial production of aromatic compounds.
1. Introduction
Synthetic biology is the engineering of microbes for the creation of new biologically based parts and novel devices and systems, as well as redesigning existing, natural biological systems (Kitney and Freemont [1]). One of its main applications is the production of high-interest molecules originally obtained by other means, such as chemical synthesis or plant extraction, using microorganisms as cell factories. For example, the insertion of a heterologous pathway from plants will allow the synthesis of a plant molecule into a microbe. Several studies from this last decade have shown successful examples of microbe engineering (Martău et al. [2], Wang et al. [3], Dudnik et al. [4], Peralta-Yahya et al. [5], Aggarwal et al. [6]). Nevertheless, this process is not straightforward and needs fine-tuning, particularly in the central metabolism. Indeed, efficient carbon fluxes through the new pathway for an interesting biosynthesis of the final product are needed, as well as precursor availability (Li et al. [7], Maurya et al. [8], Leonard et al. [9]).
One of the best examples is the use of aromatic amino acids synthesized by microorganisms (bacteria or yeasts) to produce aromatic compounds of high interest, such as phenylpropanoids (Suzuki et al. [10], Limem et al. [11], Wang et al. [12], Averesch and Krömer [13]). These molecules are derived from L-Phe and L-Tyr and are produced mainly in plants. The phenylpropanoid acids cinnamic acid (CA) and p-coumaric acid (pCA) are important building blocks allowing the biosynthesis of a great variety of secondary metabolites and natural products widely used in industry for flavoring, pharmaceuticals, and cosmetics (Vargas-Tah and Gosset [14]).
The shikimate pathway is the central metabolic route leading to the formation of L-Trp, L-Phe, and L-Tyr from simple carbon sources in microorganisms and plants (Herrmann and Weaver [15]). This metabolic pathway has been extensively studied: it is highly regulated as the accumulation of aromatic amino acids may be deleterious for the cells (Averesch and Krömer [13]). An illustration can be seen in Figure 1. In the Appendix A Section, we provide a more detailed illustration (Figure A1) based on KeGG pathways.
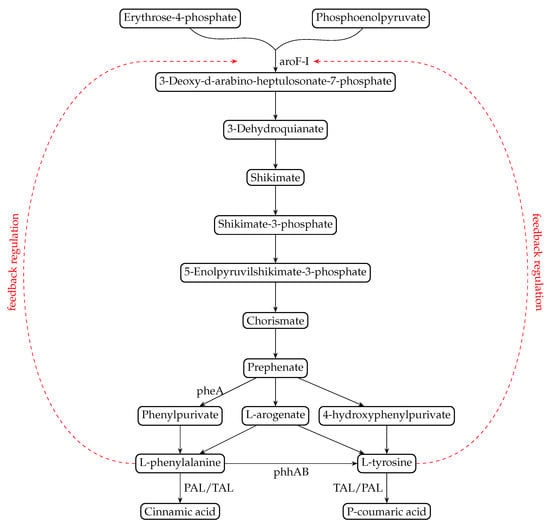
Figure 1.
An illustration of the reactions from the shikimate pathway to the production of phenolic compounds cinnamic acid and p-coumaric acid. The red arrows indicate common negative feedback regulation of DAHP synthase by aromatic amino acids.
Among the 15 enzymes that are implicated in this pathway, some, like the DAHP synthases (3-deoxy-D-arabino-heptulosonate 7-phosphate synthase), are negatively repressed by the aromatic amino acid. This phenomenon is called feedback inhibition. This regulation is widespread among microorganisms and tends to be a major bottleneck for the bioproduction of aromatic amino acids derived compounds (Jayaraman et al. [16], Wu et al. [17], Luttik et al. [18], Bilal et al. [19]). Obtaining feedback-inhibition resistant (fbr) enzymes is one of the best strategies to generate L-Phe or L-Tyr overproducer strains, and it was conducted with success in the two well-known chassis for bioproduction, Escherichia coli and Saccharomyces cerevisiae (Lütke-Eversloh and Stephanopoulos [20], Kang et al. [21], Santos et al. [22], Juminaga et al. [23], Rodriguez et al. [24]). Nevertheless, these microbial chassis are not the best adapted to produce phenylpropanoids, as these aromatic molecules are highly toxic for cells. On the contrary, bacteria of the genus Pseudomonas are useful hosts for synthetic biology as they have high-stress tolerance and an interesting metabolic versatility (Nikel et al. [25], Ankenbauer et al. [26], Schwanemann et al. [27]). On top of that, they have an efficient aromatic catabolic pathway, and tolerance towards p-coumaric acid was recently shown (Molina-Santiago et al. [28], Calero et al. [29], Mohamed et al. [30]). In their work, Calero et al. [29] clearly showed that Pseudomonas putida KT2440 was twice as tolerant to p-coumaric acid as E. coli, highlighting its suitability for the production of phenolic compounds. Previous works demonstrated that production of phenol or pCA with a correct titer could be obtained in some Pseudomonas strains, often by transforming these strains with fbr genes from Escherichia coli (aroGfbr and tyrAfbr) (Nijkamp et al. [31], Wierckx et al. [32], Calero et al. [33], Wynands et al. [34], Otto et al. [35]). Random mutagenesis experiments were also performed in these strains, and some mutations in genes from the central metabolism and/or the shikimate pathway could improve aromatic molecule production. The overproduction of the Pseudomonas DAHP synthase AroF-I gene could also improve this production (Wierckx et al. [32]), showing that this enzyme could be a good target to obtain efficient fbr enzymes from Pseudomonas. AroF-I is identified as a phospho-2-dehydro-3-deoxyheptonate aldolase (UniProt Q88KG6).
Historically, most known feedback-resistant mutants in the shikimate pathway were obtained by random mutagenesis approaches, using transposons or highly toxic chemicals, like fluoro-analogs of tyrosine or phenylalanine, or ethyl methane sulfonate, (Weaver and Herrmann [36], Lütke-Eversloh and Stephanopoulos [37]) and rational designs only appeared recently (Jayaraman et al. [16], Liu et al. [38]). While random mutagenesis is a straightforward strategy to obtain mutants with a desired phenotype, it must be associated with a laborious and time-consuming screening phase (Chen et al. [39], Rowlands [40]). Also, reverse engineering is frequently used following these methods to identify impacting mutations and introduce them in a final strain (Zhao et al. [41]). On the contrary, a rational design drastically reduces the time dedicated to screening and alleviates the need for reverse engineering.
The aim of this study was to rationally identify and evaluate the fbr mutant of the AroF-I DAHP synthase from Pseudomonas putida KT2440. First, the sensitivity of AroF-I toward the three aromatic amino acids L-Phe, L-Trp, and L-Tyr was tested in vitro, showing a negative regulation of the enzyme in the presence of L-Tyr. Then, bioinformatics studies were carried out to determine putative amino acids responsible for the negative feedback regulation of AroF-I by L-Tyr. Thus, several potential deregulated mutants of AroF-I were constructed and tested in vitro. A double mutant was found to be fully active and resistant to feedback inhibition by tyrosine. This is the first feedback-resistant DAHP enzyme designed, described, and characterized for Pseudomonas putida. A mutant strain harboring this deregulated AroF-I shows promising results for high-titer bioproduction of p-coumaric acid.
2. Results
2.1. AroF-I from Pseudomonas putida Is Highly Sensitive to and Inhibited by Tyrosine
To first evaluate if AroF-I is a feedback-inhibited enzyme, the corresponding gene (PP_2324) was cloned into the pET28 expression vector, overexpressed into E. coli and the His-tagged protein was purified (see Appendix A Figure A2). The activity of AroF-I was tested in vitro and was found to be fully active in the tested conditions (Figure 2). All the phosphoenolpyruvate was consumed in 1 h or less. Either phenylalanine, tyrosine, or tryptophane were added to the in vitro test, along with the purified AroF-I enzyme. Phenylalanine and tryptophane seemed to slightly inhibit AroF-I, whereas the inhibition by tyrosine was dramatic (Figure 2). We concluded that AroF-I is highly sensitive to tyrosine, which exerts a negative feedback inhibition on this enzyme.
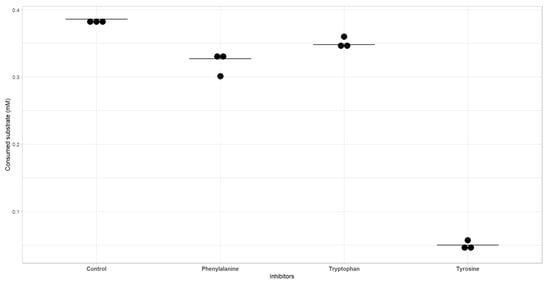
Figure 2.
Activity of AroF-I in the absence or presence of inhibitors. Dots represent each replicate as a raw value of consumed substrate (mM), and the mean is represented as a horizontal black bar.
2.2. Bioinformatics Helps in the Prediction of Allosteric Inhibition of the AroF-I Enzyme
The 3D structure of AroF-I from Pseudomonas putida KT2440 was reconstructed from its amino-acid sequence through homology modeling by SWISS-MODEL (Waterhouse et al. [42]) and used for docking with tyrosine. The protein region that is responsible for the allosteric regulation was identified by comparison with a homolog protein, AroG, a phospho-2-dehydro-3-deoxyheptonate aldolase (UniProt ID P0AB91) from Escherichia coli strain K12, known to be regulated by phenylalanine. Through a 3D structure alignment, we could identify the same structure in the AroF-I model. Analyzing different solutions obtained for the docking of AroF-I with tyrosine, we could identify a few residues that seem close enough to the molecule to interact with it. These residues are the following: 160 (P160), 164 (Q164), 190 (S190), 191 (G191), 193 (S193), and 225 (I225) (numbering corresponds to the position of the amino acid in the FASTA sequence of AroF-I).
For each one of these residues, we identified possible substitutions based on the literature and the alignment against naturally tyrosine feedback-resistant AroF enzymes. These approaches are described in the Material and Methods Section, and the results can be seen in Table 1.

Table 1.
AroF-I variant positions and amino-acid substitutions assayed in vitro.
2.3. Effect of Point Mutations on AroF-I Activity in the Presence or Absence of an Inhibitor
As described in the previous section, multiple potential mutation sites were predicted by the in silico study. Some of the best candidates were chosen, and mutations were introduced into the AroF-I enzyme. It included P160L, Q164A, S190A, G191K and S193A. As for the wildtype, the corresponding genes were cloned into the pET28a(+) expression vector, overexpressed into E. coli and the mutated His-tagged proteins were purified. Some single mutations (Q164A, S190A, and I225P) prevented the correct synthesis and/or purification of the AroF-I enzyme or shown strictly no activity, thus stopping the study at this step. The in vitro analysis was conducted on the three remaining active single mutants of AroF-I (P160L, G191K, and S193A). First, the activity in the absence of an inhibitor was assessed. As shown in Figure 3, the introduction of point mutation G191K almost completely abolished AroF-I activity. Then, this mutant was no longer considered to be an AroF-I fbr candidate. Results were contrasted for mutant AroF-I S193A, both in the absence and presence of tyrosine. Enzymatic activity without tyrosine was comparable to the WT, whereas the activity in the presence of tyrosine seemed to be slightly higher. The most interesting mutation was the AroF-I P160L. This mutant had a significantly higher activity in the presence of L-Tyr than the WT enzyme (Figure 4). Furthermore, the activity of mutant P160L without inhibitor was comparable to the WT, making AroF-I P160L the best candidate for further engineering.
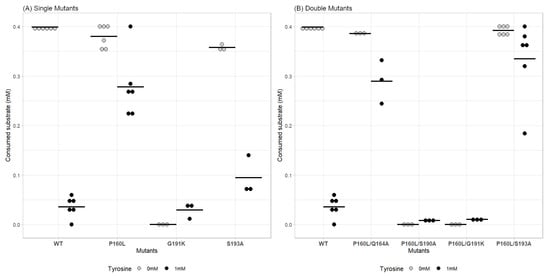
Figure 3.
Comparison of the consumed substrate (mM) for WT AroF-I vs. single mutants (A) and double mutants (B) in the absence and presence of tyrosine. Gray dots represent raw values of replicates in the absence of tyrosine; Black dots represent raw values of replicates in the presence of 1 mM tyrosine. Horizontal black bars represent the mean value of all replicates for each condition.
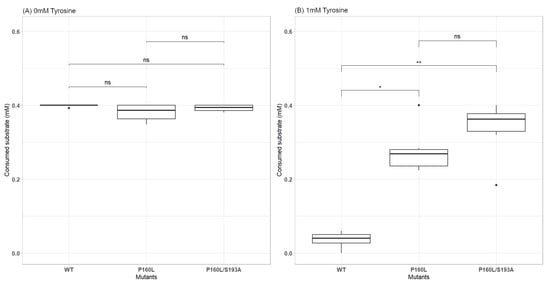
Figure 4.
Comparison of the consumed substrate (mM) for WT AroF-I vs. promising mutants in the absence (A) and presence (B) of 1 mM tyrosine. Boxplot with n = 6 for each tested condition. For statistical analysis, a Kruskal–Wallis non-parametric test with Dunn’s post hoc analysis was used. p-value adjusted using Holm’s method (significance = ns: p-value > 0.05; *: p-value < 0.05; **: p-value < 0.01). Cohen’s d test values for effect size evaluation are: WT vs. P160L = 0.78; WT vs. P160L/S193A = 1.03; P160L vs. P160L/S193A = 0.52. Values show significant differences between the compared data.
2.4. A Double Mutation Improves the Tyrosine Resistance of AroF-I and Its Enzymatic Activity
The promising results observed with AroF-I P160L led us to further work on this mutant. We thought to introduce a second mutation from the list described in the bioinformatic analysis section. All combinations were considered with no a priori as it would be interesting to observe potential synergistic effects of mutations, which are usually hard to predict (Mildvan [43], Chaparro-Riggers et al. [44]). Following the same procedure as above, we constructed several plasmids with mutated genes of AroF-I with the aim of expressing and purifying double mutants of the AroF-I enzyme. We obtained four combinations of mutations, all including P160L: P160L/Q164A; P160L/S190A; P160L/G191K; P160L/S193A). Interestingly, when compared to the single mutants, all the combinations were correctly expressed and purified (see Appendix A). The in vitro characterization was then pursued on the four double mutants obtained. First, the activity of these mutants was assayed in the absence of any inhibitor. Two out of four enzymes showed no activity (AroF-I P160L/S190A; AroF-I P160L/G191K) and were no longer studied (Figure 3). For the P160L/Q164A variant, the activity with and without tyrosine was similar to the single mutant P160L. The characteristics of this mutant are then mostly linked to the P160L mutation, and the second introduced mutation has a null or low impact on the measured activity. Regarding double mutant AroF-I P160L/S193A, the activity in the presence of tyrosine was significantly higher than the WT protein (Figure 4). Surprisingly, the combination of mutations P160L and S193A seemed to improve the resistance to tyrosine compared to the P160L single mutation. Thus, the double mutant AroF-I P160L/S193A was elected AroF-I fbr and selected to study its in vivo impact on p-coumaric acid production.
2.5. Construction of Pseudomonas putida Strains Overproducing Aromatic Amino Acids
To evaluate the potential of the AroF-I P160L/S193A enzyme for p-coumaric acid bioproduction, the gene coding for this mutant was inserted under the control of a strong constitutive promoter in the chromosome of a pCA-producing strain of Pseudomonas putida KT2440 (called Ppu201). This strain overexpresses a tyrosine ammonia lyase (TAL, EC 4.3.1.23) (integrated into the chromosome), as well as aroH, one of the three DAHP synthases found in P. putida’s chromosome. The resulting strain was named Ppu235. To assess the producing capacities of both strains, they were cultured for 24 h in a minimal medium containing glucose as the sole carbon source, and an HPLC analysis was performed on the supernatant fractions to detect and quantify the produced phenolic compounds. As shown in Figure 5, the strain harboring the AroF-I mutant showed a higher production capability, being approximately 0.66 mM Phenolics/1 OD600 nm, compared to Ppu201 producing 0.53 mM Phenolics/1 OD600 nm. The two major products detected are cinnamic acid and p-coumaric acid. Indeed, the TAL enzyme, responsible for the conversion of tyrosine to p-coumaric acid, is also able to use phenylalanine as a substrate to produce cinnamic acid. These results indicated that the Ppu235 strain produces more tyrosine and more phenylalanine than its homolog without the AroF-I mutant, indicating a deregulation of the shikimate pathway. It is worth noting that Ppu235 showed a final OD600 nm comparable with Ppu201, suggesting that the introduction of the feedback-resistant mutant did not seem to alter the overall fitness of Ppu235.
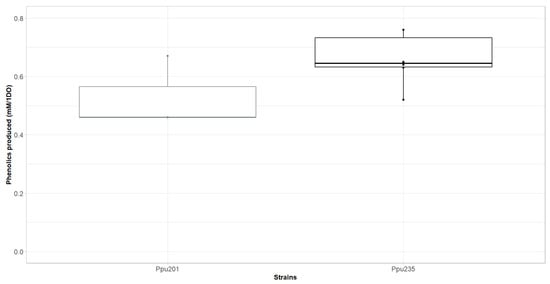
Figure 5.
In vivo production of phenolic compounds by P. putida strains expressing (Ppu235) or not (Ppu201) AroF-I fbr variant (P160L/S193A).
2.6. A Synergistic Effect of AroF-I Fbr and phhAB Improves Production of p-Coumaric Acid
As stated earlier, a Pseudomonas putida KT2440 strain with a deregulated shikimate pathway was constructed by introducing a double mutant P160L/S193A of the AroF-I enzyme into the chromosome of a previously constructed p-coumaric acid-producing strain. It is suggested that in Pseudomonads, the majority of tyrosine is produced through the conversion of phenylanine (Otto et al. [35], Wierckx et al. [45]). Therefore, we thought to overexpress the two genes phhA (PP_4490) and phhB (PP_4491) coding for the enzymes responsible for the synthesis of tyrosine from phenylalanine. The phhAB operon was cloned into a pBBR1 vector under the control of the arabinose inducible promoter araC/Pbad, creating pC2F327. This plasmid was transformed into Ppu201 and Ppu235, and the resulting strains were cultured in minimal medium MPpu with glucose as the sole carbon source, supplemented or not with 0.5% L-arabinose. A plasmid expressing eGFP under the control of the arabinose inducible promoter was used as a control (plasmid pPpu226). On the one hand, as depicted in Figure 6, Ppu235 showed a slight increase in total phenolics produced when the control plasmid was used. Furthermore, the repartition between cinnamic acid and p-coumaric was lower in Ppu235 than in Ppu201, which is in accordance with the results observed for both strains without bearing any plasmid. Induction of eGFP did not change the conclusions except that fewer phenolics were produced, which is not surprising considering the metabolic burden created by gene overexpression on plasmid (Glick [46]). On the other hand, results obtained with the expression of phhAB were very promising. When the expression of the phhAB operon was not induced, both strains produced an amount of phenolics comparable with the control plasmid. On the contrary, when arabinose was supplemented in the medium, a sharp increase in total phenolics produced was measured for Ppu235 bearing pC2F327, reaching 1.9 mM phenolics/1 OD600 nm, which is 3 times higher than with the control plasmid. In addition, pCA represented 97% of the total phenolics produced in this condition, improving by 10% the amount of pCA in total phenolics produced. However, this enhanced pCA production is correlated with a decrease in growth rate (see Appendix A Table A1). While P. putida KT2440 is known to tolerate high pCA concentrations, ruling out pCA toxicity. The observed growth reduction may be attributed to L-phenylalanine depletion and/or broader metabolic imbalances resulting from phhAB overexpression (Calero et al. [29]). Nevertheless, the strain Ppu235 expressing phhAB is a promising strain towards a high-titer and high-yield industrial production of p-coumaric acid.
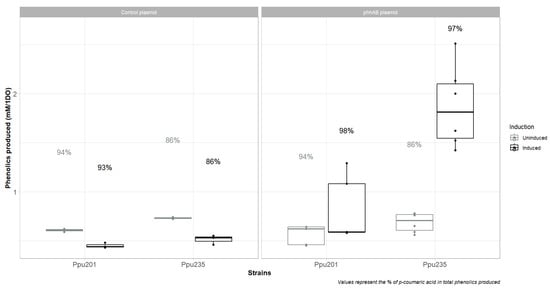
Figure 6.
In vivo production of phenolic compounds by P. putida strains expressing (Ppu235) or not (Ppu201) AroF-I fbr in combination with the overexpression of phhAB genes. Bold box plots are when phhAB is induced, gray ones when phhAB is not induced by Arabinose.
3. Materials and Methods
3.1. Bacterial Strains, Plasmids, and Growth Conditions
The bacterial strains and plasmids used in this study are listed in Table 2. P. putida strains were grown at 30 °C and E. coli strains at 37 °C either in liquid LB medium (Bertani [47]) or on solid agar LB plates (additionally containing 1.5% (w/v) agar). For the selection of plasmid transformants, 50 g/mL kanamycin (Kan) was added. After mating procedures, recombinant Pseudomonas were isolated on Yeast Extract Tryptone (YT) plates supplemented with Ampicillin (100 mg/mL) and kanamycin (50 mg/mL). The loss of the suicide plasmid was selected on YT plates containing 10% sucrose.

Table 2.
Bacterial strains and plasmids used in this study.
For enzyme production and purification, Escherichia coli strains were grown in a ZYM auto-inducer medium (Studier [51]). For p-coumaric acid production assay, P. putida KT2440 strains were grown in a minimal medium containing 4.77 g/L Na2HPO4, 2.02 g/L KH2PO4, 2.36 g/L (NH4)2SO4, 0.24 g/L MgSO4 and 5 g/L glucose, with the following trace elements: 13.9 mg/L FeSO4·7H2O, 14.7 mg/L CaCl2·2H2O, 8.6 mg/L ZnSO4·7H2O. To induce gene expression using the araC/Pbad promoter, 0.5% (m/v) arabinose were added to culture when necessary. The chemicals used in this work were obtained from Carl Roth (Karlsruhe, Germany), Merck (Darmstadt, Germany), or Sigma–Aldrich (Saint Louis, MO, USA).
3.2. Vector Construction and Strain Engineering
For plasmid constructions, DNA fragments were PCR amplified using the Platinum SuperFi high-fidelity polymerase (Thermo Fisher Scientific, Waltham, MA, USA) with corresponding overhangs to enable subsequent Gibson assembly (Gibson [52]) with the NEBuilder HiFi DNA Assembly mix (New England Biolabs, New Ipswich, NH, USA). Heat shock was used for the transformation of DNA assemblies and purified plasmids into E. coli. Plasmids were introduced in Pseudomonas putida KT2440 by conjugational transfer of mobilizable plasmids. Mating procedures were performed as follows. Overnight cultures of the E. coli donor, S17.1 strain bearing the desired plasmid, and the recipient Pseudomonas putida strain, were washed twice in MgSO4 10 mM. Equal volumes of the cultures (50 L) were mixed and incubated for 4 to 5 h at 30 °C without agitation. The mix was then harvested and resuspended in 100 L of MgSO4 10 mM before plating on a selective medium. Plates were incubated overnight at 30 °C. Replicative pBBR1-MCS2-based plasmids (Kovach et al. [49]) were used for gene expression, pET28a(+)-based plasmids (Novagen) were used for protein production and purification, and pK18mobsacB (Schäfer et al. [50])-based plasmids were used for chromosomal modification of P. putida strains. The pK18mobsacB vector is suicidal in Pseudomonas putida and can be used for gene insertion into a definite chromosomal target. When this plasmid was used for chromosomic modifications in Pseudomonas, the mating mixture for conjugational transfer was incubated overnight before following the procedure as described earlier. Eventually, for chromosomal modifications of Pseudomonas putida strains, antibiotics-resistant clones were incubated overnight at 30 °C in LB medium without selection pressure. A streak of this culture was then performed on YT sucrose 10%.
E. coli or Pseudomonas putida transformants, and chromosomally engineered Pseudomonas were screened by colony PCRs using the DreamTaq 2X Master Mix PCR (Thermo Fisher Scientific, Waltham, MA, USA). Primers were ordered as unmodified DNA oligonucleotides from Eurofins Genomics (Ebersberg, Germany). Plasmid inserts and gene deletions or insertions were confirmed by Sanger sequencing performed by Microsynth AG (Balgach, Switzerland). AroF-I mutated genes were ordered as synthetic DNA fragments from Eurofins Genomics (Ebersberg, Germany). PCR products and plasmids were purified using NucleoSpin Gel and a PCR cleanup purification kit from Macherey–Nagel (Düren, Germany) according to the manufacturer’s instructions.
3.3. Production and Purification of the AroF-I Proteins
All DNA fragments corresponding to mutated variants of AroF-I genes were amplified with a flanking overhang and cloned into a pET28a(+) backbone. Assembly mixtures were transformed into a BL21(DE3) strain and the culture was plated on LB+Agar medium with appropriate antibiotic. Positive clones were PCR-checked and sequenced to confirm successful assembly.
Auto-inducible ZYM medium was inoculated at an initial OD of 0.05 with an overnight pre-seed culture and incubated for 24 h. For the first 5 h, the temperature was set to 25 °C and then lowered to 19 °C for the remaining incubation time. Next, protein purification was performed using the Protino Ni-TED purification kit for His-tagged proteins following the manufacturer’s instructions (Macherey–Nagel, Düren, Germany). Briefly, cells were separated from the medium by cold centrifugation for 10 min at 5000× g. The pellet was resuspended in an adapted buffer with the addition of lysozyme (final concentration 1 mg/mL) and kept on ice for 30 min. Then, cells were disrupted using sonication at 30% power, pulse set to 40, and a total time of 8 min (Omni Sonic Ruptor 400, Omni International (Kennesaw, GA, USA)). Streptomycine was added to a final concentration of 0.22% (m/v), and samples were cold-centrifugated for 30 min at 15,000× g. The supernatants were kept, and the enzymes were purified. After purification, proteins were concentrated approximately 10 times in Tris-HCl 0.1 M pH 7.5 + 10% glycerol using Amicon Ultra-4 30 kD from Merck (Darmstadt, Germany). Purified proteins were checked by SDS-PAGE electrophoresis and stored at −80 °C.
3.4. Enzymatic Assay for Determination of AroF-I Activity
Enzymatic activity was assessed by measurement of substrate consumption using HPLC detection. Assays were performed in a final volume of 200 L containing 40 mM phosphate buffer pH 7, 300 M phosphoenolpyruvate (PEP), 20 g of purified enzyme and 3 M HCl. For inhibition tests, 1 mM of aromatic acid was added to the reaction mixture. The final volume was adjusted using ultrapure water. After a 2 min pre-incubation at 30 °C, reaction was started by adding 300 M of erythrose-4-phosphate (E4P) and incubated at 30 °C for 60 min. To stop the reaction, the mixture was heated to 80 °C for 5 min. Samples were then centrifugated for 10 min at 15,000× g. The supernatant was kept and filtrated on a 0.22 m membrane before subsequent HPLC analysis. The remaining PEP concentration was determined using the Agilent 1100 system (Agilent Technologies, Santa Clara, CA, USA) with a Luna OMEGA polar C18 column (Phenomenex, Torrance, CA, USA). Injection of 15 L was analyzed for each sample. The mobile phase was a 1 mM phosphoric acid solution. The rate flow was set to 0.3 mL/min, and detection occurred via UV detection at 220 nm. The total time of analysis for each sample was 15 min.
3.5. Replacement of aroH by Mutated AroF-I
The gene AroF-I P160L/S193A was amplified from the synthesized DNA fragment and cloned into the vector pK18mobsacB. The pK18mobsacB vector is suicidal in Pseudomonas putida and can be used for gene insertion into a definite chromosomal target. To perform chromosomal modification, the suicide plasmid was constructed based on pK18mobsacB bearing two homology arms of 800 bp around aroH (PP_1866) upstream and downstream the gene coding for the mutated version of AroF-I. Using this plasmid, chromosomal modification was achieved to obtain strain Ppu235 from strain Ppu201, a p-coumaric acid-producing strain obtained in our laboratory in which aroH is overexpressed under the control of a strong constitutive promoter and a tyrosine ammonia lyase is inserted into the chromosome.
3.6. Overexpression of phhAB Genes
The two genes phhA (PP_4490) and phhB (PP_4491) coding, respectively, for a Phe-4-monooxygenase and a Pterin-4-alpha-carbinolamine dehydratase were cloned into a pBBR1-MCS2 expression plasmid as an operon under the control of the inducible araC/Pbad promoter. The WT genes were amplified from purified genomic DNA with a flanking overhang to allow insertion into the plasmid backbone. The assembly mixture was then transformed into an S17.1 strain and plated LB+Agar with the appropriate antibiotic. Positive clones were PCR-checked, and amplicons were sequenced to confirm successful assembly. The final plasmid was transferred to the recipient P. putida KT2440 strain by conjugation.
3.7. Assessment of pCA Production in Batch Culture
To evaluate the production of p-coumaric acid by genetically modified Pseudomonas putida strains, batch culture in Erlenmeyer flasks was performed. First, strains were plated on an LB agar plate containing appropriate antibiotics when needed. One colony was PCR-checked and cultured overnight in 5 mL minimal medium supplemented with 5 g/L glucose at 30 °C with agitation. The next day, the pre-seed culture was used to inoculate 25 mL of minimal medium supplemented with 5 g/L glucose and appropriate antibiotic in an Erlenmeyer flask. When needed, arabinose was used to induce gene expression. Growth was followed by measuring OD at 600 nm, and p-coumaric acid production was assessed by HPLC measurement at the end of the batch culture. The analytical method used to identify and quantify p-coumaric acid and cinnamic acid was as follows: Kinetex 5 m F5 100 A 150 × 4.6 mM column (Phenomenex, Torrance, CA, USA), formic acid 0.1% and acetonitrile as mobile phases with variating gradient (0–5 min = 100% formic acid; 5–25 min = 75% formic acid + 25% acetonitrile; 25–30 min = 62% formic acid + 38% acetonitrile; 30–35 min = 100% formic acid), flow rate of 1 mL/min, oven at 40 °C, injection volume of 10 L and UV detection at 315 nm for p-coumaric acid and 28 nm for cinnamic acid.
3.8. Statistical Analysis
A Shapiro–Wilk normality test (Shapiro and Wilk [53]) and a Bartlett variance equality test (Jones et al. [54]) were first performed on data to be analyzed. As data did not meet the criteria to be analyzed via a parametric statistical analysis, a non-parametric Kruskal–Wallis test was conducted (Kruskal and Wallis [55]) followed by a Dunn’s post hoc analysis with Holm’s adjustment method for the p-value (Dunn [56]). To confirm results obtained using the Kruskal–Wallis test, a Cohen’s d measure for effect size evaluation was performed (Cohen [57]). The results of these two tests are shown in Figure 4.
3.9. Bioinformatics Protocols
Our primary objective was to identify point mutations in our target, AroF-I, that may reduce the feedback regulation of tyrosine. To achieve this, we performed a docking analysis of tyrosine within the AroF-I structure to pinpoint amino acids that are likely to play a significant role in the molecule’s binding. We then aimed to substitute these residues with amino acids exhibiting different properties, thereby blocking or diminishing the likelihood of tyrosine binding.
The PDB structure for the UniProt entry Q88KG6 (AroF-I from Pseudomonas putida KT 2440) was not available at the moment of this study, so we reconstructed it using the Swiss-Model approach (Waterhouse et al. [42]). Our structure was built with ProMod3 (version 2.0) using 1of6.4.G structure as a template. We obtained a Global Model Quality Estimate (GMQE) of 0.77, which is considered reasonable.
We used this structure to perform docking against tyrosine. We used Vina (Trott and Olson [58]) as our docking software, and we considered a large region for the docking, as we can see in Figure 7. The center of the docking box is located at the coordinates (17.214, 39.976, 59.293), with dimensions of 44, 48, and 32 ångströms along the x, y, and z axes, respectively. This choice was based on the analysis of AroG, a phospho-2-dehydro-3-deoxyheptonate aldolase (UniProt ID P0AB91) from Escherichia coli K12 (crystal structure 1kfl from PDBe), where a phenylalanine is docked in the protein. This protein is known to be regulated by phenylalanine and, based on studies by Shumilin et al. [59] and Cui et al. [60] on Escherichia coli DAHP synthases, we assumed the docking region for the tyrosine would be similar. Analyzing the docking solutions, we could identify six residues in interaction with at least one atom of the docked tyrosine: P160, Q164, S190, G191, S193, and I225. In Figure 8, we present our solutions overlapped within the protein pocket, highlighting the identified residues and some of the hypothetical bonds. Since the identified residues present putative interactions with tyrosine, we supposed that altering these residues could, in theory, inhibit the docking of this molecule.
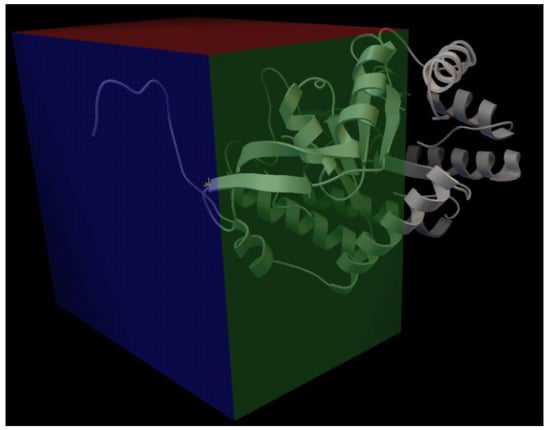
Figure 7.
Docking region used in our docking studies with the AroF-I from Pseudomonas putida KT2440. The docking box is located at the coordinates (17.214, 39.976, 59.293), with dimensions of 44, 48, and 32 along the x, y, and z axes, respectively.
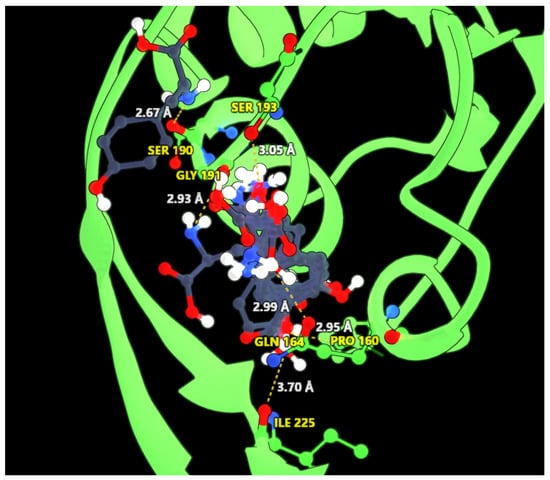
Figure 8.
Overlapped solutions of the docking of tyrosine into the AroF-I from Pseudomonas putida KT2440. In yellow we see the name and number of the residues that form a bond with at least one solution. These residues are depicted with green carbons. The dashed lines highlight the found interactions, and in white, we have the distance of the bonds.
To identify possible replacement residues for this region, we consulted the literature on known phenylalanine feedback-resistant mutations for phospho-2-dehydro-3-deoxyheptonate aldolases in Escherichia coli (Cui et al. [60], Ding et al. [61]). Being neutral and non-polar, alanine is frequently used as a replacement residue, but we also found other candidates. We employed an alternative approach based on the structural and sequence alignment of our target enzyme, AroF-I, with other enzymes known to exhibit natural feedback resistance to tyrosine. The aim was to determine which natural residues occupy the positions of those identified in our docking study. We considered UniProt entries Q9YEJ7 (AroG from Aeropyrum pernix), A0A5C0XSG1 (aroF from Pyrococcus furiosus) and Q9V1I0 (AroG from Pyrococcus abyssi) that were aligned with AroF-I and we identified different amino acids present at positions corresponding to the target residues identified in the docking approach. Finally, based primarily on amino-acid properties, we selected one candidate mutation for each position. For each target residue, we chose a replacement amino acid that was either neutral or exhibited opposing physicochemical properties compared to the original in AroF-I. The chosen mutations can be seen in Table 1.
4. Discussion
Cellular metabolic pathways, from the simple to the complex, are highly regulated in response to external nutrient changes, internal metabolite accumulation, or stress responses (Shimizu [62], Chubukov et al. [63]. Different strategies are adopted by the cell for these tight regulations: genes transcriptional and/or translational regulations, through regulatory proteins or small RNAs, and fine-tuning of enzymatic activities (Shimizu and Matsuoka [64], Nudler [65]).
One good example of tight metabolic regulation is found in fungi, bacteria, and plants, with the production of aromatic amino acids (L-tyrosine, L-phenylalanine, L-tryptophane) via the shikimate pathway. The shikimate pathway and how to optimize the metabolic fluxes to overproduce certain intermediates were extensively studied in several microbial chassis, especially since aromatic intermediates are widely used as raw materials in the medical, chemical, food, and other industries (Wu et al. [66], Jiang and Zhang [67], Cao et al. [68]).
In S. cerevisiae, the initial step of the shikimate pathway is catalyzed by two DAHP synthase isozymes, encoded by ARO4 and ARO3 and regulated through feedback inhibition by L-tyrosine and L-phenylalanine, respectively (Helmstaedt et al. [69]). In Escherichia coli three DAHP synthase isozymes exist (aroF, aroG, aroH), each one being feedback-inhibited by one of the three aromatic amino acids (Jayaraman et al. [16], Jossek et al. [70]). Regulation of DAHP synthases, the entry point in the shikimate pathway, and the mechanisms involved are well described and conserved among microorganisms (Mir et al. [71]). Some studies were already done regarding the shikimate pathway in P. putida KT2440, but most of them involve mutated enzymes from E. coli (Wierckx et al. [32], Calero et al. [33], Otto et al. [35]). To our knowledge, no evidence of such a regulation was available for DAHP synthases from P. putida even if a putative fbr mutant of AroF-I was described for P. putida S12 (Wierckx et al. [32]). Knowing that P. putida KT2440 is well suited for the industrial production of aromatic amino-acid derivatives. We thought to highlight the regulation of the shikimate pathway in this strain and engineer a DAHP synthase to obtain a phenolypropanoids overproducing strain.
Feedback-resistant mutants have initially been found using random mutagenesis and screening of the best aromatic amino-acid producers (Ger et al. [72], Ray et al. [73], Kikuchi et al. [74]). However, this process is time-consuming because promising mutants can be hard to find among all the diversity created by random mutagenesis. Nevertheless, over recent decades, the development of bioinformatics tools, combined with knowledge of protein structure, functions, and regulations, have paved the way for the rational engineering of protein (Jayaraman et al. [16], Lutz [75], Chen et al. [76], Nixon and Firestine [77]) and allows us to create variants of genes purposefully as to obtain feedback-resistant forms of DAHP synthases. We chose to apply these principles to the interesting AroF-I DAHP synthase from P. putida KT2440, with the aim of having a reasonable set of rationally designed candidates to obtain a feedback-resistant mutant of this enzyme. Using protein structure modeling, ligand interaction modeling, and knowledge from the literature (Cui et al. [60], Ding et al. [61]), several mutants of AroF-I were synthesized, then cloned and expressed in E. coli. We were confident in the results obtained using bioinformatic tools, as we successfully identified residues that had previously been described in similar DAHP synthases. Not surprisingly, some mutations proved critical for protein expression and activity, likely altering the protein’s secondary structure and preventing proper folding (Faisca et al. [78], Sen et al. [79]). Regarding these issues in protein variant expression, it may be worthwhile to conduct site-saturation experiments, as alternative amino-acid substitutions could influence the overall shape and activity of the enzyme. (Chronopoulou and Labrou [80]). Another option would be to use G energy prediction between mutated protein and wildtype as it is shown that energy variation can correlate with protein stability(Marabotti et al. [81]). Even if predictions are not perfect and do not always correlate with experimental values, these tools can help select candidates for experimental testing. Fortunately, the prediction of mutation stability has advanced significantly in recent years, and the development of new artificial intelligence models and algorithms will further enhance the reliability of these predictions. (Pucci et al. [82], Fang [83], Cheng et al. [84]). Since we explored just a small set of mutations and experimentally tested them all, we did not perform this type of prediction.
Among the few candidates we ordered, two single mutants showed better resistance to tyrosine inhibition in vitro (P160L and S193A) without the need for intensive screening. Pursuing our work further, we combined single mutations to create double mutants of AroF-I. In the end, we demonstrated that the AroF-I P160L/S193A was a fully active and fully tyrosine-resistant mutant. To our knowledge, despite a few studies of DAHP synthases in other Pseudomonas strains (Wynands et al. [34]), this is the first rationally designed and characterized mutant of a variant DAHP synthase for P. putida.
Single mutants capable of rendering a DAHP synthase feedback-resistant have never been finely enzymatically characterized. Some mutations are known to impact the allosteric regulation by tyrosine and phenylalanine, as discussed previously in this manuscript, and the binding site of the E. coli DAHP synthase is known and composed of residues from two adjacent subunits of a dimer (Shumilin et al. [59], Cui et al. [60]). However, the allosteric mechanism is not fully understood, and the possible synergic effects of double mutants have not yet, to our knowledge, been investigated. It is known that the synergistic effect of mutations may render a protein more stable, even if identifying or understanding these synergies is not straightforward (Hamborg et al. [85]). The same difficulty can be translated into the study of allosteric regulation. One way of approaching this problem may be through the aid of normal mode analysis methods. Normal mode analysis can help in understanding allostery in proteins by exposing the dynamic relationships between structural changes and functional outcomes. By analyzing the vibrational modes of proteins, we can identify allosteric sites and predict how conformational changes propagate through the protein structure (Greener and Sternberg [86], Yamato and Laprévote [87], Kolossváry [88]). Surely, these methods do not exclude in vitro or in vivo validations, but it may be a way to reduce the highly combinatorial solution space of mutations to explore. Another possibility could be the exploration of deep learning methods and protein language models that are literally revolutionizing the understanding of proteins (Ruffolo and Madani [89]). These models can extract structural and functional insights from amino-acid sequences or protein structures. This can help us to assess functional properties, and identify structural features, thereby enhancing our comprehension of protein dynamics. However, pre-trained protein language models alone are not enough since their accuracy and interpretability remain limited. However, an approach based on few-shots (few or scarce data points) used to retrain protein language models has been proven recently useful for this kind of task (Schmirler et al. [90], Zhou et al. [91], Biswas et al. [92]).
We then decided to incorporate the promising variant into a P. putida strain, already engineered to overproduce p-coumaric acid and screen the effect of a plasmid copy of phhAB. Plasmid-driven gene expression is the easiest and most common strategy for recombinant protein production, rapid screening, and strain engineering. It has been extensively used in recent decades to study enzyme activities, namely in the well-known T7 system in E. coli (Chen [93]). This system was used in this study to express, purify, and assay the catalytic parameters of the AroF-I variants and compare them to each other.
However, studying the proteins in their bacterial environment has limitations when expressed from a plasmid. It is thus preferable to opt for a chromosomal-driven strategy (Goussé et al. [94]), explaining why we adopted this strategy to express our AroF-I variant. For example, a too-high translation rate can lead to inclusion bodies or proteolytic degradation and thus a weak production (Kachroo et al. [95].). We can also be confronted with metabolic burden and/or plasmid instability. Moreover, inducing gene expression from a multi-copy plasmid is known to be a source of variability between batches when compared to chromosomal integration or single-copy plasmid (Zucca et al. [96]). This is true if considering the pCA production with our Ppu235 strain bearing the phhAB plasmid, which shows considerable variations between batches. Regarding the designed AroF-I fbr, as the aim is to express a variant of a native enzyme, we chose the native chromosomal locus. However, the choice of a different locus can also be interesting in the fine-tuning of expression level or inserting an additional copy of the gene in the future (Tyo et al. [97]). Moreover, when constructing a strain for secondary metabolite production, it is preferable to adopt a strategy for the long-term stability of the strain. The technology we developed allows the insertion of genes in the chromosome without scar or marker (e.g., antibiotic resistance (Patent [98]). Several modifications can be performed in the genome at different loci without the necessity of accumulating antibiotics or thinking about compatible plasmids. This strategy will be performed as well for the chromosomal overexpression of phhAB instead of a plasmid-driven expression.
Reviews on several microbial hosts showed that the deregulation of DHAP synthase is not sufficient and that multiple engineering strategies have to be tested to obtain a high-titer and high-yield production of aromatic amino acids and their derivatives (Bilal et al. [19], Wu et al. [66], Li et al. [99]). The shikimate pathway is highly regulated, interconnected, and central in bacterial metabolism. Thus, maximizing the fluxes towards the production of L-Phe and L-Tyr needs efforts in cutting unnecessary reactions and boosting key reactions (Bentley and Haslam [100], Liu et al. [101]). Further work will be needed on the obtained Ppu235 strain to continue improving titer and yield and make it suitable for industrial production of p-coumaric acid. For example, the derivation point around chorismate in the shikimate pathway is known to be regulated to ensure a balance between branched pathways (Kroll et al. [102], Brown and Dawes [103], Zhang et al. [104]). In Pseudomonas putida, a deregulated variant of the chorismate mutase-prephenate dehydratase pheA (PP_1769, EC 5.4.99.5), catalyzing the conversion of chorismate to prephenate, was found and used in production strains (Molina-Santiago et al. [28], Otto et al. [35]). Using this pheA fbr variant in our Ppu235 strain could enhance the production of L-Tyr and p-coumaric acid.
From an industrial perspective, an internal techno-economic study sets a target pCA titer of at least 65 mM in large-scale bioreactors. Efficient sugar conversion is essential to minimize both operating (OPEX) and capital (CAPEX) expenditures. Extrapolating from our shake-flask data, and assuming typical P. putida bioreactor densities (OD600 of 30–50) (Mokwatlo et al. [105]), our engineered strains are projected to achieve 55–92 mM pCA without any supplementation. This represents a significant improvement over previous efforts, such as the P. putida S12 strain requiring L-phenylalanine supplementation (10.6 mM) and the highly engineered yeast strain achieving 76 mM (Flourat et al. [106]). Unlike these approaches, our method combines rational enzyme engineering for feedback resistance with stable chromosomal overexpression to enhance tyrosine flux. This strategy offers a more robust and industrially relevant platform for pCA production, with the potential to meet industrial demands and elevate bioproduction into the spotlight.
Also, thinking about product purification at the step of strain construction seems important. To this extent, reducing or suppressing cinnamic acid production is crucial to the development of an efficient process. With the maximization of fluxes towards L-Phe and L-Tyr, we could end up with an accumulation of these amino acids. On the one hand, an accumulation of L-Phe would lead to a greater production of cinnamic acid. On the contrary, an over-accumulation of L-Tyr could lead to an incomplete conversion to p-coumaric acid. To avoid the first hypothesis, chromosomal expression of phhAB is a promising strategy, as described earlier in this work. Also, the use of bioinformatics tools to identify or design highly active TAL enzymes unable to use L-Phe as a substrate to form cinnamic acid seems of high priority. Several studies have reviewed works done on this enzyme to elucidate product specificity (Louie et al. [107], Watts et al. [108], Jendresen et al. [109]). Finding a highly active TAL would also have the side effect of avoiding L-Tyr accumulation and maximizing its conversion to p-coumaric acid.
In this study, we investigated the regulation of the shikimate pathway in Pseudomonas putida KT2440 with the aim of enhancing p-coumaric acid production. We successfully engineered an L-tyrosine feedback-resistant mutant of the DAHP synthase AroF-I (P160L/S193A) using rational mutagenesis and computational modeling. This approach proved advantageous over traditional random mutagenesis, leading to the identification of key residues involved in tyrosine-mediated feedback inhibition. The resulting DAHP synthase variant was incorporated into a P. putida strain previously pre-wired for p-coumaric acid production. While plasmid-based overexpression of the phhAB gene was initially used for rapid prototyping, future work will focus on chromosomal integration for long-term strain stability. In parallel, further metabolic engineering strategies are planned to maximize p-coumaric acid production. These include incorporating a deregulated chorismate mutase-prephenate dehydratase and exploring the use of highly active, L-tyrosine-specific tyrosine ammonia lyase (TAL) enzymes. By optimizing the metabolic pathway, especially with the help of computer science, we aim to develop a robust strain for industrial p-coumaric acid production.
5. Patents
A patent filed under the number WO2022238645A1 is the result of the work presented in this manuscript.
Author Contributions
Conceptualization, W.M., R.A., C.P., A.C.-P. and C.R.; methodology, W.M.; bioinformatics, R.A. and C.P.; validation, A.C.-P. and C.R.; formal analysis, W.M.; data curation, W.M. and R.A.; writing—original draft preparation, C.R.; writing—review and editing, all; visualization, W.M.; supervision, C.R.; project administration, A.C.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new datasets were created.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
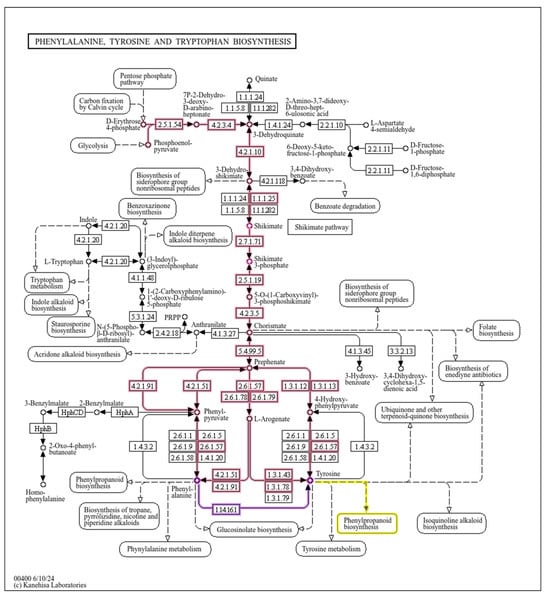
Figure A1.
An illustration of the shikimate pathway and related routes based on KeGG data [110]. In red, we have the shikimate pathway (upper part up to chorismate). Also, in red (after chorismate) and magenta, we have the phenylalanine and tyrosine biosynthesis routes. In yellow we can see the entry point to the biosynthesis of phenylpropanoids.
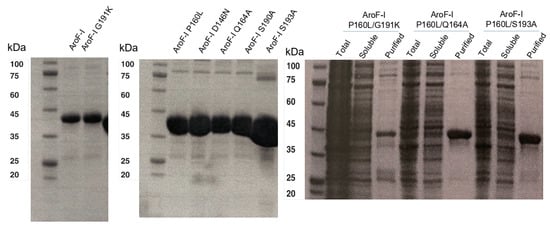
Figure A2.
SDS-PAGE gels showing purified proteins used in this study. The expected size is 37 kDa.

Table A1.
Strain growth (OD) and concentration of phenolic compounds, concentrations normalized by OD, and total phenolic compounds normalized by the OD. Grayed lines correspond to uninduced and blank lines to induced conditions. CP stands for control plasmid, and PP for phhAB plasmid. CA stands for cinnamic acid, and pCA for coumaric acid.
Table A1.
Strain growth (OD) and concentration of phenolic compounds, concentrations normalized by OD, and total phenolic compounds normalized by the OD. Grayed lines correspond to uninduced and blank lines to induced conditions. CP stands for control plasmid, and PP for phhAB plasmid. CA stands for cinnamic acid, and pCA for coumaric acid.
| Strain | OD600 | [pCA] (mM) | [CA] (mM) | pCA/1DO | CA/1DO | Total Phenolics/1DO |
|---|---|---|---|---|---|---|
| Ppu201 | 5.4 | 0.28 | 0.14 | 0.05 | 0.003 | 0.08 |
| Ppu201/CP | 3.4 | 1.95 | 0.12 | 0.57 | 0.04 | 0.61 |
| Ppu201/PP | 3.4 | 1.99 | 0.13 | 0.59 | 0.04 | 0.62 |
| Ppu235 | 4.1 | 1.94 | 0.66 | 0.47 | 0.16 | 0.63 |
| Ppu235/CP | 3.8 | 2.38 | 0.38 | 0.63 | 0.10 | 0.72 |
| Ppu235/PP | 3.7 | 2.46 | 0.37 | 0.66 | 0.10 | 0.76 |
| Ppu201 | 4.6 | 0.28 | 0.14 | 0.06 | 0.03 | 0.09 |
| Ppu201/CP | 4.6 | 1.83 | 0.15 | 0.40 | 0.03 | 0.43 |
| Ppu201/PP | 3.1 | 1.80 | 0.03 | 0.58 | 0.01 | 0.59 |
| Ppu235 | 3.4 | 1.92 | 0.66 | 0.56 | 0.20 | 0.76 |
| Ppu235/CP | 5.1 | 2.07 | 0.30 | 0.41 | 0.06 | 0.46 |
| Ppu235/PP | 1.6 | 2.21 | 0.07 | 1.38 | 0.04 | 1.42 |
| Ppu201 | 5.1 | 0.28 | 0.14 | 0.06 | 0.03 | 0.08 |
| Ppu201/CP | 3.3 | 1.92 | 0.13 | 0.58 | 0.04 | 0.62 |
| Ppu201/PP | 3.4 | 2.06 | 0.13 | 0.61 | 0.04 | 0.64 |
| Ppu235 | 3.9 | 1.93 | 0.65 | 0.49 | 0.17 | 0.66 |
| Ppu235/CP | 3.7 | 2.37 | 0.37 | 0.64 | 0.10 | 0.74 |
| Ppu235/PP | 3.6 | 2.39 | 0.37 | 0.66 | 0.10 | 0.77 |
| Ppu201 | 4.3 | 0.27 | 0.14 | 0.06 | 0.03 | 0.09 |
| Ppu201/CP | 4.1 | 1.82 | 0.14 | 0.44 | 0.03 | 0.48 |
| Ppu201/PP | 3.1 | 1.76 | 0.03 | 0.57 | 0.01 | 0.58 |
| Ppu235 | 3.8 | 1.83 | 0.65 | 0.48 | 0.17 | 0.65 |
| Ppu235/CP | 4.6 | 2.19 | 0.36 | 0.48 | 0.08 | 0.55 |
| Ppu235/PP | 1.1 | 2.26 | 0.06 | 2.05 | 0.05 | 2.10 |
| Ppu201 | 5 | 0.28 | 0.14 | 0.06 | 0.03 | 0.09 |
| Ppu201/CP | 3.5 | 1.94 | 0.13 | 0.55 | 0.04 | 0.59 |
| Ppu201/PP | 3.4 | 2.04 | 0.13 | 0.60 | 0.04 | 0.64 |
| Ppu235 | 3.9 | 2.00 | 0.64 | 0.51 | 0.17 | 0.68 |
| Ppu235/CP | 3.8 | 2.40 | 0.37 | 0.63 | 0.10 | 0.73 |
| Ppu235/PP | 3.6 | 2.44 | 0.36 | 0.68 | 0.10 | 0.78 |
| Ppu201 | 4.4 | 0.27 | 0.14 | 0.06 | 0.03 | 0.09 |
| Ppu201/CP | 4.7 | 1.90 | 0.15 | 0.41 | 0.03 | 0.44 |
| Ppu201/PP | 3.2 | 1.85 | 0.03 | 0.58 | 0.01 | 0.59 |
| Ppu235 | 3.5 | 1.98 | 0.68 | 0.56 | 0.20 | 0.76 |
| Ppu235/CP | 5.4 | 2.45 | 0.39 | 0.45 | 0.07 | 0.53 |
| Ppu235/PP | 1.6 | 2.37 | 0.07 | 1.48 | 0.04 | 1.52 |
| Ppu201 | 3.7 | 1.5 | 0.30 | 0.41 | 0.08 | 0.49 |
| Ppu235 | 4 | 1.3 | 0.80 | 0.32 | 0.20 | 0.52 |
| Ppu201/PP | 3.3 | 1.4 | 0.10 | 0.42 | 0.03 | 0.45 |
| Ppu235/PP | 3.6 | 1.8 | 0.36 | 0.50 | 0.10 | 0.60 |
| Ppu201 | 3.8 | 1.4 | 0.30 | 0.38 | 0.08 | 0.46 |
| Ppu235 | 3.9 | 1.3 | 0.74 | 0.33 | 0.19 | 0.52 |
| Ppu201/PP | 0.4 | 0.5 | 0.01 | 1.26 | 0.03 | 1.29 |
| Ppu235/PP | 0.4 | 0.8 | 0.02 | 2.08 | 0.05 | 2.13 |
| Ppu201 | 4 | 1.5 | 0.28 | 0.38 | 0.07 | 0.45 |
| Ppu235/PP | 4.2 | 1.4 | 0.76 | 0.34 | 0.18 | 0.52 |
| Ppu201/PP | 3.6 | 1.4 | 0.11 | 0.40 | 0.03 | 0.43 |
| Ppu235/PP | 4 | 1.8 | 0.32 | 0.46 | 0.08 | 0.54 |
| Ppu201 | 4 | 1.5 | 0.32 | 0.38 | 0.08 | 0.46 |
| Ppu235 | 4.1 | 1.5 | 0.74 | 0.36 | 0.18 | 0.54 |
| Ppu201/PP | 0.5 | 0.5 | 0.01 | 0.99 | 0.02 | 1.01 |
| Ppu235/PP | 0.8 | 1.1 | 0.02 | 1.40 | 0.03 | 1.43 |
| Ppu201 | 4.5 | 2.2 | 0.32 | 0.49 | 0.07 | 0.56 |
| Ppu235 | 4.6 | 2.1 | 0.74 | 0.45 | 0.16 | 0.61 |
| Ppu235/PP | 4 | 2.2 | 0.36 | 0.56 | 0.09 | 0.65 |
| Ppu201 | 5.1 | 3.0 | 0.46 | 0.58 | 0.09 | 0.67 |
| Ppu235 | 5.4 | 2.5 | 0.86 | 0.47 | 0.16 | 0.63 |
| Ppu235/PP | 0.5 | 1.2 | 0.04 | 2.43 | 0.08 | 2.51 |
References
- Kitney, R.; Freemont, P. Synthetic biology—The state of play. FEBS Lett. 2012, 586, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Martău, G.A.; Călinoiu, L.F.; Vodnar, D.C. Bio-vanillin: Towards a sustainable industrial production. Trends Food Sci. Technol. 2021, 109, 579–592. [Google Scholar] [CrossRef]
- Wang, J.; Shen, X.; Rey, J.; Yuan, Q.; Yan, Y. Recent advances in microbial production of aromatic natural products and their derivatives. Appl. Microbiol. Biotechnol. 2018, 102, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Dudnik, A.; Gaspar, P.; Neves, A.R.; Forster, J. Engineering of Microbial Cell Factories for the Production of Plant Polyphenols with Health-Beneficial Properties. Curr. Pharm. Des. 2018, 24, 2208–2225. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Yahya, P.P.; Zhang, F.; del Cardayre, S.B.; Keasling, J.D. Microbial engineering for the production of advanced biofuels. Nature 2012, 488, 320–328. [Google Scholar] [CrossRef]
- Aggarwal, N.; Pham, H.L.; Ranjan, B.; Saini, M.; Liang, Y.; Hossain, G.S.; Ling, H.; Foo, J.L.; Chang, M.W. Microbial engineering strategies to utilize waste feedstock for sustainable bioproduction. Nat. Rev. Bioeng. 2023, 2, 155–174. [Google Scholar] [CrossRef]
- Li, B.; Zhang, B.; Wang, P.; Cai, X.; Chen, Y.Y.; Yang, Y.F.; Liu, Z.Q.; Zheng, Y.G. Rerouting Fluxes of the Central Carbon Metabolism and Relieving Mechanism-Based Inactivation of l-Aspartate-α-decarboxylase for Fermentative Production of β-Alanine in Escherichia coli. Acs Synth. Biol. 2022, 11, 1908–1918. [Google Scholar] [CrossRef]
- Maurya, R.; Gohil, N.; Nixon, S.; Kumar, N.; Noronha, S.B.; Dhali, D.; Trabelsi, H.; Alzahrani, K.J.; Reshamwala, S.M.; Awasthi, M.K.; et al. Rewiring of metabolic pathways in yeasts for sustainable production of biofuels. Bioresour. Technol. 2023, 372, 128668. [Google Scholar] [CrossRef]
- Leonard, E.; Lim, K.H.; Saw, P.N.; Koffas, M.A.G. Engineering Central Metabolic Pathways for High-Level Flavonoid Production in Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 3877–3886. [Google Scholar] [CrossRef]
- Suzuki, S.; Koeduka, T.; Sugiyama, A.; Yazaki, K.; Umezawa, T. Microbial production of plant specialized metabolites. Plant Biotechnol. 2014, 31, 465–482. [Google Scholar] [CrossRef]
- Limem, I.; Guedon, E.; Hehn, A.; Bourgaud, F.; Chekir Ghedira, L.; Engasser, J.M.; Ghoul, M. Production of phenylpropanoid compounds by recombinant microorganisms expressing plant-specific biosynthesis genes. Process Biochem. 2008, 43, 463–479. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, S.; Xiao, A.; Rasmussen, M.; Skidmore, C.; Zhan, J. Metabolic engineering of Escherichia coli for the biosynthesis of various phenylpropanoid derivatives. Metab. Eng. 2015, 29, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Averesch, N.J.H.; Krömer, J.O. Metabolic Engineering of the Shikimate Pathway for Production of Aromatics and Derived Compounds—Present and Future Strain Construction Strategies. Front. Bioeng. Biotechnol. 2018, 6, 32. [Google Scholar] [CrossRef]
- Vargas-Tah, A.; Gosset, G. Production of Cinnamic and p-Hydroxycinnamic Acids in Engineered Microbes. Front. Bioeng. Biotechnol. 2015, 3, 116. [Google Scholar] [CrossRef]
- Herrmann, K.M.; Weaver, L.M. The Shikimate Pathway. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 473–503. [Google Scholar] [CrossRef]
- Jayaraman, K.; Trachtmann, N.; Sprenger, G.A.; Gohlke, H. Protein engineering for feedback resistance in 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase. Appl. Microbiol. Biotechnol. 2022, 106, 6505–6517. [Google Scholar] [CrossRef]
- Wu, J.; Howe, D.L.; Woodard, R.W. Thermotoga maritima 3-Deoxy-D-arabino-heptulosonate 7-Phosphate (DAHP) Synthase. J. Biol. Chem. 2003, 278, 27525–27531. [Google Scholar] [CrossRef]
- Luttik, M.; Vuralhan, Z.; Suir, E.; Braus, G.; Pronk, J.; Daran, J. Alleviation of feedback inhibition in Saccharomyces cerevisiae aromatic amino acid biosynthesis: Quantification of metabolic impact. Metab. Eng. 2008, 10, 141–153. [Google Scholar] [CrossRef]
- Bilal, M.; Wang, S.; Iqbal, H.M.N.; Zhao, Y.; Hu, H.; Wang, W.; Zhang, X. Metabolic engineering strategies for enhanced shikimate biosynthesis: Current scenario and future developments. Appl. Microbiol. Biotechnol. 2018, 102, 7759–7773. [Google Scholar] [CrossRef]
- Lütke-Eversloh, T.; Stephanopoulos, G. L-Tyrosine production by deregulated strains of Escherichia coli. Appl. Microbiol. Biotechnol. 2007, 75, 103–110. [Google Scholar] [CrossRef]
- Kang, S.Y.; Choi, O.; Lee, J.K.; Hwang, B.Y.; Uhm, T.B.; Hong, Y.S. Artificial biosynthesis of phenylpropanoic acids in a tyrosine overproducing Escherichia coli strain. Microb. Cell Factories 2012, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.N.S.; Koffas, M.; Stephanopoulos, G. Optimization of a heterologous pathway for the production of flavonoids from glucose. Metab. Eng. 2011, 13, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Juminaga, D.; Baidoo, E.E.K.; Redding-Johanson, A.M.; Batth, T.S.; Burd, H.; Mukhopadhyay, A.; Petzold, C.J.; Keasling, J.D. Modular Engineering of l-Tyrosine Production in Escherichia coli. Appl. Environ. Microbiol. 2012, 78, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Kildegaard, K.R.; Li, M.; Borodina, I.; Nielsen, J. Establishment of a yeast platform strain for production of p-coumaric acid through metabolic engineering of aromatic amino acid biosynthesis. Metab. Eng. 2015, 31, 181–188. [Google Scholar] [CrossRef]
- Nikel, P.I.; Martínez-García, E.; de Lorenzo, V. Biotechnological domestication of pseudomonads using synthetic biology. Nat. Rev. Microbiol. 2014, 12, 368–379. [Google Scholar] [CrossRef]
- Ankenbauer, A.; Schäfer, R.A.; Viegas, S.C.; Pobre, V.; Voß, B.; Arraiano, C.M.; Takors, R. Pseudomonas putida KT2440 is naturally endowed to withstand industrial-scale stress conditions. Microb. Biotechnol. 2020, 13, 1145–1161. [Google Scholar] [CrossRef]
- Schwanemann, T.; Otto, M.; Wierckx, N.; Wynands, B. Pseudomonas as Versatile Aromatics Cell Factory. Biotechnol. J. 2020, 15, 1900569. [Google Scholar] [CrossRef]
- Molina-Santiago, C.; Cordero, B.F.; Daddaoua, A.; Udaondo, Z.; Manzano, J.; Valdivia, M.; Segura, A.; Ramos, J.L.; Duque, E. Pseudomonas putida as a platform for the synthesis of aromatic compounds. Microbiology 2016, 162, 1535–1543. [Google Scholar] [CrossRef]
- Calero, P.; Jensen, S.I.; Bojanovič, K.; Lennen, R.M.; Koza, A.; Nielsen, A.T. Genome-wide identification of tolerance mechanisms toward p-coumaric acid in Pseudomonas putida. Biotechnol. Bioeng. 2017, 115, 762–774. [Google Scholar] [CrossRef]
- Mohamed, E.T.; Werner, A.Z.; Salvachúa, D.; Singer, C.A.; Szostkiewicz, K.; Rafael Jiménez-Díaz, M.; Eng, T.; Radi, M.S.; Simmons, B.A.; Mukhopadhyay, A.; et al. Adaptive laboratory evolution of Pseudomonas putida KT2440 improves p-coumaric and ferulic acid catabolism and tolerance. Metab. Eng. Commun. 2020, 11, e00143. [Google Scholar] [CrossRef]
- Nijkamp, K.; Westerhof, R.G.M.; Ballerstedt, H.; de Bont, J.A.M.; Wery, J. Optimization of the solvent-tolerant Pseudomonas putida S12 as host for the production of p-coumarate from glucose. Appl. Microbiol. Biotechnol. 2007, 74, 617–624. [Google Scholar] [CrossRef]
- Wierckx, N.J.P.; Ballerstedt, H.; de Bont, J.A.M.; Wery, J. Engineering of Solvent-Tolerant Pseudomonas putida S12 for Bioproduction of Phenol from Glucose. Appl. Environ. Microbiol. 2005, 71, 8221–8227. [Google Scholar] [CrossRef] [PubMed]
- Calero, P.; Jensen, S.I.; Nielsen, A.T. Broad-Host-Range ProUSER Vectors Enable Fast Characterization of Inducible Promoters and Optimization of p-Coumaric Acid Production in Pseudomonas putida KT2440. ACS Synth. Biol. 2016, 5, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Wynands, B.; Lenzen, C.; Otto, M.; Koch, F.; Blank, L.M.; Wierckx, N. Metabolic engineering of Pseudomonas taiwanensis VLB120 with minimal genomic modifications for high-yield phenol production. Metab. Eng. 2018, 47, 121–133. [Google Scholar] [CrossRef]
- Otto, M.; Wynands, B.; Lenzen, C.; Filbig, M.; Blank, L.M.; Wierckx, N. Rational Engineering of Phenylalanine Accumulation in Pseudomonas taiwanensis to Enable High-Yield Production of Trans-Cinnamate. Front. Bioeng. Biotechnol. 2019, 7, 312. [Google Scholar] [CrossRef]
- Weaver, L.M.; Herrmann, K.M. Cloning of an aroF allele encoding a tyrosine-insensitive 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase. J. Bacteriol. 1990, 172, 6581–6584. [Google Scholar] [CrossRef]
- Lütke-Eversloh, T.; Stephanopoulos, G. Feedback inhibition of chorismate mutase/prephenate dehydrogenase (TyrA) of Escherichia coli: Generation and characterization of tyrosine-insensitive mutants. Appl. Environ. Microbiol. 2005, 71, 7224–7228. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Liu, Z.; Qiao, Q.; Ni, X.; Yang, J.; Sun, G.; Li, F.; Zhou, W.; Guo, X.; et al. Engineering allosteric inhibition of homoserine dehydrogenase by semi-rational saturation mutagenesis screening. Front. Bioeng. Biotechnol. 2024, 11, 1336215. [Google Scholar] [CrossRef]
- Chen, M.M.; Snow, C.D.; Vizcarra, C.L.; Mayo, S.L.; Arnold, F.H. Comparison of random mutagenesis and semi-rational designed libraries for improved cytochrome P450 BM3-catalyzed hydroxylation of small alkanes. Protein Eng. Des. Sel. 2012, 25, 171–178. [Google Scholar] [CrossRef]
- Rowlands, R. Industrial strain improvement: Mutagenesis and random screening procedures. Enzym. Microb. Technol. 1984, 6, 3–10. [Google Scholar] [CrossRef]
- Zhao, S.; Tan, M.Z.; Wang, R.X.; Ye, F.T.; Chen, Y.P.; Luo, X.M.; Feng, J.X. Combination of genetic engineering and random mutagenesis for improving production of raw-starch-degrading enzymes in Penicillium oxalicum. Microb. Cell Factories 2022, 21, 272. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Mildvan, A.S. Inverse thinking about double mutants of enzymes. Biochemistry 2004, 43, 14517–14520. [Google Scholar] [CrossRef] [PubMed]
- Chaparro-Riggers, J.F.; Polizzi, K.M.; Bommarius, A.S. Better library design: Data-driven protein engineering. Biotechnol. J. Healthc. Nutr. Technol. 2007, 2, 180–191. [Google Scholar] [CrossRef]
- Wierckx, N.; Ruijssenaars, H.J.; de Winde, J.H.; Schmid, A.; Blank, L.M. Metabolic flux analysis of a phenol producing mutant of Pseudomonas putida S12: Verification and complementation of hypotheses derived from transcriptomics. J. Biotechnol. 2009, 143, 124–129. [Google Scholar] [CrossRef]
- Glick, B.R. Metabolic load and heterologous gene expression. Biotechnol. Adv. 1995, 13, 247–261. [Google Scholar] [CrossRef]
- Bertani, G. STUDIES ON LYSOGENESIS I: The Mode of Phage Liberation by Lysogenic Escherichia coli. J. Bacteriol. 1951, 62, 293–300. [Google Scholar] [CrossRef]
- Simon, R.; Priefer, U.; Pühler, A. A broad host range mobilization system for in vivo genetic engineering: Transposon mutagenesis in gram negative bacteria. Bio/Technology 1983, 1, 784–791. [Google Scholar] [CrossRef]
- Kovach, M.E.; Elzer, P.H.; Hill, D.S.; Robertson, G.T.; Farris, M.A.; Roop II, R.M.; Peterson, K.M. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 1995, 166, 175–176. [Google Scholar] [CrossRef]
- Schäfer, A.; Tauch, A.; Jäger, W.; Kalinowski, J.; Thierbach, G.; Pühler, A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: Selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 1994, 145, 69–73. [Google Scholar] [CrossRef]
- Studier, F.W. Protein production by auto-induction in high-density shaking cultures. Protein Expr. Purif. 2005, 41, 207–234. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.G. Synthesis of DNA fragments in yeast by one-step assembly of overlapping oligonucleotides. Nucleic Acids Res. 2009, 37, 6984–6990. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Jones, D.H.; Snedecor, G.W.; Cochran, W.G. Statistical Methods. J. Educ. Behav. Stat. 1994, 19, 304. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple Comparisons Using Rank Sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: London, UK, 2013. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Shumilin, I.A.; Zhao, C.; Bauerle, R.; Kretsinger, R.H. Allosteric Inhibition of 3-Deoxy-d-arabino-heptulosonate-7-phosphate Synthase Alters the Coordination of Both Substrates. J. Mol. Biol. 2002, 320, 1147–1156. [Google Scholar] [CrossRef]
- Cui, D.; Deng, A.; Bai, H.; Yang, Z.; Liang, Y.; Liu, Z.; Qiu, Q.; Wang, L.; Liu, S.; Zhang, Y.; et al. Molecular basis for feedback inhibition of tyrosine-regulated 3-deoxy-d-arabino-heptulosonate-7-phosphate synthase from Escherichia coli. J. Struct. Biol. 2019, 206, 322–334. [Google Scholar] [CrossRef]
- Ding, R.; Liu, L.; Chen, X.; Cui, Z.; Zhang, A.; Ren, D.; Zhang, L. Introduction of two mutations into AroG increases phenylalanine production in Escherichia coli. Biotechnol. Lett. 2014, 36, 2103–2108. [Google Scholar] [CrossRef]
- Shimizu, K. Metabolic Regulation of a Bacterial Cell System with Emphasis on Escherichia coli Metabolism. ISRN Biochem. 2013, 2013, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Chubukov, V.; Gerosa, L.; Kochanowski, K.; Sauer, U. Coordination of microbial metabolism. Nat. Rev. Microbiol. 2014, 12, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Matsuoka, Y. Feedback regulation and coordination of the main metabolism for bacterial growth and metabolic engineering for amino acid fermentation. Biotechnol. Adv. 2022, 55, 107887. [Google Scholar] [CrossRef] [PubMed]
- Nudler, E. The riboswitch control of bacterial metabolism. Trends Biochem. Sci. 2004, 29, 11–17. [Google Scholar] [CrossRef]
- Wu, S.; Chen, W.; Lu, S.; Zhang, H.; Yin, L. Metabolic Engineering of Shikimic Acid Biosynthesis Pathway for the Production of Shikimic Acid and Its Branched Products in Microorganisms: Advances and Prospects. Molecules 2022, 27, 4779. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, H. Engineering the shikimate pathway for biosynthesis of molecules with pharmaceutical activities in E. coli. Curr. Opin. Biotechnol. 2016, 42, 1–6. [Google Scholar] [CrossRef]
- Cao, M.; Gao, M.; Suástegui, M.; Mei, Y.; Shao, Z. Building microbial factories for the production of aromatic amino acid pathway derivatives: From commodity chemicals to plant-sourced natural products. Metab. Eng. 2020, 58, 94–132. [Google Scholar] [CrossRef]
- Helmstaedt, K.; Strittmatter, A.; Lipscomb, W.N.; Braus, G.H. Evolution of 3-deoxy-d-arabino-heptulosonate-7-phosphate synthase-encoding genes in the yeast Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2005, 102, 9784–9789. [Google Scholar] [CrossRef]
- Jossek, R.; Bongaerts, J.; Sprenger, G.A. Characterization of a new feedback-resistant 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase AroF of Escherichia coli. FEMS Microbiol. Lett. 2001, 202, 145–148. [Google Scholar] [CrossRef]
- Mir, R.; Jallu, S.; Singh, T.P. The shikimate pathway: Review of amino acid sequence, function and three-dimensional structures of the enzymes. Crit. Rev. Microbiol. 2013, 41, 172–189. [Google Scholar] [CrossRef]
- Ger, Y.M.; Chen, S.L.; Chiang, H.J.; Shiuan, D. A Single Ser-180 Mutation Desensitizes Feedback Inhibition of the Phenylalanine-Sensitive 3-Deoxy-D-Arabino-Heptulosonate 7-Phosphate (DAHP) Synthetase in Escherichia coli. J. Biochem. 1994, 116, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.M.; Yanofsky, C.; Bauerle, R. Mutational analysis of the catalytic and feedback sites of the tryptophan-sensitive 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase of Escherichia coli. J. Bacteriol. 1988, 170, 5500–5506. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Tsujimoto, K.; Kurahashi, O. Mutational analysis of the feedback sites of phenylalanine-sensitive 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase of Escherichia coli. Appl. Environ. Microbiol. 1997, 63, 761–762. [Google Scholar] [CrossRef] [PubMed]
- Lutz, S. Beyond directed evolution—Semi-rational protein engineering and design. Curr. Opin. Biotechnol. 2010, 21, 734–743. [Google Scholar] [CrossRef]
- Chen, H.; Ma, L.; Dai, H.; Fu, Y.; Wang, H.; Zhang, Y. Advances in Rational Protein Engineering toward Functional Architectures and Their Applications in Food Science. J. Agric. Food Chem. 2022, 70, 4522–4533. [Google Scholar] [CrossRef]
- Nixon, A.E.; Firestine, S.M. Rational and “Irrational” Design of Proteins and Their Use in Biotechnology. IUBMB Life 2000, 49, 181–187. [Google Scholar] [CrossRef]
- Faisca, P.; Travasso, R.; Ball, R.; Shakhnovich, E.I. Identifying critical residues in protein folding: Insights from ϕ-value and Pfold analysis. J. Chem. Phys. 2008, 129, 095108. [Google Scholar] [CrossRef]
- Sen, C.; Logashree, V.; Makde, R.D.; Ghosh, B. Amino acid propensities for secondary structures and its variation across protein structures using exhaustive PDB data. Comput. Biol. Chem. 2024, 110, 108083. [Google Scholar] [CrossRef]
- Chronopoulou, E.G.; Labrou, N.E. Site-saturation mutagenesis: A powerful tool for structure-based design of combinatorial mutation libraries. Curr. Protoc. Protein Sci. 2011, 63. [Google Scholar] [CrossRef]
- Marabotti, A.; Scafuri, B.; Facchiano, A. Predicting the stability of mutant proteins by computational approaches: An overview. Briefings Bioinform. 2021, 22, bbaa074. [Google Scholar] [CrossRef]
- Pucci, F.; Schwersensky, M.; Rooman, M. Artificial intelligence challenges for predicting the impact of mutations on protein stability. Curr. Opin. Struct. Biol. 2022, 72, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Fang, J. A critical review of five machine learning-based algorithms for predicting protein stability changes upon mutation. Briefings Bioinform. 2019, 21, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Mao, C.; Tang, J.; Yang, S.; Cheng, Y.; Wang, W.; Gu, Q.; Han, W.; Chen, H.; Li, S.; et al. Zero-shot prediction of mutation effects with multimodal deep representation learning guides protein engineering. Cell Res. 2024, 34, 630–647. [Google Scholar] [CrossRef] [PubMed]
- Hamborg, L.; Granata, D.; Olsen, J.G.; Roche, J.V.; Pedersen, L.E.; Nielsen, A.T.; Lindorff-Larsen, K.; Teilum, K. Synergistic stabilization of a double mutant in chymotrypsin inhibitor 2 from a library screen in E. coli. Commun. Biol. 2021, 4, 980. [Google Scholar] [CrossRef]
- Greener, J.G.; Sternberg, M.J. AlloPred: Prediction of allosteric pockets on proteins using normal mode perturbation analysis. BMC Bioinform. 2015, 16, 335. [Google Scholar] [CrossRef]
- Yamato, T.; Laprévote, O. Normal mode analysis and beyond. Biophys. Physicobiol. 2019, 16, 322–327. [Google Scholar] [CrossRef]
- Kolossváry, I. A Fresh Look at the Normal Mode Analysis of Proteins: Introducing Allosteric Co-Vibrational Modes. JACS Au 2024, 4, 1303–1309. [Google Scholar] [CrossRef]
- Ruffolo, J.A.; Madani, A. Designing proteins with language models. Nat. Biotechnol. 2024, 42, 200–202. [Google Scholar] [CrossRef]
- Schmirler, R.; Heinzinger, M.; Rost, B. Fine-tuning protein language models boosts predictions across diverse tasks. Nat. Commun. 2024, 15, 7407. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, L.; Yu, Y.; Wu, B.; Li, M.; Hong, L.; Tan, P. Enhancing efficiency of protein language models with minimal wet-lab data through few-shot learning. Nat. Commun. 2024, 15, 5566. [Google Scholar] [CrossRef]
- Biswas, S.; Khimulya, G.; Alley, E.C.; Esvelt, K.M.; Church, G.M. Low-N protein engineering with data-efficient deep learning. Nat. Methods 2021, 18, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Chen, R. Bacterial expression systems for recombinant protein production: E. coli and beyond. Biotechnol. Adv. 2012, 30, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Goussé, M.; Dell’Aglio, E.; Curien, G.; Borland, S.; Renoud, S.; Ranquet, C.; Chandor-Proust, A. E. coli chromosomal-driven expression of NADK2 from A. thaliana: A preferable alternative to plasmid-driven expression for challenging proteins. Protein Expr. Purif. 2022, 195–196, 106090. [Google Scholar] [CrossRef] [PubMed]
- Kachroo, A.H.; Jayaram, M.; Rowley, P.A. Metabolic engineering without plasmids. Nat. Biotechnol. 2009, 27, 729–731. [Google Scholar] [CrossRef]
- Zucca, S.; Pasotti, L.; Mazzini, G.; Cusella De Angelis, M.G.; Magni, P. Characterization of an inducible promoter in different DNA copy number conditions. BMC Bioinform. 2012, 13, S11. [Google Scholar] [CrossRef]
- Tyo, K.E.J.; Ajikumar, P.K.; Stephanopoulos, G. Stabilized gene duplication enables long-term selection-free heterologous pathway expression. Nat. Biotechnol. 2009, 27, 760–765. [Google Scholar] [CrossRef]
- Schild, F.; Chandor-Proust, A.; Ranquet, C. Method of Mutagenesis Without Scar; Institut National de la Propriété’ Industrielle: Paris, France, 2017. [Google Scholar]
- Li, Z.; Wang, H.; Ding, D.; Liu, Y.; Fang, H.; Chang, Z.; Chen, T.; Zhang, D. Metabolic engineering of Escherichia coli for production of chemicals derived from the shikimate pathway. J. Ind. Microbiol. Biotechnol. 2020, 47, 525–535. [Google Scholar] [CrossRef]
- Bentley, R.; Haslam, E. The Shikimate Pathway—A Metabolic Tree with Many Branche. Crit. Rev. Biochem. Mol. Biol. 1990, 25, 307–384. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, T.; Li, X.; Chen, Y.; Campbell, K.; Nielsen, J.; Chen, Y. Rewiring carbon metabolism in yeast for high level production of aromatic chemicals. Nat. Commun. 2019, 10, 4976. [Google Scholar] [CrossRef]
- Kroll, K.; Holland, C.K.; Starks, C.M.; Jez, J.M. Evolution of allosteric regulation in chorismate mutases from early plants. Biochem. J. 2017, 474, 3705–3717. [Google Scholar] [CrossRef]
- Brown, J.F.; Dawes, I.W. Regulation of chorismate mutase in Saccharomyces cerevisiae. Mol. Gen. Genet. MGG 1990, 220, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Pohnert, G.; Kongsaeree, P.; Wilson, D.B.; Clardy, J.; Ganem, B. Chorismate Mutase-Prephenate Dehydratase from Escherichia coli. J. Biol. Chem. 1998, 273, 6248–6253. [Google Scholar] [CrossRef] [PubMed]
- Mokwatlo, S.C.; Klein, B.C.; Benavides, P.T.; Tan, E.C.; Kneucker, C.M.; Ling, C.; Singer, C.A.; Lyons, R.; i Nogué, V.S.; Hestmark, K.V.; et al. Bioprocess development and scale-up for cis, cis-muconic acid production from glucose and xylose by Pseudomonas putida. Green Chem. 2024, 26, 10152–10167. [Google Scholar] [CrossRef]
- Flourat, A.L.; Combes, J.; Bailly-Maitre-Grand, C.; Magnien, K.; Haudrechy, A.; Renault, J.H.; Allais, F. Accessing p-Hydroxycinnamic Acids: Chemical Synthesis, Biomass Recovery, or Engineered Microbial Production? ChemSusChem 2021, 14, 118–129. [Google Scholar] [CrossRef]
- Louie, G.V.; Bowman, M.E.; Moffitt, M.C.; Baiga, T.J.; Moore, B.S.; Noel, J.P. Structural Determinants and Modulation of Substrate Specificity in Phenylalanine-Tyrosine Ammonia-Lyases. Chem. Biol. 2006, 13, 1327–1338. [Google Scholar] [CrossRef]
- Watts, K.T.; Mijts, B.N.; Lee, P.C.; Manning, A.J.; Schmidt-Dannert, C. Discovery of a Substrate Selectivity Switch in Tyrosine Ammonia-Lyase, a Member of the Aromatic Amino Acid Lyase Family. Chem. Biol. 2006, 13, 1317–1326. [Google Scholar] [CrossRef]
- Jendresen, C.B.; Stahlhut, S.G.; Li, M.; Gaspar, P.; Siedler, S.; Förster, J.; Maury, J.; Borodina, I.; Nielsen, A.T. Highly Active and Specific Tyrosine Ammonia-Lyases from Diverse Origins Enable Enhanced Production of Aromatic Compounds in Bacteria and Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2015, 81, 4458–4476. [Google Scholar] [CrossRef]
- KeGG Kyoto Encyclopedia of Genes and Genomes. Available online: https://www.kegg.jp/kegg/ (accessed on 15 November 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).