Transmembrane Mucin-1 Facilitates Oral Microbial Colonization in Oral Cancer
Abstract
1. Introduction
2. Oral Mucosal Defense
3. Oral Mucosal Epithelium and Oral Microbiome
4. Saliva Regulates Oral Microbiota
5. Oral Biofilm and Oral Microbiota
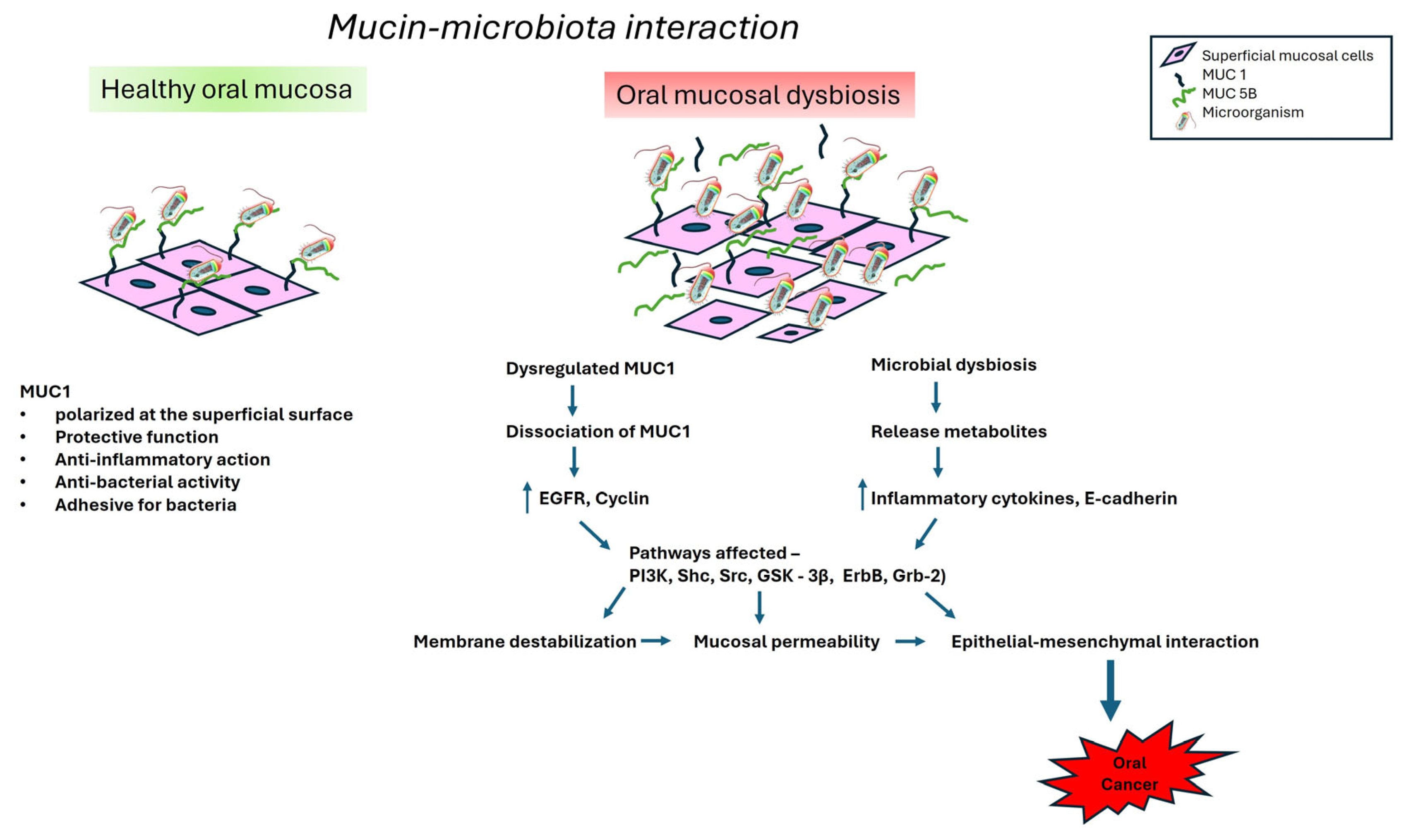
6. Interactions Between Mucin and Oral Microbiota
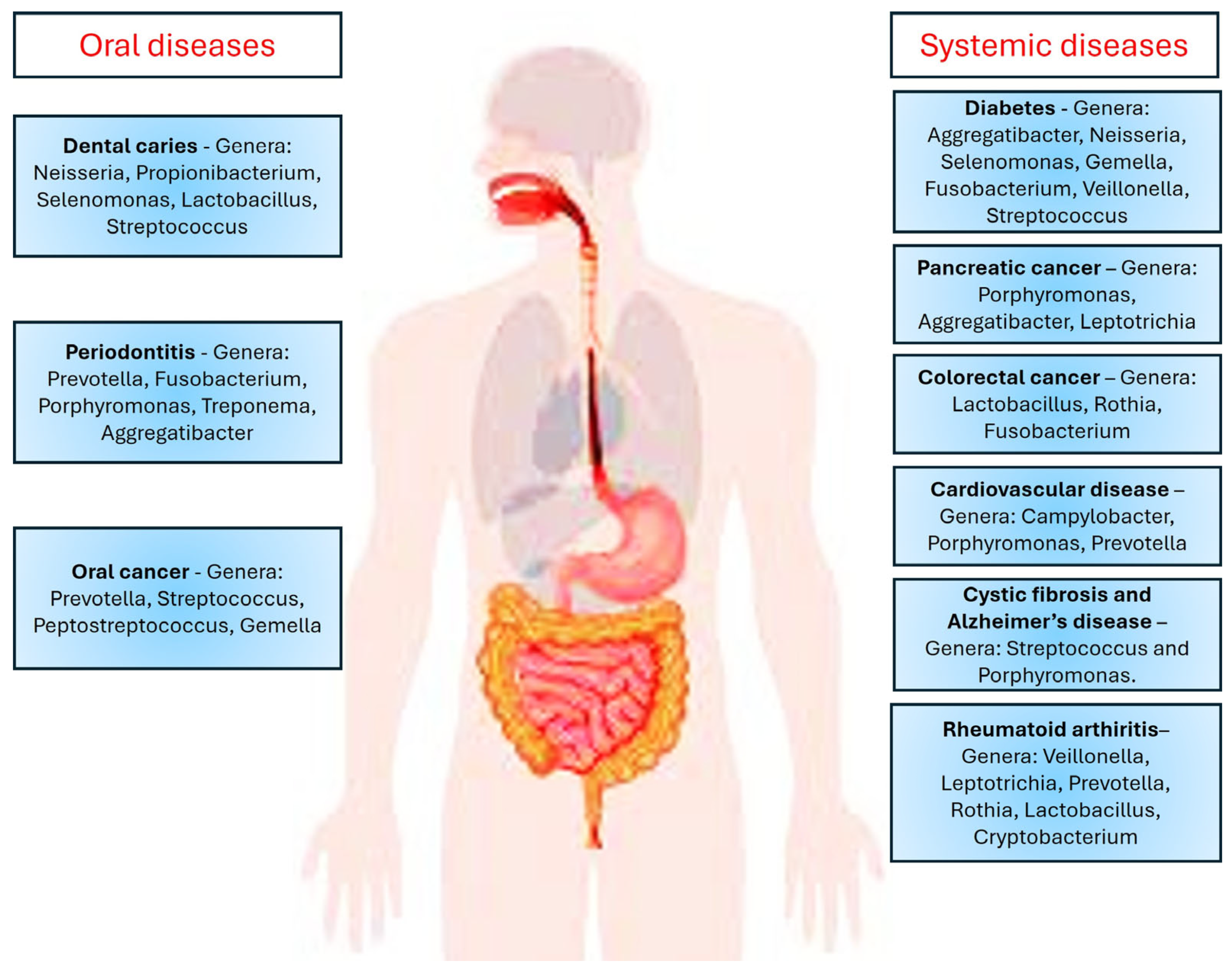
7. Involvement of Mucins and Microbes in Several Oral Diseases
7.1. Oral Infectious Diseases
7.2. Oral Precancer
7.3. Oral Cancer
8. Mucin–Oral Microbiome—A Potential Biomarkers
9. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Roi, A.; Roi, C.I.; Andreescu, N.I.; Riviş, M.; Badea, I.D.; Meszaros, N.; Rusu, L.C.; Iurciuc, S. Oral cancer histopathological subtypes in association with risk factors: A 5-year retrospective study. Rom. J. Morphol. Embryol. 2020, 61, 1213–1220. [Google Scholar] [CrossRef]
- Tarle, M.; Lukšić, I. Pathogenesis and Therapy of Oral Carcinogenesis. Int. J. Mol. Sci. 2024, 25, 6343. [Google Scholar] [CrossRef] [PubMed]
- Metsäniitty, M.; Hasnat, S.; Salo, T.; Salem, A. Oral Microbiota-A New Frontier in the Pathogenesis and Management of Head and Neck Cancers. Cancers 2021, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Delgado, R.Z.R.; Frias-Lopez, J. The Oral Microbiome and Cancer. Front. Immunol. 2020, 11, 591088. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, Q.; Peng, Y.; Wang, D.; Liu, Y. The effect of periodontal bacteria infection on incidence and prognosis of cancer: A systematic review and meta-analysis. Medicine 2020, 99, e19698. [Google Scholar] [CrossRef]
- HOMD. Human Oral Microbiome Database. Available online: http://www.homd.org/ (accessed on 10 April 2025).
- Hyvärinen, E.; Kashyap, B.; Kullaa, A.M. Oral Sources of Salivary Metabolites. Metabolites 2023, 13, 498. [Google Scholar] [CrossRef]
- Tuominen, H.; Rautava, J. Oral Microbiota and Cancer Development. Pathobiology 2021, 88, 116–126. [Google Scholar] [CrossRef]
- Asikainen, P.; Ruotsalainen, T.J.; Mikkonen, J.J.W.; Koistinen, A.P.; Bruggenkate, T.C.; Kullaa, A.M. The defence architecture of the superficial cells of oral mucosa. Med. Hypothesis 2012, 78, 790–792. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Ni, W.; Tai, G. Expression of MUC1 in different tumours and its clinical significance (Review). Mol. Clin. Oncol. 2022, 17, 161. [Google Scholar] [CrossRef]
- Thakur, A.; Tupkari, J.V.; Joy, T.; Kende, P.P.; Siwach, P.; Ahire, M.S. Expression of mucin-1 in oral squamous cell carcinoma and normal oral mucosa: An immunohistochemical study. J. Oral Maxillofac. Pathol. 2018, 22, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, B.; Kullaa, A.M. Regulation of mucin 1 expression and its relationship with oral diseases. Arch. Oral Biol. 2020, 117, 104791. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, B.; Kullaa, A. Salivary Metabolites Produced by Oral Microbes in Oral Diseases and Oral Squamous Cell Carcinoma: A Review. Metabolites 2024, 14, 277. [Google Scholar] [CrossRef] [PubMed]
- Su, S.C.; Chang, L.C.; Huang, H.D.; Peng, C.Y.; Chuang, C.Y.; Chen, Y.T.; Lu, M.Y.; Chiu, Y.W.; Chen, P.Y.; Yang, S.F. Oral microbial dysbiosis and its performance in predicting oral cancer. Carcinogenesis 2021, 42, 127–135. [Google Scholar] [CrossRef]
- Wang, K.; Miao, T.; Lu, W.; He, J.; Cui, B.; Li, J.; Li, Y.; Xiao, L. Analysis of oral microbial community and Th17-associated cytokines in saliva of patients with oral lichen planus. Microbiol. Immunol. 2015, 59, 105–113. [Google Scholar] [CrossRef]
- Agrawal, B.; Gupta, N.; Konowalchuk, J.D. MUC1 Mucin: A Putative Regulatory (Checkpoint) Molecule of T Cells. Front. Immunol. 2018, 9, 2391. [Google Scholar] [CrossRef]
- Soderholm, A.T.; Pedicord, V.A. Intestinal epithelial cells: At the interface of the microbiota and mucosal immunity. Immunology 2019, 158, 267–280. [Google Scholar] [CrossRef]
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.Y.; Ko, H.J.; Vallance, B.A. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 2018, 39, 677–696. [Google Scholar] [CrossRef]
- Groeger, S.; Meyle, J. Oral Mucosal Epithelial Cells. Front. Immunol. 2019, 10, 208. [Google Scholar] [CrossRef]
- Yamashita, Y.; Takeshita, T. The oral microbiome and human health. J. Oral Sci. 2017, 59, 201–206. [Google Scholar] [CrossRef]
- Sultan, A.S.; Kong, E.F.; Rizk, A.M.; Jabra-Rizk, M.A. The oral microbiome: A lesson in coexistence. PLoS Pathog. 2018, 14, e1006719. [Google Scholar] [CrossRef]
- Ammam, I.; Pailler-Mattéi, C.; Ouillon, L.; Nivet, C.; Vargiolu, R.; Neiers, F.; Canon, F.; Zahouani, H. Exploring the role of the MUC1 mucin in human oral lubrication by tribological in vitro studies. Sci. Rep. 2024, 14, 31019. [Google Scholar] [CrossRef]
- Lindén, S.K.; Sheng, Y.H.; Every, A.L.; Miles, K.M.; Skoog, E.C.; Florin, T.H.; Sutton, P.; McGuckin, M.A. MUC1 limits Helicobacter pylori infection both by steric hindrance and by acting as a releasable decoy. PLoS Pathog. 2009, 5, e1000617. [Google Scholar] [CrossRef]
- Sasai, M.; Yamamoto, M. Pathogen recognition receptors: Ligands and signaling pathways by Toll-like receptors. Int. Rev. Immunol. 2013, 32, 116–133. [Google Scholar] [CrossRef]
- Carraway, K.I.; Ramsauer, V.P.; Haq, B.; Carraway, C.A.C. Cell signaling through membrane mucins. Bioessays 2003, 25, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Nunes, D.P.; Troxler, R.F.; Offner, G.D. Pro-inflammatory cytokines up-regulate MUC1 gene expression in oral epithelial cells. J. Dent. Res. 2003, 82, 883–887. [Google Scholar] [CrossRef]
- Ptasiewicz, M.; Grywalska, E.; Mertowska, P.; Korona-Głowniak, I.; Poniewierska-Baran, A.; Niedźwiedzka-Rystwej, P.; Chałas, R. Armed to the Teeth-The Oral Mucosa Immunity System and Microbiota. Int. J. Mol. Sci. 2022, 23, 882. [Google Scholar] [CrossRef]
- Pathak, J.L.; Yan, Y.; Zhang, Q.; Wang, L.; Ge, L. The role of oral microbiome in respiratory health and diseases. Respir. Med. 2021, 185, 106475. [Google Scholar] [CrossRef]
- Sharma, N.; Bhatia, S.; Sodhi, A.S.; Batra, N. Oral microbiome and health. AIMS Microbiol. 2018, 4, 42–66. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, T.; Kageyama, S.; Furuta, M.; Tsuboi, H.; Takeuchi, K.; Shibata, Y.; Shimazaki, Y.; Akifusa, S.; Ninomiya, T.; Kiyohara, Y.; et al. Bacterial diversity in saliva and oral health-related conditions: The Hisayama Study. Sci. Rep. 2016, 6, 22164. [Google Scholar] [CrossRef] [PubMed]
- Kozak, M.; Pawlik, A. The Role of the Oral Microbiome in the Development of Diseases. Int. J. Mol. Sci. 2023, 24, 5231. [Google Scholar] [CrossRef]
- Hoare, A.; Soto, C.; Rojas-Celis, V.; Bravo, D. Chronic Inflammation as a Link between Periodontitis and Carcinogenesis. Mediators Inflamm. 2019, 2019, 1029857. [Google Scholar] [CrossRef]
- van Houte, J. Role of micro-organisms in caries etiology. J. Dent. Res. 1994, 73, 672–681. [Google Scholar] [CrossRef]
- van ’t Hof, W.; Veerman, E.C.; Nieuw Amerongen, A.V.; Ligtenberg, A.J. Antimicrobial defense systems in saliva. Monogr. Oral Sci. 2014, 24, 40–51. [Google Scholar] [PubMed]
- Marsh, P.D.; Do, T.; Beighton, D.; Devine, D.A. Influence of Saliva on the Oral Microbiota. Periodontology 2000 2016, 70, 80–92. [Google Scholar] [CrossRef]
- Jakubovics, N.S.; Kolenbrander, P.E. The road to ruin: The formation of disease-associated oral biofilms. Oral Dis. 2010, 16, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, B.; Padala, S.R.; Kaur, G.; Kullaa, A. Candida albicans Induces Oral Microbial Dysbiosis and Promotes Oral Diseases. Microorganisms 2024, 12, 2138. [Google Scholar] [CrossRef]
- García-Curiel, L.; Del Rocío López-Cuellar, M.; Rodríguez-Hernández, A.I.; Chavarría-Hernández, N. Toward understanding the signals of bacteriocin production by Streptococcus spp. and their importance in current applications. World J. Microbiol. Biotechnol. 2021, 37, 15. [Google Scholar] [CrossRef] [PubMed]
- Jakubovics, N.S.; Gill, S.R.; Vickerman, M.M.; Kolenbrander, P.E. Role of hydrogen peroxide in competition and cooperation between Streptococcus gordonii and Actinomyces naeslundii. FEMS Microbiol. Ecol. 2008, 66, 637–644. [Google Scholar] [CrossRef]
- Kim, D.; Koo, H. Spatial Design of Polymicrobial Oral Biofilm in Its Native Disease State. J. Dent. Res. 2020, 99, 597–603. [Google Scholar] [CrossRef]
- Huang, C.B.; Alimova, Y.; Myers, T.M.; Ebersole, J.L. Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch. Oral Biol. 2011, 56, 650–654. [Google Scholar] [CrossRef]
- Rudin, A.D.; Khamzeh, A.; Venkatakrishnan, V.; Basic, A.; Christenson, K.; Bylund, J. Short chain fatty acids released by Fusobacterium nucleatum are neutrophil chemoattractants acting via free fatty acid receptor 2 (FFAR2). Cell Microbiol. 2021, 23, e13348. [Google Scholar]
- Rudin, A.D.; Khamzeh, A.; Venkatakrishnan, V.; Persson, T.; Gabl, M.; Savolainen, O.; Forsman, H.; Dahlgren, C.; Christenson, K.; Bylund, J. Porphyromonas gingivalis Produce Neutrophil Specific Chemoattractants Including Short Chain Fatty Acids. Front. Cell. Infect. Microbiol. 2021, 10, 620681. [Google Scholar] [CrossRef]
- Nguyen, D.; Nguyen, T.K.; Rice, S.A.; Boyer, C. CO-Releasing Polymers Exert Antimicrobial Activity. Biomacromolecules 2015, 16, 2776–2786. [Google Scholar] [CrossRef]
- Takahashi, N.; Saito, K.; Schachtele, C.F.; Yamada, T. Acid tolerance and acid-neutralizing activity of Porphyromonas gingivalis, Prevotella intermedia and Fusobacterium nucleatum. Oral Microbiol. Immunol. 1997, 12, 323–328. [Google Scholar] [CrossRef]
- Erttmann, S.F.; Gekara, N.O. Hydrogen peroxide release by bacteria suppresses inflammasome-dependent innate immunity. Nat. Commun. 2019, 10, 3493. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Voltarelli, V.A.; Salinaro, A.T.; Modafferi, S.; Cuzzocrea, S.; Calabrese, E.J.; Di Paola, R.; Otterbein, L.E.; Calabrese, V. NO, CO and H2S: A trinacrium of bioactive gases in the brain. Biochem. Pharmacol. 2022, 202, 115122. [Google Scholar] [CrossRef]
- Jaffe, F.A. Pathogenicity of carbon monoxide. Am. J. Forensic Med. Pathol. 1997, 18, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.; Yaegaki, K.; Tonzetich, J. Effect of methyl mercaptan on synthesis and degradation of collagen. J. Periodontal. Res. 1996, 31, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, W.; Łukasiewicz, M.; Puppel, K. Biogenic amines: Formation, action and toxicity—A review. J. Sci. Food Agric. 2021, 101, 2634–2640. [Google Scholar] [CrossRef]
- Shatalin, K.; Shatalina, E.; Mironov, A.; Nudler, E. H2S: A universal defense against antibiotics in bacteria. Science 2011, 334, 986–990. [Google Scholar] [CrossRef]
- Dilek, N.; Papapetropoulos, A.; Toliver-Kinsky, T.; Szabo, C. Hydrogen sulfide: An endogenous regulator of the immune system. Pharmacol. Res. 2020, 161, 105119. [Google Scholar] [CrossRef]
- Uematsu, H.; Sato, N.; Hossain, M.Z.; Ikeda, T.; Hoshino, E. Degradation of arginine and other amino acids by butyrate-producing asaccharolytic anaerobic Gram-positive rods in periodontal pockets. Arch. Oral Biol. 2003, 48, 423–429. [Google Scholar] [CrossRef]
- Niederman, R.; Brunkhorst, B.; Smith, S.; Weinreb, R.N.; Ryder, M.I. Ammonia as a potential mediator of adult human periodontal infection: Inhibition of neutrophil function. Arch. Oral Biol. 1990, 35, 205S–209S. [Google Scholar] [CrossRef]
- Aruni, A.W.; Dou, Y.; Mishra, A.; Fletcher, H.M. The Biofilm Community-Rebels with a Cause. Curr. Oral Health Rep. 2015, 2, 48–56. [Google Scholar] [CrossRef]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, J.; Zhang, L.; Zhou, X.; Cisar, J.O.; Palmer, R.J., Jr. Differential Utilization of Basic Proline-Rich Glycoproteins during Growth of Oral Bacteria in Saliva. Appl. Environ. Microbiol. 2016, 82, 5249–5258. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.X.; Wu, C.M.; Ribbeck, K. Home, sweet home: How mucus accommodates our microbiota. FEBS J. 2021, 288, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, E.S.; Ribbeck, K. Salivary mucins promote the coexistence of competing oral bacterial species. ISME J. 2017, 11, 1286–1290. [Google Scholar] [CrossRef]
- Wu, C.M.; Wheeler, K.M.; Cárcamo-Oyarce, G.; Aoki, K.; McShane, A.; Datta, S.S.; Welch, J.L.M.; Tiemeyer, M.; Griffen, A.L.; Ribbeck, K. Mucin glycans drive oral microbial community composition and function. npj Biofilms Microbiomes 2023, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Takagi, J.; Aoki, K.; Turner, B.S.; Lamont, S.; Lehoux, S.; Kavanaugh, N.; Gulati, M.; Valle Arevalo, A.; Lawrence, T.J.; Kim, C.Y.; et al. Mucin O-glycans are natural inhibitors of Candida albicans pathogenicity. Nat. Chem. Biol. 2022, 18, 762–773. [Google Scholar] [CrossRef]
- Wang, B.X.; Wheeler, K.M.; Cady, K.C.; Lehoux, S.; Cummings, R.D.; Laub, M.T.; Ribbeck, K. Mucin Glycans Signal through the Sensor Kinase RetS to Inhibit Virulence-Associated Traits in Pseudomonas aeruginosa. Curr. Biol. 2021, 31, 90–102.e7. [Google Scholar] [CrossRef]
- Thornton, D.J.; Khan, N.; Mehrotra, R.; Howard, M.; Veerman, E.; Packer, N.H.; Sheehan, J.K. Salivary mucin MG1 is comprised almost entirely of different glycosylated forms of the MUC5B gene product. Glycobiology 1999, 9, 293. [Google Scholar] [CrossRef]
- Dekker, J.; Strous, G.J. Covalent oligomerization of rat gastric mucin occurs in the rough endoplasmic reticulum, is N-glycosylation-dependent and precedes initial O-glycosylation. J. Biol. Chem. 1990, 265, 18116–18122. [Google Scholar] [CrossRef]
- Corfield, A.P. Mucins: A biologically relevant glycan barrier in mucosal protection. Biochim. Biophys. Acta. 2015, 1850, 236–252. [Google Scholar] [CrossRef]
- Hollingsworth, M.A.; Swanson, B.J. Mucins in cancer: Protection and control of the cell surface. Nat. Rev. Cancer 2004, 4, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.P.; Turner, B.S.; Afdhal, N.H.; Keates, S.; Ghiran, I.; Kelly, C.P.; Ewoldt, R.H.; McKinley, G.H.; So, P.; Erramilli, S.; et al. Helicobacter pylori moves through mucus by reducing mucin viscoelasticity. Proc. Natl. Acad. Sci. USA 2009, 106, 14321–14326. [Google Scholar] [CrossRef] [PubMed]
- Norin, K.; Gustafsson, B.E.; Lindblad, B.; Midtvedt, T. The establishment of some microflora associated biochemical characteristics in feces from children during the first years of life. Acta Paediatr. Scand. 1985, 74, 207–212. [Google Scholar] [CrossRef]
- De Jong, M.H.; Van der Hoeven, J.S. The growth of oral bacteria on saliva. J. Dent. Res. 1987, 66, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Willis, C.; Cummings, J.H.; Neale, G.; Gibson, G.R. In vitro effects of mucin fermentation on the growth of human colonic sulphate-reducing bacteria. Anaerobe 1996, 2, 117–122. [Google Scholar] [CrossRef]
- Corfield, A.P.; Wagner, S.A.; Clamp, J.R.; Kriaris, M.S.; Hoskins, L.C. Mucin degradation in the human colon: Production of sialidase, sialate O-acetylesterase, N-acetylneuraminate lyase, arylesterase and glycosulfatase activities by strains of fecal bacteria. Infect. Immun. 1992, 60, 3971–3978. [Google Scholar] [CrossRef]
- Van der Hoeven, J.S.; Van den Kieboom, C.W.; Camp, P.J. Utilization of mucin by oral Streptococcus species. Antonie Van Leeuwenhoek 1990, 57, 165–172. [Google Scholar] [CrossRef]
- Kavanaugh, N.L.; Zhang, A.Q.; Nobile, C.J.; Johnson, A.D.; Ribbeck, K. Mucins Suppress Virulence Traits of Candida albicans. mBio 2014, 5, e01911–e01914. [Google Scholar] [CrossRef]
- Naglik, J.R.; Konig, A.; Hube, B.; Gaffen, S.L. Candida albicans-epithelial interactions and induction of mucosal innate immunity. Curr. Opin. Microbiol. 2017, 40, 104–112. [Google Scholar] [CrossRef]
- Hori, Y.; Sugiyama, H.; Soma, T.; Nishida, K. Expression of membrane-associated mucins in cultivated human oral mucosal epithelial cells. Cornea 2007, 26, S65–S69. [Google Scholar] [CrossRef]
- Fekete, E.; Buret, A.G. The role of mucin O-glycans in microbiota dysbiosis, intestinal homeostasis, and host-pathogen interactions. Am. J. Physiol. Gastrointest. Liver Physiol. 2023, 324, G452–G465. [Google Scholar] [CrossRef]
- Ojeda, D.; Huber, M.A.; Kerr, A.R. Oral potentially malignant disorders and oral cavity cancer. Dermatol. Clin. 2020, 38, 507–521. [Google Scholar] [CrossRef]
- Warren, R.L.; Freeman, D.J.; Pleasance, S.; Watson, P.; Moore, R.A.; Cochrane, K.; Allen-Vercoe, E.; Holt, R.A. Co-occurrence of anaerobic bacteria in colorectal carcinomas. Microbiome 2013, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Amer, A.; Galvin, S.; Healy, C.M.; Moran, G.P. The microbiome of potentially malignant oral leukoplakia exhibits enrichment for Fusobacterium, Leptotrichia, Campylobacter, and Rothia Species. Front. Microbiol. 2017, 8, 2391. [Google Scholar] [CrossRef] [PubMed]
- Amer, A.; Whelan, A.; Al-Hebshi, N.N.; Healy, C.M.; Moran, G.P. Acetaldehyde production by Rothia mucilaginosa isolates from patients with oral leukoplakia. J. Oral Microbiol. 2020, 12, 1743066. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Das, A.; Paul, R.R.; Barui, A. Cigarette smoking promotes cancer related transformation of oral epithelial cells through activation of Wnt and MAPK pathway. Future Oncol. 2019, 15, 3619–3631. [Google Scholar] [CrossRef]
- Kashyap, B.; Mikkonen, J.J.W.; Bhardwaj, T.; Dekker, H.; Schulten, E.A.J.M.; Bloemena, E.; Kullaa, A.M. Effect of smoking on MUC1 expression in oral epithelial dysplasia, oral cancer, and irradiated oral epithelium. Arch. Oral Biol. 2022, 142, 105525. [Google Scholar] [CrossRef]
- Kato, K.; Lillehoj, E.P.; Kai, H.; Kim, K.C. MUC1 expression by human airway epithelial cells mediates Pseudomonas aeruginosa adhesion. Front. Biosci. 2010, 2, 68–77. [Google Scholar]
- Zhao, H.; Chu, M.; Huang, Z.; Yang, X.; Ran, S.; Hu, B.; Zhang, C.; Liang, J. Variations in oral microbiota associated with oral cancer. Sci. Rep. 2017, 7, 11773. [Google Scholar] [CrossRef] [PubMed]
- Agner, C.E.; Wheeler, K.M.; Ribbeck, K. Mucins and Their Role in Shaping the Functions of Mucus Barriers. Annu. Rev. Cell Dev. Biol. 2018, 34, 189–215. [Google Scholar] [CrossRef]
- Yagyuu, T.; Funayama, N.; Imada, M.; Kirita, T. Effect of smoking status and programmed death-ligand 1 expression on the microenvironment and malignant transformation of oral leukoplakia: A retrospective cohort study. PLoS ONE 2021, 16, e0250359. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Mukherjee, P. MUC1: A multifaceted oncoprotein with a key role in cancer progression. Trends Mol. Med. 2014, 20, 332–342. [Google Scholar] [CrossRef]
- Nitta, T.; Sugihara, K.; Tsuyama, S.; Murata, F. Immunohistochemical study of MUC1 mucin in premalignant oral lesions and oral squamous cell carcinoma. Association with disease progression, Mode of invasion and Lymph node metastasis. Cancer 2000, 88, 245–254. [Google Scholar] [CrossRef]
- Raina, D.; Agarwal, P.; Lee, J.; Bharti, A.; McKnight, C.J.; Sharma, P.; Kharbanda, S.; Kufe, D. Characterization of the MUC1-C Cytoplasmic Domain as a Cancer Target. PLoS ONE 2015, 10, e0135156. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, M.; Ahmad, R.; Alam, M.; Uchida, Y.; Kufe, D. MUC1-C oncoprotein regulates glycolysis and pyruvate kinase M2 activity in cancer cells. PLoS ONE 2011, 6, e28234. [Google Scholar] [CrossRef]
- Bradshaw, D.J.; Homer, K.A.; Marsh, P.D.; Beighton, D. Metabolic cooperation in oral microbial communities during growth on mucin. Microbiology 1994, 140, 3407–3412. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Preston, R.; Godoy-Vitorino, F.; Jedlicka, A.; Rodríguez-Hilario, A.; González, H.; Bondy, J.; Lawson, F.; Folawiyo, O.; Michailidi, C.; Dziedzic, A.; et al. 16S rRNA amplicon sequencing identifies microbiota associated with oral cancer, human papilloma virus infection and surgical treatment. Oncotarget 2016, 7, 51320–51334. [Google Scholar] [CrossRef]
- Lee, W.H.; Chen, H.M.; Yang, S.F.; Liang, C.; Peng, C.Y.; Lin, F.M.; Tsai, L.L.; Wu, B.C.; Hsin, C.H.; Chuang, C.Y. Bacterial alterations in salivary microbiota and their association in oral cancer. Sci. Rep. 2017, 7, 16540. [Google Scholar] [CrossRef] [PubMed]
- Mok, S.F.; Karuthan, C.; Cheah, Y.K.; Ngeow, W.C.; Rosnah, Z.; Yap, S.F.; Ong, H.K.A. The oral microbiome community variations associated with normal, potentially malignant disorders and malignant lesions of the oral cavity. Malays. J. Pathol. 2017, 39, 1–15. [Google Scholar]
- Lim, Y.; Fukuma, N.; Totsika, M.; Kenny, L.; Morrison, M.; Punyadeera, C. The Performance of an Oral Microbiome Biomarker Panel in Predicting Oral Cavity and Oropharyngeal Cancers. Front. Cell. Infect. Microbiol. 2018, 8, 267. [Google Scholar] [CrossRef]
- Heng, R.; Li, D.; Shi, X.; Gao, Q.; Wei, C.; Li, X.; Li, Y.; Zhou, H. Reduced CX3CL1 Secretion Contributes to the Susceptibility of Oral Leukoplakia-Associated Fibroblasts to Candida albicans. Front. Cell. Infect. Microbiol. 2016, 6, 150. [Google Scholar]
- Dhawan, P.; Singh, A.B.; Deane, N.G.; No, Y.; Shiou, S.R.; Schmidt, C.; Neff, J.; Washington, M.K.; Beauchamp, R.D. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J. Clin. Investig. 2005, 115, 1765–1776. [Google Scholar] [CrossRef]
- Kuboniwa, M.; Hasegawa, Y.; Mao, S.; Shizukuishi, S.; Amano, A.; Lamont, R.J.; Yilmaz, O. P. gingivalis accelerates gingival epithelial cell progression through the cell cycle. Microbes Infect. 2008, 10, 122–128. [Google Scholar] [CrossRef]
- Al-Hebshi, N.N.; Nasher, A.T.; Maryoud, M.Y.; Homeida, H.E.; Chen, T.; Idris, A.M.; Johnson, N.W. Inflammatory bacteriome featuring Fusobacterium nucleatum and Pseudomonas aeruginosa identified in association with oral squamous cell carcinoma. Sci. Rep. 2017, 7, 1834. [Google Scholar] [CrossRef]
- Gallimidi, A.B.; Fischman, S.; Revach, B.; Bulvik, R.; Maliutina, A.; Rubinstein, A.M.; Nussbaum, G.; Elkin, M. Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget 2015, 6, 22613–22623. [Google Scholar] [CrossRef] [PubMed]
- Baldus, S.E.; Engelmann, K.; Hanisch, F.G. MUC1 and the MUCs: A family of human mucins with impact in cancer biology. Crit. Rev. Clin. Lab. Sci. 2004, 41, 189–231. [Google Scholar] [CrossRef]
- Gendler, S.J. MUC1, the renaissance molecule. J. Mammary Gland. Biol. 2001, 6, 339–353. [Google Scholar] [CrossRef]
- Al-Azawi, D.; Kelly, G.; Myers, E.; McDermott, E.W.; Hill, A.D.; Duffy, M.J.; Higgins, N.O. CA 15-3 is predictive of response and disease recurrence following treatment in locally advanced breast cancer. BMC Cancer 2006, 6, 220. [Google Scholar] [CrossRef]
- Zhong, X.; Lu, Q.; Zhang, Q.; He, Y.; Wei, W.; Wang, Y. Oral microbiota alteration associated with oral cancer and areca chewing. Oral Dis. 2021, 27, 226–239. [Google Scholar]
- Sarkar, P.; Malik, S.; Laha, S.; Das, S.; Bunk, S.; Ray, J.G.; Chatterjee, R.; Saha, A. Dysbiosis of Oral Microbiota During Oral Squamous Cell Carcinoma Development. Front. Oncol. 2021, 11, 614448. [Google Scholar] [CrossRef]
- Torralba, M.G.; Aleti, G.; Li, W.; Moncera, K.J.; Lin, Y.H.; Yu, Y.; Masternak, M.M.; Golusinski, W.; Golusinski, P.; Lamperska, K.; et al. Oral Microbial Species and Virulence Factors Associated with Oral Squamous Cell Carcinoma. Microb. Ecol. 2021, 82, 1030–1046. [Google Scholar] [CrossRef]
- Zhou, X.; Hao, Y.; Peng, X.; Li, B.; Han, Q.; Ren, B.; Li, M.; Li, L.; Li, Y.; Cheng, G.; et al. The Clinical Potential of Oral Microbiota as a Screening Tool for Oral Squamous Cell Carcinomas. Front. Cell. Infect. Microbiol. 2021, 11, 728933. [Google Scholar] [CrossRef] [PubMed]
- Oosterlinck, B.; Ceuleers, H.; Arras, W.; De Man, J.G.; Geboes, K.; De Schepper, H.; Peeters, M.; Lebeer, S.; Skieceviciene, J.; Hold, G.L.; et al. Mucin-microbiome signatures shape the tumor microenvironment in gastric cancer. Microbiome 2023, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Breugelmans, T.; Oosterlinck, B.; Arras, W.; Ceuleers, H.; De Man, J.; Hold, G.L.; De Winter, B.Y.; Smet, A. The role of mucins in gastrointestinal barrier function during health and disease. Lancet Gastroenterol. Hepatol. 2022, 7, 455–471. [Google Scholar] [CrossRef] [PubMed]
| Oral Microbiome | Oral Biofilm | Host Response | References |
|---|---|---|---|
| Actinomyces spp., Bacteroides spp., Corynebacteria spp., Eubacterium spp., Fusobacterium spp., Haemophilus spp., Megasphaera spp., Neisseria spp., Propionibacterium, Prevotella spp., Porphyromonas spp., Rothia spp. | Antibacterial activity | Pro-inflammatory Anti-inflammatory Chemoattractant Gut–brain interaction | [42,43,44] |
| Streptococcus mitis | Antimicrobial | Gasotransmitter | [45] |
| Porphyromonas gingivalis, Prevotella intermedia | Antibacterial activity | Chemoattractant | [46] |
| Streptococcus spp. | Regulatory function | Inhibition of inflammasomes | [47] |
| Campylobacter spp. | Bacterial survival and growth | Anti-inflammatory | [48] |
| Streptococcus spp., Lactobacillus spp. | Stimulus for the growth of most anaerobes | Toxic | [49] |
| Fusobacterium spp. | Altered biofilm composition | Decreases collagen synthesis Pro-inflammatory | [50] |
| Fusobacterium spp., Lactobacillus spp., Prevotella spp., Porphyromonas spp., Streptococcus spp., Treponema denticola | Increased resistance to antibiotics Formation of biofilms Affects cell metabolism, cell differentiation, plasmid stability, drug resistance, and signaling | Affects bacterial virulence Toxic Affects cell physiology | [51] |
| Fusobacterium spp., Parvimonas micra, Porphyromonas spp., Prevotella intermedia, Treponema denticola, Streptococcus anginosus, Desulfobacter spp., Desulfovibrio spp., Desulfomicrobium orale | Harmful at high concentrations Increased resistance to antibiotics Increased resistance to immune-mediated killing Protection from oxidative stress | Toxic at high concentrations Pro-inflammatory Anti-inflammatory Gasotransmitter | [52,53] |
| Fusobacterium spp., Porphyromonas spp., Prevotella spp., Tannerella spp., Treponema spp., Lactobacillus spp., Peptostreptococcus spp., Helicobacter pylori, Campylobacter ureolyticus, Haemophilus parainfluenzae, Streptococcus spp., Actinomyces spp., Staphylococcus spp., Rothia dentocariosa | Antibiotic resistance Inhibits neutrophil function | Toxic and impairs function of neutrophils | [54,55] |
| Oral Cavity Location | Bacterial Species | Enzymes Produced |
|---|---|---|
| Saliva, buccal mucosa, tongue, hard palate, gingiva, throat palatine tonsils, | Streptococcus anginosus | β-N-acetyl-D-glucosaminidase α- and β-D-galactosidase |
| Streptococcus mitis | β-N-acetyl-D-galactosaminidase, β-N-acetyl-D-glucosaminidase α- and β-D-galactosidase, α-L-fucosidase, neuraminidase | |
| Streptococcus mutants | β-N-acetyl-D-glucosaminidase, α- and β-D-galactosidase | |
| Streptococcus oralis | β-N-acetyl-D-galactosaminidase, β-N-acetyl-D-glucosaminidase, α- and β-D-galactosidase, α-L-fucosidase, neuraminidase, protease | |
| Streptococcus sanguinis | β-N-acetyl-D-galactosaminidase, β-N-acetyl-D-glucosaminidase, α- and β-D-galactosidase, α-L-fucosidase, protease | |
| Streptococcus sobrinus | β-N-acetyl-D-glucosaminidase, β-D-galactosidase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashyap, B.; Kullaa, A.M. Transmembrane Mucin-1 Facilitates Oral Microbial Colonization in Oral Cancer. Oral 2025, 5, 75. https://doi.org/10.3390/oral5040075
Kashyap B, Kullaa AM. Transmembrane Mucin-1 Facilitates Oral Microbial Colonization in Oral Cancer. Oral. 2025; 5(4):75. https://doi.org/10.3390/oral5040075
Chicago/Turabian StyleKashyap, Bina, and Arja M. Kullaa. 2025. "Transmembrane Mucin-1 Facilitates Oral Microbial Colonization in Oral Cancer" Oral 5, no. 4: 75. https://doi.org/10.3390/oral5040075
APA StyleKashyap, B., & Kullaa, A. M. (2025). Transmembrane Mucin-1 Facilitates Oral Microbial Colonization in Oral Cancer. Oral, 5(4), 75. https://doi.org/10.3390/oral5040075






