Abstract
Infection and sepsis remain among the leading causes of neonatal mortality. The susceptibility of newborns to infection can be attributed to their immature immune system. Regarding immune response, monocytes represent a numerically minor population of leukocytes. However, they contribute to a variety of immunological demands, such as continuous replenishment of resident macrophages under non-infectious conditions and migration to inflamed sites where they neutralize pathogens and secrete cytokines. Further functions include the presentation of antigens and T-cell activation. Cytokines coordinate host responses to bacterial and viral infections and orchestrate ongoing physiological signaling between cells of non-immune tissues. A critical event is the skewing of the cytokine repertoire to achieve a resolution of infection. In this regard, monocytes may hold a key position as deciders in addition to their phagocytic activity, securing the extinction of pathogens to prevent broader organ damage by toxins and pro-inflammatory reactions. Neonatal monocytes undergo various regulatory and metabolic changes. Thus, they are thought to be vulnerable in anticipating pro-inflammatory conditions and cause severe progressions which increase the risk of developing sepsis. Furthermore, clinical studies have shown that exposure to inflammation puts neonates at a high risk for adverse pulmonary, immunological and other organ developments, which may result in multiorgan disease. This review discusses significant functions and impairments of neonatal monocytes that are decisive for the outcome of bacterial infections.
1. Introduction
Neonates, especially if born preterm, are highly susceptible to infections by bacteria, fungi and viruses. As a result, infection remains one of the leading causes for mortality and morbidity in early human life [1,2]. Deficiencies of both innate and adaptive immunity contribute to an impaired neonatal host defense and help to explain their susceptibility [3,4]. This increased host-specific susceptibility is accompanied by increased immunopathological risk. The clinical picture in postnatal neonatal sepsis presents as systemic inflammatory response syndrome (SIRS) [5], with a consecutive shock and rapid deterioration of ventilation, circulation and metabolism. Even in cases of prenatal infection, occurring in the intrauterine cavity, the clinical features are those of systemic inflammation, termed fetal inflammatory response syndrome (FIRS). It is accompanied by a high rate of complications, such as early-onset neonatal sepsis, periventricular leukomalacia (PVL) and death, along with long-term consequences, such as bronchopulmonary dysplasia (BPD), neurodevelopmental disorders [6] and retinopathy of prematurity (ROP) [7].
In sepsis, the immune response consists of pro- and anti-inflammatory reactions. The pro-inflammatory reaction, characterized by cytokine storm, is predominant during the early phase of sepsis, whereas the anti-inflammatory reaction occurs later. This “seesaw of sepsis” must be well balanced and is crucial for survival [8].
In general, an immune response against bacteria is a complex interplay of different immune cells. Due to the immaturity of the adaptive immune system in term and especially preterm infants, the role of the innate immune defense and its components are crucial with respect to neonatal infection. This review focuses on the armamentarium of neonatal monocytes and their potential to orchestrate an adequate antibacterial immune response during sepsis as think tanks or workhorses.
2. Monocytes and Their Role in Processes of Bacterial Sepsis and Inflammation
2.1. Detection of Pathogens
The detection of pathogen-associated molecular patterns (PAMPs) via pattern recognition receptors (PRRs) is the starting point of inflammation [9]. Invading pathogens or components such as lipopolysaccharides (LPS) and lipoteichoic acid (LTA) cause the production of pro-inflammatory cytokines via the activation of PRRs, such as Toll-like receptors (TLRs) expressed by monocytes [10]. In neonates, monocyte expression of TLR-4, which detects Gram-negative bacteria and facilitates LPS signal transduction [11] is low compared to adult levels, while it increases with gestational age and explains the special susceptibility of preterm infants to Gram-negative bacteria. Moreover, TLR-4 shows a diminished reaction to LPS due to lower activation of myeloid differentiation primary response 88 (MyD88) [12] and downregulated phosphorylation of nuclear factor ‘kappa-light-chain-enhancer’ of activated B-cells (NF-kappaB) [13,14,15,16]. In a few studies, surface expression levels of TLR2, mainly involved in the detection of Gram-positive bacteria, showed no significant difference compared to adult monocytes [16], while others have reported reduced TLR2 expression on neonatal monocytes [17]. This results in deficiencies in innate immunity-associated inflammatory cytokine response, which has been widely demonstrated [18]. Besides membrane-bound receptors, such as TLRs, pathogen patterns can also be detected by intracellular PRRs called nucleotide-binding oligomerization domain receptors or NOD-like receptors (NLRs), for short [19,20,21]. NOD-1 expression is significantly lower in neonates than in adults, while NOD-2 expression was found reduced in preterm neonates only. Stimulation with NOD-1+2 agonists induced lower cytokine release compared to adults [22]. It can be assumed that the capacity of monocytes to detect pathogens is an important function in sepsis; however, altered signalling pathways in neonatal monocytes have yet to be elucidated.
2.2. Phagocytosis and Killing
After recognition, the next steps in the effective elimination of bacteria are binding, phagocytosis and intracellular degradation in the phagolysosome. Pathogen clearance is one of the monocytes’ central tasks. Monocyte counts in cord blood are comparable to those in adult blood with a tendency to be slightly higher in newborns [16,23]. Therefore, neonates do not lack cells with phagocytic capacity, and thus it is necessary to consider monocyte functionality. Besides phagocytosis, the production of bactericidal reactive oxygen species (ROS) and nitric oxide (NO) plays a crucial role in eliminating bacteria.
While few studies show lower phagocytic ability [23], we and others have shown that infection with green fluorescent protein (GFP)-labeled bacteria revealed no difference between peripheral blood monocytes from adults (PBMOs) and cord blood derived monocytes (CBMO) with regard to phagocytic capacity, phagocytic indices, degradation activity and ROS production [18,23,24,25].
2.3. Phagocytose Induced Cell Death (PICD)—Regulating the Immune Response via Depletion
PICD was first described by Zychlinsky et al. in 1992, who reported that Shigella flexneri induces apoptosis in infected macrophages [26]. Further studies showed the same effect for other bacteria and assumed that effector cell apoptosis supports the resolution of infection (reviewed in [27]).
Dysregulation or imbalance of PICD in the host is accompanied by a variety of pathophysiological consequences. If monocytes undergo abortive PICD, bacteria may be eliminated incompletely. In case of delayed or insufficient PICD, permanent or prolonged cytokine production via activated effector cells could lead to sustained inflammation and systemic damage to the host. Effector cell apoptosis is therefore tightly regulated. In this context, we discuss the early apoptosis of monocytes in adult septic patients as a means of influencing the course of disease in an advantageous way [28].
In previous studies, consecutive PICD of neonatal monocytes was compared to adult monocytes in an in vitro infection model using the most common agents of neonatal sepsis, group-B-streptococci (GBS) and Escherichia coli, as well as Candida albicans. Although CBMOs and PBMOs showed identical phagocytic and intracellular degradation properties, as mentioned above, PICD was heavily reduced in CBMOs [18,29,30]. The Fas/Fas-ligand system (CD95/C95L) as a member of the tumour necrosis factor (TNF) family was shown to be relevant for the induction of PICD and reduced PICD in CBMO. Moreover, it was associated with reduced transcription of CD95L [30]. Recent work revealed that matrix metalloprotease 9 (MMP-9), which was found to be elevated on neonatal monocytes compared to adult controls, is responsible for the shedding of CD95L and reduced cell-contact dependent PICD on infected neonatal monocytes (see Figure 1) [31,32].
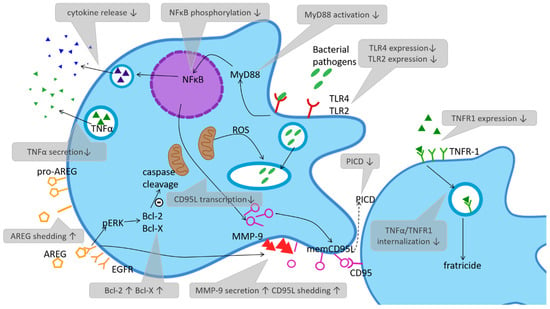
Figure 1.
Pathogen recognition, phagocytosis, killing and phagocytosis-induced cell death in cord blood derived monocytes. Alterations in neonates compared to adults are named in grey text boxes, small arrows indicate an increase or decrease. Abbreviations: NFκB (nuclear factor ‘kappa-light-chain-enhancer’ of activated B-cells), MyD88 (Myeloid differentiation primary response 88), TLR (Toll-like receptor), TNF (tumor necrosis factor), TNFR (tumor necrosis factor receptor), PICD (phagocytosis induced cell death), CD95L (CD95 ligand or Fas ligand), memCD95L (membrane-bound CD95 or Fas ligand), MMP-9 (matrix metalloproteinase 9), Bcl (B-cell lymphoma), EGFR (epidermal growth factor receptor), AREG (amphiregulin), pERK (phosphorylated extracellular signal-regulated kinase).
One molecular mechanism which may explain downregulated PICD in CBMOs is the balance of proteins belonging to the B-Cell lymphoma 2 (Bcl-2) family [33]. In comparison to PBMC, CBMC express higher levels of anti-apoptotic proteins whereas the expression of pro-apoptotic proteins is diminished [34]. Current findings identified amphiregulin (AREG) as an important player in this context. AREG is a growth factor that binds to the epidermal growth factor (EGF) receptor which can be found on the surface of monocytes and lymphocytes. Surface expression of membrane-anchored pro-AREG is increased on neonatal monocytes and cleavage/shedding of AREG was found to be highly elevated after infection with E. coli [34]. Infection induced higher phosphorylation of extracellular-signal regulated kinases (ERKs), higher expression of Bcl-2 and Bcl-XL and downregulation of cleaved caspase-3 and caspase-9 in an EGFR-dependent manner, thus inhibiting the intrinsic apoptosis pathway [34]. Data suggests that AREG shedding may cause apoptosis resistance and reduced PICD in neonatal monocytes via elevated intracellular MMP-2 and MMP-9 levels and FasL cleavage [34].
Via PICD and apoptosis regulation, monocytes play a role in preventing immune response overshooting. In this regard, their reduced function as “think tanks” in neonates leads to sustained inflammation with risk of illness in later life.
Separate analysis of monocytes infected with fluorochrome-labelled bacteria revealed reduced apoptosis in phagocytosing monocytes as compared to mates that did not phagocytose. Phagocytosing monocytes produced more TNF-α compared to bacteria-binding or non-interacting monocytes. Meanwhile, it has been observed that TNF-α is capable of inducing apoptosis in both contact and contact-independent manners. Altogether, this leads to the hypothesis that non-phagocytosing, TNF-α presenting and secreting monocytes kill their mates which do not contribute to combating pathogens in an autocrine and paracrine mechanism [35,36].
This phenomenon called “bystander kill” or “fratricide” is enabled by CD95L/CD95 and TNF-α/TNFR1 pairs of death ligand/death receptors. It is found to be reduced in neonatal monocytes. [30] Along with PBMOs, they internalize TNF-α/TNFR1 complexes, which is a prerequisite to initialize apoptosis. This effect plays a predominant role in non-phagocytosing CBMOs (see Figure 1).
TNF-α and other pro-inflammatory cytokines play a decisive role in sepsis, especially in the conversion to septic shock [6]. Supplementation of TNF-α led to a sustained detection of TNF-α on the surface of neonatal monocytes [36]. This indicates that the signalling is skewed towards anti-apoptotic effects, since TNF-α/TNFR1 complexes on the plasma membrane bind to TRAF2 (TNF receptor-associated factor 2) and activate the NF-kappaB pathway, initializing the pro-inflammatory response [37]. Additionally, it can be conjectured that TNF-α in CBMOs binds more frequently to the TNFR2 receptor. The expression pattern of the TNFR2 receptor was found to resemble that of PBMOs supporting anti-apoptotic signalling [35].
It was also found that CBMOs secrete less TNF-α and express less pro-apoptotic TNFR1 on their surface combined with TNF-α retention on the membrane and delayed TNF/TNFR1-internalization. This results in lower activation of cleaved caspase-8 and less PICD [35]. Taken together, the regulative functions of neonatal monocytes as “think tank” are skewed towards an inflammatory and anti-apoptotic phenotype that may contribute to sustained inflammation during and after infection and, through this, may build a pathophysiological explanation for enhanced infection-associated inflammatory damage in neonates.
2.4. Monocytes in Organ Destruction
In preterm neonates, sepsis and resultant sustained inflammation are associated with severe organ damage, especially in the central nervous system, lung and gut, with sequelae including bronchopulmonary dysplasia (BPD), necrotizing enterocolitis (NEC), retinopathy of prematurity (ROP), intracranial hemorrhage (ICH) and periventricular leukomalacia (PVL) [37]. Monocytes may participate in tissue injury and organ trauma in sepsis. LPS leads to activation of resident macrophages via TLR-4, which in turn (see Figure 2) initiates the production of TNF-α [16,38] (see Figure 2). This cytokine recruits circulating monocytes and macrophages via chemotaxis which leads to local inflammation and organ injury.
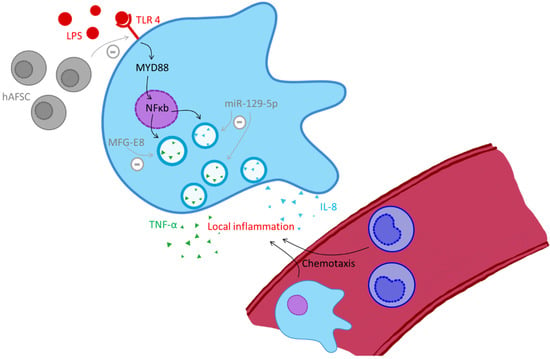
Figure 2.
Monocytes in organ destruction, black arrows indicate stimulation, grey arrows with a minus indicate inhibition. Abbreviations: hAFSC (human amniotic fluid stem cell), IL-8 (interleukin 8), LPS (lipopolysaccharide), MFG-E8 (milk fat globule–epidermal growth factor–factor 8), miR-129-5p (microRNA 129-5p), MYD88 (myeloid differentiation primary response 88), NFκ (nuclear factor ‘kappa-light-chain-enhancer’ of activated B-cells), TLR-4 (Toll-like receptor-4), TNF-α (tumor necrosis factor alpha).
The TNF-regulating RNA miR-129-5p is downregulated in neonates with sepsis. This down-regulation has been associated with increased TNF-α and interleukin-8 (IL-8) production by monocytes following LPS induction and might explain the over shooting inflammatory response. A supplementation of miR-129-5p could thus present a potential therapeutic target with anti-inflammatory effects in neonatal sepsis and should be further investigated [39].
Breastfeeding has been described as ameliorating the outcome of neonatal sepsis [40]. One contributing factor might be the presence of milk fat globule–epidermal growth factor–factor 8 (MFG-E8), also called lactadherin, in human milk. In murine neonatal sepsis, a deficiency in MFG-E8 leads to increased monocytic pro-inflammatory cytokine production (IL-6, IL-1β and TNF-α) [41] upon infection, resulting in increased serum LDH (lactate-dehydrogenase) levels, implying tissue damage, lung damage and loss of intestinal integrity, which is associated with the development of NEC with resulting increased mortality [42]. Treatment with MFG-E8 was found to improve lung injury scores and prevent the development of NEC [43,44]. In a recent study, MFG-E8 in human milk was found to decrease the production of pro-inflammatory cytokines (IL-8, TNF-α and MCP-1) and increase anti-inflammatory cytokines (IL-4) in the intestines of preterm infants, making them less prone to suffer from sepsis and NEC [45].
Over-expression of pro-inflammatory cytokines by neonatal monocytes [41] can lessen the chance of survival in sepsis [40] and cause severe sequelae.
2.5. Functions of Monocyte Subsets
In human blood, cytokines are able to convert monocytes into different subpopulations [46]. On the one hand, interferone-gamma (IFN-γ) or microbial products, such as LPS, induce the microbicidal and pro-inflammatory classical M1 macrophage. M1 macrophages are characterized by a high production of pro-inflammatory cytokines (IL-1β, IL-15, IL-18, TNF-α, IL-12) and increased surface expression of major histocompatibility complex (MHC) II and B7 family receptors (CD80/CD86), needed for T-cell interaction and antigen presentation [47]. On the other hand, polarization towards alternatively activated, anti-inflammatory macrophages occurs in response to stimulation by IL-4/IL-13, LPS/immune complexes or IL-10/transforming growth factor beta (TGF-β)/glucocorticoids. The alternatively activated subgroup is characterized by an upregulated expression of CD16, and therefore these monocytes play an important role in antibody-dependent cellular cytotoxicity (ADCC) [48]. Compared to adult peripheral blood macrophages, cord blood macrophages showed aberrant STAT1 and STAT3 (signal transducer and activator of transcription) phosphorylation upon stimulation, which is essential for differentiation into subgroups. Furthermore, CD80/CD86 presentation was reduced in both pro-inflammatory and anti-inflammatory type cord blood macrophages, resulting in a lower capacity to interact with T-cells and stimulate Treg production compared to adults [49].
Another way to discriminate monocyte subpopulations is by regarding receptor expression and functionality. The monocytes termed “classical” make up the majority, and express high levels of CD14 and only low levels or no CD16 (CD14hiCD16) [50,51]. They are further characterized by low levels of human leucocyte antigen (HLA)-DR molecules and mainly function as phagocytes with wound-healing and anti-apoptotic qualities [52].
The remaining monocytes are characterized by a higher expression of CD16 and fall into two subgroups designated as “intermediate” (CD14hiCD16+) and “non-classical” (CD14−/loCD16+). Intermediate monocytes are associated with angiogenesis, display the highest ROS production and have T-cell stimulatory properties, expressing higher levels of HLA-DR and MHCII. Non-classical monocytes stimulate T-helper cells and have pro-apoptotic qualities [52].
Adult sepsis patients have been found to have elevated levels of both CD16+ monocyte subgroups, showing an increase of intermediate monocytes especially. Furthermore, HLA-DR expression seems to be reduced during sepsis and correlates with a negative sepsis outcome [52,53,54]. Studies measuring monocyte subpopulations at different ages have found a similar phenotype in healthy neonates. Neonatal monocytes express lower levels of HLA-DR, reaching adult levels after about six months [55]. Furthermore, neonates have the highest levels of intermediate monocytes of any age group [56] also of non-classical monocytes which are even more elevated in preterm neonates [16,57,58]. Neonatal monocytes also displayed a compromised ability to activate T-cells, whereas IL-10 production was enhanced, which could, again, correlate with the high proportion of intermediate monocytes [58,59]. These results suggest that neonatal monocyte subtypes are skewed towards an immunosuppressive and anti-inflammatory phenotype which could protect from hyperinflammation during early microbial colonization but also lead to lowered anti-bacterial protective capacity in case of disease. However, it is important to discriminate between these in vitro findings and in vivo neonatal reactions to infection where an increased pro-inflammatory reaction has been observed [59]. Both hypo- and hyperinflammation can occur during the course of sepsis [8] and their regulation seems to be inadequate in neonatal monocytes.
2.6. Cytokine Production and Responsiveness
The molecular components of sepsis encompass cytokines, acute-phase proteins and plasma cascades. The cytokine profile of septic neonates is characterized by elevated levels of pro-inflammatory cytokines, such as TNF-α and IL-6, as well as the immune-regulatory cytokine IL-10 [60,61]. The levels at which these cytokines are produced vary with different stages of infection. TNF-α and IL-6 seem to be increased during the initial inflammatory response, while IL-10 and IL-4, as an anti-inflammatory signal, increase during the termination of disease [62]. Production of particularly low amounts of anti-inflammatory IL-4, IL-12 and IFN-γ has been linked to a higher risk of adverse outcomes in neonatal sepsis; additionally, IL-6 and IL-10 seem to have predictive value when it comes to determining disease outcomes, giving these two cytokines a pivotal role regarding cytokine production in neonatal sepsis [63,64,65].
Upon stimulation with microbial products, such as LPS, neonatal monocytes produce significantly more IL-6 than adult monocytes [55], and neonatal blood also contains higher levels of IL-6 compared to toddlers. This finding has been linked to decreased expression of the LPS-inducible gene Zc3h12a in newborns compared to adults. Zc3h12 has a regulatory effect on the amounts of IL-6 being produced by initiating its degradation. [66]. IL-6 activates the production of acute phase proteins in macrophages via phosphorylation of STAT3. An increase of IL-6 resulting in higher acute phase protein levels in neonates could be the result of a decrease in neonatal macrophages in the SOCS (suppressor of cytokine signalling) 3/IL-6 ratio, which is responsible for inhibiting STAT3 dependent intracellular signalling [67].
IL-10, as an immune-regulating and phagocytosis-enhancing [68] signalling peptide with anti-inflammatory potential, seems to be important in the control of inflammation [69]. It is associated with the upregulation of CD16 on macrophages, thereby promoting ADCC, and down-regulation of HLA-DR, but fails to achieve this effect during in vitro stimulation of cord blood macrophages of term and preterm neonates. The particular cellular cause of this decreased effect has yet to be determined [48]. In neonates, monocytes rather than polymorphonuclear cells (PMNs) [70,71] react with IL-10 production upon infection and therefore they are thought to be more crucial for the course of FIRS [72]. However, little is known about the exact time course of neonatal IL-10 production in comparison to adult monocytes.
Macrophage migration inhibitory factor (MIF) is another pro-inflammatory cytokine that can be found in elevated levels in neonates, especially around the time of birth, and high levels of MIF have been associated with elevated morbidity and mortality rates in septic adults. Physiologically, MIF seems to counteract the suppressed immune status of newborns, but in the face of infection it might have a detrimental effect on survival and thus also presents a potential therapeutic target [73].
Another cytokine that has been associated with the pathophysiology of neonatal sepsis is the pro-inflammatory IL-17. Il-17 recruits neutrophils and monocytes in combination with other cytokines, such as IL-8 and IL-6, and is released in reaction to bacterial contact with immunological barriers. As its production is decreased in neonates, it could contribute to an impaired immune response to extracellular pathogens and to a dysregulation of the evolving microbiome, thus resulting in bacteraemia and sepsis [74].
The immune response against different pathogens depends on lymphocyte subsets, especially T-helper (TH) cells, and their production of cytokines. IFN-γ or IL-2, known as TH1 cytokines, induce the activation and differentiation of monocytes, whereas TH2 cytokines, e.g., interleukin-10 (IL-10), promote antibody synthesis and immunoregulatory signals [75]. Studies have shown that in neonates the balance between both immune responses is shifted towards TH2 due to diminished phosphorylation of IFN-γ-R-associated STAT-1 [76], making monocyte signalling pathways hyporesponsive to IFN-γ [76]. The imbalance in polarization is necessary to maintain pregnancy because TH1 cytokines can induce pregnancy complications, including miscarriage [77,78].
Apart from their ability to influence other immune cells, such as monocytes, and thus to regulate immune responses, cytokines can also influence their primary producers and “cross-regulate” immunoreaction. IFN-γ inhibits TH2 cells [79], whereas IL-10 may bias towards TH2 differentiation, resulting in down-regulation of TH1 functions [80]. IL-10 production in neonatal leukocyte populations (CBMNC) seemed to be reduced because of an altered interaction between monocytes and T-cells [49,81]. Therefore, monocytes are involved in determining immune response. The decision about its direction requires a certain intellect in our immune system and is a further task for monocytes as thinking deciders.
Recent studies have investigated the possibility of using cytokine levels, which increase earlier in disease than acute phase proteins and are more specific to infection, as a diagnostic tool and a prognostic marker for sepsis. IL-6 and IL-10 levels especially have been found to be significantly altered in neonates and to coincide with negative sequelae [4,63,65,81].
2.7. Modulating Effector Cell Functions of Monocytes in Neonatal Sepsis
During the inflammatory response monocytes are not only essential for the innate immune response by producing cytokines and presenting phagocytic activity, they also play an essential role in activating the adaptive immune response by T-cell activation and antigen presentation [82]. To this end, numerous studies investigated the expression of molecules involved in APC (antigen-presenting cell)/T-cell communication.
Among this class of molecules is HLA-DR, an MHCII protein commonly found on monocytes that is involved in antigen presentation to T-cells. This complex is down-regulated on monocytes during sepsis, which might be indicative of an impairment in monocytic antigen presentation capacities during systemic inflammation [83]. The decreased expression of HLA-DR seems to correlate not only with a decreased lymphocyte responsiveness but also with the severity of the disease and mortality in paediatric sepsis patients [84,85]. It was associated with the anti-inflammatory secondary immune response to sepsis and is thought to prevent over-inflammation following an intense pro-inflammatory period [86] as well as possibly be involved in the long-term immunosuppression that can often be found in patients post sepsis [85]. The basal monocytic HLA-DR expression was found to be decreased in preterm [86] and term neonates [87], which suggests that even before systemic infection-related down-regulation, neonates present with an impairment in immune cell functionality. Since HLA-DR expression on monocytes is decreased in the early stages of sepsis and correlates with disease intensity and mortality, it was discussed as a biomarker for diagnosis and prognosis of sepsis [88]. However, on its own, HLA-DR expression does not seem to be sufficiently sepsis-specific and would have to be complemented by other markers, for example, CD64 expression on neutrophile granulocytes [87,88].
In an infectious setting, monocytes can activate different T-cell subtypes through cytokine production. However, in sepsis, monocytes were found to be insufficiently capable of activating T-cells [82]. Instead, immunosuppressive regulative T-cell (Treg) populations are elevated in septic patients [89,90]. These can be induced via IL-10, produced by activated monocytes [91]. Elevated Treg levels are associated with post-sepsis long-term immunosuppression and compromised immune system function. The neonatal immune system tends to be of a pathogen-tolerogenic disposition, containing high levels of Treg cells [92,93,94]. This might be necessary to allow neonates to establish a healthy commensal intestinal microbiome and to interact with foreign antigens without constant inflammatory response. However, in case of infection this immunosuppressive state supported by Tregs could become detrimental and quickly lead to severe sepsis. The T-cell imbalance in neonates, modulated by activated monocytes, has also been associated with the development of NEC [95].
In neonates the monocyte–T-cell interaction is skewed towards an immunosuppressed state which could become critical in the event of sepsis, once again highlighting the monocytes’ role as important “think tanks”.
2.8. S100-Alarmins in Neonatal Sepsis
Neonates show highly elevated blood levels of S100A8/A9 alarmins [96,97], and although it is apparent that they play an important role in the regulation of innate immunity, it remains debatable whether they enhance or attenuate inflammation and whether they benefit or harm neonates.
S100A8 and S100A9 (also referred to as MRP8 and MRP9) are Ca2+-binding proteins that are highly concentrated in the cytoplasm of granulocytes and monocytes. Together they form a heteromeric complex that is also called calprotectin [98]. S100-alarmins are released from monocytes either passively via necrosis or are actively secreted during inflammatory processes [99]. They are classified as danger-associated molecular patterns (DAMP) and act as endogenous TLR4-agonists. TLR-4 activation initiates MyD88 signalling, with activation of interleukin-1 receptor-associated kinase 1 (IRAK-1) and NF-kappaB [100], resulting in pro-inflammatory cytokine release, including TNFα, IL-6, Il-1β and macrophage inflammatory protein 2 alpha (MIP-2-alpha) [101]. The elevated serum levels in neonates result from monocyte secretion [97] and high concentrations in human breast milk [102].
In adults, an increase in S100-alarmins is mostly reported in the context of inflammation. In chronic inflammatory bowel disease, stool concentrations of calprotectin are a diagnostic tool for inflammatory activity. In rheumatoid arthritis, they cause pro-inflammatory effects and upregulate MMP3, MMP9 and MMP13 expression on phagocytes [103], along with induction of chemotaxis [104]. They have also been identified as amplifiers of inflammation promoting lethal endotoxin-induced shock in adults [100]. In neonates, however, their role has not been clarified. A recent study demonstrated their expression on monocytes to be associated with inflammatory activation, chorioamnionitis, FIRS (fetal inflammatory response syndrome) and elevated inflammatory proteins [104]. Contrary to that, other studies report a more regulatory, protective function for S100-alarmins in newborns [105]. High amounts of alarmins alter MyD88 signalling in a way that prevents hyperinflammation without impinging on pathogen defence in neonatal sepsis [101] and prevent the expansion of an inflammatory monocyte population that causes hyperinflammation and endotoxic shock in neonatal mice [96]. A study from 2014 supports this observation and demonstrates the TLR-4 hyporesponsiveness of phagocytes with decreased TNFα release and enhanced survival of mice in septic shock upon S100-alarmin treatment. This stress tolerance was also seen in human cord blood derived monocytes, whose inflammatory LPS response was impaired compared to adult monocytes, while depletion and pharmacological inhibition of S100A8/A9 reversed this inhibition of pro-inflammation, supporting the hypothesis that alarmins act as an endogenous tolerizing factor [99]. This tolerizing quality might also come to play in the neonatal development of a healthy gut microbiome, as a recent study suggests. Enterally absorbed S100A8/S100A9 deriving from breast milk stimulated lamina propria macrophages to support the expansion of Tregs and in turn allowed eubiont bacteria to colonize. A healthy gut microbiome is essential to avoid the development of gut-origin sepsis [106]. It was also shown that mechanistic target of rapamycin (mTOR) suppression in CBMOs is linked to the known high levels of S100A8/A9 alarmins in cord blood serum. Treating PBMOs with cord blood serum or alarmins inhibited mTOR signalling and glycolysis in a similar manner. S100A8/A9 treatment also inhibits the metabolic shift towards glycolysis upon inflammatory stimulation by mTOR inhibition in human CBMOs [107].
Taking these findings together, the massive release of S100-alarmins by monocytes points towards an important regulatory role for monocytes in neonatal immunity.
2.9. Immunometabolism of Neonatal Monocytes
The metabolism of activated monocytes and macrophages has been extensively discussed [108], however, research has been mainly focused on monocytes of adults, while little is known about the situation in neonates.
Glycolysis is a fast though ineffective way to generate adenosine triphosphate (ATP) and other metabolites. ATP is elevated during inflammation, acts as a signal of danger and can initiate and prolong immune responses. Activated inflammatory monocytes upregulate glycolytic activity and glucose transporter 1 (GLUT-1) expression to increase glucose uptake via mTOR and Akt signalling [109]. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) plays a role in the translational regulation of inflammatory chemokines, such as TNF [110].
One of the main metabolic shifts during an inflammatory response moves from oxidative phosphorylation (OxPhos) towards glycolysis and fatty acid synthesis (FAS), which is known as the Warburg effect [111]. This shift towards glycolysis is reduced in preterm monocytes due to low mTOR signalling in macrophages and CBMOs fail to increase glucose uptake in response to infection when compared to PBMOs [112,113]. CBMOs reacted with only an increase in glycolysis, along with lower TNF and elevated IL-6 secretion [114].
Recent studies have tried to elucidate the involvement of mTOR signalling in neonatal immunometabolism and PICD. Leucine pre-treatment induced mTOR-activation on E. coli-infected monocytes, leading to lower levels of Bcl-2 and raising PICD of CBMOs to levels comparable to PBMOs. This occurred only on CBMOs despite lower surface expression of LAT-1 (L-amino acid-transporter) than on PBMOs. Although leucine did not change the phagocytic properties or glycolysis rates of CBMOs, it did increase OxPhos on infected cells in an mTOR-dependent manner [115].
On a genetic and epigenetic level, several genes for glucose metabolism were found lowered in CBMOs compared to PBMOs: the primary rate-limiting glucose transporter GLUT-1, phosphofructokinase M, mitochondrial pyruvate carrier, pyruvate carboxylase and malate dehydrogenase 2 were all decreased in neonatal monocytes, leading to broad metabolic defects, especially regarding glycolysis [107].
Neonatal monocytes show lower expression of genes related to OxPhos, fatty acid oxidation (FAO) and glycolysis [116]. Another area of metabolic alteration is lipid metabolism and the balance between FAO and FAS. FAO is described as mostly anti-inflammatory apart from NLRP-3 (NLR family pyrin domain containing-3) inflammasome activation in macrophages, while FAS favours inflammation in innate immune cells and is critical for differentiation and phagocytic activity [116]. Compared to adults, neonates dispose of reduced energy reserves—FAO especially is limited due to diminished adipose tissue, making the neonatal immune response more disease tolerant. The reduced neonatal anti-inflammatory immune response correlates with a higher bacterial load threshold in neonates (50 CFU/mL vs. 1 CFU/mL in adults). In neonatal sepsis, genes involved in lipid metabolism are upregulated [117]. In addition, neonatal monocytes express higher levels of peroxisome proliferator-activated receptor gamma (PPARγ), which is a nuclear receptor involved in lipid metabolism, as well as inhibition and termination of inflammation [117].
Profiling of human sepsis patients undergoing immunoparalysis has revealed a transitory defect in glycolysis and oxidative metabolism [118]. This suggests that the alterations in neonatal immunometabolism might be problematic for pathogen defence and could be a potential target in nutrition and pharmacotherapy of septic neonates [118].
The availability of ketone bodies for brain development is critical for the neonate and is in danger of being “maxed out” energetically by ketone body consumption after infection [118,119]. To this end, ICU applies enteral nutrition, resembling intrauterine nutrition (carbohydrate-rich/fatty acid-low).
Taken together, these observations point to a differential regulation of metabolism, and its influence on immune responses via cytokine release and cell survival strongly hints towards a regulatory thinking function of monocytes in neonatal sepsis.
3. Conclusions
Besides their role as phagocytosing cells with a well-known function of eliminating bacteria, monocytes are crucial deciders in regulating immunoreaction. Mediation of apoptosis, modulation of cytokine responsiveness and calculated interactions with lymphocytes affect the outcome of sepsis in a particular manner. According to our opening question, monocytes are indispensable for both functions: as think tanks and workhorses.
In neonates, the ability of monocytes to work seems to be fully developed, whereas their role as mastermind is immature. This leads to phenomes, such as prolonged inflammation and organ damage, with risk of sequelae.
A deeper understanding of monocyte regulating functions opens up new vistas with regard to therapeutic targets, such as pharmacological influence on cytokines or death ligands. Therefore, further investigations into monocytes as think tanks may help to fight neonatal sepsis and its complications.
Author Contributions
Conceptualization T.O., S.D., L.O., C.D. and N.-U.H.; Writing—original draft preparation: L.O., C.D. and N.-U.H.; Writing—review and editing: S.D. and C.G.; Project administration: T.O.; Supervision S.D.; Visualization: L.O., C.D. and N.-U.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
For this review, no data sets have been used.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, L.; Oza, S.; Hogan, D.; Chu, Y.; Perin, J.; Zhu, J.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of under-5 mortality in 2000–2015: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016, 388, 3027–3035. [Google Scholar] [CrossRef]
- Fleischmann-Struzek, C.; Goldfarb, D.M.; Schlattmann, P.; Schlapbach, L.J.; Reinhart, K.; Kissoon, N. The global burden of paediatric and neonatal sepsis: A systematic review. Lancet Respir Med. 2018, 6, 223–230. [Google Scholar] [CrossRef]
- Jong, d.E.; Strunk, T.; Burgner, D.; Lavoie, P.M.; Currie, A. The phenotype and function of preterm infant monocytes: Implications for susceptibility to infection. J. Leukoc. Biol. 2017, 102, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Strunk, T.; Hibbert, J.; Doherty, D.; Nathan, E.; Simmer, K.; Richmond, P.; Currie, A.; Burgner, D. Impaired Cytokine Responses to Live Staphylococcus epidermidis in Preterm Infants Precede Gram-positive, Late-onset Sepsis. Clin. Infect. Dis. 2021, 72, 27. [Google Scholar] [CrossRef]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef]
- Cai, S.; Thompson, D.K.; Anderson, P.J.; Yang, J.Y. Short- and Long-Term Neurodevelopmental Outcomes of Very Preterm Infants with Neonatal Sepsis: A Systematic Review and Meta-Analysis. Children 2019, 6, 131. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Romero, R.; Yeo, L.; Diaz-Primera, R.; Marin-Concha, J.; Para, R.; Lopez, A.M.; Pacora, N. Gomez-Lopez, P.; Yoon, B.H.; et al. The fetal inflammatory response syndrome: The origins of a concept, pathophysiology, diagnosis, and obstetrical implications. Semin Fetal Neonatal Med. 2020, 25, 101146. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Coopersmith, C.M.; McDunn, J.W.; Ferguson, T.A. The sepsis seesaw: Tilting toward immunosuppression. Nat. Med. 2009, 15, 496–497. [Google Scholar] [CrossRef]
- Janeway, C.A. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant Biol. 1989, 54, 1–13. [Google Scholar] [CrossRef]
- Underhill, D.M.; Ozinsky, A. Toll-like receptors: Key mediators of microbe detection. Curr. Opin. Immunol. 2002, 14, 103–110. [Google Scholar] [CrossRef]
- Viemann, D.; Dubbel, G.; Schleifenbaum, S.; Harms, E.; Sorg, C.; Roth, J. Expression of toll-like receptors in neonatal sepsis. Pediatr Res. 2005, 58, 654–659. [Google Scholar] [CrossRef]
- Sadeghi, K.; Berger, A.; Langgartner, M.; Prusa, A.; Hayde, M.; Herkner, K.; Pollak, A.; Spittler, A.; Förster-Waldl, E. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J. Infect. Dis. 2007, 195, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Yua, S.; Zhanga, W.; Yua, J.; Feng, S.; Guob, T.; Lua, H. Inhibiting effect of Radix Hedysari Polysaccharide (HPS) on endotoxin-induced uveitis in rats. Immunopharmacology 2014, 21, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Terashima-Hasegawa, M.; Ashino, T.; Kawazoe, Y.; Shiba, T.; Manabe, A.; Numazawa, S. Inorganic polyphosphate protects against lipopolysaccharide-induced lethality and tissue injury through regulation of macrophage recruitment. Biochem. Pharmacol. 2019, 159, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Ichiyama, T.; Isumi, H.; Nakata, M.; Sase, M.; Furukawa, S. NF-kappaB activation in peripheral blood mononuclear cells in neonatal asphyxia. Clin. Exp. Immunol. 2004, 132, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Wisgrill, L.; Groschopf, A.; Herndl, E.; Sadeghi, K.; Spittler, A.; Berger, A.; Forster-Waldl, E. Reduced TNF-alpha response in preterm neonates is associated with impaired nonclassic monocyte function. J. Leukoc. Biol. 2016, 100, 607–612. [Google Scholar] [CrossRef]
- Hegge, I.; Niepel, F.; Lange, A.; Vogelgesang, A.; Heckmann, M.; Ruhnau, J. Functional analysis of granulocyte and monocyte subpopulations in neonates. Mol. Cell Pediatr. 2019, 1, 5. [Google Scholar] [CrossRef]
- Dreschers, S.; Saupp, P.; Hornef, M.; Prehn, A.; Platen, C.; Morschhauser, J.; Orlikowsky, T.W. Reduced PICD in Monocytes Mounts Altered Neonate Immune Response to Candida albicans. PLoS ONE. 2016, 11, e0166648. [Google Scholar]
- Sun, J.; Katz, S.; Dutta, B.; Wang, Z.; Fraser, I.D. Genome-wide siRNA screen of genes regulating the LPS-induced TNF-α response in human macrophages. Sci. Data. 2017, 4, 170007. [Google Scholar] [CrossRef] [PubMed]
- Inohara, N.; Chamaillard, M.; McDonald, C.; Nuñez, G. NOD-LRR proteins: Role in host-microbial interactions and inflammatory disease. Annu. Rev. Biochem. 2005, 74, 355–383. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Warner, N.; Viani, K.; Nuñez, G. Function of Nod-like receptors in microbial recognition and host defense. Immunol. Rev. 2009, 227, 106–128. [Google Scholar] [CrossRef]
- Chen, S.; Zuo, Y.; Huang, L.; Sherchan, P.; Zhang, J.; Yu, Z.; Peng, J.; Zhang, J.; Zhao, L.; Doycheva, D.; et al. The MC 4 receptor agonist RO27–3225 inhibits NLRP1-dependent neuronal pyroptosis via the ASK1/JNK/p38 MAPK pathway in a mouse model of intracerebral haemorrhage. Br. J. Pharmacol. 2019, 176, 1341–1356. [Google Scholar] [CrossRef]
- Silveira-Lessa, A.L.; Quinello, C.; Lima, L.; Redondo, A.C.C.; Ceccon, M.; Carneiro-Sampaio, M.; Palmeira, P. TLR expression, phagocytosis and oxidative burst in healthy and septic newborns in response to Gram-negative and Gram-positive rods. Hum. Immunol. 2016, 77, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Gille, C.; Leiber, A.; Mundle, J.; Spring, B.; Abele, H.; Spellerberg, B.; Hartmann, H.; Poets, F.C.; Orlikowsky, T.W. Phagocytosis and postphagocytic reaction of cord blood and adult blood monocyte after infection with green fluorescent protein-labeled Escherichia coli and group B Streptococci. Cytometry. B Clin. Cytom. 2009, 76, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Dreschers, S.; Gille, C.; Haas, M.; Seubert, F.; Platen, C.; Orlikowsky, T.W. Reduced internalization of TNF-a/TNFR1 down-regulates caspase dependent phagocytosis induced cell death (PICD) in neonatal monocytes. PLoS ONE 2017, 12, e0182415. [Google Scholar] [CrossRef] [PubMed]
- Zychlinsky, A.; Prevost, M.C.; Sansonetti, P.J. Shigella flexneri induces apoptosis in infected macrophages. Nature 1992, 358, 167–169. [Google Scholar] [CrossRef]
- DeLeo, F.R. Modulation of phagocyte apoptosis by bacterial pathogens. Apoptosis 2004, 9, 399–413. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Routsi, C.; Plachouras, D.; Markaki, V.; Raftogiannis, M.; Zervakis, D.; Koussoulas, V.; Orfanos, S.; Kotanidou, A.; Armaganidis, A. Early apoptosis of blood monocytes in the septic host: Is it a mechanism of protection in the event of septic shock? Crit. Care 2006, 10, R76. [Google Scholar] [CrossRef]
- Gille, C.; Leiber, A.; Spring, B.; Kempf, V.A.; Loeffler, J.; Poets, C.F.; Orlikowsky, T.W. Diminished phagocytosis-induced cell death (PICD) in neonatal monocytes upon infection with Escherichia coli. Pediatr. Res. 2008, 63, 33–38. [Google Scholar] [CrossRef]
- Gille, C.; Dreschers, S.; Leiber, A.; Lepiorz, F.; Krusch, M.; Grosse-Opphoff, J.; Spring, B.; Haas, M.; Urschitz, M.; Poets, C.F.; et al. The CD95/CD95L pathway is involved in phagocytosis-induced cell death of monocytes and may account for sustained inflammation in neonates. Pediatr. Res. 2013, 73, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Dreschers, S.; Platen, C.; Ludwig, A.; Gille, C.; Köstlin, N.; Orlikowsky, T.W. Metalloproteinases TACE and MMP-9 Differentially Regulate Death Factors on Adult and Neonatal Monocytes After Infection with Escherichia coli. Int. J. Mol. Sci. 2019, 20, 1399. [Google Scholar] [CrossRef]
- Leiber, A.; Graf, B.; Spring, B.; Rudner, J.; Köstlin, N.; Orlikowsky, T.W.; Poets, C.F.; Gille, C. Neonatal monocytes express antiapoptotic pattern of Bcl-2 proteins and show diminished apoptosis upon infection with Escherichia coli. Pediatr. Res. 2014, 76, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Platen, C.; Dreschers, S.; Reiss, K.L.; Wappler, J.; Orlikowsky, T.W. Amphiregulin Regulates Phagocytosis-Induced Cell Death in Monocytes via EGFR and Matrix Metalloproteinases. Mediat. Inflamm. 2018, 2018, 4310419. [Google Scholar] [CrossRef] [PubMed]
- Platen, C.; Dreschers, S.; Wappler, J.; Ludwig, A.; Düsterhöft, S.; Reiss, L.K.; Orlikowsky, T.W. Amphiregulin Regulates Phagocytosis-Induced Cell Death in Monocytes via EGFR and the Bcl-2 Protein Family. Mediat. Inflamm. 2019, 2019, 1603131. [Google Scholar] [CrossRef]
- Dreschers, S.; Gille, C.; Haas, M.; Grosse-Ophoff, J.; Schneider, M.; Leiber, A.; Bühring, H.-J.; Orlikowsky, T.W. Infection–induced Bystander-Apoptosis of Monocytes Is TNF-alpha-mediated. PLoS ONE. 2013, 8, e53589. [Google Scholar] [CrossRef] [PubMed]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-alpha signaling in macrophages. Crit. Rev. Eukaryot. Gene. Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Humberg, A.; Fortmann, I.; Siller, B.; Kopp, M.V.; Herting, E.; Gopel, W.; Hartel, C.; German Neonatal Network, German Center for Lung Research and Priming Immunity at the Beginning of Life (PRIMAL) Consortium. Preterm birth and sustained inflammation: Consequences for the neonate. Semin. Immunopathol. 2020, 4, 451–468. [Google Scholar] [CrossRef]
- Ito, S.; Tanaka, Y.; Oshino, R.; Okado, S.; Hori, M.; Isobe, K.I. GADD34 suppresses lipopolysaccharide-induced sepsis and tissue injury through the regulation of macrophage activation. Cell Death Dis. 2016, 7, e2219. [Google Scholar] [CrossRef]
- Li, M.; Huang, X.; Zhuo, Q.; Zhang, J.; Ju, X. Clinical significance of miR-129–5p in patients with neonatal sepsis and its regulatory role in the lipopolysaccharide-induced inflammatory response. Bosn. J. Basic Med. Sci. 2020. [Google Scholar] [CrossRef]
- Carbone, F.; Montecucco, F.; Sahebkar, A. Current and emerging treatments for neonatal sepsis. Expert Opin. Pharm. 2020, 21, 549–556. [Google Scholar] [CrossRef]
- Aziz, M.; Jacob, A.; Matsuda, A.; Wu, R.; Zhou, M.; Dong, W.; Yang, W.L.; Wang, P. Pre-treatment of recombinant mouse MFG-E8 downregulates LPS-induced TNF-alpha production in macrophages via STAT3-mediated SOCS3 activation. PLoS ONE 2011, 6, e27685. [Google Scholar] [CrossRef]
- Shen, H.; Lei, Y.; He, X.; Liu, D.; He, Z. Role of lactadherin in intestinal barrier integrity in experimental neonatal necrotizing enterocolitis. J. Cell Biochem. 2019, 2, 19509–19517. [Google Scholar] [CrossRef]
- Hansen, L.W.; Yang, W.L.; Bolognese, A.C.; Jacob, A.; Chen, T.; Prince, J.M.; Nicastro, J.M.; Coppa, G.F.; Wang, P. Treatment with milk fat globule epidermal growth factor-factor 8 (MFG-E8) reduces inflammation and lung injury in neonatal sepsis. Surgery 2017, 162, 349–357. [Google Scholar] [CrossRef]
- Hansen, L.W.; Khader, A.; Yang, W.L.; Jacob, A.; Chen, T.; Nicastro, J.M.; Coppa, G.F.; Prince, J.M.; Wang, P. Deficiency in milk fat globule-epidermal growth factor-factor 8 exacerbates organ injury and mortality in neonatal sepsis. J. Pediatric Surg. 2017, 52, 1520–1527. [Google Scholar] [CrossRef]
- Asaro, J.A.; Khan, Z.; Brewer, M.; Klose, K.; Pesce, C.; Schanler, R.J.; Codipilly, C.N. Relationship Between Milk Fat Globule-Epidermal Growth Factor 8 and Intestinal Cytokines in Infants Born Preterm. J. Pediatr. 2021, 230, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef]
- Orlikowsky, T.; Wang, Z.Q.; Dudhane, A.; Horowitz, H.; Conti, B.; Hoffmann, M.K. Two distinct pathways of human macrophage differentiation are mediated by interferon-gamma and interleukin-10. Mmunology 1997, 191, 104–108. [Google Scholar]
- Gille, C.; Spring, B.; Tewes, L.J.; Loffler, J.; Dannecker, G.E.; Hoffmann, M.K.; Eichner, M.; Poets, C.F.; Orlikowsky, T.W. Diminished response to interleukin-10 and reduced antibody-dependent cellular cytotoxicity of cord blood monocyte-derived macrophages. Pediatr. Res. 2006, 60, 152–157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dreschers, S.; Ohl, K.; Schulte, N.; Tenbrock, K.; Orlikowsky, T.W. Impaired functional capacity of polarised neonatal macrophages. Sci. Rep. 2020, 10, 624. [Google Scholar] [CrossRef]
- Passlick, B.; Flieger, D.; Ziegler-Heitbrock, H.W. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 1089, 74, 2527–2534. [Google Scholar] [CrossRef]
- Wong, K.L.; Yeap, W.H.; Yi Tai, J.J.; Ong, S.W.; Dang, T.M.; Wong, S.C. The three human monocyte subsets: Implications for health and disease. Immunol. Res. 2011, 53, 41–57. [Google Scholar] [CrossRef]
- Ozanska, A.; Szymczak, D.; Rybka, J. Pattern of human monocyte subpopulations in health and disease. Scand. J. Immunol. 2020, 92, e12883. [Google Scholar] [CrossRef] [PubMed]
- da Mota, N.V.F.; Brunialti, M.K.C.; Santos, S.S.; Machado, F.R.; Assuncao, M.; Azevedo, L.C.P.; Salomao, R. Immunophenotyping of Monocytes During Human Sepsis Shows Impairment in Antigen Presentation: A Shift Toward Nonclassical Differentiation and Upregulation of FCgammaRi-Receptor. Shock 2018, 50, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.; Mazzei, A.; Palumbo, C.; Verri, T.; Lobreglio, G. Flow Cytometric Analysis of Monocytes Polarization and Reprogramming from Inflammatory to Immunosuppressive Phase During Sepsis. EJIFCC 2019, 30, 371–384. [Google Scholar]
- Nguyen, M.; Leuridan, E.; Zhang, T.; De Wit, D.; Willems, F.; Van Damme, P.; Goldman, M.; Goriely, S. Acquisition of adult-like TLR4 and TLR9 responses during the first year of life. PLoS ONE 2010, 5, e10407. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, D.; Teodosio, C.; van den Bossche, V.B.L.; Perez-Andres, M.; Arriba, S.; Munoz-Bellvis, L.; Romero, A.; Blanco, J.F.; Remesal, A.; Puig, N.; et al. Distribution of subsets of blood monocytic cells throughout life. J. Allergy. Clin. Immunol. 2019, 144, 320–323. [Google Scholar] [CrossRef]
- Murphy, F.J.; Reen, D.J. Differential expression of function-related antigens on newborn and adult monocyte subpopulations. Immunology 1996, 89, 587–591. [Google Scholar] [CrossRef]
- Skrzeczynska-Moncznik, J.; Bzowska, M.; Loseke, S.; Grage-Griebenow, E.; Zembala, M.; Pryjma, J. Peripheral blood CD14high CD16+ monocytes are main producers of IL-10. Scand. J. Immunol. 2008, 67, 152–159. [Google Scholar] [CrossRef]
- Brook, B.; Harbeson, D.; Ben-Othman, R.; Viemann, D.; Kollmann, T.R. Newborn susceptibility to infection vs. disease depends on complex in vivo interactions of host and pathogen. Semin. Immunopathol. 2017, 36, 615–625. [Google Scholar] [CrossRef]
- Sugitharini, V.; Prema, A.; Berla Thangam, E. Inflammatory mediators of systemic inflammation in neonatal sepsis. Inflamm. Res. 2013, 62, 1025–1034. [Google Scholar] [CrossRef]
- Ye, Q.; Du, L.Z.; Shao, W.X.; Shang, S.Q. Utility of cytokines to predict neonatal sepsis. Pediatr. Res. 2017, 81, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Khaertynov, K.S.; Boichuk, S.V.; Khaiboullina, S.F.; Anokhin, V.A.; Andreeva, A.A.; Lombardi, V.C.; Satrutdinov, M.A.; Agafonova, E.A.; Rizvanov, A.A. Comparative Assessment of Cytokine Pattern in Early and Late Onset of Neonatal Sepsis. J. Immunol. Res. 2017, 2017, 8601063. [Google Scholar] [CrossRef] [PubMed]
- Leal, Y.A.; Alvarez-Nemegyei, J.; Lavadores-May, A.I.; Giron-Carrillo, J.L.; Cedillo-Rivera, R.; Velazquez, J.R. Cytokine profile as diagnostic and prognostic factor in neonatal sepsis. J. Matern. Fetal. Neonatal. Med. 2019, 32, 2830–2836. [Google Scholar] [CrossRef] [PubMed]
- Ebenebe, C.U.; Hesse, F.; Blohm, M.E.R.; Jung, R.; Kunzmann, S.; Singer, D. Diagnostic accuracy of interleukin-6 for early-onset sepsis in preterm neonates. J. Matern. Fetal. Neonatal. Med. 2021, 34, 253–258. [Google Scholar] [CrossRef]
- Rodrıguez-Gaspar, M.; Santolaria, F.; Lopez, A.J.; Gonzalez-Reimers, E.; Milena, A.; Rodrıguez-Rodrıguez, E.; Gomez-Sirvent, J.L. Prognostic value of cytokines in SIRS general medical patients. Cytokine 2001, 15, 232–236. [Google Scholar] [CrossRef]
- Chen, X.F.; Wu, J.; Zhang, Y.D.; Zhang, C.X.; Chen, X.T.; Sun, J.H.; Chen, T.X.; Sun, J.H. Role of Zc3h12a in enhanced IL-6 production by newborn mononuclear cells in response to lipopolysaccharide. Pediatr. Neonatol. 2018, 59, 288–295. [Google Scholar] [CrossRef]
- Chen, X.F.; Wu, J.; Zhang, Y.D.; Zhang, C.X.; Chen, X.T.; Zhao, W.; Chen, T.X. Role of SOCS3 in enhanced acute-phase protein genes by neonatal macrophages in response to IL-6. J. Microbiol. Immunol. Infect. 2021, 54, 206–212. [Google Scholar] [CrossRef]
- Lingnau, M.; Höflich, C.; Volk, H.-D.; Sabat, R.; Döcke, W.-D. Interleukin-10 enhances the CD14-dependent phagocytosis of bacteria and apoptotic cells by human monocytes. Human Immunol. 2007, 68, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Mazer, M.; Unsinger, J.; Drewry, A.; Walton, A.; Osborne, D.; Blood, T.; Hotchkiss, R.; Remy, K.E. IL-10 Has Differential Effects on the Innate and Adaptive Immune Systems of Septic Patients. J. Immunol. 2019, 203, 2088–2099. [Google Scholar] [CrossRef]
- Wu, H.P.; Chu, C.M.; Kao, K.C.; Huang, S.H.; Chuang, D.Y. High Interleukin-10 Expression in Type 2 T Helper Cells in Septic Patients. Immunol. Investig. 2017, 46, 385–394. [Google Scholar] [CrossRef]
- Fabri, A.; Kandara, K.; Coudereau, R.; Gossez, M.; Abraham, P.; Monard, C.; Cour, M.; Rimmele, T.; Argaud, L.; Monneret, G.; et al. Characterization of Circulating IL-10-Producing Cells in Septic Shock Patients: A Proof of Concept Study. Front. Immunl. 2020, 11, 615009. [Google Scholar] [CrossRef]
- Davidson, D.; Miskolci, V.; Clark, D.C.; Dolmaian, G.; Vancurova, I. Interleukin-10 production after pro-inflammatory stimulation of neutrophils and monocytic cells of the newborn. Neonatology 2007, 92, 127–133. [Google Scholar] [CrossRef]
- Roger, T.; Schneider, A.; Weier, M.; Sweep, F.C.; le Roy, D.; Bernhagen, J.; Calandra, T.; Giannoni, E. High expression levels of macrophage migration inhibitory factor sustain the innate immune responses of neonates. Proc. Natl. Acad. Sci. USA 2016, 113, E997–E1005. [Google Scholar] [CrossRef]
- Lawrence, S.M.; Ruoss, J.L.; Wynn, J.L. IL-17 in neonatal health and disease. Am. J. Reprod. Immunol. 2018, 79, e12800. [Google Scholar] [CrossRef]
- Cherwinski, H.M.; Schumacher, J.H.; Brown, K.D.; Mosmann, T.R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J. Exp. Med. 1987, 166, 1229–1244. [Google Scholar] [CrossRef] [PubMed]
- Maródi, L.; Goda, K.; Palicz, A.; Szabó, G. Cytokine receptor signalling in neonatal macrophages: Defective STAT-1 phosphorylation in response to stimulation with IFN-gamma. Clin. Exp. Immunol. 2001, 26, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Marodi, L. Neonatal innate immunity to infectious agents. Infect. Immun. 2006, 4, 1999–2006. [Google Scholar] [CrossRef]
- Marodi, L. Down-regulation of Th1 responses in human neonates. Clin. Exp. Immunol. 2002, 128, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sung, N.; Gilman-Sachs, A.; Kwak-Kim, J. T Helper (Th) Cell Profiles in Pregnancy and Recurrent Pregnancy Losses: Th1/Th2/Th9/Th17/Th22/Tfh Cells. Front. Immunol. 2020, 11, 2025. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F.; Fitch, F.W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J. Immunol. 1988, 140, 4245–4252. [Google Scholar]
- Fiorentino, D.F.; Zlotnik, A.; Vieira, P.; Mosmann, T.R.; Howard, M.; Moore, K.W.; O’Garra, A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 1991, 146, 3444–3451. [Google Scholar] [PubMed]
- Coakley, J.D.; Breen, E.P.; Moreno-Olivera, A.; Al-Harbi, A.I.; Melo, A.M.; O’Connell, B.; McManus, R.; Doherty, D.G.; Ryan, T. Dysregulated T helper type 1 (Th1) and Th17 responses in elderly hospitalised patients with infection and sepsis. PLoS ONE 2019, 14, e0224276. [Google Scholar] [CrossRef] [PubMed]
- Manzoli, T.F.; Troster, E.J.; Ferreira-Ferranti, J.; Mirtes-Sales, M. Prolonged suppression of monocytic human leukocyte antigen–DR expression correlates with mortality in pediatric septic patients in a pediatric tertiary Intensive Care Unit. J. Crit. Care 2016, 33, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Boeddha, N.P.; Kerklaan, D.; Dunbar, A.; van Puffelen, E.; Nagtzaam, N.M.A.; Vanhorebeek, I.; Van den Berghe, G.; Hazelzet, J.A.; Joosten, K.F.; Verbruggen, S.C.; et al. HLA-DR Expression on Monocyte Subsets in Critically Ill Children. Pediatr. Infect. Dis. J. 2018, 37, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Genel, F.; Atlihan, F.; Ozsu, F.; Ozbek, E. Monocyte HLA-DR expression as predictor of poor outcome in neonates with late onset neonatal sepsis. J Infect. 2010, 60, 224–228. [Google Scholar] [CrossRef]
- Schefold, J.C.; Porz, L.; Uebe, B.; Poehlmann, H.; von Haehling, S.; Jung, A.; Unterwalder, N.; Meisel, C. Diminished HLA-DR expression on monocyte and dendritic cell subsets indicating impairment of cellular immunity in pre-term neonates: A prospective observational analysis. J. Perinat. Med. 2015, 43, 609–618. [Google Scholar] [CrossRef]
- Juskewitch, J.E.; Abraham, R.S.; League, S.C.; Jenkins, S.M.; Smith, C.Y.; Enders, F.T.; Grebe, S.K.; Carey, W.A.; Huskins, W.C. Monocyte HLA-DR expression and neutrophil CD64 expression as biomarkers of infection in critically ill neonates and infants. Pediatr. Res. 2015, 78, 683–690. [Google Scholar] [CrossRef]
- Pradhan, R.; Jain, P.; Paria, A.; Saha, A.; Sahoo, J.; Sen, A.; Mukherjee, S.; Som, T.; Hazra, A.; Warner, N.; et al. Ratio of neutrophilic CD64 and monocytic HLA-DR: A novel parameter in diagnosis and prognostication of neonatal sepsis. Cytom. B Clin Cytom. 2016, 90, 295–302. [Google Scholar] [CrossRef]
- Venet, F.; Pachot, A.; Debard, A.I.; Bohe, J.; Bienvenu, J.; Lepape, A.; Monneret, G. Increased percentage of CD4+CD25+ regulatory T cells during septic shock is due to the decrease of CD4+CD25- lymphocytes. Crit. Care Med. 2004, 32, 2329–2331. [Google Scholar] [CrossRef]
- Nascimento, D.C.; Melo, P.H.; Piñeros, A.R.; Ferreira, R.G.; Colón, D.F.; Donate, P.B.; Castanheira, F.V.; Gozzi, A.; Czaikoski, P.G.; Niedbala, W.; et al. IL-33 contributes to sepsis-induced long-term immunosuppression by expanding the regulatory T cell population. Nat. Commun. 2017, 8, 14919. [Google Scholar] [CrossRef]
- Wolk, K.; Docke, W.; von Baehr, V.; Volk, H.; Sabat, R. Comparison of monocyte functions after LPS- or IL-10-induced reorientation: Importance in clinical immunoparalysis. Pathobiology 1999, 67, 253–256. [Google Scholar] [CrossRef]
- Hikita, N.; Cho, Y.; Tachibana, D.; Hamazaki, T.; Koyama, M.; Tokuhara, D. Cell surface antigens of neonatal monocytes are selectively impaired in basal expression, but hyperresponsive to lipopolysaccharide and zymosan. J. Reprod. Immunol. 2019, 136, 102614. [Google Scholar] [CrossRef]
- Zahran, A.M.; Saad, K.; Abdel-Raheem, Y.F.; Elsayh, K.I.; El-Houfey, A.A.; Aboul-Khair, M.D.; Alblihed, M.A. Characterization of Regulatory T Cells in Preterm and Term Infants. Arch. Immunol. Ther. Exp. 2019, 67, 49–54. [Google Scholar] [CrossRef]
- Lee, J.G.; Jaeger, K.E.; Seki, Y.; Lim, Y.W.; Cunha, C.; Vuchkovska, A.; Nelson, A.J.; Nikolai, A.; Kim, D.; Nishimura, M.; et al. Human CD36hi monocytes induce Foxp3+ CD25+ T cells with regulatory functions from CD4 and CD8 subsets. Immunology 2021, 163, 293–309. [Google Scholar] [CrossRef]
- Pang, Y.; Du, X.; Xu, X.; Wang, M.; Li, Z. Monocyte activation and inflammation can exacerbate Treg/Th17 imbalance in infants with neonatal necrotizing enterocolitis. Int. Immunopharmacol. 2018, 59, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, A.S.; Pirr, S.; Fehlhaber, B.; Mellinger, L.; Burgmann, J.; Busse, M.; Ginzel, M.; Friesenhagen, J.; von Kockritz-Blickwede, M.; Ulas, T.; et al. In neonates S100A8/S100A9 alarmins prevent the expansion of a specific inflammatory monocyte population promoting septic shock. FASEB J. 2017, 31, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Ehrchen, J.M.; Sunderkötter, C.; Foell, D.; Vogl, T.; Roth, J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J. Leukoc. Biol. 2009, 86, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Pruenster, M.; Vogl, T.; Roth, J.; Sperandio, M. S100A8/A9: From basic science to clinical application. Pharmacol. Ther. 2016, 167, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Austermann, J.; Friesenhagen, J.; Fassl, S.K.; Petersen, B.; Ortkras, T.; Burgmann, J.; Barczyk-Kahlert, M.; Faist, E.; Zedler, S.; Pirr, S.; et al. Alarmins MRP8 and MRP14 induce stress tolerance in phagocytes under sterile inflammatory conditions. Cell Rep. 2014, 9, 2112–2123. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.; Tenbrock, K.; Ludwig, S.; Leukert, N.; Ehrhardt, C.; van Zoelen, M.A.; Nacken, W.; Foell, D.; van der Poll, C.; Sorg, C.; et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. N. Nat. Med. 2007, 13, 1042–1049. [Google Scholar] [CrossRef]
- Ulas, T.; Pirr, S.; Fehlhaber, B.; Bickes, M.S.; Loof, T.G.; Vogl, T.; Mellinger, L.; Heinemann, A.S.; Burgmann, J.; Schöning, J.; et al. S100-alarmin-induced innate immune programming protects newborn infants from sepsis. Nat. Immunol. 2017, 18, 622–632. [Google Scholar] [CrossRef]
- Pirr, S.; Richter, M.; Fehlhaber, B.; Pagel, J.; Hartel, C.; Roth, J.; Vogl, T.; Viemann, D. High Amounts of S100-Alarmins Confer Antimicrobial Activity on Human Breast Milk Targeting Pathogens Relevant in Neonatal Sepsis. Front. Immunol. 2017, 8, 1822. [Google Scholar] [CrossRef]
- Van Lent, P.L.; Grevers, L.C.; Blom, A.B.; Arntz, O.J.; van de Loo, F.A.; van den Kraan, P.; Abdollahi-Roodsaz, S.; Srikrishna, G.; Freeze, H.; Sloetjes, A.; et al. Stimulation of chondrocyte-mediated cartilage destruction by S100A8 in experimental murine arthritis. Arthritis Rheum. 2008, 58, 3776–3787. [Google Scholar] [CrossRef]
- Golubinskaya, V.; Puttonen, H.; Fyhr, I.M.; Rydbeck, H.; Hellstrom, A.; Jacobsson, B.; Nilsson, H.; Mallard, C.; Savman, K. Expression of S100A Alarmins in Cord Blood Monocytes Is Highly Associated with Chorioamnionitis and Fetal Inflammation in Preterm Infants. Front. Immunol. 2020, 11, 1194. [Google Scholar] [CrossRef]
- Viemann, D. S100-Alarmins Are Essential Pilots of Postnatal Innate Immune Adaptation. Front. Immunol. 2020, 11, 688. [Google Scholar] [CrossRef] [PubMed]
- Willers, M.; Ulas, T.; Vollger, L.; Vogl, T.; Heinemann, A.S.; Pirr, S.; Pagel, J.; Fehlhaber, B.; Halle, O.; Schoning, J.; et al. S100A8 and S100A9 Are Important for Postnatal Development of Gut Microbiota and Immune System in Mice and Infants. Gastroenterology 2020, 159, 2130–2145. [Google Scholar] [CrossRef] [PubMed]
- Dreschers, S.; Ohl, K.; Lehrke, M.; Möllmann, J.; Denecke, B.; Costa, I.; Vogl, T.; Viemann, D.; Roth, J.; Orlikowsky, T.; et al. Impaired cellular energy metabolism in cord blood macrophages contributes to abortive response toward inflammatory threats. Nat. Commun. 2019, 10, 1685. [Google Scholar] [CrossRef] [PubMed]
- Diskin, C.; Palsson-McDermott, E.M. Metabolic Modulation in Macrophage Effector Function. Front. Immunol. 2018, 9, 270. [Google Scholar] [CrossRef]
- Freemerman, A.J.; Johnson, A.R.; Sacks, G.N.; Milner, J.J.; Kirk, E.L.; Troester, M.A.; Macintyre, A.N.; Goraksha-Hicks, P.; Rathmell, J.C.; Makowski, L. Metabolic reprogramming of macrophages: Glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J. Biol. Chem. 2014, 289, 7884–7896. [Google Scholar] [CrossRef]
- Millet, P.; Vachharajani, V.; McPhail, L.; Yoza, B.; McCall, C.E. GAPDH Binding to TNF-alpha mRNA Contributes to Posttranscriptional Repression in Monocytes: A Novel Mechanism of Communication between Inflammation and Metabolism. J. Immunol. 2016, 196, 6. [Google Scholar] [CrossRef]
- Warburg, O.; Wind, F.; Negelein, E. The Metabolism of Tumors in the Body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef]
- Huang, S.C.; Smith, A.M.; Everts, B.; Colonna, M.; Pearce, E.L.; Schilling, J.D.; Pearce, E.J. Metabolic Reprogramming Mediated by the mTORC2-IRF4 Signaling Axis Is Essential for Macrophage Alternative Activation. Immunity 2016, 45, 817–830. [Google Scholar] [CrossRef]
- Kan, B.; Michalski, C.; Fu, H.; Au, H.H.T.; Lee, K.; Marchant, E.A.; Cheng, M.F.; Anderson-Baucum, E.; Aharoni-Simon, M.; Tilley, P.; et al. Cellular metabolism constrains innate immune responses in early human ontogeny. Nat. Commun. 2018, 16, 4822. [Google Scholar] [CrossRef]
- Maoldomhnaigh, C.Ó.; Cox, D.J.; Phelan, J.J.; Malone, F.D.; Keane, J.; Basdeo, S.A. The Warburg Effect Occurs Rapidly in Stimulated Human Adult but Not Umbilical Cord Blood Derived Macrophages. Front. Immunol. 2021, 12, 657261. [Google Scholar] [CrossRef]
- Dreschers, S.; Ohl, K.; Mollmann, J.; Tenbrock, K.; Orlikowsky, T.W. Leucine Reconstitutes Phagocytosis-Induced Cell Death in E. coli-Infected Neonatal Monocytes-Effects on Energy Metabolism and mTOR Signaling. Int. J. Mol. Sci. 2021, 22, 4271. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.C.; Scicluna, B.P.; Arts, R.J.W.; Gresnigt, M.S.; Lachmandas, E.; Giamarellos-Bourboulis, E.J.; Kox, M.; Manjeri, G.R.; Wagenaars, J.A.L.; Cremer, O.L.; et al. Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in se. Nat. Immunol. 2016, 17, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Harbeson, D.; Francis, F.; Bao, W.; Amenyogbe, N.A.; Kollmann, T. Energy Demands of Early Life Drive a Disease Tolerant Phenotype and Dictate Outcome in Neonatal Bacterial Sepsis. Front. Immunol. 2018, 9, 1918. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.L.; Dickinson, P.; Forster, T.; Craigon, M.; Ross, A.; Khondoker, M.R.; France, R.; Ivens, A.; Lynn, D.J.; Orme, J.; et al. Identification of a human neonatal immune-metabolic network associated with bacterial infection. Nat. Commun. 2014, 14, 4649. [Google Scholar] [CrossRef]
- Conti, M.G.; Angelidou, A.; Diray-Arce, J.; Smolen, K.K.; Lasky-Su, J.; De Curtis, M.; Levy, O. Immunometabolic approaches to prevent, detect, and treat neonatal sepsis. Pediatr. Res. 2020, 87, 399–405. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).