Role of Glutathione in Neutrophil Chemotaxis in Periodontitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
- Healthy volunteers were recruited from staff and students (22 ± 5 years old) of the School of Dentistry, University of Birmingham and Birmingham Dental Hospital.
- Pilot study: Periodontitis patients (n = 4) were recruited from periodontitis new patient clinics at the University of Birmingham’s School of Dentistry and Birmingham Dental Hospital. Age- (± 5 years) and sex-matched healthy controls (n = 4) were recruited from the staff of the University of Birmingham’s School of Dentistry. Demographic data for these donors is shown in Table 1. Donors gave medical history details including age, sex, ethnicity and smoking status at recruitment. Periodontitis patients underwent detailed pocket charting. Measurements that were recorded in this clinical study included the percentages of sites of bleeding on probing (% bleeding) and the presence of plaque percentages (plaque index PI). Periodontal disease was classified as severe periodontitis based on Eke et al. (2012) [32] case definitions (severe periodontitis: >2 interproximal sites with PPD > 5 mm, loss of attachment and bleeding on probing). Controls were not examined for periodontal health but were asked for their latest classification by their general dental practitioner. Thus, they may have had some level of gingivitis. The exclusion criteria were patients lacking the capacity to consent, smokers and those with other chronic inflammatory conditions such as diabetes, arthritis and chronic kidney disease.
2.2. Neutrophil Cell Isolation
2.3. Neutrophil Cell Viability
2.4. Glutathione Assays
2.5. Neutrophil Cell Chemotaxis
2.6. Quantitative Proteomics
2.7. Statistical Analysis
3. Results
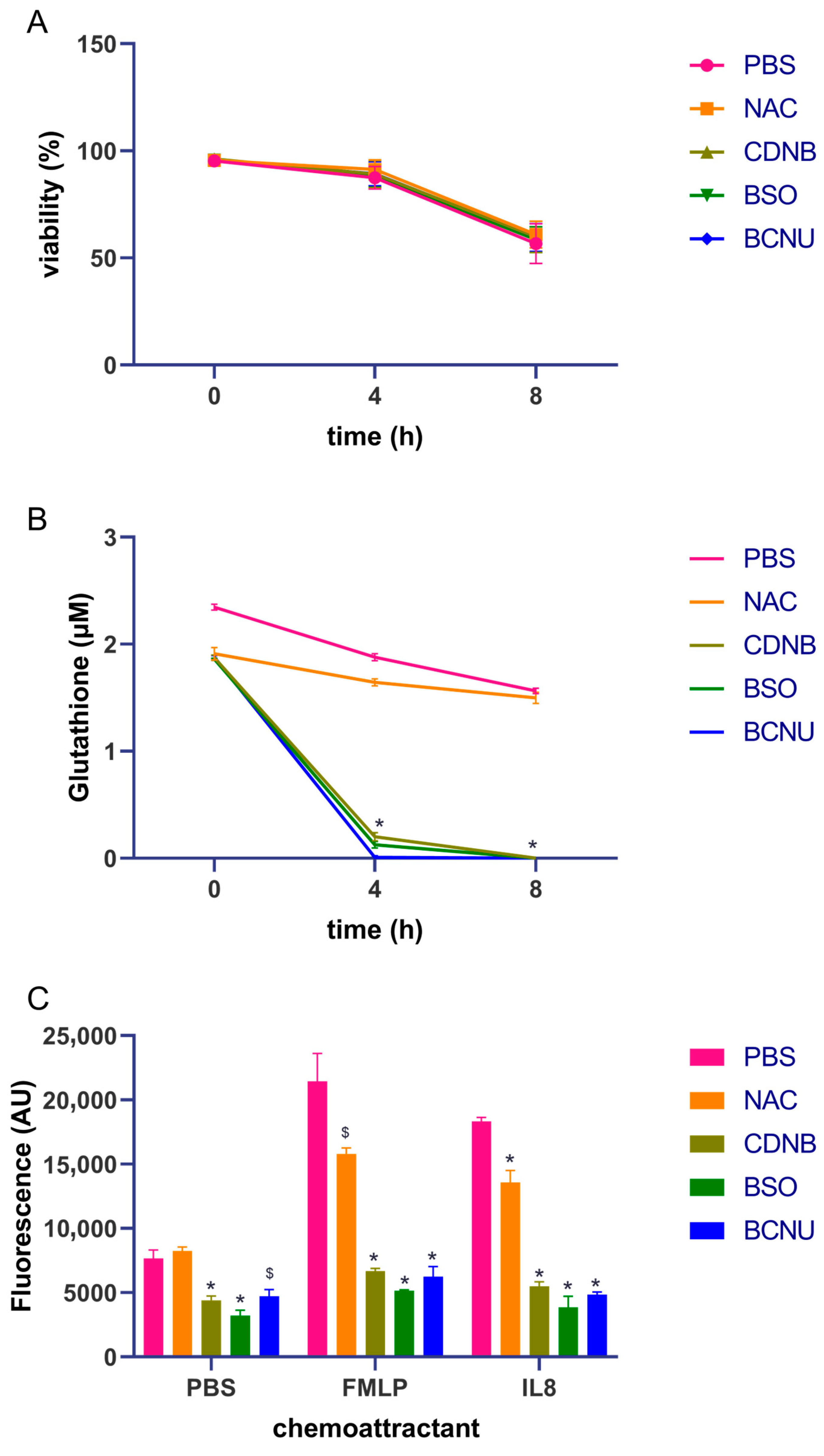
3.1. Gluthathione Modulation in Healthy Neutrophils
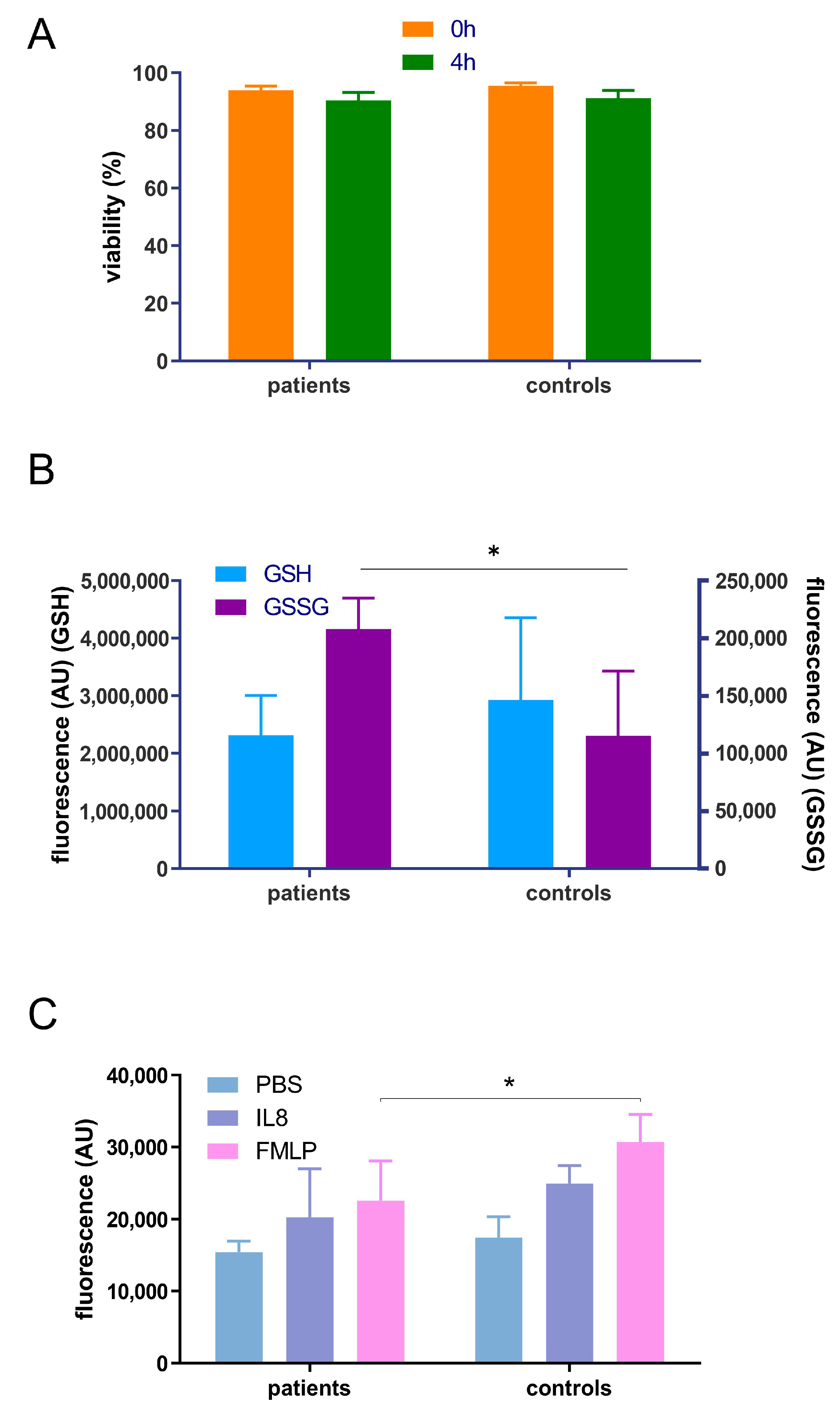
3.2. Pilot Study to Determione the Effect of Periodontitis on Neutrophil Chemotaxis, Glutathione Status and Proteome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hasturk, H.; Kantarci, A. Activation and resolution of periodontal inflammation and its systemic impact. Periodontol. 2000 2015, 69, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.T.; Cochran, D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J. Periodontol. 2003, 74, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72–80. [Google Scholar]
- Gamonal, J.; Acevedo, A.; Bascones, A.; Jorge, O.; Silva, A. Characterization of cellular infiltrate, detection of chemokine receptor CCR5 and interleukin-8 and RANTES chemokines in adult periodontitis. J. Periodontal Res. 2001, 36, 194–203. [Google Scholar] [CrossRef]
- Hajishengallis, E.; Hajishengallis, G. Neutrophil homeostasis and periodontal health in children and adults. J. Dent. Res. 2014, 93, 231–237. [Google Scholar] [CrossRef]
- Dias, I.H.; Matthews, J.B.; Chapple, I.L.; Wright, H.J.; Dunston, C.R.; Griffiths, H.R. Activation of the neutrophil respiratory burst by plasma from periodontitis patients is mediated by pro-inflammatory cytokines. J. Clin. Periodontol. 2011, 38, 1–7. [Google Scholar] [CrossRef]
- Matthews, J.B.; Wright, H.J.; Roberts, A.; Cooper, P.R.; Chapple, I.L. Hyperactivity and reactivity of peripheral blood neutrophils in chronic periodontitis. Clin. Exp. Immunol. 2007, 147, 255–264. [Google Scholar] [CrossRef]
- Matthews, J.B.; Wright, H.J.; Roberts, A.; Ling-Mountford, N.; Cooper, P.R.; Chapple, I.L. Neutrophil hyper-responsiveness in periodontitis. J. Dent. Res. 2007, 86, 718–722. [Google Scholar] [CrossRef]
- Roberts, H.M.; Ling, M.R.; Insall, R.; Kalna, G.; Spengler, J.; Grant, M.M.; Chapple, I.L. Impaired neutrophil directional chemotactic accuracy in chronic periodontitis patients. J. Clin. Periodontol. 2015, 42, 1–11. [Google Scholar] [CrossRef]
- Ling, M.R.; Chapple, I.L.; Matthews, J.B. Neutrophil superoxide release and plasma C-reactive protein levels pre- and post-periodontal therapy. J. Clin. Periodontol. 2016, 43, 652–658. [Google Scholar] [CrossRef]
- Weiss, S.J. Tissue destruction by neutrophils. N. Engl. J. Med. 1989, 320, 365–376. [Google Scholar] [CrossRef]
- Chapman, A.L.; Hampton, M.B.; Senthilmohan, R.; Winterbourn, C.C.; Kettle, A.J. Chlorination of bacterial and neutrophil proteins during phagocytosis and killing of Staphylococcus aureus. J. Biol. Chem. 2002, 277, 9757–9762. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.R.; Palmer, L.J.; Chapple, I.L. Neutrophil extracellular traps as a new paradigm in innate immunity: Friend or foe? Periodontol. 2000 2013, 63, 165–197. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Bowerman, B. Symmetry breaking in biology. Cold Spring Harb. Perspect. Biol. 2010, 2, a003475. [Google Scholar] [CrossRef] [PubMed]
- Wang, F. The signaling mechanisms underlying cell polarity and chemotaxis. Cold Spring Harb. Perspect. Biol. 2009, 1, a002980. [Google Scholar] [CrossRef]
- Harbord, M.W.; Marks, D.J.; Forbes, A.; Bloom, S.L.; Day, R.M.; Segal, A.W. Impaired neutrophil chemotaxis in Crohn’s disease relates to reduced production of chemokines and can be augmented by granulocyte-colony stimulating factor. Aliment. Pharmacol. Ther. 2006, 24, 651–660. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Dent, G.; Ward, J.; Angco, G.; Nong, G.; Nomura, N.; Hirata, K.; Djukanovic, R. Impaired neutrophil chemotaxis in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2007, 175, 473–479. [Google Scholar] [CrossRef]
- Janetopoulos, C.; Firtel, R.A. Directional sensing during chemotaxis. FEBS Lett. 2008, 582, 2075–2085. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Andrew, N.; Insall, R.H. Chemotaxis in shallow gradients is mediated independently of PtdIns 3-kinase by biased choices between random protrusions. Nat. Cell Biol. 2007, 9, 193–200. [Google Scholar] [CrossRef]
- Dickinson, B.C.; Chang, C.J. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Sakai, J.; Li, J.; Subramanian, K.K.; Mondal, S.; Bajrami, B.; Hattori, H.; Jia, Y.; Dickinson, B.C.; Zhong, J.; Ye, K.; et al. Reactive oxygen species-induced actin glutathionylation controls actin dynamics in neutrophils. Immunity 2012, 37, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Niethammer, P.; Grabher, C.; Look, A.T.; Mitchison, T.J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 2009, 459, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.M.; Gabrielsen, M.; Chim, Y.H.; Munro, J.; McGhee, E.J.; Sumpton, D.; Eaton, P.; Anderson, K.I.; Yin, H.; Olson, M.F. Polarized cell motility induces hydrogen peroxide to inhibit cofilin via cysteine oxidation. Curr. Biol. 2015, 25, 1520–1525. [Google Scholar] [CrossRef]
- Johansson Mali’n, T.; Lindberg, S.; Astot, C. Novel glutathione conjugates of phenyl isocyanate identified by ultra-performance liquid chromatography/electrospray ionization mass spectrometry and nuclear magnetic resonance. J. Mass Spectrom. 2014, 49, 68–79. [Google Scholar] [CrossRef]
- Sies, H. Glutathione and its role in cellular functions. Free Radic. Biol. Med. 1999, 27, 916–921. [Google Scholar] [CrossRef]
- Meister, A. Selective modification of glutathione metabolism. Science 1983, 220, 472–477. [Google Scholar] [CrossRef]
- Dias, I.H.; Chapple, I.L.; Milward, M.; Grant, M.M.; Hill, E.; Brown, J.; Griffiths, H.R. Sulforaphane restores cellular glutathione levels and reduces chronic periodontitis neutrophil hyperactivity in vitro. PLoS ONE 2013, 8, e66407. [Google Scholar] [CrossRef]
- Harlan, J.M.; Levine, J.D.; Callahan, K.S.; Schwartz, B.R.; Harker, L.A. Glutathione redox cycle protects cultured endothelial cells against lysis by extracellularly generated hydrogen peroxide. J. Clin. Investig. 1984, 73, 706–713. [Google Scholar] [CrossRef]
- Grant, M.M.; Brock, G.R.; Matthews, J.B.; Chapple, I.L. Crevicular fluid glutathione levels in periodontitis and the effect of non-surgical therapy. J. Clin. Periodontol. 2010, 37, 17–23. [Google Scholar] [CrossRef]
- Brock, G.R.; Butterworth, C.J.; Matthews, J.B.; Chapple, I.L. Local and systemic total antioxidant capacity in periodontitis and health. J. Clin. Periodontol. 2004, 31, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Page, R.C.; Wei, L.; Thornton-Evans, G.; Genco, R.J. Update of the Case Definitions for Population-Based Surveillance of Periodontitis. J. Periodontol. 2012, 83, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Pasha, S.; Inui, T.; Chapple, I.; Harris, S.; Holcombe, L.; Grant, M.M. The Saliva Proteome of Dogs: Variations within and between Breeds and between Species. Proteomics 2018, 18, 1700293. [Google Scholar] [CrossRef] [PubMed]
- Woodi, M.; Mondal, A.K.; Padmanabhan, B.; Rajagopalan, K.P. Analysis of protein posttranslational modifications by mass spectrometry: With special reference to haemoglobin. Indian J. Clin. Biochem. 2009, 24, 23–29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Nilsa, R.D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef]
- Kumar, R.S.; Prakash, S. Impaired neutrophil and monocyte chemotaxis in chronic and aggressive periodontitis and effects of periodontal therapy. Indian J. Dent. Res. 2012, 23, 69–74. [Google Scholar] [CrossRef]
- Dahiya, P.; Kamal, R.; Gupta, R.; Bhardwaj, R.; Chaudhary, K.; Kaur, S. Reactive oxygen species in periodontitis. J. Indian Soc. Periodontol. 2013, 17, 411–416. [Google Scholar] [CrossRef]
- Szczur, K.; Xu, H.; Atkinson, S.; Zheng, Y.; Filippi, M.D. Rho GTPase CDC42 regulates directionality and random movement via distinct MAPK pathways in neutrophils. Blood 2006, 108, 4205–4213. [Google Scholar] [CrossRef]
- Thomas, H.B.; Moots, R.J.; Edwards, S.W.; Wright, H.L. Whose Gene Is It Anyway? The Effect of Preparation Purity on Neutrophil Transcriptome Studies. PLoS ONE 2015, 10, e0138982. [Google Scholar] [CrossRef]
- Roberts, H.; White, P.; Dias, I.; McKaig, S.; Veeramachaneni, R.; Thakker, N.; Grant, M.; Chapple, I. Characterization of neutrophil function in Papillon-Lefevre syndrome. J. Leukoc. Biol. 2016, 100, 433–444. [Google Scholar] [CrossRef]
- Pan, K.T.; Chen, Y.Y.; Pu, T.H.; Chao, Y.S.; Yang, C.Y.; Bomgarden, R.D.; Rogers, J.C.; Meng, T.C.; Khoo, K.H. Mass spectrometry-based quantitative proteomics for dissecting multiplexed redox cysteine modifications in nitric oxide-protected cardiomyocyte under hypoxia. Antioxid. Redox Signal. 2014, 20, 1365–1381. [Google Scholar] [CrossRef] [PubMed]


| Control Group | Periodontitis Group | |
|---|---|---|
| Number | 4 | 4 |
| Mean age (range) | 40.5 ± 4.5 years old | 40.75 ± 2.5 years old |
| Sex (% female) | 50% | 50% |
| Number of teeth | - | 30 ± 2 |
| Percentage bleeding | - | 18 + ±7% |
| Percentage plaque surfaces | - | 27 ± 21% |
| PPD Mean ± SD (mm) | - | 3 ± 1 mm |
| Max PPD (mm) | - | 10 ± 3 mm |
| Teeth > 4 mm | - | 16 ± 6 |
| CAL Mean ± SD | - | 3 ± 1 mm |
| % sites > 4 mm | - | 23 ± 11% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binti Badlishah Sham, N.I.; Grant, M.M. Role of Glutathione in Neutrophil Chemotaxis in Periodontitis. Oral 2023, 3, 526-538. https://doi.org/10.3390/oral3040043
Binti Badlishah Sham NI, Grant MM. Role of Glutathione in Neutrophil Chemotaxis in Periodontitis. Oral. 2023; 3(4):526-538. https://doi.org/10.3390/oral3040043
Chicago/Turabian StyleBinti Badlishah Sham, Nurul Iman, and Melissa M. Grant. 2023. "Role of Glutathione in Neutrophil Chemotaxis in Periodontitis" Oral 3, no. 4: 526-538. https://doi.org/10.3390/oral3040043
APA StyleBinti Badlishah Sham, N. I., & Grant, M. M. (2023). Role of Glutathione in Neutrophil Chemotaxis in Periodontitis. Oral, 3(4), 526-538. https://doi.org/10.3390/oral3040043







