Proposal of a Cephalometric Method in Computed Tomography to Mandibular Analysis in Infants with Pierre Robin Sequence Treated by Fast and Early Mandibular Osteo-Distraction: Pilot Study
Abstract
1. Introduction
2. Methods
2.1. Patients
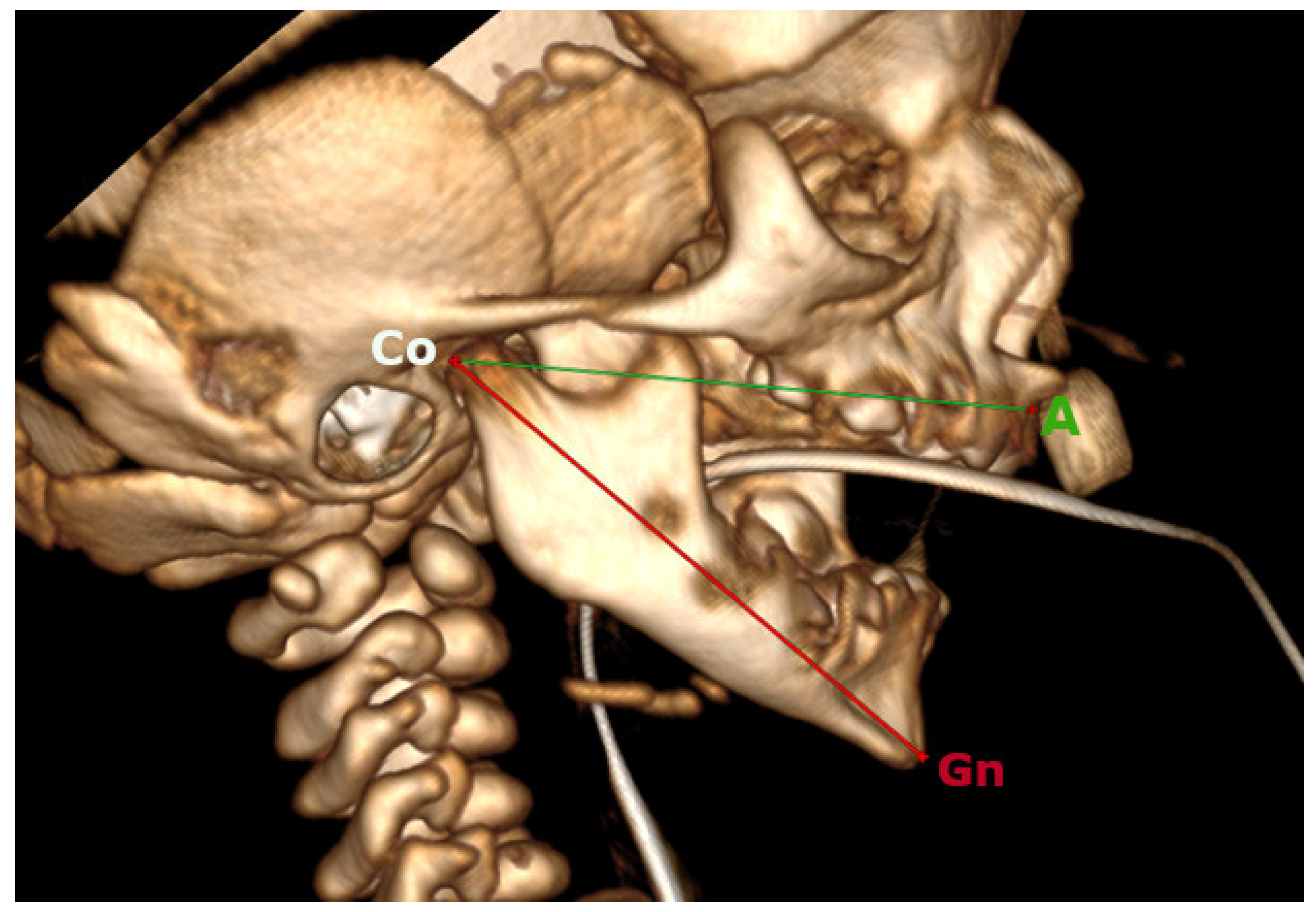
2.2. Cephalometrics Analysis
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elliott, M.A.; Studen-Pavlovich, D.A.; Ranalli, D.N. Prevalence of selected pediatric conditions in children with Pierre Robin sequence. Pediatr. Dent. 1995, 17, 106–111. [Google Scholar]
- Santoro, M.; Coi, A.; Barišić, I.; Pierini, A.; Addor, M.; Baldacci, S.; Ballardini, E.; Boban, L.; Braz, P.; Cavero-Carbonell, C.; et al. Epidemiology of Pierre-Robin sequence in Europe: A population-based EUROCAT study. Paediatr. Perinat. Epidemiol. 2021, 35, 530–539. [Google Scholar] [CrossRef]
- Scott, A.R.; Tibesar, R.J.; Sidman, J.D. Pierre Robin Sequence: Evaluation, Management, Indications for Surgery, and Pitfalls. Otolaryngol. Clin. N. Am. 2012, 45, 695–710. [Google Scholar] [CrossRef]
- Bronshtein, M.; Blazer, S.; Zalel, Y.; Zimmer, E.Z. Ultrasonographic diagnosis of glossoptosis in fetuses with Pierre Robin sequence in early and mid pregnancy. Am. J. Obstet. Gynecol. 2005, 193, 1561–1564. [Google Scholar] [CrossRef]
- Cicchetti, R.; Cascone, P.; Caresta, E.; Papoff, P.; Miano, S.; Cerasaro, C.; Ramieri, V.; Midulla, F.; Moretti, C. Mandibular distraction osteogenesis for neonates with Pierre Robin sequence and airway obstruction. J. Matern. Fetal Neonatal Med. 2012, 25 (Suppl. 4), 133–135. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.T.; Woo, A.S. Pierre Robin Sequence. Clin. Plast. Surg. 2019, 46, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Galluccio, G.; Mazzoli, V.; Vernucci, R.; Silvestri, A.; Barbato, E. Neonatal Functional Treatment for Pierre Robin Sequence. Turk. J. Orthod. 2019, 32, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Côté, A.; Fanous, A.; Almajed, A.; Lacroix, Y. Pierre Robin sequence: Review of diagnostic and treatment challenges. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 451–464. [Google Scholar] [CrossRef]
- Almajed, A.; Viezel-Mathieu, A.; Gilardino, M.S.; Flores, R.L.; Tholpady, S.S.; Côté, A. Outcome Following Surgical Interventions for Micrognathia in Infants with Pierre Robin Sequence: A Systematic Review of the Literature. Cleft Palate-Craniofacial J. 2017, 54, 32–42. [Google Scholar] [CrossRef]
- Kurian, C.; Ehsan, Z. Sleep and respiratory outcomes in neonates with Pierre Robin sequence: A concise review. Sleep Breath. 2019, 24, 1–5. [Google Scholar] [CrossRef]
- Cascone, P.; Papoff, P.; Arangio, P.; Vellone, V.; Calafati, V.; Silvestri, A. Fast and early mandibular osteodistraction (FEMOD) in severe Pierre Robin Sequence. J. Cranio-Maxillofacial Surg. 2014, 42, 1364–1370. [Google Scholar] [CrossRef]
- Susarla, S.M.; Vasilakou, N.; Kapadia, H.; Egbert, M.; Hopper, R.A.; Evans, K.N. Defining mandibular morphology in Robin sequence: A matched case-control study. Am. J. Med Genet. Part A 2017, 173, 1831–1838. [Google Scholar] [CrossRef]
- Krimmel, M.; Kluba, S.; Breidt, M.; Bacher, M.; Dietz, K.; Buelthoff, H.; Reinert, S. Three-dimensional assessment of facial development in children with pierre robin sequence. J. Craniofacial Surg. 2009, 20, 2055–2060. [Google Scholar] [CrossRef] [PubMed]
- Breugem, C.C.; Evans, K.N.; Poets, C.F.; Suri, S.; Picard, A.; Filip, C.; Paes, E.C.; Mehendale, F.V.; Saal, H.M.; Basart, H.; et al. Best Practices for the Diagnosis and Evaluation of Infants with Robin Sequence: A Clinical Consensus Report. JAMA Pediatr. 2016, 170, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Brons, S.; Meulstee, J.W.; Loonen, T.G.; Nada, R.M.; Kuijpers, M.A.; Bronkhorst, E.M.; Bergé, S.J.; Maal, T.J.; Kuijpers-Jagtman, A.M. Three-dimensional facial development of children with unilateral cleft lip and palate during the first year of life in comparison with normative average faces. PeerJ 2019, 7, e7302. [Google Scholar] [CrossRef]
- Brons, S.; Meulstee, J.W.; Nada, R.M.; Kuijpers, M.A.R.; Bronkhorst, E.M.; Bergé, S.J.; Maal, T.J.J.; Kuijpers-Jagtman, A.M.; Arakeri, G. Uniform 3D meshes to establish normative facial averages of healthy infants during the first year of life. PLoS ONE 2019, 14, e0217267. [Google Scholar] [CrossRef] [PubMed]
- Krimmel, M.; Breidt, M.; Bacher, M.; Müller-Hagedorn, S.; Dietz, K.; Bülthoff, H.; Reinert, S.; Kluba, S. Three-dimensional normal facial growth from birth to the age of 7 Years. Plast. Reconstr. Surg. 2015, 136, 490e–501e. [Google Scholar] [CrossRef]
- Van Der Haven, I.; Mulder, J.W.; Van Der Wal, K.G.H.; Hage, J.J.; De Lange-De Klerk, E.S.M.; Haumann, T.J. The jaw index: New guide defining micrognathia in newborns. Cleft Palate-Craniofacial J. 1997, 34, 240–241. [Google Scholar] [CrossRef]
- Liu, Y.P.; Behrents, R.G.; Buschang, P.H. Mandibular growth, remodeling, and maturation during infancy and early childhood. Angle Orthod. 2010, 80, 97–105. [Google Scholar] [CrossRef]
- O’Sullivan, E.; van de Lande, L.S.; El Ghoul, K.; Koudstaal, M.J.; Schievano, S.; Khonsari, R.H.; Dunaway, D.J.; Zafeiriou, S. Growth patterns and shape development of the paediatric mandible—A 3D statistical model. Bone Rep. 2022, 16, 101528. [Google Scholar] [CrossRef]
- McNamara, J.A. A method of cephalometric evaluation. Am. J. Orthod. 1984, 86, 449–469. [Google Scholar] [CrossRef]
- Wong, R.; Chau, A.; Hägg, U. 3D CBCT McNamara’s cephalometric analysis in an adult southern Chinese population. Int. J. Oral Maxillofac. Surg. 2011, 40, 920–925. [Google Scholar] [CrossRef]
- Dos Santos, R.M.G.; De Martino, J.M.; Haiter Neto, F.; Passeri, L.A. Cone-beam computed tomography-based three-dimensional McNamara cephalometric analysis. J. Craniofacial Surg. 2018, 29, 895–899. [Google Scholar] [CrossRef]
- Santos, R.M.G.; De Martino, J.M.; Haiter Neto, F.; Passeri, L.A. Cone beam computed tomography-based cephalometric norms for Brazilian adults. Int. J. Oral Maxillofac. Surg. 2018, 47, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Barreto, M.S.; da Silva Barbosa, I.; Miranda Leite-Ribeiro, P.; de Araújo, T.M.; Almeida Sarmento, V. Accuracy of the measurements from multiplanar and sagittal reconstructions of CBCT. Orthod. Craniofacial Res. 2019, 23, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.M.; Al Bishri, A.; Haroun Mohamed, A. Distraction osteogenesis as followed by CT scan in Pierre Robin sequence. J. Cranio-Maxillofac. Surg. 2011, 39, 412–419. [Google Scholar] [CrossRef]
- Meyers, A.B.; Zei, M.G.; Denny, A.D. Imaging neonates and children with Pierre Robin sequence before and after mandibular distraction osteogenesis: What the craniofacial surgeon wants to know. Pediatr. Radiol. 2015, 45, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Sam, A.; Currie, K.; Oh, H.; Flores-Mir, C.; Lagravére-Vich, M. Reliability of different three-dimensional cephalometric landmarks in cone-beam computed tomography: A systematic review. Angle Orthod. 2018, 89, 317–332. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Li, F.; Wu, W.; Hao, J.; Luo, D.; Wang, H. Condylar positions before and after bilateral mandibular distraction osteogenesis in children with Pierre Robin sequence. Int. J. Oral Maxillofac. Surg. 2018, 47, 57–63. [Google Scholar] [CrossRef]
- Mimouni, G.; Merlob, P.; Mimouni, F.B.; Bin-Nun, A. The goniomaxillar length/goniomandibular length ratio in normal newborn infants: A clinical tool for defining chin position abnormalities. Am. J. Med. Genet. Part A 2020, 185, 46–49. [Google Scholar] [CrossRef]
- Susarla, S.M.; Evans, K.N.; Kapadia, H.; Vasilakou, N.; Egbert, M.A.; Hopper, R.A. Distraction Osteogenesis Normalizes Mandibular Body-Symphysis Morphology in Infants with Robin Sequence. J. Oral Maxillofac. Surg. 2018, 76, 169–179. [Google Scholar] [CrossRef] [PubMed]
| CoA | CoGn Min | CoGn Max | Ratio Md/Mx Min | Ratio Md/Mx Max |
|---|---|---|---|---|
| 35 | 36 | 38 | 1.03 | 1.08 |
| 40 | 42 | 44 | 1.05 | 1.10 |
| 45 | 48 | 50 | 1.07 | 1.12 |
| 50 | 54 | 57 | 1.09 | 1.14 |
| 55 | 61 | 64 | 1.11 | 1.16 |
| 60 | 68 | 71 | 1.13 | 1.18 |
| 65 | 75 | 78 | 1.15 | 1.19 |
| 70 | 82 | 85 | 1.17 | 1.21 |
| 75 | 90 | 94 | 1.19 | 1.23 |
| 80 | 97 | 100 | 1.21 | 1.25 |
| 85 | 105 | 108 | 1.24 | 1.27 |
| 90 | 113 | 116 | 1.26 | 1.29 |
| 95 | 122 | 125 | 1.28 | 1.32 |
| 100 | 130 | 133 | 1.30 | 1.33 |
| 105 | 138 | 141 | 1.31 | 1.34 |
| Patient | Sex | T1 (Days of Born) | FEMOD (Days of Born) | T2 (Days of Born) |
|---|---|---|---|---|
| 1 | F | 2 | 14 | 1581 |
| 2 | M | 13 | 15 | 85 |
| 3 | M | 71 | 89 | 778 |
| 4 | F | 77 | 97 | 401 |
| 5 | M | 7 | 14 | 668 |
| 6 | F | 6 | 21 | 267 |
| Patient | T1. CoA (mm) | T1. CoGn (mm) | Expected T1. CoGn (mm) | Difference Measured T1. CoGn–Expected T1. CoGn (mm) | p Value Expected T1. CoGn Vs. Measured T1. CoGn | T1 Md/Mx ratio | Expected T1. Md/Mx Ratio | p Value Expected T1. Md/Mx Ratio Vs. Measured T1 Md/Mx Ratio |
|---|---|---|---|---|---|---|---|---|
| 1 | 34.9 | 32.4 | 36 | −3.6 | 0.028 * | 0.93 | 1.03 | 0.029 * |
| 2 | 42.1 | 38.6 | 44 | −5.4 | 0.92 | 1.06 | ||
| 3 | 43.6 | 42.6 | 46 | −3.4 | 0.98 | 1.06 | ||
| 4 | 42.0 | 41.5 | 44 | −2.5 | 0.99 | 1.06 | ||
| 5 | 42.5 | 37.1 | 44 | −6.9 | 0.87 | 1.06 | ||
| 6 | 35.0 | 33.0 | 36 | −3.0 | 0.94 | 1.03 |
| Patient | T2. CoA (mm) | T2. CoGn (mm) | Expected T2. CoGn (mm) | Difference Measured T2. CoGn–Expected T2.CoGn (mm) | p Value Expected T2. CoGn Vs. Measured T2. CoGn | T2 Md/Mx Ratio | Expected T2. Md/Mx Ratio | p Value Expected T2. Md/Mx Ratio Vs. Measured T2 Md/Mx Ratio |
|---|---|---|---|---|---|---|---|---|
| 1 | 61.4 | 70.45 | 70 | 0.5 | 0.400 | 1.15 | 1.14 | 0.461 |
| 2 | 47.2 | 47.4 | 51 | −3.6 | 1.01 | 1.08 | ||
| 3 | 58.9 | 68.2 | 68 | 0.2 | 1.16 | 1.16 | ||
| 4 | 52.9 | 58.8 | 58 | 0.8 | 1.11 | 1.11 | ||
| 5 | 65.4 | 76.9 | 76 | 0.9 | 1.18 | 1.17 | ||
| 6 | 47.3 | 59.5 | 52 | 7.5 | 1.26 | 1.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imondi, F.; De Stefano, A.A.; Podda, R.; Horodynski, M.; Vernucci, R.A.; Mazzoli, V.; Cascone, P.; Galluccio, G. Proposal of a Cephalometric Method in Computed Tomography to Mandibular Analysis in Infants with Pierre Robin Sequence Treated by Fast and Early Mandibular Osteo-Distraction: Pilot Study. Oral 2025, 5, 58. https://doi.org/10.3390/oral5030058
Imondi F, De Stefano AA, Podda R, Horodynski M, Vernucci RA, Mazzoli V, Cascone P, Galluccio G. Proposal of a Cephalometric Method in Computed Tomography to Mandibular Analysis in Infants with Pierre Robin Sequence Treated by Fast and Early Mandibular Osteo-Distraction: Pilot Study. Oral. 2025; 5(3):58. https://doi.org/10.3390/oral5030058
Chicago/Turabian StyleImondi, Francesca, Adriana Assunta De Stefano, Rachele Podda, Martina Horodynski, Roberto Antonio Vernucci, Valentina Mazzoli, Piero Cascone, and Gabriella Galluccio. 2025. "Proposal of a Cephalometric Method in Computed Tomography to Mandibular Analysis in Infants with Pierre Robin Sequence Treated by Fast and Early Mandibular Osteo-Distraction: Pilot Study" Oral 5, no. 3: 58. https://doi.org/10.3390/oral5030058
APA StyleImondi, F., De Stefano, A. A., Podda, R., Horodynski, M., Vernucci, R. A., Mazzoli, V., Cascone, P., & Galluccio, G. (2025). Proposal of a Cephalometric Method in Computed Tomography to Mandibular Analysis in Infants with Pierre Robin Sequence Treated by Fast and Early Mandibular Osteo-Distraction: Pilot Study. Oral, 5(3), 58. https://doi.org/10.3390/oral5030058







