Effects of Mandibular Advancement Device on Cardiovascular and Respiratory Parameters in OSA Patients
Abstract
1. Introduction
2. Materials and Methods
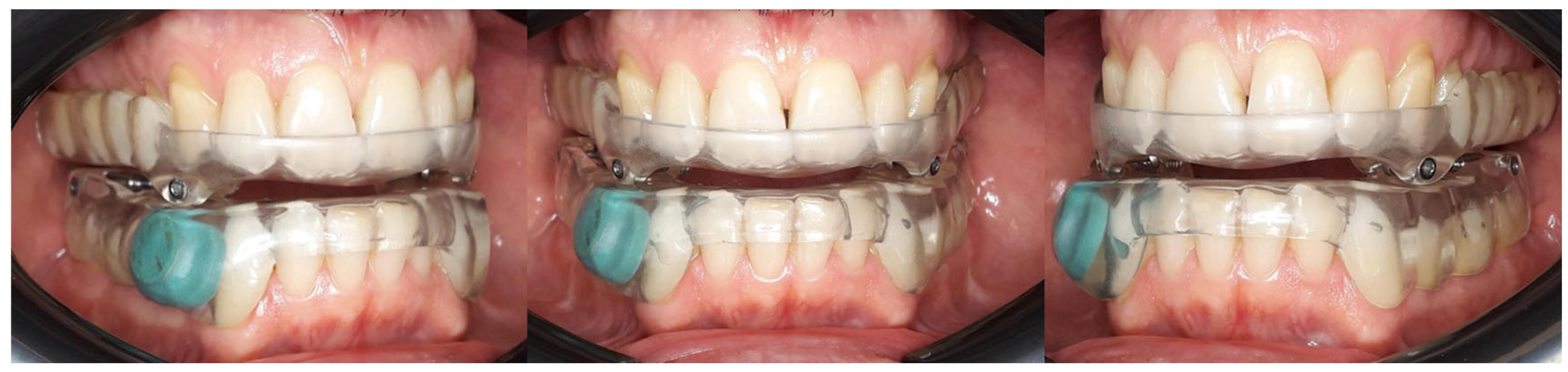
2.1. Study Population
2.2. Methods and Parameters
2.3. Statistical Analysis
3. Results
| Mean HR (T0) | Mean HR (T1) | Minimum HR (T0) | Minimum HR (T1) | Maximum HR (T0) | Maximum HR (T1) | ODI (T0) | ODI (T1) | AHI (T0) | AHI (T1) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Number of values | 64 | 64 | 64 | 64 | 64 | 64 | 64 | 64 | 64 | 64 |
| Mean | 61.47 | 59.75 | 43.84 | 48.00 | 131.3 | 107.3 | 19.7 | 4.65 | 27.7 | 6.22 |
| Std. Deviation | 7.329 | 8.84 | 9.68 | 8.17 | 41.24 | 28.57 | 9.42 | 3.21 | 12.3 | 4.05 |
| Std. Error of Mean | 0.91 | 1.106 | 1.211 | 1.02 | 5.155 | 3.57 | 1.17 | 0.4 | 1.54 | 0.5 |
| Lower 95% CI of mean | 59.64 | 57.54 | 41.42 | 45.96 | 121.01 | 100.2 | 17.3 | 3.85 | 24.6 | 5.21 |
| Upper 95% CI of mean | 63.30 | 61.96 | 46.26 | 50.04 | 141.6 | 114.4 | 22.01 | 5.46 | 30.7 | 7.23 |
| Passed normality test | No | No | No | Yes | No | No | No | No | No | No |
| p value | 0.05 | 0.001 | 0.001 | 0.001 | 0.002 |
| (N = 64) | Mean Difference | p Value * | Significance After Bonferroni Correction(α = 0.01) |
|---|---|---|---|
| Mean HR | −1.72 | 0.047 | Not significant |
| Minimum HR | 4.16 | 0.001 | significant |
| Maximum HR | −24 | 0.0001 | significant |
| ODI | −15.05 | 0.0001 | significant |
| AHI | −21.48 | 0.0001 | significant |
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faber, J.; Faber, C.; Faber, A.P. Obstructive sleep apnea in adults. Dental Press. J. Orthod. 2019, 24, 99–109. [Google Scholar] [CrossRef]
- Malhotra, A.; White, D.P. Obstructive sleep apnoea. Lancet 2002, 360, 237–245. [Google Scholar] [CrossRef]
- Azagra-Calero, E.; Espinar-Escalona, E.; Barrera-Mora, J.M.; Llamas-Carreras, J.M.; Solano-Reina, E. Obstructive sleep apnea syndrome (OSAS). Review of the literature. Med. Oral. Patol. Oral. Cir. Bucal 2012, 17, e925–e929. [Google Scholar] [CrossRef]
- Rundo, J.V. Obstructive sleep apnea basics. Cleve Clin. J. Med. 2019, 86 (Suppl. S1), 2–9. [Google Scholar] [CrossRef]
- Punjabi, N.M. The epidemiology of adult obstructive sleep apnea. Proc. Am. Thorac. Soc. 2008, 5, 136–143. [Google Scholar] [CrossRef]
- Malhotra, A.; Huang, Y.; Fogel, R.; Lazic, S.; Pillar, G.; Jakab, M.; Kikinis, R.; White, D.P. Aging influences on pharyngeal anatomy and physiology: The predisposition to pharyngeal collapse. Am. J. Med. 2006, 119, 72.e9–72.14. [Google Scholar] [CrossRef] [PubMed]
- Fietze, I.; Laharnar, N.; Obst, A.; Ewert, R.; Felix, S.B.; Garcia, C.; Glaser, S.; Glos, M.; Schmidt, C.O.; Stubbe, B.; et al. Prevalence and association analysis of obstructive sleep apnea with gender and age differences—Results of SHIP-Trend. J. Sleep Res. 2019, 28, e12770. [Google Scholar] [CrossRef] [PubMed]
- Messineo, L.; Bakker, J.P.; Cronin, J.; Yee, J.; White, D.P. Obstructive sleep apnea and obesity: A review of epidemiology, pathophysiology and the effect of weight-loss treatments. Sleep Med. Rev. 2024, 78, 101996. [Google Scholar] [CrossRef]
- Ciavarella, D.; Lorusso, M.; Campobasso, A.; Cazzolla, A.P.; Montaruli, G.; Burlon, G.; Lo Muzio, E.; Laurenziello, M.; Tepedino, M. Craniofacial morphology in Obstructive Sleep Apnea patients. J. Clin. Exp. Dent. 2023, 15, e999–e1006. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, M.R.; Borel, A.L.; Machan, E.; Grunstein, R. Sleep apnoea and metabolic dysfunction. Eur. Respir. Rev. 2013, 22, 353–364. [Google Scholar] [CrossRef]
- Olaithe, M.; Bucks, R.S.; Hillman, D.R.; Eastwood, P.R. Cognitive deficits in obstructive sleep apnea: Insights from a meta-review and comparison with deficits observed in COPD, insomnia, and sleep deprivation. Sleep Med. Rev. 2018, 38, 39–49. [Google Scholar] [CrossRef]
- Hartenbaum, N.; Collop, N.; Rosen, I.; Phillips, B. Truckers with OSA, should they be driving? J. Occup. Environ. Med. 2006, 48, 871–872. [Google Scholar] [CrossRef]
- O’Donnell, C.; O’Mahony, A.M.; McNicholas, W.T.; Ryan, S. Cardiovascular manifestations in obstructive sleep apnea: Current evidence and potential mechanisms. Pol. Arch. Intern. Med. 2021, 131, 550–560. [Google Scholar] [CrossRef]
- Gonzalez-Aquines, A.; Martinez-Roque, D.; Baltazar Trevino-Herrera, A.; Chavez-Luevanos, B.E.; Guerrero-Campos, F.; Gongora-Rivera, F. Obstructive sleep apnea syndrome and its relationship with ischaemic stroke. Rev. Neurol. 2019, 69, 255–260. [Google Scholar] [PubMed]
- Linz, D.; McEvoy, R.D.; Cowie, M.R.; Somers, V.K.; Nattel, S.; Levy, P.; Kalman, J.M.; Sanders, P. Associations of Obstructive Sleep Apnea with Atrial Fibrillation and Continuous Positive Airway Pressure Treatment: A Review. JAMA Cardiol. 2018, 3, 532–540. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Dong, Z.; Fan, J.; Nie, S.; Wei, Y. Effect of continuous positive airway pressure on long-term cardiovascular outcomes in patients with coronary artery disease and obstructive sleep apnea: A systematic review and meta-analysis. Respir. Res. 2018, 19, 61. [Google Scholar] [CrossRef] [PubMed]
- Gagnadoux, F.; Bequignon, E.; Prigent, A.; Micoulaud-Franchi, J.A.; Chambe, J.; Texereau, J.; Alami, S.; Roche, F. The PAP-RES algorithm: Defining who, why and how to use positive airway pressure therapy for OSA. Sleep Med. Rev. 2024, 75, 101932. [Google Scholar] [CrossRef] [PubMed]
- Azarbarzin, A.; Sands, S.A.; Younes, M.; Taranto-Montemurro, L.; Sofer, T.; Vena, D.; Alex, R.M.; Kim, S.W.; Gottlieb, D.J.; White, D.P.; et al. The Sleep Apnea-Specific Pulse-Rate Response Predicts Cardiovascular Morbidity and Mortality. Am. J. Respir. Crit. Care Med. 2021, 203, 1546–1555. [Google Scholar] [CrossRef]
- Azarbarzin, A.; Zinchuk, A.; Wellman, A.; Labarca, G.; Vena, D.; Gell, L.; Messineo, L.; White, D.P.; Gottlieb, D.J.; Redline, S.; et al. Cardiovascular Benefit of Continuous Positive Airway Pressure in Adults with Coronary Artery Disease and Obstructive Sleep Apnea without Excessive Sleepiness. Am. J. Respir. Crit. Care Med. 2022, 206, 767–774. [Google Scholar] [CrossRef]
- Somers, V.K.; Dyken, M.E.; Clary, M.P.; Abboud, F.M. Sympathetic neural mechanisms in obstructive sleep apnea. J. Clin. Investig. 1995, 96, 1897–1904. [Google Scholar] [CrossRef]
- Souza, H.C.D.; Philbois, S.V.; Veiga, A.C.; Aguilar, B.A. Heart Rate Variability and Cardiovascular Fitness: What We Know so Far. Vasc. Health Risk Manag. 2021, 17, 701–711. [Google Scholar] [CrossRef]
- Glos, M.; Penzel, T.; Schoebel, C.; Nitzsche, G.R.; Zimmermann, S.; Rudolph, C.; Blau, A.; Baumann, G.; Jost-Brinkmann, P.G.; Rautengarten, S.; et al. Comparison of effects of OSA treatment by MAD and by CPAP on cardiac autonomic function during daytime. Sleep Breath. 2016, 20, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef]
- Ciavarella, D.; Ferrara, D.; Fanelli, C.; Montaruli, G.; Burlon, G.; Laurenziello, M.; Lo Russo, L.; Esperouz, F.; Tepedino, M.; Lorusso, M. Evaluation of sleep position shifts in patients with obstructive sleep apnea syndrome with the use of a mandibular advancement device. Front. Dent. Med. 2025, 6, 1524334. [Google Scholar]
- Cohen, J. Statistical Power Analysis. Curr. Dir. Psychol. Sci. 1992, 1, 98–101. [Google Scholar] [CrossRef]
- Arnaud, C.; Bochaton, T.; Pepin, J.L.; Belaidi, E. Obstructive sleep apnoea and cardiovascular consequences: Pathophysiological mechanisms. Arch. Cardiovasc. Dis. 2020, 113, 350–358. [Google Scholar] [CrossRef]
- Floras, J.S. Sleep apnea and cardiovascular risk. J. Cardiol. 2014, 63, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dobrosielski, D.A.; Papandreou, C.; Patil, S.P.; Salas-Salvado, J. Diet and exercise in the management of obstructive sleep apnoea and cardiovascular disease risk. Eur. Respir. Rev. 2017, 26, 160110. [Google Scholar] [CrossRef] [PubMed]
- Bratton, D.J.; Gaisl, T.; Wons, A.M.; Kohler, M. CPAP vs Mandibular Advancement Devices and Blood Pressure in Patients With Obstructive Sleep Apnea: A Systematic Review and Meta-analysis. JAMA 2015, 314, 2280–2293. [Google Scholar] [CrossRef]
- Dissanayake, H.U.; Colpani, J.T.; Sutherland, K.; Loke, W.; Mohammadieh, A.; Ou, Y.H.; de Chazal, P.; Cistulli, P.A.; Lee, C.H. Obstructive sleep apnea therapy for cardiovascular risk reduction-Time for a rethink? Clin. Cardiol. 2021, 44, 1729–1738. [Google Scholar] [CrossRef]
- de Vries, G.E.; Wijkstra, P.J.; Houwerzijl, E.J.; Kerstjens, H.A.M.; Hoekema, A. Cardiovascular effects of oral appliance therapy in obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med. Rev. 2018, 40, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Kasai, T.; Bradley, T.D. Obstructive sleep apnea and heart failure: Pathophysiologic and therapeutic implications. J. Am. Coll. Cardiol. 2011, 57, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Munzel, T.; Hahad, O.; Gori, T.; Hollmann, S.; Arnold, N.; Prochaska, J.H.; Schulz, A.; Beutel, M.; Pfeiffer, N.; Schmidtmann, I.; et al. Heart rate, mortality, and the relation with clinical and subclinical cardiovascular diseases: Results from the Gutenberg Health Study. Clin. Res. Cardiol. 2019, 108, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Tjugen, T.B.; Flaa, A.; Kjeldsen, S.E. The prognostic significance of heart rate for cardiovascular disease and hypertension. Curr. Hypertens. Rep. 2010, 12, 162–169. [Google Scholar] [CrossRef]
- Bratton, D.J.; Stradling, J.R.; Barbe, F.; Kohler, M. Effect of CPAP on blood pressure in patients with minimally symptomatic obstructive sleep apnoea: A meta-analysis using individual patient data from four randomised controlled trials. Thorax 2014, 69, 1128–1135. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciavarella, D.; Ferrara, D.; Fanelli, C.; Esperouz, F.; Burlon, C.; Burlon, G.; Lo Russo, L.; Tepedino, M.; Lorusso, M. Effects of Mandibular Advancement Device on Cardiovascular and Respiratory Parameters in OSA Patients. Oral 2025, 5, 62. https://doi.org/10.3390/oral5030062
Ciavarella D, Ferrara D, Fanelli C, Esperouz F, Burlon C, Burlon G, Lo Russo L, Tepedino M, Lorusso M. Effects of Mandibular Advancement Device on Cardiovascular and Respiratory Parameters in OSA Patients. Oral. 2025; 5(3):62. https://doi.org/10.3390/oral5030062
Chicago/Turabian StyleCiavarella, Domenico, Donatella Ferrara, Carlotta Fanelli, Fariba Esperouz, Carlotta Burlon, Giuseppe Burlon, Lucio Lo Russo, Michele Tepedino, and Mauro Lorusso. 2025. "Effects of Mandibular Advancement Device on Cardiovascular and Respiratory Parameters in OSA Patients" Oral 5, no. 3: 62. https://doi.org/10.3390/oral5030062
APA StyleCiavarella, D., Ferrara, D., Fanelli, C., Esperouz, F., Burlon, C., Burlon, G., Lo Russo, L., Tepedino, M., & Lorusso, M. (2025). Effects of Mandibular Advancement Device on Cardiovascular and Respiratory Parameters in OSA Patients. Oral, 5(3), 62. https://doi.org/10.3390/oral5030062










