Diagnostic and Prognostic Predictors for the Success of Pulpotomy in Permanent Mature Posterior Teeth with Moderate to Severe Pulpitis: A Scoping Review
Abstract
:1. Introduction
1.1. Challenges Associated with the Diagnosis of Irreversible Pulpitis
1.2. The Need for Updated Definitions of Pulpitis
1.3. The Shift towards Vital Pulp Therapy
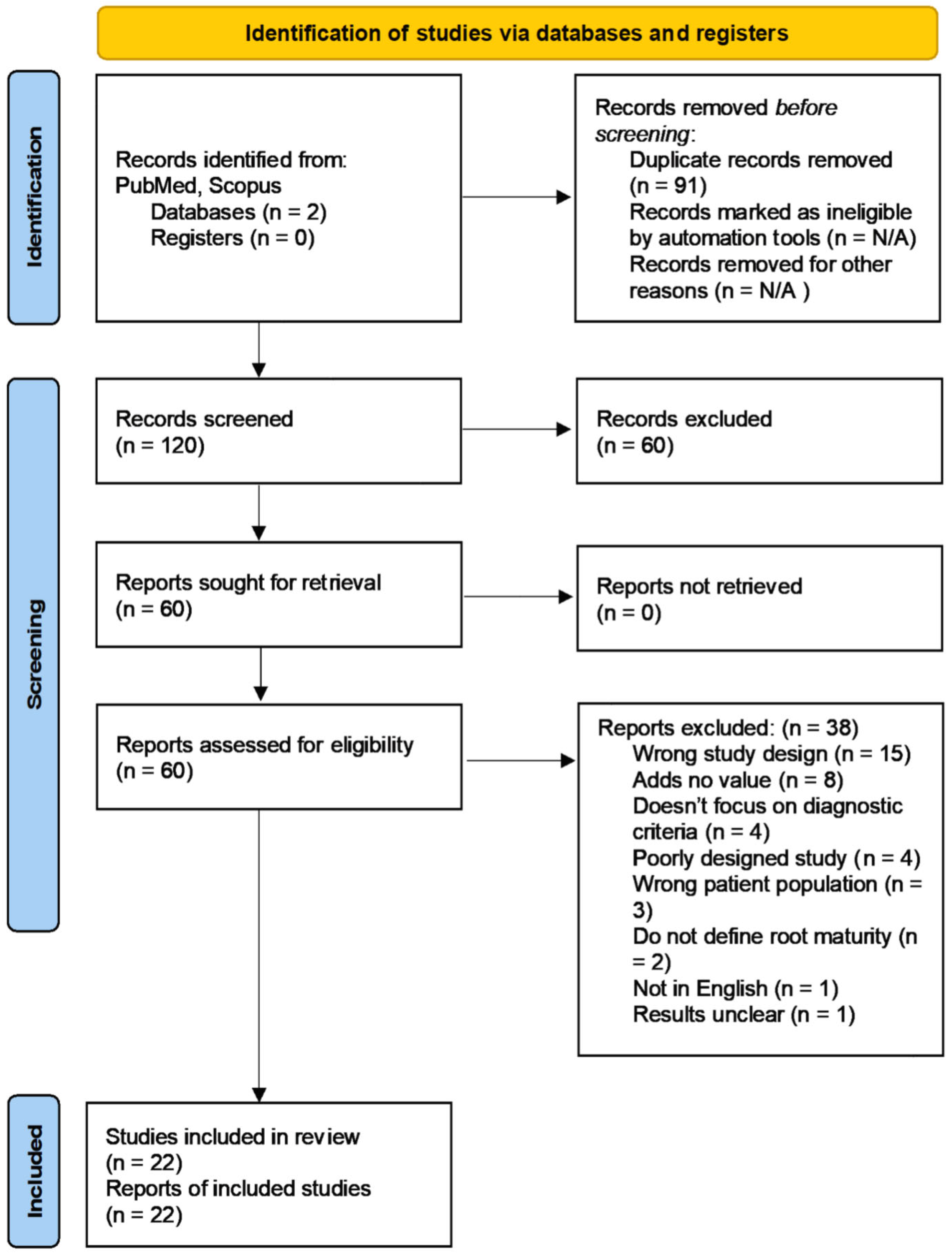
2. Materials and Methods
- “Irreversible pulpitis” OR “moderate pulpitis” OR “moderate inflammat*” OR “severe pulpitis” OR “severe inflammat*” OR “acute pulpitis” OR “pulpal inflammat*”
- AND
- “Vital pulp therapy” OR VPT OR Pulpotomy OR “Vital pulp treatment”
- AND
- (((Permanent OR adult) AND (teeth OR tooth OR molar* OR dentition OR premolar*)) OR “Dentition, Permanent”[Mesh])
- AND
- Success OR Retention OR Vitality OR Outcome OR Pain OR Survival
3. Results
3.1. Diagnostic Factors Examined
3.1.1. Presenting Signs and Symptoms
3.1.2. Periapical Diagnosis
3.1.3. Inflammation; Bleeding Time and Biomarkers
3.2. Prognostic Factors Examined
3.2.1. Patient Age
3.2.2. Medical Status
3.2.3. Caries Depth, Activity, and Location
3.2.4. Restorative Factors
4. Discussion
4.1. Outcomes and Success Criteria
4.2. Partial versus Complete Pulpotomy
4.3. Age and Medical Status as Prognostic Factors
4.4. Presenting Signs and Symptoms as Diagnostic Factors
4.5. Does the Apical Status Affect Prognosis?
4.6. Depth of Caries as a Prognostic Factor
4.7. Inflammation: Degree of Bleeding, Bleeding Time, and Concentration of Biomarkers
4.8. The Importance of a Definitive Restoration
4.9. Pulp Capping Material
4.10. Potential Risks and Complications Associated with Pulpotomies
5. Conclusions
- Future studies should focus on assessing how different medical conditions or medications may affect the outcome of VPT, particularly as medically compromised patients may be more likely to benefit from such a treatment option;
- From the studies analyzed in this scoping review, it is evident that bleeding time is likely not a predictor of success or failure of pulpotomy [15,18,35,46,51]. Historically, the bleeding time has been perceived to be a predictor of the extent of inflammation, with longer bleeding times indicating an irreversibly inflamed pulp that would not remain vital [35]. However, the authors recognize that a practical limit for the clinician may be adopted;
- Biomarkers are a potentially promising quantitative method for identifying the extent of pulpal inflammation, and therefore guiding treatment decisions [48,95]. Future studies should consider enrolling high numbers of participants to identify a biomarker with high specificity and sensitivity for pulpotomy outcomes.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, J.; Zhang, T.; Zhao, D.; Haapasalo, M.; Shen, Y. Characteristics of endodontic emergencies during coronavirus disease 2019 outbreak in Wuhan. J. Endod. 2020, 46, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Currie, C.C.; Stone, S.J.; Durham, J. Pain and problems: A prospective cross-sectional study of the impact of dental emergencies. J. Oral Rehabil. 2015, 42, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.; Rasaiah, S.; Hamzah Ahmed, S.; Breckons, M.; Stone, S.J.; Currie, C.C.; Durham, J.; Whitworth, J. The financial and quality of life impact of urgent dental presentations: A cross-sectional study. Int. Endod. J. 2023, 56, 697–709. [Google Scholar] [CrossRef] [PubMed]
- McDougal, R.A.; Delano, E.O.; Caplan, D.; Sigurdsson, A.; Trope, M. Success of an alternative for interim management of irreversible pulpitis. J. Am. Dent. Assoc. 2004, 135, 1707–1712. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Eghbal, M.J.; Fazlyab, M.; Baghban, A.A.; Ghoddusi, J. Five-year results of vital pulp therapy in permanent molars with irreversible pulpitis: A non-inferiority multicenter randomized clinical trial. Clin. Oral Investig. 2015, 19, 335–341. [Google Scholar] [CrossRef]
- Murdoch-Kinch, C.A.; McLean, M.E. Minimally invasive dentistry. J. Am. Dent. Assoc. 2003, 134, 87–95. [Google Scholar] [CrossRef]
- Santos, J.M.; Pereira, J.F.; Marques, A.; Sequeira, D.B.; Friedman, S. Vital pulp therapy in permanent mature posterior teeth with symptomatic irreversible pulpitis: A systematic review of treatment outcomes. Medicina 2021, 57, 573. [Google Scholar] [CrossRef]
- American Association of Endodontists. AAE Position Statement on Vital Pulp Therapy. Available online: https://f3f142zs0k2w1kg84k5p9i1o-wpengine.netdna-ssl.com/wp-content/uploads/2021/05/VitalPulpTherapyPositionStatement_v2.pdf (accessed on 4 May 2023).
- American Association of Endodontists. Glossary of Endodontic Terms. Available online: https://www.aae.org/specialty/clinical-resources/glossary-endodontic-terms/ (accessed on 5 May 2022).
- Langeland, K. Tissue response to dental caries. Endod. Dent. Traumatol. 1987, 3, 149–171. [Google Scholar] [CrossRef]
- Tomson, P.L.; Vilela Bastos, J.; Jacimovic, J.; Jakovljevic, A.; Pulikkotil, S.J.; Nagendrababu, V. Effectiveness of pulpotomy compared with root canal treatment in managing non-traumatic pulpitis associated with spontaneous pain: A systematic review and meta-analysis. Int. Endod. J. 2022, 56, 355–369. [Google Scholar] [CrossRef]
- Wolters, W.J.; Duncan, H.F.; Tomson, P.L.; Karim, I.E.; McKenna, G.; Dorri, M.; Stangvaltaite, L.; van der Sluis, L.W.M. Minimally invasive endodontics: A new diagnostic system for assessing pulpitis and subsequent treatment needs. Int. Endod. J. 2017, 50, 825–829. [Google Scholar] [CrossRef]
- Mejàre, I.; Cvek, M. Partial pulpotomy in young permanent teeth with deep carious lesions. Endod. Dent. Traumatol. 1993, 9, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Cvek, M. A clinical report on partial pulpotomy and capping with calcium hydroxide in permanent incisors with complicated crown fracture. J. Endod. 1978, 4, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Careddu, R.; Duncan, H.F. A prospective clinical study investigating the effectiveness of partial pulpotomy after relating preoperative symptoms to a new and established classification of pulpitis. Int. Endod. J. 2021, 54, 2156–2172. [Google Scholar] [CrossRef]
- Ather, A.; Patel, B.; Gelfond, J.A.L.; Ruparel, N.B. Outcome of pulpotomy in permanent teeth with irreversible pulpitis: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 19664. [Google Scholar] [CrossRef]
- American Association of Endodontists. Endodontic Diagnosis. Available online: https://www.aae.org/specialty/wp-content/uploads/sites/2/2017/07/endodonticdiagnosisfall2013.pdf (accessed on 5 May 2022).
- Uesrichai, N.; Nirunsittirat, A.; Chuveera, P.; Srisuwan, T.; Sastraruji, T.; Chompu-Inwai, P. Partial pulpotomy with two bioactive cements in permanent teeth of 6- to 18-year-old patients with signs and symptoms indicative of irreversible pulpitis: A noninferiority randomized controlled trial. Int. Endod. J. 2019, 52, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Eghbal, M.J.; Shahravan, A.; Saberi, E.; Baghban, A.A.; Parhizkar, A. Outcomes of root canal therapy or full pulpotomy using two endodontic biomaterials in mature permanent teeth: A randomized controlled trial. Clin. Oral Investig. 2022, 26, 3287–3297. [Google Scholar] [CrossRef] [PubMed]
- Taha, N.A.; Abuzaid, A.M.; Khader, Y.S. A randomized controlled clinical trial of pulpotomy versus root canal therapy in mature teeth with irreversible pulpitis: Outcome, quality of life, and patients’ satisfaction. J. Endod. 2023, 49, 624–631.e622. [Google Scholar] [CrossRef]
- Seltzer, S.; Bender, I.B.; Ziontz, M. The dynamics of pulp inflammation: Correlations between diagnostic data and actual histologic findsings in the pulp. Oral Surg. Oral Med. Oral Pathol. 1963, 16, 846–977. [Google Scholar] [CrossRef]
- Mejàre, I.A.; Axelsson, S.; Davidson, T.; Frisk, F.; Hakeberg, M.; Kvist, T.; Norlund, A.; Petersson, A.; Portenier, I.; Sandberg, H.; et al. Diagnosis of the condition of the dental pulp: A systematic review. Int. Endod. J. 2012, 45, 597–613. [Google Scholar] [CrossRef]
- Ricucci, D.; Loghin, S.; Siqueira, J.F. Correlation between clinical and histologic pulp diagnoses. J. Endod. 2014, 40, 1932–1939. [Google Scholar] [CrossRef]
- Yamasaki, M.; Kumazawa, M.; Kohsaka, T.; Nakamura, H.; Kameyama, Y. Pulpal and periapical tissue reactions after experimental pulpal exposure in rats. J. Endod. 1994, 20, 13–17. [Google Scholar] [CrossRef]
- Bjørndal, L.; Ricucci, D. Pulp inflammation: From the reversible pulpitis to pulp necrosis during caries progression. In The Dental Pulp: Biology, Pathology, and Regenerative Therapies; Goldberg, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 125–139. ISBN 978-3-642-55160-4. [Google Scholar]
- Van Hassel, H.J. Physiology of the human dental pulp. Oral Surg. Oral Med. Oral Pathol. 1971, 32, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Heyeraas, K.J.; Berggreen, E. Interstitial fluid pressure in normal and inflamed pulp. Crit. Rev. Oral Biol. Med. 1999, 10, 328–336. [Google Scholar] [CrossRef]
- Duncan, H.F.; Galler, K.M.; Tomson, P.L.; Simon, S.; El-Karim, I.; Kundzina, R.; Krastl, G.; Dammaschke, T.; Fransson, H.; Markvart, M.; et al. European society of endodontology position statement: Management of deep caries and the exposed pulp. Int. Endod. J. 2019, 52, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Eghbal, M.J. Treatment outcomes of pulpotomy in permanent molars with irreversible pulpitis using biomaterials: A multi-center randomized controlled trial. Acta Odontol. Scand. 2013, 71, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Duncan, H.F.; Kirkevang, L.L.; Peters, O.A.; El-Karim, I.; Krastl, G.; Del Fabbro, M.; Chong, B.S.; Galler, K.M.; Segura-Egea, J.J.; Kebschull, M.; et al. Treatment of pulpal and apical disease: The european society of endodontology (ESE) S3-level clinical practice guideline. Int. Endod. J. 2023, 56, 238–295. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.L.; Mann, V.; Rahbaran, S.; Lewsey, J.; Gulabivala, K. Outcome of primary root canal treatment: Systematic review of the literature—Part 2. influence of clinical factors. Int. Endod. J. 2008, 41, 6–31. [Google Scholar] [CrossRef]
- Sjögren, U.; Hägglund, B.; Sundqvist, G.; Wing, K. Factors affecting the long-term results of endodontic treatment. J. Endod. 1990, 16, 498–504. [Google Scholar] [CrossRef]
- Ricucci, D.; Russo, J.; Rutberg, M.; Burleson, J.A.; Spångberg, L.S. A prospective cohort study of endodontic treatments of 1369 root canals: Results after 5 years. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, 825–842. [Google Scholar] [CrossRef]
- Zhang, M.; Xiong, Y.; Wang, X.; Wang, Y.; Cai, Y.; Xu, J.; Zhang, C.; Li, J. Factors affecting the outcome of full pulpotomy in permanent posterior teeth diagnosed with reversible or irreversible pulpitis. Sci. Rep. 2022, 12, 20280. [Google Scholar] [CrossRef]
- Aldeen, R.Z.; Aljabban, O.; Almanadili, A.; Alkurdi, S.; Eid, A.; Mancino, D.; Haikel, Y.; Kharouf, N. The influence of carious lesion and bleeding time on the success of partial pulpotomy in permanent molars with irreversible pulpitis: A prospective study. Bioengineering 2023, 10, 700. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Lara y Tajonar, R.G.; Vergara-Tinoco, J.V.; Dammaschke, T.; Domínguez-Pérez, R.A. A pilot feasibility study to establish full pulpotomy in mature permanent teeth with symptomatic irreversible pulpitis as a routine treatment in mexican public healthcare services. Healthcare 2022, 10, 2350. [Google Scholar] [CrossRef]
- Galani, M.; Tewari, S.; Sangwan, P.; Mittal, S.; Kumar, V.; Duhan, J. Comparative evaluation of postoperative pain and success rate after pulpotomy and root canal treatment in cariously exposed mature permanent molars: A randomized controlled trial. J. Endod. 2017, 43, 1953–1962. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, P.A. Clinical strategies for managing endodontic pain. Endod. Top. 2002, 3, 78–92. [Google Scholar] [CrossRef]
- Philip, N.; Suneja, B. Minimally invasive endodontics: A new era for pulpotomy in mature permanent teeth. Br. Dent. J. 2022, 233, 1035–1041. [Google Scholar] [CrossRef]
- Lin, L.M.; Ricucci, D.; Saoud, T.M.; Sigurdsson, A.; Kahler, B. Vital pulp therapy of mature permanent teeth with irreversible pulpitis from the perspective of pulp biology. Aust. Endod. J. 2020, 46, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Beauquis, J.; Setbon, H.M.; Dassargues, C.; Carsin, P.; Aryanpour, S.; Van Nieuwenhuysen, J.-P.; Leprince, J.G. Short-term pain evolution and treatment succes of pulpotomy as irreversible pulpitis permanent treatment: A non-randomized clinical study. J. Clin. Med. 2022, 11, 787. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, J.F.; Norooz, S.; Callahan, D.; Mohajeri, A. Survey of vital pulp therapy treatment in permanent dentition being taught at U.S. dental schools. J. Endod. 2022, 48, 1107–1112. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Asgary, S.; Eghbal, M.J. The effect of pulpotomy using a calcium-enriched mixture cement versus one-visit root canal therapy on postoperative pain relief in irreversible pulpitis: A randomized clinical trial. Odontology 2010, 98, 126–133. [Google Scholar] [CrossRef]
- Airsang, A.J.; Shankaregowda, A.M.; Meena, N.; Lingaiah, U.; Lakshminarasimhaiah, V.; Harti, S. Comparative assessment of complete pulpotomy in mature permanent teeth with carious exposure using calcium silicate cement: A randomized clinical trial. World J. Dent. 2022, 13, S135–S143. [Google Scholar] [CrossRef]
- Elmas, S.; Kotan, D.A.; Odabaş, M.E. Two-year outcomes of coronal pulpotomy in young permanent molars with clinical signs indicative of irreversible pulpitis. Pediatr. Dent. 2023, 45, 46–52. [Google Scholar] [PubMed]
- Ramani, A.; Sangwan, P.; Tewari, S.; Duhan, J.; Mittal, S.; Kumar, V. Comparative evaluation of complete and partial pulpotomy in mature permanent teeth with symptomatic irreversible pulpitis: A randomized clinical trial. Int. Endod. J. 2022, 55, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kumar, V.; Logani, A.; Chawla, A.; Mir, R.A.; Sharma, S.; Kalaivani, M. Association between concentration of active MMP-9 in pulpal blood and pulpotomy outcome in permanent mature teeth with irreversible pulpitis—A preliminary study. Int. Endod. J. 2021, 54, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Taha, N.A.; Abdelkhader, S.Z. Outcome of full pulpotomy using biodentine in adult patients with symptoms indicative of irreversible pulpitis. Int. Endod. J. 2018, 51, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Aravind, A.; Kumar, V.; Sharma, S.; Chawla, A.; Logani, A. Influence of occlusal and proximal caries on the outcome of full pulpotomy in permanent mandibular molar teeth with partial irreversible pulpitis: A prospective study. Int. Endod. J. 2021, 54, 1699–1707. [Google Scholar] [CrossRef]
- Anta, S.; Diouma, N.; Ousmane, N.S.; Fatou, L.B.; Florence, F.; Babacar, T. Evaluation of complete pulpotomy with biodentine on mature permanent molars with signs and symptoms of symptomatic irreversible pulpitis: 12-months follow-up. J. Endod. 2022, 48, 312–319. [Google Scholar] [CrossRef]
- Jassal, A.; Nawal, R.R.; Yadav, S.; Talwar, S.; Yadav, S.; Duncan, H.F. Outcome of partial and full pulpotomy in cariously exposed mature molars with symptoms indicative of irreversible pulpitis: A randomized controlled trial. Int. Endod. J. 2022, 56, 331–344. [Google Scholar] [CrossRef]
- Baranwal, H.; Mittal, N.; Yadav, J.; Rani, P.; Kumar, N. Outcome of partial pulpotomy verses full pulpotomy using biodentine in vital mature permanent molar with clinical symptoms indicative of irreversible pulpitis: A randomized clinical trial. J. Conserv. Dent. 2022, 25, 317–323. [Google Scholar]
- Taha, N.A.; Abdulkhader, S.Z. Full pulpotomy with biodentine in symptomatic young permanent teeth with carious exposure. J. Endod. 2018, 44, 932–937. [Google Scholar] [CrossRef]
- Hussain, M.I.; Bashar, A.M. Outcome of mineral trioxide aggregate pulpotomy for mature permanent molars with symptoms indicative of irreversible pulpitis. Mymensingh Med. J. 2022, 31, 223–229. [Google Scholar] [CrossRef]
- Kumar, V.; Juneja, R.; Duhan, J.; Sangwan, P.; Tewari, S. Comparative evaluation of platelet-rich fibrin, mineral trioxide aggregate, and calcium hydroxide as pulpotomy agents in permanent molars with irreversible pulpitis: A randomized controlled trial. Contemp. Clin. Dent. 2016, 7, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Ehsani, S. Permanent molar pulpotomy with a new endodontic cement: A case series. J. Conserv. Dent. 2009, 12, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Taha, N.A.; Khazali, M.A. Partial pulpotomy in mature permanent teeth with clinical signs indicative of irreversible pulpitis: A randomized clinical trial. J. Endod. 2017, 43, 1417–1421. [Google Scholar] [CrossRef]
- Ørstavik, D.; Kerekes, K.; Eriksen, H.M. The periapical index: A scoring system for radiographic assessment of apical periodontitis. Dent. Traumatol. 1986, 2, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Kirkevang, L.L.; Vaeth, M.; Wenzel, A. Ten-year follow-up of root filled teeth: A radiographic study of a Danish population. Int. Endod. J. 2014, 47, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Frisk, F.; Hugosson, A.; Kvist, T. Is apical periodontitis in root filled teeth associated with the type of restoration? Acta Odontol. Scand. 2015, 73, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Strindberg, L.Z. The dependence of the results of pulp therapy on certain factors; an analytic study based on radiographic and clinical follow-up examinations. Acta Odontol. Scand. 1956, 14, 1–175. [Google Scholar]
- Duncan, H.F.; Nagendrababu, V.; El-Karim, I.; Dummer, P.M.H. Outcome measures to assess the effectiveness of endodontic treatment for pulpitis and apical periodontitis for use in the development of european society of endodontology S3-level clinical practice guidelines: A consensus-based development. Int. Endod. J. 2021, 54, 2184–2194. [Google Scholar] [CrossRef]
- Tan, H.; Peres, K.; Peres, M. Retention of teeth and oral health–related quality of life. J. Dent. Res. 2016, 95, 1350–1357. [Google Scholar] [CrossRef]
- Donnermeyer, D.; Dammaschke, T.; Lipski, M.; Schäfer, E. Effectiveness of diagnosing pulpitis: A systematic review. Int. Endod. J. 2022, 56, 296–325. [Google Scholar] [CrossRef] [PubMed]
- Cushley, S.; Duncan, H.F.; Lappin, M.J.; Tomson, P.L.; Lundy, F.T.; Cooper, P.; Clarke, M.; El Karim, I.A. Pulpotomy for mature carious teeth with symptoms of irreversible pulpitis: A systematic review. J. Dent. 2019, 88, 103158. [Google Scholar] [CrossRef] [PubMed]
- Taha, N.A.; Al-khatib, H. 4-year follow-up of full pulpotomy in symptomatic mature permanent teeth with carious pulp exposure using a stainproof calcium silicate–based material. J. Endod. 2022, 48, 87–95. [Google Scholar] [CrossRef] [PubMed]
- George, R. Is partial pulpotomy in cariously exposed posterior permanent teeth a viable treatment option? Evid. Based Dent. 2020, 21, 112–113. [Google Scholar] [CrossRef] [PubMed]
- Duncan, H.F. Present status and future directions—Vital pulp treatment and pulp preservation strategies. Int. Endod. J. 2022, 55, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.Z.; Daud, S.; Nambiar, P.; Razak, F.A.; Ab-Murat, N.; Saub, R.; Bakri, M.M. Correlation between numbers of cells in human dental pulp and age: Implications for age estimation. Arch. Oral Biol. 2017, 80, 51–55. [Google Scholar] [CrossRef]
- Morse, D.R. Age-related changes of the dental pulp complex and their relationship to systemic aging. Oral Surg. Oral Med. Oral Pathol. 1991, 72, 721–745. [Google Scholar] [CrossRef]
- Elmsmari, F.; Ruiz, X.-F.; Miró, Q.; Feijoo-Pato, N.; Durán-Sindreu, F.; Olivieri, J.G. Outcome of partial pulpotomy in cariously exposed posterior permanent teeth: A systematic review and meta-analysis. J. Endod. 2019, 45, 1296–1306.e1293. [Google Scholar] [CrossRef]
- Linsuwanont, P.P.; Wimonsutthikul, K.D.D.S.; Pothimoke, U.D.D.S.; Santiwong, B.P. Treatment outcomes of mineral trioxide aggregate pulpotomy in vital permanent teeth with carious pulp exposure: The retrospective study. J. Endod. 2016, 43, 225–230. [Google Scholar] [CrossRef]
- Asgary, S.; Eghbal, M.J.; Ghoddusi, J. Two-year results of vital pulp therapy in permanent molars with irreversible pulpitis: An ongoing multicenter randomized clinical trial. Clin. Oral Investig. 2014, 18, 635–641. [Google Scholar] [CrossRef]
- Hussain, M.I.; Bashar, A.K.M.; Zakir Hossain Shikder, A.H.M. Alternative management of acute irreversible pulpitis of an adult HIV-positive patient: A case report. Bangladesh Med. Res. Counc. Bull. 2021, 47, 230–234. [Google Scholar] [CrossRef]
- Stashenko, P.; Wang, C.Y.; Riley, E.; Wu, Y.; Ostroff, G.; Niederman, R. Reduction of infection-stimulated periapical bone resorption by the biological response modifier PGG glucan. J. Dent. Res. 1995, 74, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Langeland, K. Pulp histology and physiology. In Pathways of the Pulp; Cohen, S., Burns, R., Eds.; CV Mosby: St Louis, MO, USA, 1976; pp. 203–290. [Google Scholar]
- Kanagasingam, S.; Lim, C.X.; Yong, C.P.; Mannocci, F.; Patel, S. Diagnostic accuracy of periapical radiography and cone beam computed tomography in detecting apical periodontitis using histopathological findings as a reference standard. Int. Endod. J. 2017, 50, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Hashem, D.; Mannocci, F.; Patel, S.; Manoharan, A.; Brown, J.E.; Watson, T.F.; Banerjee, A. Clinical and radiographic assessment of the efficacy of calcium silicate indirect pulp capping: A randomized controlled clinical trial. J. Dent. Res. 2015, 94, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Demant, S.; Dabelsteen, S.; Bjørndal, L. A macroscopic and histological analysis of radiographically well-defined deep and extremely deep carious lesions: Carious lesion characteristics as indicators of the level of bacterial penetration and pulp response. Int. Endod. J. 2021, 54, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Ricucci, D.; Siqueira, J.F.; Li, Y.; Tay, F.R. Vital pulp therapy: Histopathology and histobacteriology-based guidelines to treat teeth with deep caries and pulp exposure. J. Dent. 2019, 86, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Sundqvist, G.; Figdor, D. Life as an endodontic pathogen. Endod. Top. 2003, 6, 3–28. [Google Scholar] [CrossRef]
- Matsuo, T.; Nakanishi, T.; Shimizu, H.; Ebisu, S. A clinical study of direct pulp capping applied to carious-exposed pulps. J. Endod. 1996, 22, 551–556. [Google Scholar] [CrossRef]
- Waterhouse, P.J.; Nunn, J.H.; Whitworth, J.M. Prostaglandin E2 and treatment outcome in pulp therapy of primary molars with carious exposures. Int. J. Paediatr. Dent. 2002, 12, 116–123. [Google Scholar] [CrossRef]
- Leong, D.J.X.; Yap, A.U. Vital pulp therapy in carious pulp–exposed permanent teeth: An umbrella review. Clin. Oral Investig. 2021, 25, 6743–6756. [Google Scholar] [CrossRef]
- Aguilar, P.M.D.D.S.; Linsuwanont, P.D.D.S.M.P. Vital pulp therapy in vital permanent teeth with cariously exposed pulp: A systematic review. J. Endod. 2011, 37, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Hafez, A.A.; Cox, C.F.; Tarim, B.; Otsuki, M.; Akimoto, N. An in vivo evaluation of hemorrhage control using sodium hypochlorite and direct capping with a one- or two-component adhesive system in exposed nonhuman primate pulps. Quintessence Int. 2002, 33, 261–272. [Google Scholar] [PubMed]
- Vostatek, S.F.; Kanellis, M.J.; Weber-Gasparoni, K.; Gregorsok, R.L. Sodium hypochlorite pulpotomies in primary teeth: A retrospective assessment. Pediatr. Dent. 2011, 33, 327–332. [Google Scholar] [PubMed]
- Ballal, N.; Duncan, H.F.; Wiedemeier, D.B.; Rai, N.; Jalan, P.; Bhat, V.; Belle, V.S.; Zehnder, M. MMP-9 levels and NaOCl lavage in randomized trial on direct pulp capping. J. Dent. Res. 2021, 101, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Özgür, B.; Uysal, S.; Güngör, H.C. Partial pulpotomy in immature permanent molars after carious exposures using different hemorrhage control and capping materials. Pediatr. Dent. 2017, 39, 364–370. [Google Scholar] [PubMed]
- Tunç, E.Ş.; Şaroğlu, I.; Sarı, Ş.; Günhan, Ö. The effect of sodium hypochlorite application on the success of calcium hydroxide pulpotomy in primary teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, e22–e26. [Google Scholar] [CrossRef] [PubMed]
- Zanini, M.; Meyer, E.; Simon, S. Pulp inflammation diagnosis from clinical to inflammatory mediators: A systematic review. J. Endod. 2017, 43, 1033–1051. [Google Scholar] [CrossRef]
- Al-Ali, M.; Camilleri, J. The scientific management of deep carious lesions in vital teeth using contemporary materials—A narrative review. Front. Dent. Med. 2022, 3, 1048137. [Google Scholar] [CrossRef]
- Ballal, V.; Rao, S.; Bagheri, A.; Bhat, V.; Attin, T.; Zehnder, M. MMP-9 in dentinal fluid correlates with caries lesion depth. Caries Res. 2017, 51, 460–465. [Google Scholar] [CrossRef]
- Mente, J.; Petrovic, J.; Gehrig, H.; Rampf, S.; Michel, A.; Schürz, A.; Pfefferle, T.; Saure, D.; Erber, R. A prospective clinical pilot study on the level of matrix metalloproteinase-9 in dental pulpal blood as a marker for the state of inflammation in the pulp tissue. J. Endod. 2016, 42, 190–197. [Google Scholar] [CrossRef]
- Trope, M.D.M.D. Regenerative Potential of Dental Pulp. J. Endod. 2008, 34, S13–S17. [Google Scholar] [CrossRef]
- Munoz-Sanchez, M.-L.; Linas, N.; Decerle, N.; Nicolas, E.; Hennequin, M.; Cousson, P.-Y. A combination of full pulpotomy and chairside CAD/CAM endocrown to treat teeth with deep carious lesions and pulpitis in a single session: A preliminary study. Int. J. Environ. Res. Public Health 2020, 17, 6340. [Google Scholar] [CrossRef] [PubMed]
- Mente, J.; Hufnagel, S.; Leo, M.; Michel, A.; Gehrig, H.; Panagidis, D.; Saure, D.; Pfefferle, T. Treatment outcome of mineral trioxide aggregate or calcium hydroxide direct pulp capping: Long-term results. J. Endod. 2014, 40, 1746–1751. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Yu, V.S.H.; Lim, K.C.; Tan, B.C.K.; Neo, C.L.J.; Shen, L.; Messer, H.H. Long-term pulpal and restorative outcomes of pulpotomy in mature permanent teeth. J. Endod. 2020, 46, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Barthel, C.R.; Rosenkranz, B.; Leuenberg, A.; Roulet, J.-F. Pulp capping of carious exposures: Treatment outcome after 5 and 10 years: A retrospective study. J. Endod. 2000, 26, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.C. Microleakage of intermediate restorative materials. J. Endod. 1990, 16, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Tewari, S.; Tewari, S. Assessment of coronal microleakage in intermediately restored endodontic access cavities. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 93, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J. Scanning electron microscopic evaluation of the material interface of adjacent layers of dental materials. Dent. Mater. 2011, 27, 870–878. [Google Scholar] [CrossRef]
- Sluyk, S.; Moon, P.; Hartwell, G. Evaluation of setting properties and retention characteristics of mineral trioxide aggregate when used as a furcation perforation repair material. J. Endod. 1998, 24, 768–771. [Google Scholar] [CrossRef]
- Ha, W.N.; Nicholson, T.; Kahler, B.; Walsh, L.J. Methodologies for measuring the setting times of mineral trioxide aggregate and Portland cement products used in dentistry. Acta Biomater. Odontol. Scand. 2016, 2, 25–30. [Google Scholar] [CrossRef]
- Tsujimoto, M.; Tsujimoto, Y.; Ookubo, A.; Shiraishi, T.; Watanabe, I.; Hayashi, Y. Timing for composite resin placement on mineral trioxide aggregate. J. Endod. 2013, 39, 1167–1170. [Google Scholar] [CrossRef]
- Zanini, M.; Hennequin, M.; Cousson, P.Y. Which procedures and materials could be applied for full pulpotomy in permanent mature teeth? a systematic review. Acta Odontol. Scand. 2019, 77, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Bjørndal, L.; Simon, S.; Tomson, P.L.; Duncan, H.F. Management of deep caries and the exposed pulp. Int. Endod. J. 2019, 52, 949–973. [Google Scholar] [CrossRef] [PubMed]
- Delfino, M.M.; Jampani, J.L.d.A.; Lopes, C.S.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M.; Sasso-Cerri, E.; Cerri, P.S. Participation of fibroblast growth factor-1 and interleukin-10 in connective tissue repair following subcutaneous implantation of bioceramic materials in rats. Int. Endod. J. 2023, 56, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Hassanizadeh, R.; Torabzadeh, H.; Eghbal, M.J. Treatment outcomes of 4 vital pulp therapies in mature molars. J. Endod. 2018, 44, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour, S.; Gaudin, A.; Peters, O.A. A critical analysis of research methods and experimental models to study biocompatibility of endodontic materials. Int. Endod. J. 2022, 55, 346–369. [Google Scholar] [CrossRef]
- Mejare, I.; Hasselgren, G.; Hammarstrom, L.E. Effect of formaldehyde containing drugs on human dental pulp evaluated by enzyme histochemical technique. Scand. J. Dent. Res. 1976, 84, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Parirokh, M. Mineral trioxide aggregate: A comprehensive literature review—Part II: Leakage and biocompatibility investigations. J. Endod. 2010, 36, 190–202. [Google Scholar] [CrossRef]
- Asgary, S.; Eghbal, M.J.; Bagheban, A.A. Long-term outcomes of pulpotomy in permanent teeth with irreversible pulpitis: A multi-center randomized controlled trial. Am. J. Dent. 2017, 30, 151–155. [Google Scholar]
- Chueh, L.H.; Chiang, C.P. Histology of Irreversible pulpitis premolars treated with mineral trioxide aggregate pulpotomy. Oper. Dent. 2010, 35, 370–374. [Google Scholar] [CrossRef]
- McCabe, P.S.; Dummer, P.M.H. Pulp canal obliteration: An endodontic diagnosis and treatment challenge. Int. Endod. J. 2012, 45, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, I.; Kerekes, K. Long-term prognosis of traumatized permanent anterior teeth showing calcifying processes in the pulp cavity. Scand. J. Dent. Res. 1977, 85, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.; Andreasen, F.M.; Bergenholtz, G.; Andreasen, J.O.; Norén, J.G. Incidence of pulp necrosis subsequent to pulp canal obliteration from trauma of permanent incisors. J. Endod. 1996, 22, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Tronstad, L. Root resorption—Etiology, terminology and clinical manifestations. Dent. Traumatol. 1988, 4, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Felman, D.; Parashos, P. Coronal tooth discoloration and white mineral trioxide aggregate. J. Endod. 2013, 39, 484–487. [Google Scholar] [CrossRef]
- Camilleri, J. Color stability of white mineral trioxide aggregate in contact with hypochlorite solution. J. Endod. 2014, 40, 436–440. [Google Scholar] [CrossRef]
- Taha, N.A.; Al-Rawash, M.H.; Imran, Z.A. Outcome of full pulpotomy in mature permanent molars using 3 calcium silicate-based materials: A parallel, double blind, randomized controlled trial. Int. Endod. J. 2022, 55, 416–429. [Google Scholar] [CrossRef]
- Aravind, A.; Rechithra, R.; Sharma, R.; Rana, A.; Sharma, S.; Kumar, V.; Chawla, A.; Logani, A. Response to pulp sensibility tests after full pulpotomy in permanent mandibular teeth with symptomatic irreversible pulpitis: A retrospective data analysis. J. Endod. 2022, 48, 80–86. [Google Scholar] [CrossRef]
- Kirkwood, B.R.; Sterne, J.A.C. Essential Medical Statistics, 2nd ed.; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]

| Diagnosis | Signs and Symptoms | Recommended Management |
|---|---|---|
| Initial pulpitis | Heightened but not lengthened response to cold, no tenderness to percussion and no spontaneous pain. | Indirect pulp capping |
| Mild pulpitis | Heightened and lengthened reaction to cold, warm, or sweet that lasts no longer than 20 s. The tooth may or may not be tender to percussion. | Indirect pulp capping |
| Moderate pulpitis | Heightened and prolonged reaction to cold that lasts for longer than 20 s. The tooth may or may not have tenderness to percussion. Spontaneous dull pain can be suppressed by simple analgesics. | Coronal or partial pulpotomy |
| Severe pulpitis | Severe spontaneous pain with hot and cold, sharp or dull throbbing pain that is usually worse when lying down. The tooth is tender to percussion and palpation. | Coronal pulpotomy (note that pulpectomy is indicated if hemostasis cannot be achieved, or no bleeding is noted from one or more of the canals) |
| Author, Year | Sample Size (SS), Follow-Up (F/U), Drop-Out (D/O) | Partial or Complete Pulpotomy Type (PP, CP, Respectively), Material(s) Used | Age (Yrs), Time to Rest’n | Other Exclusions | Pulpitis Diagnosis, Periapical Diagnosis | Inflammation: Bleeding Time (mins), Agent, Other Indicators | Definition of Success Including Radiographic Appearance. P/O Pain. Success Rate & Recall Period | Diagnostic/Prognostic Factors Examined for Success (Statistical Significance Indicated by p < 0.05) |
|---|---|---|---|---|---|---|---|---|
| Asgary [5] 2015 | SS: 407 F/U: 271 D/O: 33% | CP (compared with RCT) CEM or MTA | 9–65 1 week | Active systemic disease, physical or mental disability | Spontaneous pain or pain exacerbated with hot and cold stimuli that lasts for a few seconds to several hours (lingering) compared to control teeth. With/without periapical involvement. | No limit Saline | Asymptomatic, no abscess, swelling, sinus tract, redness or tenderness. Modified Strindberg criteria for radiographic success. P/O pain: reported in an earlier report [44]. Success: 71.3% 5 years. | Pulp capping material, age, pre-operative PARL, outcome compared with RCT (p > 0.05) |
| Careddu [15] 2021 | SS: 51 F/U: 41 D/O: 20% | PP Biodentine | 14–60 1 week | No pulp exposure | Wolter’s classification and AAE classification. Absence/presence of TTP; radiographic appearance not mentioned. | Limit ≤ 6 min 2.5% NaOCl | Responsive, non-lingering to cold testing, asymptomatic, no TTP, no PARL. Also considered unresponsive but successful as a sub-category. P/O pain: whilst P/O was generally absent at 24 h, at 7 days >50% of pts reported increased sensitivity to cold that gradually subsided within 3 months. Success: 90% overall for PP; 88% for moderate & 60% for severe pulpitis 12 months. | Mild pulpitis had a better outcome than severe (p < 0.05). TTP, bleeding time, RP or IP, moderate or mild pulpitis (p > 0.05) |
| Uesrichai [18] 2019 | SS: 27 F/U: 27 D/O: 0% | PP MTA or Biodentine | 7.11–16.11 = 11.4) Immediate | ASA class ≥ 3, necrotic or partially necrotic on exposure, exposure size >1 mm but <5 mm, no pulp exposure | Spontaneous pain with sharp and lingering pain to cold testing. No prominent PARL included but PDL widening or condensing osteitis included. | Limit ≤ 10 min 2.5% NaOCl | No S&S of pulpitis, abnormal mobility or fistula. Positive response to cold. Improvement of early periapical changes, absence of PARL, IRR and ERR. P/O pain not reported. Success: ~85% (range 8 months to 5.75 yrs). | Not mentioned |
| Taha [20] 2023 | SS: 30 F/U: 29 D/O: 3% | CP Biodentine | 15–50 = 29.9) Immediate | Tooth not in occlusion | History of spontaneous pain lasting for a few seconds to several hours, exacerbation of pain by hot & cold fluids, radiating pain. Normal apical tissues, symptomatic or asymptomatic AP included. | Limit ≤ 10 min 2.5% NaOCl | No spontaneous pain (except first few days P/O), functional and asymptomatic, no TTP or tenderness to palpation. Soft tissue appears normal, no mobility, no pathosis (IRR, ERR, furcal pathosis or new PARL). P/O pain: CP had a lower mean pain score than RCT at 24 h (p < 0.05) but no difference at 2, 3, 5 & 7 days (p > 0.05). CP had lower analgesic use than RCT (p < 0.05). Pt satisfaction recorded: higher for CP in terms of duration of treatment, intraoperative pain, pleasantness & cost (p < 0.05). QOL assessment: improvements for RCT & CP. Success: 93% 12 months. | Outcome compared with RCT (p > 0.05) |
| Aldeen [35] 2023 | SS: 40 F/U: 36 D/O: 10% | PP MTA | 18–25 Immediate | Partially necrotic | Spontaneous pain or pain exacerbated by cold stimuli lasting for a few seconds to several hours (lingering) compared to controls. No prominent PARL. | Limit ≤ 6 min 2.5% NaOCl | Response to cold WNL, no abnormal mobility, fistulae, PARL, IRR or ERR. P/O pain not reported. Success: 88.9% 12 months. | Caries depth and activity, bleeding time (p > 0.05) |
| Sánchez-Lara y Tajonar [36] 2022 | SS: 41 F/U: 41 D/O: 0% | CP MTA | 17–78 = 24.6 ± 15.8) Immediate | Mild or moderate pain (<7 out of 10) except when there was deep caries, pathologic medically compromised | Spontaneous pain or pain exacerbated by thermal stimuli lasting for a few secs to several hours (lingering compared to control). No PARL. | Limit ≤ 10 min 2.5% NaOCl | No persistent or spontaneous pain, no TTP, tenderness to palpation, sinus tract, discoloration, swelling, abnormal mobility or PDs. No PARL, furcal pathosis, IRR or ERR. P/O pain: 78.8% of pts had no pain at 24 h, at 7 days 97.5% had no pain. Pt satisfaction recorded (97.5% satisfied at 24 h). Success: 97.6% 12 months. | Not mentioned |
| Asgary [29] 2013 | SS: 413 F/U: 346 D/O: 16% | CP CEM or MTA | 9–65 ( = 27 ± 8.5) 1 week | Active systemic disease, physical or mental disability, or pregnant or nursing | Spontaneous pain for a few seconds to several hours with extensive caries, pain exacerbated by hot or cold fluids and/or radiating pain. With/without periapical involvement. | No limit Saline | Asymptomatic, no abscess, swelling, sinus tract, redness or tenderness. Modified Strindberg criteria for radiographic success P/O pain: no significant difference in pain intensity after MTA or CEM CP (recorded every 24 h for 7 days) Success: 93.9% 12 months | Pulp capping material (p > 0.05) |
| Beauquis [41] 2022 | SS: 44 F/U: 35 D/O: 20% | CP (compared with RCT) Biodentine | ≥18 = 34.8) Immediate (or within 4 weeks) | Systemic conditions | Spontaneous, radiating pain that lingers after removal of cold stimulus. PAI 1, 2 or 3 included. | Limit not quantified Saline | Absence of S&S, no ERR, IRR or furcal bone loss. PAI 1 or 2, maintenance of PAI 3 or drop in score if pre-operative PAI > 3. P/O pain: no difference in reduction of pain at any time period (24 h & 7 days) between RCT & CP. Success: 76% 12 months. | Outcome compared with RCT (p > 0.05) |
| Airsang [45] 2022 | SS: 60 F/U: 53 D/O: 12% | CP NeoMTA or Biodentine | 18–35 = 26) 2 weeks | No pulp exposure, no significant medical history | AAE Glossary definition; exaggerated and prolonged response to EPT and cold. No AP including PDL widening. | Limit <10 min 2.5% NaOCl | Not well-defined. Asymptomatic, no TTP, swelling, fistulae, sinus tract, integrity of rest’n, ERR, IRR, furcal or periapical pathosis. P/O pain not reported. Success: 86% 12 months (materials combined). | Pulp capping material (p > 0.05) |
| Elmas [46] 2023 | SS: 25 F/U: 25 D/O: 0% | CP MTA | 9–14 = 10.8) 3 days | Contributory medical history, no pulp exposure, necrotic or partially necrotic on exposure | AAE Glossary definition; spontaneous pain or pain exacerbated by cold stimuli lasting for much longer than control teeth. PAI 1, 2, 3 or 4 included. | No limit (range 3–25 min) Saline | No pain, TTP, sinus tract, swelling, IRR or ERR. No new furcal pathosis or PARL, reduction in PAI score. P/O pain: at 2 days, 64.6% of cases had complete pain relief, 31.2% scored 2/8 & 4.2% scored 4/8. No pts took analgesics in this period. Success: 96% 12 months. | PAI score, bleeding time (p > 0.05) |
| Ramani [47] 2022 | SS: 93 F/U: 88 D/O: 5% | PP or CP MTA | 18–40 = 23.3 ± 4.9) 1 week | Systemically healthy, analgesic intake in the past week, antibiotics in the past month, partial necrosis on exposure | AAE Glossary definition; history of spontaneous pain or lingering pain that could be reproduced by cold testing. Normal apical tissues (PAI ≤ 2, nil TTP). | Limit ≤ 6 min 3% NaOCl | Absence of S&S, no TTP, PAI < 3, no furcal involvement, IRR or ERR P/O pain: 97.7% of pts reported pain at 24 h, none reported moderate to severe pain at day 7. In the CP group, analgesics were needed by fewer pts & mean consumption was also less than for PP (p < 0.05). Success: 97.7% PP, 98.8% CP 12 months. | PP or CP outcome (p > 0.05) |
| Sharma [48] 2021 | SS: 40 F/U: 40 D/O: 0% | CP MTA | 16–35 = 25.3 ± 6.2) Immediate | Systemically unhealthy, history of taking long-acting NSAIDs in the past week | AAE Glossary definition of symptomatic irreversible pulpitis. Normal apical tissues (PAI ≤ 2). | Limit ≤ 10 min 2.5% NaOCl | No S&S, IRR, ERR or furcal pathosis. PAI < 3. P/O pain not reported. Success: 88% 12 months. | Concentration of MMP-9 levels on treatment outcome and pre-treatment diagnosis (p < 0.05), bleeding time and MMP-9 concentration (p > 0.05) |
| Taha [49] 2018 | SS: 64 F/U: 60 D/O: 6% | CP Biodentine | 19–69 = 33.2) Immediate or 2 weeks later | Contributory medical history, no pulp exposure, partially necrotic on exposure | AAE Glossary definition; spontaneous pain or pain exacerbated by cold stimuli lasting for a few seconds to several hours (lingering) compared to control teeth. PAI 1, 2, 3 or 4 included. | Limit ≤ 6 min 2.5% NaOCl | No history of spontaneous pain or discomfort (except 2 days P/O), no TTP or tenderness to palpation, swelling, sinus tract or new pathosis (furcal, periapical or resorption). Normal mobility and PDs. PAI 1 or 2 or reduction in pre-operative PAI score. P/O pain: at 2 days, 93% of pts reported complete pain relief (the rest reported mild pain 1–2/10). Success: 97% 12 months. | Not mentioned |
| R [50] 2021 | SS: 80 F/U: 80 D/O: 0% | CP MTA | 16–35 Immediate | Extremely deep caries | ESE definition; episodes of spontaneous, radiating pain that lingered after removal of stimulus. No PARL. | Limit ≤ 10 min 2.5% NaOCl | No history of spontaneous pain, swelling or discomfort on chewing. Functional, PAI ≤ 2, no IRR. P/O pain not reported. Success: 94% 12 months. | Location of caries (occlusal versus proximal) (p > 0.05) |
| Anta [51] 2022 | SS: 66 F/U: 52 D/O: 21% | CP Biodentine | 20–47 = 26 ± 8) 1 week | Not in “good general health” | Spontaneous, nocturnal and provoked and exacerbated by hot and cold foods and/or radiating pain. Responded positively to cold and EPT. PAI 1 or 2 included. | Limit ≤ 5 min Saline | Asymptomatic, non-mobile, no TTP, defective rest’n, sinus tract, IRR or ERR. PAI 1 or 2 with no increase in PAI score. P/O pain not reported. Success: 87% 12 months. | Age and PAI score (p < 0.05). Location of caries, mechanical or carious pulp exposure, pre-operative pain (moderate or severe), bleeding time, pre-operative tenderness to percussion and treatment time (p > 0.05) |
| Jassal [52] 2022 | SS: 50 F/U: 49 D/O: 2% | PP or CP Biodentine | ≥18 = 24.8 ± 5) 2 weeks | Not medically healthy, no pulp exposure, necrotic or partially necrotic on exposure | Spontaneous pain, heightened or lingering response to thermal or EPT, nocturnal pain. Normal apical tissues (PAI 1, nil TTP). | Limit ≤ 10 min 2.5% NaOCl | No spontaneous pain or discomfort, heightened response to hot/cold, TTP or tenderness to palpation. P/O pain: no difference in pain reduction at any time periods between CP & CP (24 h, 48 h & 1 week), analgesic use was low. Success: 88% (PP), 91.6% (CP) 12 months. | PP or CP outcome (p > 0.05) |
| Baranwal [53] 2022 | SS: 61 F/U: 54 D/O: 11% | PP or CP Biodentine | 18–40 1 week | No pulp exposure, partial necrosis on exposure | With/without spontaneous sharp or dull pain, lingering pain with hot or cold. With/without periapical involvement defined by PAI. | Limit ≤ 10 min 3% NaOCl | Absence of S&S, no ERR, IRR, furcal or new periapical pathosis. Resolved or reduced size of PARL. P/O pain not reported. Success: 80.7% (PP), 92.8% (CP) 12 months. | Pre-operative PARL, CP or PP outcome (p > 0.05) |
| Taha [54] 2018 | SS: 17 F/U: 17 D/O: 0% | CP Biodentine | 9–17 Immediate | Not mentioned | AAE Glossary definition; spontaneous pain or pain exacerbated by cold stimuli lasting for a few seconds to several hours (lingering) compared to control teeth. PAI scores recorded. | Limit ≤ 6 min 2.5% NaOCl | No history of spontaneous pain or discomfort (except first few days P/O), functional and asymptomatic, grade I mobility, no swelling or sinus tract. No IRR, ERR, PAI < 3 or reduction in PAI score. P/O pain: at 2 days, all pts had complete pain relief & none required analgesics. Success: 94% 12 months. | Not mentioned |
| Hussain [55] 2022 | SS: 20 F/U: 17 D/O: 15% | CP MTA | 25–55 Immediate | Contributory medical history, necrotic or partially necrotic on exposure | Exaggerated response with cold & heat lingering for >15 s. No PARL. | Limit ≤ 5 min Saline | No history of spontaneous pain (except 1 week P/O), TTP, tenderness to palpation, swelling, ERR, IRR or PARL. Functional and responsive to EPT. P/O pain: at 2 days, all cases reported complete relief of pain and no pts had required analgesics. Success: 94% 12 months. | Not mentioned |
| Kumar [56] 2016 | SS: 54 F/U: 48 D/O: 11% | CP PRF, MTA or CaOH | 14–32 24 h | Systemic disease, opioid or steroid therapy, taking antibiotics | Spontaneous, lingering pain exacerbated by hot and cold fluids and/or radiating pain. No periapical involvement. | “Several minutes” (not quantified) Saline | No abscess, swelling, sinus tract or tenderness. Modified Strindberg criteria for radiographic success (success had normal lamina dura. Widened PDL was considered a failure). P/O pain: no difference between the materials at 24 h or 7 days. Success: 37.5% for CaOH, 44.4% for MTA & 35.7% for PRF 12 months. | Pulp capping material (p > 0.05) |
| Asgary [57] 2009 | SS: 12 F/U: 12 D/O: 0% | CP NEC | 14–62 Immediate | Medical contraindication | Moderate to severe pain, history of lingering pain. Periapical status not mentioned. | 5 min Saline | No mobility, no TTP, asymptomatic, normal periodontium radiographically. P/O pain not reported. Success: 92% (μ 15.8 months). | Not mentioned |
| Taha [58] 2017 | SS: 50 F/U: 49 D/O: 2% | PP MTA or CaOH | 20–52 = 30.3 ± 9.6) 1 week | Contributory medical history | Severe spontaneous lingering pain that could be reproduced by cold testing. Periapical status not mentioned. | Limit ≤ 6 min 2.5% NaOCl | No history of spontaneous pain or discomfort (except first few days P/O), functional and asymptomatic, positive response to cold, no TTP or tenderness to palpation, grade I mobility with normal soft tissues. No IRR or ERR, PAI < 3. P/O pain not reported. Success: 85% MTA, 43% CaOH 2 years. | Pulp capping material (p < 0.05) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McHugh, H.; Wright, P.P.; Peters, C.I.; Peters, O.A. Diagnostic and Prognostic Predictors for the Success of Pulpotomy in Permanent Mature Posterior Teeth with Moderate to Severe Pulpitis: A Scoping Review. Oral 2023, 3, 545-571. https://doi.org/10.3390/oral3040045
McHugh H, Wright PP, Peters CI, Peters OA. Diagnostic and Prognostic Predictors for the Success of Pulpotomy in Permanent Mature Posterior Teeth with Moderate to Severe Pulpitis: A Scoping Review. Oral. 2023; 3(4):545-571. https://doi.org/10.3390/oral3040045
Chicago/Turabian StyleMcHugh, Helen, Patricia P. Wright, Christine I. Peters, and Ove A. Peters. 2023. "Diagnostic and Prognostic Predictors for the Success of Pulpotomy in Permanent Mature Posterior Teeth with Moderate to Severe Pulpitis: A Scoping Review" Oral 3, no. 4: 545-571. https://doi.org/10.3390/oral3040045
APA StyleMcHugh, H., Wright, P. P., Peters, C. I., & Peters, O. A. (2023). Diagnostic and Prognostic Predictors for the Success of Pulpotomy in Permanent Mature Posterior Teeth with Moderate to Severe Pulpitis: A Scoping Review. Oral, 3(4), 545-571. https://doi.org/10.3390/oral3040045







