Abstract
The objective of this study is the development of an in vitro cell culture model of pigmented gingival keratinocytes to provide a unique tool to assess oral care products such as toothpaste and evaluate whether pigmented gingival cells might be less susceptible than unpigmented cells to cytotoxicity by any toothpaste. Sepia melanin at various concentrations was added to primary human gingival keratinocyte (HGK) monolayers to identify the concentration at which melanin is sufficiently phagocytosed in the absence of cytotoxicity; this concentration was subsequently used to generate pigmented HGK model. Extracts from three commercial adult toothpastes (Crest 3D White, Sensodyne, and Colgate Optic) at different dilutions were evaluated in pigmented and unpigmented HGKs for cytotoxicity over a 24 h duration by MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay. Results demonstrated that HGKs showed a concentration-dependent uptake of sepia melanin with a significant linear correlation of pigment uptake. Moreover, the melanin was distributed perinuclearly in the cells, that was similar to the distribution of physiological gingiva in vivo. Further experiments were conducted with 25 µg/mL sepia melanin as higher concentrations induced cytotoxicity. Evaluation of three commercial toothpastes on unpigmented and pigmented HGKs showed no differential effects at any dilution. In summary, a model of pigmented HGKs with the ability to create a controlled level of pigmentation was demonstrated. Examination of extracts from three commercial toothpastes revealed similar cytotoxicity to both pigmented and unpigmented HGKs. In conclusion, this study shows that the artificially pigmented HGK model is an easy and low-cost tool that mimics the in vivo gingival pigmentation. Moreover, the evaluated toothpastes showed similar cytotoxicity to pigmented and unpigmented HGKs, suggesting that the presence of melanin did not impart any protective effects. Further studies to employ this pigmented HGK model to evaluate a large number of oral care products and include repeated exposures and longer duration are warranted.
1. Introduction
The gingival epithelium is the first line of barrier against pathogens [1] and is also the most frequently pigmented tissue of the oral cavity [2,3,4]. Melanin that imparts protective benefits in the oral cavity is synthesized by gingival melanocytes, exported to gingival keratinocytes, and distributed perinuclearly, which results in a dark gingival epithelium leading to visible pigmentation [5,6,7,8]. Gingival pigmentation can be physiological (racial) or pathological, dependent on endogenous or exogenous factors such as tobacco smoking [9,10]. It has a higher prevalence in dark-skinned individuals [11], with a frequency of 54% in blacks and 21% in whites. Removal of dental plaque biofilms is necessary to maintain good oral hygiene and prevent tooth decay and the development of gingival inflammation and periodontitis [12]. Toothpaste, a standard oral care product, is ubiquitously used as an effective aid for oral hygiene on a daily basis by every race/ethnicity. Yet, the gingival tissue that contacts these products in the mouth is not unpigmented in most cases, owing to the greater frequency of the population with dark gingiva due to genetic or external factors. Thus, shades of brown-black pigmentation occur in gingiva in the ethnic population (African-American, Hispanic, and Indian). The contributing factor in the visible spectrum of gingival pigmentation is the differential uptake and distribution of melanin pigment around the nucleus of keratinocytes, and the pivotal role of keratinocytes in regulating melanin distribution and the final visible pigmentation has been demonstrated [13]. With the rapid global growth of the market of oral care products for cosmetic or medical use [14], their safety and biocompatibility in ethnic populations is an unmet need. Evaluation of the biocompatibility of oral care products on keratinocytes (containing melanin pigment and pigment-free) is imperative as they are the first cells to contact oral care products. Since the keratinocytes not only act as a barrier to external pathogens and chemicals but are also the key cells to receive the exported melanin from gingival melanocytes and distribute them around the nucleus [7,15], cytotoxicity to keratinocytes might disrupt the homeostasis of the keratinocyte–melanocyte unit in the oral epithelium and cause untoward effects.
Toothpaste comprises surfactants, abrasives, and active compounds as primary ingredients with humectants, preservatives, binders, colors, and flavoring agents [16,17]. Surfactants are incorporated in toothpastes for their cleaning and foaming efficacy [18]. Toothpastes are meant to be used intra-orally in conjunction with toothbrushes to provide adequate teeth cleaning and should not damage the soft or hard tissues in the oral cavity with continued use. Despite this, certain components of toothpastes (such as the surfactant sodium lauryl sulfate, SLS) are capable of inducing damage; hence, the use of alternative natural surfactants instead of SLS has been recommended for child toothpastes [19]. Likewise, desirable features of toothpastes for the elderly comprise low cytotoxicity and low amounts of abrasives to avoid soft tissue damage, dentin wear, and dry mouth [20]. Previous studies have shown that pigmented cells are protected from adverse effects compared to unpigmented cells [21], and the presence of melanin diminished the cytotoxicity action of chemotherapeutic drugs [22]. Hence, cells containing melanin (either artificially fed or by endogenous biosynthesis, such as in melanocytes) might display lower cytotoxicity than cells lacking melanin. Accordingly, the biocompatibility of a specific toothpaste might be nonidentical in various ethnicities with different degrees of gingival pigmentation and warrants consideration.
Compared to melanocytes that are commercially available from light-, medium-, or dark-skinned neonatal and adult donors, keratinocytes (skin or gingiva) from different ethnic donors are not commercially available. Keratinocytes used in previous studies [13,23,24,25] primarily for the study of differential responses with melanocyte cultures in terms of melanin distribution were isolated from light and dark skin. To our knowledge, there are no reports on the use of keratinocytes isolated from light and dark gingival tissue. The genetic variability between donors, the disease history of donors, and the cumbersome isolation procedures necessitate the use of a single keratinocyte model that can be pigmented to varying degrees to represent the different spectrums of pigmentations of the gingiva to offer convenience and reproducibility. Notably, the commercially available gingival keratinocytes (primary or immortalized) are unpigmented, and in the absence of donor information, it is difficult to ascertain whether they were isolated from unpigmented or pigmented gingival tissue. The latter is also a possibility, as the pigmentation after in vitro cultivation is diminished due to dilution amongst daughter cells; hence, the cells received from vendors might have become depigmented. A similar occurrence has been noted with human retinal pigment epithelial cells (ARPE-19) that lack any detectable melanin in vitro cultures [26] or with primary RPE cells that, despite containing melanin in vitro, rapidly lose their pigmentation levels in subsequent passages [27,28], thus introducing variability in the cell model per se. Consequently, the use of artificially pigmented single-cell models that overcome these challenges of variation in the degree of baseline pigmentation has become an attractive alternative.
Sepia melanin is a melanoprotein extracted from the ink gland of the cephalopod Sepia officinalis and is considered a standard model that mimics natural melanin more closely than synthetic melanin [29,30]. Moreover, it has been used as a model melanin in binding studies [31,32]. In multiple prior studies, sepia melanin, synthetic melanin, or melanosomes have been employed to generate melanized models of different cells. For instance, sepia melanin has been used to pigment dermal dendritic cells [33] and retinal pigment epithelial (RPE) cells [34,35], while synthetic melanin was used to pigment HaCaT cells [36,37], PC12 neural cells [38,39], and RPE cells [40]. In other studies, human melanosomes have been used to pigment human epidermal keratinocytes [41,42] or ARPE-19 cells [43], while porcine melanosomes were used to pigment ARPE-19 cells [44]. We have previously used synthetic melanin to artificially pigment HaCaT cells [37]. However, it does not result in sufficiently dark pellets compared to sepia melanin; this was also observed in another study, where keratinocytes demonstrated lower uptake of synthetic melanin compared to isolated melanosomes [42]. Herein, we chose sepia melanin as it is commercially available and has high reproducibility compared to melanosomes, which require tedious isolation and purification methods. Additionally, the physicochemical properties of sepia melanin have been well-characterized previously [45,46,47,48] and shown to have a uniform dispersion of spherical melanosomes, while melanosomes isolated from different mammalian cells lack a well-defined characterization.
Hence, this study had a two-fold objective: (i) to develop an in vitro model of artificially pigmented human gingival keratinocytes (HGKs) and (ii) to evaluate whether commercial oral care products (toothpastes) exhibit differential biocompatibility to pigmented and unpigmented HGKs. We hypothesize that pigmented HGKs exhibit lower cytotoxicity to toothpastes than unpigmented HGKs, due to the presence of melanin.
2. Materials and Methods
2.1. Materials
Melanin derived from Sepia officinalis (Cat# M2649, >99% purity), hereafter referred to as sepia melanin, was procured from Millipore-Sigma (St. Louis, MO, USA). MTS reagent was procured from Promega Corporation (Madison, WI, USA). Hank’s buffered salt saline (HBSS; Hyclone™), penicillin-streptomycin (10,000 U/mL), sodium hydroxide (NaOH) solution, and Dulbecco’s phosphate-buffered saline (DPBS) were obtained from Thermo Fisher Scientific (Waltham, MA, USA). The three kinds of commercial toothpastes, Crest 3D White, Sensodyne, and Colgate Optic White (detailed compositions are listed under Table 1), were procured from a local store.

Table 1.
Compositions of three commercial toothpastes used in this study.
2.2. Cell Culture
Primary human gingival keratinocytes (HGKs) were procured from American-type culture collection (ATCC® PCS-200-014; lot# 80523333) and cultured using dermal cell basal medium (ATCC® PCS-200-030™) supplemented with keratinocyte growth kit (ATCC® PCS-200-040™) and 1% penicillin-streptomycin antibiotics. The cells were incubated at 37 °C in a humidified environment in 5% CO2 and 95% air. Trypsin-EDTA (ATCC® PCS-999-003) and trypsin neutralizing solution (ATCC® PCS-999-004) were used to detach cultures. For all experiments, cells were used between passages 2–6.
2.3. Melanin Feeding Procedure
2.3.1. Phagocytosis of Melanin
Varying concentrations of sepia melanin were initially examined to identify the concentration at which a significant melanin uptake occurs in the absence of cytotoxicity; this optimized concentration was used in subsequent experiments. HGKs (1.9 × 105 cells per well in 2 mL culture medium) were grown in a six-well plate for 24 h to full confluency. After 24 h period, various concentrations (25–400 µg/mL) of sepia melanin diluted from a 2 mg/mL stock solution in HBSS (bath sonicated to prevent aggregation) were added to confluent cultures. Cultures were maintained for 24 h to allow phagocytosis of melanin granules. After 24 h, the cultures were imaged using bright-field microscopy (for visualization of uptake of melanin particles) and phase-contrast microscopy (to evaluate any cytotoxicity to cell monolayer).
2.3.2. Spectrophotometric Analysis of Melanin
A total of 24 h post-melanin feeding, cells were detached, centrifuged, and washed in Dulbecco’s phosphate-buffered saline. At this step, the cells in each group were manually counted using a hematocytometer. After this, the cell pellets were resuspended in 1N NaOH and heated at 70 °C for 30 min to dissolve melanin pigment. A total of 100 µL of lysates was transferred to a 96-well plate, and the absorbance of melanin was determined at 405 nm using a Versamax™ microplate reader. A standard curve was generated using sepia melanin that was similarly dissolved in NaOH at various concentrations (Figure S1) and used to estimate melanin amounts reported as pg melanin/cell.
2.4. Experiments on Pigmented HGK Model
2.4.1. Preparation of Toothpaste-Conditioned Medium (TCM)
Three commercial adult toothpastes, Crest 3D White, Sensodyne, and Colgate Optic White, were used in this study. The TCM preparation method was similar to that reported in a previous study [49]. Each toothpaste was weighed and resuspended in culture medium in a sterile tube at 50% w/v and vigorously vortexed to mix the slurry, followed by centrifugation (13,000 rpm, 30 min). The supernatants were recovered and considered as 50% w/v TCM. For all experiments, only freshly prepared TCM was used.
2.4.2. Generation of In Vitro Sepia Melanin-Loaded HGK Model
To compare the response of different kinds of toothpaste on melanin-loaded and unloaded HGKs, cells growing in culture were split into two T-25 tissue culture flasks identically (to keep the same passage). After 72 h culture, 25 µg/mL sepia melanin was added to the second flask, and both T-25 flasks were maintained in the CO2 incubator for 24 h. After 24 h, cells from pigmented and unpigmented cultures were detached and replated (1.45 × 104 cells/well in 0.2 mL culture medium) onto a 96-well tissue culture plate and maintained for 24 h.
2.4.3. Viability of Pigmented and Unpigmented HGKs after Treatment with TCM
TCM of all three toothpastes was diluted using culture medium to ratios of 1:50, 1:100, 1:250, 1:500, and 1:1000 (equivalent to 1%, 0.5%, 0.2%, 0.1%, and 0.05% w/v, respectively) and added to pigmented and unpigmented HGKs that were already cultured in the 96-well plate for 24 h (as reported in Section 2.4.2 above). The cultures were maintained for another 24 h. After this, 20 µL of MTS reagent was combined with 100 µL of culture medium, and the plate was incubated at 37 °C. Subsequently, the absorbance of 100 µL aliquots was recorded at 490 nm using a microplate reader and expressed as a percentage of control to denote cell viabilities.
2.5. Statistical Analysis
Data are presented as mean ± SD. A p-value at a level of <0.05 was regarded as statistically significant. The normality of data was evaluated by the Shapiro–Wilk test, and normality was accepted at p > 0.05. An unpaired, two-tailed student’s t-test was employed to compare differences between the two groups. One-way analysis of variance (ANOVA) with Dunnett’s test was used for comparing multiple groups. The correlation analysis was evaluated by the Pearson’s correlation test. For all statistical analyses, GraphPad Prism software 9.5.0 (San Diego, CA, USA) was used.
3. Results
3.1. Effects of Melanin Loading on HGKs
Qualitative observation of HGKs showed that the cell density was lower for cells treated with sepia melanin at 100, 200, and 400 µg/mL concentrations compared to the untreated control (0 µg/mL) group, with effects more pronounced at the highest concentration of 400 µg/mL where cellular morphology appeared altered, and a lower number of cells remained intact (Figure 1A). Examination of cells under bright-field microscopy showed a concentration-dependent uptake of melanin particles with a perinuclear distribution (Figure 1B).
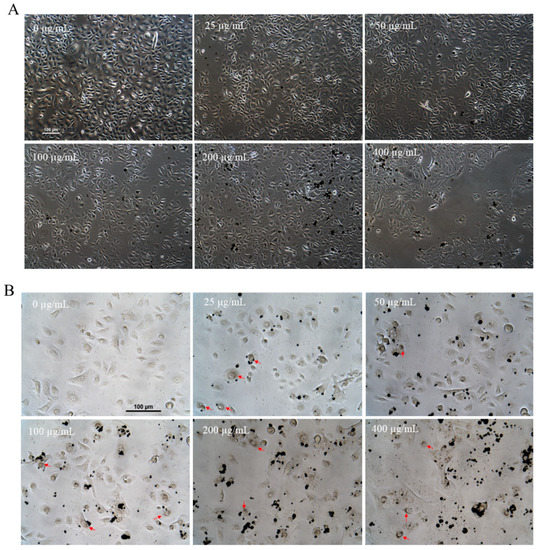
Figure 1.
(A) Representative phase-contrast micrographs of HGKs incubated with sepia melanin (0–400 µg/mL) for 24 h. (B) Representative bright-field images of HGKs after incubation with sepia melanin at various concentrations for 24 h; red arrows denote aggregation of melanin in the perinuclear zone in HGKs.
Cell counts further showed that the mean viabilities of HGKs after exposure to 25 and 50 µg/mL sepia melanin were 97.73% and 98.33%, respectively, and did not differ from the control group. However, significant cytotoxicity was noted at concentrations > 50 µg/mL; cell survival was diminished by 13.69% (p = 0.0489), 32% (p = 0.0002), and 32.15% (p < 0.0001) at 100, 200, and 400 µg/mL concentrations, respectively (Figure 2A).
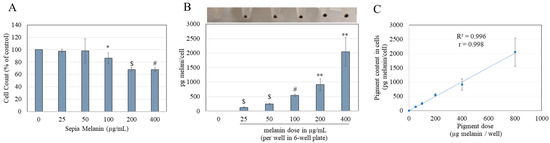
Figure 2.
(A) Viability of HGKs after a 24 h incubation with sepia melanin (0–400 µg/mL) estimated by manual cell count. (B) Melanin amounts in HGKs determined spectrophotometrically after sepia melanin feeding with corresponding photos of cell pellets. (C) Linear correlation between the incubated sepia melanin amounts and cellular pigmentation; R2 and r denote the coefficient of determination and Pearson correlation coefficient, respectively. The significant correlation was obtained with p < 0.0001; (* p < 0.05, ** p < 0.01, $ p < 0.001, and # p < 0.0001 vs. control group, unpaired t-test), and all data are average of at least three independent experiments.
Visual inspection of cell pellets showed darker coloration with increased concentrations of sepia melanin (photo panel; Figure 2B). Quantitation of melanin content in HGKs revealed a significant concentration-dependent uptake of sepia melanin; melanin contents were 129.75, 245.54, 538.87, 910.69, and 2048.16 pg/cell at 25, 50, 100, 200, and 400 µg/mL concentrations, respectively (Figure 2B).
Linear correlation (R2 = 0.996) was achieved for applied concentrations of sepia melanin to that of the melanin content in cells; the correlation coefficient (r = 0.998) was also found to be statistically significant (p < 0.0001), indicative of the ease of controlling pigmentation in cells (Figure 2C). Based on these results, 25 µg/mL sepia melanin was selected for melanin feeding in subsequent experiments as it generated significant melanin content without altering cell count.
3.2. Effects of TCM on Pigmented and Unpigmented HGKs
The scheme of the generation of pigmented and unpigmented HGKs is shown in Figure 3A. HGKs pigmented with 25 µg/mL sepia melanin did not show any changes in viability compared to unpigmented cells as evaluated by MTS assay (Figure S2). Next, results revealed no significant differences between the cytotoxic responses of unpigmented and pigmented HGKs at any concentration to Crest 3D white toothpaste (Figure 3B), Sensodyne toothpaste (Figure 3C), and Colgate Optic White toothpaste (Figure 3D).
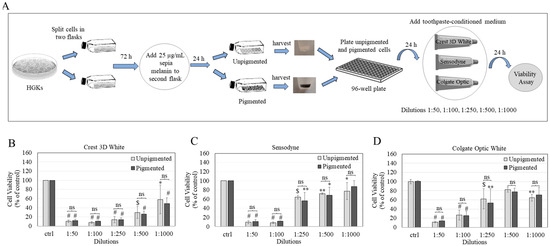
Figure 3.
(A) Scheme to show the steps involved in developing pigmented HGK culture model and its use to examine the cytotoxicity of commercial toothpastes. Viabilities of unpigmented and pigmented HGKs after 24 h treatment with conditioned medium of (B) Crest 3D white toothpaste, (C) Sensodyne toothpaste, and (D) Colgate Optic toothpaste. Data for (B,C) are the average of three independent experiments (n = 3); data for (D) are the average of values combined from two independent experiments (n = 4) (* p < 0.05, ** p < 0.01, $ p < 0.001, and # p < 0.0001 vs. control (ctrl) group by One-way ANOVA with Dunnett’s test); dilutions of each toothpaste were compared for unpigmented and pigmented cell groups by unpaired t-test, and no significance (ns) was found (p > 0.05).
The results of IC50 values for each toothpaste in unpigmented and pigmented HGKs (Table 2) further confirmed no significant difference in cytotoxicity between both groups. However, Crest toothpaste displayed the greatest cytotoxicity to unpigmented HGKs; the cytotoxicity was significantly greater than Sensodyne (p = 0.001) and Colgate toothpaste (p = 0.0196). In pigmented HGKs, Crest toothpaste continued to demonstrate greater cytotoxicity that was also significantly greater than both Sensodyne (p = 0.0121) and Colgate toothpaste (p = 0.0336) (Table 2).

Table 2.
IC50 values of cytotoxicity of toothpastes in unpigmented and pigmented HGKs.
4. Discussion
To our knowledge, this is the first study that reports a model of artificially pigmented HGKs and its use to examine the cytotoxicity of oral care products. Keratinocytes in culture have been shown to accumulate phagocytosed melanin [50]. After keratinocytes phagocytose melanin, it accumulates in the perinuclear zone forming supranuclear caps, characteristic of the distribution found in the in vivo gingiva tissue [5,51], which was also observed in our study, thus establishing the physiological relevance of the pigmented HGK model. We selected sepia melanin at 25 µg/mL to generate an optimized pigmented model that was used for further testing; a similar concentration of sepia melanin has been used in a previous study that generated pigmented dermal dendritic cells [33]. Previously, it was demonstrated that retinal cells loaded with melanosomes at the confluent stage retained phagocytosed melanin stably for many days [44], as loading in confluent cells prevents pigment dilution by cell division. In our experiments that comprised a duration of 48 h after cells were initially loaded with melanin (24 h culture and 24 h treatment with product), we did not anticipate any significant loss of melanin from cells that may affect the response of the experimental model to test products. Owing to the occurrence of binding of drugs and chemicals to melanin [52], it is necessary to identify any potential confounders in our model where melanin might bind to exogenous additives present in the culture medium. Streptomycin is a common antibiotic that is routinely added to the medium of cell cultures. Wiernek et al. [53] incubated synthetic melanin (1 mg/mL) with varying streptomycin concentrations (0.1–10 mM) for 24 h in a cell-free system and showed concentration-dependent binding of streptomycin to melanin. In another study [31], sepia melanin showed greater binding than synthetic melanin to a drug (memantine); the authors concluded that the melanin type and incubation medium influence melanin binding. In our experiments, sepia melanin at a concentration of 25 µg/mL (40 times lower than the concentration of synthetic melanin of study [53]) was added to HGKs that were cultured in a medium supplemented with streptomycin at a concentration of 0.17 mM. Hence, we speculate that the likelihood of any significant binding of streptomycin to sepia melanin that may have interfered in the response of cells to toothpaste dilutions might be minimal, although future studies are warranted to specifically address this. Our standard curve included cell matrix effects that accounted for the background of cells without melanin loading. The use of subcultured cells (after melanin loading) to evaluate the cytotoxicity of TCM also excluded the presence of extracellular melanin in the culture medium that might have interacted with toothpaste and interfered with results [39].
Our results of the greatest cytotoxicity by Crest 3D toothpaste might be attributable to the detergent sodium lauryl sulfate (SLS). However, another toothpaste, Sensodyne, contained Cocamidopropyl betaine (CAPB) with sodium methyl cocoyl taurate. In contrast, Colgate Optic White contained CAPB with SLS. Prior reports that have studied the effects of toothpaste extracts on cells have used an exposure time of 2 min as it is the standard brushing time [54,55]. However, an exposure duration of 24 h has also been used previously [56]. We selected a treatment of 24 h with TCM as it represents residual toothpaste in the oral cavity post-brushing. Mouthwashes are also used alongside toothpaste as part of oral care. A commercial mouthwash (composition listed in Table S1) at different dilutions was also preliminarily examined and found to have similar cytotoxicity to pigmented and unpigmented HGKs after a 24 h treatment (Figure S3).
Interestingly, a previous study also showed that pigmented and unpigmented PC12 neural cells had similar cytotoxicity to amyloid-β [39]. Hence, it seems that melanin-loaded cells might not always protect against toxic insults, but it should be emphasized that the protective effects depend on treatment duration. However, our results showed no differential effects of toothpaste on unpigmented or pigmented gingival cells; whether similar effects occur in the oral cavity where saliva, mucus layer, and microbiota are present warrants future investigation. Interestingly, darkly pigmented skin has a higher barrier function than lightly pigmented skin, owing to which SLS is less irritating to dark-skinned individuals [57,58,59]; whether this is also applicable in the case of dark gingiva and light gingiva is unknown. Further studies to examine the response of SLS-containing toothpaste on pigmented HGKs’ barrier function will also be essential, as some toothpastes are known to alter gingival barrier function [60]. Additionally, the in vivo amounts of melanin in gingiva have yet to be quantified. Hence, we cannot compare the amounts of melanin loaded in HGKs in this study to physiological numbers. Nevertheless, the in vivo amounts of melanin have been reported to range from 0 to 1.25 mg/mL in the human epidermis [61], while another in vitro study suggested that melanocytes from Caucasians (light skin) and African-Americans (dark skin) can be estimated to contain melanin in the range of 25 pg/cell and 250 pg/cell, respectively [62]. This study postulates a first-screen proof-of-principle model to evaluate the impact of oral hygiene products on gingival cells artificially pigmented by melanin and provides a convenient way to control pigmentation to mimic different degrees of gingival pigmentation index.
The results of this study should be considered in light of some limitations. First, a limited set of toothpastes were evaluated on pigmented/unpigmented HGKs. Moreover, the cellular responses of HGKs (pigmented/unpigmented) on toothpastes were assessed only at a single time-point (24 h). Longer time points might be needed to ascertain if pigmented HGKs might be protected from cytotoxicity compared to unpigmented counterparts. For example, a previous study evaluated the cytotoxicity of child toothpaste to gingival fibroblasts after a 72 h duration [63]; hence, a 72 h duration can be employed in further studies. Another limitation is that the HGK pigmented model of this study is a 2D monolayer culture model; thus, it cannot truly recapitulate the multilayered gingival tissue that is achievable in 3D organotypic models that have been used to evaluate the biocompatibility of different oral care materials [64,65]. Moreover, the oral cavity has a complex microenvironment with the presence of microbiota, saliva flow, and mucus layer, which were absent in the in vitro model used in this study. Future studies with pigmented HGK model and biofilms will allow examining the host–biofilm interactions, as has been shown in previous reports that have evaluated the effects of therapeutic agents using such a model, although the keratinocytes used in them were unpigmented [66,67,68]. We propose that the pigmented HGK model with cultured biofilms (single/poly species) can be used as the next step to examine the effects of oral care product biocompatibility and antibacterial efficacies. Finally, the pigmented model we obtained in this study cannot mimic the non-uniform distribution of melanin pigmentation as found in the realistic scenario. For example, it has been shown that gingival pigmentation in vivo may not always be present as a uniform generalized pigmentation; rather, some individuals have gingival pigmentation that can appear as patches or ribbon-like bands [69,70]. Nonetheless, our study provides a promising tool for initial screening using the novel in vitro model of pigmented HGKs.
In conclusion, the pigmented HGK model can have clinical applications to identify biocompatible oral care products that may be better suited for specific racial/ethnic groups to aid in personalized oral care. There is an abundance of over-the-counter (OTC) products that are marketed for oral care. Although this study focused on evaluating a limited number of toothpastes, further studies that include the examination of a large number of commercial toothpastes (cosmetic and medical-grade) and other oral care products will significantly help expand the suitability of this model. This model can also be employed to examine the biocompatibility of novel experimental toothpaste formulations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/oral3020021/s1, Figure S1. Standard curve for sepia melanin standard samples (0–200 μg; 0–2 μg/μL) according to the measured absorbances at 405 nm. Each data point is the mean ± SD of samples in duplicates. Figure S2. MTS assay absorbances of unpigmented HGKs and HGKs pigmented with 25 µg/mL sepia melanin; both groups were insignificant (p > 0.05) as analyzed by unpaired t-test; data are mean ± SD of three independent experiments. Figure S3. Viabilities of unpigmented and pigmented HGKs after treatment with mouthrinse at various dilutions for 24 h; Mouthrinse (considered 100%) was diluted using culture medium to ratios of 1:2, 1:5, 1:10, and 1:1000 to yield concentrations of 50%, 20%, 10%, and 0.1% v/v, respectively; data are mean ± SD of one experiment with duplicate determinations. Table S1. Composition of mouthrinse used in this study.
Funding
This research was funded by unrestricted funds available from the Stony Brook Foundation. The funder had no role in the design of the study, experimental data collection or analysis, manuscript preparation, or the decision to publish.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available from the corresponding author upon reasonable request.
Acknowledgments
The author acknowledges the use of lab resources under Sanford Simon (Department of Biochemistry and Cell Biology, Stony Brook University).
Conflicts of Interest
The author declares no conflict of interest.
References
- Groeger, S.E.; Meyle, J. Epithelial barrier and oral bacterial infection. Periodontology 2015, 69, 46–67. [Google Scholar] [CrossRef] [PubMed]
- Dummett, C.O.; Barens, G. Pigmentation of the oral tissues: A review of the literature. J. Periodontol. 1967, 38, 369–378. [Google Scholar] [CrossRef]
- Moneim, R.A.A.; El Deeb, M.; Rabea, A.A. Gingival pigmentation (cause, treatment and histological preview). Future Dent. J. 2017, 3, 1–7. [Google Scholar] [CrossRef]
- Masilana, A.; Khammissa, R.A.; Lemmer, J.; Feller, L. Physiological oral melanin pigmentation in a South African sample: A clinical study. J. Investig. Clin. Dent. 2017, 8, e12258. [Google Scholar] [CrossRef]
- Schroeder, H.E. Melanin containing organelles in cells of the human gingiva: II. Keratinocytes. J. Periodontal Res. 1969, 4, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Feller, L.; Masilana, A.; Khammissa, R.A.; Altini, M.; Jadwat, Y.; Lemmer, J. Melanin: The biophysiology of oral melanocytes and physiological oral pigmentation. Head Face Med. 2014, 10, 8. [Google Scholar] [CrossRef]
- Jha, N.; Ryu, J.J.; Wahab, R.; Al-Khedhairy, A.A.; Choi, E.H.; Kaushik, N.K. Treatment of oral hyperpigmentation and gummy smile using lasers and role of plasma as a novel treatment technique in dentistry: An introductory review. Oncotarget 2017, 8, 20496. [Google Scholar] [CrossRef]
- Bergamaschi, O.; Kon, S.; Doine, A.; Ruben, M. Melanin repigmentation after gingivectomy: A 5-year clinical and transmission electron microscopic study in humans. Int. J. Periodontics Restor. Dent. 1993, 13, 85–92. [Google Scholar]
- Rotbeh, A.; Kazeminia, M.; Rajati, F. Global prevalence of oral pigmentation and its related factors: A systematic review and meta-analysis. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, e411–e424. [Google Scholar] [CrossRef]
- Rosebush, M.S.; Briody, A.N.; Cordell, K.G. Black and brown: Non-neoplastic pigmentation of the oral mucosa. Head Neck Pathol. 2019, 13, 47–55. [Google Scholar] [CrossRef]
- Peeran, S.W.; Ramalingam, K.; Peeran, S.A.; Altaher, O.B.; Alsaid, F.M.; Mugrabi, M.H. Gingival pigmentation index proposal of a new index with a brief review of current indices. Eur. J. Dent. 2014, 8, 287–290. [Google Scholar] [CrossRef]
- Nightingale, K.; Chinta, S.; Agarwal, P.; Nemelivsky, M.; Frisina, A.; Cao, Z.; Norman, R.; Fisch, G.; Corby, P. Toothbrush efficacy for plaque removal. Int. J. Dent. Hyg. 2014, 12, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Minwalla, L.; Zhao, Y.; Boissy, R.E.; Le Poole, I.C.; Wickett, R.R. Keratinocytes play a role in regulating distribution patterns of recipient melanosomes in vitro. J. Investig. Dermatol. 2001, 117, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Oral Care Market Size & Share to Surpass $51.37 Billion by 2030. Available online: https://www.globenewswire.com/en/news-release/2023/04/11/2644137/0/en/Oral-Care-Market-Size-Share-to-Surpass-51-37-Billion-by-2030-Vantage-Market-Research.html (accessed on 14 May 2023).
- Natesan, S.C.; Ramakrishnan, B.P.; Krishnapillai, R.; Thomas, P. Biophysiology of oral mucosal melanocytes. J. Health Sci. Res. 2019, 10, 47–51. [Google Scholar] [CrossRef]
- Vranic, E.; Lacevic, A.; Mehmedagic, A.; Uzunovic, A. Formulation ingredients for toothpastes and mouthwashes. Bosn. J. Basic Med. Sci. 2004, 4, 51. [Google Scholar] [CrossRef]
- Lippert, F. An introduction to toothpaste-its purpose, history and ingredients. In Toothpastes; Karger Publishers: Basel, Switzerland, 2013; Volume 23, pp. 1–14. [Google Scholar]
- Forward, G.C.; James, A.H.; Barnett, P.; Jackson, R.J. Gum health product formulations: What is in them and why? Periodontology 1997, 15, 32–39. [Google Scholar] [CrossRef]
- Jang, S.-O.; Shim, Y.-S.; Choi, Y.-R. Evaluation of cytotoxicity of child toothpaste. Sci. Adv. Mater. 2016, 8, 331–335. [Google Scholar] [CrossRef]
- Souza-Rodrigues, R.D.; Ferreira, S.d.S.; D’Almeida-Couto, R.S.; Lachowski, K.M.; Sobral, M.Â.P.; Marques, M.M. Choice of toothpaste for the elderly: An in vitro study. Braz. Oral Res. 2015, 29, S1806. [Google Scholar] [CrossRef]
- Kvam, E.; Dahle, J. Pigmented melanocytes are protected against ultraviolet-A-induced membrane damage. J. Investig. Dermatol. 2003, 121, 564–569. [Google Scholar] [CrossRef]
- Svensson, S.P.; Lindgren, S.; Powell, W.; Green, H. Melanin inhibits cytotoxic effects of doxorubicin and daunorubicin in MOLT 4 cells. Pigment. Cell Res. 2003, 16, 351–354. [Google Scholar] [CrossRef]
- Cardinali, G.; Bolasco, G.; Aspite, N.; Lucania, G.; Lotti, L.V.; Torrisi, M.R.; Picardo, M. Melanosome transfer promoted by keratinocyte growth factor in light and dark skin-derived keratinocytes. J. Investig. Dermatol. 2008, 128, 558–567. [Google Scholar] [CrossRef]
- Ebanks, J.P.; Koshoffer, A.; Wickett, R.R.; Schwemberger, S.; Babcock, G.; Hakozaki, T.; Boissy, R.E. Epidermal keratinocytes from light vs. dark skin exhibit differential degradation of melanosomes. J. Investig. Dermatol. 2011, 131, 1226–1233. [Google Scholar] [CrossRef]
- Man, M.-Q.; Lin, T.-K.; Santiago, J.L.; Celli, A.; Zhong, L.; Huang, Z.-M.; Roelandt, T.; Hupe, M.; Sundberg, J.P.; Silva, K.A. Basis for enhanced barrier function of pigmented skin. J. Investig. Dermatol. 2014, 134, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.; Aotaki-Keen, A.; Putkey, F.; Hjelmeland, L. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp. Eye Res. 1996, 62, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Boulton, M.E. Studying melanin and lipofuscin in RPE cell culture models. Exp. Eye Res. 2014, 126, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Boulton, M.; Marshall, J. Repigmentation of human retinal pigment epithelial cells in vitro. Exp. Eye Res. 1985, 41, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A. Melanogenesis in the ink gland of Sepia officinalis. Pigment. Cell Res. 2003, 16, 517–522. [Google Scholar] [CrossRef]
- Magarelli, M.; Passamonti, P.; Renieri, C. Purification, characterization and analysis of sepia melanin from commercial sepia ink (Sepia Officinalis). Rev. CES Med. Vet. Y Zootec. 2010, 5, 18–28. [Google Scholar]
- Koeberle, M.J.; Hughes, P.M.; Skellern, G.G.; Wilson, C.G. Binding of memantine to melanin: Influence of type of melanin and characteristics. Pharm. Res. 2003, 20, 1702–1709. [Google Scholar] [CrossRef]
- Schroeder, R.L.; Double, K.L.; Gerber, J.P. Using Sepia melanin as a PD model to describe the binding characteristics of neuromelanin–A critical review. J. Chem. Neuroanat. 2015, 64, 20–32. [Google Scholar] [CrossRef]
- Müller, M.; Elsässer, H.P. Alterations in the secretory pattern of dermal dendritic cells following melanin uptake. Cell Tissue Res. 2013, 352, 599–610. [Google Scholar] [CrossRef]
- Song, Q.; Risco, R.; Latina, M.; Berthiaume, F.; Nahmias, Y.; Yarmush, M. Selective targeting of pigmented retinal pigment epithelial (RPE) cells by a single pulsed laser irradiation: An in vitro study. Opt. Express 2008, 16, 10518–10528. [Google Scholar] [CrossRef]
- Seagle, B.-L.L.; Gasyna, E.M.; Mieler, W.F.; Norris, J.R. Photoprotection of human retinal pigment epithelium cells against blue light-induced apoptosis by melanin free radicals from Sepia officinalis. Proc. Natl. Acad. Sci. USA 2006, 103, 16644–16648. [Google Scholar] [CrossRef]
- Yan, X.; Wang, T.-M.; Ming, Y.-C.; Yeh, Y.-M.; Chen, T.-Y.; Pang, J.-H.S. Melanin uptake reduces cell proliferation of human epidermal keratinocytes. J. Cosmet. Dermatol. Sci. Appl. 2015, 5, 300. [Google Scholar] [CrossRef]
- Goenka, S.; Simon, S.R. Depigmenting effect of Xanthohumol from hop extract in MNT-1 human melanoma cells and normal human melanocytes. Biochem. Biophys. Rep. 2021, 26, 100955. [Google Scholar] [CrossRef]
- Östergren, A.; Lindquist, N.G.; Brittebo, E.B. Differential effects of dopamine melanin on norharman-induced toxicity in PC12 cells. J. Neural Transm. 2007, 114, 909–918. [Google Scholar] [CrossRef]
- Östergren, A.; Svensson, A.L.; Lindquist, N.G.; Brittebo, E.B. Dopamine melanin-loaded PC12 cells: A model for studies on pigmented neurons. Pigment. Cell Res. 2005, 18, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Eves, P.; Smith-Thomas, L.; Hedley, S.; Wagner, M.; Balafa, C.; Neil, S.M. A comparative study of the effect of pigment on drug toxicity in human choroidal melanocytes and retinal pigment epithelial cells. Pigment. Cell Res. 1999, 12, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.-Y.; Choi, N.; Lee, J.U.; Lee, E.J.; Kim, J.Y.; Choi, W.J.; Oh, S.H.; Sung, J.-H. Marliolide derivative induces melanosome degradation via Nrf2/p62-mediated autophagy. Int. J. Mol. Sci. 2021, 22, 3995. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-I.; Sohn, K.-C.; Hong, D.-K.; Lee, Y.; Kim, C.D.; Yoon, T.-J.; Park, J.W.; Jung, S.; Lee, J.-H.; Lee, Y.H. Melanosome uptake is associated with the proliferation and differentiation of keratinocytes. Arch. Dermatol. Res. 2014, 306, 59–66. [Google Scholar] [CrossRef]
- Olchawa, M.M.; Szewczyk, G.M.; Zadlo, A.C.; Krzysztynska-Kuleta, O.I.; Sarna, T.J. The effect of aging and antioxidants on photoreactivity and phototoxicity of human melanosomes: An in vitro study. Pigment. Cell Melanoma Res. 2021, 34, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Hellinen, L.; Hagström, M.; Knuutila, H.; Ruponen, M.; Urtti, A.; Reinisalo, M. Characterization of artificially re-pigmented ARPE-19 retinal pigment epithelial cell model. Sci. Rep. 2019, 9, 13761. [Google Scholar] [CrossRef] [PubMed]
- De la Calle, I.; Soto-Gómez, D.; Pérez-Rodríguez, P.; López-Periago, J.E. Particle size characterization of sepia ink eumelanin biopolymers by SEM, DLS, and AF4-MALLS: A comparative study. Food Anal. Methods 2019, 12, 1140–1151. [Google Scholar] [CrossRef]
- Mbonyiryivuze, A.; Nuru, Z.; Ngom, B.D.; Mwakikunga, B.W.; Dhlamini, S.M.; Park, E.; Maaza, M. Morphological and chemical composition characterization of commercial sepia melanin. Am. J. Nanomater. 2015, 3, 22–27. [Google Scholar] [CrossRef]
- Mbonyiryivuze, A.; Mwakikunga, B.W.; Dhlamini, S.M.; Maaza, M. Fourier transform infrared spectroscopy for sepia melanin. Phys. Mater. Chem. 2015, 3, 25–29. [Google Scholar]
- Katritzky, A.R.; Akhmedov, N.G.; Denisenko, S.N.; Denisko, O.V. 1H NMR spectroscopic characterization of solutions of Sepia melanin, Sepia melanin free acid and human hair melanin. Pigment. Cell Res. 2002, 15, 93–97. [Google Scholar] [CrossRef]
- Qi, C.; Peng, X.; Yuan, S.; Zhang, M.; Xu, X.; Cheng, X. Evaluation of the Antibacterial and Anti-Inflammatory Effects of a Natural Products-Containing Toothpaste. Front. Cell. Infect. Microbiol. 2022, 98, 827643. [Google Scholar] [CrossRef]
- Ando, H.; Niki, Y.; Yoshida, M.; Ito, M.; Akiyama, K.; Kim, J.H.; Yoon, T.J.; Lee, J.H.; Matsui, M.S.; Ichihashi, M. Keratinocytes in culture accumulate phagocytosed melanosomes in the perinuclear area. Pigment. Cell Melanoma Res. 2010, 23, 129–133. [Google Scholar] [CrossRef]
- Yussif, N.M.; Koranyb, N.; Abbassc, M. Evidence of the effect of intraepidermic vitamin C injection on melanocytes and keratinocytes in gingival tissues: In vivo study. Dentistry 2017, 7, 2161-1122. [Google Scholar] [CrossRef]
- Bridelli, M.; Ciati, A.; Crippa, P. Binding of chemicals to melanins re-examined: Adsorption of some drugs to the surface of melanin particles. Biophys. Chem. 2006, 119, 137–145. [Google Scholar] [CrossRef]
- Wiernek, B.K.; Pilawa, B.; Zdybel, M.; Buszman, E.; Wrześniok, D. Interaction of free radicals of DOPA-melanin-streptomycin complexes with paramagnetic oxygen O2. J. Appl. Biomed. 2014, 12, 161–169. [Google Scholar] [CrossRef]
- Gallagher, A.; Sowinski, J.; Bowman, J.; Barrett, K.; Lowe, S.; Patel, K.; Bosma, M.L.; Creeth, J.E. The effect of brushing time and dentifrice on dental plaque removal in vivo. Am. Dent. Hyg. Assoc. 2009, 83, 111–116. [Google Scholar]
- Winterfeld, T.; Schlueter, N.; Harnacke, D.; Illig, J.; Margraf-Stiksrud, J.; Deinzer, R.; Ganss, C. Toothbrushing and flossing behaviour in young adults—A video observation. Clin. Oral Investig. 2015, 19, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Camargo, S.E.A.; Milhan, N.V.M.; Saraiva, F.d.O.; Oliveira, J.R.d.; Oliveira, L.D.d.; Camargo, C.H.R. Are Desensitizing Toothpastes Equally Biocompatible and Effective Against Microorganisms? Braz. Dent. J. 2017, 28, 604–611. [Google Scholar] [CrossRef]
- Hicks, S.P.; Swindells, K.J.; Middelkamp-Hup, M.A.; Sifakis, M.A.; González, E.; González, S. Confocal histopathology of irritant contact dermatitis in vivo and the impact of skin color (black vs. white). J. Am. Acad. Dermatol. 2003, 48, 727–734. [Google Scholar] [CrossRef]
- Berardesca, E.; Maibach, H.I. Racial differences in sodium lauryl sulphate induced cutaneous irritation: Black and white. Contact Dermat. 1988, 18, 65–70. [Google Scholar] [CrossRef]
- Lee, E.; Kim, S.; Lee, J.; Cho, S.A.; Shin, K. Ethnic differences in objective and subjective skin irritation response: An international study. Ski. Res. Technol. 2014, 20, 265–269. [Google Scholar] [CrossRef]
- Groeger, S.; Schott, S.; Windhorst, A.; Meyle, J. Effects of Toothpaste on the Gingival Barrier Function in vitro. Oral Health Dent. Manag. 2016, 15, 3–4. [Google Scholar]
- Kalia, S.; Zhao, J.; Zeng, H.; McLean, D.; Kollias, N.; Lui, H. Melanin quantification by in vitro and in vivo analysis of near-infrared fluorescence. Pigment. Cell Melanoma Res. 2018, 31, 31–38. [Google Scholar] [CrossRef]
- Haywood, R.M.; Lee, M.; Linge, C. Synthetic melanin is a model for soluble natural eumelanin in UVA-photosensitised superoxide production. J. Photochem. Photobiol. B Biol. 2006, 82, 224–235. [Google Scholar] [CrossRef]
- Pecci-Lloret, M.P.; López-García, S.; Rodríguez-Lozano, F.J.; Álvarez-Novoa, P.; García-Bernal, D. In Vitro Biocompatibility of Several Children’s Toothpastes on Human Gingival Fibroblasts. Int. J. Environ. Res. Public Health 2022, 19, 2954. [Google Scholar] [CrossRef] [PubMed]
- Klausner, M.; Handa, Y.; Aizawa, S. In vitro three-dimensional organotypic culture models of the oral mucosa. In Vitro Cell. Dev. Biol. Anim. 2021, 57, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, B.; Yang, J.; Roohpour, N. Biologic evaluation of devices with chronic exposure using 3D human gingival model. Front. Bioeng. Biotech. 2016, 4, 01671. [Google Scholar]
- Brown, J.L.; Johnston, W.; Delaney, C.; Rajendran, R.; Butcher, J.; Khan, S.; Bradshaw, D.; Ramage, G.; Culshaw, S. Biofilm-stimulated epithelium modulates the inflammatory responses in co-cultured immune cells. Sci. Rep. 2019, 9, 15779. [Google Scholar] [CrossRef]
- Millhouse, E.; Jose, A.; Sherry, L.; Lappin, D.F.; Patel, N.; Middleton, A.M.; Pratten, J.; Culshaw, S.; Ramage, G. Development of an in vitro periodontal biofilm model for assessing antimicrobial and host modulatory effects of bioactive molecules. BMC Oral Health 2014, 14, 80. [Google Scholar] [CrossRef]
- Peyyala, R.; Kirakodu, S.; Novak, K.; Ebersole, J.L. Epithelial interleukin-8 responses to oral bacterial biofilms. Clin. Vaccine Immunol. 2011, 18, 1770–1772. [Google Scholar] [CrossRef]
- John, S.; Arun, K.; Talwar, A.; Ittycheria, P.G.; Cherian, S.A.; Clements, J. Assessment of Parameters Influencing Physiologic Gingival Pigmentation Using a Novel Classification System. Avicenna J. Dent. Res. 2019, 11, 111–115. [Google Scholar] [CrossRef]
- Batra, P.; Daing, A.; Azam, I.; Miglani, R.; Bhardwaj, A. Impact of altered gingival characteristics on smile esthetics: Laypersons’ perspectives by Q sort methodology. Am. J. Orthod. Dentofac. Orthop. 2018, 154, 82–90.e2. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).