Nonlinear Finite Element Analysis of Bone–Implant Contact in Three Short Dental Implant Models with Varying Osseointegration Percentages
Abstract
1. Introduction
2. Materials and Methods
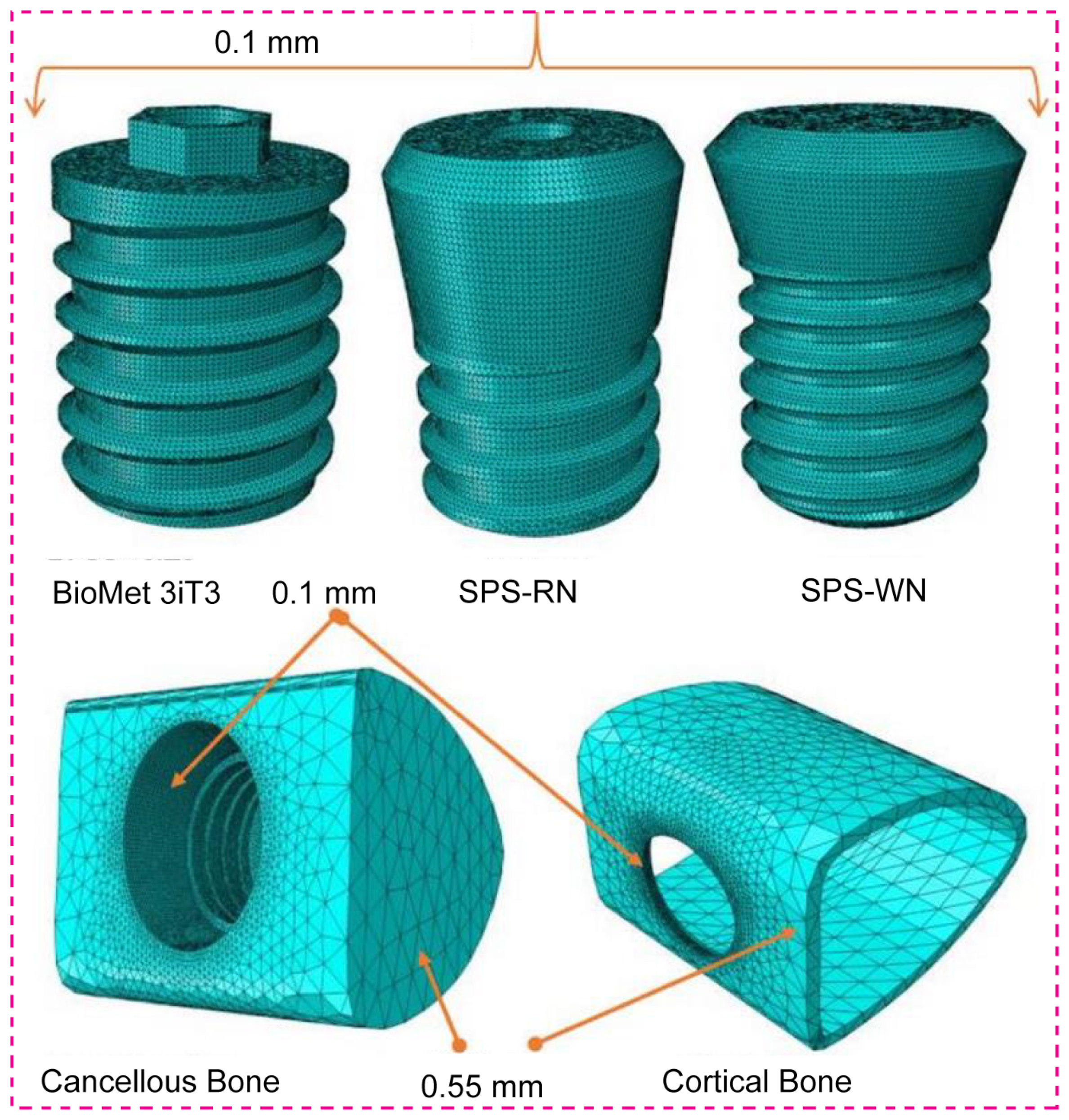
2.1. CAD Geometry Design
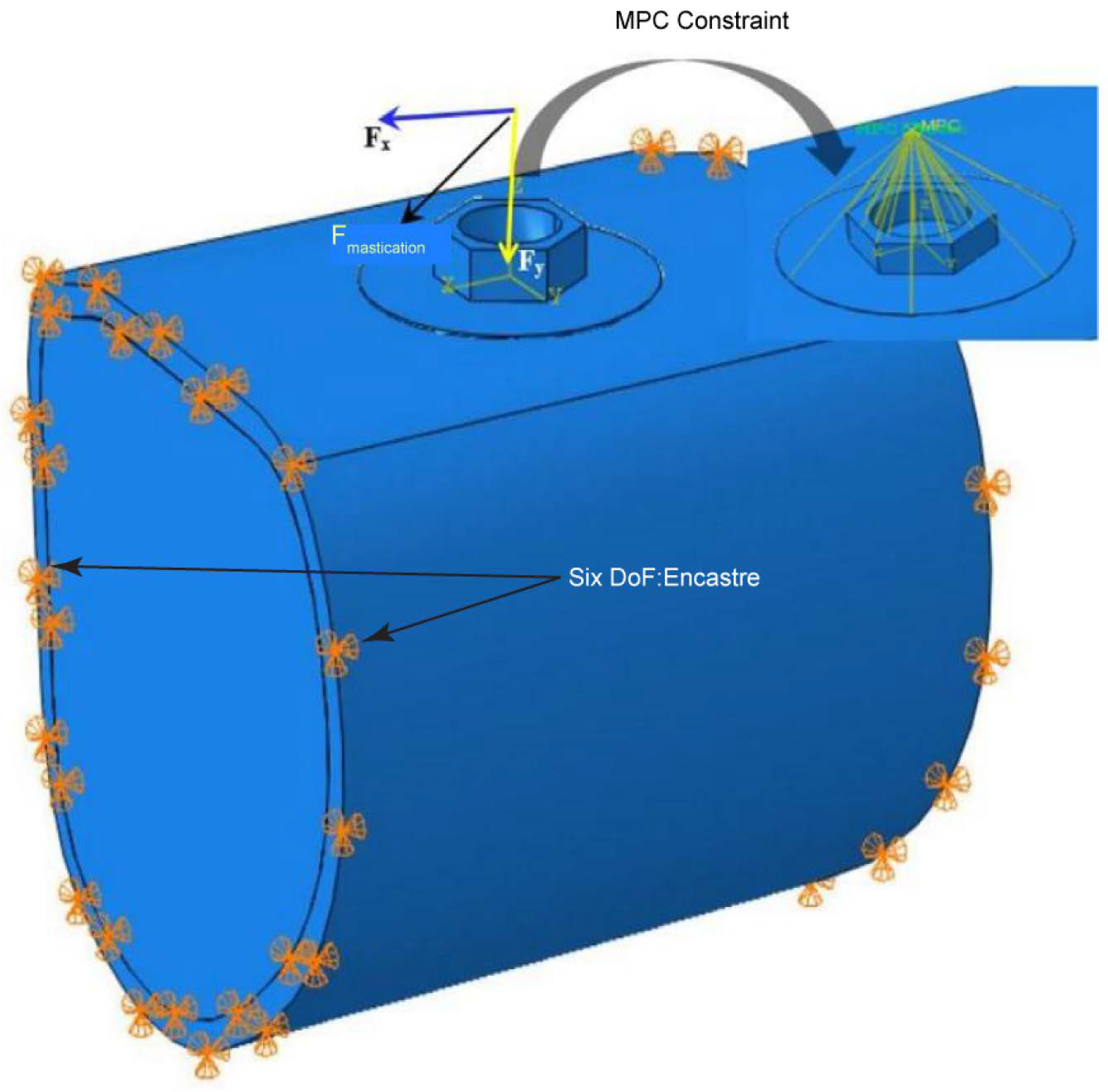
2.2. Three-Dimensional Finite Element Analysis (FEA)
2.2.1. Material Properties
2.2.2. FE Mesh and Contact Definition
3. Results
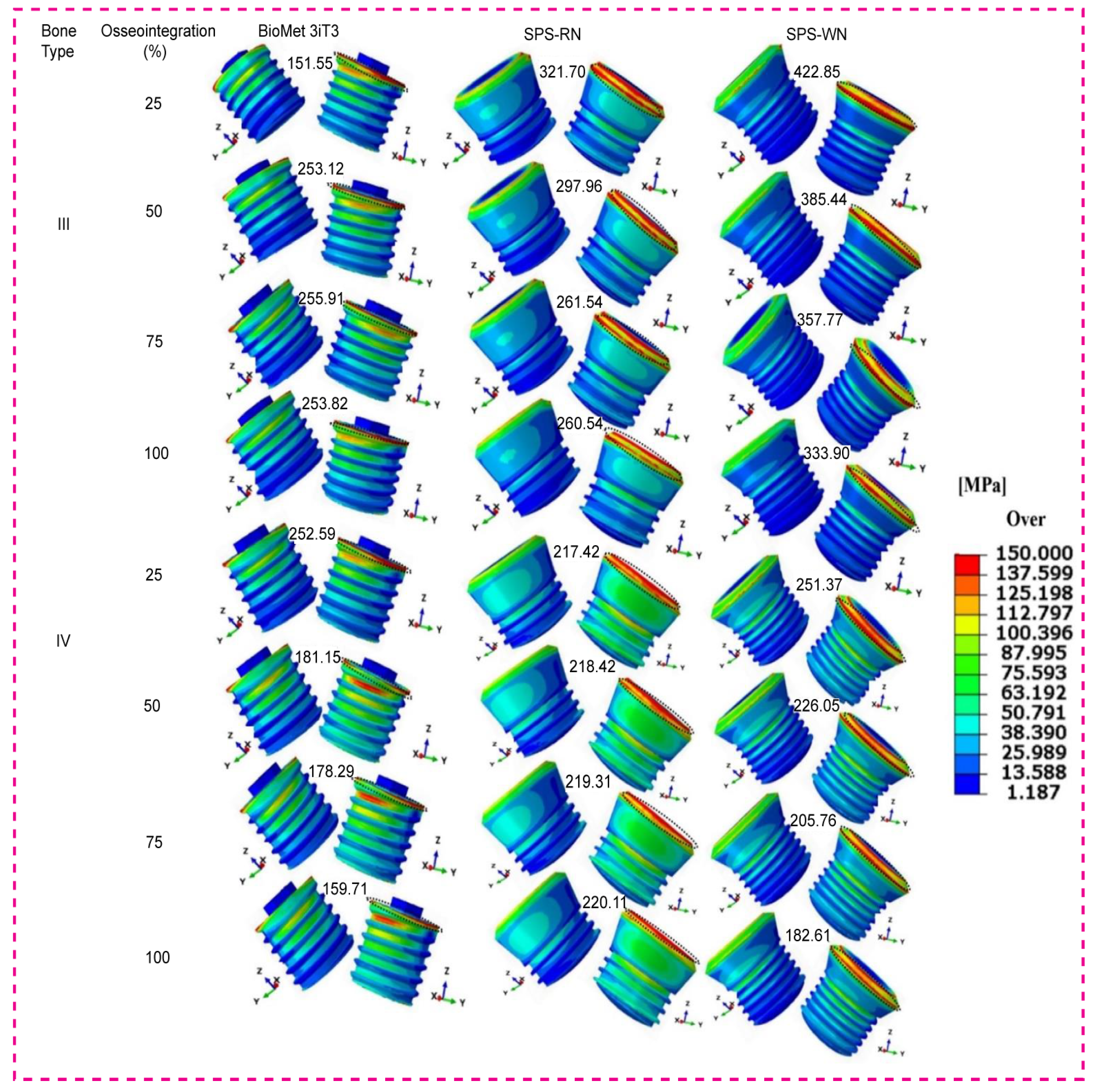
3.1. Maximum Stress and Maximum Bone Strain
3.1.1. BioMet 3iT3 Short Implant
3.1.2. Standard Plus Short (SPS) Implants with Regular Neck (SRN)
3.1.3. Standard Plus Short (SPS) Implants with Wide Neck (SWN)
3.2. Maximum Shear Stresses Along the Three-Plane
3.3. Maximum von Mises Stress at the Bone–Implant Contact (BIC)
3.4. Maximum von Mises Stress in Three Types of Short Dental Implants
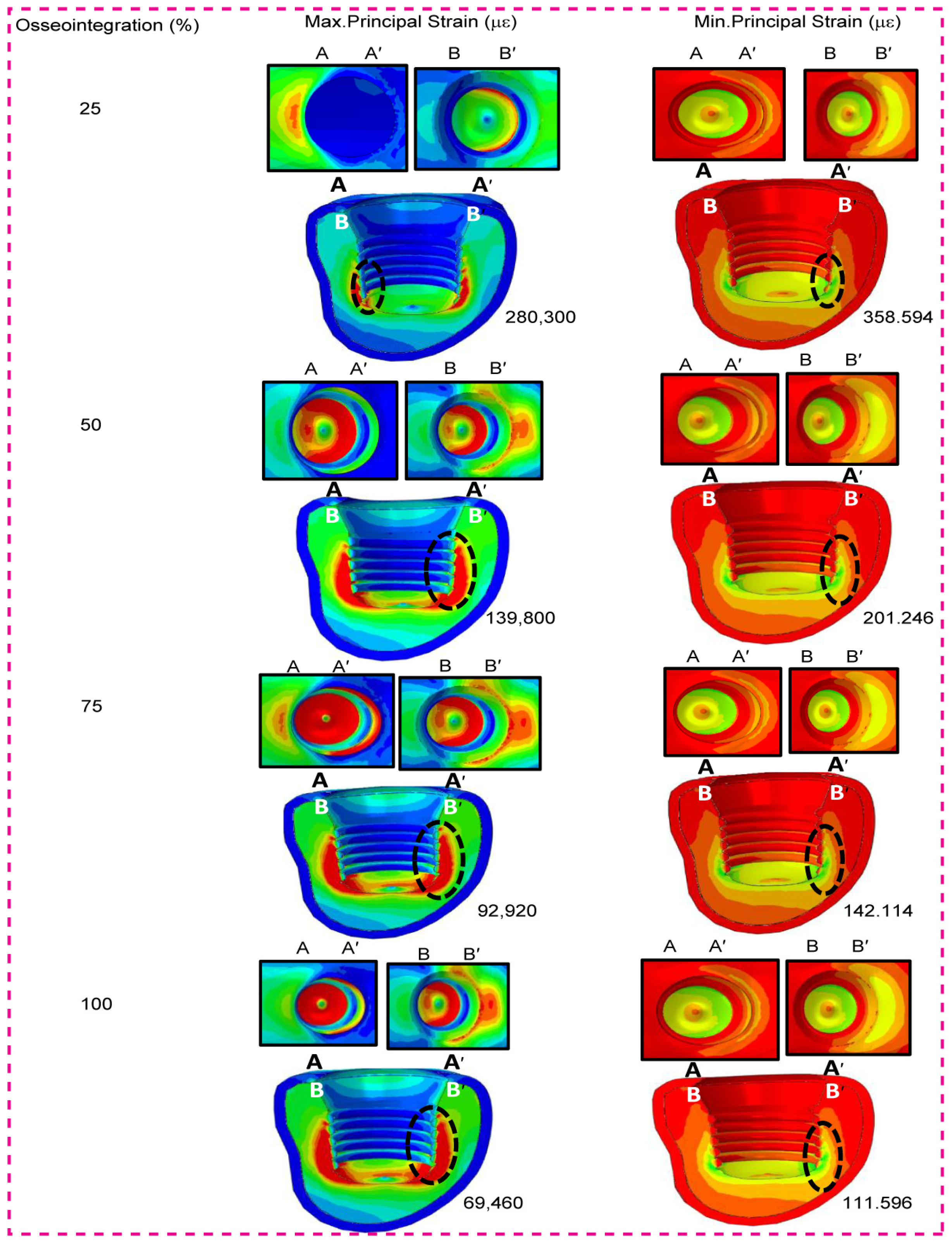
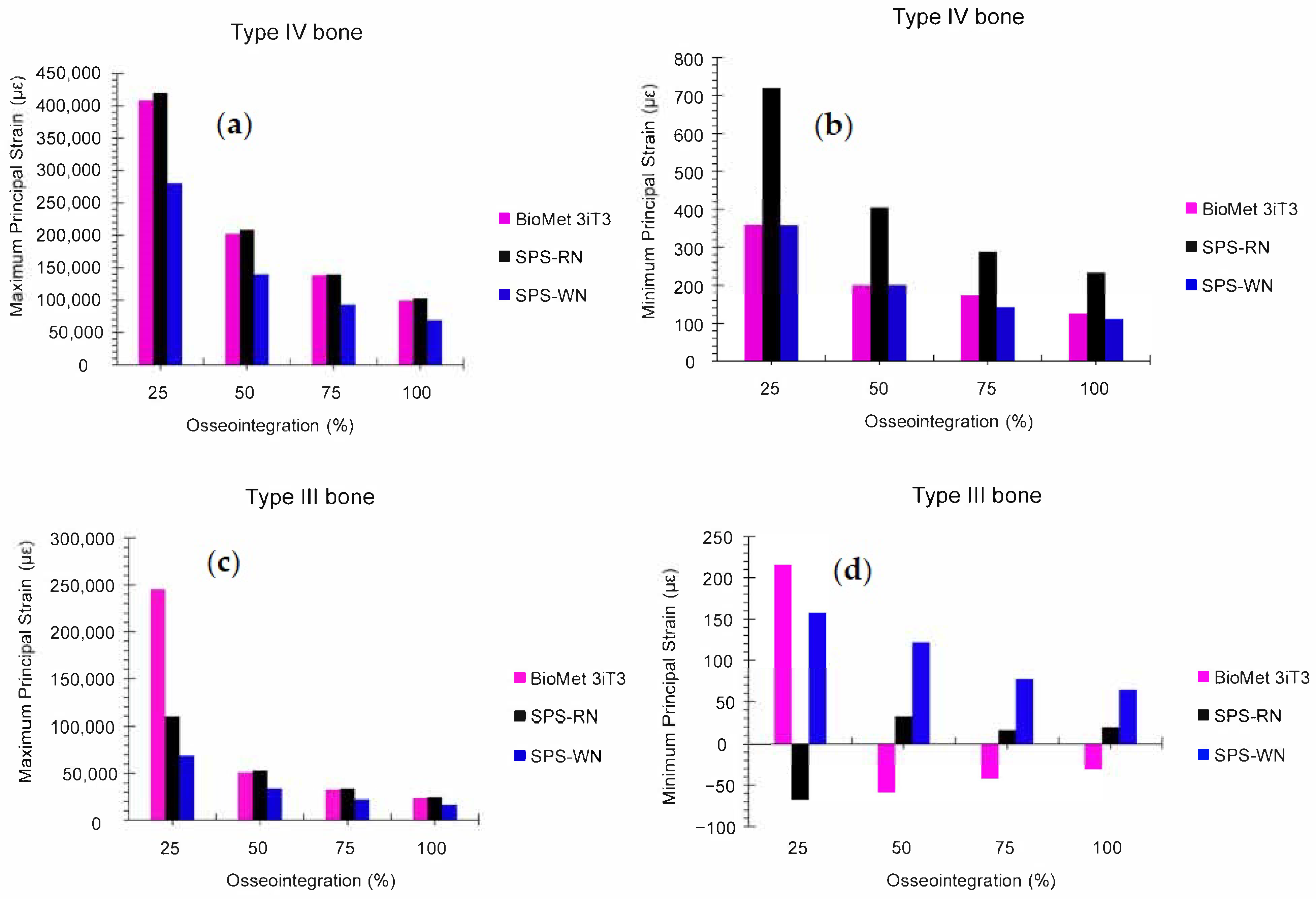
3.5. Low-Density Cancellous Bone Maximum and Minimum Principal Strain
3.6. High-Density Cancellous Bone Maximum and Minimum Principal Strain
3.7. Low- and High-Density Cancellous Bone Maximum and Minimum Principal Strain in Three Types of Short Dental Implants
4. Discussion
5. Conclusions
- ▪
- Osseointegration improves implant stability: Across all implant types and bone densities, increasing osseointegration results in a substantial decrease in both von Mises stress and principal strains at the bone–implant interface, indicating better load distribution and a reduced likelihood of localized destruction of the bone.
- ▪
- The SPS-WN implant exhibits superior biomechanical performance, with the lowest maximum and minimum principal strains at all phases of osseointegration, particularly at 100% integration. This shows that the SPS-WN implant design is more successful at reducing mechanical stress while increasing stability, making it ideal for individuals with variable bone densities.
- ▪
- The effect of bone density on stress distribution: All implants showed higher initial stress and strain in low-density cancellous bone (bone type IV). This shows how important it is to achieve complete osseointegration to reduce these effects. The SPS-WN implant was particularly successful in lowering stress in low-density bone, indicating its potential for use in patients with poor bone quality.
- ▪
- BioMet 3iT3 implant needs careful consideration: The BioMet 3iT3 implant had greater starting strain values, but it benefitted from enhanced osseointegration, resulting in considerable stress and strain reductions. However, the fact that it consistently shows higher strain values than the SPS-WN model suggests that it might not work as well when bone quality is low or osseointegration is weak.
- ▪
- The critical role of complete osseointegration: The research emphasizes the need for 100% osseointegration for proper implant function. Incomplete osseointegration is linked to increased stress concentrations and a higher likelihood of implant failure, especially in less dense bones.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rane, A.V.; Abitha, V.K.; Sisanth, K.S.; Kanny, K. Introduction to Polymer Materials for Implants. In Polymeric Materials for Biomedical Implants; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1–29. [Google Scholar]
- Michalakis, K.; Misci, S.; Abdallah, A.; Vasilaki, D.; Hirayama, H. Implant Supportive Maintenance for Fixed Prosthetic Rehabilitations: The Patient with the Complete Arch Fixed Implant–Supported Rehabilitation: Prosthetic Concepts to Optimize Maintenance Protocols. In Saving Dental Implants; Wiley: Hoboken, NJ, USA, 2024; pp. 357–380. [Google Scholar] [CrossRef]
- Xu, X.; Zuo, J.; Zeng, H.; Zhao, Y.; Fan, Z. Improving Osseointegration Potential of 3D Printed PEEK Implants with Biomimetic Periodontal Ligament Fiber Hydrogel Surface Modifications. Adv. Funct. Mater. 2024, 34, 2308811. [Google Scholar] [CrossRef]
- Lemos, C.A.A.; Ferro-Alves, M.L.; Okamoto, R.; Mendonça, M.R.; Pellizzer, E.P. Short Dental Implants versus Standard Dental Implants Placed in the Posterior Jaws: A Systematic Review and Meta-Analysis. J. Dent. 2016, 47, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Trombelli, L.; Farina, R.; Tomasi, C.; Vignoletti, F.; Paolantoni, G.; Giordano, F.; Ortensi, L.; Simonelli, A. Factors Affecting Radiographic Marginal Bone Resorption at Dental Implants in Function for at Least 5 Years: A Multicenter Retrospective Study. Clin. Oral Implants Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Khater, A.G.A.; Gehrke, S.A.; Inchingolo, F.; Tari, S.R. Animal Models for Investigating Osseointegration: An Overview of Implant Research over the Last Three Decades. J. Funct. Biomater. 2024, 15, 83. [Google Scholar] [CrossRef] [PubMed]
- Villalobos-Tinoco, J.; Fischer, N.G.; Jurado, C.A.; Sayed, M.E.; Feregrino-Mendez, M.; de la Mata Garcia, O.; Tsujimoto, A. Combining a Single Implant and a Veneer Restoration in the Esthetic Zone. Int. J. Esthet. Dent. 2020, 15, 428–439. [Google Scholar]
- Tseng, K.-F.; Shiu, S.-T.; Hung, C.-Y.; Chan, Y.-H.; Chee, T.-J.; Huang, P.-C.; Lai, P.-C.; Feng, S.-W. Osseointegration Potential Assessment of Bone Graft Materials Loaded with Mesenchymal Stem Cells in Peri-Implant Bone Defects. Int. J. Mol. Sci. 2024, 25, 862. [Google Scholar] [CrossRef]
- Luo, F.; Mo, Y.; Jiang, J.; Wen, J.; Ji, Y.; Li, L.; Wan, Q. Advancements in Dental Implantology: The Alveolar Ridge Split Technique for Enhanced Osseointegration. Clin. Implant Dent. Relat. Res. 2024, 26, 1012–1031. [Google Scholar] [CrossRef]
- Mohammadi, A.; Dehkordi, N.R.; Mahmoudi, S.; Rafeie, N.; Sabri, H.; Valizadeh, M.; Poorsoleiman, T.; Jafari, A.; Mokhtari, A.; Khanjarani, A. Effects of Drugs and Chemotherapeutic Agents on Dental Implant Osseointegration: A Narrative Review. Curr. Rev. Clin. Exp. Pharmacol. Former. Curr. Clin. Pharmacol. 2024, 19, 42–60. [Google Scholar] [CrossRef]
- Šromová, V.; Sobola, D.; Kaspar, P. A Brief Review of Bone Cell Function and Importance. Cells 2023, 12, 2576. [Google Scholar] [CrossRef]
- Choukroun, E.; Parnot, M.; Surmenian, J.; Gruber, R.; Cohen, N.; Davido, N.; Simonpieri, A.; Savoldelli, C.; Afota, F.; El Mjabber, H. Bone Formation and Maintenance in Oral Surgery: The Decisive Role of the Immune System—A Narrative Review of Mechanisms and Solutions. Bioengineering 2024, 11, 191. [Google Scholar] [CrossRef]
- Menchini-Fabris, G.B.; Toti, P.; Crespi, G.; Covani, U.; Crespi, R. Distal Displacement of Maxillary Sinus Anterior Wall versus Conventional Sinus Lift with Lateral Access: A 3-Year Retrospective Computerized Tomography Study. Int. J. Environ. Res. Public Health 2020, 17, 7199. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, Z.H.; Bhangoo, H.S.; Meyer, A.J.; Rao, G.V.; Buttar, N.S. Full-Thickness Endoscopic and Combined Laparoscopic-Endoscopic Techniques. In Advanced Techniques for Endoscopic Resection in the GI Tract; CRC Press: Boca Raton, FL, USA, 2024; pp. 387–402. [Google Scholar]
- Bornstein, M.M.; Al-Nawas, B.; Kuchler, U.; Tahmaseb, A. Consensus Statements and Recommended Clinical Procedures Regarding Contemporary Surgical and Radiographic Techniques in Implant Dentistry. Int. J. Oral Maxillofac. Implants 2014, 29, 78. [Google Scholar] [CrossRef] [PubMed]
- Hossain, N.; Islam, M.A.; Ahmed, M.M.S.; Chowdhury, M.A.; Mobarak, M.H.; Rahman, M.M.; Hossain, M.D.H. Advances and Significances of Titaniumin Dental Implant Applications. Results Chem. 2024, 7, 101394. [Google Scholar] [CrossRef]
- Liang, L.; Wu, X.; Yan, Q.; Shi, B. Are Short Implants (≤8.5 Mm) Reliable in the Rehabilitation of Completely Edentulous Patients: A Systematic Review and Meta-Analysis. J. Prosthet. Dent. 2024, 131, 826–832. [Google Scholar] [CrossRef]
- Strauss, F.J.; Gil, A.; Smirani, R.; Rodriguez, A.; Jung, R.; Thoma, D. The Use of Digital Technologies in Peri-Implant Soft Tissue Augmentation–A Narrative Review on Planning, Measurements, Monitoring and Aesthetics. Clin. Oral Implants Res. 2024, 35, 922–938. [Google Scholar] [CrossRef]
- Truong, T.-D.-N.; Pradhan, A.M.S.; Nguyen, T.-T.; Tran, M.-H.; Nguyen, C.-K.; Ho, D.-D.; Huynh, T.-C. Bone-Implant Osseointegration Monitoring Using Electro-Mechanical Impedance Technique and Convolutional Neural Network: A Numerical Study. J. Nondestruct. Eval. 2024, 43, 10. [Google Scholar] [CrossRef]
- Alemayehu, D.B.; Huang, S.-J.; Koricho, E.G. Experimental and FEM Analysis of Three Carbon Steel Characterization under Quasi-Static Strain Rate for Bumper Beam Application. In MATEC Web of Conferences, Proceedings of the 2nd International Conference on Precision Machinery and Manufacturing Technology (ICPMMT 2017), Kending, Taiwan, 19–21 May 2017; EDP Sciences: Les Ulis, France, 2017; Volume 123. [Google Scholar]
- Alemayehu, D.B.; Masahiro, T. Enhanced Energy Absorption with Bioinspired Composite Triply Periodic Minimal Surface Gyroid Lattices Fabricated via Fused Filament Fabrication (FFF). J. Manuf. Mater. Process. 2024, 8, 86. [Google Scholar] [CrossRef]
- Alemayehu, D.B.; Jeng, Y.R. Three-Dimensional Finite Element Investigation into Effects of Implant Thread Design and Loading Rate on Stress Distribution in Dental Implants and Anisotropic Bone. Materials 2021, 14, 6974. [Google Scholar] [CrossRef]
- Alemayehu, D.B.; Todoh, M.; Huang, S.J. Advancing 3D Dental Implant Finite Element Analysis: Incorporating Biomimetic Trabecular Bone with Varied Pore Sizes in Voronoi Lattices. J. Funct. Biomater. 2024, 15, 94. [Google Scholar] [CrossRef]
- Alemayehu, D.B. Design and Development of Shell and Tube Heat Exchanger for Harar Brewery Company Pasteurizer Application (Mechanical and Thermal Design). Am. J. Eng. Res. 2013, 2, 99–109. [Google Scholar]
- Alshoaibi, A.M.; Fageehi, Y.A. Simulation of Quasi-Static Crack Propagation by Adaptive Finite Element Method. Metals 2021, 11, 98. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Yuan, X.; Ren, M.; Chen, X.; Luo, L.; Zheng, L.; Liu, Y. Three-Dimensional Finite Element Analysis of Stress Distribution on Short Implants with Different Bone Conditions and Osseointegration Rates. BMC Oral Health 2023, 23, 220. [Google Scholar] [CrossRef] [PubMed]
- Hisam, M.J.; Lim, J.Y.; Kurniawan, D.; Nor, F.M. Stress Distribution Due to Loading on Premolar Teeth Implant: A Three Dimensional Finite Element Analysis. Procedia Manuf. 2015, 2, 218–223. [Google Scholar] [CrossRef][Green Version]
- Ye, Z.; Ye, H.; Wu, Y.; Jiang, Z.; Yao, H.; Xu, X.; Zhang, Y.; Du, W.; Li, W.; Zheng, Y. Effect of Bone Mass Density and Alveolar Bone Resorption on Stress in Implant Restoration of Free-End Edentulous Posterior Mandible: Finite Element Analysis of Double-Factor Sensitivity. Ann. Anat.-Anat. Anz. 2024, 253, 152210. [Google Scholar] [CrossRef]
- Kurniawan, D.; Nor, F.M.; Lee, H.Y.; Finite, J.Y.L. Finite Element Analysis of Bone—Implant Biomechanics: Refinement through Featuring Various Osseointegration Conditions. Int. J. Oral Maxillofac. Surg. 2012, 41, 1090–1096. [Google Scholar] [CrossRef]
- Williams, J.L. Anisotropic Elasticity of Cortical and Cancellous Bone in the Posterior Mandible Increases Peri-Implant Stress and Strain under Oblique Loading. Clin. Oral Implant. Res. 1995, 12, 648–657. [Google Scholar]
- Lee, C.C.; Lin, S.C.; Kang, M.J.; Wu, S.W.; Fu, P.Y. Effects of Implant Threads on the Contact Area and Stress Distribution of Marginal Bone. J. Dent. Sci. 2010, 5, 156–165. [Google Scholar] [CrossRef]
- Farré-Pagès, N.; Augé-Castro, M.L.; Alaejos-Algarra, F.; Mareque-Bueno, J.; Ferrés-Padró, E.; Hernández-Alfaro, F. Relation between Bone Density and Primary Implant Stability. Med. Oral Patol. Oral Cir. Bucal 2011, 16, e62–e67. [Google Scholar] [CrossRef]
- Diao, Q.; Zeng, Y.; Chen, J. The Applications and Latest Progress of Ceramic 3D Printing. Addit. Manuf. Front. 2024, 3, 200113. [Google Scholar] [CrossRef]
- Liu, X.; Pang, F.; Li, Y.; Jia, H.; Cui, X.; Yue, Y.; Yang, X.; Yang, Q. Effects of Different Positions and Angles of Implants in Maxillary Edentulous Jaw on Surrounding Bone Stress under Dynamic Loading: A Three-Dimensional Finite Element Analysis. Comput. Math. Methods Med. 2019, 2019, 8074096. [Google Scholar] [CrossRef]
- Salavati, H.; Pullens, P.; Ceelen, W.; Debbaut, C. The Effect of a Necrotic Core on the Interstitial Fluid Pressure in Solid Tumors. In Proceedings of the 17th International Symposium on Computer Methods in Biomechanics and Biomedical Engineering and 5th Conference on Imaging and Visualization, Bonn, Germany, 7–9 September 2021. [Google Scholar]
- Dechow, P.C.; Nail, G.A. Elastic Properties of Human Supraorbital and Mandibular Bone. Am. J. Phys. Anthropol. 1993, 90, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Raheem, A.; Dash, M.; Kumar, P.; Elsebahy, A.; Singh, H.; Manivasagam, G.; Nanda, H.S. Surface Engineering of Orthopedic Implants for Better Clinical Adoption. J. Mater. Chem. B 2024. [Google Scholar] [CrossRef]
- Antoniac, I.; Valeanu, N.; Niculescu, M.; Antoniac, A.; Robu, A.; Popescu, L.; Manescu, V.; Anusca, D.; Enachescu, C.I. Outcomes of Birmingham Hip Resurfacing Based on Clinical Aspects and Retrieval Analysis of Failed Prosthesis. Materials 2024, 17, 3965. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rath, B.; Tingart, M.; Eschweiler, J. Role of Implants Surface Modification in Osseointegration: A Systematic Review. J. Biomed. Mater. Res. Part A 2020, 108, 470–484. [Google Scholar] [CrossRef]
- Bjelić, D.; Finšgar, M. The Role of Growth Factors in Bioactive Coatings. Pharmaceutics 2021, 13, 1083. [Google Scholar] [CrossRef] [PubMed]
- Laubach, M.; Suresh, S.; Herath, B.; Wille, M.-L.; Delbrück, H.; Alabdulrahman, H.; Hutmacher, D.W.; Hildebrand, F. Clinical Translation of a Patient-Specific Scaffold-Guided Bone Regeneration Concept in Four Cases with Large Long Bone Defects. J. Orthop. Transl. 2022, 34, 73–84. [Google Scholar] [CrossRef] [PubMed]



| Bone Type | Cortical Bone Thickness | Cancellous Bone Density |
|---|---|---|
| III | 1 mm | High density |
| IV | 1 mm | Low density |
| Properties | High-Density Cancellous Bone | Low-Density Cancellous Bone | Cortical Bone | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25% | 50% | 75% | 100% | 25% | 50% | 75% | 100% | 25% | 50% | 75% | 100% | |
| Ex (MPa) | 287 | 574 | 861 | 1148 | 57.5 | 115 | 172.5 | 230 | 3150 | 6300 | 9450 | 12,600 |
| Ey (MPa) | 52.5 | 105 | 157.5 | 210 | 10.5 | 21 | 31.5 | 42 | 3150 | 6300 | 9450 | 12,600 |
| Ez (MPa) | 287 | 574 | 861 | 1148 | 57.5 | 115 | 172.5 | 230 | 4850 | 9700 | 14,550 | 19,400 |
| vxy | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.3 | 0.3 | 0.3 | 0.3 |
| vxz | 0.32 | 0.32 | 0.32 | 0.32 | 0.32 | 0.32 | 0.32 | 0.32 | 0.253 | 0.253 | 0.253 | 0.253 |
| vyz | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.253 | 0.253 | 0.253 | 0.253 |
| Gxy (MPa) | 17 | 34 | 51 | 68 | 3.5 | 7 | 10.5 | 14 | 1212.5 | 2425 | 3637.5 | 4850 |
| Gxz (MPa) | 108.5 | 217 | 325.5 | 434 | 21.75 | 43.5 | 65.2 | 87 | 1425 | 2850 | 4275 | 5700 |
| Gyz (MPa) | 17 | 34 | 51 | 68 | 3.5 | 7 | 10.5 | 14 | 1425 | 2850 | 4275 | 5700 |
| Model | Brand | Dimensions (Diameter × Length) | Description |
|---|---|---|---|
| Model I | Biomet 3iT3 Short | 5 mm (D) × 4 mm (L) | Biomet 3iT3 implant from Zimmer Biomet Dental, USA |
| Model II | SRN-Straumann® SPS | 4.1 mm (D) × 4 mm (L) | Standard Regular Neck Straumann Standard Plus Short implant from Holding AG, Switzerland |
| Model III | SWN-Straumann® SPS | 4.8 mm (D) × 4 mm (L) | Standard Wide Neck Straumann Standard Plus Short implant from Holding AG, Switzerland |
| Implant Model | Implant | Cancellous Bone | Cortical Bone | Assembly | ||||
|---|---|---|---|---|---|---|---|---|
| No. of Nodes | No. of Elements | No. of Nodes | No. of Elements | No. of Nodes | No. of Elements | No. of Nodes | No. of Elements | |
| BioMet 3iT3 | 73,108 | 391,448 | 47,751 | 244,083 | 5594 | 21,672 | 126,453 | 657,203 |
| SPS-RN | 67,363 | 363,525 | 30,876 | 149,142 | 4974 | 19,015 | 103,213 | 531,682 |
| SPS-WN | 88,074 | 478,307 | 49,333 | 249,052 | 7667 | 30,075 | 145,074 | 757,434 |
| Bone Type | Osseointegeration (%) | Highest Stress | Highest Strain | ||
|---|---|---|---|---|---|
| Cortical (MPa) | Cancellous (MPa) | Cortical | Cancellous | ||
| III | 25 | 158.4 | 19.23 | 0.02559 | 0.1060 |
| 50 | 166.4 | 18.48 | 0.0139 | 0.05091 | |
| 75 | 174.3 | 17.79 | 0.009997 | 0.03264 | |
| 100 | 182.1 | 17.15 | 0.008047 | 0.02358 | |
| IV | 25 | 303.0 | 18.62 | 0.04905 | 0.4088 |
| 50 | 309.9 | 18.44 | 0.02379 | 0.2024 | |
| 75 | 316.2 | 18.36 | 0.01552 | 0.1387 | |
| 100 | 323.8 | 18.10 | 0.01189 | 0.09931 | |
| Bone Type | Osseointegration (%) | Highest Stress | Highest Strain | ||
|---|---|---|---|---|---|
| Cortical (MPa) | Cancellous (MPa) | Cortical | Cancellous | ||
| III | 25 | 137.0 | 38.48 | 0.04126 | 0.1099 |
| 50 | 134.7 | 36.6 | 0.01988 | 0.05293 | |
| 75 | 134.1 | 34.88 | 0.01280 | 0.03402 | |
| 100 | 136.6 | 33.32 | 0.009295 | 0.02463 | |
| IV | 25 | 330.1 | 26.12 | 0.08977 | 0.4199 |
| 50 | 319.1 | 25.91 | 0.04436 | 0.2084 | |
| 75 | 311.5 | 25.53 | 0.02967 | 0.1393 | |
| 100 | 300.1 | 25.45 | 0.02171 | 0.1027 | |
| Bone Type | Osseointegration (%) | Highest Stress | Highest Strain | ||
|---|---|---|---|---|---|
| Cortical (MPa) | Cancellous (MPa) | Cortical | Cancellous | ||
| III | 25 | 135.5 | 14.78 | 0.04750 | 0.06905 |
| 50 | 127.1 | 14.27 | 0.02200 | 0.03396 | |
| 75 | 120.2 | 13.79 | 0.01368 | 0.02228 | |
| 100 | 114.3 | 13.34 | 0.009631 | 0.01645 | |
| IV | 25 | 399.1 | 15.73 | 0.09706 | 0.2803 |
| 50 | 369.0 | 15.72 | 0.04523 | 0.1398 | |
| 75 | 344.0 | 15.70 | 0.02831 | 0.09292 | |
| 100 | 322.7 | 15.68 | 0.02004 | 0.06946 | |
| Implant Model | Osseointegeration (%) | Cancellous Bone | Cortical Bone | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IV | III | IV | III | ||||||||||
| Sxy (MPa) | Sxz (MPa) | Syz (MPa) | Sxy (MPa) | Sxz (MPa) | Syz (MPa) | Sxy (MPa) | Sxz (MPa) | Syz (MPa) | Sxy (MPa) | Sxz (MPa) | Syz (MPa) | ||
| BioMet 3iT3 | 25 | 0.9718 | 4.056 | 0.4626 | 1.620 | 6.760 | 0.7709 | 97.53 | 55.37 | 61.19 | 58.52 | 33.22 | 36.71 |
| 50 | 1.606 | 6.695 | 0.7667 | 1.806 | 7.309 | 1.190 | 99.33 | 57.82 | 64.44 | 55.93 | 27.74 | 25.22 | |
| 75 | 1.620 | 6.776 | 0.8093 | 1.755 | 7.012 | 1.161 | 112.9 | 57.79 | 43.43 | 58.22 | 32.19 | 26.80 | |
| 100 | 1.581 | 6.567 | 0.7586 | 1.706 | 6.737 | 1.134 | 102.8 | 67.93 | 71.01 | 60.43 | 36.15 | 28.38 | |
| SPS-RN | 25 | 1.552 | 4.733 | 0.9097 | 1.744 | 8.276 | 1.409 | 78.34 | 95.35 | 74.07 | 37.96 | 50.72 | 34.14 |
| 50 | 1.546 | 4.684 | 0.9037 | 1.715 | 7.634 | 1.351 | 77.27 | 95.44 | 73.78 | 39.20 | 50.53 | 35.09 | |
| 75 | 1.555 | 4.488 | 0.9003 | 1.686 | 7.172 | 1.299 | 77.20 | 96.29 | 74.55 | 40.32 | 50.31 | 36.55 | |
| 100 | 1.533 | 4.516 | 0.8918 | 1.658 | 7.312 | 1.252 | 75.28 | 95.37 | 75.97 | 41.36 | 50.06 | 37.89 | |
| SPS-WN | 25 | 1.105 | 5.034 | 0.6854 | 1.266 | 6.566 | 1.039 | 62.77 | 106.9 | 37.46 | 34.44 | 60.75 | 18.85 |
| 50 | 1.102 | 5.010 | 0.6851 | 1.242 | 6.306 | 1.014 | 61.05 | 93.30 | 37.13 | 32.98 | 57.03 | 18.83 | |
| 75 | 1.099 | 4.984 | 0.6847 | 1.219 | 6.064 | 0.9903 | 59.47 | 90.01 | 36.80 | 31.63 | 54.30 | 18.81 | |
| 100 | 1.095 | 4.961 | 0.6839 | 1.197 | 6.059 | 0.9675 | 57.96 | 59.41 | 36.47 | 30.38 | 51.90 | 18.78 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alemayehu, D.B.; Todoh, M.; Huang, S.-J. Nonlinear Finite Element Analysis of Bone–Implant Contact in Three Short Dental Implant Models with Varying Osseointegration Percentages. Oral 2024, 4, 505-524. https://doi.org/10.3390/oral4040040
Alemayehu DB, Todoh M, Huang S-J. Nonlinear Finite Element Analysis of Bone–Implant Contact in Three Short Dental Implant Models with Varying Osseointegration Percentages. Oral. 2024; 4(4):505-524. https://doi.org/10.3390/oral4040040
Chicago/Turabian StyleAlemayehu, Dawit Bogale, Masahiro Todoh, and Song-Jeng Huang. 2024. "Nonlinear Finite Element Analysis of Bone–Implant Contact in Three Short Dental Implant Models with Varying Osseointegration Percentages" Oral 4, no. 4: 505-524. https://doi.org/10.3390/oral4040040
APA StyleAlemayehu, D. B., Todoh, M., & Huang, S.-J. (2024). Nonlinear Finite Element Analysis of Bone–Implant Contact in Three Short Dental Implant Models with Varying Osseointegration Percentages. Oral, 4(4), 505-524. https://doi.org/10.3390/oral4040040







