Biotechnological Advances in Vanillin Production: From Natural Vanilla to Metabolic Engineering Platforms
Abstract
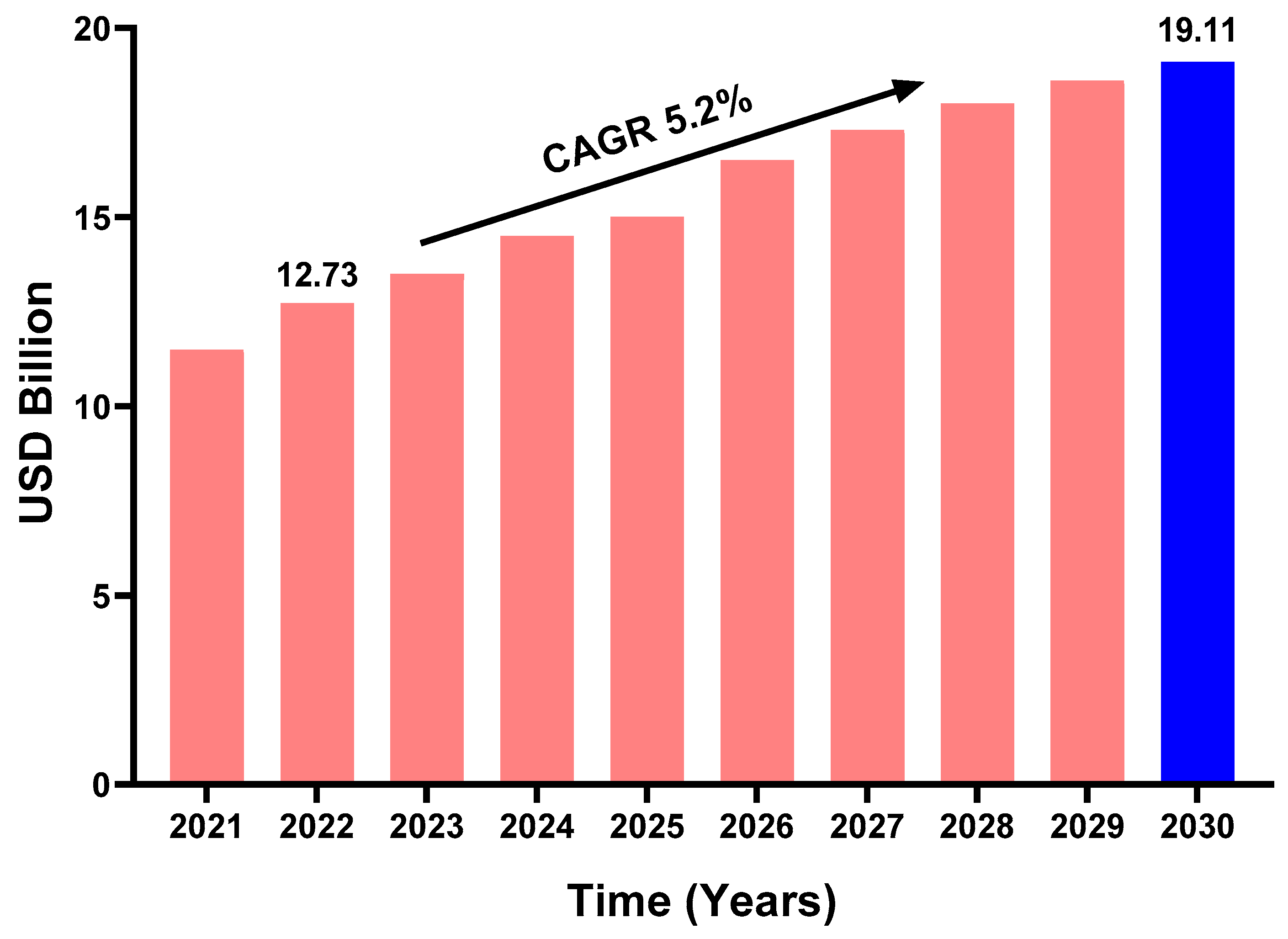
1. Introduction
2. Industrial Applications of Vanillin
2.1. Food and Beverage Industry Applications
2.2. Applications in Perfume/Cosmetics
2.3. Bioactivities/Pharmaceutical and Agrochemical Applications
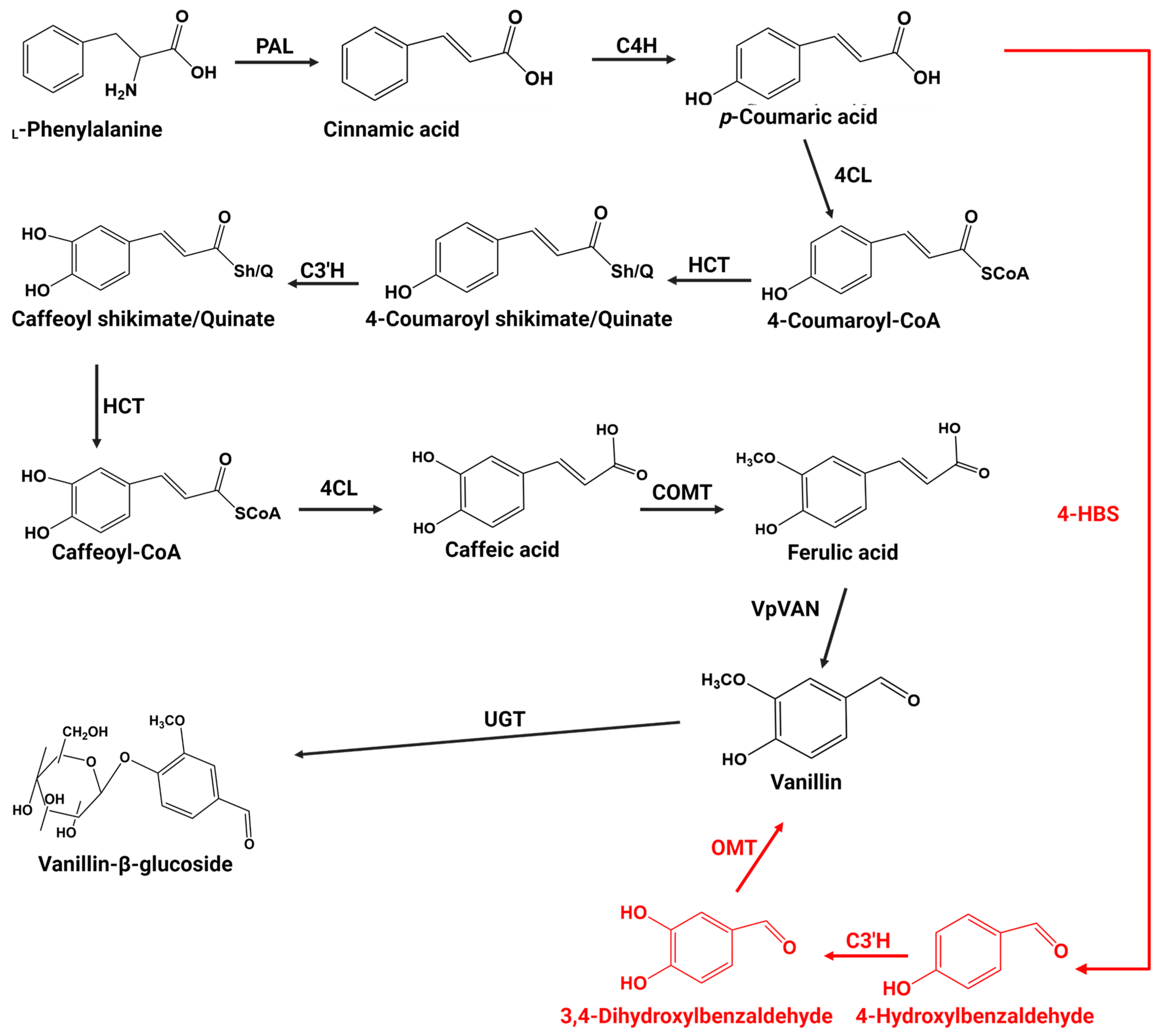
3. Vanillin Biosynthesis in Vanilla Plants
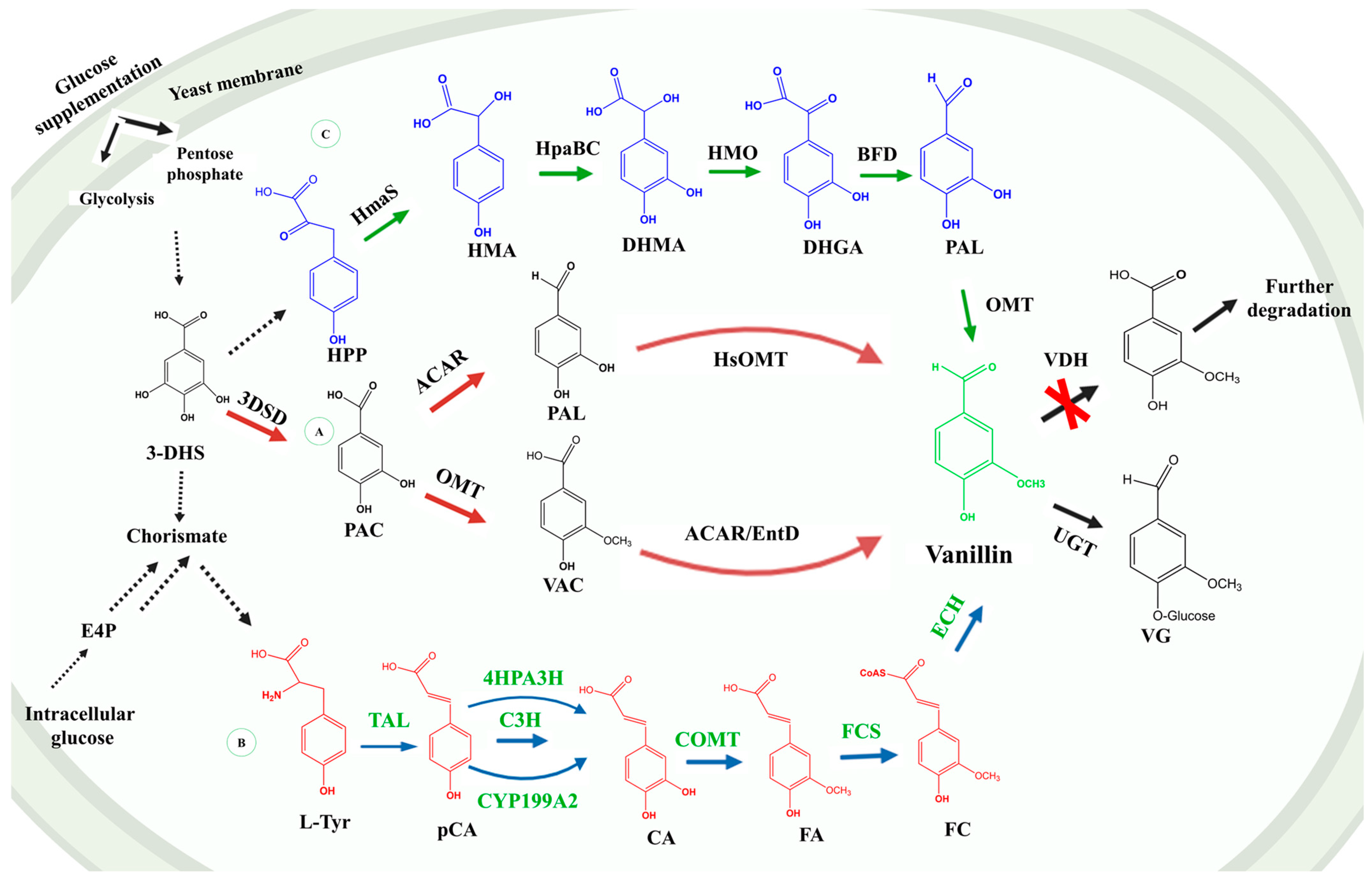
4. Engineering Prokaryotic and Eukaryotic Expression Systems for Sustainable Vanillin Production
4.1. Engineering Prokaryotic Microorganisms for Vanillin Production
4.1.1. Engineering Escherichia coli
4.1.2. Engineering Pseudomonas sp.
4.1.3. Engineering Corynebacterium glutamicum
4.1.4. Engineering Pediococcus acidilactici
4.2. Engineering Eukaryotic Organisms for Vanillin Production
4.2.1. Engineering Saccharomyces cerevisiae and Schizosaccharomyces pombe
4.2.2. Engineering Plant Tissues for the Production of Vanillin
5. Current Challenges and Potential Strategies for Enhancing Microbial-Based Vanillin Production Systems
6. Green Biology: Harnessing Microalgae as Natural Producers of Vanillin Precursors and Promising Hosts for Biotechnological Vanillin Production
6.1. Microalgae as Natural Producers of Vanillin Precursors
6.2. Engineering and Modulation of Robust Microalgal-Based Vanillin Production Systems
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Engineering Platform | Genes (Origin) | Bioengineering Strategies Transformation Insertion Type Mutations Method | Substrates (g/L) | Complexity | Product | Volume of Culture (mL) | Fermentation Time (h) | Yield (g/L) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Escherichia coli BL21(DE3) | IEM (Pseudomonas nitroreducens Jin1) | Heat shock | Episomal | None | 49.2 Isoeugenol | 2 | Vanillin | NI | 16 | 38.3 | [57] |
| Escherichia coli BL21(DE3) | IEM (Pseudomonas putida IE27) | NI | Genome integration | None | 37.7 Isoeugenol | 2 | Vanillin | 10 | 48 | 28.3 | [113] |
| Escherichia coli BL21 (DE3) | IEM (microbial metagenome) | NI | NI | None | 16.4 Isoeugenol | 2 | Vanillin | 15 | 48 | 2.2 | [114] |
| Escherichia coli JM109 | fcs and ech (Pseudomonas fluorescens BF13) | NI | NI | None | 0.64 Ferulic acid | 4 | Vanillin | NI | 6 | 0.53 | [12] |
| Escherichia coli FR13 | fcs and ech (Pseudomonas fluorescens BF13) | NI | Genome integration | None | 4.5 Ferulic acid | 4 | Vanillin | 15 | 24 | 4.3 | [115] |
| Escherichia coli BW25113 | PobA (Pseudomonas aeruginosa), CAR (Mycobacterium marinum), sfp (Bacillus subtilis), and comt (Arabidopsis thaliana) | NI | NI | None | 1 3,4-dihydroxy benzoyl alcohol. | 4 | Vanillyl alcohol | 20 | 48 | 0.24 | [56] |
| Escherichia coli K-12 MG1655 | CAR (Nocardia iowensis), sfp (Bacillus subtilis), comt (Homo sapiens), DSD (Bacillus thuringiensis) | P1 bacteriophage transduction | Genome integration | Deletion of yqhC-dkgA genes | Protocatechuic acid | 4 | Vanillate | 50 | 72 | 0.004 | [55] |
| Pseudomonas fluorescens BF13 | fcs and ech (Pseudomonas fluorescens BF13) | Heat shock | NI | Deletion of vdh gene | 0.48 Ferulic acid | 4 | Vanillin | 2000 | 24 | 1.27 | [29] |
| Escherichia coli | tal (Saccharothrix espanaensis), sam5 (actinomycete), comt (Arabidopsis thaliana), sam8 (Saccharothrix espanaensis), fcs, and ech (Streptomyces sp.) | Co-transformation | Genome integration | Deletion of TyrR gene | L-Tyrosine | 6 | Vanillin | 50 | 12 | 0.013–0.097 | [54] |
| Escherichia coli MG1655 RARE | LCC (unknown prokaryote), TPADO (Comamonas sp.), DCDDH (Comamonas sp.), O-MT (Rattus norvegicus), and CAR (Nocardia iowensis). | Heat shock | NI | None | Monomer terephthalic acid | 6 | Vanillin | 10 | 24 | 0.11 | [116] |
| Escherichia coli BW25113 | PobA (Pseudomonas aeruginosa), CAR (Mycobacterium marinum), sfp (Bacillus subtilis), and COMT (Arabidopsis thaliana) | Heat shock | NI | Deletion of aroE gene | ND | 6 | Vanillyl alcohol | 50 and 3000 | 2 and 24 | 3.89 and 0.55 | [58] |
| Escherichia coli | fcs and ech (Amycolatopsis sp.) | NI | NI | None | Glucose | 6 | Vanillin | NI | 48 | 0.50 | [117] |
| Pediococcus acidilactici BD16 | fcs and ech (Amycolatopsis sp.) | Heat shock | Episomal | None | 0.21 Ferulic Acid per mg of recombinant cell biomass | 4 | Vanillin | 100 | 0.33 | 0.47 | [65] |
| Pediococcus acidilactici BD16 | fcs and ech (Pediococcus acidilactici BD16) | Heat shock | Episomal | None | Rice bran | 4 | Vanillin | 50 | 24 | 4.01 | [66] |
| Pseudomonas putida KT2440 | Pp-SpuC and ATA (Chromobacterium), adh (Bacillus) | Electroporation | Genome integration | None | Lignin-derived substrates | 4 | Vanillin and vanillylamine | 50 and 100 | 24 | 0.10 and 0.13 | [118] |
| Pseudomonas putida KT2440 | fcs and ech (Pseudomonas putida) | Electroporation | Genome integration | Deletion of vdh and modABC genes | 1.94 Ferulic acid | 4 | Vanillin | 5 | 3 | 1.66 | [59] |
| Corynebacterium glutamicum ATCC 21420 | vanAB (Corynebacterium efficiens NBRC 100395) | Electroporation | Genome integration | None | 3.1 Ferulic Acid | 4 | Protocatechuic acid | 400 | 24 | 1.06 | [62] |
| Corynebacterium glutamicum PV-IYΔ0324 | comt (Rattus norvegicus) and CAR (Nocardia iowensis) | NI | Genome integration | Deletion of NCgl0324 gene | Endogenous 4-Hydroxylbenzoic acid | 6 | Vanillin | 25 | 48 | 0.31 | [63] |
| Amycolatopsis sp. ATCC 39116 | fcs and ech (Amycolatopsis sp.) | Direct mycelium transformation | Genome integration | Deletion of vdh gene | 5 Glucose | 6 | Vanillin | 2000 | 20 | 22.3 | [119] |
| Saccharomyces cerevisiae and Schizosaccharomyces pombe | 3DSD (Podospora pauciseta), ACAR (Nocardia sp.), O-MT (Homo sapiens), and UGT (Arabidopsis thaliana) | Lithium acetate/polyethylene glycol/single carrier DNA | Genome integration | Deletion of adh6 gene | Glucose | 6 | Vanillin | 5 | 48 | 0.065 and 0.045 | [70] |
| Saccharomyces cerevisiae VAN286 | UGT (Arabidopsis thaliana) | NI | NI | Deletion of pdc1 and gdh1 genes | Glucose | 6 | Vanillin-β-D-glucoside | NI | 90 | 0.50 | [72] |
| Saccharomyces cerevisiae | Sam8 (Actinomycete), sam5/ hpaB/ CYP199A2(Saccharothrix espanaensis/Pseudomonas aeruginosa/Rhodopseudomonas palustris), comt (Arabidopsis thaliana), fcs, and ech (Streptomyces sp.). | Lithium acetate/polyethylene glycol/single carrier DNA | Genome integration | Deletion of Aro4 and Aro7 genes | 0.4 ferulic acid | 6 | Vanillin | 50 | 96 | 0.008 | [76] |
| Saccharomyces cerevisiae S288c and CEN.PK | ACAR (Neurospora sp.), EntD (Escherichia coli), UGT (Arabidopsis thaliana), OMT (Homo sapiens), and 3DSD (Podospora pauciseta) | Lithium acetate/polyethylene glycol/single carrier DNA | Genome integration | Deletion of Bgl1 and adh6 genes | 20 Glucose | 6 | Vanillin-β-D-glucoside | 50 | 45 | 2 | [79] |
| Saccharomyces cerevisiae BY4741 | AroZ (Podospora anserina), OMT (Homo sapiens), CAR (Segniliparus Rotundus), GapC (Clostridium acetobutylicum), Pos5c (S. cerevisiae), Sfp (Bacillus subtilis), EntD (E. coli), MetK (E. coli), hpaB (Pseudomonas aeruginosa), hpaC (Salmonella enterica), Xfpk (Bifidobacterium breve), CkPta (Clostridium kluyveri), HmaS (A. orientalis), HMO (S. coelicolor A3), and BFD (P. putida KT2440) | Electroporation | Combinaison of both episomal expression and genome integration | Deletion of adh6, adh7, gre3, gcy1, ydl124w, ypr1, ari1, ydr541c, aad3, hfd1, AKR, and AL DR family genes | Glucose | 4 | Vanillin | 10 | 120 | 0.36 | [20] |
| Saccharomyces cerevisiae BY4742 | 4CL (Petroselinum crispum), Ech (Pseudomonas putida KT2440), VpVAN (V. planifolia), hpaB(Pseudomonas aeruginosa), hpaC (Salmonella enterica), PobA (Pseudomonas putida KT2440), COMT (Arabidopsis thaliana), METHER (Arabidopsis thaliana), and PsVAO (Penicillium simplicissimum) | NI | Genome integration | Deletion of adh6, adh7, bdh2, bdh1, hfd1, pdc5, aro10, pha2, pgm1, trp2, pad1, ahfd1, AKR, and ALDR family genes | lignocellulosic biomass | 6 | Vanillin | 50 | 24 | 0.29 | [77] |
| Oryza sativa | VpVAN (V. planifolia) | Agrobacterium-mediated transformation | Genome integration | None | Endogenous ferulic Acid | 4 | Vanillin | NI | NI | 0.00054 | [81] |
| Capsicum frutescens | VpVAN (V. planifolia) | Microparticle bombardment | Genome integration | None | Endogenous ferulic Acid | 4 | Vanillin | NI | NI | 0.00057 | [80] |
References
- Spence, C. Odour hedonics and the ubiquitous appeal of vanilla. Nat. Food 2022, 3, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Furuya, T.; Miura, M.; Kuroiwa, M.; Kino, K. High-yield production of vanillin from ferulic acid by a coenzyme-independent decarboxylase/oxygenase two-stage process. New Biotechnol. 2015, 32, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liaqat, F.; Sun, J.; Khazi, M.I.; Xie, R.; Zhu, D. Advances in the vanillin synthesis and biotransformation: A review. Renew. Sustain. Energy Rev. 2024, 189, 113905. [Google Scholar] [CrossRef]
- Gallage, N.J.; Hansen, E.H.; Kannangara, R.; Olsen, C.E.; Motawia, M.S.; Jørgensen, K.; Holme, I.; Hebelstrup, K.; Grisoni, M.; Møller, B.L. Vanillin formation from ferulic acid in Vanilla planifolia is catalysed by a single enzyme. Nat. Commun. 2014, 5, 4037. [Google Scholar] [CrossRef] [PubMed]
- Taira, J.; Toyoshima, R.; Ameku, N.; Iguchi, A.; Tamaki, Y. Vanillin production by biotransformation of phenolic compounds in fungus, Aspergillus luchuensis. AMB Express 2018, 8, 40. [Google Scholar] [CrossRef]
- Krings, U.; Berger, R.G. Biotechnological production of flavours and fragrances. Appl. Microbiol. Biotechnol. 1998, 49, 1–8. [Google Scholar] [CrossRef]
- Gallage, N.J.; Moller, B.L. Vanillin-bioconversion and bioengineering of the most popular plant flavor and its de novo biosynthesis in the vanilla orchid. Mol. Plant 2015, 8, 40–57. [Google Scholar] [CrossRef]
- Omokhua-Uyi, A.G.; Van Staden, J. Natural product remedies for COVID-19: A focus on safety. S. Afr. J. Bot. 2021, 139, 386–398. [Google Scholar] [CrossRef]
- SNS, Vanillin Market Size, Share and Segmentation by Product Type (Natural, Synthetic Vanillin), by End-Use (Food & Beverage, Fragrance, Pharmaceutical), by Regions and Global Market Forecast 2023–2030. Microorganisms. 2022. Available online: https://www.snsinsider.com/reports/vanillin-market-2244 (accessed on 25 November 2024).
- Hocking, M.B. Vanillin: Synthetic Flavoring from Spent Sulfite Liquor. J. Chem. Educ. 1997, 74, 1055. [Google Scholar] [CrossRef]
- Zhou, N.; Thilakarathna, W.P.D.W.; He, Q.S.; Rupasinghe, H.P.V. A Review: Depolymerization of Lignin to Generate High-Value Bio-Products: Opportunities, Challenges, and Prospects. Front. Energy Res. 2022, 9, 758744. [Google Scholar] [CrossRef]
- Barghini, P.; Di Gioia, D.; Fava, F.; Ruzzi, M. Vanillin production using metabolically engineered Escherichia coli under non-growing conditions. Microb. Cell Fact. 2007, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.; Guerreiro, C.E.; Belo, I. Generation of Flavors and Fragrances Through Biotransformation and De Novo Synthesis. Food Bioprocess. Technol. 2018, 11, 2217–2228. [Google Scholar] [CrossRef]
- Muheim, A.; Lerch, K. Towards a high-yield bioconversion of ferulic acid to vanillin. Appl. Microbiol. Biotechnol. 1999, 51, 456–461. [Google Scholar] [CrossRef]
- Chou, T.H.; Ding, H.Y.; Hung, W.J.; Liang, C.H. Antioxidative characteristics and inhibition of alpha-melanocyte-stimulating hormone-stimulated melanogenesis of vanillin and vanillic acid from Origanum vulgare. Exp. Dermatol. 2010, 19, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Mitra, A. Flavoring extracts of Hemidesmus indicus roots and Vanilla planifolia pods exhibit in vitro acetylcholinesterase inhibitory activities. Plant Foods Hum. Nutr. 2013, 68, 247–253. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, L.; Zhao, S.; Huang, Z.; Li, C.; Jiang, S.; Li, Q.; Gu, P. Biosynthesis of vanillin by different microorganisms: A review. World J. Microbiol. Biotechnol. 2022, 38, 40. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, Y.; Xue, J.; Liao, Y.; Fu, X.; Zhu, C.; Li, J.; Zeng, L.; Yang, Z. Biosynthetic Pathway and Bioactivity of Vanillin, a Highly Abundant Metabolite Distributed in the Root Cortex of Tea Plants (Camellia sinensis). J. Agric. Food Chem. 2024, 72, 1660–1673. [Google Scholar] [CrossRef]
- Xu, L.; Liaqat, F.; Khazi, M.I.; Sun, J.; Zhu, D. Natural deep eutectic solvents-based green extraction of vanillin: Optimization, purification, and bioactivity assessment. Front. Nutr. 2024, 10, 1279552. [Google Scholar] [CrossRef]
- Mo, Q.; Yuan, J. Minimal aromatic aldehyde reduction (MARE) yeast platform for engineering vanillin production. Biotechnol. Biofuels Bioprod. 2024, 17, 4. [Google Scholar] [CrossRef]
- Jassey, V.E.J.; Hamard, S.; Lepère, C.; Céréghino, R.; Corbara, B.; Küttim, M.; Leflaive, J.; Leroy, C.; Carrias, J.-F. Photosynthetic microorganisms effectively contribute to bryophyte CO2 fixation in boreal and tropical regions. ISME Commun. 2022, 2, 64. [Google Scholar] [CrossRef]
- Cordero, B.F.; Couso, I.; León, R.; Rodríguez, H.; Vargas, M.A. Enhancement of carotenoids biosynthesis in Chlamydomonas reinhardtii by nuclear transformation using a phytoene synthase gene isolated from Chlorella zofingiensis. Appl. Microbiol. Biotechnol. 2011, 91, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Shamriz, S.; Ofoghi, H. Expression of Recombinant PfCelTOS Antigen in the Chloroplast of Chlamydomonas reinhardtii and its Potential Use in Detection of Malaria. Mol. Biotechnol. 2019, 61, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhuang, J.; Ni, S.; Luo, H.; Zheng, K.; Li, X.; Lan, C.; Zhao, D.; Bai, Y.; Jia, B.; et al. Overexpressing CrePAPS Polyadenylate Activity Enhances Protein Translation and Accumulation in Chlamydomonas reinhardtii. Mar. Drugs 2022, 20, 276. [Google Scholar] [CrossRef]
- Passariello, C.L.; Marchionni, S.; Carcuro, M.; Casali, G.; Della Pasqua, A.; Hrelia, S.; Malaguti, M.; Lorenzini, A. The Mediterranean Athlete’s Nutrition: Are Protein Supplements Necessary? Nutrients 2020, 12, 3681. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Gheisar, M.; Hosseindoust, A.; Kim, I.H. Evaluating the effect of microencapsulated blends of organic acids and essential oils in broiler chickens diet. J. Appl. Poult. Res. 2015, 24, 511–519. [Google Scholar] [CrossRef]
- Pereira, A.M.; de Lurdes Nunes Enes Dapkevicius, M.; Borba, A.E.S. Alternative pathways for hydrogen sink originated from the ruminal fermentation of carbohydrates: Which microorganisms are involved in lowering methane emission? Anim. Microbiome 2022, 4, 5. [Google Scholar] [CrossRef]
- Malone, S.L.; Oh, Y.; Arndt, K.A.; Burba, G.; Commane, R.; Contosta, A.R.; Goodrich, J.P.; Loescher, H.W.; Starr, G.; Varner, R.K. Gaps in network infrastructure limit our understanding of biogenic methane emissions for the United States. Biogeosciences 2022, 19, 2507–2522. [Google Scholar] [CrossRef]
- Di Gioia, D.; Luziatelli, F.; Negroni, A.; Ficca, A.G.; Fava, F.; Ruzzi, M. Metabolic engineering of Pseudomonas fluorescens for the production of vanillin from ferulic acid. J. Biotechnol. 2011, 156, 309–316. [Google Scholar] [CrossRef]
- Lee, J.; Cho, J.Y.; Lee, S.Y.; Lee, K.W.; Lee, J.; Song, J.Y. Vanillin protects human keratinocyte stem cells against ultraviolet B irradiation. Food Chem. Toxicol. 2014, 63, 30–37. [Google Scholar] [CrossRef]
- Loh, Y.H.; Wu, Q.; Chew, J.L.; Vega, V.B.; Zhang, W.; Chen, X.; Bourque, G.; George, J.; Leong, B.; Liu, J.; et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat. Genet. 2006, 38, 431–440. [Google Scholar] [CrossRef]
- Paul, V.; Rai, D.C.; Ramyaa Lakshmi, T.S.; Srivastava, S.K.; Tripathi, A.D. A comprehensive review on vanillin: Its microbial synthesis, isolation and recovery. Food Biotechnol. 2021, 35, 22–49. [Google Scholar] [CrossRef]
- Arya, S.S.; Rookes, J.E.; Cahill, D.M.; Lenka, S.K. Vanillin: A review on the therapeutic prospects of a popular flavouring molecule. Adv. Tradit. Med. 2021, 21, 1–17. [Google Scholar] [CrossRef]
- Mani, A.; Ahamed, A.; Ali, D.; Alarifi, S.; Akbar, I. Dopamine-Mediated Vanillin Multicomponent Derivative Synthesis via Grindstone Method: Application of Antioxidant, Anti-Tyrosinase, and Cytotoxic Activities. Drug Des. Devel. Ther. 2021, 15, 787–802. [Google Scholar] [CrossRef] [PubMed]
- Olatunde, A.; Mohammed, A.; Ibrahim, M.A.; Tajuddeen, N.; Shuaibu, M.N. Vanillin: A food additive with multiple biological activities. Eur. J. Med. Chem. Rep. 2022, 5, 100055. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Soares, A.K.; de Sousa, D.P. Overview of the Role of Vanillin on Redox Status and Cancer Development. Oxid. Med. Cell Longev. 2016, 2016, 9734816. [Google Scholar] [CrossRef]
- Kim, H.J.; Hwang, I.K.; Won, M.H. Vanillin, 4-hydroxybenzyl aldehyde and 4-hydroxybenzyl alcohol prevent hippocampal CA1 cell death following global ischemia. Brain Res. 2007, 1181, 130–141. [Google Scholar] [CrossRef]
- Yan, X.; Liu, D.F.; Zhang, X.Y.; Liu, D.; Xu, S.Y.; Chen, G.X.; Huang, B.X.; Ren, W.Z.; Wang, W.; Fu, S.P.; et al. Vanillin Protects Dopaminergic Neurons against Inflammation-Mediated Cell Death by Inhibiting ERK1/2, P38 and the NF-κB Signaling Pathway. Int. J. Mol. Sci. 2017, 18, 389. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, H.-O.; Cho, Y.-J.; Kim, J.; Chun, J.; Choi, J.; Lee, Y.; Jung, W.H. A vanillin derivative causes mitochondrial dysfunction and triggers oxidative stress in Cryptococcus neoformans. PLoS ONE 2014, 9, e89122. [Google Scholar] [CrossRef]
- Hariono, M.; Abdullah, N.; Damodaran, K.; Kamarulzaman, E.E.; Mohamed, N.; Hassan, S.S.; Shamsuddin, S.; Wahab, H.A. Potential new H1N1 neuraminidase inhibitors from ferulic acid and vanillin: Molecular modelling, synthesis and in vitro assay. Sci. Rep. 2016, 6, 38692. [Google Scholar] [CrossRef]
- Zhao, D.; Jiang, Y.; Sun, J.; Li, H.; Huang, M.; Sun, X.; Zhao, M. Elucidation of The Anti-Inflammatory Effect of Vanillin In Lps-Activated THP-1 Cells. J. Food Sci. 2019, 84, 1920–1928. [Google Scholar] [CrossRef]
- Yun, H.M.; Kim, E.; Kwon, Y.J.; Park, K.R. Vanillin Promotes Osteoblast Differentiation, Mineral Apposition, and Antioxidant Effects in Pre-Osteoblasts. Pharmaceutics 2024, 16, 485. [Google Scholar] [CrossRef] [PubMed]
- Gendron, D. Vanillin: A Promising Biosourced Building Block for the Preparation of Various Heterocycles. Front. Chem. 2022, 10, 949355. [Google Scholar] [CrossRef] [PubMed]
- Lou, M.; Li, S.; Jin, F.; Yang, T.; Song, R.; Song, B. Pesticide Engineering from Natural Vanillin: Recent Advances and a Perspective. Engineering 2024. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, X.; Feng, Y.; Sun, J.; Jiang, Y.; Zhang, W.; Xin, F.; Jiang, M. Current Status, Challenges, and Prospects for the Biological Production of Vanillin. Fermentation 2023, 9, 389. [Google Scholar] [CrossRef]
- Funk, C.; Brodelius, P.E. Phenylpropanoid Metabolism in Suspension Cultures of Vanilla planifolia Andr. 1: IV. Induction of Vanillic Acid Formation. Plant Physiol. 1992, 99, 256–262. [Google Scholar] [CrossRef]
- Schoch, G.; Goepfert, S.; Morant, M.; Hehn, A.; Meyer, D.; Ullmann, P.; Werck-Reichhart, D. CYP98A3 from Arabidopsis thaliana is a 3’-hydroxylase of phenolic esters, a missing link in the phenylpropanoid pathway. J. Biol. Chem. 2001, 276, 36566–36574. [Google Scholar] [CrossRef]
- Kundu, A. Vanillin biosynthetic pathways in plants. Planta 2017, 245, 1069–1078. [Google Scholar] [CrossRef]
- Podstolski, A.; Havkin-Frenkel, D.; Malinowski, J.; Blount, J.W.; Kourteva, G.; Dixon, R.A. Unusual 4-hydroxybenzaldehyde synthase activity from tissue cultures of the vanilla orchid Vanilla planifolia. Phytochemistry 2002, 61, 611–620. [Google Scholar] [CrossRef]
- Liaqat, F.; Xu, L.; Khazi, M.I.; Ali, S.; Rahman, M.U.; Zhu, D. Extraction, purification, and applications of vanillin: A review of recent advances and challenges. Ind. Crops Prod. 2023, 204, 117372. [Google Scholar] [CrossRef]
- Zhao, M.L.; Cai, W.S.; Zheng, S.Q.; Zhao, J.L.; Zhang, J.L.; Huang, Y.; Hu, Z.L.; Jia, B. Metabolic Engineering of the Isopentenol Utilization Pathway Enhanced the Production of Terpenoids in Chlamydomonas reinhardtii. Mar. Drugs 2022, 20, 577. [Google Scholar] [CrossRef]
- Converti, A.; Aliakbarian, B.; Domínguez, J.M.; Bustos Vázquez, G.; Perego, P. Microbial production of biovanillin. Braz. J. Microbiol. 2010, 41, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Torre, P.; De Faveri, D.; Perego, P.; Ruzzi, M.; Barghini, P.; Gandolfi, R.; Converti, A. Bioconversion of ferulate into vanillin by Escherichia coli strain JM109/pBB1 in an immobilized-cell reactor. Ann. Microbiol. 2004, 54, 517–527. [Google Scholar]
- Ni, J.; Tao, F.; Du, H.; Xu, P. Mimicking a natural pathway for de novo biosynthesis: Natural vanillin production from accessible carbon sources. Sci. Rep. 2015, 5, 13670. [Google Scholar] [CrossRef] [PubMed]
- Kunjapur, A.M.; Hyun, J.C.; Prather, K.L. Deregulation of S-adenosylmethionine biosynthesis and regeneration improves methylation in the E. coli de novo vanillin biosynthesis pathway. Microb. Cell Fact. 2016, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Shen, X.; Wang, J.; Wang, J.; Zhang, R.; Rey, J.F.; Yuan, Q.; Yan, Y. Establishing an Artificial Pathway for De Novo Biosynthesis of Vanillyl Alcohol in Escherichia coli. ACS Synth. Biol. 2017, 6, 1784–1792. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, X.; Lu, X.; He, Y.; Ma, B.; Xu, Y. Efficient Biosynthesis of Vanillin from Isoeugenol by Recombinant Isoeugenol Monooxygenase from Pseudomonas nitroreducens Jin1. Appl. Biochem. Biotechnol. 2021, 193, 1116–1128. [Google Scholar] [CrossRef]
- Yang, M.; Meng, H.; Li, X.; Wang, J.; Shen, X.; Sun, X.; Yuan, Q. Coculture engineering for efficient production of vanillyl alcohol in Escherichia coli. aBIOTECH 2022, 3, 292–300. [Google Scholar] [CrossRef]
- Graf, N.; Altenbuchner, J. Genetic engineering of Pseudomonas putida KT2440 for rapid and high-yield production of vanillin from ferulic acid. Appl. Microbiol. Biotechnol. 2014, 98, 137–149. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, H.U.; Kim, T.Y.; Park, J.S.; Kim, S.-S.; Lee, S.Y. Metabolic engineering of Corynebacterium glutamicum for L-arginine production. Nat. Commun. 2014, 5, 4618. [Google Scholar] [CrossRef]
- Chen, C.; Pan, J.; Yang, X.; Guo, C.; Ding, W.; Si, M.; Zhang, Y.; Shen, X.; Wang, Y. Global Transcriptomic Analysis of the Response of Corynebacterium glutamicum to Vanillin. PLoS ONE 2016, 11, e0164955. [Google Scholar] [CrossRef]
- Okai, N.; Masuda, T.; Takeshima, Y.; Tanaka, K.; Yoshida, K.I.; Miyamoto, M.; Ogino, C.; Kondo, A. Biotransformation of ferulic acid to protocatechuic acid by Corynebacterium glutamicum ATCC 21420 engineered to express vanillate O-demethylase. AMB Express 2017, 7, 130. [Google Scholar] [CrossRef]
- Kim, H.S.; Choi, J.A.; Kim, B.Y.; Ferrer, L.; Choi, J.M.; Wendisch, V.F.; Lee, J.H. Engineered Corynebacterium glutamicum as the Platform for the Production of Aromatic Aldehydes. Front. Bioeng. Biotechnol. 2022, 10, 880277. [Google Scholar] [CrossRef] [PubMed]
- Mora, D.; Fortina, M.G.; Parini, C.; Manachini, P.L. Identification of Pediococcus acidilactici and Pediococcus pentosaceus based on 16S rRNA and ldhD gene-targeted multiplex PCR analysis. FEMS Microbiol. Lett. 1997, 151, 231–236. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaur, B.; Chakraborty, D.; Kumar, B. Metabolic engineering of Pediococcus acidilactici BD16 for production of vanillin through ferulic acid catabolic pathway and process optimization using response surface methodology. Appl. Microbiol. Biotechnol. 2014, 98, 8539–8551. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Selvam, A.; Kaur, B.; Wong, J.W.C.; Karthikeyan, O.P. Application of recombinant Pediococcus acidilactici BD16 (fcs+/ech+) for bioconversion of agrowaste to vanillin. Appl. Microbiol. Biotechnol. 2017, 101, 5615–5626. [Google Scholar] [CrossRef]
- Scheper, A.F.; Schofield, J.; Bohara, R.; Ritter, T.; Pandit, A. Understanding glycosylation: Regulation through the metabolic flux of precursor pathways. Biotechnol. Adv. 2023, 67, 108184. [Google Scholar] [CrossRef]
- Bryan, L.; Clynes, M.; Meleady, P. The emerging role of cellular post-translational modifications in modulating growth and productivity of recombinant Chinese hamster ovary cells. Biotechnol. Adv. 2021, 49, 107757. [Google Scholar] [CrossRef]
- Liu, Z.L.; Ma, M.; Song, M. Evolutionarily engineered ethanologenic yeast detoxifies lignocellulosic biomass conversion inhibitors by reprogrammed pathways. Mol. Genet. Genom. 2009, 282, 233–244. [Google Scholar]
- Hansen, E.H.; Møller, B.L.; Kock, G.R.; Bünner, C.M.; Kristensen, C.; Jensen, O.R.; Okkels, F.T.; Olsen, C.E.; Motawia, M.S.; Hansen, J. De novo biosynthesis of vanillin in fission yeast (Schizosaccharomyces pombe) and baker’s yeast (Saccharomyces cerevisiae). Appl. Env. Microbiol. 2009, 75, 2765–2774. [Google Scholar] [CrossRef]
- Moehs, C.P.; Allen, P.V.; Friedman, M.; Belknap, W.R. Cloning and expression of solanidine UDP-glucose glucosyltransferase from potato. Plant J. 1997, 11, 227–236. [Google Scholar] [CrossRef]
- Brochado, A.R.; Matos, C.; Møller, B.L.; Hansen, J.; Mortensen, U.H.; Patil, K.R. Improved vanillin production in baker’s yeast through in silico design. Microb. Cell Factories 2010, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Zamzuri, N.A.A.; Abd-Aziz, S. Biovanillin from agro wastes as an alternative food flavour. J. Sci. Food Agric. 2013, 93, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, H.; Wang, X.; Zhang, X.; Hou, J.; Wang, L.; Gao, N.; Bao, X. High vanillin tolerance of an evolved Saccharomyces cerevisiae strain owing to its enhanced vanillin reduction and antioxidative capacity. J. Ind. Microbiol. Biotechnol. 2014, 41, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Wang, X.; Bao, X.; Wei, T.; Hou, J.; Liu, W.; Shen, Y. Newly identified genes contribute to vanillin tolerance in Saccharomyces cerevisiae. Microb. Biotechnol. 2021, 14, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Wang, M.; Zhou, C.; Zhao, J.; Zhang, G. De novo biosynthesis of vanillin in engineered Saccharomyces cerevisiae. Chem. Eng. Sci. 2022, 263, 118049. [Google Scholar] [CrossRef]
- Xin, X.; Zhang, R.-K.; Liu, S.-C.; He, Z.-J.; Liu, R.-Y.; Lan, H.-N.; Liu, Z.-H.; Li, B.-Z.; Yuan, Y.-J. Engineering yeast to convert lignocellulose into vanillin. Chem. Eng. J. 2024, 485, 149815. [Google Scholar] [CrossRef]
- Chen, R.; Gao, J.; Yu, W.; Chen, X.; Zhai, X.; Chen, Y.; Zhang, L.; Zhou, Y.J. Engineering cofactor supply and recycling to drive phenolic acid biosynthesis in yeast. Nat. Chem. Biol. 2022, 18, 520–529. [Google Scholar] [CrossRef]
- Strucko, T.; Magdenoska, O.; Mortensen, U.H. Benchmarking two commonly used Saccharomyces cerevisiae strains for heterologous vanillin-β-glucoside production. Metab. Eng. Commun. 2015, 2, 99–108. [Google Scholar] [CrossRef]
- Chee, M.J.; Lycett, G.W.; Khoo, T.J.; Chin, C.F. Bioengineering of the Plant Culture of Capsicum frutescens with Vanillin Synthase Gene for the Production of Vanillin. Mol. Biotechnol. 2017, 59, 1–8. [Google Scholar] [CrossRef]
- Arya, S.S.; Mahto, B.K.; Sengar, M.S.; Rookes, J.E.; Cahill, D.M.; Lenka, S.K. Metabolic Engineering of Rice Cells with Vanillin Synthase Gene (VpVAN) to Produce Vanillin. Mol. Biotechnol. 2022, 64, 861–872. [Google Scholar] [CrossRef]
- Diamond, A.; Barnabé, S.; Desgagné-Penix, I. Is a spice missing from the recipe? The intra-cellular localization of vanillin biosynthesis needs further investigations. Plant Biol. 2022, 25, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Barros-Rios, J.; Kourteva, G.; Rao, X.; Chen, F.; Shen, H.; Liu, C.; Podstolski, A.; Belanger, F.; Havkin-Frenkel, D.; et al. A re-evaluation of the final step of vanillin biosynthesis in the orchid Vanilla planifolia. Phytochemistry 2017, 139, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, L.; Huo, Y.-X.; Guo, S. Strategies for improving the production of bio-based vanillin. Microb. Cell Factories 2023, 22, 147. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Zhang, H.; Chen, Y.; Wei, L.; Liu, J.; Nielsen, J.; Chen, Y.; Xu, N. Relieving metabolic burden to improve robustness and bioproduction by industrial microorganisms. Biotechnol. Adv. 2024, 74, 108401. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, Y.; Kang, Z.; Cheng, J.; Xiong, X.; Hu, C.Y.; Meng, Y. Engineering a Feruloyl–Coenzyme A Synthase for Bioconversion of Phenylpropanoid Acids into High-Value Aromatic Aldehydes. J. Agric. Food Chem. 2022, 70, 9948–9960. [Google Scholar] [CrossRef]
- Pattrick, C.A.; Webb, J.P.; Green, J.; Chaudhuri, R.R.; Collins, M.O.; Kelly, D.J. Proteomic Profiling, Transcription Factor Modeling, and Genomics of Evolved Tolerant Strains Elucidate Mechanisms of Vanillin Toxicity in Escherichia coli. mSystems 2019, 4. [Google Scholar] [CrossRef]
- Fujimaki, S.; Sakamoto, S.; Shimada, S.; Kino, K.; Furuya, T. Engineering a coenzyme-independent dioxygenase for one-step production of vanillin from ferulic acid. Appl. Environ. Microbiol. 2024, 90, e0023324. [Google Scholar] [CrossRef]
- Zeng, X.; Danquah, M.K.; Chen, X.D.; Lu, Y. Microalgae bioengineering: From CO2 fixation to biofuel production. Renew. Sustain. Energy Rev. 2011, 15, 3252–3260. [Google Scholar] [CrossRef]
- Kselíková, V.; Singh, A.; Bialevich, V.; Čížková, M.; Bišová, K. Improving microalgae for biotechnology—From genetics to synthetic biology—Moving forward but not there yet. Biotechnol. Adv. 2022, 58, 107885. [Google Scholar] [CrossRef]
- Hammerschmidt, N.; Tscheliessnig, A.; Sommer, R.; Helk, B.; Jungbauer, A. Economics of recombinant antibody production processes at various scales: Industry-standard compared to continuous precipitation. Biotechnol. J. 2014, 9, 766–775. [Google Scholar] [CrossRef]
- Cichonski, J.; Chrzanowski, G. Microalgae as a Source of Valuable Phenolic Compounds and Carotenoids. Molecules 2022, 27, 8852. [Google Scholar] [CrossRef] [PubMed]
- Rico, M.; López, A.; Santana-Casiano, J.M.; Gonzàlez, A.G.; Gonzàlez-Dàvila, M. Variability of the phenolic profile in the diatom Phaeodactylum tricornutum growing under copper and iron stress. Limnol. Oceanogr. 2013, 58, 144–152. [Google Scholar] [CrossRef]
- Del Mondo, A.; Sansone, C.; Brunet, C. Insights into the biosynthesis pathway of phenolic compounds in microalgae. Comput. Struct. Biotechnol. J. 2022, 20, 1901–1913. [Google Scholar] [CrossRef] [PubMed]
- Bhuvana, P.; Sangeetha, P.; Anuradha, V.; Ali, M.S. Spectral characterization of bioactive compounds from microalgae: N. oculata and C. vulgaris. Biocatal. Agric. Biotechnol. 2019, 19, 101094. [Google Scholar] [CrossRef]
- Tripathi, U.; Ramachandra Rao, S.; Ravishankar, G.A. Biotransformation of phenylpropanoid compounds to vanilla flavor metabolites in cultures of Haematococcus pluvialis. Process Biochem. 2002, 38, 419–426. [Google Scholar] [CrossRef]
- Liyanage, N.S.; Awwad, F.; Gonçalves dos Santos, K.C.; Jayawardena, T.U.; Mérindol, N.; Desgagné-Penix, I. Navigating Amaryllidaceae alkaloids: Bridging gaps and charting biosynthetic territories. J. Exp. Bot. 2024, erae187. [Google Scholar] [CrossRef]
- Desgagné-Penix, I. Biosynthesis of alkaloids in Amaryllidaceae plants: A review. Phytochem. Rev. 2021, 20, 409–431. [Google Scholar] [CrossRef]
- Tzin, V.; Galili, G. New Insights into the Shikimate and Aromatic Amino Acids Biosynthesis Pathways in Plants. Mol. Plant 2010, 3, 956–972. [Google Scholar] [CrossRef]
- Yang, W.; Tang, H.; Ni, J.; Wu, Q.; Hua, D.; Tao, F.; Xu, P. Characterization of two Streptomyces enzymes that convert ferulic acid to vanillin. PLoS ONE 2013, 8, e67339. [Google Scholar] [CrossRef]
- Ryu, J.-Y.; Seo, J.; Park, S.; Ahn, J.-H.; Chong, Y.; Sadowsky, M.J.; Hur, H.-G. Characterization of an Isoeugenol Monooxygenase (Iem) from Pseudomonas nitroreducens Jin1 That Transforms Isoeugenol to Vanillin. Biosci. Biotechnol. Biochem. 2013, 77, 289–294. [Google Scholar] [CrossRef]
- Jeong, B.-r.; Jang, J.; Jin, E. Genome engineering via gene editing technologies in microalgae. Bioresour. Technol. 2023, 373, 128701. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.K.; Das, A.; Kumari, R.; Kajla, S. Recent progress and challenges in CRISPR-Cas9 engineered algae and cyanobacteria. Algal Res. 2023, 71, 103068. [Google Scholar] [CrossRef]
- Slattery, S.S.; Diamond, A.; Wang, H.; Therrien, J.A.; Lant, J.T.; Jazey, T.; Lee, K.; Klassen, Z.; Desgagné-Penix, I.; Karas, B.J.; et al. An Expanded Plasmid-Based Genetic Toolbox Enables Cas9 Genome Editing and Stable Maintenance of Synthetic Pathways in Phaeodactylum tricornutum. ACS Synth. Biol. 2018, 7, 328–338. [Google Scholar] [CrossRef]
- Diamond, A.; Diaz-Garza, A.M.; Li, J.; Slattery, S.S.; Merindol, N.; Fantino, E.; Meddeb-Mouelhi, F.; Karas, B.J.; Barnabé, S.; Desgagné-Penix, I. Instability of extrachromosomal DNA transformed into the diatom Phaeodactylum tricornutum. Algal Res. 2023, 70, 102998. [Google Scholar] [CrossRef]
- Roig-Merino, A.; Urban, M.; Bozza, M.; Peterson, J.D.; Bullen, L.; Büchler-Schäff, M.; Stäble, S.; van der Hoeven, F.; Müller-Decker, K.; McKay, T.R.; et al. An episomal DNA vector platform for the persistent genetic modification of pluripotent stem cells and their differentiated progeny. Stem Cell Rep. 2022, 17, 143–158. [Google Scholar] [CrossRef]
- Wade-Martins, R.; James, M.R.; Frampton, J. Long-term stability of large insert genomic DNA episomal shuttle vectors in human cells. Nucleic Acids Res. 1999, 27, 1674–1682. [Google Scholar] [CrossRef]
- Chen, Y.; Partow, S.; Scalcinati, G.; Siewers, V.; Nielsen, J. Enhancing the copy number of episomal plasmids in Saccharomyces cerevisiae for improved protein production. FEMS Yeast Res. 2012, 12, 598–607. [Google Scholar] [CrossRef]
- Suttangkakul, A.; Sirikhachornkit, A.; Juntawong, P.; Puangtame, W.; Chomtong, T.; Srifa, S.; Sathitnaitham, S.; Dumrongthawatchai, W.; Jariyachawalid, K.; Vuttipongchaikij, S. Evaluation of strategies for improving the transgene expression in an oleaginous microalga Scenedesmus acutus. BMC Biotechnol. 2019, 19, 4. [Google Scholar] [CrossRef]
- Cagney, M.H.; O’Neill, E.C. Strategies for producing high value small molecules in microalgae. Plant Physiol. Biochem. 2024, 214, 108942. [Google Scholar] [CrossRef]
- Einhaus, A.; Baier, T.; Kruse, O. Molecular design of microalgae as sustainable cell factories. Trends Biotechnol. 2024, 42, 728–738. [Google Scholar] [CrossRef]
- Naduthodi, M.I.S.; Claassens, N.J.; D’Adamo, S.; van der Oost, J.; Barbosa, M.J. Synthetic Biology Approaches To Enhance Microalgal Productivity. Trends Biotechnol. 2021, 39, 1019–1036. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Okada, Y.; Yoshida, T.; Nagasawa, T. Vanillin production using Escherichia coli cells over-expressing isoeugenol monooxygenase of Pseudomonas putida. Biotechnol. Lett. 2008, 30, 665–670. [Google Scholar] [CrossRef]
- Zhao, L.; Xie, Y.; Chen, L.; Xu, X.; Zhao, C.X.; Cheng, F. Efficient biotransformation of isoeugenol to vanillin in recombinant strains of Escherichia coli by using engineered isoeugenol monooxygenase and sol-gel chitosan membrane. Process Biochem. 2018, 71, 76–81. [Google Scholar] [CrossRef]
- Luziatelli, F.; Brunetti, L.; Ficca, A.G.; Ruzzi, M. Maximizing the Efficiency of Vanillin Production by Biocatalyst Enhancement and Process Optimization. Front. Bioeng. Biotechnol. 2019, 7, 279. [Google Scholar] [CrossRef] [PubMed]
- Sadler, J.C.; Wallace, S. Microbial synthesis of vanillin from waste poly(ethylene terephthalate). Green Chem. 2021, 23, 4665–4672. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Li, C.; Kim, J.-E.; Lee, S.-H.; Yoon, J.-Y.; Choi, M.-S.; Seo, W.-T.; Yang, J.-K.; Kim, J.-Y.; Kim, S.-W. Production of Vanillin by Metabolically Engineered Escherichia coli. Biotechnol. Lett. 2005, 27, 1829–1832. [Google Scholar] [CrossRef]
- Manfrão-Netto, J.H.C.; Lund, F.; Muratovska, N.; Larsson, E.M.; Parachin, N.S.; Carlquist, M. Metabolic engineering of Pseudomonas putida for production of vanillylamine from lignin-derived substrates. Microb. Biotechnol. 2021, 14, 2448–2462. [Google Scholar] [CrossRef]
- Fleige, C.; Meyer, F.; Steinbüchel, A. Metabolic Engineering of the Actinomycete Amycolatopsis sp. Strain ATCC 39116 towards Enhanced Production of Natural Vanillin. Appl. Environ. Microbiol. 2016, 82, 3410–3419. [Google Scholar] [CrossRef]


| Homologues of Vanillin biosynthesis Enzymes | Conserved Domains Database | Function | Component | Process | Target Biochemical Mechanisms |
|---|---|---|---|---|---|
| Putative Vanillin synthetase | 214853, 425470, 239068, 197621. | Enables cysteine-type endopeptidase activity | Extracellular space and lysosome | Involved in protein catabolic process | Retro-aldol-type reaction [4] |
| Putative Feruloyl-CoA synthetase | 341284; | Enables acyl-activating enzyme activity | NI | Catalyze the ATP-dependent acylation of fatty acids in a two-step reaction | CoA-dependent non-β-oxidation reaction [100] |
| Putative Enoyl-CoA Aldolase/hydratase | 474030 119339 | Enables enoyl-CoA hydratase activity | Mitochondrion | Involved in fatty acid beta-oxidation | CoA-dependent non-β-oxidation reaction [100] |
| Putative Isoeugenol Monooxygenase | 442887 | Enables carotenoid dioxygenase activity | NI | Involved in carotene catabolic process | Oxidative cleavage [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tazon, A.W.; Awwad, F.; Meddeb-Mouelhi, F.; Desgagné-Penix, I. Biotechnological Advances in Vanillin Production: From Natural Vanilla to Metabolic Engineering Platforms. BioChem 2024, 4, 323-349. https://doi.org/10.3390/biochem4040017
Tazon AW, Awwad F, Meddeb-Mouelhi F, Desgagné-Penix I. Biotechnological Advances in Vanillin Production: From Natural Vanilla to Metabolic Engineering Platforms. BioChem. 2024; 4(4):323-349. https://doi.org/10.3390/biochem4040017
Chicago/Turabian StyleTazon, Arnold William, Fatima Awwad, Fatma Meddeb-Mouelhi, and Isabel Desgagné-Penix. 2024. "Biotechnological Advances in Vanillin Production: From Natural Vanilla to Metabolic Engineering Platforms" BioChem 4, no. 4: 323-349. https://doi.org/10.3390/biochem4040017
APA StyleTazon, A. W., Awwad, F., Meddeb-Mouelhi, F., & Desgagné-Penix, I. (2024). Biotechnological Advances in Vanillin Production: From Natural Vanilla to Metabolic Engineering Platforms. BioChem, 4(4), 323-349. https://doi.org/10.3390/biochem4040017







