Analgesic Efficacy of Postoperative Ibuprofen in Third Molar Surgery: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection Criteria
2.2. Search Using Databases
2.3. Assessment of Bias
2.4. Data Extraction
2.5. Statistical Analysis
3. Results
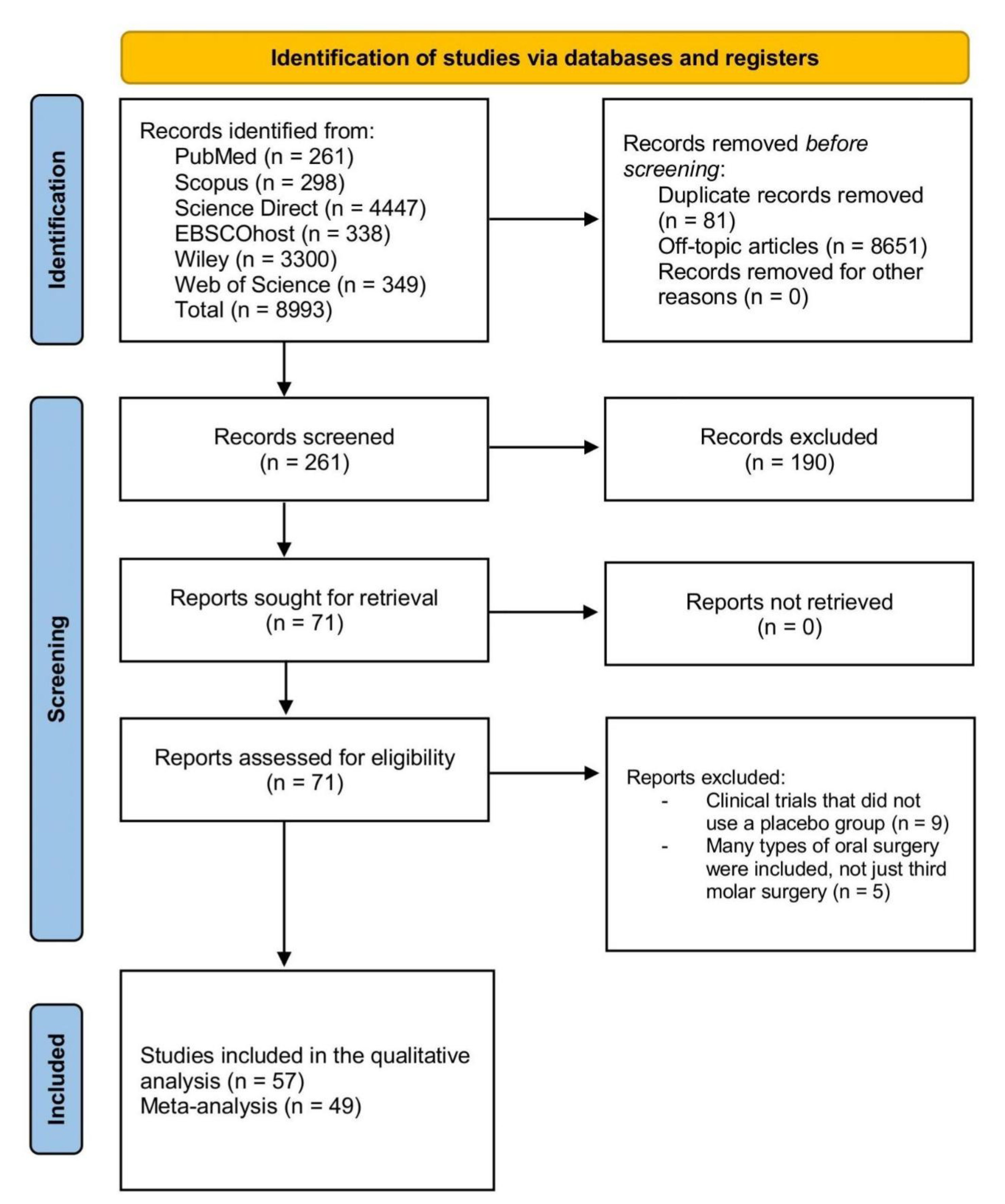
3.1. Database Search
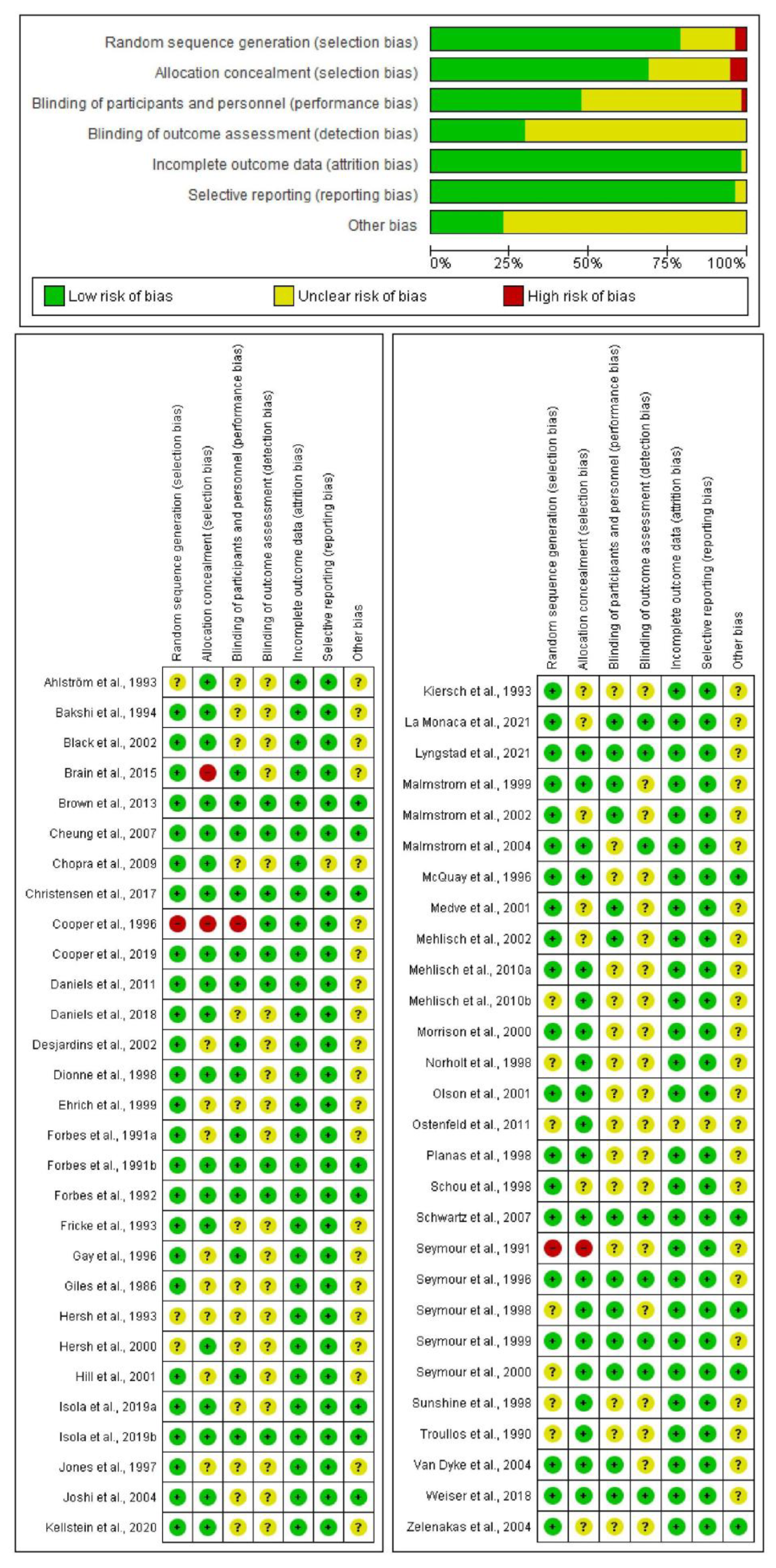
3.2. Evaluation of Bias
3.3. Qualitative Analysis
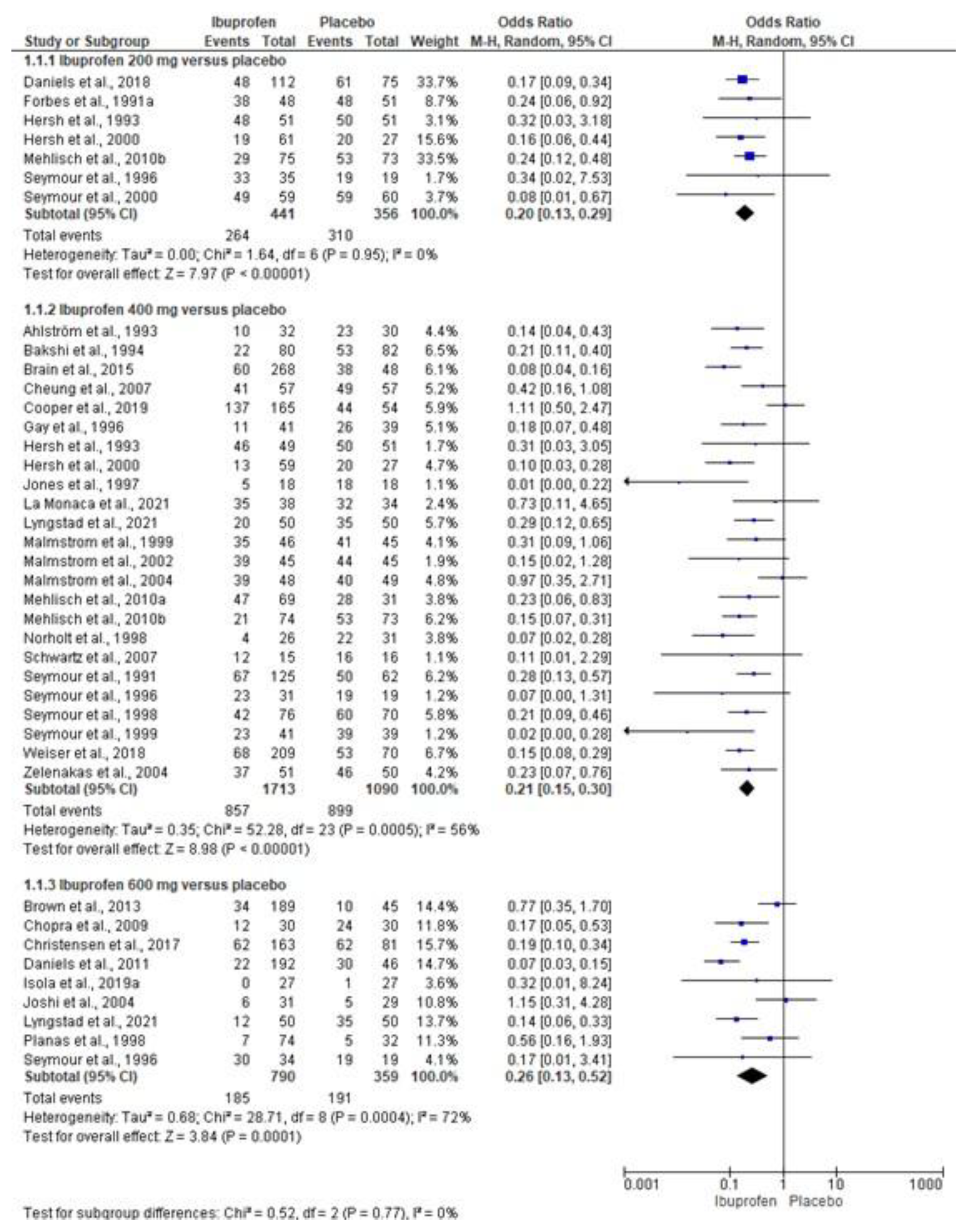
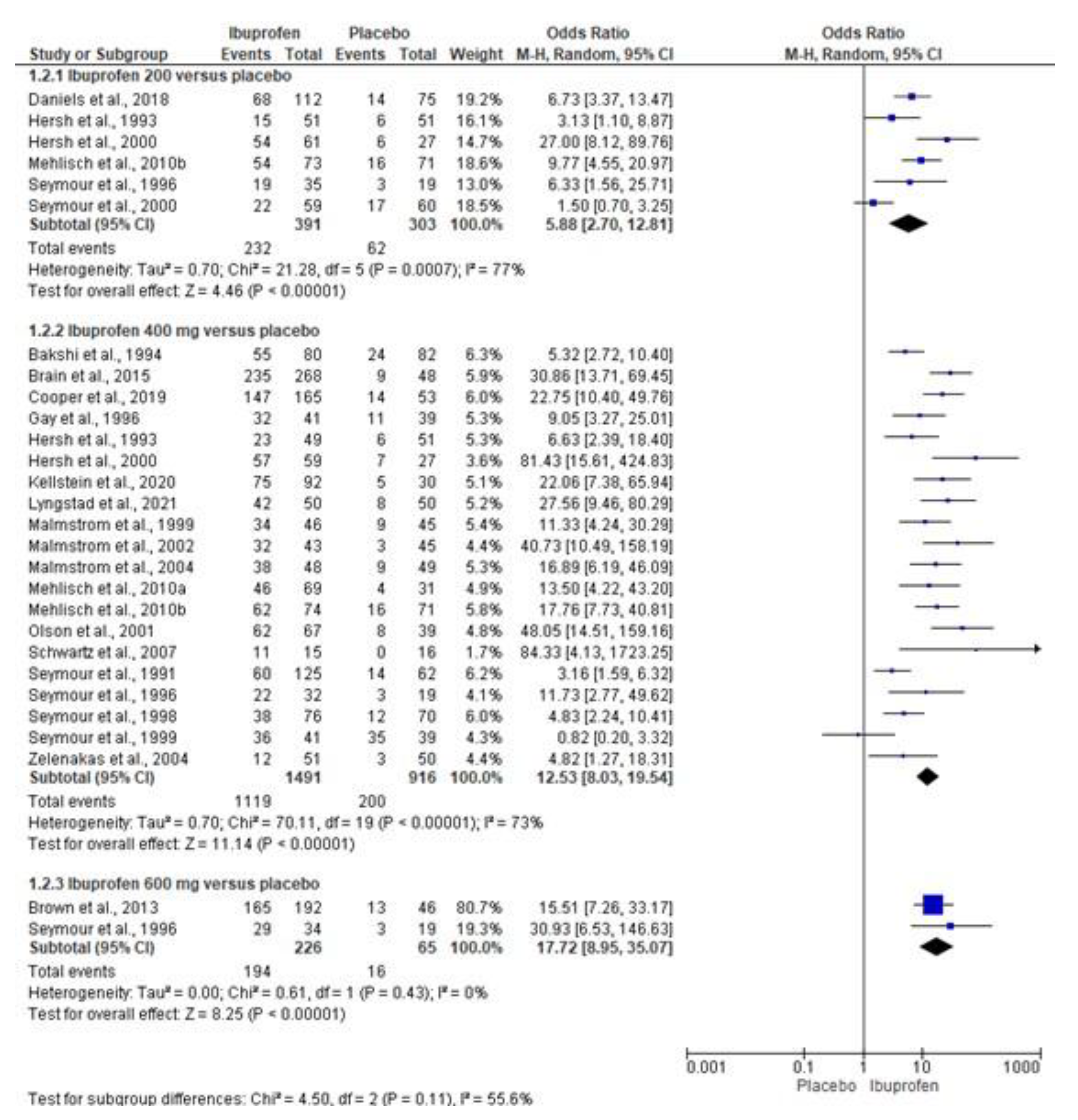
3.4. Quantitative Analysis
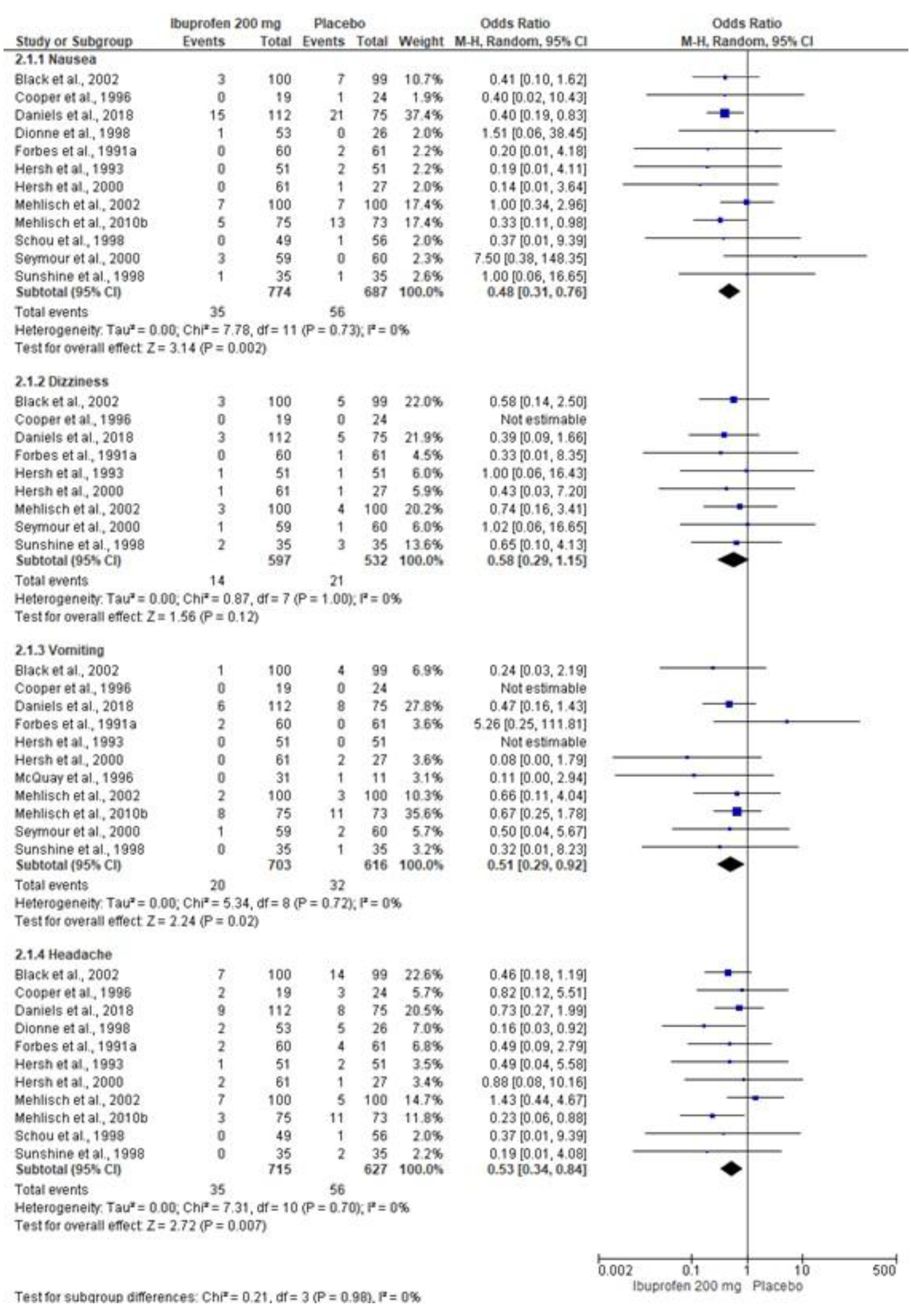
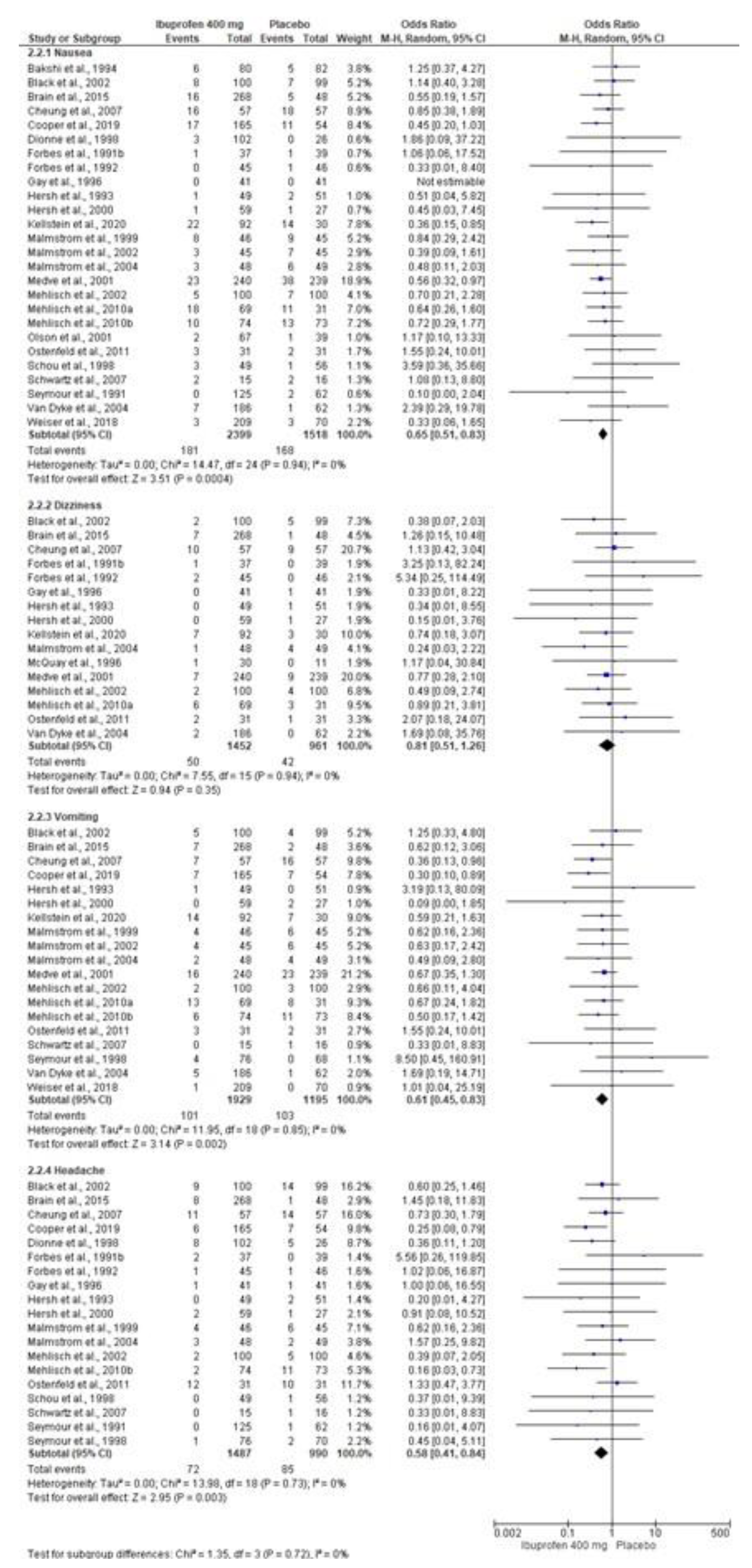
3.5. Adverse Effects
3.6. Assessment of Publication Bias
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Borad Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chakraborty, P.K.; Shai, A.; Tilak, P.B.D.; Kumar, A.; Kamdar, A.; Niranjana, A.; Kisave, P.N. Comparative Analgesic Efficacy of Intramuscular Dexamethasone, Ketorolac, Tramadol, and Butorphanol with Regard to Postoperative Pain After Mandibular Third Molar Surgery. J. Pharm. Bioallied Sci. 2024, 16 (Suppl. S2), S1378–S1380. [Google Scholar] [CrossRef]
- Latifi, F.; Choobsaz, P.; Yousefi-Koma, A.A.; Yousefi-Koma, H.; Mirtaleb, M.H. Comparison of the analgesic effects of single-dose 75 mg oral pregabalin versus single-dose 400 mg oral ibuprofen after impacted third mandibular molar surgery: A randomized, double-blind, split-mouth clinical trial. Dent. Med. Probl. 2023, 60, 619–625. [Google Scholar] [CrossRef]
- Palaia, G.; Tenore, G.; Tribolati, L.; Russo, C.; Gaimari, G.; Del Vecchio, A.; Romeo, U. Evaluation of wound healing and postoperative pain after oral mucosa laser biopsy with the aid of compound with chlorhexidine and sodium hyaluronate: A randomized double blind clinical trial. Clin. Oral Investig. 2019, 23, 3141–3151. [Google Scholar] [CrossRef] [PubMed]
- Pippi, R. Post-Surgical Clinical Monitoring of Soft Tissue Wound Healing in Periodontal Implant Surgery. Int. J. Med. Sci. 2017, 14, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.K.R.; Bhola, N. Efficacy of Kinesio taping in post operative sequalae after surgical removal of mandibular third molars: A split mouth randomized control study. BMC Oral Health 2023, 23, 964. [Google Scholar] [CrossRef] [PubMed]
- Thuruthel, M.J.; Surej Kumar, L.K.; Kurien, N.M.; Tharakan, M. Efficacy of gelatamp in controlling the postoperative sequelae following mandibular posterior teeth extraction—Asplit-mouth study. J. Oral Biol. Craniofacial Res. 2023, 13, 96–103. [Google Scholar] [CrossRef]
- Gursoytrak, B.; Kocaturk, Ö.; Koparal, M.; Gulsun, B. Comparison of Dexmedetomidine Ketamine for Managing Postoperative Symptoms After Third-Molar Surgery. J. Oral Maxillofac. Surg. 2021, 79, 532–536. [Google Scholar] [CrossRef]
- Patil, C.; Jadhav, A.K.R.; Bhola, N.; Borle, R.M.; Mishra, A. Piezosurgery vs bur in impacted mandibular third molar surgery: Evaluation of postoperative sequelae. J. Oral Biol. Craniofacial Res. 2019, 9, 259–262. [Google Scholar] [CrossRef]
- Aloy-Prósper, A.; Pellicer-Chover, H.; Balaguer-Martínez, J.; Llamas-Monteagudo, O.; Peñarrocha-Diago, M. Patient compliance to postoperative instructions after third molar surgery comparing traditional verbally written form versus the effect of a postoperative phone call follow-up a: Arandomized clinical study. J. Clin. Exp. Dent. 2020, 12, e909–e915. [Google Scholar] [CrossRef]
- Shenoi, R.S.; Rajguru, J.G.; Parate, S.R.; Ingole, P.D.; Khandaitkar, S.R.; Karmarkar, J.S. Compliance of postoperative instructions following the surgical extraction of impacted lower third molars Indian. J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2021, 32, 87–91. [Google Scholar] [CrossRef]
- Alvira-González, J.; Gay-Escoda, C. Compliance of postoperative instructions following the surgical extraction of impacted lower third molars: A randomized clinical trial. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e224–e230. [Google Scholar] [CrossRef]
- Camps-Font, O.; Sábado-Bundó, H.; Toledano-Serrabona, J.; Valmaseda-de-la-Rosa, N.; Figueiredo, R.; Valmaseda-Castellón, E. Antibiotic prophylaxis in the prevention of dry socket surgical site infection after lower third molar extraction: A network meta-analysis. Int. J. Oral Maxillofac. Surg. 2024, 53, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Torof, E.; Morrissey, H.; Ball, P.A. The Role of Antibiotic Use in Third Molar Tooth Extractions: A Systematic Review and Meta-Analysis. Medicina 2023, 59, 422. [Google Scholar] [CrossRef] [PubMed]
- Varvara, G.; Bernardi, S.; Cutilli, T.; Bianchi, S.; Sinjari, B.; Piattelli, M. Anti-inflammatory steroid use in impacted third molar surgery: A systematic review. J. Biol. Regul. Homeost. Agents 2017, 31, 1095–1099. [Google Scholar] [PubMed]
- Pergolizzi, J.V.; Breve, F.; Magnusson, P.; LeQuang, J.K.; Varassi, G. Current and emerging COX inhibitors for treating postoperative pain following oral surgery. Expert Opin. Pharmacother. 2023, 24, 347–358. [Google Scholar] [CrossRef]
- Silva, P.U.J.; Meneses-Santos, D.; Vieira, W.A.; Ramacciato, J.C.; Silva, R.P.D.; Silva, M.C.P.D.; Rode, S.M.; Paranhos, L.R. Preemptive use of intravenous ibuprofen to reduce postoperative pain after lower third molar surgery: A systematic review of randomized controlled trials. Clinics 2021, 76, e2780. [Google Scholar] [CrossRef]
- Cetira Filho, E.L.; Carvalho, F.S.R.; de Barros Silva, P.G.; Barbosa, D.A.F.; Alves Pereira, K.M.; Ribeiro, T.R.; Costa, F.W.G. Preemptive use of oral nonsteroidal anti-inflammatory drugs for the relief of inflammatory events after surgical removal of lower third molars: Asystematic review with meta-analysis of placebo-controlled randomized clinical trials. J. Cranio Maxillofac. Surg. 2020, 48, 293–307. [Google Scholar] [CrossRef]
- Gounari, M.M.; Tsaousi, G.; Zouloumis, L.; Kouvelas, D.; Pourzitaki, C. Efficacy and safety of parenteral and local application of tramadol in mandibular third molar extraction: A qualitative systematic review of current evidence. Oral Maxillofac. Surg. 2024, 28, 499–513. [Google Scholar] [CrossRef]
- Selvido, D.I.; Bhattarai, B.P.; Niyomtham, N.; Riddhabhaya, A.; Vongsawan, K.; Pairuchvej, V.; Wongsirichat, N. Review of dexamethasone administration for management of complications in postoperative third molar surgery. J. Korean Assoc. Oral Maxillofac. Surg. 2021, 47, 341–350. [Google Scholar] [CrossRef]
- Franco-de la Torre, L.; Figueroa-Fernández, N.P.; Franco-González, D.L.; Alonso-Castro, Á.J.; Rivera-Luna, F.; Isiordia-Espinoza, M.A.A. Meta-Analysis of the Analgesic Efficacy of Single-Doses of Ibuprofen Compared to Traditional Non-Opioid Analgesics Following Third Molar Surgery. Pharmaceuticals 2021, 14, 360. [Google Scholar] [CrossRef]
- Klinger, R.; Stuhlreyer, J.; Schwartz, M.; Schmitz, J.; Colloca, L. Clinical Use of Placebo Effects in Patients With Pain Disorders. Int. Rev. Neurobiol. 2018, 139, 107–128. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann. Intern. Med. 2009, 151, W65–W94. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 5.1.0; The Cochrane Collaboration: Oxford, UK, 2011; Available online: http://www.cochranehandbook.org (accessed on 9 September 2024).
- Leonardo, R. PICO: Model for clinical questions. Evid.-Based Med. Pract. 2018, 3, 2. [Google Scholar]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.M.; Kim, J.Y.; Kwon, B.S.; Kim, K.N. Risk of bias for randomized controlled trials in Journal of Clinical Monitoring Computing. J. Clin. Monit. Comput. 2023, 37, 103–111. [Google Scholar] [CrossRef]
- Isiordia-Espinoza, M.A.; Franco-González, M.A.; Alonso-Castro, Á.J.; Franco-de la Torre, L. Analgesic effectiveness safety of celecoxib versus non-opioid active controls after third molar surgery: Ameta-analytical evaluation. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, e1–e9. [Google Scholar] [CrossRef]
- Isiordia-Espinoza, M.A.; Alonso-Castro, Á.J.; Serafín-Higuera, N.; Castañeda-Santana, D.I.; de la Rosa Coronado, M.; Bologna-Molina, R.E. Postoperative administration of ketorolac compared to other drugs for pain control after third molar surgery: Ameta-analysis of double-blind randomized clinical trials. Br. J. Clin. Pharmacol. 2022, 88, 2591–2604. [Google Scholar] [CrossRef]
- Jones, A.; Steel, D. Evaluating the quality of medical evidence in real-world contexts. J. Eval. Clin. Pract. 2018, 24, 950–956. [Google Scholar] [CrossRef]
- Atkins, D.; Eccles, M.; Flottorp, S.; Guyatt, G.H.; Henry, D.; Hill, S.; Liberati, A.; O’Connell, D.; Oxman, A.D.; Phillips, B.; et al. Systems for grading the quality of evidence and the strength of recommendations I: Critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv. Res. 2004, 4, 38. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE Working Group GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Whitley, E.; Ball, J. Statistics review 3: Hypothesis testing and P values. Crit. Care 2002, 6, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Citrome, L.; Ketter, T.A. When does a difference make a difference? Interpretation of number needed to treat number needed to harm likelihood to be helped or harmed. Int. J. Clin. Pract. 2013, 67, 407–411. [Google Scholar] [CrossRef]
- Andrade, C. The numbers needed to treat harm, (NNT; NNH) statistics: What they tell us what they do not. J. Clin. Psychiatry 2015, 76, e330–e333. [Google Scholar] [CrossRef]
- Risk Reduction Calculator. Available online: http://araw.mede.uic.edu/cgi-bin/nntcalc.pl (accessed on 24 October 2024).
- Ahlström, U.; Bakshi, R.; Nilsson, P.; Wåhlander, L. The analgesic efficacy of diclofenac dispersible ibuprofen in postoperative pain after dental extraction. Eur. J. Clin. Pharmacol. 1993, 44, 587–588. [Google Scholar] [CrossRef]
- Bakshi, R.; Frenkel, G.; Dietlein, G.; Meurer-Witt, B.; Schneider, B.; Sinterhauf, U. A placebo-controlled comparative evaluation of diclofenac dispersible versus ibuprofen in postoperative pain after third molar surgery. J. Clin. Pharmacol. 1994, 34, 225–230. [Google Scholar] [CrossRef]
- Black, P.; Max, M.B.; Desjardins, P.; Norwood, T.; Ardia, A.; Pallotta, T. A randomized double-blind, placebo-controlled comparison of the analgesic efficacy, onset of action, and tolerability of ibuprofen arginate and ibuprofen in postoperative dental pain. Clin. Ther. 2002, 24, 1072–1089. [Google Scholar] [CrossRef]
- Brain, P.; Leyva, R.; Doyle, G.; Kellstein, D. Onset of analgesia efficacy of ibuprofen sodium in postsurgical dental pain: A randomized placebo-controlled study versus standard ibuprofen. Clin. J. Pain 2015, 31, 444–450. [Google Scholar] [CrossRef]
- Brown, J.D.; Daniels, S.E.; Bandy, D.P.; Ko, A.T.; Gammaitoni, A.; Mehta, A.; Boice, J.A.; Losada, M.C.; Peloso, P.M. Evaluation of multiday analgesia with etoricoxib in a double-blind randomized controlled trial using the postoperative third-molar extraction dental pain model. Clin. J. Pain 2013, 29, 492–498. [Google Scholar] [CrossRef]
- Cheung, R.; Krishnaswami, S.; Kowalski, K. Analgesic efficacy of celecoxib in postoperative oral surgery pain: A single-dose, two-center, randomized, double-blind, active- and placebo-controlled study. Clin. Ther. 2007, 29, 2498–2510. [Google Scholar] [CrossRef]
- Chopra, D.; Rehan, H.S.; Mehra, P.; Kakkar, A.K. A randomized, double-blind, placebo-controlled study comparing the efficacy and safety of paracetamol, serratiopeptidase, ibuprofen and betamethasone using the dental impaction pain model. Int. J. Oral Maxillofac. Surg. 2009, 38, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.; Paluch, E.; Jayawardena, S.; Daniels, S.; Meeves, S. Analgesic Efficacy of a New Immediate-Release/Extended-Release Formulation of Ibuprofen: Results From Single- and Multiple-Dose Postsurgical Dental Pain Studies. Clin. Pharmacol. Drug Dev. 2017, 6, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.A.; Cowan, A.; Tallarida, R.J.; Hargreaves, K.; Roszkowski, M.; Jamali, F.; Borenstein, M.; Lucyk, D.; Fielding, A.F.; Smith, B.; et al. The Analgesic Interaction of Misoprostol with Nonsteroidal Anti-Inflammatory Drugs. Am. J. Ther. 1996, 3, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.A.; Desjardins, P.; Brain, P.; Paredes-Diaz, A.; Troullos, E.; Centofanti, R.; An, B. Longer analgesic effect with naproxen sodium than ibuprofen in post-surgical dental pain: A randomized, double-blind, placebo-controlled, single-dose trial. Curr. Med. Res. Opin. 2019, 35, 2149–2158. [Google Scholar] [CrossRef]
- Daniels, S.E.; Bandy, D.P.; Christensen, S.E.; Boice, J.; Losada, M.C.; Liu, H.; Mehta, A.; Peloso, P.M. Evaluation of the dose range of etoricoxib in an acute pain setting using the postoperative dental pain model. Clin. J. Pain 2011, 27, 1–8. [Google Scholar] [CrossRef]
- Daniels, S.E.; Atkinson, H.C.; Stanescu, I.; Frampton, C. Analgesic Efficacy of an Acetaminophen/Ibuprofen Fixed-dose Combination in Moderate to Severe Postoperative Dental Pain: A Randomized, Double-blind, Parallel-group, Placebo-controlled Trial. Clin. Ther. 2018, 40, 1765–1776.e5. [Google Scholar] [CrossRef]
- Desjardins, P.; Black, P.; Papageorge, M.; Norwood, T.; Shen, D.D.; Norris, L.; Ardia, A. Ibuprofen arginate provides effective relief from postoperative dental pain with a more rapid onset of action than ibuprofen. Eur. J. Clin. Pharmacol. 2002, 58, 387–394. [Google Scholar] [CrossRef]
- Dionne, R.A.; McCullagh, L. Enhanced analgesia and suppression of plasma beta-endorphin by the S(+)-isomer of ibuprofen. Clin. Pharmacol. Ther. 1998, 63, 694–701. [Google Scholar] [CrossRef]
- Ehrich, E.W.; Dallob, A.; De Lepeleire, I.; Van Hecken, A.; Riendeau, D.; Yuan, W.; Porras, A.; Wittreich, J.; Seibold, J.R.; De Schepper, P.; et al. Characterization of rofecoxib as a cyclooxygenase-2 isoform inhibitor and demonstration of analgesia in the dental pain model. Clin. Pharmacol. Ther. 1999, 65, 336–347. [Google Scholar] [CrossRef]
- Forbes, J.A.; Beaver, W.T.; Jones, K.F.; Kehm, C.J.; Smith, W.K.; Gongloff, C.M.; Zeleznock, J.R.; Smith, J.W. Effect of caffeine on ibuprofen analgesia in postoperative oral surgery pain. Clin. Pharmacol. Ther. 1991, 49, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.A.; Edquist, I.A.; Smith, F.G.; Schwartz, M.K.; Beaver, W.T. Evaluation of bromfenac, aspirin, and ibuprofen in postoperative oral surgery pain. Pharmacotherapy 1991, 11, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.A.; Beaver, W.T.; Jones, K.F.; Edquist, I.A.; Gongloff, C.M.; Smith, W.K.; Smith, F.G.; Schwartz, M.K. Analgesic efficacy of bromfenac, ibuprofen, and aspirin in postoperative oral surgery pain. Clin. Pharmacol. Ther. 1992, 51, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Fricke, J.R.; Halladay, S.C.; Francisco, C.A. Efficacy and safety of naproxen sodium and ibuprofen for pain relief after oral surgery. Curr. Ther. Res. 1993, 54, 619–627. [Google Scholar] [CrossRef]
- Gay, C.; Planas, E.; Donado, M.; Martínez, J.M.; Artigas, R.; Torres, F.; Mauleón, D.; Carganico, G. Analgesic Efficacy of Low Doses of Dexketoprofen in the Dental Pain Model. Clin. Drug Investig. 1996, 11, 320–330. [Google Scholar] [CrossRef]
- Giles, A.D.; Hill, C.M.; Shepherd, J.P.; Stewart, D.J.; Pickvance, N.J. A single dose assessment of an ibuprofen/codeine combination in postoperative dental pain. Int. J. Oral Maxillofac. Surg. 1986, 15, 727–732. [Google Scholar] [CrossRef]
- Hersh, E.V.; Cooper, S.; Betts, N.; Wedell, D.; MacAfee, K.; Quinn, P.; Lamp, C.; Gaston, G.; Bergman, S.; Henry, E. Single dose and multidose analgesic study of ibuprofen and meclofenamate sodium after third molar surgery. Oral Surg. Oral Med. Oral Pathol. 1993, 76, 680–687. [Google Scholar] [CrossRef]
- Hersh, E.V.; Levin, L.M.; Cooper, S.A.; Doyle, G.; Waksman, J.; Wedell, D.; Hong, D.; Secreto, S.A. Ibuprofen liquigel for oral surgery pain. Clin. Ther. 2000, 22, 1306–1318. [Google Scholar] [CrossRef]
- Hill, C.M.; Balkenohl, M.; Thomas, D.W.; Walker, R.; Mathé, H.; Murray, G. Pregabalin in patients with postoperative dental pain. Eur. J. Pain 2001, 5, 119–124. [Google Scholar] [CrossRef]
- Isola, G.; Matarese, M.; Ramaglia, L.; Iorio-Siciliano, V.; Cordasco, G.; Matarese, G. Efficacy of a drug composed of herbal extracts on postoperative discomfort after surgical removal of impacted mandibular third molar: A randomized, triple-blind, controlled clinical trial. Clin. Oral Investig. 2019, 23, 2443–2453. [Google Scholar] [CrossRef]
- Isola, G.; Matarese, M.; Ramaglia, L.; Cicciù, M.; Matarese, G. Evaluation of the efficacy of celecoxib ibuprofen on postoperative pain swelling mouth opening after surgical removal of impacted third molars: A randomized controlled clinical trial. Int. J. Oral Maxillofac. Surg. 2019, 48, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.; Seymour, R.A.; Hawkesford, J.E. Are the pharmacokinetics of ibuprofen important determinants for the drug’s efficacy in postoperative pain after third molar surgery? Br. J. Oral Maxillofac. Surg. 1997, 35, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Parara, E.; Macfarlane, T.V. A double-blind randomised controlled clinical trial of the effect of preoperative ibuprofen, diclofenac, paracetamol with codeine and placebo tablets for relief of postoperative pain after removal of impacted third molars. Br. J. Oral Maxillofac. Surg. 2004, 42, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Kellstein, D.; Leyva, R. Evaluation of Fixed-Dose Combinations of Ibuprofen and Acetaminophen in the Treatment of Postsurgical Dental Pain: A Pilot, Dose-Ranging, Randomized Study. Drugs RD 2020, 20, 237–247. [Google Scholar] [CrossRef]
- Kiersch, T.A.; Halladay, S.C.; Koschik, M. A double-blind, randomized study of naproxen sodium, ibuprofen, and placebo in postoperative dental pain. Clin. Ther. 1993, 15, 845–854. [Google Scholar]
- La Monaca, G.; Pranno, N.; Annibali, S.; Polimeni, A.; Pompa, G.; Vozza, I.; Cristalli, M.P. Comparative analgesic effects of single-dose preoperative administration of paracetamol (acetaminophen) 500 mg plus codeine 30 mg ibuprofen 400 mg on pain after third molar surgery. J. Evid. Based Dent. Pract. 2021, 21, 101611. [Google Scholar] [CrossRef]
- Lyngstad, G.; Skjelbred, P.; Swanson, D.M.; Skoglund, L.A. Analgesic effect of oral ibuprofen 400, 600, and 800 mg; paracetamol 500 and 1000 mg; and paracetamol 1000 mg plus 60 mg codeine in acute postoperative pain: A single-dose, randomized, placebo-controlled, and double-blind study. Eur. J. Clin. Pharmacol. 2021, 77, 1843–1852. [Google Scholar] [CrossRef]
- Malmstrom, K.; Daniels, S.; Kotey, P.; Seidenberg, B.C.; Desjardins, P.J. Comparison of rofecoxib and celecoxib, two cyclooxygenase-2 inhibitors, in postoperative dental pain: A randomized, placebo- and active-comparator-controlled clinical trial. Clin. Ther. 1999, 21, 1653–1663. [Google Scholar] [CrossRef]
- Malmstrom, K.; Fricke, J.R.; Kotey, P.; Kress, B.; Morrison, B. A comparison of rofecoxib versus celecoxib in treating pain after dental surgery: A single-center, randomized, double-blind, placebo- and active-comparator-controlled, parallel-group, single-dose study using the dental impaction pain model. Clin. Ther. 2002, 24, 1549–1560. [Google Scholar] [CrossRef]
- Malmstrom, K.; Sapre, A.; Couglin, H.; Agrawal, N.G.; Mazenko, R.S.; Fricke, J.R., Jr. Etoricoxib in acute pain associated with dental surgery: A randomized, double-blind, placebo- and active comparator-controlled dose-ranging study. Clin. Ther. 2004, 26, 667–679. [Google Scholar] [CrossRef]
- McQuay, H.J.; Angell, K.; Carroll, D.; Moore, R.A.; Juniper, R.P. Ibuprofen compared with ibuprofen plus caffeine after third molar surgery. Pain 1996, 66, 247–251. [Google Scholar] [CrossRef]
- Medve, R.A.; Wang, J.; Karim, R. Tramadol and acetaminophen tablets for dental pain. Anesth. Prog. 2001, 48, 79–81. [Google Scholar]
- Mehlisch, D.R.; Ardia, A.; Pallotta, T. A controlled comparative study of ibuprofen arginate versus conventional ibuprofen in the treatment of postoperative dental pain. J. Clin. Pharmacol. 2002, 42, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Mehlisch, D.R.; Aspley, S.; Daniels, S.E.; Bandy, D.P. Comparison of the analgesic efficacy of concurrent ibuprofen and paracetamol with ibuprofen or paracetamol alone in the management of moderate to severe acute postoperative dental pain in adolescents and adults: A randomized, double-blind, placebo-controlled, parallel-group, single-dose, two-center, modified factorial study. Clin. Ther. 2010, 32, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Mehlisch, D.R.; Aspley, S.; Daniels, S.E.; Southerden, K.A.; Christensen, K.S. A single-tablet fixed-dose combination of racemic ibuprofen/paracetamol in the management of moderate to severe postoperative dental pain in adult and adolescent patients: A multicenter, two-stage, randomized, double-blind, parallel-group, placebo-controlled, factorial study. Clin. Ther. 2010, 32, 1033–1049. [Google Scholar] [CrossRef]
- Morrison, B.W.; Fricke, J.; Brown, J.; Yuan, W.; Kotey, P.; Mehlisch, D. The optimal analgesic dose of rofecoxib: Overview of six randomized controlled trials. J. Am. Dent. Assoc. 1939, 131, 1729–1737. [Google Scholar] [CrossRef]
- Nørholt, S.E.; Aagaard, E.; Svensson, P.; Sindet-Pedersen, S. Evaluation of trismus bite force pressure algometry after third molar surgery: A placebo-controlled study of ibuprofen. J. Oral Maxillofac. Surg. 1998, 56, 420–429. [Google Scholar] [CrossRef]
- Olson, N.Z.; Otero, A.M.; Marrero, I.; Tirado, S.; Cooper, S.; Doyle, G.; Jayawardena, S.; Sunshine, A. Onset of analgesia for liquigel ibuprofen 400 mg acetaminophen 1000 mg ketoprofen 25 mg placebo in the treatment of postoperative dental pain. J. Clin. Pharmacol. 2001, 41, 1238–1247. [Google Scholar] [CrossRef]
- Ostenfeld, T.; Price, J.; Albanese, M.; Bullman, J.; Guillard, F.; Meyer, I.; Leeson, R.; Costantin, C.; Ziviani, L.; Nocini, P.F.; et al. A randomized controlled study to investigate the analgesic efficacy of single doses of the cannabinoid receptor-2 agonist, G.W.8.4.2.1.6.6.; ibuprofen or placebo in patients with acute pain following third molar tooth extraction. Clin. J. Pain 2011, 27, 668–676. [Google Scholar] [CrossRef]
- Planas, M.E.; Gay-Escoda, C.; Bagán, J.V.; Santamaría, J.; Peñarrocha, M.; Donado, M.; Puerta, J.L.; García-Magaz, I.; Ruíz, J.; Ortiz, P. Oral metamizol (1 g 2 g) versus ibuprofen placebo in the treatment of lower third molar surgery pain: Randomised double-blind multi-centre study Cooperative Study Group. Eur. J. Clin. Pharmacol. 1998, 53, 405–409. [Google Scholar] [CrossRef]
- Schou, S.; Nielsen, H.; Nattestad, A.; Hillerup, S.; Ritzau, M.; Branebjerg, P.E.; Bugge, C.; Skoglund, L.A. Analgesic dose-response relationship of ibuprofen 50, 100, 200, and 400 mg after surgical removal of third molars: A single-dose, randomized, placebo-controlled, and double-blind study of 304 patients. J. Clin. Pharmacol. 1998, 38, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.I.; Kotey, P.N.; Fricke, J.R., Jr.; Gottesdiener, K. MK-0703 (a Cyclooxygenase-2 inhibitor) in Acute Pain Associated with Dental Surgery: A Randomized, Double-Blind, Placebo- and Active Comparator-Controlled Dose-Ranging Study. Am. J. Ther. 2007, 14, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Seymour, R.A.; Hawkesford, J.E.; Weldon, M.; Brewster, D. An evaluation of different ibuprofen preparations in the control of postoperative pain after third molar surgery. Br. J. Clin. Pharmacol. 1991, 31, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Seymour, R.A.; Ward-Booth, P.; Kelly, P.J. Evaluation of different doses of soluble ibuprofen ibuprofen tablets in postoperative dental pain. Br. J. Oral Maxillofac. Surg. 1996, 34, 110–114. [Google Scholar] [CrossRef]
- Seymour, R.A.; Frame, J.; Negus, T.W.; Hawkesford, J.E.; Marsden, J.; Matthew, I.R. The comparative efficacy of aceclofenac ibuprofen in postoperative pain after third molar surgery. Br. J. Oral Maxillofac. Surg. 1998, 36, 375–379. [Google Scholar] [CrossRef]
- Seymour, R.A.; Hawkesford, J.E.; Hill, C.M.; Frame, J.; Andrews, C. The efficacy of a novel adenosine agonist (WAG994) in postoperative dental pain. Br. J. Clin. Pharmacol. 1999, 47, 675–680. [Google Scholar] [CrossRef]
- Seymour, R.A.; Watkinson, H.; Hawkesford, J.E.; Moore, U. The efficacy of buffered ketoprofen in postoperative pain after third molar surgery. Eur. J. Clin. Pharmacol. 2000, 55, 801–806. [Google Scholar] [CrossRef]
- Sunshine, A.; Olson, N.Z.; Marrero, I.; Tirado, S. Onset duration of analgesia for low-dose ketoprofen in the treatment of postoperative dental pain. J. Clin. Pharmacol. 1998, 38, 1155–1164. [Google Scholar] [CrossRef]
- Troullos, E.S.; Hargreaves, K.M.; Butler, D.P.; Dionne, R.A. Comparison of nonsteroidal anti-inflammatory drugs ibuprofen flurbiprofen with methylprednisolone placebo for acute pain swelling trismus. J. Oral Maxillofac. Surg. 1990, 48, 945–952. [Google Scholar] [CrossRef]
- Van Dyke, T.; Litkowski, L.J.; Kiersch, T.A.; Zarringhalam, N.M.; Zheng, H.; Newman, K. Combination oxycodone 5 mg/ibuprofen 400 mg for the treatment of postoperative pain: A double-blind, placebo- and active-controlled parallel-group study. Clin. Ther. 2004, 26, 2003–2014. [Google Scholar] [CrossRef]
- Weiser, T.; Richter, E.; Hegewisch, A.; Muse, D.D.; Lange, R. Efficacy safety of a fixed-dose combination of ibuprofen caffeine in the management of moderate to severe dental pain after third molar extraction. Eur. J. Pain 2018, 22, 28–38. [Google Scholar] [CrossRef]
- Zelenakas, K.; Fricke, J.R., Jr.; Jayawardene, S.; Kellstein, D. Analgesic efficacy of single oral doses of lumiracoxib ibuprofen in patients with postoperative dental pain. Int. J. Clin. Pract. 2004, 58, 251–256. [Google Scholar] [CrossRef]
- Moore, R.A.; Derry, S.; Wiffen, P.J.; Banerjee, S.; Karan, R.; Glimm, E.; Wiksten, A.; Aldington, D.; Eccleston, C. Estimating relative efficacy in acute postoperative pain: Network meta-analysis is consistent with indirect comparison to placebo alone. Pain 2018, 159, 2234–2244. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.R.; Straube, S.; Paine, J.; Derry, S.; McQuay, H.J. Minimum efficacy criteria for comparisons between treatments using individual patient meta-analysis of acute pain trials: Examples of etoricoxib, paracetamol, ibuprofen, and ibuprofen/paracetamol combinations after third molar extraction. Pain 2011, 152, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Bushra, R.; Aslam, N. An overview of clinical pharmacology of Ibuprofen. Oman Med. J. 2010, 25, 155–1661. [Google Scholar] [CrossRef] [PubMed]
- Rocca, G.D.; Chiarandini, P.; Pietropaoli, P. Analgesia in PACU: Nonsteroidal anti-inflammatory drugs. Curr. Drug Targets 2005, 6, 781–787. [Google Scholar] [CrossRef]
- Halpern, S.M.; Fitzpatrick, R.; Volans, G.N. Ibuprofen toxicity. A review of adverse reactions and overdose. Advers. Drug React. Toxicol. Rev. 1993, 12, 107–128. [Google Scholar]

| Analgesic Efficacy | Ibuprofen | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 200 mg | 400 mg | 600 mg | |||||||
| n | NNT | CIs | n | NNT | CIs | n | NNT | CIs | |
| The number of patients who took rescue analgesics | 797 | 3.7 | 3 to 4.7 | 2803 | 3.1 | 2.8 to 3.4 | 1149 | 3.4 | 2.8 to 4.2 |
| Overall evaluation | 694 | 2.6 | 2.2 to 3.1 | 2407 | 1.9 | 1.8 to 2 | 291 | 1.6 | 1.4 to 2 |
| Adverse effects | n | NNH | CIs | n | NNH | CIs | n | NNH | CIs |
| Nausea | 1461 | 27.6 | 16.3 to 89.8 | 3917 | 28.4 | 18.4 to 61.6 | - | - | - |
| Vomiting | 1319 | 42.6 | 22.3 to 477.7 | 3124 | 29.6 | 19 to 66.3 | 716 | 17 | 10 to 50.8 |
| Headache | 1342 | 24.8 | 14.8 to 76.9 | 2477 | 26.7 | 17.2 to 59.3 | 716 | 12.3 | 7.7 to 31.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora-Falcón, I.J.; Amador-Beas, I.A.; Bologna-Molina, R.; Molina-Frechero, N.; Aragón-Martínez, O.H.; Serafín-Higuera, N.; López-Verdín, S.; Isiordia-Espinoza, M.A. Analgesic Efficacy of Postoperative Ibuprofen in Third Molar Surgery: A Meta-Analysis. Oral 2025, 5, 72. https://doi.org/10.3390/oral5030072
Mora-Falcón IJ, Amador-Beas IA, Bologna-Molina R, Molina-Frechero N, Aragón-Martínez OH, Serafín-Higuera N, López-Verdín S, Isiordia-Espinoza MA. Analgesic Efficacy of Postoperative Ibuprofen in Third Molar Surgery: A Meta-Analysis. Oral. 2025; 5(3):72. https://doi.org/10.3390/oral5030072
Chicago/Turabian StyleMora-Falcón, Itzel Joselyn, Iván Agustín Amador-Beas, Ronell Bologna-Molina, Nelly Molina-Frechero, Othoniel Hugo Aragón-Martínez, Nicolás Serafín-Higuera, Sandra López-Verdín, and Mario Alberto Isiordia-Espinoza. 2025. "Analgesic Efficacy of Postoperative Ibuprofen in Third Molar Surgery: A Meta-Analysis" Oral 5, no. 3: 72. https://doi.org/10.3390/oral5030072
APA StyleMora-Falcón, I. J., Amador-Beas, I. A., Bologna-Molina, R., Molina-Frechero, N., Aragón-Martínez, O. H., Serafín-Higuera, N., López-Verdín, S., & Isiordia-Espinoza, M. A. (2025). Analgesic Efficacy of Postoperative Ibuprofen in Third Molar Surgery: A Meta-Analysis. Oral, 5(3), 72. https://doi.org/10.3390/oral5030072