Evaluating the Health Implications of Kombucha Fermented with Gardenia jasminoides Teas: A Comprehensive Analysis of Antioxidant, Antimicrobial, and Cytotoxic Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Tea Selection and Kombucha Fermentation
2.2. Antioxidant Assay
2.3. Quantification of 3,5-Dicaffeoylquinic Acid in GJ Kombucha Ferments
2.4. Microbiological Profile
2.5. Antimicrobial Assay
2.6. Anticancer Assays
3. Results
3.1. DPPH Assay
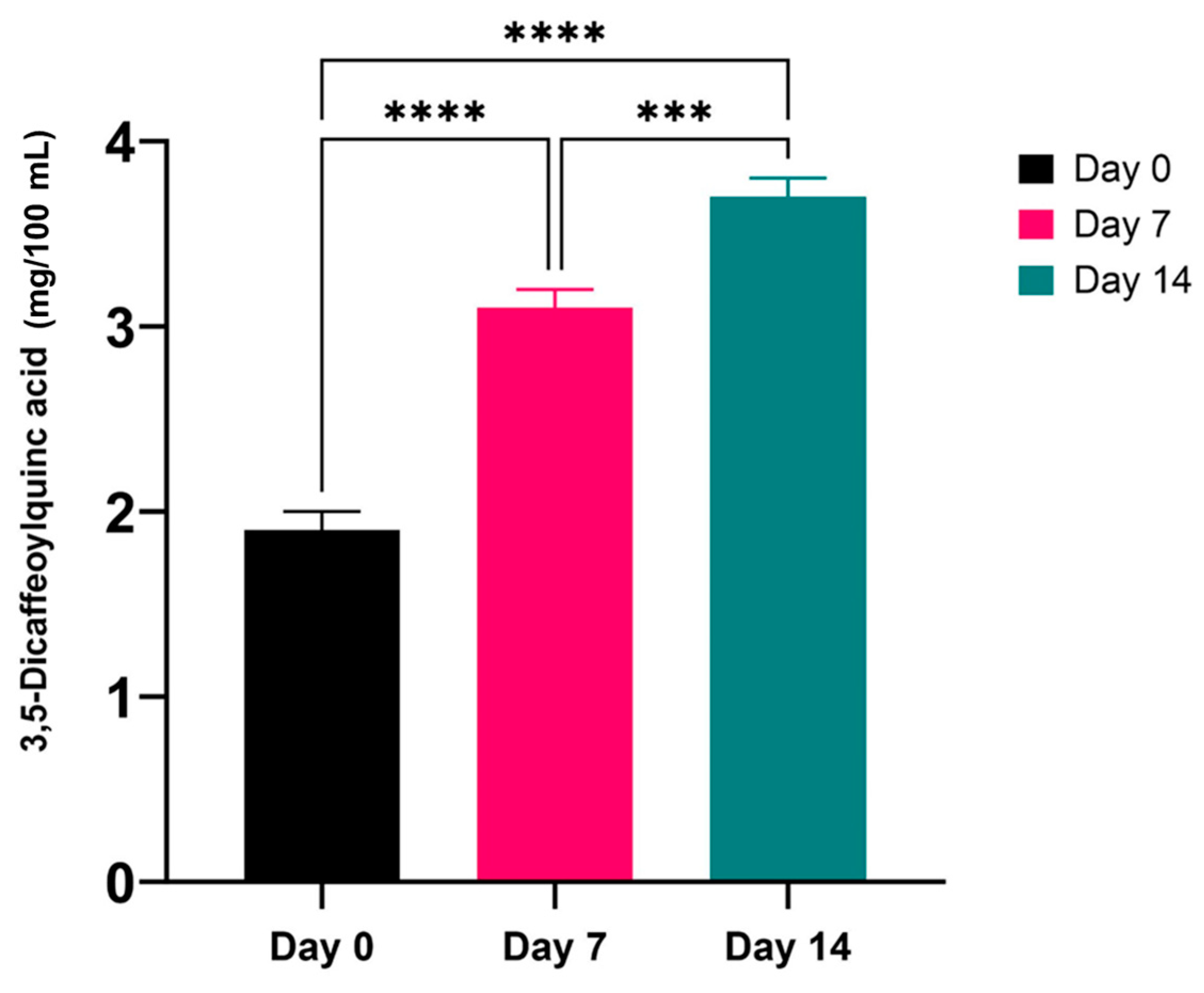
3.2. Quantification of 3,5-Dicaffeoylquinic Acid in GJ Kombucha Ferments
3.3. Microbiological Profile
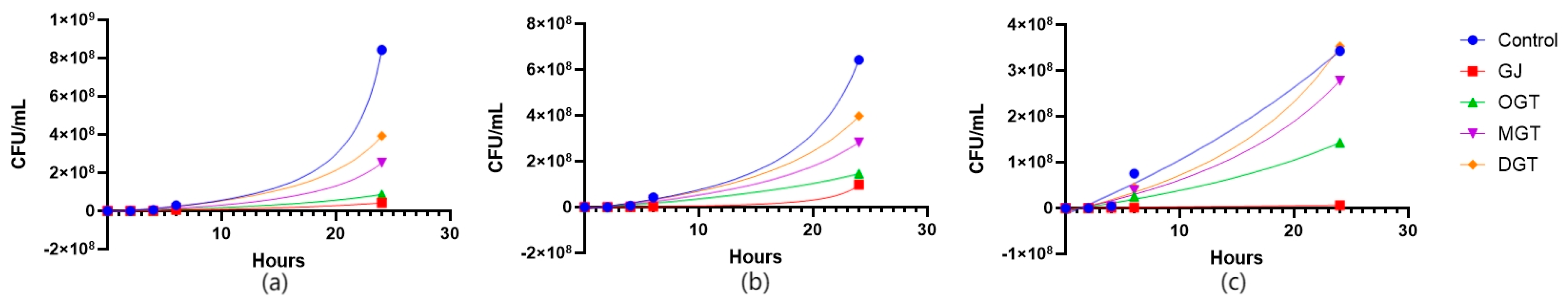
3.4. Kill Time
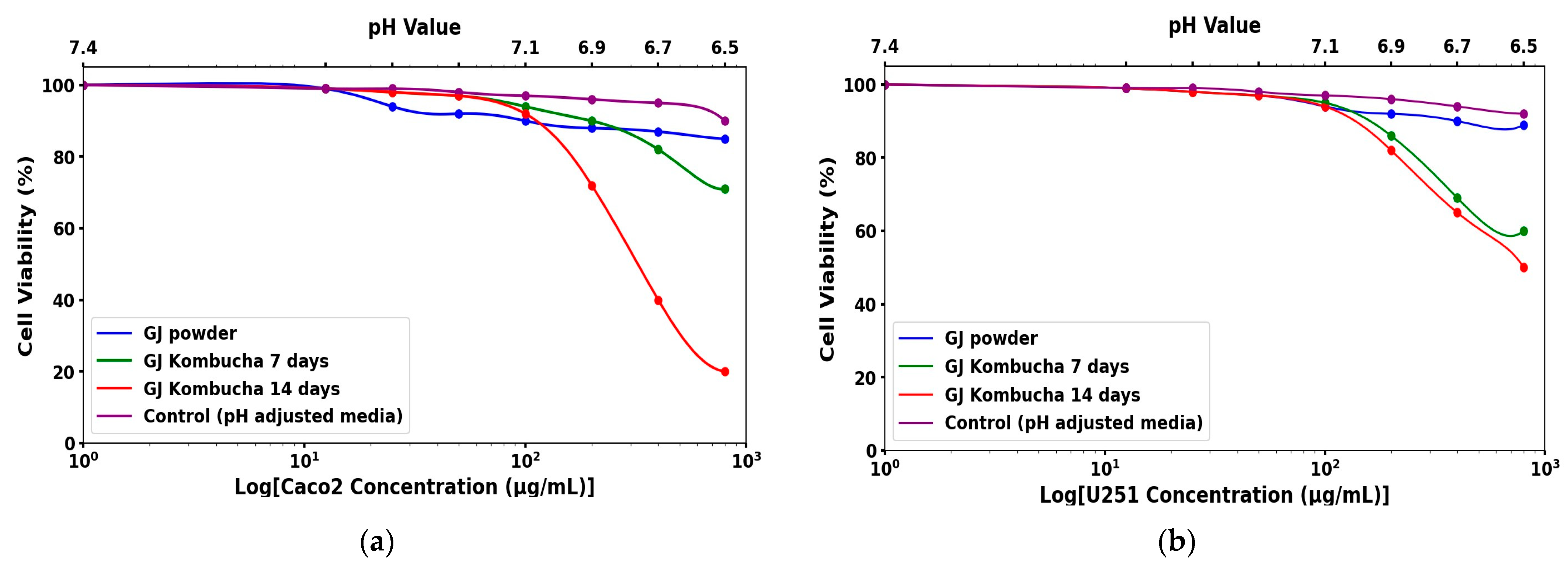
3.5. Differential Cytotoxic Effects of GJ Kombucha on Caco-2 and U251 Cancer Cell Lines
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Presenza, L.; Teixeira, B.F.; Galvão, J.A.; de Souza Vieira, T.M.F. Technological strategies for the use of plant-derived compounds in the preservation of fish products. Food Chem. 2023, 419, 136069. [Google Scholar] [CrossRef] [PubMed]
- Moura, A.; Leclercq, A.; Vales, G.; Tessaud-Rita, N.; Bracq-Dieye, H.; Thouvenot, P.; Madec, Y.; Charlier, C.; Lecuit, M. Phenotypic and genotypic antimicrobial resistance of L. monocytogenes: An observational study in France. Lancet Reg. Health–Eur. 2024, 37, 100800. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cai, J.; Chen, X.; Guo, B.; Liu, J.; Qiu, G.; Li, H. The connection between the antibiotic resistome and nitrogen-cycling microorganisms in paddy soil is enhanced by application of chemical and plant-derived organic fertilizers. Environ. Res. 2024, 243, 117880. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, G.R.; Swetschinski, L.R.; Weaver, N.D.; Ikuta, K.S.; Mestrovic, T.; Gray, A.P.; Chung, E.; Wool, E.E.; Han, C.; Hayoon, A.G.; et al. The burden of antimicrobial resistance in the Americas in 2019: A cross-country systematic analysis. Lancet Reg. Health–Am. 2023, 25, 100561. [Google Scholar] [CrossRef]
- Li, Y.C.; Tanapichatsakul, C.; Pripdeevech, P.; Hwang, T.L.; Kuo, P.C.; Tzen, J.T. Characterisation of teaghrelin-like principles from Assam tea cultivated in Thailand. Nat. Prod. Res. 2021, 36, 305–311. [Google Scholar] [CrossRef]
- Morishita, Y.; Ikeda, K.; Matsuno, H.; Ito, H.; Tai, A. Identification of degranulation inhibitors from rooibos (Aspalathus linearis) tea in rat basophilic leukaemia cells. Nat. Prod. Res. 2019, 33, 1472–1476. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef]
- Banik, B.K.; Das, A. Natural Products as Anticancer Agents; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Shrihastini, V.; Muthuramalingam, P.; Adarshan, S.; Sujitha, M.; Chen, J.T.; Shin, H.; Ramesh, M. Plant Derived Bioactive Compounds, Their Anti-Cancer Effects and In Silico Approaches as an Alternative Target Treatment Strategy for Breast Cancer: An Updated Overview. Cancers 2021, 13, 6222. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Kałduńska, J.; Dec, K.; Kawczuga, D.; Janda, K. Antioxidant properties of small-molecule non-enzymatic compounds. Pol. Merkur. Lek. 2020, 48, 128–132. [Google Scholar]
- Xiong, R.G.; Zhou, D.D.; Cheng, J.; Wu, S.X.; Saimaiti, A.; Huang, S.Y.; Liu, Q.; Shang, A.; Li, H.B.; Li, S. Preparation and evaluation of liquorice (Glycyrrhiza uralensis) and ginger (Zingiber officinale) kombucha beverage based on antioxidant capacities, phenolic compounds and sensory qualities. Int. J. Gastron. Food Sci. 2024, 35, 100869. [Google Scholar] [CrossRef]
- Taupiqurrohman, O.; Hastuti, L.P.; Oktavia, D.; Al-Najjar, B.O.; Yusuf, M.; Suryani, Y.; Gaffar, S. From Fermentation to Cancer Prevention: The Anticancer Potential of Kombucha. Phytomedicine Plus 2024, 4, 100633. [Google Scholar] [CrossRef]
- Ivanišová, E.; Meňhartová, K.; Terentjeva, M.; Harangozo, Ľ.; Kántor, A.; Kačániová, M. The Evaluation of Chemical, Antioxidant, Antimicrobial and Sensory Properties of Kombucha Tea Beverage. J. Food Sci. Technol. 2020, 57, 1840–1846. [Google Scholar] [CrossRef]
- Bishop, P.; Pitts, E.R.; Budner, D.; Thompson-Witrick, K.A. Kombucha: Biochemical and microbiological impacts on the chemical and flavor profile. Food Chem. Adv. 2022, 1, 100025. [Google Scholar] [CrossRef]
- Antolak, H.; Piechota, D.; Kucharska, A. Kombucha Tea—A Double Power of Bioactive Compounds from Tea and Symbiotic Culture of Bacteria and Yeasts (SCOBY). Antioxidants 2021, 10, 1541. [Google Scholar] [CrossRef]
- Kapp, J.M.; Sumner, W. Kombucha: A Systematic Review of the Empirical Evidence of Human Health Benefit. Ann. Epidemiol. 2019, 30, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Fortune Business Insights. Kombucha Market Size, Share & COVID-19 Impact Analysis, By Type (Flavored, Non-Flavored), By Distribution Channel (Supermarkets & Hypermarkets, Online Retail, Health & Natural Food Stores), By Application (Non-Alcoholic Beverages, Alcoholic Beverages), and Regional Forecast, 2020–2027. 2020. Available online: https://www.fortunebusinessinsights.com/industry-reports/kombucha-market-100230 (accessed on 20 November 2024).
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.-P.; Taillandier, P. Understanding Kombucha Tea Fermentation: A Review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef]
- Dartora, B.; Hickert, L.R.; Fabricio, M.F.; Ayub, M.A.Z.; Furlan, J.M.; Wagner, R.; Perez, K.J.; Sant’Anna, V. Understanding the Effect of Fermentation Time on Physicochemical Characteristics, Sensory Attributes, and Volatile Compounds in Green Tea Kombucha. Food Res. Int. 2023, 174, 113569. [Google Scholar] [CrossRef] [PubMed]
- Jayabalan, R.; Malini, K.; Sathishkumar, M.; Swaminathan, K.; Yun, S.-E. Biochemical characteristics of tea fungus produced during kombucha fermentation. Food Sci. Biotechnol. 2010, 19, 843–847. [Google Scholar] [CrossRef]
- Xu, L.; Shi, Q.; Yan, S.M.; Yang, Q.; Fu, H.Y.; She, Y.B. Fusion of elemental profiles and chemometrics: Discrimination of organic and conventional green teas. Microchem. J. 2019, 149, 104006. [Google Scholar] [CrossRef]
- Sornkayasit, K.; Jumnainsong, A.; Srijampa, S.; Ruknarong, L.; Buddhisa, S.; Thanonkeo, P.; Sutthanut, K.; Thukhammee, W.; Wattanathorn, J.; Leelayuwat, C.; et al. Immunomodulatory potentials of modified kombucha with pineapple by-products in aging: An ex vivo study. J. Funct. Foods 2024, 112, 105933. [Google Scholar] [CrossRef]
- Vitas, J.S.; Cvetanovic, A.D.; Maškovič, P.Z.; Švarc-Gajič, J.V.; Malbaša, R.V. Chemical composition and biological activity of novel types of kombucha beverages with yarrow. J. Funct. Foods 2018, 44, 95–102. [Google Scholar] [CrossRef]
- Zubaidah, E.; Dewantari, F.J.; Novitasari, F.R.; Srianta, I.; Blanc, P.J. Potential of snake fruit (Salacca zalacca (Gaerth.) Voss) for the development of a beverage through fermentation with the Kombucha consortium. Biocatal. Agric. Biotechnol. 2018, 13, 198–203. [Google Scholar] [CrossRef]
- Ayed, L.; Ben Abid, S.; Hamdi, M. Development of a beverage from red grape juice fermented with the Kombucha consortium. Ann. Microbiol. 2017, 67, 111–121. [Google Scholar] [CrossRef]
- Watawana, M.I.; Jayawardena, N.; Waisundara, V.Y. Enhancement of the functional properties of coffee through fermentation by “tea fungus” (kombucha). J. Food Process. Preserv. 2015, 39, 2596–2603. [Google Scholar] [CrossRef]
- Watawana, M.I.; Jayawardena, N.; Ranasinghe, S.J.; Waisundara, V.Y. Evaluation of the effect of different sweetening agents on the polyphenol contents and antioxidant and starch hydrolase inhibitory properties of Kombucha. J. Food Process. Preserv. 2017, 41, e12752. [Google Scholar] [CrossRef]
- Sknepnek, A.; Tomić, S.; Miletić, D.; Lević, S.; Čolić, M.; Nedović, V.; Nikšić, M. Fermentation Characteristics of Novel Coriolus versicolor and Lentinus edodes Kombucha Beverages and Immunomodulatory Potential of Their Polysaccharide Extracts. Food Chem. 2021, 342, 128344. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Zheng, Z.; Pi, Z.; Liu, Z.; Song, F. Online microdialysis-ultra performance liquid chromatography–mass spectrometry method for comparative pharmacokinetic investigation on iridoids from Gardenia jasminoides Ellis in rats with different progressions of type 2 diabetic complications. J. Pharm. Biomed. Anal. 2017, 140, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.J.; Lim, K.H.; Jung, H.J.; Park, E.H. Anti-inflammatory evaluation of gardenia extract, geniposide and genipin. J. Ethnopharmacol. 2006, 103, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Li, K.D.; Wang, Q.S.; Zhang, W.W.; Zhang, W.Y.; Fu, S.N.; Xu, D.; Wu, J.R.; Zhai, J.B.; Cui, Y.L. Gardenia fructus antidepressant formula for depression in diabetes patients: A systematic review and meta-analysis. Complement. Ther. Med. 2020, 48, 102248. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, H.; Tian, X.; Zhao, C.; Cai, L.; Liu, Y.; Jia, L.; Yin, H.X.; Chen, C. Antioxidant potential of crocins and ethanol extracts of Gardenia jasminoides ELLIS and Crocus sativus L.: A relationship investigation between antioxidant activity and crocin contents. Food Chem. 2008, 109, 484–492. [Google Scholar] [CrossRef]
- Kuratsune, H.; Umigai, N.; Takeno, R.; Kajimoto, Y.; Nakano, T. Effect of crocetin from Gardenia Jasminoides Ellis on sleep: A pilot study. Phytomedicine 2010, 17, 840–843. [Google Scholar] [CrossRef]
- Huang, H.; Zhu, Y.; Fu, X.; Zou, Y.; Li, Q.; Luo, Z. Integrated natural deep eutectic solvent and pulse-ultrasonication for efficient extraction of crocins from ardenia fruits (Gardenia jasminoides Ellis) and its bioactivities. Food Chem. 2022, 380, 132216. [Google Scholar] [CrossRef]
- Chen, L.; Li, M.; Yang, Z.; Tao, W.; Wang, P.; Tian, X.; Li, X.; Wang, W. Gardenia jasminoides Ellis: Ethnopharmacology, Phytochemistry, and Pharmacological and Industrial Applications of an Important Traditional Chinese Medicine. J. Ethnopharmacol. 2020, 257, 112829. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Zhang, X.; Lu, Y.; Wang, F.; Xu, Z.; Liu, S.; Zheng, H.; Liu, X. Geniposide Inhibits NLRP3 Inflammasome Activation via Autophagy in BV-2 Microglial Cells Exposed to Oxygen–Glucose Deprivation/Reoxygenation. Int. Immunopharmacol. 2020, 84, 106547. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhao, Y.; Zhou, X.; Peng, C.; Yan, X.; Zou, T. Geniposide Improves Depression by Promoting the Expression of Synapse-Related Proteins through the Creb1/Six3os1 Axis. Gene 2023, 877, 147564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, Y.; Zhong, X.; Guo, Q.; Wang, H.; Gao, J.; Bai, T.; Ren, L.; Guo, Y.; Jiao, X.; et al. Geniposide acutely stimulates insulin secretion in pancreatic β-cells by regulating GLP-1 receptor/cAMP signaling and ion channels. Mol. Cell. Endocrinol. 2016, 430, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.P.; Zhang, Y.G.; Li, H.N.; Kong, H.T.; Zhang, S.S.; Zhang, X.M.; Li, X.B.; Liu, K.C.; Han, L.W.; Tian, Q.P. Discovery and identification of antithrombotic chemical markers in Gardenia Fructus by herbal metabolomics and zebrafish model. J. Ethnopharmacol. 2020, 253, 112679. [Google Scholar] [CrossRef]
- Ma, T.T.; Li, X.F.; Li, W.X.; Yang, Y.; Huang, C.; Meng, X.M.; Zhang, L.; Li, J. Geniposide alleviates inflammation by suppressing MeCP2 in mice with carbon tetrachloride-induced acute liver injury and LPS-treated THP-1 cells. Int. Immunopharmacol. 2015, 29, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Liu, M.; Ji, Y.; Ma, M.; Chen, K.; Liang, T.; Liu, C. Genipin reverses HFD-induced liver damage and inhibits UCP2-mediated pyroptosis in mice. Cell. Physiol. Biochem. 2018, 49, 1885–1897. [Google Scholar] [CrossRef]
- Xi, L.; Qian, Z.; Shen, X.; Wen, N.; Zhang, Y. Crocetin prevents dexamethasone-induced insulin resistance in rats. Planta Med. 2005, 71, 917–922. [Google Scholar] [CrossRef]
- Guo, Z.L.; Li, M.X.; Li, X.L.; Wang, P.; Wang, W.G.; Du, W.Z.; Yang, Z.Q.; Chen, S.F.; Wu, D.; Tian, X.Y. Crocetin: A systematic review. Front. Pharmacol. 2022, 12, 745683. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Cai, X.; Zhang, Q.; de Freitas, V.; Mateus, N.; He, J.; Fernandes, I. Gastrointestinal Absorption, Anti-Proliferative, and Anti-Inflammatory Effect of the Major Carotenoids of Gardenia jasminoides Ellis on Cancer Cells. Food Funct. 2017, 8, 1672–1679. [Google Scholar] [CrossRef]
- Kim, H.I.; Hong, S.H.; Ku, J.M.; Kim, M.J.; Ju, S.W.; Chang, S.W.; Cheon, C.; Ko, S.G. Gardenia jasminoides Enhances CDDP-Induced Apoptosis of Glioblastoma Cells via the AKT/mTOR Pathway While Protecting Astrocytes from Death. Nutrients 2020, 12, 196. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, X.; Xu, H.; Gong, Y.; Yuan, F.; Gao, Y. On-line HPLC-ABTS screening and HPLC-DAD-MS/MS identification of free radical scavengers in Gardenia (Gardenia jasminoides Ellis) fruit extracts. Food Chem. 2010, 123, 521–528. [Google Scholar] [CrossRef]
- Liu, Z.; Mohsin, A.; Wang, Z.; Zhu, X.; Zhuang, Y.; Cao, L.; Guo, M.; Yin, Z. Enhanced biosynthesis of chlorogenic acid and its derivatives in methyl-jasmonate-treated Gardenia jasminoides cells: A study on metabolic and transcriptional responses of cells. Front. Bioeng. Biotechnol. 2021, 8, 604957. [Google Scholar] [CrossRef]
- Xu, J.G.; Hu, Q.P.; Liu, Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef]
- Sokary, S.; Al-Asmakh, M.; Zakaria, Z.; Bawadi, H. The therapeutic potential of matcha tea: A critical review on human and animal studies. Curr. Res. Food Sci. 2023, 6, 100396. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Matsuoka, Y.; Sakai, K.; Fujiya, N.; Fujii, H.; Mano, J.I. Catechins in green tea powder (matcha) are heat-stable scavengers of acrolein, a lipid peroxide-derived reactive carbonyl species. Food Chem. 2021, 355, 129403. [Google Scholar] [CrossRef] [PubMed]
- Starzomska, D.; Gajdzińska, N.; Basiura, K.; Salwa, A.; Rymaszewska, K.; Puchała, J. The Advantage of Matcha Green Tea over Other Drinks: A Comprehensive Review. Qual. Sport 2024, 32, 55912. [Google Scholar] [CrossRef]
- Kochman, J.; Jakubczyk, K.; Antoniewicz, J.; Mruk, H.; Janda, K. Health Benefits and Chemical Composition of Matcha Green Tea: A Review. Molecules 2020, 26, 85. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Kochman, J.; Kwiatkowska, A.; Kałduńska, J.; Dec, K.; Kawczuga, D.; Janda, K. Antioxidant Properties and Nutritional Composition of Matcha Green Tea. Foods 2020, 9, 483. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.H.; Kim, J.H.; Kim, Y.J. Comparative Studies on the Antioxidant Capacities and Catechin Profiles of Conventional and Organic Green Tea. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 475–480. [Google Scholar] [CrossRef]
- Tarhan Kuzu, K.; Aykut, G.; Tek, S.; Yatmaz, E.; Germec, M.; Yavuz, I.; Turhan, I. Production and Characterization of Kombucha Tea from Different Sources of Tea and Its Kinetic Modeling. Processes 2023, 11, 2100. [Google Scholar] [CrossRef]
- Marsh, A.J.; O’Sullivan, O.; Hill, C.; Ross, R.P.; Cotter, P.D. Sequence-based analysis of the bacterial and fungal compositions of multiple kombucha (tea fungus) samples. Food Microbiol. 2014, 38, 171–178. [Google Scholar] [CrossRef]
- Daneluz, J.; da Silva, G.F.; Duarte, J.; Turossi, T.C.; dos Santos, V.; Baldasso, C.; Daneluz, A.C. Membrane Separation Process of Microfiltration Applied to the Filtration of Kombuchas. Food Chem. Adv. 2023, 3, 100451. [Google Scholar] [CrossRef]
- Chen, Z.; Bertin, R.; Froldi, G. EC50 estimation of antioxidant activity in DPPH assay using several statistical programs. Food Chem. 2013, 138, 414–420. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Tefon Öztürk, B.E.; Eroğlu, B.; Delik, E.; Çiçek, M.; Çiçek, E. Comprehensive Evaluation of Three Important Herbs for Kombucha Fermentation. Food Technol. Biotechnol. 2023, 61, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Pankey, G.A.; Ashcraft, D.S.; Dornelles, A. Comparison of 3 Etest® methods and time-kill assay for determination of antimicrobial synergy against carbapenemase-producing Klebsiella species. Diagn. Microbiol. Infect. Dis. 2013, 77, 220–226. [Google Scholar] [CrossRef]
- Esentürk-Güzel, İ.; Durgun, M.E.; Özsoy, Y.; Güngör, S. Drug Release, Susceptibility and Time-Kill Assays to Develop Novel Anti-Infective Drugs. Encycl. Infect. Immun. 2022, 4, 640–651. [Google Scholar]
- Zhou, H.; Zhang, S.; Chen, L.; Liu, Y.; Shen, L.; Zhang, J. Effective therapeutic verification of crocin I, geniposide, and Gardenia (Gardenia jasminoides Ellis) on type 2 diabetes mellitus in vivo and in vitro. Foods 2023, 12, 1668. [Google Scholar] [CrossRef]
- Zhang, L.; Ai, Y.; Chen, Y.; Li, C.; Li, P.; Chen, J.; Jiang, L.; Pan, Y.; Sun, A.; Yang, Y.; et al. Elucidation of geniposide and crocin accumulation and their biosynthesis-related key enzymes during Gardenia jasminoides fruit growth. Plants 2023, 12, 2209. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, D.U.; Jeong, C.S. Gardenia jasminoides Ellis ethanol extract and its constituents reduce the risks of gastritis and reverse gastric lesions in rats. Food Chem. Toxicol. 2009, 47, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Uddin, R.; Saha, M.R.; Subhan, N.; Hossain, H.; Jahan, I.A.; Akter, R.; Alam, A. HPLC-analysis of polyphenolic compounds in Gardenia jasminoides and determination of antioxidant activity by using free radical scavenging assays. Adv. Pharm. Bull. 2014, 4, 273. [Google Scholar] [PubMed]
- Xiao, W.; Li, S.; Wang, S.; Ho, C.T. Chemistry and bioactivity of Gardenia jasminoides. J. Food Drug Anal. 2017, 25, 43–61. [Google Scholar] [CrossRef]
- Forester, S.C.; Lambert, J.D. The role of antioxidant versus pro-oxidant effects of green tea polyphenols in cancer prevention. Mol. Nutr. Food Res. 2011, 55, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Jayabalan, R.; Marimuthu, S.; Swaminathan, K. Changes in content of organic acids and tea polyphenols during kombucha tea fermentation. Food Chem. 2007, 102, 392–398. [Google Scholar] [CrossRef]
- Assamica Agro. Organic Green Tea VS. Conventional Green Tea, Is There Any Difference? Available online: https://www.bluemoonteas.com/blogs/article/organic-green-tea-vs-conventional-green-tea (accessed on 14 January 2023).
- Truong, V.-L.; Jeong, W.-S. Cellular Defensive Mechanisms of Tea Polyphenols: Structure-Activity Relationship. Int. J. Mol. Sci. 2021, 22, 9109. [Google Scholar] [CrossRef]
- Timothy, B.; Emma, D. Black Tea Flavonoids: A Focus on Thearubigins and Their Potential Roles in Diet & Health. Nutr. Food Technol. 2020, 6, 1–8. [Google Scholar]
- Ortiz-Islas, S.; Espinosa-Leal, C.A.; González-Rodríguez, T.; García-Lara, S. Enhancing the Antioxidant Activity of Tea (Camellia sinensis) Through Common Herbal Infusions. Foods 2024, 13, 3284. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, C.; Ma, C.; Ma, B.; Wang, J.; Zhou, B.; Xia, T. Comparative Analysis of Chemical Constituents and Antioxidant Activity in Tea-Leaves Microbial Fermentation of Seven Tea-Derived Fungi from Ripened Pu-erh Tea. LWT 2021, 142, 111006. [Google Scholar] [CrossRef]
- Ma, C.Q.; Li, X.H.; Xia, T. Comparison of Characteristic Components in Tea-Leaves Fermented by Aspergillus pallidofulvus PT-3, Aspergillus sesamicola PT-4 and Penicillium manginii PT-5 Using LC-MS Metabolomics and HPLC Analysis. Food Chem. 2021, 350, 129228. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Hu, W.; Tang, S.; Meng, L.; Huang, Z.; Xu, X.; Xia, X.; Azi, F.; Dong, M. Isolation and Identification of Starmerella davenportii Strain Do18 and Its Application in Black Tea Beverage Fermentation. Food Sci. Hum. Well. 2020, 9, 355–362. [Google Scholar] [CrossRef]
- Pan, X.; Niu, G.; Liu, H. Microwave-Assisted Extraction of Tea Polyphenols and Tea Caffeine from Green Tea Leaves. Chem. Eng. Process. 2003, 42, 129–133. [Google Scholar] [CrossRef]
- Chakravorty, S.; Bhattacharya, S.; Chatzinotas, A.; Chakraborty, W.; Bhattacharya, D.; Gachhui, R. Kombucha Tea Fermentation: Microbial and Biochemical Dynamics. Int. J. Food Microbiol. 2016, 220, 63–72. [Google Scholar] [CrossRef]
- Dueñas, M.; Fernández, D.; Hernández, T.; Estrella, I.; Muñoz, R. Bioactive Phenolic Compounds of Cowpeas (Vigna sinensis L). Modifications by Fermentation with Natural Microflora and with Lactobacillus plantarum ATCC 14917. J. Sci. Food Agric. 2005, 85, 297–304. [Google Scholar] [CrossRef]
- Liu, M.; Xie, H.; Ma, Y.; Li, H.; Li, C.; Chen, L.; Jiang, B.; Nian, B.; Guo, T.; Zhang, Z.; et al. High Performance Liquid Chromatography and Metabolomics Analysis of Tannase Metabolism of Gallic Acid and Gallates in Tea Leaves. J. Agric. Food Chem. 2020, 68, 4946–4954. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; He, C.; Chen, Y.; Ho, C.-T.; Wu, X.; Huang, Y.; Gao, Y.; Hou, A.; Li, Z.; Wang, Y.; et al. UPLC-QQQ-MS/MS-Based Widely Targeted Metabolomic Analysis Reveals the Effect of Solid-State Fermentation with Eurotium cristatum on the Dynamic Changes in the Metabolite Profile of Dark Tea. Food Chem. 2022, 378, 131999. [Google Scholar] [CrossRef] [PubMed]
- de Noronha, M.C.; Cardoso, R.R.; dos Santos D′Almeida, C.T.; do Carmo, M.A.V.; Azevedo, L.; Maltarollo, V.G.; Júnior, J.I.R.; Eller, M.R.; Cameron, L.C.; Ferreira, M.S.L.; et al. Black Tea Kombucha: Physicochemical, Microbiological and Comprehensive Phenolic Profile Changes During Fermentation, and Antimalarial Activity. Food Chem. 2022, 384, 132515. [Google Scholar] [CrossRef]
- Bortolomedi, B.M.; Paglarini, C.S.; Brod, F.C.A. Bioactive compounds in kombucha: A review of substrate effect and fermentation conditions. Food Chem. 2022, 385, 132719. [Google Scholar] [CrossRef]
- Klawpiyapamornkun, T.; Uttarotai, T.; Wangkarn, S.; Sirisa-ard, P.; Kiatkarun, S.; Tragoolpua, Y.; Bovonsombut, S. Enhancing the Chemical Composition of Kombucha Fermentation by Adding Indian Gooseberry as a Substrate. Fermentation 2023, 9, 291. [Google Scholar] [CrossRef]
- Nishi, A.A.; Kumar, P. Hypolipidemic effect of chlorogenic acid in a hypercholesterolemic rat model. Int. J. Pharm. Bio Sci. 2013, 4, B582–B586. [Google Scholar]
- Ali, N.; Rashid, S.; Nafees, S.; Hasan, S.K.; Shahid, A.; Majed, F.; Sultana, S. Protective effect of Chlorogenic acid against Methotrexate induced oxidative stress inflammation and apoptosis in rat liver: An experimental approach. Chem. Biol. Interact. 2017, 272, 80–91. [Google Scholar] [CrossRef]
- Huang, W.Y.; Fu, L.; Li, C.Y.; Xu, L.P.; Zhang, L.X.; Zhang, W.M. Quercetin, hyperin, and chlorogenic acid improve endothelial function by antioxidant, anti-inflammatory, and ACE inhibitory effects. J. Food Sci. 2017, 82, 1239. [Google Scholar] [CrossRef]
- Andreson, M.; Kazantseva, J.; Kuldjärv, R.; Malv, E.; Vaikma, H.; Kaleda, A.; Kütt, M.L.; Vilu, R. Characterisation of chemical, microbial and sensory profiles of commercial kombuchas. Int. J. Food Microbiol. 2022, 373, 109715. [Google Scholar] [CrossRef] [PubMed]
- Landis, E.A.; Fogarty, E.; Edwards, J.C.; Popa, O.; Eren, A.M.; Wolfe, B.E. Microbial diversity and interaction specificity in kombucha tea fermentations. Msystems 2022, 7, e00157-22. [Google Scholar] [CrossRef]
- Al-Mohammadi, A.R.; Ismaiel, A.A.; Ibrahim, R.A.; Moustafa, A.H.; Abou Zeid, A.; Enan, G. Chemical constitution and antimicrobial activity of kombucha fermented beverage. Molecules 2021, 26, 5026. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Tan, Q.; Tang, Q.; Tong, Z.; Yang, M. Research progress on alternative kombucha substrate transformation and the resulting active components. Front. Microbiol. 2023, 14, 1254014. [Google Scholar] [CrossRef]
- Cvetković, D.; Ranitović, A.; Savić, D.; Joković, N.; Tomić, A.; Pezo, L.; Markov, S. Survival of wild strains of lactobacilli during Kombucha fermentation and their contribution to functional characteristics of beverage. Pol. J. Food Nutr. Sci. 2019, 69, 407–415. [Google Scholar] [CrossRef]
- Kim, H.; Hur, S.; Lim, J.; Jin, K.; Yang, T.H.; Keehm, I.S.; Kim, S.W.; Kim, T.; Kim, D. Enhancement of the phenolic compounds and antioxidant activities of Kombucha prepared using specific bacterial and yeast. Food Biosci. 2023, 56, 103431. [Google Scholar] [CrossRef]
- Wahnou, H.; Limami, Y.; Oudghiri, M. Flavonoids and Flavonoid-Based Nanoparticles for Osteoarthritis and Rheumatoid Arthritis Management. BioChem 2024, 4, 38–61. [Google Scholar] [CrossRef]
- Wahnou, H.; Liagre, B.; Sol, V.; El Attar, H.; Attar, R.; Oudghiri, M.; Duval, R.E.; Limami, Y. Polyphenol-Based Nanoparticles: A Promising Frontier for Enhanced Colorectal Cancer Treatment. Cancers 2023, 15, 3826. [Google Scholar] [CrossRef] [PubMed]



| Beverage | t (Fermentation)/day | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 14 | 0 | 14 | 0 | 14 | 0 | 14 | |
| N (Microorganism)/(CFU/mL) | ||||||||
| ACC | TMB | Yeast | Lactobacillus | |||||
| GJ | (2.3 ± 0.2) × 103 | (3.2 ± 0.1) × 106 | (6.7 ± 0.2) × 103 | (9.2 ± 0.4) × 105 | (1.7 ± 0.1) × 103 | (6.7 ± 0.7) × 106 | (7.4 ± 0.3) × 103 | (5.8 ± 0.2) × 106 |
| MGT | (2.3 ± 0.4) × 103 | (1.0 ± 0.2) × 107 | (6.7 ± 0.2) × 103 | (3.2 ± 0.4) × 107 | (1.7 ± 0.3) × 103 | (2.7 ± 0.7) × 107 | (7.4 ± 0.3) × 103 | (1.8 ± 0.1) × 107 |
| OGT | (2.3 ± 0.1) × 103 | (3.0 ± 0.3) × 107 | (6.7 ± 0.2) × 103 | (3.7 ± 0.4) × 107 | (1.7 ± 0.1) × 103 | (2.9 ± 0.6) × 107 | (7.4 ± 0.2) × 103 | (2.1 ± 0.1) × 107 |
| DGT | (2.3 ± 0.4) × 103 | (3.0 ± 0.3) × 107 | (6.7 ± 0.2) × 103 | (4.2 ± 0.4) × 107 | (1.7 ± 0.1) × 103 | (3.7 ± 0.4) × 107 | (7.4 ± 0.4) × 103 | (2.8 ± 0.1) × 107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thenuwara, G.; Cui, X.; Yao, Z.; Javed, B.; Naik, A.S.; Tian, F. Evaluating the Health Implications of Kombucha Fermented with Gardenia jasminoides Teas: A Comprehensive Analysis of Antioxidant, Antimicrobial, and Cytotoxic Properties. BioChem 2024, 4, 350-370. https://doi.org/10.3390/biochem4040018
Thenuwara G, Cui X, Yao Z, Javed B, Naik AS, Tian F. Evaluating the Health Implications of Kombucha Fermented with Gardenia jasminoides Teas: A Comprehensive Analysis of Antioxidant, Antimicrobial, and Cytotoxic Properties. BioChem. 2024; 4(4):350-370. https://doi.org/10.3390/biochem4040018
Chicago/Turabian StyleThenuwara, Gayathree, Xu Cui, Zhen Yao, Bilal Javed, Azza Silotry Naik, and Furong Tian. 2024. "Evaluating the Health Implications of Kombucha Fermented with Gardenia jasminoides Teas: A Comprehensive Analysis of Antioxidant, Antimicrobial, and Cytotoxic Properties" BioChem 4, no. 4: 350-370. https://doi.org/10.3390/biochem4040018
APA StyleThenuwara, G., Cui, X., Yao, Z., Javed, B., Naik, A. S., & Tian, F. (2024). Evaluating the Health Implications of Kombucha Fermented with Gardenia jasminoides Teas: A Comprehensive Analysis of Antioxidant, Antimicrobial, and Cytotoxic Properties. BioChem, 4(4), 350-370. https://doi.org/10.3390/biochem4040018








