Oral Rehabilitation Following Surgical Treatment of Mandibular Ameloblastoma: Case Report and Comprehensive Literature Review
Abstract
1. Introduction
- Conservative surgery encompasses surgical enucleation, curettage, and cryotherapy. These techniques are generally used for less aggressive forms of the tumor like unicystic and peripheral ameloblastoma. These methods need required a reduced surgical time but may be associated with higher recurrence rates and the potential need for re-intervention.
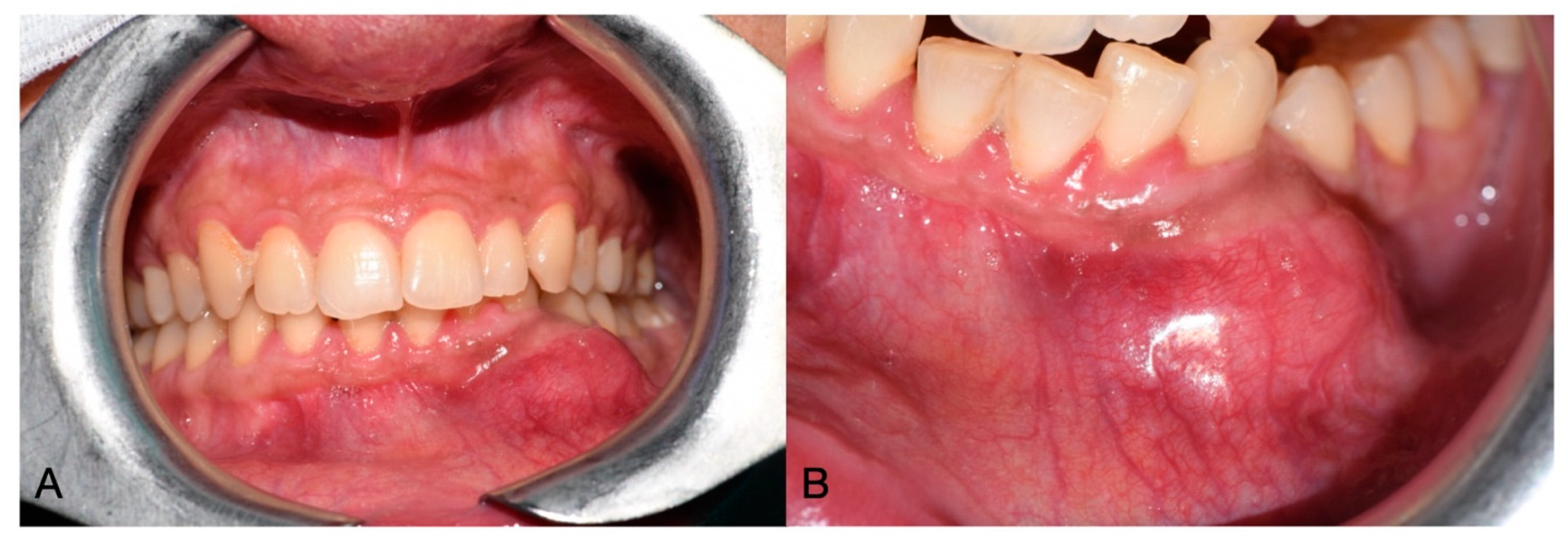
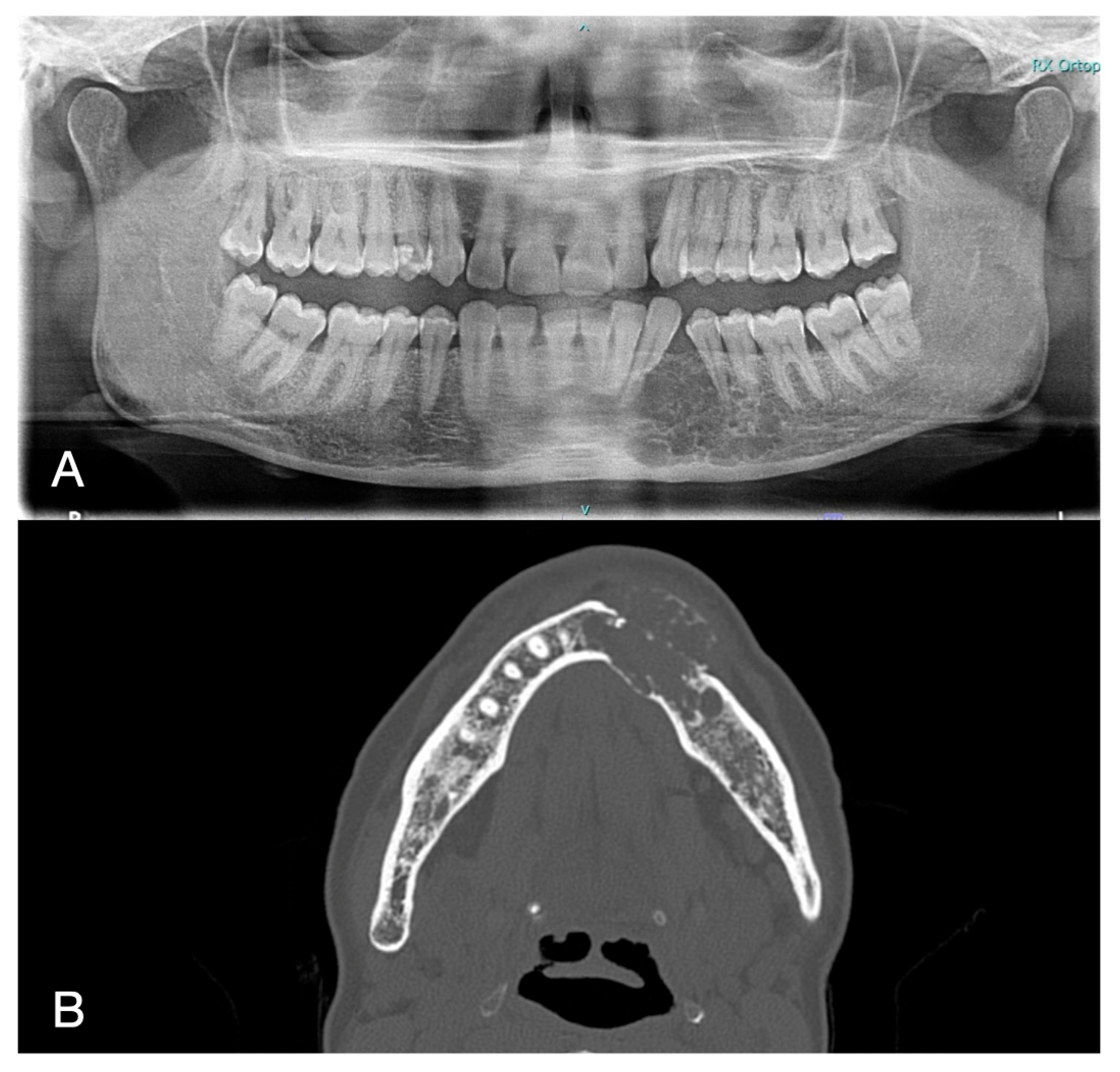
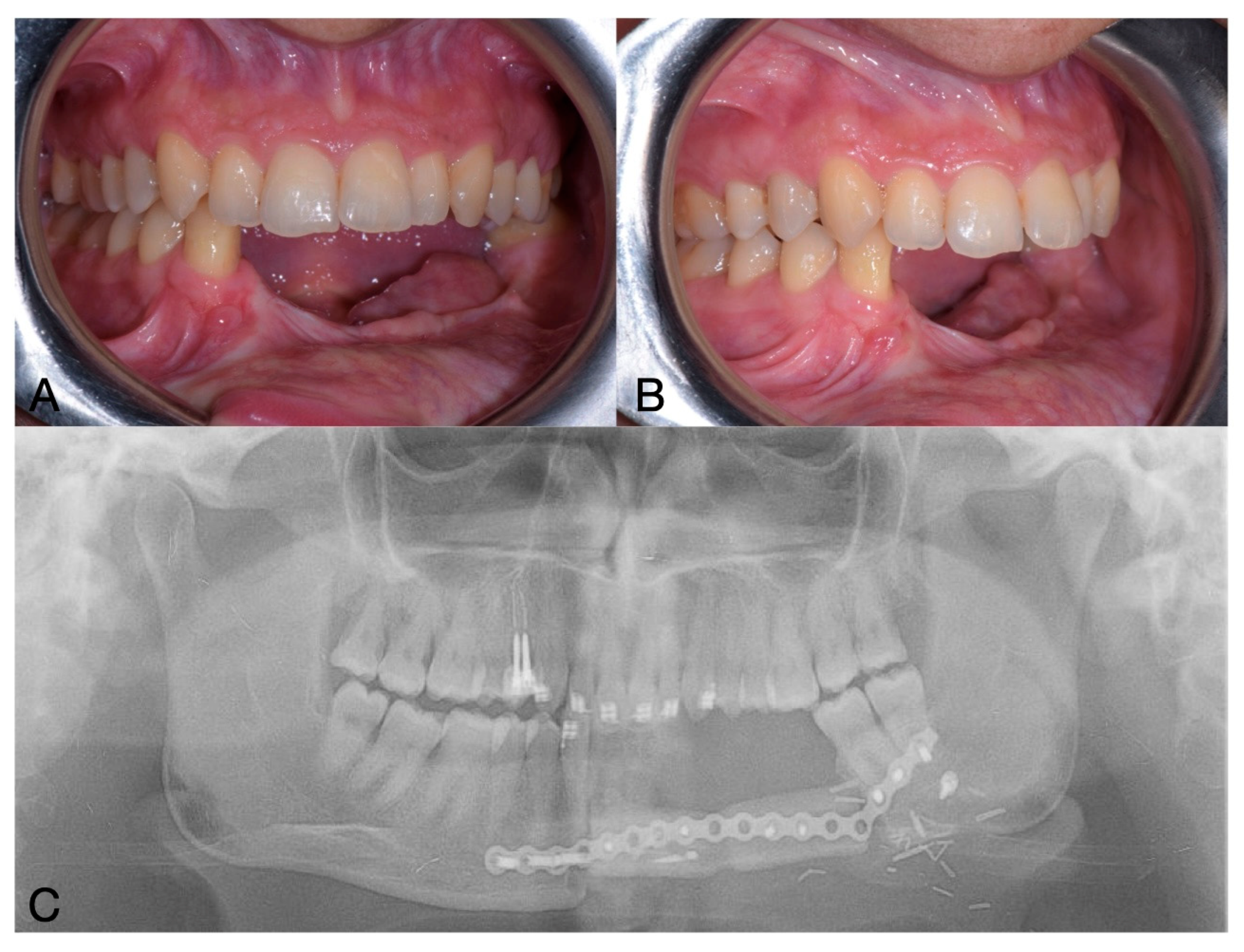
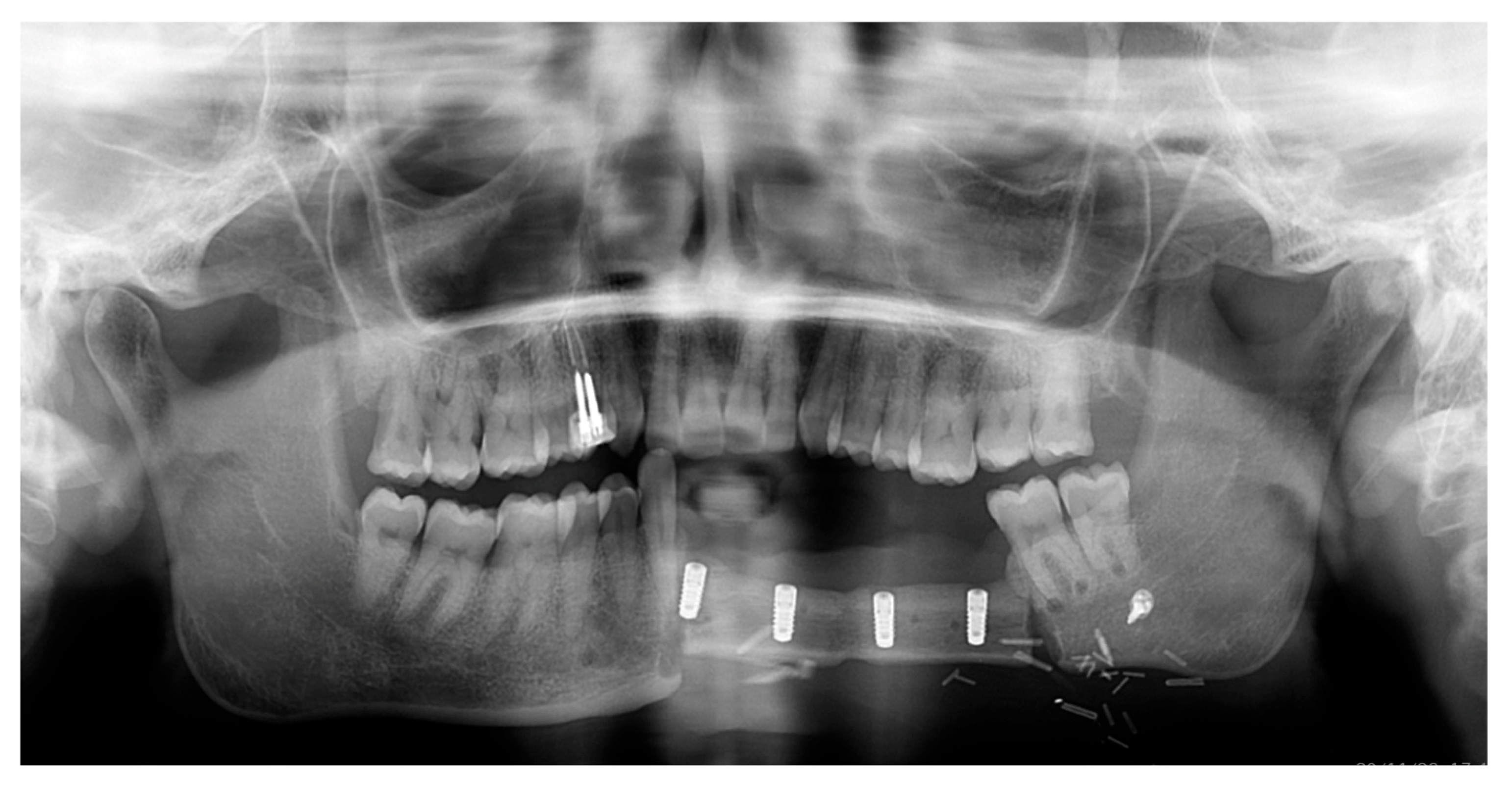
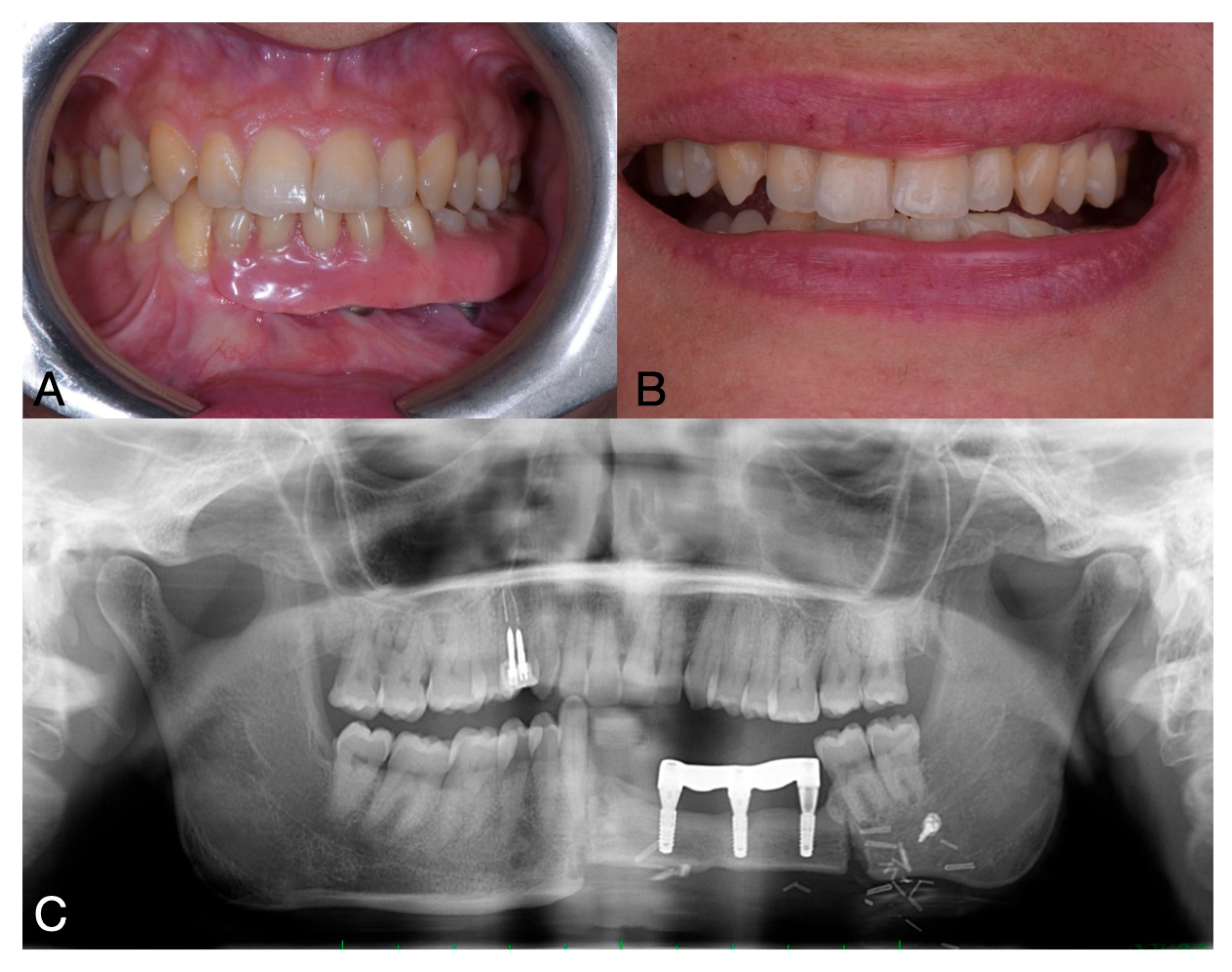
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torres-Lagares, D.; Infante-Cossío, P.; Hernández-Guisado, J.M.; Gutiérrez-Pérez, J.L. Mandibular ameloblastoma. A review of the literature and presentation of six cases. Med. Oral Patol. Oral Cir. Bucal 2005, 10, 231–238, (In English, Spanish). [Google Scholar] [PubMed]
- Hendra, F.N.; Van Cann, E.M.; Helder, M.N.; Ruslin, M.; de Visscher, J.G.; Forouzanfar, T.; de Vet, H.C.W. Global incidence and profile of ameloblastoma: A systematic review and meta-analysis. Oral Dis. 2020, 26, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.A.; Ng, C.W.B.; Kwa, C.T.; Sim, Q.X.C. Ameloblastoma: A succinct review of the classification, genetic understanding and novel molecular targeted therapies. Surgeon 2021, 19, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Kreppel, M.; Zöller, J. Ameloblastoma—Clinical, radiological, and therapeutic findings. Oral Dis. 2018, 24, 63–66. [Google Scholar] [CrossRef]
- Bianchi, B.; Ferri, A.; Ferrari, S.; Leporati, M.; Copelli, C.; Ferri, T.; Sesenna, E. Mandibular resection and reconstruction in the management of extensive ameloblastoma. J. Oral Maxillofac. Surg. 2013, 71, 528–537. [Google Scholar] [CrossRef]
- Cadavid, A.M.H.; Araujo, J.P.; Coutinho-Camillo, C.M.; Bologna, S.; Junior, C.A.L.; Lourenço, S.V. Ameloblastomas: Current aspects of the new WHO classification in an analysis of 136 cases. Surg. Exp. Pathol. 2019, 2, 17. [Google Scholar] [CrossRef]
- Bwambale, P.; Yahaya, J.J.; Owor, G.; Wabinga, H. Histopathological patterns and biological characteristics of ameloblastoma: A retrospective cross-sectional study. J. Taibah Univ. Med. Sci. 2022, 17, 96–104. [Google Scholar] [CrossRef]
- Hendra, F.N.; Natsir Kalla, D.S.; Van Cann, E.M.; de Vet, H.C.W.; Helder, M.N.; Forouzanfar, T. Radical vs conservative treatment of intraosseous ameloblastoma: Systematic review and meta-analysis. Oral Dis. 2019, 25, 1683–1696. [Google Scholar] [CrossRef]
- Pappalardo, M.; Tsao, C.K.; Tsang, M.L.; Zheng, J.; Chang, Y.M.; Tsai, C.Y. Long-term outcome of patients with or without osseointegrated implants after resection of mandibular ameloblastoma and reconstruction with vascularized bone graft: Functional assessment and quality of life. J. Plast. Reconstr. Aesthetic Surg. 2018, 71, 1076–1085. [Google Scholar] [CrossRef]
- Hertog, D.; Bloemena, E.; Aartman, I.H.A.; van-der-Waal, I. Histopathology of ameloblastoma of the jaws; some critical observations based on a 40 years single institution experience. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e76–e82. [Google Scholar] [CrossRef]
- Filizzola, A.I.; Bartholomeu-Dos-Santos, T.C.R.; Pires, F.R. Ameloblastomas: Clinicopathological features from 70 cases diagnosed in a single Oral Pathology service in an 8-year period. Med. Oral Patol. Oral Cir. Bucal 2014, 19, e556–e561. [Google Scholar] [CrossRef]
- Sham, E.; Leong, J.; Maher, R.; Schenberg, M.; Leung, M.; Mansour, A.K. Mandibular ameloblastoma: Clinical experience and literature review. ANZ J. Surg. 2009, 79, 739–744. [Google Scholar] [CrossRef]
- Effiom, O.A.; Ogundana, O.M.; Akinshipo, A.O.; Akintoye, S.O. Ameloblastoma: Current etiopathological concepts and management. Oral Dis. 2018, 24, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Dugast, S.; Longis, J.; Anquetil, M.; Corre, P.; Komarova, S.; Bertin, H. Assessing Dental Implant Success: A Systematic Review and Meta-Analysis of Primary Versus Secondary Implantation in Free Bone Flap Reconstruction for Malignant Tumors. Head Neck 2025, 47, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.F.; Rodriguez, E.D.; Wei, F.C.; Tsai, C.Y.; Chang, Y.M. Aesthetic and Functional Mandibular Reconstruction with Immediate Dental Implants in a Free Fibular Flap and a Low-Profile Reconstruction Plate: Five-Year Follow-up. Ann. Plast. Surg. 2015, 74, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.H.; Eo, M.Y.; Myoung, H.; Kim, M.J.; Kim, S.M. Implant-supported fixed and removable prostheses in the fibular mandible. Int. J. Implant. Dent. 2020, 6, 44. [Google Scholar] [CrossRef]
- Neal, T.W.; Williams, F.C.; Carr, B.R.; Pankey, T.; Teigen, K.; Kim, R.Y. Immediate Implants in Fibulas: Does the Implant to Fibula Osteotomy Distance Impact Early Implant Failure? J. Oral Maxillofac. Surg. 2024, 82, 1620–1626. [Google Scholar] [CrossRef]
- Wallace, C.G.; Chang, Y.M.; Tsai, C.Y.; Wei, F.C. Harnessing the potential of the free fibula osteoseptocutaneous flap in mandible reconstruction. Plast. Reconstr. Surg. 2010, 125, 305–314. [Google Scholar] [CrossRef]
- Raffaelli, S.D.; Neal, T.W.; Jelmini, J.J.; Kim, R.Y.; Williams, F.C. Evaluation of Postoperative Peri-Implant Reactive Tissues Following Implant Supported Prosthetic Rehabilitation in Fibula Free Flaps. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2024, 138, e107–e112. [Google Scholar] [CrossRef]
- Anne-Gaëlle, B.; Samuel, S.; Julie, B.; Renaud, L.; Pierre, B. Dental implant placement after mandibular reconstruction by microvascular free fibula flap: Current knowledge and remaining questions. Oral Oncol. 2011, 47, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Jaquiéry, C.; Rohner, D.; Kunz, C.; Bucher, P.; Peters, F.; Schenk, R.K.; Hammer, B. Reconstruction of maxillary and mandibular defects using prefabricated microvascular fibular grafts and osseointegrated dental implants—A prospective study. Clin. Oral Implant. Res. 2004, 15, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, M.J.; Choi, W.S.; Yoon, P.Y.; Ahn, K.M.; Myung, H.; Hwang, S.J.; Seo, B.M.; Choi, J.Y.; Choung, P.H.; et al. Concomitant reconstruction of mandibular basal and alveolar bone with a free fibular flap. Int. J. Oral. Maxillofac. Surg. 2004, 33, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, W.M.; Werning, J.W.; Fernandes, R.; Malyapa, R.S.; Mendenhall, N.P. Ameloblastoma. Am. J. Clin. Oncol. Cancer Clin. Trials 2007, 30, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Gortzak, R.A.T.; Latief, B.S.; Lekkas, C.; Slootweg, P.J. Growth characteristics of large mandibular ameloblastomas: Report of 5 cases with implications for the approach to surgery. Int. J. Oral Maxillofac. Surg. 2006, 35, 691–695. [Google Scholar] [CrossRef]
- Zwahlen, R.A.; Grätz, K.W. Maxillary ameloblastomas: A review of the literature and of a 15-year database. J. Cranio-Maxillofac. Surg. 2002, 30, 273–279. [Google Scholar] [CrossRef]
- Brown, N.A.; Betz, B.L. Ameloblastoma: A Review of Recent Molecular Pathogenetic Discoveries. Biomark. Cancer 2015, 7, 19–24. [Google Scholar] [CrossRef]
- Almeida, R.D.A.C.; Andrade, E.S.D.S.; Barbalho, J.C.; Vajgel, A.; Vasconcelos, B.C.D.E. Recurrence rate following treatment for primary multicystic ameloblastoma: Systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2016, 45, 359–367. [Google Scholar] [CrossRef]
- Kreutzer, K.; Steffen, C.; Nahles, S.; Koerdt, S.; Heiland, M.; Rendenbach, C.; Beck-Broichsitter, B. Removal of patient-specific reconstruction plates after mandible reconstruction with a fibula free flap: Is the plate the problem? Int. J. Oral Maxillofac. Surg. 2022, 51, 182–190. [Google Scholar] [CrossRef]
- Cuesta-Gil, M.; Ochandiano Caicoya, S.; Riba-García, F.; Ruiz, B.D.; Navarro Cuéllar, C.; Navarro Vila, C. Oral Rehabilitation With Osseointegrated Implants in Oncologic Patients. J. Oral Maxillofac. Surg. 2009, 67, 2485–2496. [Google Scholar] [CrossRef]
- McClary, A.C.; West, R.B.; McClary, A.C.; Pollack, J.R.; Fischbein, N.J.; Holsinger, C.F.; Sunwoo, J.; Colevas, A.D.; Sirjani, D. Ameloblastoma: A clinical review and trends in management. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 1649–1661. [Google Scholar] [CrossRef]
- Kumar, B.P.; Venkatesh, V.; Kumar, K.A.J.; Yadav, B.Y.; Mohan, S.R. Mandibular Reconstruction: Overview. J. Maxillofac. Oral Surg. 2016, 15, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Rendenbach, C.; Steffen, C.; Hanken, H.; Schluermann, K.; Henningsen, A.; Beck-Broichsitter, B.; Kreutzer, K.; Heiland, M.; Precht, C. Complication rates and clinical outcomes of osseous free flaps: A retrospective comparison of CAD/CAM versus conventional fixation in 128 patients. Int. J. Oral Maxillofac. Surg. 2019, 48, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Bucky, L.P. Multi-Specialty Facial Plastic Surgery. Facial Plast. Surg. 2003, 19, 001–002. [Google Scholar] [CrossRef]
- Zlotolow, L.M.; Huryn, J.M.; Piro, J.D.; Lenchewski, E.; Hidalgo, D.A. Osseointegrated Implants and Functional Prosthetic Rehabilitation in Microvascular Fibula Free Flap Reconstructed Mandibles. Am. J. Surg. 1992, 164, 677–681. [Google Scholar] [CrossRef]
- Hidalgo, D.A. Fibula free flap: A new method of mandible reconstruction. Plast. Reconstr. Surg. 1989, 84, 71–79. [Google Scholar] [CrossRef]
- Illand, C.; Destruhaut, F.; Porporatti, A.L.; Wulfman, C.; Naveau, A.; Rignon-Bret, C. Implant Survival Rate in Mandible Reconstructed with Free Fibula Flaps After Oral Tumors: A Systematic Review and Meta-Analysis. Int. J. Oral. Maxillofac. Implant. 2023, 38, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Roumanas, E.D.; Garrett, N.; Blackwell, K.E.; Freymiller, E.; Abemayor, E.; Wong, W.K.; Beumer, J.; Fueki, K.; Fueki, W.; Kapur, K.K. Masticatory and swallowing threshold performances with conventional and implant-supported prostheses after mandibular fibula free-flap reconstruction. J. Prosthet. Dent. 2006, 96, 289–297. [Google Scholar] [CrossRef]
- Dean, A.; Alamillos, F.; Heredero, S.; Redondo-Camacho, A.; Guler, I.; Sanjuan, A. Fibula free flap in maxillomandibular reconstruction. Factors related to osteosynthesis plates’ complications. J. Cranio-Maxillofac. Surg. 2020, 48, 994–1003. [Google Scholar] [CrossRef]
- Urken, M.L.; Buchbinder, D.; Weinberg, H.; Vickery, C.; Sheiner, A.; Parker, R.; Schaefer, J.; Som, P.; Shapiro, A.; Lawson, W.; et al. Functional evaluation following microvascular oromandibular reconstruction of the oral cancer patient: A comparative study of reconstructed and nonreconstructed patients. Laryngoscope 1991, 101, 935–950. [Google Scholar] [CrossRef]
- Duyck, J.; Van Oosterwyck, H.; Vander Sloten, J.; De Cooman, M.; Puers, R.; Naert, I. Magnitude and distribution of occlusal forces on oral implants supporting fixed prostheses: An in vivo study. Clin. Oral Implant. Res. 2000, 11, 465–475. [Google Scholar] [CrossRef]
- Bede, S.Y.H.; Ismael, W.K.; Hashim, E.A. Reconstruction plate-related complications in mandibular continuity defects. Oral Maxillofac. Surg. 2019, 23, 193–199. [Google Scholar] [CrossRef]
- Wijbenga, J.G.; Schepers, R.H.; Werker, P.M.N.; Witjes, M.J.H.; Dijkstra, P.U. A systematic review of functional outcome and quality of life following reconstruction of maxillofacial defects using vascularized free fibula flaps and dental rehabilitation reveals poor data quality. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 1024–1036. [Google Scholar] [CrossRef]
- Qiao, X.; Shi, J.; Liu, J.; Liu, J.; Guo, Y.; Zhong, M. Recurrence Rates of Intraosseous Ameloblastoma Cases with Conservative or Aggressive Treatment: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 647200. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.N. Quality of life for head and neck cancer patients—Has treatment planning altered? Oral Oncol 2009, 45, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Hanasono, M.M.; Zevallos, J.P.; Skoracki, R.J.; Yu, P. A Prospective Analysis of Bony versus Soft-Tissue Reconstruction for Posterior Mandibular Defects. Plast. Reconstr. Surg. 2010, 125, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Toto, J.M.; Chang, E.I.; Agag, R.; Devarajan, K.; Patel, S.A.; Topham, N.S. Improved operative efficiency of free fibula flap mandible reconstruction with patient-specific, computer-guided preoperative planning. Head Neck 2015, 37, 1660–1664. [Google Scholar] [CrossRef]
- Rendenbach, C.; Sellenschloh, K.; Gerbig, L.; Morlock, M.M.; Beck-Broichsitter, B.; Smeets, R.; Heiland, M.; Huber, G.; Hanken, H. CAD–CAM plates versus conventional fixation plates for primary mandibular reconstruction: A biomechanical in vitro analysis. J. Cranio-Maxillofac. Surg. 2017, 45, 1878–1883. [Google Scholar] [CrossRef]
- Al-Bustani, S.; Austin, G.K.; Ambrose, E.C.; Miller, J.; Hackman, T.G.; Halvorson, E.G. Miniplates versus reconstruction bars for oncologic free fibula flap mandible reconstruction. Ann. Plast. Surg. 2016, 77, 314–317. [Google Scholar] [CrossRef]
- Zavattero, E.; Fasolis, M.; Garzino-Demo, P.; Berrone, S.; Ramieri, G.A. Evaluation of plate-related complications and efficacy in fibula free flap mandibular reconstruction. J. Craniofacial Surg. 2014, 25, 397–399. [Google Scholar] [CrossRef]
- Bujtár, P.; Simonovics, J.; Váradi, K.; Sándor, G.K.B.; Avery, C.M.E. The biomechanical aspects of reconstruction for segmental defects of the mandible: A finite element study to assess the optimisation of plate and screw factors. J. Cranio-Maxillofac. Surg. 2014, 42, 855–862. [Google Scholar] [CrossRef]
- Heberer, S.; Nelson, K. Clinical Evaluation of a Modified Method of Vestibuloplasty Using an Implant-Retained Splint. J. Oral Maxillofac. Surg. 2009, 67, 624–629. [Google Scholar] [CrossRef]
- Schoen, P.J.; Raghoebar, G.M.; Bouma, J.; Reintsema, H.; Burlage, F.R.; Roodenburg, J.L.N.; Vissink, A. Prosthodontic rehabilitation of oral function in head-neck cancer patients with dental implants placed simultaneously during ablative tumour surgery: An assessment of treatment outcomes and quality of life. Int. J. Oral Maxillofac. Surg. 2008, 37, 8–16. [Google Scholar] [CrossRef]
- Chodankar, R.N.; Acharya, A.; Patil, R. Oral Rehabilitation of Segmental Mandibulectomy with Extensive Fibrosis- A Daunting Obstacle for the Maxillofacial Prosthodontist. J. Clin. Diagn. Res. 2023, 17, ZD12–ZD14. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goudarzi, S.; Cinquini, C.; Izzetti, R.; Nisi, M.; Priami, M.; Brevi, B.C.; Bruschini, L.; Lorenzetti, F.; Santarelli, S.; Barone, A. Oral Rehabilitation Following Surgical Treatment of Mandibular Ameloblastoma: Case Report and Comprehensive Literature Review. Oral 2025, 5, 57. https://doi.org/10.3390/oral5030057
Goudarzi S, Cinquini C, Izzetti R, Nisi M, Priami M, Brevi BC, Bruschini L, Lorenzetti F, Santarelli S, Barone A. Oral Rehabilitation Following Surgical Treatment of Mandibular Ameloblastoma: Case Report and Comprehensive Literature Review. Oral. 2025; 5(3):57. https://doi.org/10.3390/oral5030057
Chicago/Turabian StyleGoudarzi, Sepideh, Chiara Cinquini, Rossana Izzetti, Marco Nisi, Mattia Priami, Bruno Carlo Brevi, Luca Bruschini, Fulvio Lorenzetti, Simonetta Santarelli, and Antonio Barone. 2025. "Oral Rehabilitation Following Surgical Treatment of Mandibular Ameloblastoma: Case Report and Comprehensive Literature Review" Oral 5, no. 3: 57. https://doi.org/10.3390/oral5030057
APA StyleGoudarzi, S., Cinquini, C., Izzetti, R., Nisi, M., Priami, M., Brevi, B. C., Bruschini, L., Lorenzetti, F., Santarelli, S., & Barone, A. (2025). Oral Rehabilitation Following Surgical Treatment of Mandibular Ameloblastoma: Case Report and Comprehensive Literature Review. Oral, 5(3), 57. https://doi.org/10.3390/oral5030057







