The Future Is Bright for Polyoxometalates
Abstract
:1. Introduction
2. Polyoxometalates against Emerging Health Pollutants
3. Antibacterial Activity of Polyoxometalates
Mechanisms of Action of Polyoxometalates against Bacteria
4. Anticancer Activity of Polyoxometalates
Mechanisms of Action of Polyoxometalates against Cancer Cells
5. Antiviral Activity of Polyoxometalates
Mechanisms of Action of Polyoxometalates against Viral Infection
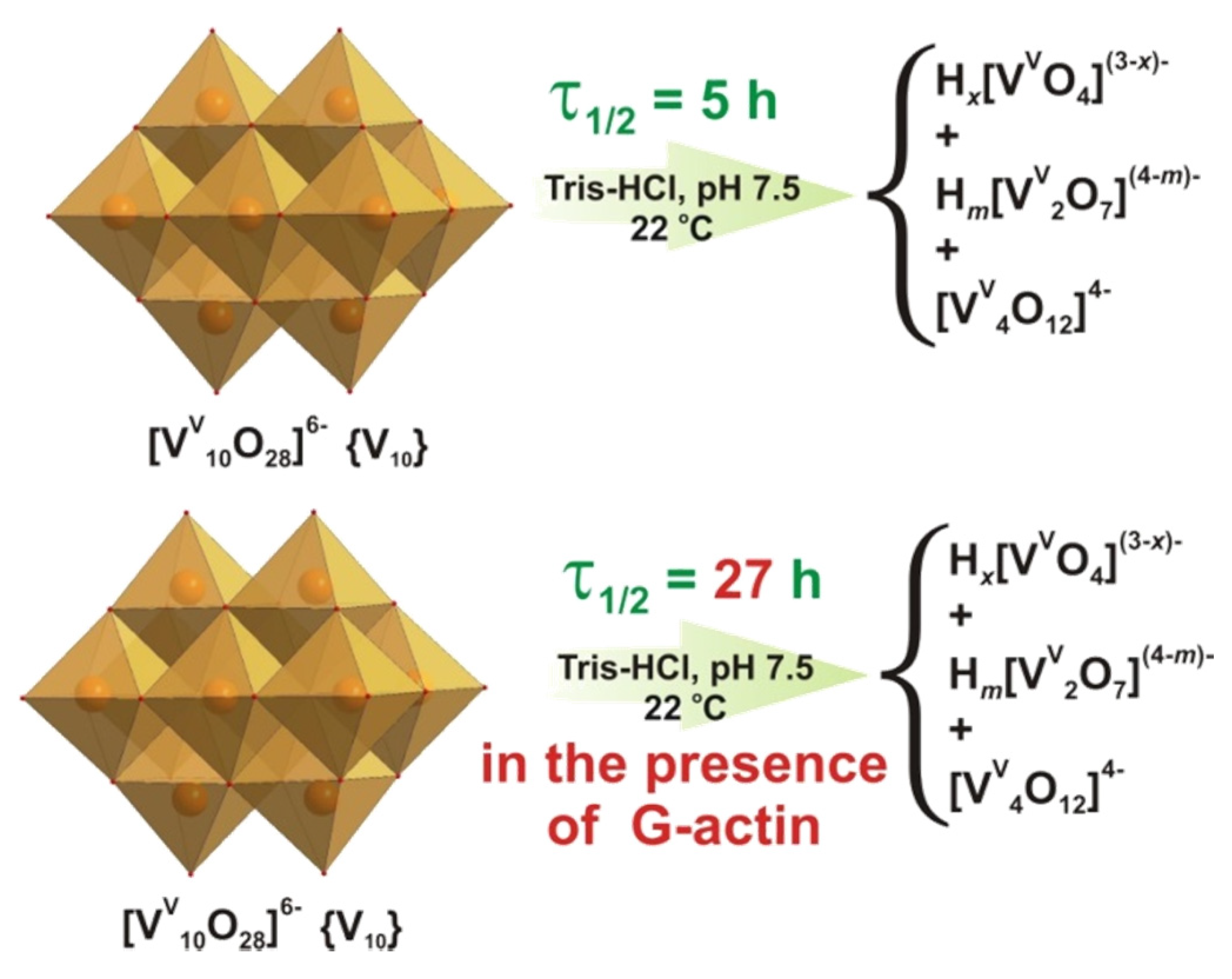
6. POMs Stability and Speciation in Solution
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A549 | Human lung cancer cells |
| ALP | Alkaline phosphatase |
| As4W40 | [KAs4W40O140]27− |
| ATPase | Adenosine triphosphatase |
| BWCN | (Himi)2[Bi2W20O66(OH)4CO2(H2O)6Na4 (H2O)14]∙17H2O |
| BW9VIW2VMn | Ce2H3[BW9VIW2VMn(H2O)O39] |
| Ca2+-ATPase | Calcium adenosine triphosphatase |
| CTS-Ca3V10O28 | Chitosan-Ca3V10O28(NH4)6 |
| Cu3(PW9O34)2 | [Cu3(PW9O34)2]12− |
| gp120 | Glycoprotein expressed by HIV |
| HCB | Hepatitis B virus |
| HCMV | Human cytomegalovirus |
| HCV | Hepatitis C virus |
| HDAC | Histone deacetylase |
| HeLa | Human cervical cancer cells |
| Hep-2 | Human Larynx carcinoma cell line |
| HepG2 | Human hepato-cellular carcinoma |
| HIV | Human immunodeficiency virus |
| HUVEC | Human umbilical vein endothelial cells |
| IC50 | Half inhibitory concentration |
| K-562 | Human myelogenous leukemia |
| MCF-7 | Human breast cancer cells |
| MDA-MB-231 | Human breast adenocarcinoma cell line |
| MDCK | Madin–Darby canine kidney |
| mecA | Gene that codes for PBP2′ |
| MnV11 | K5MnV11O33∙10H2O |
| MnV13 | K7MnV13O38∙18H2O |
| Na-lipidP5W29 | [(C16H33)2NCONH(CH2)3SiNaP5W29O110] |
| Nb10 | [Nb10O28]6− |
| POMos | Polyoxomolybdates |
| POMs | Polyoxometalates |
| PONbs | Polyoxoniobates |
| POTs | Polyoxotungstates |
| POVs | Polyoxovanadates |
| PVA/PEI | poly(vinylalcohol)/polyethylenimine |
| PW11 | [PW11O39]7− |
| P2W18 | K6[P2W18O62]∙14H2O |
| P5W29 | [NaP5W29O107]14− |
| P5W30 | [NaP5W30O110]14− |
| PW10Ti2 | [PW10Ti2O40]7− |
| S180 | Murine sarcoma cells |
| SARS-V | Severe acute respiratory syndrome virus |
| SARS-CoV | Severe acute respiratory syndrome coronavirus |
| SERCA | Sarco(endo)plasmatic membrane calcium ATPase |
| SiW9Nb3O40 | Cs2K4Na[SiW9Nb3O40] |
| SiVW11 | [SiVW11O40]7− |
| Sb9W21 | [KSb9W21O86]18− |
| SR | Survival rate |
| TeW6 | [TeW6O24]6− |
| U-87 | Human brain-like glioblastoma cells |
| U251 | Human malignant glioma cells |
| VRSA | Vancomycin-resistant Staphylococcus aureus |
| V10 | [V10O28]6− |
| V18O42 | [V18O42(H2O)]12− |
References
- Bijelic, A.; Aureliano, M.; Rompel, A. The antibacterial activity of polyoxometalates: Structures, antibiotic effects and future perspectives. Chem. Commun. 2018, 54, 1153–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bijelic, A.; Aureliano, M.; Rompel, A. Polyoxometalates as Potential Next-Generation Metallodrugs in the Combat against Cancer. Angew. Chem. Int. Ed. 2019, 58, 2980–2999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, Y. Hetero and lacunary polyoxovanadate chemistry: Synthesis, reactivity and structural aspects. Coord. Chem. Rev. 2011, 255, 2270–2280. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Yan, S.; Wang, H.; Hu, Z.; Wan, X.; Huo, M. Aerobic oxidation of starch catalyzed by isopolyoxovanadate Na4Co(H2O)6V10O28. Carbohydr. Polym. 2015, 117, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, L.; Parida, K.M. Dramatic activities of vanadate intercalated bismuth doped LDH for solar light photocatalysis. Phys. Chem. Chem. Phys. 2014, 16, 16985–16996. [Google Scholar] [CrossRef] [PubMed]
- Bijelic, A.; Rompel, A. The use of polyoxometalates in protein crystallography—An attempt to widen a well-known bottleneck. Coord. Chem. Rev. 2015, 299, 22–38. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Wang, C.; Dong, S.; Li, Y.; Zhang, D.; Han, L.W. In vitro study on the antitumor activity of several new polyoxometalates. In Proceedings of the 2011 International Conference on Human Health and Biomedical Engineering, Jilin, China, 19–22 August 2011; pp. 594–597. [Google Scholar]
- Wang, L.; Yu, K.; Zhou, B.-B.; Su, Z.-H.; Gao, S.; Chu, L.-L.; Liu, J.-R.; Wang, L. The inhibitory effects of a new cobalt-based polyoxometalate on the growth of human cancer cells. Dalt. Trans. 2014, 43, 6070. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-T.; Zhu, C.-Y.; Wu, Z.-Y.; Jiang, M.; Yan, C.-W. Synthesis, crystal structures and anticancer activities of two decavanadate compounds. Transit. Met. Chem. 2010, 35, 597–603. [Google Scholar] [CrossRef]
- Zhang, B.; Qiu, J.; Wu, C.; Li, Y.; Liu, Z. Anti-tumor and immunomodulatory activity of iron hepta-tungsten phosphate oxygen clusters complex. Int. Immunopharmacol. 2015, 29, 293–301. [Google Scholar] [CrossRef]
- Dianat, S.; Bordbar, A.K.; Tangestaninejad, S.; Yadollahi, B.; Zarkesh-Esfahani, S.H.; Habibi, P. ctDNA binding affinity and in vitro antitumor activity of three Keggin type polyoxotungstates. J. Photochem. Photobiol. B Biol. 2013, 124, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Gao, H.; Yan, M.; Li, S.; Li, X.; Dai, Z.; Liu, S. Polyoxometalate-Based Organic-Inorganic Hybrids as Antitumor Drugs. Small 2015, 11, 2938–2945. [Google Scholar] [CrossRef]
- Bâlici, Ş.; Şuşman, S.; Rusu, D.; Nicula, G.Z.; Soriţău, O.; Rusu, M.; Biris, A.S.; Matei, H. Differentiation of stem cells into insulin-producing cells under the influence of nanostructural polyoxometalates. J. Appl. Toxicol. 2016, 36, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Fiene, A.; Li, W.; Hanck, T.; Brylev, K.A.; Fedorov, V.E.; Lecka, J.; Haider, A.; Pietzsch, H.J.; Zimmermann, H.; et al. Polyoxometalates—Potent and selective ecto-nucleotidase inhibitors. Biochem Pharmacol. 2015, 93, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.H.; Al-Oweini, R.; Haider, A.; Kortz, U.; Iqbal, J. Cytotoxicity and enzyme inhibition studies of polyoxometalates and their chitosan nanoassemblies. Toxicol. Rep. 2014, 1, 341–352. [Google Scholar]
- Iqbal, J.; Barsukova-Stuckart, M.; Ibrahim, M.; Ali, S.U.; Khan, A.A.; Kortz, U. Polyoxometalates as potent inhibitors for acetyl and butyrylcholinesterases and as potential drugs for the treatment of Alzheimer’s disease. Med. Chem. Res. 2012, 22, 1224–1228. [Google Scholar] [CrossRef]
- Stephan, H.; Kubeil, M.; Emmerling, F.; Müller, C.E. Polyoxometalates as Versatile Enzyme Inhibitors. Eur. J. Inorg. Chem. 2012, 2013, 1585–1594. [Google Scholar] [CrossRef]
- Turner, T.L.; Nguyen, V.H.; McLauchlan, C.C.; Dymon, Z.; Dorsey, B.M.; Hooker, J.D.; Jones, M. Inhibitory effects of decavanadate on several enzymes and Leishmania tarentolae In Vitro. J. Inorg. Biochem. 2012, 108, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Sanseverino, I.; Navarro-Cuenca, A.; Loos, R.; Marinov, D.; Lettieri, T. State of the Art on the Contribution of Water to Antimicrobial Resistance; JRC Technical Report; Publications Office of the European Union: Luxembourg, 2018. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Vaz-Moreira, I.; Della Giustina, S.V.; Llorca, M.; Barceló, D.; Schubert, S.; Berendonk, T.U.; Michael-Kordatou, I.; Fatta-Kassinos, D.; Martinez, J.L.; et al. Antibiotic residues in final effluents of European wastewater treatment plants and their impact on the aquatic environment. Environ. Int. 2020, 140, 105733. [Google Scholar] [CrossRef] [PubMed]
- Gomez Cortes, L.; Marinov, D.; Sanseverino, I.; Navarro-Cuenca, A.; Niegowska, M.; Porcel Rodriguez, E.; Lettieri, T. Selection of Substances for the 3rd Watch List under the Water Framework Directive; JRC Technical Report; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar] [CrossRef]
- Rhule, J.; Hill, C.; Judd, D.; Schinazi, R. Polyoxometalates in Medicine. Chem. Rev. 1998, 98, 327–358. [Google Scholar] [CrossRef] [PubMed]
- Hasenknopf, B. Polyoxometalates: Introduction to a class of inorganic compounds and their biomedical applications. Front. Biosci. 2005, 10, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamase, T. Anti-tumor, -viral, and -bacterial activities of polyoxometalates for realizing an inorganic drug. J. Mater. Chem. 2005, 15, 4773. [Google Scholar] [CrossRef]
- Sivakumar, R.; Thomas, J.; Yoon, M. Polyoxometalate-Based Molecular/Nano Composites: Advances in Environmental Remediation by Photocatalysis and Biomimetic Approaches to Solar Energy Conversion. J. Photochem. Photobiol. C 2012, 13, 277–298. [Google Scholar] [CrossRef]
- Omwoma, S.; Gore, C.T.; Ji, Y.; Hu, C.; Song, Y.F. Environmentally Benign Polyoxometalate Materials. Coord. Chem. Rev. 2015, 286, 17–29. [Google Scholar] [CrossRef]
- Lai, S.Y.; Ng, K.H.; Cheng, C.K.; Nur, H.; Nurhadi, M.; Arumugam, M. Photocatalytic Remediation of Organic Waste over Keggin-Based Polyoxometalate Materials: A Review. Chemosphere 2021, 263, 128244. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Xue, K.; Wang, J.; Yan, Y.; Peng, Y.; Yang, T.; Hu, Y.; Wang, W. Nitrogen-deficient g-C3Nx/POMs porous nanosheets with P–N heterojunctions capable of the efficient photocatalytic degradation of ciprofloxacin. Chemosphere 2020, 259, 127465. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, T.; Wang, J.; Wang, Y.; Chen, Z.; Liu, B.; Ji, H.; Wang, W.; Zhang, G.; Li, Y. Fabrication of g-C3N4/PW12/TiO2 composite with significantly enhanced photocatalytic performance under visible light. J. Alloy. Compd. 2021, 860, 157924. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, Y.; Sarakha, M.; Mailhot, G. Enhancement of the photocatalytic activity of decatungstate, W10O324−, for the oxidation of sulfasalazine/sulfapyridine in the presence of hydrogen peroxide. J. Photochem. Photobiol. A Chem. 2021, 404, 112890. [Google Scholar] [CrossRef]
- Silva, E.S.D.; Sarakha, M.; Burrows, H.D.; Wong-Wah-Chung, P. Decatungstate anion as an efficient photocatalytic species for the transformation of the pesticide 2-(1-naphthyl)acetamide in aqueous solution. J. Photochem. Photobiol. A 2017, 334, 61–73. [Google Scholar] [CrossRef]
- Li, G.; Zhang, K.; Li, C.; Gao, R.; Cheng, Y.; Hou, L.; Wang, Y. Solvent-free method to encapsulate polyoxometalate into metal-organic frameworks as efficient and recyclable photocatalyst for harmful sulfamethazine degrading in water. Appl. Catal. B Environ. 2019, 245, 753–759. [Google Scholar] [CrossRef]
- López, Y.C.; Viltres, H.; Gupta, N.K.; Acevedo-Peña, P.; Leyva, C.; Ghaffari, Y.; Gupta, A.; Kim, S.; Bae, J.; Kim, K.S. Transition metal-based metal–organic frameworks for environmental applications: A review. Env. Chem. Lett. 2021, 19, 1295–1334. [Google Scholar] [CrossRef]
- Majdafshar, M.; Piryaei, M.; Abolghasemi, M.M.; Rafiee, E. Polyoxometalate-based ionic liquid coating for solid phase microextraction of triazole pesticides in water samples. Sep. Sci. Technol. 2019, 54, 1553–1559. [Google Scholar] [CrossRef]
- Martinetto, Y.; Pegot, B.; Roch-Marchal, C.; Cottyn-Boitte, B.; Floquet, S. Designing functional polyoxometalate-based ionic liquid crystals and ionic liquids. Eur. J. Inorg. Chem. 2020, 2020, 228–247. [Google Scholar] [CrossRef] [Green Version]
- Misra, A.; Zambrzycki, C.; Kloker, G.; Kotyrba, A.; Anjass, M.H.; Castillo, I.F.; Mitchell, S.G.; Güttel, R.; Streb, C. Water Purification and Microplastics Removal Using Magnetic Polyoxometalate-Supported Ionic Liquid Phases (magPOM-SILPs). Angew. Chem. Int. Ed. 2020, 59, 1601–1605. [Google Scholar] [CrossRef] [Green Version]
- Worthington, R.J.; Melander, C. Combination approaches to combat multidrug-resistant bacteria. Trends Biotechnol. 2013, 31, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Tajima, Y.; Nagasawa, Z.; Tadano, J. A factor found in aged tungstate solution enhanced the antibacterial effect of beta-lactams on methicillin-resistant Staphylococcus aureus. Microbiol. Immunol. 1993, 37, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Raynaud, M.; Chermann, J.C.; Plata, F.; Mathé, G. Inhibitors of viruses of the leukemia and murine sarcoma group. Biological inhibitor CJMR. Comptes Rendus Hebd. Seances L’academie des Sci. Ser. D 1971, 272, 2038–2040. [Google Scholar]
- Mukherjee, H.N. Treatment of Cancer of the Intestinal Tract with a Complex Compound of Phosphotungstic Phosphomolybdic Acids and Caffeine. J. Indian Med. Assoc. 1965, 44, 477–479. [Google Scholar] [PubMed]
- Ling, L.L.; Schneider, T.; Peoples, A.J.; Spoering, A.L.; Engels, I.; Conlon, B.P.; Mueller, A.; Schäberle, T.F.; Hughes, D.E.; Epstein, S.; et al. A new antibiotic kills pathogens without detectable resistance. Nature 2015, 517, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Fiers, W.D.; Craighead, M.; Singh, I. Teixobactin and Its Analogues: A New Hope in Antibiotic Discovery. ACS Infect. Dis. 2017, 3, 688–690. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Farzana, R.; Iqra, P.; Hunaiza, T. Antioxidant and antimicrobial effects of polyoxometalates. Microbiol. Curr. Res. 2018, 2, 7–11. [Google Scholar]
- Misra, A.; Franco Castillo, I.; Müller, D.P.; González, C.; Eyssautier-Chuine, S.; Ziegler, A.; de laFuente, J.M.; Mitchell, S.G.; Streb, C. Polyoxometalate-Ionic Liquids (POM-ILs) as Anticorrosion and Antibacterial Coatings for Natural Stones. Angew. Chem. Int. Ed. 2018, 57, 14926–14931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, M.R.; Colliard, I.; Rahman, T.; Miyaishi, T.C.; Harper, B.; Harper, S.; Nyman, M. Hybrid Polyoxometalate Salt Adhesion by Butyltin Functionalization. ACS Appl Mater Interfaces 2021, 13, 19497–19506. [Google Scholar] [CrossRef] [PubMed]
- Rajkowska, K.; Koziróg, A.; Otlewska, A.; Piotrowska, M.; Atrián-Blasco, E.; Franco-Castillo, I.; Mitchell, S.G. Antifungal Activity of Polyoxometalate-Ionic Liquids on Historical Brick. Molecules 2020, 25, 5663. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Liu, Y.; Li, M.; Liu, L.; Yu, Q.; Wu, L. Amelioration of enteric dysbiosis by polyoxotungstates in mice gut. J. Inorg. Biochem. 2022, 226, 111654. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Yu, Q.; Liu, Y.; Yin, P. Bacterial hyperpolarization modulated by polyoxometalates for solutions of antibiotic resistance. J. Inorg. Biochem. 2021, 220, 111463. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, K.; Li, M.; Hu, C.; Yin, P. Sustained release of Ag+ confined inside polyoxometalates for long-lasting bacterial resistance. Chem. Commun. 2020, 56, 5287–5290. [Google Scholar] [CrossRef]
- Gonzalez, A.; Galvez, N.; Clemente-Leon, M.; Dominguez-Vera, J.M. Electrochromic polyoxometalate material as a sensor of bacterial activity. Chem. Commun. 2015, 51, 10119–10122. [Google Scholar] [CrossRef]
- Chen, S.; Wu, G.; Long, D.; Liu, Y. Preparation, characterization and antibacterial activity of chitosan-Ca3V10O28 complex membrane. Carbohydr. Polym. 2006, 64, 92–97. [Google Scholar] [CrossRef]
- Yamase, T.; Fukuda, N.; Tajima, Y. Synergistic effect of polyoxotungstates in combination with beta-lactam antibiotics on antibacterial activity against methicillin-resistant Staphylococcus aureus. Biol. Pharm. Bull. 1996, 19, 459–465. [Google Scholar] [CrossRef] [Green Version]
- Balici, S.; Niculae, M.; Pall, E.; Rusu, M.; Rusu, D.; Matei, H. Antibiotic-Like Behaviour of Polyoxometalates. In vitro comparative study: Seven polyoxotungstates-nine antibiotics against gram-positive and gram-negative bacteria. Rev. Chim. 2016, 67, 485–490. [Google Scholar]
- Fang, Y.; Xing, C.; Liu, J.; Zhang, Y.; Li, M.; Han, Q. Supermolecular film crosslinked by polyoxometalate and chitosan with superior antimicrobial effect. Int. J. Biol. Macromol. 2020, 154, 732–738. [Google Scholar] [CrossRef]
- Fukuda, N.; Yamase, T.; Tajima, Y. Inhibitory effect of polyoxotungstates on the production of penicillin- binding proteins and β-lactamase against methicillin-resistant Staphylococcus aureus. Biol. Pharm. Bull. 1999, 22, 463–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gumerova, N.; Al-Sayed, E.; Krivosudský, L.; Čipčić-Paljetak, H.; Verbanac, D.; Rompel, A. Antibacterial Activity of Polyoxometalates Against Moraxella catarrhalis. Front. Chem. 2018, 6, 336–345. [Google Scholar] [CrossRef]
- Inoue, M.; Segawa, K.; Matsunaga, S.; Matsumoto, N.; Oda, M.; Yamase, T. Antibacterial activity of highly negative charged polyoxotungstates, K27[KAs4W40O140] and K18[KSb9W21O86], and Keggin-structural polyoxotungstates against Helicobacter pylori. J. Inorg. Biochem. 2005, 99, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Suzuki, T.; Fujita, Y.; Oda, M.; Matsumoto, N.; Yamase, T. Enhancement of antibacterial activity of beta-lactam antibiotics by P2W18O62]6-, [SiMo12O40]4-, and [PTi2W10O40]7− against methicillin-resistant and vancomycin-resistant Staphylococcus aureus. J. Inorg. Biochem. 2006, 100, 1225–1233. [Google Scholar] [CrossRef]
- Samart, N.; Arhouma, Z.; Kumar, S.; Murakami, H.A.; Crick, D.C.; Crans, D.C. Decavanadate inhibits microbacterial growth more potently than other oxovanadates. Front. Chem. 2018, 6, 519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Missina, J.M.; Gavinho, B.; Postal, K.; Santana, F.S.; Valdameri, G.; De Souza, E.M.; Hughes, D.L.; Ramirez, M.I.; Soares, J.F.; Nunes, G.G. Effects of Decavanadate Salts with Organic and Inorganic Cations on Escherichia coli, Giardia intestinalis, and Vero Cells. Inorg. Chem. 2018, 57, 11930–11941. [Google Scholar] [CrossRef]
- Marques-Da-Silva, D.; Fraqueza, G.; Lagoa, R.; Vannathan, A.A.; Mal, S.S.; Aureliano, M. Polyoxovanadate inhibition of: Escherichia coli growth shows a reverse correlation with Ca2+-ATPase inhibition. New J. Chem. 2019, 43, 17577–17587. [Google Scholar] [CrossRef]
- Fukuda, N.; Yamase, T. In Vitro Antibacterial Activity of Vanadate and Vanadyl Compounds against Streptococcus pneumoniae. Biol. Pharm. Bull. 1997, 20, 927–930. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Bassil, B.S.; Lin, Z.; Haider, A.; Alfaro-Espinoza, G.; Ullrich, M.S.; Silvestru, C.; Kortz, U. Organoantimony(III)-Containing Tungstoarsenates(III): From Controlled Assembly to Biological Activity. Chem. Eur. J. 2015, 21, 15600–15606. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Lin, Z.; Bassil, B.S.; Alfaro-Espinoza, G.; Ullrich, M.S.; Li, M.X.; Silvestru, C.; Kortz, U. Tetra-Antimony(III)-Bridged 18-Tungsto-2-Arsenates(V), [(LSbIII)4(A-α-AsVW9O34)2]10– (L = Ph, OH): Turning Bioactivity On and Off by Ligand Substitution. Inorg. Chem. 2016, 55, 3718–3720. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zou, Y.L.; Zhang, L.; Liu, J.X.; Song, C.Y.; Chai, D.F.; Gao, G.G.; Qiu, Y.F. Polyoxometalate cobalt–gatifloxacin complex with DNA binding and antibacterial activity. J. Coord. Chem. 2014, 67, 2257–2270. [Google Scholar] [CrossRef]
- Yang, F.-C.; Wu, K.-H.; Lin, W.-P.; Hu, M.-K. Preparation and antibacterial efficacy of bamboo charcoal/polyoxometalate biological protective material. Microporous Mesoporous Mater. 2009, 118, 467–472. [Google Scholar] [CrossRef]
- Wu, K.H.; Yu, P.Y.; Yang, C.C.; Wang, G.P.; Chao, C.M. Preparation and characterization of polyoxometalate-modified poly(vinyl alcohol)/polyethyleneimine hybrids as a chemical and biological self-detoxifying material. Polym. Degrad. Stab. 2009, 94, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.-L.; Kremer, L.; Herrmann, S.; Mitchell, S.G.; Bondarenko, O.M.; Kahru, A.; Streb, C. Antimicrobial Activity of Polyoxometalate Ionic Liquids against Clinically Relevant Pathogens. ChemPlusChem 2017, 82, 867–871. [Google Scholar] [CrossRef]
- Fraqueza, G.; Ohlin, C.A.; Casey, W.H.; Aureliano, M. Sarcoplasmic reticulum calcium ATPase interactions with decaniobate, decavanadate, vanadate, tungstate and molybdate. J. Inorg. Biochem. 2012, 107, 82–89. [Google Scholar] [CrossRef]
- Fraqueza, G.; Carvalho, L.A.; de Marques, E.B.; Marques, M.P.M.; Maia, L.; Ohlin, C.A.; Casey, W.H.; Aureliano, M. Decavanadate, decaniobate, tungstate and molybdate interactions with sarcoplasmic reticulum Ca2+-ATPase: Quercetin prevents cysteine oxidation by vanadate but does not reverse ATPase inhibition. Dalt. Trans. 2012, 41, 12749. [Google Scholar] [CrossRef] [Green Version]
- Gumerova, N.; Krivosudský, L.; Fraqueza, G.; Breibeck, J.; Al-Sayed, E.; Tanuhadi, E.; Bijelic, A.; Fuentes, J.; Aureliano, M.; Rompel, A. The P-type ATPase inhibiting potential of polyoxotungstates. Metallomics 2018, 10, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Fraqueza, G.; Fuentes, J.; Krivosudský, L.; Dutta, S.; Mal, S.S.; Roller, A.; Giester, G.; Rompel, A.; Aureliano, M. Inhibition of Na+/K+- and Ca2+-ATPase activities by phosphotetradecavanadate. J. Inorg. Biochem. 2019, 197, 110700. [Google Scholar] [CrossRef]
- Fang, Y.; Xing, C.; Zhan, S.; Zhao, M.; Li, M.; Liu, H. A polyoxometalate-modified magnetic nanocomposite: A promising antibacterial material for water treatment. J. Mater. Chem. B 2019, 7, 1933–1944. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhou, F.; Yue, H.; Hua, J.; Ma, P. A nano-linear zinc-substituted phosphomolybdate with reactive oxygen species catalytic ability and antibacterial activity. J. Mol. Struct. 2019, 1198, 126865. [Google Scholar] [CrossRef]
- Missina, J.M.; Leme, L.B.P.; Postal, K.; Santana, F.S.; Hughes, D.L.; de Sá, E.L.; Ribeiro, R.R.; Nunes, G.G. Accessing decavanadate chemistry with tris(hydroxymethyl)aminomethane, and evaluation of methylene blue bleaching. Polyhedron 2020, 180, 114414. [Google Scholar] [CrossRef]
- Harduin-Lepers, A.; Mollicone, R.; Delannoy, P.; Oriol, R. The animal sialyltransferases and sialyltransferase-related genes: A phylogenetic approach. Glycobiology 2005, 15, 805–817. [Google Scholar] [CrossRef]
- Negishi, M.; Pedersen, L.G.; Petrotchenko, E.; Shevtsov, S.; Gorokhov, A.; Kakuta, Y.; Pedersen, L.C. Structure and function of sulfotransferases. Arch. Biochem. Biophys. 2001, 390, 149–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seko, A.; Yamase, T.; Yamashita, K. Polyoxometalates as effective inhibitors for sialyl- and sulfotransferases. J. Inorg. Biochem. 2009, 103, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Tan, R.; Cao, J.; Yang, Y.; Kong, C.; Du, J.; Zhu, S.; Zhang, Y.; Lu, J.; Huang, B.; et al. Discovery of polyoxometalate-based HDAC inhibitors with profound anticancer activity in vitro and in vivo. Eur. J. Med. Chem. 2011, 46, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Raza, R.; Matin, A.; Sarwar, S.; Barsukova-Stuckart, M.; Ibrahim, M.; Kortz, U.; Iqbal, J. Polyoxometalates as potent and selective inhibitors of alkaline phosphatases with profound anticancer and amoebicidal activities. Dalt. Trans. 2012, 41, 14329. [Google Scholar] [CrossRef] [PubMed]
- León, I.E.; Porro, V.; Astrada, S.; Egusquiza, M.G.; Cabello, C.I.; Bollati-Fogolin, M.; Etchevery, S.B. Polyoxometalates as antitumor agents: Bioactivity of a new polyoxometalate with copper on a human osteosarcoma model. Chem. Biol. Interact. 2014, 222, 87–96. [Google Scholar] [CrossRef]
- She, S.; Bian, S.; Huo, R.; Chen, K.; Huang, Z.; Zhang, J.; Hao, J.; Wei, Y. Degradable Organically-Derivatized Polyoxometalate with Enhanced Activity against Glioblastoma Cell Line. Sci. Rep. 2016, 6, 33529. [Google Scholar] [CrossRef] [Green Version]
- Cheng, M.; Li, N.; Wang, N.; Hu, K.; Xiao, Z.; Wu, P.; Wei, Y. Synthesis, structure and antitumor studies of a novel decavanadate complex with a wavelike two-dimensional network. Polyhedron 2018, 155, 313–319. [Google Scholar] [CrossRef]
- Qi, W.; Zhang, B.; Qi, Y.; Guo, S.; Tian, R.; Sun, J.; Zhao, M. The Anti-Proliferation Activity and Mechanism of Action of K12[V18O42(H2O)].6H2O on Breast Cancer Cell Lines. Molecules 2018, 22, 1535. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Gan, H.; Zhao, Y.; Li, W.; Wu, Y.; Yan, X.; Wang, Y.; Li, J.; Li, J.; Wang, X. Self-Assembly and Antitumor Activity of a Polyoxovanadate-Based Coordination Nanocage. Chem. A Eur. J. 2019, 25, 15326–15332. [Google Scholar] [CrossRef]
- Sun, T.; Cui, W.; Yan, M.; Qin, G.; Guo, W.; Gu, H.; Liu, S.; Wu, Q. Target Delivery of a Novel Antitumor Organoplatinum(IV)-Substituted Polyoxometalate Complex for Safer and More Effective Colorectal Cancer Therapy in vivo. Adv. Mater. 2016, 28, 7397–7404. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Yang, Y.; Liu, S.; Lu, J.; Huang, B.; Zhang, Y. HDAC inhibitor PAC-320 induces G2/M cell cycle arrest and apoptosis in human prostate cancer. Oncotarget 2018, 9, 512–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, Y.; Zhang, C.; Gu, Z.; Du, J.; Guo, Z.; Dong, X.; Xie, J.; Zhang, G.; Liu, X.; Zhao, Y. Polyoxometalate-Based Radiosensitization Platform for Treating Hypoxic Tumors by Attenuating Radioresistance and Enhancing Radiation Response. ACS Nano 2017, 11, 7164–7176. [Google Scholar] [CrossRef] [PubMed]
- Boulmier, A.; Feng, X.; Oms, O.; Mialane, P.; Rivière, E.; Shin, C.J.; Yao, J.; Kubo, T.; Furuta, T.; Oldfield, E.; et al. Anticancer Activity of Polyoxometalate-Bisphosphonate Complexes: Synthesis, Characterization, in vitro and in vivo Results. Inorg. Chem. 2017, 56, 7558–7565. [Google Scholar] [CrossRef]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; Rompel, A.; Crans, D.C. Polyoxovanadates with emerging biomedical activities. Coord. Chem. Rev. 2021, 447, 214143. [Google Scholar] [CrossRef]
- Guedes, G.; Wang, S.; Santos, H.A.; Sousa, F.L. Polyoxometalate Composites in Cancer Therapy and Diagnostics. Eur. J. Inorg. Chem. 2020, 2020, 2121–2132. [Google Scholar] [CrossRef]
- Guedes, G.; Wang, S.; Fontana, F.; Figueiredo, P.; Linden, J.; Correia, A.; Pinto, R.J.B.; Hietala, S.; Sousa, F.L.; Santos, H.A. Dual-Crosslinked Dynamic Hydrogel Incorporating {Mo-154} with pH and NIR Responsiveness for Chemo-Photothermal Therapy. Adv. Mater. 2021, 33, 2007761. [Google Scholar] [CrossRef]
- Karimian, D.; Yadollahi, B.; Mirkhani, V. Dual functional hybrid-polyoxometalate as a new approach for multidrug delivery. Microporous Mesoporous Mater. 2017, 247, 23–30. [Google Scholar] [CrossRef]
- Nienberg, C.; Garmann, C.; Gratz, A.; Bollacke, A.; Götz, C.; Jose, J. Identification of a Potent Allosteric Inhibitor of Human Protein Kinase CK2 by Bacterial Surface Display Library Screening. Pharmaceuticals 2017, 10, 6. [Google Scholar] [CrossRef] [Green Version]
- Prudent, R.; Moucadel, V.; Laudet, B.; Barette, C.; Lafanechère, L.; Hasenknopf, B.; Li, J.; Bareyt, S.; Lacote, E.; Thorimbert, S.; et al. Identification of Polyoxometalates as Nanomolar Noncompetitive Inhibitors of Protein Kinase CK2. Chem. Biol. 2008, 15, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Pimpão, C.; da Silva, I.V.; Mósca, A.F.; Pinho, J.O.; Gaspar, M.M.; Gumerova, N.I.; Rompel, A.; Aureliano, M.; Soveral, G. The aquaporin-3-inhibiting potential of polyoxotungstates. Int. J. Mol. Sci. 2020, 21, 2467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soveral, G.; Casini, A. Aquaporin modulators: A patent review (2010–2015). Expert Opin. Ther. Pat. 2016, 27, 49–62. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, C.; Fraqueza, G.; Carabineiro, S.A.C.; Aureliano, M. The Ca2+-ATPase inhibition potential of gold (I,III) compounds. Inorganics 2020, 8, 49. [Google Scholar] [CrossRef]
- Berrocal, M.; Cordoba-Granados, J.J.; Carabineiro, S.A.C.; Gutierrez-Merino, C.; Aureliano, M.; Mata, A.M. Gold Compounds Inhibit the Ca2+-ATPase Activity of Brain PMCA and Human Neuroblastoma SH-SY5Y Cells and Decrease Cell Viability. Metals 2021, 11, 1934. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Flütsch, A.; Schroeder, T.; Grütter, M.G.; Patzke, G.R. HIV-1 protease inhibition potential of functionalized polyoxometalates. Bioorg. Med. Chem. Lett. 2011, 21, 1162–1166. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Xiang, Y.; Wang, J.; Qi, Y.; Li, J.; Niu, J.; Zhong, J. Inhibition of hepatitis C virus infection by polyoxometalates. Antivir. Res. 2013, 100, 392–398. [Google Scholar] [CrossRef]
- Witvrouw, M.; Weigold, H.; Pannecouque, C.; Schols, D.; De Clercq, E.; Holan, G. Potent anti-HIV (type 1 and type 2) activity of polyoxometalates: Structure-activity relationship and mechanism of action. J. Med. Chem. 2000, 43, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Francese, R.; Civra, A.; Rittà, M.; Donalisio, M.; Argenziano, M.; Cavalli, R.; Mougharbel, A.; Kortz, U.; Lembo, D. Anti-zika virus activity of polyoxometalates. Antivir. Res. 2019, 163, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Xu, K.; Qi, Y.; Zhong, J.; Zhang, K.; Li, J.; Wang, E.; Wu, Z.; Kan, G.Z. Broad-spectrum antiviral property of polyoxometalate localized on a cell surface. ACS Appl. Mater. Interfaces 2014, 6, 9785–9789. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Zhang, W.; Li, B.; Zhu, Y.; Hu, Q.; Yang, Y.; Zhang, X.; Yan, H.; Zeng, Y. Inhibition of human immunodeficiency virus type 1 entry by a keggin polyoxometalate. Viruses 2018, 10, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Zhang, H.; Qi, Y.; Wang, J.; Li, J.; Niu, J. Antiviral effects of a niobium-substituted heteropolytungstate on hepatitis B virus-transgenic mice. Drug Dev. Res. 2019, 80, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qi, Y.; Ding, Y.; Wang, J.; Li, Q.; Zhang, J.; Jiang, Y.; Chi, X.; Li, J.; Niu, J. Synthesis, characterization and biological activity of a niobium-substituted-heteropolytungstate on hepatitis B virus. Bioorg. Med. Chem. Lett. 2012, 22, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Amini, E.; Kheiri, M.T.; Mehrbod, P.; Shahidi, M.; Zabihi, E. Anti-influenza Activity of a Novel Polyoxometalate Derivative (POM-4960). Int. J. Mol. Cell. Med. 2012, 1, 21–29. [Google Scholar]
- Shigeta, S.; Mori, S.; Yamase, T.; Yamamoto, N.; Yamamoto, N. Anti-RNA virus activity of polyoxometalates. Biomed. Pharmacother. 2006, 60, 211–219. [Google Scholar] [CrossRef]
- Chanh, C.T.; Dreesman, G.R.; Kennedy, R.C. Monoclonal anti-idiotypic antibody mimics the CD4 receptor and binds human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 1987, 84, 3891–3895. [Google Scholar] [CrossRef] [Green Version]
- Douglas, T.; Young, M. Host-guest encapsulation of materials by assembled virus protein cages. Nature 1998, 393, 152–155. [Google Scholar] [CrossRef]
- Yamase, T. Polyoxometalates Active against Tumors, Viruses, and Bacteria. Prog. Mol. Subcell. Biol. 2013, 54, 65–116. [Google Scholar] [CrossRef]
- Liu, J.; Mei, W.; Li, Y.; Wang, E.; Ji, L.; Tao, P. Antiviral Activity of Mixed-Valence Rare Earth Borotungstate Heteropoly Blues against Influenza Virus in Mice. Antivir. Chem. Chemother. 2000, 11, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Shao, C.; Wang, J.-P.; Yang, G.-C.; Su, Z.-M.; Hu, D.-H.; Sun, C.-C. Interactions of [Mo6O19]2− and its derivatives substituted with organic groups inhibitor with SARS-CoV 3CLpro by molecular modeling. Gaodeng Xuexiao Huaxue Xuebao/Chem. J. Chin. Univ. 2008, 29, 165–169. [Google Scholar]
- Hu, D.; Shao, C.; Guan, W.; Su, Z.; Sun, J. Studies on the interactions of Ti-containing polyoxometalates (POMs) with SARS-CoV 3CLpro by molecular modeling. J. Inorg. Biochem. 2007, 101, 89–94. [Google Scholar] [CrossRef]
- Aureliano, M.; Ohlin, C.A.; Vieira, M.O.; Marques, M.P.M.; Casey, W.H.; Batista de Carvalho, L.A.E. Characterization of decavanadate and decaniobate solutions by Raman spectroscopy. Dalton Trans. 2016, 45, 7391–7399. [Google Scholar] [CrossRef] [Green Version]
- Gumerova, N.I.; Rompel, A. Polyoxometalates in solution: Speciation under spotlight. Chem. Soc. Rev. 2020, 49, 7568–7601. [Google Scholar] [CrossRef]
- Soares, S.S.; Henao, F.; Aureliano, M.; Gutiérrez-Merino, C. Vanadate-induced necrotic death in neonatal rat cardiomyocytes through mitochondrial membrane depolarization. Chem. Res. Toxicol. 2008, 21, 607–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gândara, R.M.C.; Soares, S.S.; Martins, H.; Gutiérrez-Merino, C.; Aureliano, M. Vanadate oligomers: In vivo effects in hepatic vanadium accumulation and stress markers. J. Inorg. Biochem. 2005, 99, 1238–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; McLauchlan, C.C.; Rompel, A.; Crans, D.C. Polyoxovanadates interactions with proteins: An overview. Coord. Chem. Rev. 2022, 454, 214344. [Google Scholar] [CrossRef]
- Soares, S.S.; Gutiérrez-Merino, C.; Aureliano, M. Decavanadate induces mitochondrial membrane depolarization and inhibits oxygen consumption. J. Inorg. Biochem. 2007, 101, 789–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, S.; Manuel, M.; Tiago, T.; Duarte, R.O.; Martins, J.; Gutiérrez-Merino, C.; Moura, J.J.G.; Aureliano, M. Decavanadate interactions with actin inhibit G-actin polymerization and stabilize decameric vanadate species. J. Inorg. Biochem. 2006, 100, 1734–1743. [Google Scholar] [CrossRef] [Green Version]
- Aureliano, M. Decavanadate: A journey in a search of a role. Dalton Trans. 2009, 42, 9093–9100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aureliano, M.; Fraqueza, G.; Ohlin, C.A. Ion pumps as biological targets for decavanadate. Dalton Trans. 2013, 42, 11770–11777. [Google Scholar] [CrossRef] [PubMed]
- Crans, D.C.; Smee, J.J.; Gaidamauskas, E.; Yang, L. The chemistry and biochemistry of vanadium and the biological activities exerted by vanadium compounds. Chem. Rev. 2004, 104, 849–902. [Google Scholar] [CrossRef]
- Al-Qatati, A.; Fontes, F.L.; Barisas, B.G.; Zhang, D.; Roess, D.A.; Crans, D.C. Raft localization of Type I Fcε receptor and degranulation of RBL-2H3 cells exposed to decavanadate, a structural model for V2O5. Dalton Trans. 2013, 42, 11912–11920. [Google Scholar] [CrossRef] [PubMed]
- Aureliano, M.; Crans, D.C. Decavanadate (V10O286−) and oxovanadates: Oxometalates with many biological activities. J. Inorg. Biochem. 2009, 103, 536–546. [Google Scholar] [CrossRef]
- Sciortino, G.; Aureliano, M.; Garribba, E. Rationalizing the decavanadate(V) and oxidovanadium(IV) binding to G-actin and the competition with decaniobate(V) and ATP. Inorg. Chem. 2021, 60, 334–344. [Google Scholar] [CrossRef]
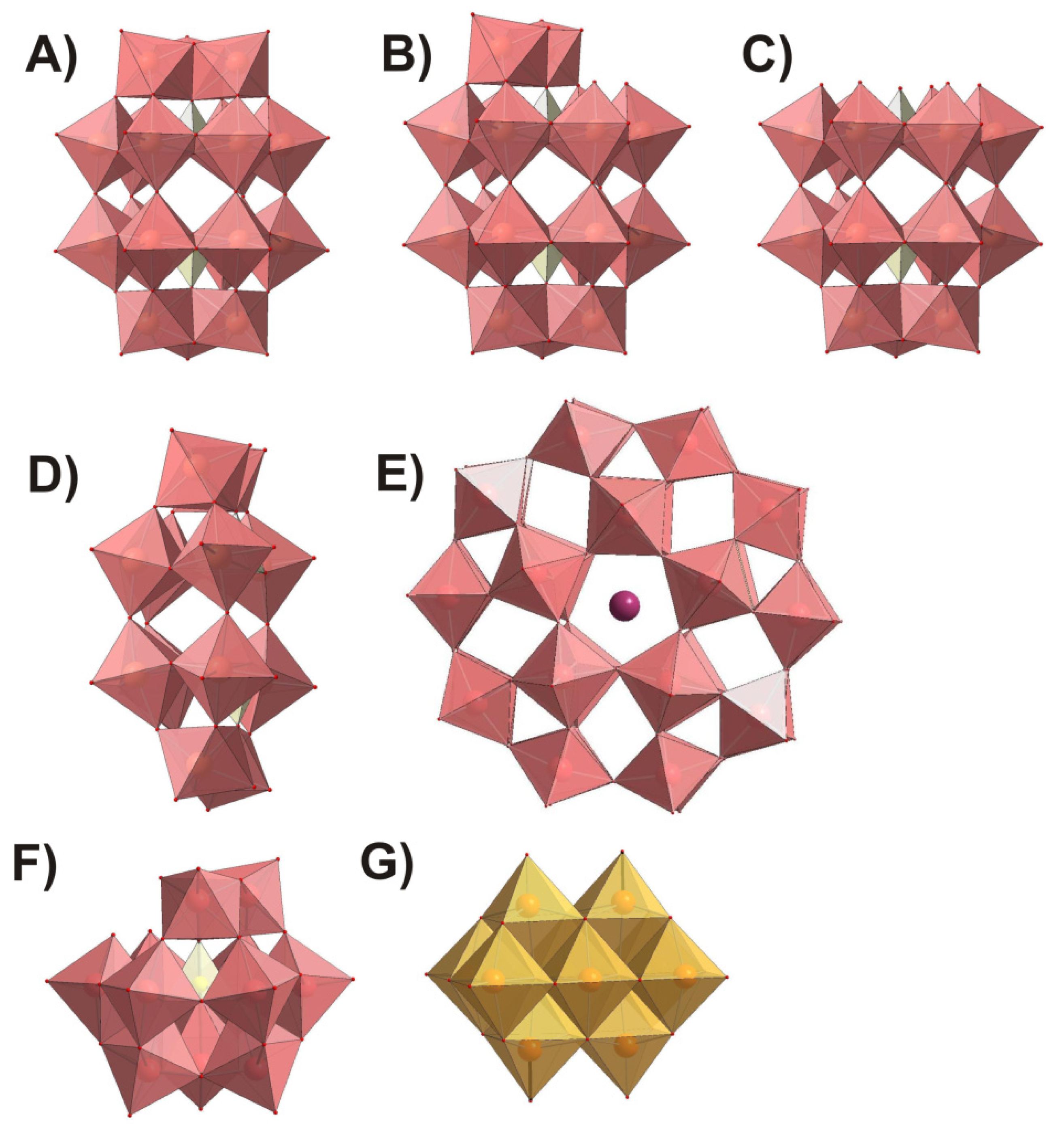
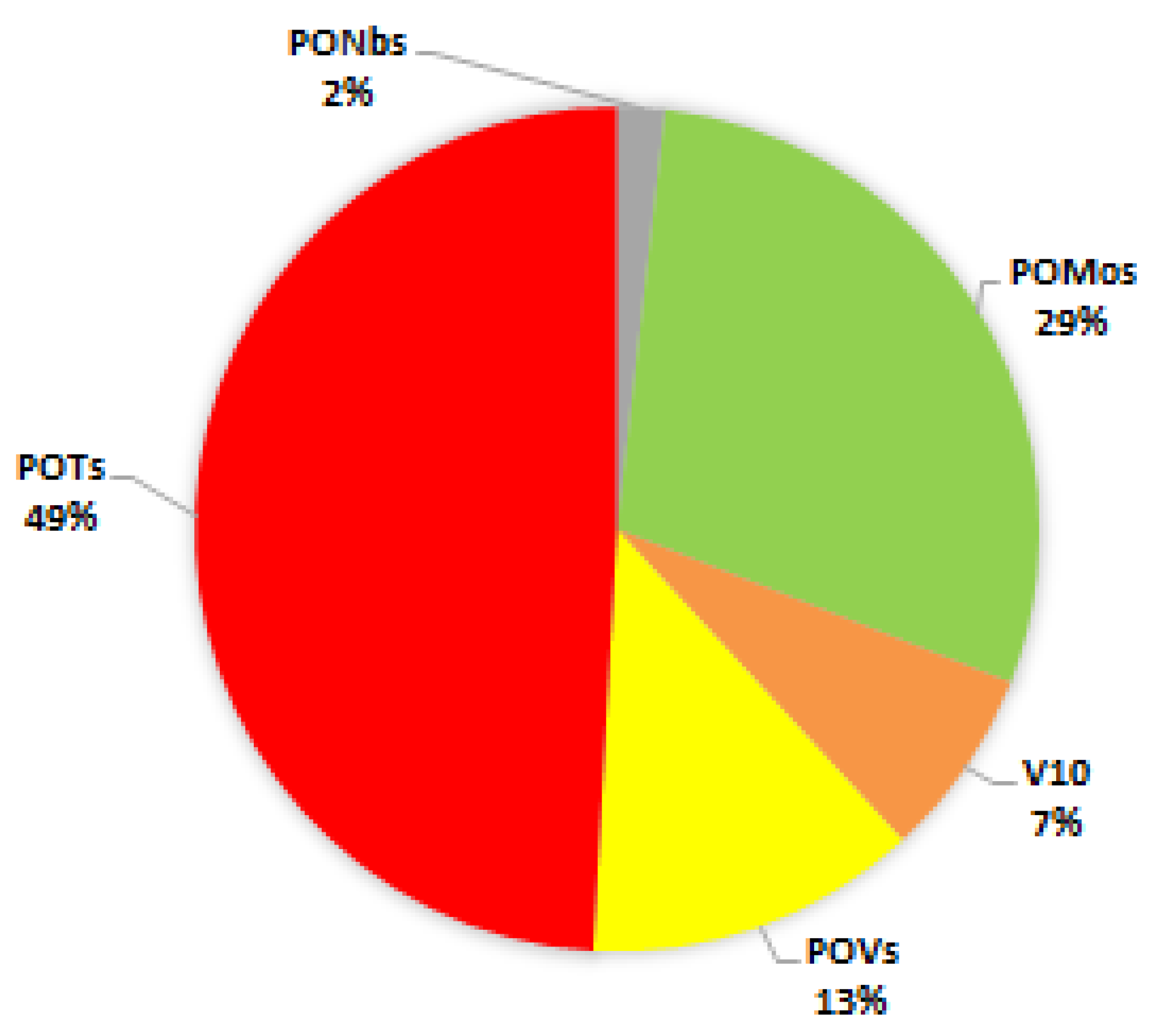
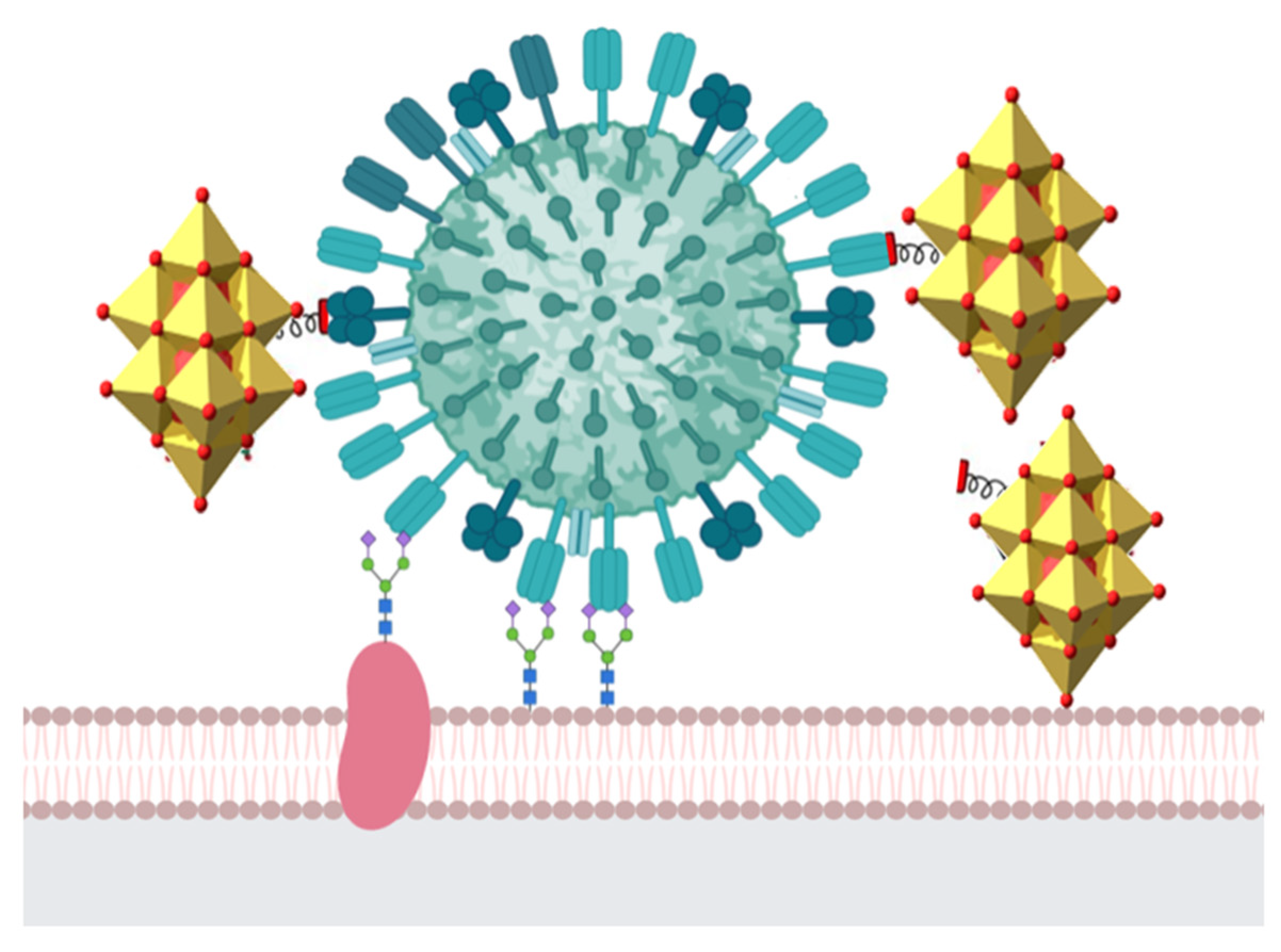
| POM/POM-Hybrid | MIC(µg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| S. aureus | E. coli | B. subtilis | P. aeruginosa | Paenibacillus sp | Vibrio sp | Ref. | |
| Polyoxovanadates | |||||||
| [V10O28]6− | 50 | 50 | - | - | - | - | [52] |
| [V4O12]4− | 8000 | [56] | |||||
| Polyoxotungstates | |||||||
| [PW12O40]3− | 3200 | [53] | |||||
| [SiW12O40]4− | 3200 | ||||||
| [BW12O40]5− | 800 | [53] | |||||
| [PTi2W10O40]7− | 12,800 | [53] | |||||
| Polyoxomolybdates | [53] | ||||||
| [P2Mo5O23]6− | 6400 | [56] | |||||
| [MnMo9O32]6− | 1600 | [56] | |||||
| [Eu(MoO4)(H2O)16(Mo7O24)4]14− | 400 | [56] | |||||
| Organic-inorganic-POM: | |||||||
| [(PhSbIII){Na(H2O)}AsIII2W19O67(H2O)]11− | - | 500 | 125 | - | 250 | 125 | [64] |
| [(PhSbIII)2AsIII2W19O67(H2O)]10− | - | 250 | 62.5 | - | 125 | 62.5 | [64] |
| [(PhSbIII)3(B-α-AsIIIW9O33)2]12− | - | 125 | 62.5 | - | 62.5 | 31.3 | [64] |
| [(PhSbIII)4(A-α-AsVW9O34)2]10– | - | 62.5 | 15.6 | - | 15.6 | 15.6 | [65] |
| Quinolone-based drug-POM: | |||||||
| [CoII(C19FH22N3O4)3][C19FH23N3O4][HSiW12O40] | 2.52 | 2.42 | - | - | - | - | [66] |
| Nanocomposite: | |||||||
| Bamboo charcoal-POM: | |||||||
| BC/POM | 4 | 4 | 4 | 4 | - | - | [67] |
| Polymer-POM: | |||||||
| PVA/PEI-POM: | |||||||
| PVA-PEI-H5PV2Mo10O40 | 0.02 | 2 | 0.2 | 0.02 | - | - | [68] |
| Chitosan-POM: | |||||||
| CTS-Ca3V10O28 | 12.5 | 12.5 | - | - | - | - | [52] |
| Polyoxometalate ionic liquids: | |||||||
| [N(C6H13)4]8[α-SiW11O39] | 10 | 1000 | - | 1000 | - | - | [69] |
| [N(C7H15)4]8[α-SiW11O39] | 2 | 25 | - | 100 | - | - | [69] |
| [N(C8H17)4]8[α-SiW11O39] | 5 | 50 | - | 100 | - | - | [69] |
| Polyoxotungstates | Virus | Animal | Survival Rate (SR) | Dose (Mode of Administration) | Ref. |
|---|---|---|---|---|---|
| K7[PW10Ti2O40] | HSV-2 | mouse | 97% | 25.0 mg/kg | [114] |
| Ce2H3[BW9VIW2VMn(H2O)O39] | FM1 | mouse | 90% | 100 mg/kg (o.a.) | [115] |
| Ce2H3[BW9VIW2VMn(H2O)O39] | FM1 | mouse | 90% | 10 mg/kg (i.p.) | [115] |
| Clinically approved drugs | |||||
| Acyclovir | HSV-2 | mouse | 33% | 50.0 mg/kg | [114] |
| Ribavirin | FM1 | mouse | 70% | 200 mg/kg (o.a) | [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aureliano, M. The Future Is Bright for Polyoxometalates. BioChem 2022, 2, 8-26. https://doi.org/10.3390/biochem2010002
Aureliano M. The Future Is Bright for Polyoxometalates. BioChem. 2022; 2(1):8-26. https://doi.org/10.3390/biochem2010002
Chicago/Turabian StyleAureliano, Manuel. 2022. "The Future Is Bright for Polyoxometalates" BioChem 2, no. 1: 8-26. https://doi.org/10.3390/biochem2010002
APA StyleAureliano, M. (2022). The Future Is Bright for Polyoxometalates. BioChem, 2(1), 8-26. https://doi.org/10.3390/biochem2010002






