Can New Remineralizing Agents Serve as Fluoride Alternatives in Caries Prevention? A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Review Objectives
2.2. Eligibility Criteria
2.2.1. Inclusion Criteria
- Population: A healthy population (no general disease) with initial stages of dental caries.
- Interventions: Studies involving interventions with definite enamel remineralizing agents: amorphous calcium phosphate (ACP), polyphosphate systems, STMP, functionalized β-tricalcium phosphate, calcium sodium phosphosilicate (CSP), casein phosphopeptide–amorphous calcium phosphate (CPP-ACP), self-assembling polypeptide, P11-4, nano-hydroxyapatite, and fluoride.
- Outcomes: Laboratory studies: definite dg criteria: remineralization of enamel-scanning electron microscopy, spectroscopy, microhardness test, light microscopy, profilometry, transverse microhardness microradiography, integrated mineral loss, light microscopy, photothermal radiometry, and microcomputed tomography (MCT); Clinical studies: RCT, CONSORT document, and trial registration.
- Study design: Randomized controlled trials (RCT, CONSORT document, trial registration), in vitro, in vivo, and in situ studies.
- Language: English only.
- Period of search: From the end of August to 28 October 2024.
- Publication type: Peer-reviewed studies only.
2.2.2. Exclusion Criteria
- Unhealthy population.
- Studies reported the effect affecting dentine.
- Studies not reporting the isolated effect of a definite enamel-remineralizing effect. Also, studies that do not use a definite enamel-remineralizing effect.
- The outcomes were measured without the use of the outcome criteria.
- Studies not published in English.
- Repetitive data.
2.3. Information Sources and Search Strategy
2.4. Study Selection, Data Extraction, and Synthesis
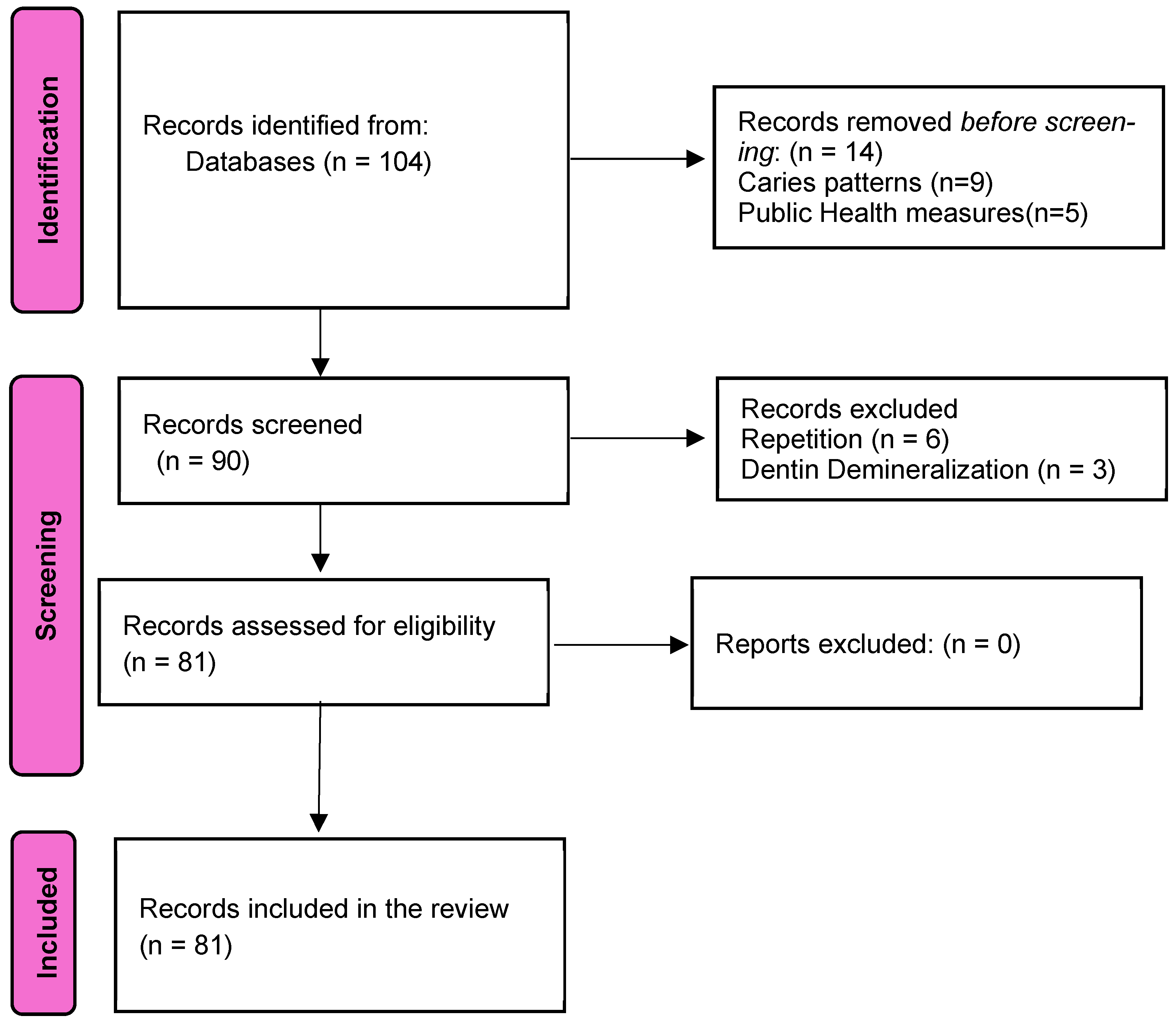
3. Results
| The Name of the Agent | Remin/Demin | Biofilm | Adverse Effect | Commercial Product |
|---|---|---|---|---|
| Fluoride | Remin↑/Demin↓ A high fluoride dose results in surface-zone remineralization and only prevents fuller and homogenous remineralization of the lesion [5]. Incorporation of fluoride into the tooth surface is at a very low level with no “hardening” of the tooth [19]. Those remineralizing properties of fluorides and their mechanisms of inhibiting demineralization have not yet been fully understood [19]. | Reduces saccharolytic organisms and inhibits pathways of sugar fermentation [20,21]; also, it prevents demineralization without affecting biofilm composition and growth inhibits various bacterial enzymes that are necessary for cell growth, sugar transport, and energy metabolism (e.g., enolase, F-ATPase, sulfatase, catalase, phosphatases, and phosphoglucomutase) [21]. | Fluorosis | A wide range of commercial products available |
| Amorphous calcium phosphate | Insufficiently remineralized sub-surface lesions with dental calculus depositions on teeth [7,22]. | No studies are available. | No | EnamelonTM toothpaste (https://www.enamelon.com, accessed on the 30 September 2024) |
| Sodium trimetaphosphate (STMP) | Strongly binds to phosphate on enamel (which blocks the release of calcium and phosphate from the crystal) and leads to the formation of a layer on the enamel surface that limits acid ion diffusion, but calcium and phosphate diffusion is not affected; was able to minimize mineral loss even in the presence of low F concentrations [7,22]. | ↓The number of Str. mutans cells, and ↓biofilm metabolism (without F) [23]. Also, STMP+F led to the formation of a less compact biofilm [24,25]. STMP alone had a reducing effect, mainly on the metabolism and the extracellular matrix components of the biofilms [26]. | No | Oral-B Pro Expert toothpaste (https://www.oralb.co.uk; https://oralb.com/en-us, accessed on the 30 September 2024) |
| Functionalized β-tricalcium phosphate (f β-TCP) | Boost F-ion activity on the tooth surface, with remineralization driven mostly by salivary Ca2+ and PO43− ions [7,22]. f-TCP creates barriers to prevent interactions between fluoride and calcium, delivering minerals and fluoride to the teeth’ surface [21]. The minerals produced by the combination of fluoride and fTCP have greater acid resistance potential than F, β-TCP, and f-TCP alone [27]. | f-TCP, in addition to AgNO3 and NaF, reduced the damage of dentine caries caused by cariogenic biofilm [28]. | No | 3M™ Clinpro™ 5000 1.1% Sodium Fluoride (https://www.solventum.com/en-us/home/f/b00005767/, accessed on the 30 September 2024) 3M™ Clinpro™ XT Varnish Durable Fluoride-Releasing Coating 3M (https://www.3m.com/3M/sl_SI/p/d/v000180763, accessed on the 30 September 2024) |
| Calcium sodium phosphosilicate (Bioglass) | When it interacts with saliva, it releases Na+, Ca2+, and PO43− ions and deposits a crystalline hydroxycarbonate apatite layer like the structure of the natural tooth minerals [7,22]. Remin↑/Demine↓ [28] | It has demonstrated antimicrobial effects against various microbial genera, including S. gordonii, V. parvula, P. aeruginosa, and MRSA (methicillin-resistant, Staphylococcus aureus) [29]. | No | NovaMin toothpaste, Sensodyne (https://www.sensodyne.com/en-ca/products/repair-and-protect-original, accessed on the 30 September 2024) Oravive Revitalizing Toothpaste With Novamin, GSK (https://www.instacart.com/products/22049256-oravive-toothpaste-revitalizing-classic-regular-strength-double-mint-4-00-oz, accessed on the 30 September 2024) |
| Casein phosphopeptide–amorphous calcium phosphate (CPP-ACP) | ↑Remin of subsurface lesions, supplying a high concentration of calcium and phosphate ions [28]. | ↓ The number of Str. Mutans at a higher rate than fluoride toothpaste alone [30,31,32]. | It could not be used in people with an allergy to lactose. | MI Paste cremes, Recaldent, Trident White sugar-free gum, MI Paste One toothpaste MI Varnish, GC (https://www.gc.dental/europe/en/products, accessed on the 30 September 2024) (https://www.gc.dental/america/products, accessed on the 30 September 2024) |
| Self-assembling peptides P11-4 | Forms a 3D scaffold in carious lesions and promotes the new formation of hydroxyapatite crystals [33]. | The self-assembling peptide P11-4 exhibited an inhibitory influence on S. Mutans, which may lead to changes in the formation of cariogenic bacteria biofilm [34]. | No | Curodont Repair, Straumann (https://professional.vvardis.com/product/curodont-repair, accessed on the 30 September 2024) (https://professional.vvardis.us/products/#repair, accessed on the 30 September 2024) |
| Nano-hydroxyapatite | It induces remineralization of initial caries lesions by filling micropores in demineralized tooth surfaces, where it acts as a crystal nucleus and promotes crystal deposition and growth by continuously attracting large amounts of calcium and phosphate ions from the surrounding remineralization solution [5]. | HAP reduces the initial plaque formation on enamel surfaces [19]. It acts as an acid buffer and reservoir for calcium and phosphate ions in plaque, leading to significant reduction and inhibition of plaque formation without killing bacteria [19]. The high potential of HAP to adsorb to bacterial cell walls facilitates an antibiofilm effect by inducing coaggregation of bacteria within the HAP particles, aiding biofilm removal from the tooth surfaces, and hindering oral biofilm formation [5]. | No | Pro-Mineralizer Toothpaste, Great Oral Health; Kinder Karex Zahnpasta, Dr. Kurt Wolff GmbH and Co. KG, Bielefeld (https://www.karex.com/en-de/products/kinder-karex-toothpaste, accessed on the 30 September 2024) APAGARD® M-plus toothpaste (https://www.sangi-eu.com/en/apagard-m-plus/mpc125n, accessed on the 30 September 2024) (https://www.sangi-co.com/en/products/apagard_mplus/index.html, accessed on the 30 September 2024) Desensin® oral rinse, DENTAID technologies (https://www.dentaid.com/en/countries, accessed on the 30 September 2024) |
4. Discussion
4.1. Amorphous Calcium Phosphate
4.2. Polyphosphate Systems (Sodium Trimetaphosphate, Calcium Glycerophosphate, Sodium Hexametaphosphate)
4.3. Functionalized β-Tricalcium Phosphate
4.4. Calcium Sodium Phosphosilicate
4.5. Casein Phosphopeptide–Amorphous Calcium Phosphate (CPP-ACP)
4.6. Self-Assembling Polypeptide
4.7. Nano-Hydroxyapatite (nHA, Crystalline)
4.8. Main Findings and Limitations
4.8.1. Main Findings
4.8.2. Limitations of Evidence Included
Study Design and Sample Size
Methodology
Lack of Standardized Outcomes
Bias and Confounding Factors
4.8.3. Limitations of the Review Processes
4.8.4. Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Frencken, J.E.; Peters, M.C.; Manton, D.J.; Leal, S.C.; Gordan, V.V.; Eden, E. Minimal Intervention Dentistry (MID) for managing dental caries a review: Report of a FDI task group. Int. Dent. J. 2012, 62, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Tafere, Y.; Chanie, S.; Dessie, T.; Gedamu, H. Assessment of prevalence of dental caries and the associated factors among patients attending dental clinic in Debre Tabor general hospital: A hospital-based crosssectional study. BMC Oral Health 2018, 18, 119–126. [Google Scholar] [CrossRef]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 20, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Amaechi, B.T.; Abdul Azees, P.A.; Alshareif, D.O.; Shehata, M.A.; de Carvalho Sampaio Lima, P.P.; Abdollahi, A.; Kalkhorani, P.S.; Evans, V. Comparative efficacy of a hydroxyapatite and a fluoride toothpaste for prevention and remineralization of dental caries in children. BDJ Open 2019, 5, 18–29. [Google Scholar] [CrossRef]
- Kazeminia, M.; Abdi, A.; Shohaimi, S.; Jalali, R.; Vaisi-Raygani, A.; Salari, N.; Mohammadi, M. Dental caries in primary and permanent teeth in children’s worldwide, 1995 to 2019: A systematic review and meta-analysis. Head Face Med. 2020, 16, 22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Philip, N. State of the Art Enamel Remineralization Systems: The Next Frontier in Caries Management. Caries Res. 2019, 53, 284–295. [Google Scholar] [CrossRef]
- Wen, P.Y.F.; Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M. Global Burden and Inequality of Dental Caries, 1990 to 2019. J. Dent. Res. 2022, 101, 392–399. [Google Scholar] [CrossRef]
- Dentistry TUKSo. Comprehensive Management of Dental Caries Practice Guidelines; Temple University Kornberg School of Dentistry: Philadelphia, PA, USA, 2019. [Google Scholar]
- Campos, P.H.; Gimenez, T.; Rocha, R.S.; Caneppele, T.M.F.; Guaré, R.O.; Lussi, A.; Bresciani, E.; Diniz, M.B. Prevalence of White Spot Caries Lesions in Primary Teeth in Preschool Children: Systematic Review and Meta-analysis. Curr. Pediatr. Rev. 2022, 18, 33–46. [Google Scholar] [CrossRef]
- He, S.; Yon, M.J.Y.; Liu, F.; Lo, E.C.M.; Yiu, C.K.Y.; Chu, C.H.; Lam, P.P.Y. Prevalence of caries Patterns in the 21st Century Preschool Children: A Systemic Review and Meta-Analysis. J. Evid. Based Dent. Pract. 2024, 24, 101992. [Google Scholar] [CrossRef]
- Yazdanbakhsh, E.; Bohlouli, B.; Patterson, S.; Amin, M. Community water fluoride cessation and rate of caries-related pediatric dental treatments under general anesthesia in Alberta, Canada. Can. J. Pub. Health 2024, 115, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Iheozor-Ejiofor, Z.; Walsh, T.; Lewis, S.R.; Riley, P.; Boyers, D.; Clarkson, J.E.; Worthington, H.V.; Glenny, A.M.; O’Malley, L. Water fluoridation for the prevention of dental caries. Cochrane Database Syst. Rev. 2024, 10, CD010856. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abrams, S.; Beltran-Aguilar, E.; Martinez-Mier, E.A.; Kumar, J.; Slade, G.D.; Gooch, B. Water Fluoridation: Safety, Effectiveness and Value in Oral Health: A Symposium at the 2014 Annual Meeting of the American and Canadian Associations for Dental Research. J. Can. Dent. Assoc. 2015, 80, f16. [Google Scholar] [PubMed]
- Goodwin, M.; Walsh, T.; Whittaker, W.; Emsley, R.; Kelly, M.P.; Sutton, M.; Tickle, M.; Pretty, I.A. The CATFISH study: An evaluation of a water fluoridation program in Cumbria, UK. Community Dent. Oral Epidemiol. 2024, 52, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.I.; Pitts, N.B.; Tellez, M.; Banerjee, A.; Deery, C.; Douglas, G.; Eggertsson, H.; Ekstrand, K.; Ellwood, R.; Gomez, J.; et al. The International Caries Classification and Management System (ICCMS™) An Example of a Caries Management Pathway. BMC Oral Health 2015, 15, 9–22. [Google Scholar] [CrossRef]
- Malcangi, G.; Patano, A.; Morolla, R.; De Santis, M.; Piras, F.; Settanni, V.; Mancini, A.; Di Venere, D.; Inchingolo, F.; Inchingolo, A.D.; et al. Analysis of Dental Enamel Remineralization: A Systematic Review of Technique Comparisons. Bioengineering 2023, 10, 472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Westphaln, K.K.; Regoeczi, W.; Masotya, M.; Vazquez-Westphaln, B.; Lounsbury, K.; McDavid, L.; Lee, H.L.; Johnson, J.; Ronis, S.D. From Arksey and O’Malley and Beyond: Customizations to enhance a team-based, mixed approach to scoping review methodology. Methods X 2021, 8, 101375. [Google Scholar] [CrossRef]
- Meyer, F.; Schulze Zur Wiesche, E.; Amaechi, B.T.; Limeback, H.; Enax, J. Caries Etiology and Preventive Measures. Eur. J. Dent. 2024, 18, 766–776. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- López-López, A.; Mira, A. Shifts in Composition and Activity of Oral Biofilms After Fluoride Exposure. Microb. Ecol. 2020, 80, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Thurnheer, T.; Belibasakis, G.N. Effect of sodium fluoride on oral biofilm microbiota and enamel demineralization. Arch. Oral Biol. 2018, 89, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Grohe, B.; Mittler, S. Advanced non-fluoride approaches to dental enamel remineralization: The next level in enamel repair management. Biomat. Biosyst. 2021, 4, 100029. [Google Scholar] [CrossRef] [PubMed]
- Cavazana, T.P.; Hosida, T.Y.; Pessan, J.P.; Sampaio, C.; Monteiro, D.R.; Delbem, A.C.B. Activity of sodium trimetaphosphate, associated or not with fluoride, on dual-species biofilms. Biofouling 2019, 35, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Enax, J.; Epple, M.; Amaechi, B.T.; Simader, B. Cariogenic Biofilms: Development, Properties, and Biomimetic Preventive Agents. Dent. J. 2021, 9, 88. [Google Scholar] [CrossRef]
- Amarante, V.O.Z.; Delbem, A.C.B.; Sampaio, C.; de Morais, L.A.; de Camargo, E.R.; Monteiro, D.R.; Pessan, J.P.; Hosida, T.Y. Activity of Sodium Trimetaphosphate Nanoparticles on Cariogenic-Related Biofilms In Vitro. Nanomaterials 2022, 13, 170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zen, I.; Delbem, A.C.B.; Martins, T.P.; de Morais, L.A.; Sampaio, C.; Hosida, T.Y.; Monteiro, D.R.; Pessan, J.P. Evaluation of Solutions Containing Fluoride, Sodium Trimetaphosphate, Xylitol, and Erythritol, Alone or in Different Associations, on Dual-Species Biofilms. Int. J. Mol. Sci. 2023, 24, 12910. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, O.L.; Niu, J.Y.; Yin, I.X.; Yu, O.Y.; Mei, M.L.; Chu, C.H. Bioactive Materials for Caries Management: A Literature Review. Dent. J. 2023, 11, 59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, O.Y.; Zhao, I.S.; Mei, M.L.; Lo, E.C.; Chu, C.H. Effect of Silver Nitrate and Sodium Fluoride with Tri-Calcium Phosphate on Streptococcus mutans and Demineralised Dentine. Int. J. Mol. Sci. 2018, 19, 1288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, P.; Garcia, B.L.; Kotsakis, G.A. Comparison of antibacterial and antibiofilm activity of bioactive glass compounds S53P4 and 45S5. BMC Microbiol. 2022, 22, 212–224. [Google Scholar] [CrossRef]
- Philip, N.; Leishman, S.J.; Bandara, H.M.H.N.; Walsh, L.J. Casein Phosphopeptide-Amorphous Calcium Phosphate Attenuates Virulence and Modulates Microbial Ecology of Saliva-Derived Polymicrobial Biofilms. Car. Res. 2019, 53, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Sionov, R.V.; Tsavdaridou, D.; Aqawi, M.; Zaks, B.; Steinberg, D.; Shalish, M. Tooth mousse containing casein phosphopeptide-amorphous calcium phosphate prevents biofilm formation of Streptococcus mutans. BMC Oral Health 2021, 21, 136–146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Widyarman, A.S.; Udawatte, N.S.; Theodorea, C.F.; Apriani, A.; Richi, M.; Astoeti, T.E.; Seneviratne, C.J. Casein phosphopeptide-amorphous calcium phosphate fluoride treatment enriches the symbiotic dental plaque microbiome in children. J. Dent. 2021, 106, 103582. [Google Scholar] [CrossRef] [PubMed]
- Shaalan, O.; Fawzy El-Sayed, K.; Abouauf, E. Evaluation of the remineralization potential of self-assembling peptide P11-4 with fluoride compared to fluoride varnish in the management of incipient carious lesions: A randomized controlled clinical trial. Clin. Oral. Investig. 2024, 28, 438–448. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gayas, Z.; Azher, U.; Paul, S.T.; Selvan, A.; Reddy, C.D.; Raghu, D.; Uday, V. Comparative Evaluation of Antimicrobial Efficacy of Fluoride-Based and Self-Assembling Peptide P11-4-based Tooth Remineralization Agents on Streptococcus mutans: A Microbiological Study. Contemp. Clin. Dent. 2023, 14, 141–144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schemehorn, B.R.; Wood, G.D.; Winston, A.E. Laboratory enamel solubility reduction and fluoride uptake from enamelon dentifrice. J. Clin. Dent. 1999, 10, 9–12. [Google Scholar] [PubMed]
- Reynolds, E.C. Calcium phosphate-based remineralization systems: Scientific evidence? Aust. Dent. J. 2008, 53, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yang, H.; Luo, T.; Hua, F.; He, H. Application of Amorphous Calcium Phosphate Agents in the Prevention and Treatment of Enamel Demineralization. Front. Bioeng. Biotechnol. 2022, 10, 853436. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lippert, F.; Gill, K.K. Carious lesion remineralizing potential of fluoride- and calcium-containing toothpastes: A laboratory study. J. Am. Dent. Assoc. 2019, 150, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zheng, W.; Zhao, Y.; Shi, Y.; Wang, Y.; Sun, H.; Xu, X. Advancing dentin remineralization: Exploring amorphous calcium phosphate and its stabilizers in biomimetic approaches. Dent. Mater. 2024, 40, 1282–1295. [Google Scholar] [CrossRef]
- Buzalaf, M.A.R.; Pessan, J.P. New Preventive Approaches Part I: Functional Peptides and Other Therapies to Prevent Tooth Demineralization. Monogr. Oral. Sci. 2017, 26, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Freire, I.R.; Pessan, J.P.; Amaral, J.G.; Martinhon, C.C.R.; Cunha, R.F.; Delbem, A.C.B. Anticaries effect of low-fluoride dentifrices with phosphates in children: A randomized, controlled trial. J. Dent. 2016, 50, 37–42. [Google Scholar] [CrossRef]
- Danelon, M.; Garcia, L.G.; Pessan, J.P.; Passarinho, A.; Camargo, E.R.; Delbem, A.C.B. Effect of Fluoride Toothpaste Containing Nano-Sized Sodium Hexametaphosphate on Enamel Remineralization: An in Situ Study. Caries Res. 2019, 53, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Dipalma, G.; Inchingolo, A.D.; Guglielmo, M.; Morolla, R.; Palumbo, I.; Riccaldo, L.; Mancini, A.; Palermo, A.; Malcangi, G.; Inchingolo, A.M.; et al. Nanotechnology and Its Application in Dentistry: A Systematic Review of Recent Advances and Innovations. J. Clin. Med. 2024, 13, 5268. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takeshita, E.M.; Exterkate, R.A.M.; Delbem, A.C.B.; ten Cate, J.M. Evaluation of Different Fluoride Concentrations Supplemented with Trimetaphosphate on Enamel De- and Remineralization in vitro. Caries. Res. 2011, 45, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Zaze, A.C.S.F.; Dias, A.P.; Sassaki, K.T.; Delbem, B.A.C. The effects of low-fluoride toothpaste supplemented with calcium glycerophosphate on enamel demineralization. Clin. Oral. Investig. 2014, 18, 1619–1624. [Google Scholar] [CrossRef]
- Camara, D.M.; Pessan, J.P.; Francati, T.M.; Souza, J.A.S.; Delbem, B.A.l. Fluoride toothpaste supplemented with sodium hexametaphosphate reduces enamel demineralization in vitro. Clin. Oral Investig. 2016, 20, 1981–1985. [Google Scholar] [CrossRef]
- Mohammadipour, H.S.; Maghrebi, Z.F.; Ramezanian, N.; Ahrari, F.; Daluyi, R.A. The effects of sodium hexametaphosphate combined with other remineralizing agents on the staining and microhardness of early enamel caries: An in vitro modified pH-cycling model. Dent. Res. J. 2019, 12, 398–406. [Google Scholar] [PubMed] [PubMed Central]
- Gonçalves, F.M.C.; Delbem, A.C.B.; Gomes, L.F.; Emerenciano, N.G.; Pessan, J.P.; Romero, G.D.A.; Cannon, M.L.; Danelon, M. Effect of fluoride, casein phosphopeptide-amorphous calcium phosphate and sodium trimetaphosphate combination treatment on the remineralization of caries lesions: An in vitro study. Arch. Oral. Biol. 2021, 122, 105001. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.E.; Delbem, A.C.B.; Báez-Quintero, L.C.; Danelon, M.; Sampaio, C.; Monteiro, D.R.; Wiegand, A.; Pessan, J.P. Effect of fluoride gels with nano-sized sodium trimetaphosphate on the in vitro remineralization of caries lesions. J. Appl. Oral Sci. 2023, 31, e20230155. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paiva, M.F.; Delbem, A.C.B.; Veri, I.V.; Sampaio, C.; Wiegand, A.; Pessan, J.P. Fluoride varnishes supplemented with nano-sized sodium trimetaphosphate reduce enamel erosive wear in vitro. J. Dent. 2023, 138, 104726. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.S.; Scaffa, P.M.C.; Giacomini, M.C.; Vidal, C.M.P.; Honório, H.M.; Wang, L. Sodium Trimetaphosphate as a Novel Strategy for Matrix Metalloproteinase Inhibition and Dentin Remineralization. Caries Res. 2018, 52, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.S.; Candia Scaffa, P.M.; Giacomini, M.C.; Rabelo Buzalaf, M.A.; Honório, H.M.; Wang, L. Use of sodium trimetaphosphate in the inhibition of dentin matrix metalloproteinases and as a remineralizing agent. J. Dent. 2018, 68, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Nunes, G.P.; Danelon, M.; Pessan, J.P.; Capalbo, L.C.; Junior, N.A.N.; Matos, A.A.; Souza, J.A.S.; Buzalaf, M.A.R.; Delbem, A.C.B. Fluoride and trimetaphosphate association as a novel approach for remineralization and antiproteolytic activity in dentin tissue. Arch. Oral Biol. 2022, 142, 105508. [Google Scholar] [CrossRef] [PubMed]
- Karlinsey, R.L.; Mackey, A.C.; Walker, E.R.; Frederick, K.E. Preparation, characterization and in vitro efficacy of an acid-modified β-TCP material for dental hard-tissue remineralization. Acta Biomater. 2010, 6, 969–978. [Google Scholar] [CrossRef]
- Chen, K.J.; Gao, S.S.; Duangthip, D.; Lo, E.C.M.; Chu, C.H. Randomized Clinical Trial on Sodium Fluoride with Tricalcium Phosphate. J. Dent. Res. 2021, 100, 66–73. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, J.; Zhang, Y.; Yin, I.X.; Yu, O.Y.; Chan, A.K.Y.; Chu, C.H. Preventing Dental Caries with Calcium-Based Materials: A Concise Review. Inorganics 2024, 12, 253. [Google Scholar] [CrossRef]
- Salamara, O.; Papadimitriou, A.; Mortensen, D.; Twetman, S.; Koletsi, D.; Gizani, S. Effect of fluoride varnish with functionalized tri-calcium phosphate on post-orthodontic white spot lesions: An investigator-blinded controlled trial. Quintessence Int. 2020, 51, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Bhat, D.V.; Awchat, K.L.; Singh, P.; Jha, M.; Arora, K.; Mitra, M. Evaluation of Remineralizing Potential of CPP-ACP, CPP-ACP + F and β TCP + F and Their Effect on Microhardness of Enamel Using Vickers Microhardness Test: An In Vitro Study. Int. J. Clin. Pediatr. Dent. 2022, 15, 221–225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Handa, A.; Chengappa, D.; Sharma, P.; Handa, J.K. Effectiveness of Clinpro Tooth Crème in comparison with MI Varnish with RECALDENT™ for treatment of white spot lesions: A randomized controlled trial. Clin. Oral Investig. 2023, 27, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Alexandrino, L.D.; de Melo Alencar, C.; Silva da Silveira, A.D.; Alves, E.B.; Silva, C.M. Randomized clinical trial of the effect of NovaMin and CPP-ACPF in combination with dental bleaching. J. Appl. Oral. Sci. 2017, 25, 335–340. [Google Scholar] [CrossRef]
- Khijmatgar, S.; Reddy, U.; John, S.; Badavannavar, A.N.; Souza, D.T. Is there evidence for Novamin application in remineralization?: A Systematic review. J. Oral Biol. Craniofac. Res. 2020, 10, 87–92. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Joshi, S.; Vaidya, N.; Gupta, B.; Pustake, B.; Shinde, G.; Pharande, S. A Comparative Evaluation of Arginine Complex Combined With Flouride and Two Standard Non-Fluoridated Remineralizing Agents: An In Vitro Study. Cureus 2024, 16, e60118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reema, S.; Lahiri, P.; Roy, S. Review of Casein Phosphopeptides-Amorphous Calcium Phosphate. Chin. J. Dent. Res. 2014, 17, 7–14. [Google Scholar] [PubMed]
- Sleibi, A.; Tappuni, A.R.; Baysan, A. Reversal of Root Caries with Casein Phosphopeptide-Amorphous Calcium Phosphate and Fluoride Varnish in Xerostomia. Car. Res. 2021, 55, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.; Qian, F.; Bowers, R.D. CPP-ACP paste’s effect on salivary conditions in patients with removable dentures. J. Prosthodont. 2024, 33, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Leite, K.L.F.; Martins, M.L.; Monteiro, A.S.N.; Vieira, T.I.; Alexandria, A.K.; Rocha, G.M.; Fonseca-Gonçalves, A.; Pithon, M.M.; Cavalcanti, Y.W.; Maia, L.C. In-vitro effect of a single application of CPP-ACP pastes and different fluoridated solutions on the prevention of dental caries around orthodontic brackets. Dent. Press J. Orthod. 2024, 28, e2321383. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sitthisettapong, T.; Phantumvanit, P.; Huebner, C.; Derouen, T. Effect of CPP-ACP paste on dental caries in primary teeth: A randomized trial. J. Dent. Res. 2012, 91, 847–852. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oliveira, G.M.; Ritter, A.V.; Heymann, H.O.; Swift, E., Jr.; Donovan, T.; Brock, G.; Wright, T. Remineralization effect of CPP-ACP and fluoride for white spot lesions in vitro. J. Dent. 2014, 42, 1592–1602. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhadoria, N.; Gunwal, M.K.; Kukreja, R.; Maran, S.; Devendrappa, S.N.; Singla, S. An In Vitro Evaluation of Remineralization Potential of Functionalized Tricalcium Phosphate Paste and CPP-ACPF on Artificial White Spot Lesion in Primary and Permanent Enamel. Int. J. Clin. Pediatr. Dent. 2020, 13, 579–584. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Basheer, B.; Alqahtani, A.A.; Abdullah Alowairdhi, A.; Nuri Alohali, S. Analyzing the Effectiveness of Different Delivery Methods for Remineralization Agents in Pediatric Dental Health: A Systematic Review. Cureus 2024, 16, e76577. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Llena, C.; Leyda, A.M.; Forner, L. CPP-ACP and CPP-ACFP versus fluoride varnish in remineralisation of early caries lesions. A prospective study. Eur. J. Paediatr. Dent. 2015, 16, 181–186. [Google Scholar] [PubMed]
- Pithon, M.M.; Dos Santos, M.J.; Andrade, C.S.; Leão Filho, J.C.; Braz, A.K.; de Araujo, R.E.; Tanaka, O.M.; Fidalgo, T.K.; Dos Santos, A.M.; Maia, L.C. Effectiveness of varnish with CPP-ACP in prevention of caries lesions around orthodontic brackets: An OCT evaluation. Eur. J. Orthod. 2015, 37, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Savas, S.; Kavrìk, F.; Kucukyìlmaz, E. Evaluation of the remineralization capacity of CPP-ACP containing fluoride varnish by different quantitative methods. J. Appl. Oral Sci. 2016, 24, 198–203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wierichs, R.J.; Stausberg, S.; Lausch, J.; Meyer-Lueckel, H.; Esteves-Oliveira, M. Caries-Preventive Effect of NaF, NaF plus TCP, NaF plus CPP-ACP, and SDF Varnishes on Sound Dentin and Artificial Dentin Caries in vitro. Car. Res. 2018, 52, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Varma, V.; Hegde, K.S.; Bhat, S.S.; Sargod, S.S.; Rao, H.A. Comparative Evaluation of Remineralization Potential of Two Varnishes Containing CPP-ACP and Tricalcium Phosphate: An In Vitro Study. Int. J. Clin. Pediatr. Dent. 2019, 12, 233–236. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Attiguppe, P.; Malik, N.; Ballal, S.; Naik, S.V. CPP-ACP and Fluoride: A Synergism to Combat Caries. Int. J. Clin. Pediatr. Dent. 2019, 12, 120–125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akgun, O.M.; Haman Bayari, S.; Ide, S.; Guven Polat, G.; Yildirim, C.; Orujalipoor, I. Evaluation of the protective effect on enamel demineralization of CPP-ACP paste and ROCS by vibrational spectroscopy and SAXS: An in vitro study. Microsc. Res. Tech. 2021, 84, 2977–2987. [Google Scholar] [CrossRef] [PubMed]
- Altınışık, H.; Kedici Alp, C. Evaluation of Enamel Surfaces Treated with a Desensitizing Agent Containing CPP-ACP Before or After In-Office Bleaching. Int. J. Period. Rest. Dent. 2023, 7, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Almansouri, N.; Bakry, A.S.; Abbassy, M.A.; Linjawi, A.I.; Hassan, A.H. Evaluation of Resin Infiltration, Fluoride and the Biomimetic Mineralization of CPP-ACP in Protecting Enamel after Orthodontic Inter-Proximal Enamel Reduction. Biomimetics 2023, 8, 82. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Erkmen Almaz, M.; Ulusoy, N.B.; Akbay Oba, A.; Dokumaci, A. Remineralization effect of NaF, NaF with TCP, NaF with CPP-ACP and NaF with CXP varnishes on newly erupted first permanent molars: A randomized controlled trial. Int. J. Dent. Hyg. 2024, 22, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Al-Nerabieah, Z.; AlKhouli, M.; Dashash, M. Preventive efficacy of 38% silver diamine fluoride and CPP-ACP fluoride varnish on molars affected by molar incisor hypomineralization in children: A randomized controlled trial. F1000Research 2024, 12, 1052–1088. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rechmann, P.; Bekmezian, S.; Rechmann, B.M.T.; Chaffee, B.W.; Featherstone, J.D.B. MI Varnish and MI Paste Plus in a caries prevention and remineralization study: A randomized controlled trial. Clin. Oral. Investig. 2018, 22, 2229–2239. [Google Scholar] [CrossRef] [PubMed]
- Enax, J.; Amaechi, B.T.; Farah, R.; Liu, J.A.; Schulze Zur Wiesche, E.; Meyer, F. Remineralization Strategies for Teeth with Molar Incisor Hypomineralization (MIH): A Literature Review. Dent. J. 2023, 11, 80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- İlisulu, S.C.; Gürcan, A.T.; Şişmanoğlu, S. Remineralization efficiency of three different agents on artificially produced enamel lesions: A micro-CT study. J. Esthet. Restor. Dent. 2024, 36, 1536–1546. [Google Scholar] [CrossRef] [PubMed]
- Silvertown, J.D.; Wong, B.P.Y.; Sivagurunathan, K.S.; Abrams, S.H.; Kirkham, J.; Amaechi, B.T. Remineralization of natural early caries lesions in vitro by P11 -4 monitored with photothermal radiometry and luminescence. J. Investig. Clin. Dent. 2017, 8, e12257. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.S.; Nekkanti, S. Remineralization Potential of a Novel Biomimetic Material (Self-assembling Peptide P11-4) on Early Enamel Caries: An In Vitro Study. J. Contemp. Dent. Pract. 2023, 24, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Alkilzy, M.; Qadri, G.; Splieth, C.H.; Santamaría, R.M. Biomimetic Enamel Regeneration Using Self-Assembling Peptide P11-4. Biomimetics 2023, 8, 290. [Google Scholar] [CrossRef]
- Inces, G.S.; Ermisr, B.R. The in situ potential of synthetic nano-hydroxyapatite for tooth enamel repair. Bioinsp. Biomim. Nanobiomat. 2021, 10, 78–86. [Google Scholar] [CrossRef]
- Pushpalatha, C.; Gayathri, V.S.; Sowmya, S.V.; Augustine, D.; Alamoudi, A.; Zidane, B.; Hassan Mohammad Albar, N.; Bhandi, S. Nanohydroxyapatite in dentistry: A comprehensive review. Saudi Dent. J. 2023, 35, 741–752. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pepla, E.; Besharat, L.K.; Palaia, G.; Tenore, G.; Migliau, G. Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: A review of literature. Ann. Stomatol. 2014, 5, 108–114. [Google Scholar] [CrossRef]
- Florea, A.-D.; Pop, L.C.; Benea, H.-R.-C.; Tomoaia, G.; Racz, C.-P.; Mocanu, A.; Dobrota, C.-T.; Balint, R.; Soritau, O.; Tomoaia-Cotisel, M. Remineralization Induced by Biomimetic Hydroxyapatite Toothpastes on Human Enamel. Biomimetics 2023, 8, 450. [Google Scholar] [CrossRef]
- Alajlan, S.; Baysan, A. The effect of nano-hydroxyapatite on white spot lesions: A systematic review and meta-analysis. J. Dent. 2024, 151, 105402. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan-Wong, K.; Enax, J.; Meyer, F.; Ganss, B. The use of hydroxyapatite toothpaste to prevent dental caries. Odontol. 2022, 110, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Paszynska, E.; Pawinska, M.; Enax, J.; Meyer, F.; Schulze zur Wiesche, E.; May, T.W.; Amaechi, B.T.; Limeback, H.; Hernik, A.; Otulakowska-Skrzynska, J.; et al. Caries-preventing effect of a hydroxyapatite-toothpaste in adults: A 18-month double-blinded randomized clinical trial. Front. Public Health 2023, 11, 1199728. [Google Scholar] [CrossRef] [PubMed]
- Amaechi, B.T.; Farah, R.; Liu, J.A.; Phillips, T.S.; Perozo, B.I.; Kataoka, Y.; Meyer, F.; Enax, J. Remineralization of molar incisor hypomineralization (MIH) with a hydroxyapatite toothpaste: An in-situ study. BDJ Open 2022, 8, 33–43. [Google Scholar] [CrossRef]
- Carey, C.M. Remineralization of Early Enamel Lesions with Apatite-Forming Salt. Dent. J. 2023, 11, 182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fontana, M.; Gonzalez-Cabezas, C.; Tenuta, L. Evidence-based approaches and considerations for nonrestorative treatments within modern caries management. JADA 2024, 155, 1000–1011. [Google Scholar] [CrossRef]
- Pawinska, M.; Paszynska, E.; Amaechi, B.T.; Meyer, F.; Enax, J.; Limeback, H. Clinical evidence of caries prevention by hydroxyapatite: An updated systematic review and meta-analysis. J. Dent. 2024, 151, 105429. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gudkina, J.; Amaechi, B.T.; Abrams, S.H.; Brinkmane, A. Can New Remineralizing Agents Serve as Fluoride Alternatives in Caries Prevention? A Scoping Review. Oral 2025, 5, 47. https://doi.org/10.3390/oral5030047
Gudkina J, Amaechi BT, Abrams SH, Brinkmane A. Can New Remineralizing Agents Serve as Fluoride Alternatives in Caries Prevention? A Scoping Review. Oral. 2025; 5(3):47. https://doi.org/10.3390/oral5030047
Chicago/Turabian StyleGudkina, Jekaterina, Bennett T. Amaechi, Stephen H. Abrams, and Anda Brinkmane. 2025. "Can New Remineralizing Agents Serve as Fluoride Alternatives in Caries Prevention? A Scoping Review" Oral 5, no. 3: 47. https://doi.org/10.3390/oral5030047
APA StyleGudkina, J., Amaechi, B. T., Abrams, S. H., & Brinkmane, A. (2025). Can New Remineralizing Agents Serve as Fluoride Alternatives in Caries Prevention? A Scoping Review. Oral, 5(3), 47. https://doi.org/10.3390/oral5030047