1. Introduction
Craniofacial microsomia (CFM) is a congenital developmental disorder that involves the anatomic structures derived from the first and second pharyngeal arches during the 4th and 6th weeks of embryogenesis and is the second most common congenital craniofacial disorder after cleft lips and palates, with an incidence from 1:3500 to 1:5600–1:20,000 births [
1,
2,
3]. A recent consensus paper has advised using the term craniofacial microsomia for this condition and discarding the other denominations used over the years (hemifacial microsomia, first and second pharyngeal arch anomalies, oculo-auriculo-vertebral spectrum, Goldenhar syndrome) [
4].
A number of genetic and environmental factors, as well as external factors like exposure to teratogenic agents (thalidomide, retinoic acid, cocaine, nicotine, and hormonal therapy), were reported as aetiologic factors. The pathogenetic theory most accepted nowadays is a disruption during the migration and differentiation of the neural crest cells between the 30th and the 45th day of embryogenesis [
5]. The damage may also involve anatomic structures beyond the first and second pharyngeal arches.
As all the structures derived from the first and the second pharyngeal arches may be involved, the phenotype of the CFM is extremely variable with a various spectrum of severity [
6]. The clinical picture is characterized by a facial asymmetry that is determined by the progressive hypoplastic growth of the affected side with respect to the contralateral; reduced volume of the affected hemimandible, with short mandibular ramus; hypoplasia of condyle, mandibular fossa, and temporo-mandibular joint that may even be absent; and midline deviation to the affected side. Open gonial angle on the affected side, convex profile with mandibular retrusion. Unilateral skeletal class II, steep mandibular plane, and canting of the occlusal and mandibular plane as the result of the hemimandibular hypoplasia are common clinical features too [
7,
8]. Patients with severe mandibular hypoplasia have shown a significant delay in dental development on the affected side [
9].
Among the several classifications proposed, the OMENS and the OMENS+ classification systems allow a comprehensive evaluation of the anatomic structures involved and of the extra-cranial malformations associated, if present [
10,
11].
Most of the patients affected by CFM require a multidisciplinary approach for the number of systems involved and for the complexity of the anatomic damage. The actual treatment options for facial asymmetry include myofunctional therapy, orthopedic, functional, or orthodontic-surgical treatment, mandibular distraction osteogenesis (MDO), and facial soft tissue reconstruction [
5,
12,
13]. During the age of growth, the multidisciplinary treatment of the dentofacial deformity aims to improve mandibular and facial symmetry, increasing the vertical dimension of the affected side and of soft tissues associated, to correct the dental occlusion and canting, to simulate a TMJ function on the affected side, and to improve facial and dental aesthetics, with a strict interaction between orthodontist and maxillo-facial surgeon [
5,
14,
15]. Among the orthodontic strategies, the orthopedic-functional approach is based on asymmetric activators, variously designed, that work with an asymmetrical function to support the vertical development of the affected side. As regards the enhancement of the growth symmetry, good outcomes were reported in the literature in patients with mild and moderate severity (M1-2/S1, according to the OMENS classification) [
5,
15].
It is still unclear if the affected side grows at a lower rate, making the asymmetry progressive, or at the same rate as the non-affected side; understanding the mandibular growth of the CFM side may help to set up the orthodontic-functional and the surgical protocols [
16]. According to Aiyar et al., the existing literature consists mainly of case reports with low scientific value. They reported that the tendency to relapse was lower if treatments continued until skeletal maturity, outcomes and effects were better in mild to moderate cases, and morphologic changes in the condylar head are still not studied [
14].
This study aimed to evaluate the effects of functional therapy on the mandibular growth in our case series by using a simple method to measure the mandibular height on the panoramic radiographs of patients diagnosed with CFM. We have compared the measures of the mandibular and condylar height before and after the functional therapy to evaluate the effects of the functional therapy on the growth of the mandibular ramus affected by CFM; we analyzed if the functional therapy was able to obtain a growth difference between the affected side and the healthy side and if this was significant.
2. Materials and Methods
This retrospective longitudinal study was performed examining the medical records of patients affected by CFM and treated in the Unit of Orthodontics of our department during the period 2010–2020. Criteria for the inclusion in the study were patients diagnosed with CFM who were in the age of growth, underwent functional therapy, and had complete pre- and post-treatment documentation. All patients included in this study were treated with the Asymmetric Functional Activator (AFA), a type of hybrid appliance specifically designed for treatment of CFM that combines the biteblock part of the Andresen with the vestibular shields of the Frankel appliance on the affected side [
15,
16,
17].
The exclusion criteria were adult CFM patients, other malocclusions and syndromic; those who had previously undergone maxillo-facial surgery; patients affected by other types of mandibular hypoplasia, such as pseudo-CFM (HH condyle-coronoid collapse) and hemimandibular hypoplasia; incomplete treatments; patients treated with other appliances; and those who had not had pre- and post-treatment panoramic radiographs.
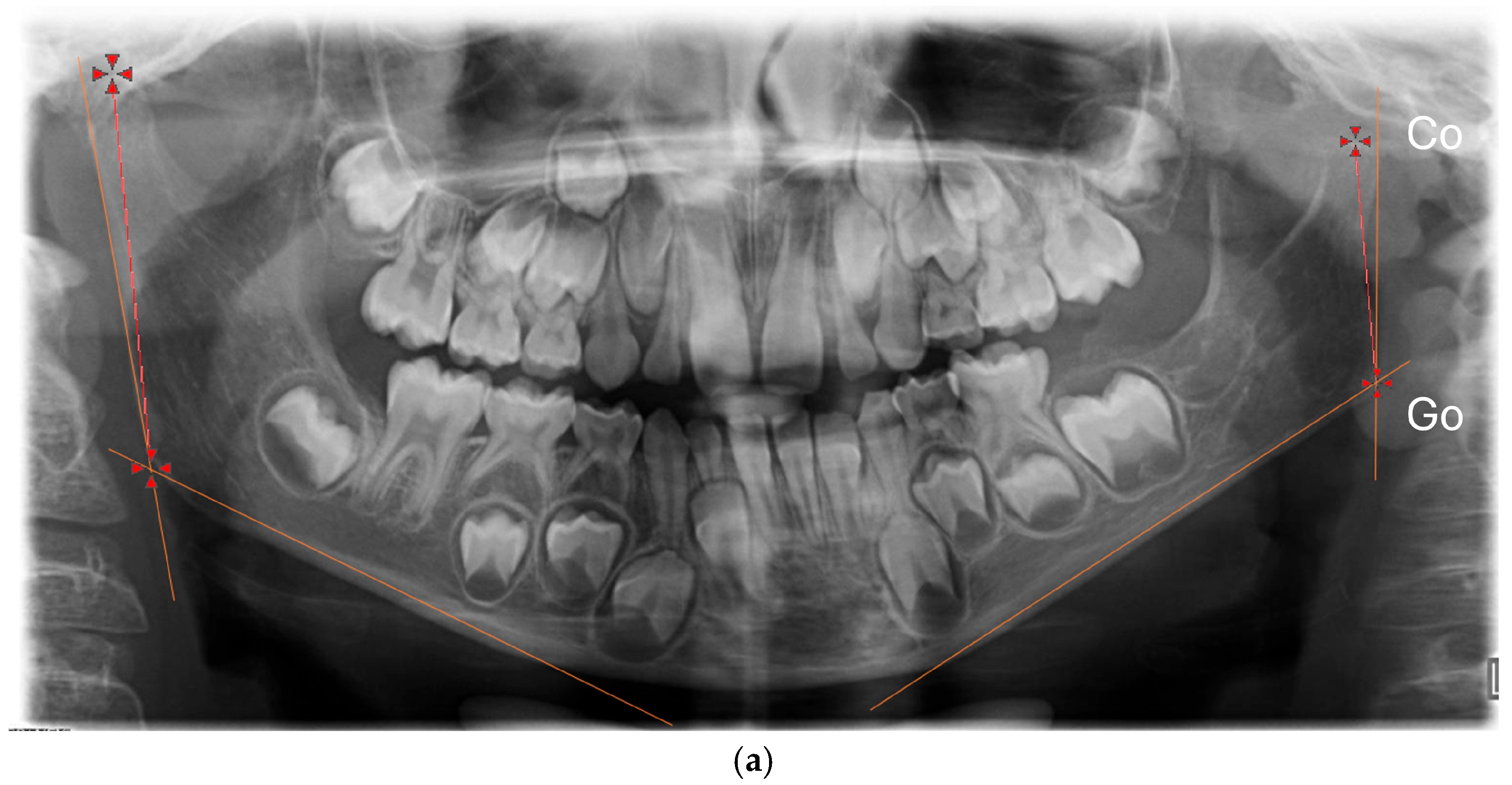
For each patient, sex, side of the CFM, degree of severity, classified according to the OMENS system, and age at the start and at the end of the functional treatment were collected. We collected the panoramic radiographs of patients at the beginning (T0) and at the end (T1) of the functional therapy. All the radiographs were digitized, together with a 5 cm ruler as reference. The “Meazure” measurement free software (version 4.0) was used to calibrate every digital image; after calibration, two measures were taken on the right and on the left side (
Figure 1):
The vertical height of the mandibular ramus (Co-Go), from the condylion (the uppermost posterior point of the condyle) to the gonion (the lowest and posterior point on the gonial angle) [
16,
17,
18].
The vertical length of the condyle, from the condylion to a vertical point on the plane passing by the sigmoid notch (Co-IS) (
Figure 1).
Raw data of each patient were grouped and analyzed by means of descriptive statistics. From the raw data, we calculated the absolute difference in millimeters between the non-affected and the affected side and the growth rate as a function of the growth difference in relation to the treatment time to minimize the effects of the different ages of beginning of the functional therapy and of the different treatment times.
One operator (EL) has performed all the measurements; to assess the intra-examiner reliability, the same operator repeated the measurements after 1 month. A second investigator (MM) has performed the entire protocol for the assessment of the inter-rater reliability.
The purpose of the inferential statistical analysis has been to compare the measurements of the height of the mandibular ramus and of the condylar height between the affected and the non-affected side at T0 and at T1; pre-post comparison was performed between the length measurements, the difference between measurements, and the growth rate in each side. We tested as a null hypothesis that there were no statistically significant differences between the two sides in T0 and T1.
Normality of the distribution was tested with the Shapiro–Wilk test; Student’s t-test for matched pairs was applied when distributions were normal; otherwise, the Wilcoxon signed-rank test was used for non-normal distributions. The
p-value for statistical significance was set to 0.05. All the statistical analysis was performed with the free statistical software “R” version 4.2.0. (
https://www.r-project.org, accessed on 14 June 2024). Post hoc power analysis was performed using the G*Power software (version 3.1.9.7; Franz Faul, University of Kiel, Kiel, Germany) [
19].
3. Results
Following the inclusion and exclusion criteria, out of 30 patients affected by CFM, 14 were selected: 10 male (71.4%) and 4 female patients (28.5%). The mean age at the start of treatment (T0) was 8.8 years old + 3, while in T1 it was 11.8 years old ±3.1. The affected side was the left in 9 patients (64.2%) and right in 5 (
Table 1).
Table 2 displays the OMENS classification of the sample.
The ICC for intra-rater repeatability ranged between 0.87 (affected Co-IS) and 0.92 (healthy Co-Go), revealing very good to excellent reliability of the measurements. The ICC for inter-rater reliability highlighted good reliability (from 0.79 for the affected Co-Is to 0.88 for the healthy Co-Go).
Table 3 and
Table 4 display the descriptive statistics. As regards the Co-Go distance, the mean in T0 was 53.9 mm (SD 13.9) on the CFM side and increased at the end of the functional therapy (T1 mean = 62.2 m (SD 15.6); on the healthy side, the mean distance in T0 was 69.0 mm (SD 15.6) and 72.5 mm (SD 16.3) in T1. The CFM side grew more than the healthy side, both in the absolute difference and the growth rate (
Table 3). The boxplots of measurements in T0 and in T1, as well as the boxplot of the absolute differences, display graphically those results (
Figure 2).
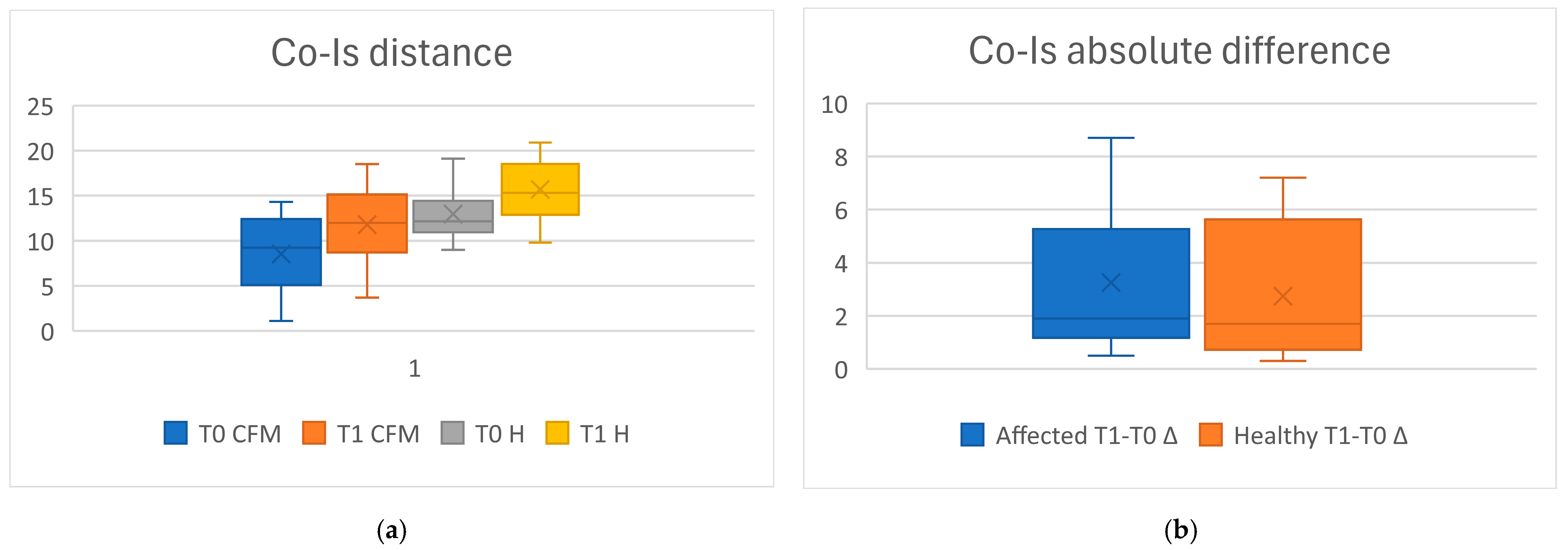
In T0, the mean of the Co-IS distance was 8.1 mm (SD 4.1) on the CFM side, and in T1, 12.8 (SD 3.1); on the healthy side, the mean distance in T0 was 11.6 (SD 4.4), and in T1, 15.6 (SD 3.6). The CFM side grew more than the healthy side, both as the absolute difference, while the growth rate was similar (
Table 4). The results of the measurements, as well as the differences between the affected and the healthy side, were also displayed with box plots (
Figure 3).
As regards the comparisons of the Co-Go measurements with inferential statistics (
Table 5), the differences between the measurements of the CFM and those of the healthy side were statistically significant in both T0 (
p < 0.000) and T1 (
p < 0.000); the mean differences between T0 and T1 were statistically relevant both in the CFM (
p < 0.000) and in the healthy side (
p = 0.0041), and both the absolute difference and the growth rate between the affected and the healthy side were significant (
p = 0.016).
As regards the comparisons of the Co-IS measurements, the differences between the measurements of the CFM and those of the healthy side were statistically significant in both T0 (p < 0.000) and T1 (p < 0.000); the mean differences between T0 and T1 were statistically significant in both the CFM (p < 0.000) and the healthy side (p = 0.001), while the absolute difference (p = 0.5416) and the growth rate between the affected and the healthy side were not significant (p = 7148).
As the sample size required to have a 5% level of significance, a power of 0.80, and a Cohen effect size, calculated as the effect size able to detect a difference of at least 2× the standard deviation of the variables, of d = 0.5, was 20 subjects, we performed a post hoc power analysis with our sample size that showed a power of 0.66.
4. Discussion
The aim of this paper was to test a simple method to measure the vertical dimension of the mandibular ramus and of the condyle on panoramic radiographs and to evaluate whether functional therapy may have a role in guiding the growth of the affected side in patients affected by craniofacial microsomia.
From a technical point of view, 2D imaging has several limitations that may affect measurements on panoramic radiographs: magnification and distortions of the anatomic structures may depend on the distance from the focal layer or on the incorrect positioning of patients, while the identification of the landmarks may be difficult due to the anatomic overlap of the bones. In asymmetric patients, the magnification ratio between the two sides may even be more evident and can generate distortions in the horizontal direction. Di Blasio et al. demonstrated that vertical measurements on panoramic radiographs are a reliable parameter to assess the mandibular asymmetry, as their reliability was higher than the horizontal measurements [
18]. Therefore, we have chosen to evaluate only the vertical measurements on panoramic radiographs, following the recent literature [
16,
18].
Nowadays, the validity of measurements and the visualization of the side affected by the deformity can be improved by using the reconstructions of the Cone Beam CT, which also allow for performing a three-dimensional evaluation of the skull or horizontal measurements or precisely measuring the growth of the alveolar processes or canting of the maxillary occlusal plane. But the use of CBCT in children for orthodontic diagnosis may be unethical and postponed until patients need three-dimensional evaluations (for example, for surgical reasons) or when the severity of the deformation requires a comprehensive craniofacial diagnosis.
From the descriptive statistics, we observed a prevalence of male subjects (M:F = 10:4), as reported by the literature, and the left side was prevalent in most of the cases (9 out of 14). The mean age at the start of the therapy was around 8.8 years; the low mean age in T0 was expected, as treatments started very early in order to take advantage of the prepubertal peak of growth; the duration of the functional therapy was around 3.3 years.
We observed a significant difference in the vertical height of the ramus and of the condylar process in T0. Kaprio et al. reported that, in mild CFM cases, the affected/non-affected ratio of the vertical height of the ramus was statistically significant and does not change during the development of the dentition; according to their findings, CFM does not have a progressive nature, and they suggest postponing surgical treatments until skeletal maturity is reached [
16] Other papers reported a statistically significant difference between the affected and non-affected sides, also using different measurements on the panoramic radiograph or in posterior-anterior cephalograms [
20,
21,
22].
After treatment, the measurements increased and the vertical dimensions changed on both sides due to the combined action of functional therapy and peaks of growth. The T1-T0 absolute difference and the growth ratio were statistically significant, indicating a different response in the two sides, although the vertical difference between the two sides was still statistically significant in T1.
Many authors have reported that the statistically significant initial differences in the ramal height between the affected and non-affected sides are still present at the end of the treatment, but they reported a similar growth rate in the treated and in the control group [
23,
24]. The functional therapy in patients of growth age has tried to hyper-stimulate the lack of growth on the affected side and has helped to delay the progression of the asymmetry, although this was not able to entirely correct the pathological phenotype. This clinical finding is important to evaluate the burden of care, considering also the initial severity of the asymmetry and the very long treatment time that may not be able to avoid surgical intervention. A close collaboration with maxillo-facial surgeons may allow the development of treatment timing personalized based on the severity of the condition, according to the OMENS+ classification.
The comparisons of the absolute growth difference and of the growth ratio were significant for the Co-Go, while the same comparisons were not significant for the Co-IS. The reason for these results may be related to the stimulation of the vertical growth of the alveolar processes more than a specific growth from the sigmoid notch to the gonial angle. This point would explain the reduced growth in patients with greater damage at the level of the TMJ. This hypothesis surely needs a specific further investigation to be confirmed, but our findings agree with other papers: Meazzini et al. reported that the main effect of the functional therapy is dento-alveolar, and patients treated with functional therapy had a stable occlusal plane and slowed down relapse of the vertical mandibular asymmetry compared with patients treated with distraction osteogenesis [
25]. The combination of functional therapy, natural growth, and changes in masticatory loading may have influenced the entire facial growth too [
26].
According to Poole, the downward vertical growth of the maxilla on the affected side is inhibited by the mandibular position; consequently, the occlusal plane on the affected side is canted upward [
27]. Therefore, the correction of the canted occlusal plane by enhancing the vertical dimension of the affected side is an important therapeutic target. Our results agree with the paper by Cassi et al., who reported that functional therapy with AFA improves the mandibular symmetry and canting of the occlusal plane after functional therapy with AFA and that a very early start of treatment, patient compliance, and duration of the therapy of about 30 months may be the factors for treatment success [
17].
In fact, the different approach in CFM treatment is related to the earlier start of the therapy and to the longer duration with respect to conventional orthopedic-functional treatment. More clinical data are needed to establish if CFM patients may benefit from early intervention, as reported by literature, or delayed intervention may still be useful to prevent or reduce the surgical correction of the bony bases.
Despite the statistically significant differences observed, this study has several limitations, firstly because of the retrospective nature of the study, because such treatments of CFM are often long, and longitudinal studies may need a long period to collect cases with complete documentation. Findings, in fact, may be biased from the many confounding factors that may affect this type of study. The limited number of samples was determined by the low prevalence of the disease and by the inclusion criteria and has affected the post-hoc power of this study; we can define this research as a preliminary study useful to gather other patients, as only an increase in the sample may reach an adequate power. The findings of this study are affected by the limitations of 2D imaging, but, for the clinical and ethical considerations stated above, only a multicentric approach would gather enough data to setup a study using CBCT.
Future studies may focus on the design of devices able to further stimulate the growth development of the condylar process or able to promote a three-dimensional development; the correlation of the therapeutic outcomes with the severity of the condition; or establishing a precise cutoff that helps clinicians determine whether functional therapy may sufficiently improve the malformation without an orthodontic-surgical approach.
5. Conclusions
There is a statistically significant difference in the ramus length between the affected side and the healthy side; the difference in the growth of the Co-Go length on the affected side is statistically significant compared to the healthy side, but functional therapy does not completely correct the difference in the vertical dimension of the ramus.
Thus, although the functional therapy may have a therapeutic effect on the growth of the side affected by CFM, the clinical characteristics of the malformation remain at the end of the therapy and may need surgery to improve mandibular symmetry.
Even though growth influences the overall height of the ramus, the growth of the condylar process was not statistically relevant; the severity of the condylar damage may suggest a role in the therapeutic response to functional therapy that may need further investigation.
Author Contributions
Writing—original draft preparation, investigation, data curation, E.L.; writing—reviewing and editing, M.M. and R.A.V.; data curation, formal analysis, R.D.G.; investigation, conceptualization, G.G.; methodology, supervision, E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by our department and by the local University Ethical Board, prot. n. 4663, 29 September 2017.
Informed Consent Statement
Not applicable. Because we have conducted a study on radiographs and patients were completely anonymized, in fact none of us who conducted the study was able to identify the patients on the basis of the data collected. When patients were treated, they signed an informed consent for the use of clinical data in anonymous form, on the basis of the UE law GDPR 2016/679.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available because all the data analyzed were reported in this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Young, A.; Spinner, A. Hemifacial Microsomia. In Operative Oral and Maxillofacial Surgery, 2nd ed.; StatPearls: Treasure Island, FL, USA, 2023; pp. 685–690. [Google Scholar] [CrossRef]
- Kuu-Karkku, L.; Suominen, A.; Svedström-Oristo, A. Craniofacial microsomia—More than a structural malformation. Orthod. Craniofac. Res. 2023, 26, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Sun, H.; Bian, Q.; Liu, Z.; Wang, X. The etiology, clinical features, and treatment options of hemifacial microsomia. Oral Dis. 2023, 29, 2449–2462. [Google Scholar] [CrossRef]
- Renkema, R.W. European Guideline Craniofacial Microsomia. J. Craniofac. Surg. 2020, 31 (Suppl. S8), 2385–2484. [Google Scholar] [CrossRef] [PubMed]
- López, D.F.; Acosta, D.M.; Rivera, D.A.; Mejía, C.M. Hemifacial microsomia: Treatment alternatives—A systematic review of literature. J. Clin. Pediatr. Dent. 2022, 46, 15–30. [Google Scholar] [CrossRef]
- Yang, I.-H.; Chung, J.H.; Yim, S.; Cho, I.-S.; Lim, S.-W.; Kim, K.; Kim, S.; Choi, J.-Y.; Lee, J.-H.; Kim, M.-J.; et al. Distribution and phenotypes of hemifacial microsomia and its association with other anomalies. Korean J. Orthod. 2020, 50, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Ahiko, N.; Baba, Y.; Tsuji, M.; Suzuki, S.; Kaneko, T.; Kindaichi, J.; Moriyama, K. Investigation of Maxillofacial Morphology and Dental Development in Hemifacial Microsomia. Cleft. Palate Craniofac. J. 2015, 52, 203–209. [Google Scholar] [CrossRef]
- Chen, Y.F.; Vinayahalingam, S.; Bergé, S.; Liao, Y.F.; Maal, T.; Xi, T. Is the pattern of mandibular asymmetry in mild craniofacial microsomia comparable to non-syndromic class II asymmetry? Clin. Oral Investig. 2022, 26, 4603–4613. [Google Scholar] [CrossRef]
- Ongkosuwito, E.M.; De Gijt, P.; Wattel, E.; Carels, C.E.L.; Kuijpers-Jagtman, A.M. Dental development in hemifacial microsomia. J. Dent. Res. 2010, 89, 1368–1372. [Google Scholar] [CrossRef]
- Vento, A.R.; Labrie, R.A.; Mulliken, J.B. The O.M.E.N.S. classification of hemifacial microsomia. Cleft. Palate Craniofac. J. 1991, 28, 68–77. [Google Scholar] [CrossRef]
- Horgan, J.E.; Padwa, B.L.; Labrie, R.A.; Mulliken, J.B. OMENS-Plus: Analysis of craniofacial and extracraniofacial anomalies in hemifacial microsomia. Cleft. Palate Craniofac. J. 1995, 32, 405–412. [Google Scholar] [CrossRef]
- Dowgierd, K.; Pokrowiecki, R.; Myśliwiec, A.; Krakowczyk, Ł. Use of a Fibula Free Flap for Mandibular Reconstruction in Severe Craniofacial Microsomia in Children with Obstructive Sleep Apnea. J. Clin. Med. 2023, 12, 1124. [Google Scholar] [CrossRef]
- Lech, D.; Matysek, J.; Maksymowicz, R.; Strączek, C.; Marguła, R.; Krakowczyk, Ł.; Kozakiewicz, M.; Dowgierd, K. Maxillofacial Microvascular Free-Flap Reconstructions in Pediatric and Young Adult Patients—Outcomes and Potential Factors Influencing Success Rate. J. Clin. Med. 2024, 13, 2015. [Google Scholar] [CrossRef] [PubMed]
- Aiyar, A.; Pedersen, T.K.; Resnick, C.M.; Nørholt, S.E.; Verna, C.; Stoustrup, P.B. Management of unilateral craniofacial microsomia with orthopaedic functional appliances: A systematic literature review. Orthod. Craniofac. Res. 2024, 27, 131–140. [Google Scholar] [CrossRef]
- Silvestri, A.; Natali, G.; Iannetti, G. Functional therapy in hemifacial microsomia: Therapeutic protocol for growing children. J. Oral Maxillofac. Surg. 1996, 54, 271–278. [Google Scholar] [CrossRef]
- Kaprio, L.; Grann, A.; Leikola, J.; Saarikko, A.; Kurimo, J.; Kiukkonen, A. Non-progressive mandibular changes in children with Type I and II craniofacial microsomia. Orthod. Craniofac. Res. 2024, 27 (Suppl. S1), 122–130. [Google Scholar] [CrossRef] [PubMed]
- Cassi, D.; Magnifico, M.; Gandolfinini, M.; Kasa, I.; Mauro, G.; Di Blasio, A. Early orthopaedic treatment of hemifacial microsomia. Case Rep. Dent. 2017, 2017, 1–6. [Google Scholar] [CrossRef]
- Di Blasio, C.; Di Blasio, A.; Pedrazzi, G.; Anghinoni, M.; Sesenna, E. How Does the Mandible Grow After Early High Condylectomy? J. Craniofac. Surg. 2015, 26, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Tokura, T.A.; Miyazaki, A.; Igarashi, T.; Dehari, H.; Kobayashi, J.I.; Miki, Y.; Ogi, K.; Sonoda, T.; Yotsuyanagi, T.; Hiratsuka, H. Quantitative Evaluation of Cephalometric Radiographs of Patients with Hemifacial Microsomia. Cleft Palate Craniofac. J. 2019, 56, 711–719. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Kim, B.S.; Han, W.; Yan, Y.; Wang, X.; Li, X.; Zhang, Y.; Chai, G. Quantitative structural analysis of hemifacial microsomia mandibles in different age groups. Front. Pediatr. 2023, 11, 1157607. [Google Scholar] [CrossRef]
- Ko, E.W.C.; Chen, P.K.T.; Lo, L.J. Comparison of the adult three-dimensional craniofacial features of patients with unilateral craniofacial microsomia with and without early mandible distraction. Int. J. Oral. Maxillofac. Surg. 2017, 46, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Ongkosuwito, E.; van Vooren, J.; van Neck, J.; Wattel, E.; Wolvius, E.; van Adrichem, L.; Kuijpers-Jagtman, A. Changes of mandibular ramal height, during growth in unilateral hemifacial microsomia patients and unaffected controls. J. Craniomaxillofac. Surg. 2013, 41, 92–97. [Google Scholar] [CrossRef]
- Renkema, R.W.; van Beelen, I.; Koudstaal, M.J.; Caron, C.J.J.M. The effect of natural growth on chin point deviation in patients with unilateral craniofacial microsomia: A retrospective study. J. Craniomaxillofac. Surg. 2022, 50, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Meazzini, M.C.; Mazzoleni, F.; Bozzetti, A.; Brusati, R. Does functional appliance treatment truly improve stability of mandibular vertical distraction osteogenesis in hemifacial microsomia? J. Craniomaxillofac. Surg. 2008, 36, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Landi, F.; Çetin, I.E.; Profico, A.; Buzi, C.; Dutel, H.; Khonsari, R.H.; O’HIggins, P.; Moazen, M. Functional adaptation of the infant craniofacial system to mechanical loadings arising from masticatory forces. Proc. R. Soc. B. Biol. Sci. 2024, 291, 20240654. [Google Scholar] [CrossRef]
- Poole, M.D. Hemifacial microsomia. World J. Surg. 1989, 13, 396–400. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).