Abstract
High frequencies of MYD88L265P mutation are observed in IgM monoclonal gammopathies, and specifically in Waldenström macroglobulinemia (WM), indicating this mutation as a potential disease biomarker. Given the fact that MYD88L265P mutation has been described as a key driver mutation, has increased our understanding of the biology behind MYD88 signaling and helped us to identify the functional components which could be targeted. On the other hand, the absence of the MYD88L265P mutation in patients with IgM monoclonal gammopathies has been associated with a higher risk of transformation to aggressive lymphomas, resistance to several therapies, and shorter overall survival. The present review focuses on the molecular mechanisms that shape the signaling pattern in MYD88WT cells, as well as on the clinical implications and therapeutic challenges of WM patients that harbor the MYD88WT genotype.
1. Introduction
Mature B-cell neoplasms are clonal tumors of B-cells characterized as a group of diseases with a highly heterogeneous profile, both biologically and clinically. Depending on the entity, the clinical course may range from an indolent to an aggressive disease. Mature B-cell neoplasms constitute more than 90% of lymphoid neoplasms and, based on histology and immunophenotype, they account for 34 different entities, including diffuse large B-cell lymphoma (DLBCL), chronic lymphocytic lymphoma (CLL), Burkitt lymphoma (BL), lymphoplasmacytic lymphoma (LPL)/Waldenström macroglobulinemia (WM), splenic marginal zone lymphoma (SMZL), nodal marginal zone lymphoma (NMZL), mantle cell lymphoma (MCL), follicular lymphoma (FL), and hairy cell leukemia (HCL) [1]. They exhibit a broad spectrum of characteristic cytogenetic abnormalities and genetic aberrations, which are partly characteristic among different B-cell neoplasms but are (most of the time) not specific enough for a definitive diagnosis. Some of the cytogenetic abnormalities include recurrent translocations such as t(11;14) (q13;q32) seen in >95% cases of MCL, t(14;18) (q32;q21) seen in 90% cases of FL, t(8;14) (q24;q32) seen in 80% cases of BL, and 6q deletion (del6q) seen in 27% cases of WM [2,3,4,5] while genetic aberrations include gene mutations, such as BRAF V600E in HCL, immunoglobulin heavy chain gene (IGHV) in CLL, and MYD88 L265P in WM [6,7].
IgM monoclonal gammopathy is a heterogeneous group of B-cell/plasma cell clonal diseases that includes a range of conditions from monoclonal gammopathy of undetermined significance to Waldenström macroglobulinemia, IgM multiple myeloma, and less common, other B-cell neoplasms secreting IgM.
Studies by Treon et al. and other researchers suggested the MYD88L265P mutation is present in >90% of WM, and that it could be important for the differential diagnosis of WM [8] vs. plasma cell malignancies. This mutation is also present in various other B-cell neoplasms such as SMZL, CLL, and DLBCL, but at a lower frequency [9,10,11,12,13]. Studies have shown that WM patients lacking the MYD88L265P may be less responsive to Bruton’s tyrosine kinase (BTK) inhibitors [14], which may also be associated with a lower number of tumor cells and lower International Prognostic Scoring System score at presentation [11]. In the most recent WHO nomenclature and classification, MYD88 wild-type (MYD88WT) does not exclude the diagnosis of WM; however, it may be associated with a genetic profile other than MYD88L265P WM. Hence, the prognostic impact of MYD88WT genotype [11] requires further study. In this review, we aim to explore the latest findings on the MYD88WT genotype in various B-cell neoplasms, focusing on its role in tumor biology and its association with therapeutic challenges.
2. MYD88: Role, Pathway, Origin of Mutation
MYD88 plays an important role in the functional integrity of the innate immune response. The MYD88 gene was first described in the 1990s as a primary differentiation response gene which is upregulated during IL6-induced terminal differentiation and growth arrest. It encodes for a protein called myeloid differentiation primary response 88 (MYD88), located in the cytosol, which is involved in the signaling pathways within immune cells triggered by Toll-like receptors (TLRs) and interleukin-1 receptors (IL-1Rs). The MYD88 gene is located on human chromosome 3p22.2. It spans approximately 11.7 kilobases and consists of five exons [15]. In normal physiology, MYD88 acts as an adaptor of inflammatory signaling via the canonical NF-κB pathway. The MYD88 protein contains a death domain (DD), an intermediate linker domain (ID), and a Toll/IL-1 receptor (TIR) domain at the C-terminus. The DD enables protein–protein interactions; the absence of ID has been associated with the inability of MYD88 to support signaling [16] while the TIR domain mediates the downstream signaling cascade by interacting with TLRs and IL-1Rs. These domains are essential for MYD88’s function in innate immune signaling [17,18]. Upon activation of TLRs or IL-1Rs, MYD88 is recruited to the receptor complex, leading to the formation of a signaling complex known as the Myddosome. This complex acts as a platform for the recruitment of downstream signaling molecules; activated MYD88 recruits IL-1 receptor-associated kinases (IRAKs), a serine-threonine kinase, and together they phosphorylate IRAK1 and IRAK2 which, in turn, interact with TNF receptor-associated factor 6 (TRAF6), initiating the activation of various signaling pathways, including transforming growth factor beta-activated kinase 1 (TAK1), mitogen-activated protein kinase (MAPK), and TAK1-binding protein (TAB) [18,19]. Activation of MYD88-dependent signaling pathways leads to the production of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-6 (IL-6), as well as the expression of co-stimulatory molecules necessary for an effective immune response [19]. Ngo et al. were the first to identify that inhibition of MYD88 signaling via a non-synonymous, gain-of-function mutation in MYD88 gene, leading to an amino acid change of leucine to proline at position 265 (NM_002468.5) (in the TIR domain), resulted in decreased NFκB activity and enhanced survival of activated B-cell-type diffuse large-cell lymphoma cell lines [12]. Other recurrent mutations in MYD88 have also been reported, although the impact of these mutations is still under investigation due to their low prevalence [20]. As previously mentioned, MYD88 DD and ID are responsible for downstream signal propagation via IRAKs, whereas the TIR domain integrates signals from upstream TLR and IL1R [21,22,23]. In the case of the MYD88L265P mutation, the TIR domain of MYD88, where this mutation resides, is highly activated compared to the MYD88WT, and this increases downstream signaling and formation of the Myddosome complex. It has been shown that mutated MYD88 recruits IRAK1 and, together with IRAK4, promotes the survival of activated B-cell-(ABC)-diffuse large B-cell lymphoma (DLBCL) cell lines [12]. Hence it has been hypothesized that MYD88L265P occurs in B-cell neoplasms where there is a strong selection for aberrant NFκB signaling [23]. Since MYD88L265P constitutively activates the NFκB pathway, it is contemplated as an important oncogenic driver in B-cell lymphomas [12,24,25]. Non-L265P mutations (M232T, S243N, S222R, and T294P) have an intermediate effect on NFκB pathway signaling compared to the MYD88WT, which shows the lowest activity [12]. In addition to NFκB activation, L265P induces B-cell proliferation, which is accompanied with the induction of TNFAIP3 [26]. However, although studies have shown that L265P triggers the anti-apoptotic NFκB signaling that, in turn, enables cell survival during B-cell development, is not capable of providing a continuous B-cell clonal selection on its own, and for this reason, a second somatic mutation is required [26]. In WM, these mutations usually reside in genes such as CXCR4, which is the second most mutated gene, TNFAIP3, CD79 A/B, and ARID1 A/B [27,28]. In WM, patients who harbor the L265P mutation have also been reported to bear a mutation in the 196 tyrosine residue of CD79B gene, leading to a better response to BTK-based therapies [29].
In addition to NFκB pathway signaling, the BCR pathway also plays an important role in B-cell survival and proliferation and the oncogenesis of various B-cell lymphomas in combination with MYD88 mutations [30]. Within the BCR signaling cascade, BTK acts as an integral protein which forms complexes with MYD88L265P but not MYD88WT cells [31]. Furthermore, the level of phosphorylated BTK is higher in WM cells with L265P mutation than lymphoma cells with WT MYD88 [31]. Therefore, inhibition of BTK would result in the disruption of the MYD88L265P complex but would not significantly affect the MYD88WT cells.
3. MYD88 Mutation Detection Assays
Currently used methods to detect MYD88L265P mutation most often involve allele-specific polymerase chain reaction (AS-PCR), ddPCR and Sanger sequencing, or use of NGS-based protocols in unsorted or sorted (for Sanger sequencing or NGS) bone marrow (BM) aspirates of patients with IgM monoclonal gammopathies [32,33,34,35,36,37,38]. The sensitivity of the molecular assay for the detection of MYD88L265P should not exceed a detection limit of 10−3. It has been shown that conventional polymerase chain reaction (PCR) and Sanger sequencing–based methods for MYD88 mutational detection have a low sensitivity of 25%, and although fairly described, should be considered especially when used in non-selected B-cells [39,40]. Non-L265P MYD88 mutations have also been identified in patients with WM, including S219C, M232T, and S243N [41,42]. Furthermore, the evaluation of cell-free DNA (cfDNA) for the mutational characterization and monitoring of disease burden has recently been described in several hematological malignancies, including IgM monoclonal gammopathies, and has shown remarkable results [43,44,45]. It is a less invasive, patient-friendly test that could provide a good diagnostic yield, even comparable to BM, but the challenges in the detection sensitivity should be evaluated. Data so far have shown that only highly sensitive techniques such as ddPCR or Cast-PCR should be used for the detection of MYD88L265P mutation in cfDNA [36,38]. However, all these techniques, although promising, need to be standardized and implemented in prospective studies before they can be used in clinical practice; therefore, the current recommendation for molecular analysis is to perform BM aspiration at diagnosis [3].
4. MYD88 Mutation Status in B-Cell Neoplasms
L265P mutation was first reported in DLBCL [46]. The study by Ngo et al. found that MYD88 mutations are more frequently seen in the activated B-cell-like subtype of DLBCL (ABC-DLBCL) at a frequency of 29% of cases, rather than the germinal center B-cell-like subtype (GCB-DLBCL) where the mutated cases are rare to absent [12]. The mutation is also frequent in the primary DLBCL of the central nerve system (70%), in primary cutaneous PCDLBCL leg-type (54%), and in testicular DLBCL (74%) [30].
Lymphoplasmacytic lymphoma (LPL) is a B-cell neoplasm characterized by the abnormal growth and clonal proliferation of small mature B lymphocytes and plasma cells in the bone marrow and lymphoid tissues. WM is a distinct clinical entity of LPL characterized by the presence of lymphoplasmacytic bone marrow infiltration and the secretion of monoclonal IgM immunoglobulin [1] WM represents about 95% of LPL cases, based on the presence of monoclonal IgM, while other types of LPL produce either IgA or IgG monoclonal immunoglobulins (non-IgM LPL) [47]. Treon et al. ware the first to identify the presence of L265P mutation in 91% of WM patients compared to the frequency of 25% seen in non-IgM LPL [8].
The presence of disease-associated symptoms distinguishes the symptomatic from the asymptomatic/smoldering WM [48], whereas those patients with an IgM serum protein of less 3 g/dL and a BM infiltration of less than 10% but no symptoms, are classified as IgM monoclonal gammopathy of undetermined significance (MGUS) which is considered the pre-malignant phase of WM (or rarely IgM myeloma) [49,50]. Patients with IgM-MGUS are at higher risk for developing WM, DLBCL, and mucosa-associated lymphoid tissue (MALT) lymphoma, as well as chronic lymphocytic leukemia. MYD88L265P is found in 50–80% of patients with IgM-MGUS and cannot be used to differentiate WM (symptomatic or asymptomatic) from IgM-MGUS [32,33,34,35,51]. Studies have shown that IgM-MGUS patients with MYD88L265P are at greater risk of progression to WM [8,33,34,52,53] while the mutation has not been found in IgG or IgA MGUS [34,52]. On the other hand, in IgM myeloma, which is commonly characterized by the presence of t(11;14) translocation in clonal plasma cells, a typical cytogenetic feature of multiple myeloma (MM), MYD88L265P mutation is absent.
Marginal-zone lymphoma (MZL) is an indolent disease comprising 7% of all non-Hodgkin lymphomas [54]. There are three subtypes of MZL: MALT lymphoma, nodal marginal-zone lymphoma (NMZL), and splenic marginal-zone lymphoma (SMZL). Two small series report the presence of L265P mutation in 4–21% of MZL cases [1,9,55]. Interestingly, those with MYD88L265P were also more likely to present with an IgM paraprotein [9].
Finally, the MYD88L265P mutation seems to be absent in primary mediastinal B-cell lymphoma [12,20,56] and primary cutaneous follicle center lymphoma, and it is rarely present in hairy cell leukemia (1.1%) [10,57,58,59], Burkitt lymphoma (1.5%) [12], follicular lymphoma (1.9%), [23,57,60,61,62] and CLL (2.5%) [23,55,57,63,64,65].
5. MYD88WT Genotype in IgM Monoclonal Gammopathies
Patients with MYD88WT genotype have not been studied extensively due to the low prevalence of this genotype; hence, the effect of this genotype on the disease outcome of patients with IgM monoclonal gammopathies is still unclear. While most WM cases have mutated MYD88 gene, 5–10% do not carry MYD88 mutations. Some studies show that WT WM patients may have a shorter overall survival (OS) (10-year OS of 73% in WT versus 97% in mutated patients) [66,67] while other studies indicate that the OS is not affected in this subgroup of patients [28,68]. Treon et al. suggested that although MYD88WT patients with suspected WM fulfil the WHO criteria for WM diagnosis, around 30% have an alternate diagnosis [69] such as IgM MM, where the predominant plasma cell compartment and the high IgM levels are the main characteristics [70]. A study by Lee et al. showed that DLBCL patients with L265P had a statistically significant inferior overall survival compared to DLBCL patients with the WT genotype [57]. In other subtypes of B-NHL, such as CLL and SMZL, MYD88L265P is associated with superior survival compared to WT MYD88 [71,72,73]. In IgM-MGUS patients, although the presence of MYD88L265P mutation has been associated with greater risk of progression to WM [34,52], most IgM-MGUS patients never progress to WM or other lymphoproliferative disorders, so this mutation cannot be considered a unique pathogenic factor in WM, and other WM precursors might exist rather than the transformation from IgM-MGUS [51,74]. In contrast to the “classic” IgM-MGUS cases that typically evolve to WM or even MZL, IgM-MGUS cases with a plasma cell infiltrate, rather than a predominant B-cell clone, may serve as precursors to IgM MM [69]. A study by Treon et al. showed that among patients with suspected MYD88WT WM, 10% had findings consistent with IgM myeloma characterized by predominant plasma cell clone and a significantly higher IgM level compared to MYD88WT WM patients [69].
Few studies have compared the clinical and laboratory features of MYD88WT versus MYD88L265P cases in WM. Patkar et al. found that WT patients had lower hemoglobin and IgM paraprotein levels, lower tumor burden in the bone marrow, lower prognostic score, higher total leukocyte counts (TLC), and higher platelet counts compared to MYD88 mutated cases [11]. Treon et al., in a study which included 150 patients with B-cell neoplasms, also showed lower serum IgM levels, TLC, and bone marrow infiltration, but also an association with older age in WT patients. Given the low prevalence of WT genotype across patients with IgM monoclonal gammopathies, some experts in the WM field consider the disease with this genotype to be an entirely separate clinicopathological entity, distinct from the typical WM associated with MYD88 gene mutation, and are proposing that the presence of the L265P mutation should be considered as a WM-defining feature [66]. However, since the WM disease characteristics and severity assessed by the IPSS-WM, the bone marrow involvement, and patients’ performance status are similar between the two subgroups, the diagnosis could not be other than an active WM with a different genotype status. Therefore, more studies on MYD88WT patients need to be conducted, wherein the combination of high throughput molecular assays, such as single-cell RNA seq analysis and a close follow-up of these patients, will lead to a better understanding of this genetically distinct subgroup of patients at both the biological and the clinical level.
In terms of the genomic landscape of WM patients harboring the MYD88WT genotype, Hunter et al. provided the first—and, to date, only—study, aiming to explore the genomic and transcriptomic characteristics of WM WT patients in a cohort of patients that included 18 MYD88WT patients [75]. Data from this analysis were compared with previous genome and transcriptome data from MYD88L265P WM patients [8,42,69,76]. This analysis in WT WM patients identified the presence of somatic mutations in NFκB-related genes, in genes that impact epigenomic dysregulation, and in genes that impair DNA damage repair. Transcriptionally, MYD88WT patients showed similarities to the MYD88L265P patients, justifying the many overlapping disease characteristics noted between the two subsets of patients [66,69]. Transcriptomic studies have also shown that MYD88WT WM clonal cells represent an earlier stage of B-cell differentiation compared to the MYD88L265P clonal cells [76] which is also consistent with the lower rate of IgH somatic hypermutation previously described in MYD88WT WM patients [35].
WM patients with MYD88WT have also been shown to have an increased risk of disease transformation and resistance to ibrutinib monotherapy [69,75,77]. A study by Treon et al. showed a higher incidence of disease transformation to DLBCL in MYD88WT WM patients, which also contributed to 36% of the death events observed in these patients [69]. Furthermore, this study showed that associated DLBCL events in MYD88WT patients were also associated with shortened survival. In terms of response to ibrutinib therapy, IgM and hemoglobin responses were more frequent and deeper in MYD88L265P WM cases, and significantly lower in MYD88WT WM cases [78]. Response to therapy was also affected by the CXCR4 mutational status, where patients with the CXCR4WT genotype achieved better response rates compared to those with the CXCR4WHIM genotype. Given the above data, it is suggested that patients with WT genotype should be followed closely due to the higher risk of histological transformation and higher resistance to BTK-based therapies [68,69,75,79,80].
6. MYD88WT and Related Genes
NGS data from Hunter et al.’s study on MYD88WT patients showed that the majority of the genes with distinct mutational patterns affected pathways of NFkB signaling, epigenomic regulators, and DNA damage response [75]. The mutations found in NFκB-related genes, which have also been found in aggressive B-cell lymphomas, were rare or absent in the L265P-mutated subset of patients, and included TBL1XR1, PTPN13, MALT1, BCL10, NFKB1, NFKB2, NFKBIB, NFKBIZ, and UDRL1F [81,82,83]. TBL1XR1, a gene frequently mutated in the WT patients, encodes transducin-β–like 1 X-linked receptor 1, which is a transcriptional regulator that interacts with nuclear hormone receptor corepressors [84] and which may play a regulatory role in the NFκB pathway and Wnt-mediated transcription. Deletions and mutations of TBL1XR1 have also been reported in acute lymphoblastic leukemias [85,86], however, the specific mechanisms by which TBL1XR1 mutations contribute to tumorigenesis are yet to be discovered. Furthermore, mutations in genes such as NFKBIB, NFKB2, and MALT1 were also identified in this study, i.e., genes that have also been linked to promoting ibrutinib resistance in MCL patients [87]. In terms of the mutations observed in epigenomic regulator genes closely linked to the MYD88WT patients, KDM6A, KMT2C, and KMT2D have also been found to be the most frequently mutated epigenetic regulators in several types of cancer [88,89,90,91,92] highlighting their role in tumorigenesis. On the other hand, structural alterations, such as the deletion of chromosome 6q (chr6q), mostly observed in MYD88L265P-mutated WM patients (30–50%), seem to be absent in WT WM patients [41,42].
One other important mechanism in lymphomagenesis is the DNA damage repair (DDR) pathway, and studies have shown that lymphoma patients often display mutations in genes involved in DDR pathways [93,94,95]. DDR pathways have been shown to affect sensitivity to alkylating agents [96,97,98,99], and a study by Li et al. demonstrated that inhibition of PAK4 and NAMPT by KPT-9274, a compound which affects the DDR pathway, sensitizes WM cells to the activity of alkylating agents, such as melphalan or bendamustine [100]. So far, little is known about the role of DDR genes, and specifically mutations of the TP53 gene, in the development of WM. About 8% of WM patients bear TP53 mutations and studies have shown an association with poor survival [41,101,102] and an increase in frequency after the first-line of therapy [103]. TP53 mutations are mostly associated with mutated MYD88 and CXCR4 [28,102,104]; however, Hunter et al. showed that MYD88WT patients are also presented with mutations in DDR genes, including TP53, and this subset of patients is considered to be an ultra-high-risk disease group [75].
Studies have shown that some WM patients with WT genotype can also harbor mutations that promote WHIM-like signaling in the CXCR4 gene [42,105], although these almost always occur in those with a MYD88L265P mutation [42,66,105]. Although the frequency of MYD88WT/CXCR4MUT WM patients is very low, it seems to also be accompanied with mutations affecting NFκB signaling [75,106]. Mutations in NFkB-related genes observed in a subset of MYD88WT patients are mainly observed downstream of the BTK pathway, involving genes such as CARD11, BCL10, MALT1, and PTPN13 [75].
Finally, transcriptomic sequencing in WM patients revealed that gene expression in the MYD88WT patient cohort was quite heterogeneous compared to the MYD88L265P patient cohort, indicating a diversity in the pathogenesis of this population [76]. Specifically, a downregulation of genes associated with NFκB signaling was observed in these patients, including genes such as IL6, IRAK2, TNFAIP3, NFKBIZ, TIRAP, PIM1, and PIM2. In addition, Hunter et al. suggested that the upregulation of members of the PIK3 pathway observed in these patients, accompanied with increased promoter methylation, create a rationale for assigning PIK3 inhibitors and demethylating agents as targets for future preclinical studies [76].
7. MYD88WT and Therapeutic Implications
When it comes to therapy, there are several treatment options for patients with symptomatic WM, mainly based on monoclonal antibodies targeting CD20 (rituximab and newer ones) in combination with alkylators (cyclophosphamide, bendamustine) and, less often, 20S proteasome inhibitors (PIs) (bortezomib and carfilzomib) and BTK inhibitors (ibrutinib, acalabrutinb, zanubrutinib) [78,107,108,109]. IgM-MGUS patients, regardless of MYD88 mutation status, usually do not require treatment [110]. Treatment of IgM MM usually includes regimens that are used for non-IgM MM patients [111]. Treatment of WM patients is highly personalized depending on their clinical features, preferences, and comorbidities, as well as the efficacy and toxicity profile of the various regimens [112,113].
The MYD88 mutation status is also important given the interest in therapies targeting the components of pathways activated by the L265P mutation (Figure 1). As previously mentioned, MYD88 is preferentially complexed to phosphorylated BTK (pBTK) in WM cells harboring the L265P mutation, a complex which is observed less in lymphoma cells with the WT genotype. Furthermore, the same study shows that overexpression of MYD88 WT did not show enhanced BTK activation; hence, the use of ibrutinib, an inhibitor of BTK kinase activity, resulted in decreased MYD88-BTK complexing in MYD88 L265P-expressing cells and not in MYD88 WT-expressing cells. Studies have shown that activation of BTK via signaling through the B-cell receptor or other signaling axes might contribute clinically relevant pro-survival signals in patients harboring the MYD88WT genotype [114]. The data from an extended follow-up study in relapsed/refractory WM patients, where ibrutinib was used as a monotherapy, showed that among patients with MYD88L265P mutation but no mutations in the CXCR4 gene, responses rates were very high, with a 75% progression-free survival rate (PFS) at a follow-up of almost 5 years compared to and 3.5 years seen in patients with MYD88L265P/CXCR4MUT, and a median PFS of just 5 months in patients with MYD88WT genotype [115,116]. Additionally, no patient harboring the MYD88WT signature achieved partial response (PR) or better in this study [78]. The presence of rare non-L265P MYD88 mutations does not seem to affect response to therapy using ibrutinib [14,113]. In terms of BTK inhibitors, the use of acalabrutinib, which is a more selective BTK inhibitor compared to ibrutinib, showed better response rates, but none of the patients achieved a very good partial response rate (VGPR) [117]. In another prospective study, the use of zanubrutinib, a potent second-generation BTK inhibitor, which has shown reduced off-target effects and a better BTK occupancy compared to ibrutinib, has shown better response rates in MYD88WT WM patients [118]. This study was part of the non-randomized arm of the ASPEN trial, comprising WM patients with only MYD88WT genotype, where zanubrutinib led to an outstanding 50% major response rate (MRR), including a 27% response rate with VGPR. Furthermore, its ongoing efficacy is highlighted by the fact that at 18 months follow-up, the median PFS and OS was not reached for these patients. The efficacy of BTK inhibitors in MYD88WT WM patients is indicated in Table 1.
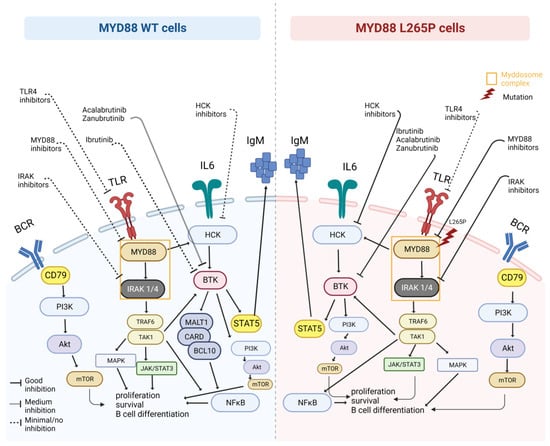
Figure 1.
MYD88WT therapeutic challenges. Representation of the MYD88L265P-mutated and MYD88WT cells and related pathways that can be targeted by specific drugs (created with BioRender.com, accessed on 7 August 2023).

Table 1.
Data from BTK-based therapies on MYD88WT WM patients.
Given the above data, knowledge regarding the MYD88 and CXCR4 mutation status of each patient seems to be important for the use of BTK-based therapy, especially in cases of ibrutinib monotherapy [78,120]; however, this may not be the case for all BTKis as data on non-covalent BKTis such as pirtobrutinib in patients with MYD88WT are still lacking [121]. It is notable, however, that data from the phase 3 iNNOVATE study indicate that the combination of ibrutinib and rituximab is not affected by the absence of MYD88 mutations [122]. In both the iNNOVATE and ASPEN trials, the evaluation of MYD88 mutational status was conducted centrally in NeoGenomics laboratory (NeoGenomics, Aliso Viejo, CA, USA).
Available data indicate that the presence (or absence) of MYD88L265P does not affect the efficacy of chemoimmunotherapy regimens (BR or dexamethasone, rituximab, and cyclophosphamide (DRC) +/− bortezomib) [123,124], thus, based on these observations, it seems reasonable to prioritize chemoimmunotherapy rather than BTKi monotherapy in WT WM patients. If BTKi therapy is considered, and if available, then second-generation BTKis (zanubrutinib, acalabrutinib) may be preferable over ibrutinib. Otherwise, a combination of ibrutinib with rituximab is an approved option, independently of MYD88 mutational status, based on the subgroup analysis of the iNNOVATE study.
Overall, BTKi-based therapies are more effective in MYD88L265P patients; however, second-generation BTKis seem to improve response rates in MYD88WT WM patients compared to the first-generation BTKi therapy. These ongoing studies need to be extended and more patients need to be included in order to obtain a clearer view of the efficacy of these therapies, especially in MYD88WT patients.
8. Conclusions and Future Perspectives
MYD88WT IgM monoclonal gammopathies have become a diagnostic and treatment challenge in the era of small-molecule targeting therapies. Data from studies have shown that patients with MYD88WT genotype appear to have a higher risk of transformation, shorter survival, and resistance to BTK-based therapy. Furthermore, the available data indicate that this is quite a heterogeneous group of lymphomas, which may include WM and its precursor conditions but also other lymphomas and plasma cell neoplasms. The genomic and transcriptomic data support the contention that WT WM and WM with MYD88L265P share some common characteristics despite their differences, and this is translated to a similar clinical course with most non-BTKi therapies. Thus, absence of MY88L265P should not exclude the diagnosis of WM. Ongoing research will further refine the special characteristics of MYD88WT WM/IgM monoclonal gammopathies by further dissecting the genetic characteristics of the clonal cells in an attempt to clarify the reasons behind the distinct clinical outcomes observed between the different BTK- and non-BTK-based therapies in patients, and provide mechanistic insights as an opportunity to develop more personalized therapeutic strategies.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Guerrera, M.L.; Tsakmaklis, N.; Xu, L.; Yang, G.; Demos, M.; Kofides, A.; Chan, G.G.; Manning, R.J.; Liu, X.; Chen, J.G.; et al. MYD88 mutated and wild-type Waldenström’s Macroglobulinemia: Characterization of chromosome 6q gene losses and their mutual exclusivity with mutations in CXCR4. Haematologica 2018, 103, e408–e411. [Google Scholar] [CrossRef] [PubMed]
- García-Sanz, R.; Dogliotti, I.; Zaccaria, G.M.; Ocio, E.M.; Rubio, A.; Murillo, I.; Escalante, F.; Aguilera, C.; García-Mateo, A.; García de Coca, A.; et al. 6q deletion in Waldenström macroglobulinaemia negatively affects time to transformation and survival. Br. J. Haematol. 2021, 192, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Khac, F.; Lambert, J.; Chapiro, E.; Grelier, A.; Mould, S.; Barin, C.; Daudignon, A.; Gachard, N.; Struski, S.; Henry, C.; et al. Chromosomal aberrations and their prognostic value in a series of 174 untreated patients with Waldenström's macroglobulinemia. Haematologica 2013, 98, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Green, M.R.; Gentles, A.J.; Nair, R.V.; Irish, J.M.; Kihira, S.; Liu, C.L.; Kela, I.; Hopmans, E.S.; Myklebust, J.H.; Ji, H.; et al. Hierarchy in somatic mutations arising during genomic evolution and progression of follicular lymphoma. Blood 2013, 121, 1604–1611. [Google Scholar] [CrossRef]
- Tiacci, E.; Trifonov, V.; Schiavoni, G.; Holmes, A.; Kern, W.; Martelli, M.P.; Pucciarini, A.; Bigerna, B.; Pacini, R.; Wells, V.A.; et al. BRAF mutations in hairy-cell leukemia. N. Engl. J. Med. 2011, 364, 2305–2315. [Google Scholar] [CrossRef]
- Damle, R.N.; Wasil, T.; Fais, F.; Ghiotto, F.; Valetto, A.; Allen, S.L.; Buchbinder, A.; Budman, D.; Dittmar, K.; Kolitz, J.; et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood 1999, 94, 1840–1847. [Google Scholar] [CrossRef]
- Treon, S.P.; Xu, L.; Yang, G.; Zhou, Y.; Liu, X.; Cao, Y.; Sheehy, P.; Manning, R.J.; Patterson, C.J.; Tripsas, C.; et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N. Engl. J. Med. 2012, 367, 826–833. [Google Scholar] [CrossRef]
- Hamadeh, F.; MacNamara, S.P.; Aguilera, N.S.; Swerdlow, S.H.; Cook, J.R. MYD88 L265P mutation analysis helps define nodal lymphoplasmacytic lymphoma. Mod. Pathol. 2015, 28, 564–574. [Google Scholar] [CrossRef]
- Ondrejka, S.L.; Lin, J.J.; Warden, D.W.; Durkin, L.; Cook, J.R.; Hsi, E.D. MYD88 L265P somatic mutation: Its usefulness in the differential diagnosis of bone marrow involvement by B-cell lymphoproliferative disorders. Am. J. Clin. Pathol. 2013, 140, 387–394. [Google Scholar] [CrossRef]
- Patkar, N.; Subramanian, P.G.; Deshpande, P.; Ghodke, K.; Tembhare, P.; Mascarenhas, R.; Muranjan, A.; Chaudhary, S.; Bagal, B.; Gujral, S.; et al. MYD88 mutant lymphoplasmacytic lymphoma/Waldenström macroglobulinemia has distinct clinical and pathological features as compared to its mutation negative counterpart. Leuk. Lymphoma 2015, 56, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.N.; Young, R.M.; Schmitz, R.; Jhavar, S.; Xiao, W.; Lim, K.H.; Kohlhammer, H.; Xu, W.; Yang, Y.; Zhao, H.; et al. Oncogenically active MYD88 mutations in human lymphoma. Nature 2011, 470, 115–119. [Google Scholar] [CrossRef] [PubMed]
- García-Abellás, P.; Ferrer Gómez, A.; Bueno Sacristán, D.; Piris Villaespesa, M.; Talavera Yagüe, M.; Reguero Callejas, M.E.; García-Cosío, M. Lymphoplasmacytic lymphoma and marginal zone lymphoma involving bone marrow: A diagnostic dilemma. Useful clinicopathological features to accurate the diagnosis. EJHaem 2022, 3, 1181–1187. [Google Scholar] [CrossRef]
- Treon, S.P.; Xu, L.; Hunter, Z. MYD88 Mutations and Response to Ibrutinib in Waldenström's Macroglobulinemia. N. Engl. J. Med. 2015, 373, 584–586. [Google Scholar] [CrossRef]
- Toshchakov, V.; Jones, B.W.; Perera, P.Y.; Thomas, K.; Cody, M.J.; Zhang, S.; Williams, B.R.; Major, J.; Hamilton, T.A.; Fenton, M.J.; et al. TLR4, but not TLR2, mediates IFN-beta-induced STAT1alpha/beta-dependent gene expression in macrophages. Nat. Immunol. 2002, 3, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.; Janssens, S.; Brissoni, B.; Olivos, N.; Beyaert, R.; Tschopp, J. Inhibition of interleukin 1 receptor/Toll-like receptor signaling through the alternatively spliced, short form of MyD88 is due to its failure to recruit IRAK-4. J. Exp. Med. 2003, 197, 263–268. [Google Scholar] [CrossRef]
- Wesche, H.; Gao, X.; Li, X.; Kirschning, C.J.; Stark, G.R.; Cao, Z. IRAK-M is a novel member of the Pelle/interleukin-1 receptor-associated kinase (IRAK) family. J. Biol. Chem. 1999, 274, 19403–19410. [Google Scholar] [CrossRef]
- Medzhitov, R.; Preston-Hurlburt, P.; Kopp, E.; Stadlen, A.; Chen, C.; Ghosh, S.; Janeway, C.A., Jr. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 1998, 2, 253–258. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Dubois, S.; Viailly, P.J.; Bohers, E.; Bertrand, P.; Ruminy, P.; Marchand, V.; Maingonnat, C.; Mareschal, S.; Picquenot, J.M.; Penther, D.; et al. Biological and Clinical Relevance of Associated Genomic Alterations in MYD88 L265P and non-L265P-Mutated Diffuse Large B-Cell Lymphoma: Analysis of 361 Cases. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 2232–2244. [Google Scholar] [CrossRef]
- Zhan, C.; Qi, R.; Wei, G.; Guven-Maiorov, E.; Nussinov, R.; Ma, B. Conformational dynamics of cancer-associated MyD88-TIR domain mutant L252P (L265P) allosterically tilts the landscape toward homo-dimerization. Protein Eng. Des. Sel. PEDS 2016, 29, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Vyncke, L.; Bovijn, C.; Pauwels, E.; Van Acker, T.; Ruyssinck, E.; Burg, E.; Tavernier, J.; Peelman, F. Reconstructing the TIR Side of the Myddosome: A Paradigm for TIR-TIR Interactions. Structure 2016, 24, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, W.; Deng, Q.; Li, L.; Hsi, E.D.; Young, K.H.; Zhang, M.; Li, Y. MYD88 L265P Mutation in Lymphoid Malignancies. Cancer Res. 2018, 78, 2457–2462. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M.; Hodge, L.S.; Secreto, F.J.; Manske, M.; Braggio, E.; Price-Troska, T.; Ziesmer, S.; Li, Y.; Johnson, S.H.; Hart, S.N.; et al. Activation of TAK1 by MYD88 L265P drives malignant B-cell Growth in non-Hodgkin lymphoma. Blood Cancer J. 2014, 4, e183. [Google Scholar] [CrossRef]
- Rousseau, S.; Martel, G. Gain-of-Function Mutations in the Toll-Like Receptor Pathway: TPL2-Mediated ERK1/ERK2 MAPK Activation, a Path to Tumorigenesis in Lymphoid Neoplasms? Front. Cell Dev. Biol. 2016, 4, 50. [Google Scholar] [CrossRef]
- Wang, J.Q.; Jeelall, Y.S.; Beutler, B.; Horikawa, K.; Goodnow, C.C. Consequences of the recurrent MYD88(L265P) somatic mutation for B cell tolerance. J. Exp. Med. 2014, 211, 413–426. [Google Scholar] [CrossRef]
- Hunter, Z.R.; Yang, G.; Xu, L.; Liu, X.; Castillo, J.J.; Treon, S.P. Genomics, Signaling, and Treatment of Waldenström Macroglobulinemia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 994–1001. [Google Scholar] [CrossRef]
- Varettoni, M.; Zibellini, S.; Defrancesco, I.; Ferretti, V.V.; Rizzo, E.; Malcovati, L.; Gallì, A.; Porta, M.G.D.; Boveri, E.; Arcaini, L.; et al. Pattern of somatic mutations in patients with Waldenström macroglobulinemia or IgM monoclonal gammopathy of undetermined significance. Haematologica 2017, 102, 2077–2085. [Google Scholar] [CrossRef]
- Wilson, W.H.; Young, R.M.; Schmitz, R.; Yang, Y.; Pittaluga, S.; Wright, G.; Lih, C.J.; Williams, P.M.; Shaffer, A.L.; Gerecitano, J.; et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat. Med. 2015, 21, 922–926. [Google Scholar] [CrossRef]
- de Groen, R.A.L.; Schrader, A.M.R.; Kersten, M.J.; Pals, S.T.; Vermaat, J.S.P. MYD88 in the driver’s seat of B-cell lymphomagenesis: From molecular mechanisms to clinical implications. Haematologica 2019, 104, 2337–2348. [Google Scholar] [CrossRef]
- Yang, G.; Zhou, Y.; Liu, X.; Xu, L.; Cao, Y.; Manning, R.J.; Patterson, C.J.; Buhrlage, S.J.; Gray, N.; Tai, Y.T.; et al. A mutation in MYD88 (L265P) supports the survival of lymphoplasmacytic cells by activation of Bruton tyrosine kinase in Waldenström macroglobulinemia. Blood 2013, 122, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Poulain, S.; Roumier, C.; Decambron, A.; Renneville, A.; Herbaux, C.; Bertrand, E.; Tricot, S.; Daudignon, A.; Galiègue-Zouitina, S.; Soenen, V.; et al. MYD88 L265P mutation in Waldenstrom macroglobulinemia. Blood 2013, 121, 4504–4511. [Google Scholar] [CrossRef]
- Varettoni, M.; Arcaini, L.; Zibellini, S.; Boveri, E.; Rattotti, S.; Riboni, R.; Corso, A.; Orlandi, E.; Bonfichi, M.; Gotti, M.; et al. Prevalence and clinical significance of the MYD88 (L265P) somatic mutation in Waldenstrom’s macroglobulinemia and related lymphoid neoplasms. Blood 2013, 121, 2522–2528. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Hunter, Z.R.; Yang, G.; Zhou, Y.; Cao, Y.; Liu, X.; Morra, E.; Trojani, A.; Greco, A.; Arcaini, L.; et al. MYD88 L265P in Waldenström macroglobulinemia, immunoglobulin M monoclonal gammopathy, and other B-cell lymphoproliferative disorders using conventional and quantitative allele-specific polymerase chain reaction. Blood 2013, 121, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, C.; Sebastián, E.; Chillón, M.C.; Giraldo, P.; Mariano Hernández, J.; Escalante, F.; González-López, T.J.; Aguilera, C.; de Coca, A.G.; Murillo, I.; et al. MYD88 L265P is a marker highly characteristic of, but not restricted to, Waldenström’s macroglobulinemia. Leukemia 2013, 27, 1722–1728. [Google Scholar] [CrossRef]
- Drandi, D.; Genuardi, E.; Dogliotti, I.; Ferrante, M.; Jiménez, C.; Guerrini, F.; Schirico, M.L.; Mantoan, B.; Muccio, V.; Lia, G.; et al. Highly sensitive MYD88(L265P) mutation detection by droplet digital polymerase chain reaction in Waldenström macroglobulinemia. Haematologica 2018, 103, 1029–1037. [Google Scholar] [CrossRef]
- Bagratuni, T.; Ntanasis-Stathopoulos, I.; Gavriatopoulou, M.; Mavrianou-Koutsoukou, N.; Liacos, C.; Patseas, D.; Kanellias, N.; Migkou, M.; Ziogas, D.C.; Eleutherakis-Papaiakovou, E.; et al. Detection of MYD88 and CXCR4 mutations in cell-free DNA of patients with IgM monoclonal gammopathies. Leukemia 2018, 32, 2617–2625. [Google Scholar] [CrossRef]
- Bagratuni, T.; Markou, A.; Patseas, D.; Mavrianou-Koutsoukou, N.; Aktypi, F.; Liacos, C.I.; Sklirou, A.D.; Theodorakakou, F.; Ntanasis-Stathopoulos, I.; Gavriatopoulou, M.; et al. Determination of MYD88L265P mutation fraction in IgM monoclonal gammopathies. Blood Adv. 2022, 6, 189–199. [Google Scholar] [CrossRef]
- Wang, C.Z.; Lin, J.; Qian, J.; Shao, R.; Xue, D.; Qian, W.; Xiao, G.F.; Deng, Z.Q.; Yang, J.; Li, Y.; et al. Development of high-resolution melting analysis for the detection of the MYD88 L265P mutation. Clin. Biochem. 2013, 46, 385–387. [Google Scholar] [CrossRef]
- Dogliotti, I.; Jiménez, C.; Varettoni, M.; Talaulikar, D.; Bagratuni, T.; Ferrante, M.; Pérez, J.; Drandi, D.; Puig, N.; Gilestro, M.; et al. Diagnostics in Waldenström’s macroglobulinemia: A consensus statement of the European Consortium for Waldenström’s Macroglobulinemia. Leukemia 2023, 37, 388–395. [Google Scholar] [CrossRef]
- Treon, S.P.; Xu, L.; Guerrera, M.L.; Jimenez, C.; Hunter, Z.R.; Liu, X.; Demos, M.; Gustine, J.; Chan, G.; Munshi, M.; et al. Genomic Landscape of Waldenström Macroglobulinemia and Its Impact on Treatment Strategies. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Hunter, Z.R.; Xu, L.; Yang, G.; Zhou, Y.; Liu, X.; Cao, Y.; Manning, R.J.; Tripsas, C.; Patterson, C.J.; Sheehy, P.; et al. The genomic landscape of Waldenstrom macroglobulinemia is characterized by highly recurring MYD88 and WHIM-like CXCR4 mutations, and small somatic deletions associated with B-cell lymphomagenesis. Blood 2014, 123, 1637–1646. [Google Scholar] [CrossRef] [PubMed]
- Mithraprabhu, S.; Spencer, A. Circulating tumour DNA analysis in multiple myeloma. Oncotarget 2017, 8, 90610–90611. [Google Scholar] [CrossRef] [PubMed]
- Oberle, A.; Brandt, A.; Voigtlaender, M.; Thiele, B.; Radloff, J.; Schulenkorf, A.; Alawi, M.; Akyüz, N.; März, M.; Ford, C.T.; et al. Monitoring multiple myeloma by next-generation sequencing of V(D)J rearrangements from circulating myeloma cells and cell-free myeloma DNA. Haematologica 2017, 102, 1105–1111. [Google Scholar] [CrossRef]
- Rustad, E.H.; Coward, E.; Skytøen, E.R.; Misund, K.; Holien, T.; Standal, T.; Børset, M.; Beisvag, V.; Myklebost, O.; Meza-Zepeda, L.A.; et al. Monitoring multiple myeloma by quantification of recurrent mutations in serum. Haematologica 2017, 102, 1266–1272. [Google Scholar] [CrossRef]
- Alizadeh, A.A.; Eisen, M.B.; Davis, R.E.; Ma, C.; Lossos, I.S.; Rosenwald, A.; Boldrick, J.C.; Sabet, H.; Tran, T.; Yu, X.; et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000, 403, 503–511. [Google Scholar] [CrossRef]
- Cao, X.; Medeiros, L.J.; Xia, Y.; Wang, X.; Thomas, S.K.; Loghavi, S.; Li, X.; Shah, J.J.; Gustafson, S.A.; Weber, D.M.; et al. Clinicopathologic features and outcomes of lymphoplasmacytic lymphoma patients with monoclonal IgG or IgA paraprotein expression. Leuk. Lymphoma 2016, 57, 1104–1113. [Google Scholar] [CrossRef]
- Campo, E.; Swerdlow, S.H.; Harris, N.L.; Pileri, S.; Stein, H.; Jaffe, E.S. The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood 2011, 117, 5019–5032. [Google Scholar] [CrossRef]
- Owen, R.G.; Treon, S.P.; Al-Katib, A.; Fonseca, R.; Greipp, P.R.; McMaster, M.L.; Morra, E.; Pangalis, G.A.; San Miguel, J.F.; Branagan, A.R.; et al. Clinicopathological definition of Waldenstrom’s macroglobulinemia: Consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin. Oncol. 2003, 30, 110–115. [Google Scholar] [CrossRef]
- Kyle, R.A.; Durie, B.G.; Rajkumar, S.V.; Landgren, O.; Blade, J.; Merlini, G.; Kröger, N.; Einsele, H.; Vesole, D.H.; Dimopoulos, M.; et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia 2010, 24, 1121–1127. [Google Scholar] [CrossRef]
- Landgren, O.; Staudt, L. MYD88 L265P somatic mutation in IgM MGUS. N. Engl. J. Med. 2012, 367, 2255–2256, author reply 2256–2257. [Google Scholar] [CrossRef]
- Varettoni, M.; Zibellini, S.; Arcaini, L.; Boveri, E.; Rattotti, S.; Pascutto, C.; Mangiacavalli, S.; Gotti, M.; Pochintesta, L.; Paulli, M.; et al. MYD88 (L265P) mutation is an independent risk factor for progression in patients with IgM monoclonal gammopathy of undetermined significance. Blood 2013, 122, 2284–2285. [Google Scholar] [CrossRef] [PubMed]
- Varettoni, M.; Zibellini, S.; Boveri, E.; Klersy, C.; Candido, C.; Rattotti, S.; Ferretti, V.V.; Defrancesco, I.; Mangiacavalli, S.; Nizzoli, M.E.; et al. A risk-stratification model based on the initial concentration of the serum monoclonal protein and MYD88 mutation status identifies a subset of patients with IgM monoclonal gammopathy of undetermined significance at high risk of progression to Waldenström macroglobulinaemia or other lymphoproliferative disorders. Br. J. Haematol. 2019, 187, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Reid, R.; Friedberg, J.W. Management of marginal zone lymphoma. Oncology 2013, 27, 840,842,844. [Google Scholar] [PubMed]
- Insuasti-Beltran, G.; Gale, J.M.; Wilson, C.S.; Foucar, K.; Czuchlewski, D.R. Significance of MYD88 L265P Mutation Status in the Subclassification of Low-Grade B-Cell Lymphoma/Leukemia. Arch. Pathol. Lab. Med. 2015, 139, 1035–1041. [Google Scholar] [CrossRef]
- Gebauer, N.; Hardel, T.T.; Gebauer, J.; Bernard, V.; Merz, H.; Feller, A.C.; Rades, D.; Biersack, H.; Lehnert, H.; Thorns, C. Activating mutations affecting the NF-kappa B pathway and EZH2-mediated epigenetic regulation are rare events in primary mediastinal large B-cell lymphoma. Anticancer Res. 2014, 34, 5503–5507. [Google Scholar]
- Lee, J.H.; Jeong, H.; Choi, J.W.; Oh, H.; Kim, Y.S. Clinicopathologic significance of MYD88 L265P mutation in diffuse large B-cell lymphoma: A meta-analysis. Sci. Rep. 2017, 7, 1785. [Google Scholar] [CrossRef]
- Jallades, L.; Baseggio, L.; Sujobert, P.; Huet, S.; Chabane, K.; Callet-Bauchu, E.; Verney, A.; Hayette, S.; Desvignes, J.P.; Salgado, D.; et al. Exome sequencing identifies recurrent BCOR alterations and the absence of KLF2, TNFAIP3 and MYD88 mutations in splenic diffuse red pulp small B-cell lymphoma. Haematologica 2017, 102, 1758–1766. [Google Scholar] [CrossRef]
- Staiger, A.M.; Ott, M.M.; Parmentier, S.; Rosenwald, A.; Ott, G.; Horn, H.; Griese, E.U. Allele-specific PCR is a powerful tool for the detection of the MYD88 L265P mutation in diffuse large B cell lymphoma and decalcified bone marrow samples. Br. J. Haematol. 2015, 171, 145–148. [Google Scholar] [CrossRef]
- Onaindia, A.; Medeiros, L.J.; Patel, K.P. Clinical utility of recently identified diagnostic, prognostic, and predictive molecular biomarkers in mature B-cell neoplasms. Mod. Pathol. 2017, 30, 1338–1366. [Google Scholar] [CrossRef]
- Hung, S.S.; Meissner, B.; Chavez, E.A.; Ben-Neriah, S.; Ennishi, D.; Jones, M.R.; Shulha, H.P.; Chan, F.C.; Boyle, M.; Kridel, R.; et al. Assessment of Capture and Amplicon-Based Approaches for the Development of a Targeted Next-Generation Sequencing Pipeline to Personalize Lymphoma Management. J. Mol. Diagn. JMD 2018, 20, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Okosun, J.; Bödör, C.; Wang, J.; Araf, S.; Yang, C.Y.; Pan, C.; Boller, S.; Cittaro, D.; Bozek, M.; Iqbal, S.; et al. Integrated genomic analysis identifies recurrent mutations and evolution patterns driving the initiation and progression of follicular lymphoma. Nat. Genet. 2014, 46, 176–181. [Google Scholar] [CrossRef]
- Baer, C.; Dicker, F.; Kern, W.; Haferlach, T.; Haferlach, C. Genetic characterization of MYD88-mutated lymphoplasmacytic lymphoma in comparison with MYD88-mutated chronic lymphocytic leukemia. Leukemia 2017, 31, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Lin, C.T.; Agathangelidis, A.; Lin, L.I.; Kuo, Y.Y.; Tien, H.F.; Ghia, P. Distinct molecular genetics of chronic lymphocytic leukemia in Taiwan: Clinical and pathogenetic implications. Haematologica 2017, 102, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Ali, Y.B.; Foad, R.M.; Abdel-Wahed, E. Lack of Associations between TLR9 and MYD88 Gene Polymorphisms and Risk of Chronic Lymphocytic Leukemia. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 3245–3250. [Google Scholar] [CrossRef]
- Treon, S.P.; Cao, Y.; Xu, L.; Yang, G.; Liu, X.; Hunter, Z.R. Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenstrom macroglobulinemia. Blood 2014, 123, 2791–2796. [Google Scholar] [CrossRef]
- Chakraborty, R.; Novak, A.J.; Ansell, S.M.; Muchtar, E.; Kapoor, P.; Hayman, S.R.; Dispenzieri, A.; Buadi, F.K.; Lacy, M.Q.; King, R.L.; et al. First report of MYD88 L265P somatic mutation in IgM-associated light-chain amyloidosis. Blood 2016, 127, 2936–2938. [Google Scholar] [CrossRef]
- Abeykoon, J.P.; Paludo, J.; King, R.L.; Ansell, S.M.; Gertz, M.A.; LaPlant, B.R.; Halvorson, A.E.; Gonsalves, W.I.; Dingli, D.; Fang, H.; et al. MYD88 mutation status does not impact overall survival in Waldenström macroglobulinemia. Am. J. Hematol. 2018, 93, 187–194. [Google Scholar] [CrossRef]
- Treon, S.P.; Gustine, J.; Xu, L.; Manning, R.J.; Tsakmaklis, N.; Demos, M.; Meid, K.; Guerrera, M.L.; Munshi, M.; Chan, G.; et al. MYD88 wild-type Waldenstrom Macroglobulinaemia: Differential diagnosis, risk of histological transformation, and overall survival. Br. J. Haematol. 2018, 180, 374–380. [Google Scholar] [CrossRef]
- Avet-Loiseau, H.; Garand, R.; Lodé, L.; Harousseau, J.L.; Bataille, R. Translocation t(11;14)(q13;q32) is the hallmark of IgM, IgE, and nonsecretory multiple myeloma variants. Blood 2003, 101, 1570–1571. [Google Scholar] [CrossRef]
- Qin, S.C.; Xia, Y.; Miao, Y.; Zhu, H.Y.; Wu, J.Z.; Fan, L.; Li, J.Y.; Xu, W.; Qiao, C. MYD88 mutations predict unfavorable prognosis in Chronic Lymphocytic Leukemia patients with mutated IGHV gene. Blood Cancer J. 2017, 7, 651. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parry, M.; Rose-Zerilli, M.J.; Ljungström, V.; Gibson, J.; Wang, J.; Walewska, R.; Parker, H.; Parker, A.; Davis, Z.; Gardiner, A.; et al. Genetics and Prognostication in Splenic Marginal Zone Lymphoma: Revelations from Deep Sequencing. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 4174–4183. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.A. Waldenström macroglobulinemia: 2019 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2019, 94, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.; Braggio, E. The MYDas touch of next-gen sequencing. Blood 2013, 121, 2373–2374. [Google Scholar] [CrossRef]
- Hunter, Z.R.; Xu, L.; Tsakmaklis, N.; Demos, M.G.; Kofides, A.; Jimenez, C.; Chan, G.G.; Chen, J.; Liu, X.; Munshi, M.; et al. Insights into the genomic landscape of MYD88 wild-type Waldenström macroglobulinemia. Blood Adv. 2018, 2, 2937–2946. [Google Scholar] [CrossRef]
- Hunter, Z.R.; Xu, L.; Yang, G.; Tsakmaklis, N.; Vos, J.M.; Liu, X.; Chen, J.; Manning, R.J.; Chen, J.G.; Brodsky, P.; et al. Transcriptome sequencing reveals a profile that corresponds to genomic variants in Waldenström macroglobulinemia. Blood 2016, 128, 827–838. [Google Scholar] [CrossRef]
- Puente, X.S.; Pinyol, M.; Quesada, V.; Conde, L.; Ordóñez, G.R.; Villamor, N.; Escaramis, G.; Jares, P.; Beà, S.; González-Díaz, M.; et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature 2011, 475, 101–105. [Google Scholar] [CrossRef]
- Treon, S.P.; Tripsas, C.K.; Meid, K.; Warren, D.; Varma, G.; Green, R.; Argyropoulos, K.V.; Yang, G.; Cao, Y.; Xu, L.; et al. Ibrutinib in previously treated Waldenström’s macroglobulinemia. N. Engl. J. Med. 2015, 372, 1430–1440. [Google Scholar] [CrossRef]
- Zanwar, S.; Abeykoon, J.P.; Durot, E.; King, R.; Perez Burbano, G.E.; Kumar, S.; Gertz, M.A.; Quinquenel, A.; Delmer, A.; Gonsalves, W.; et al. Impact of MYD88(L265P) mutation status on histological transformation of Waldenström Macroglobulinemia. Am. J. Hematol. 2020, 95, 274–281. [Google Scholar] [CrossRef]
- Wang, Y.; Gali, V.L.; Xu-Monette, Z.Y.; Sano, D.; Thomas, S.K.; Weber, D.M.; Zhu, F.; Fang, X.; Deng, M.; Zhang, M.; et al. Molecular and genetic biomarkers implemented from next-generation sequencing provide treatment insights in clinical practice for Waldenström macroglobulinemia. Neoplasia 2021, 23, 361–374. [Google Scholar] [CrossRef]
- Schmitz, R.; Wright, G.W.; Huang, D.W.; Johnson, C.A.; Phelan, J.D.; Wang, J.Q.; Roulland, S.; Kasbekar, M.; Young, R.M.; Shaffer, A.L.; et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2018, 378, 1396–1407. [Google Scholar] [CrossRef] [PubMed]
- Chapuy, B.; Stewart, C.; Dunford, A.J.; Kim, J.; Kamburov, A.; Redd, R.A.; Lawrence, M.S.; Roemer, M.G.M.; Li, A.J.; Ziepert, M.; et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat. Med. 2018, 24, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Aguilar, A.; Idbaih, A.; Boisselier, B.; Habbita, N.; Rossetto, M.; Laurenge, A.; Bruno, A.; Jouvet, A.; Polivka, M.; Adam, C.; et al. Recurrent mutations of MYD88 and TBL1XR1 in primary central nervous system lymphomas. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 5203–5211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Chang, Q.; Zeng, L.; Gu, J.; Brown, S.; Basch, R.S. TBLR1 regulates the expression of nuclear hormone receptor co-repressors. BMC Cell Biol. 2006, 7, 31. [Google Scholar] [CrossRef]
- Parker, H.; An, Q.; Barber, K.; Case, M.; Davies, T.; Konn, Z.; Stewart, A.; Wright, S.; Griffiths, M.; Ross, F.M.; et al. The complex genomic profile of ETV6-RUNX1 positive acute lymphoblastic leukemia highlights a recurrent deletion of TBL1XR1. Genes Chromosomes Cancer 2008, 47, 1118–1125. [Google Scholar] [CrossRef]
- Zhang, J.; Mullighan, C.G.; Harvey, R.C.; Wu, G.; Chen, X.; Edmonson, M.; Buetow, K.H.; Carroll, W.L.; Chen, I.M.; Devidas, M.; et al. Key pathways are frequently mutated in high-risk childhood acute lymphoblastic leukemia: A report from the Children’s Oncology Group. Blood 2011, 118, 3080–3087. [Google Scholar] [CrossRef]
- Balaji, S.; Ahmed, M.; Lorence, E.; Yan, F.; Nomie, K.; Wang, M. NF-κB signaling and its relevance to the treatment of mantle cell lymphoma. J. Hematol. Oncol. 2018, 11, 83. [Google Scholar] [CrossRef]
- Bailey, P.; Chang, D.K.; Nones, K.; Johns, A.L.; Patch, A.M.; Gingras, M.C.; Miller, D.K.; Christ, A.N.; Bruxner, T.J.; Quinn, M.C.; et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016, 531, 47–52. [Google Scholar] [CrossRef]
- Biankin, A.V.; Waddell, N.; Kassahn, K.S.; Gingras, M.C.; Muthuswamy, L.B.; Johns, A.L.; Miller, D.K.; Wilson, P.J.; Patch, A.M.; Wu, J.; et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012, 491, 399–405. [Google Scholar] [CrossRef]
- Hoadley, K.A.; Yau, C.; Wolf, D.M.; Cherniack, A.D.; Tamborero, D.; Ng, S.; Leiserson, M.D.M.; Niu, B.; McLellan, M.D.; Uzunangelov, V.; et al. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell 2014, 158, 929–944. [Google Scholar] [CrossRef]
- van Haaften, G.; Dalgliesh, G.L.; Davies, H.; Chen, L.; Bignell, G.; Greenman, C.; Edkins, S.; Hardy, C.; O’Meara, S.; Teague, J.; et al. Somatic mutations of the histone H3K27 demethylase gene UTX in human cancer. Nat. Genet. 2009, 41, 521–523. [Google Scholar] [CrossRef]
- Waddell, N.; Pajic, M.; Patch, A.M.; Chang, D.K.; Kassahn, K.S.; Bailey, P.; Johns, A.L.; Miller, D.; Nones, K.; Quek, K.; et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015, 518, 495–501. [Google Scholar] [CrossRef]
- Carrassa, L.; Colombo, I.; Damia, G.; Bertoni, F. Targeting the DNA damage response for patients with lymphoma: Preclinical and clinical evidences. Cancer Treat. Rev. 2020, 90, 102090. [Google Scholar] [CrossRef] [PubMed]
- Derenzini, E.; Agostinelli, C.; Imbrogno, E.; Iacobucci, I.; Casadei, B.; Brighenti, E.; Righi, S.; Fuligni, F.; Ghelli Luserna Di Rorà, A.; Ferrari, A.; et al. Constitutive activation of the DNA damage response pathway as a novel therapeutic target in diffuse large B-cell lymphoma. Oncotarget 2015, 6, 6553–6569. [Google Scholar] [CrossRef] [PubMed]
- de Miranda, N.F.; Peng, R.; Georgiou, K.; Wu, C.; Falk Sörqvist, E.; Berglund, M.; Chen, L.; Gao, Z.; Lagerstedt, K.; Lisboa, S.; et al. DNA repair genes are selectively mutated in diffuse large B cell lymphomas. J. Exp. Med. 2013, 210, 1729–1742. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, P.; Jonkers, J. The effects of deregulated DNA damage signalling on cancer chemotherapy response and resistance. Nat. Rev. Cancer 2012, 12, 587–598. [Google Scholar] [CrossRef]
- Enoch, T.; Norbury, C. Cellular responses to DNA damage: Cell-cycle checkpoints, apoptosis and the roles of p53 and ATM. Trends Biochem. Sci. 1995, 20, 426–430. [Google Scholar] [CrossRef]
- Fan, S.; el-Deiry, W.S.; Bae, I.; Freeman, J.; Jondle, D.; Bhatia, K.; Fornace, A.J., Jr.; Magrath, I.; Kohn, K.W.; O’Connor, P.M. p53 gene mutations are associated with decreased sensitivity of human lymphoma cells to DNA damaging agents. Cancer Res. 1994, 54, 5824–5830. [Google Scholar] [PubMed]
- Yarde, D.N.; Oliveira, V.; Mathews, L.; Wang, X.; Villagra, A.; Boulware, D.; Shain, K.H.; Hazlehurst, L.A.; Alsina, M.; Chen, D.T.; et al. Targeting the Fanconi anemia/BRCA pathway circumvents drug resistance in multiple myeloma. Cancer Res. 2009, 69, 9367–9375. [Google Scholar] [CrossRef]
- Li, N.; Lopez, M.A.; Linares, M.; Kumar, S.; Oliva, S.; Martinez-Lopez, J.; Xu, L.; Xu, Y.; Perini, T.; Senapedis, W.; et al. Dual PAK4-NAMPT Inhibition Impacts Growth and Survival, and Increases Sensitivity to DNA-Damaging Agents in Waldenström Macroglobulinemia. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 369–377. [Google Scholar] [CrossRef]
- Krzisch, D.; Guedes, N.; Boccon-Gibod, C.; Baron, M.; Bravetti, C.; Davi, F.; Armand, M.; Smagghe, L.; Caron, J.; Bernard, O.A.; et al. Cytogenetic and molecular abnormalities in Waldenström’s macroglobulinemia patients: Correlations and prognostic impact. Am. J. Hematol. 2021, 96, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Poulain, S.; Roumier, C.; Bertrand, E.; Renneville, A.; Caillault-Venet, A.; Doye, E.; Geffroy, S.; Sebda, S.; Nibourel, O.; Nudel, M.; et al. TP53 Mutation and Its Prognostic Significance in Waldenstrom’s Macroglobulinemia. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 6325–6335. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.S.; Opat, S.; D’Sa, S.; Jurczak, W.; Lee, H.P.; Cull, G.; Owen, R.G.; Marlton, P.; Wahlin, B.E.; Sanz, R.G.; et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenström macroglobulinemia: The ASPEN study. Blood 2020, 136, 2038–2050. [Google Scholar] [CrossRef] [PubMed]
- Gustine, J.N.; Tsakmaklis, N.; Demos, M.G.; Kofides, A.; Chen, J.G.; Liu, X.; Munshi, M.; Guerrera, M.L.; Chan, G.G.; Patterson, C.J.; et al. TP53 mutations are associated with mutated MYD88 and CXCR4, and confer an adverse outcome in Waldenström macroglobulinaemia. Br. J. Haematol. 2019, 184, 242–245. [Google Scholar] [CrossRef]
- Xu, L.; Hunter, Z.R.; Tsakmaklis, N.; Cao, Y.; Yang, G.; Chen, J.; Liu, X.; Kanan, S.; Castillo, J.J.; Tai, Y.T.; et al. Clonal architecture of CXCR4 WHIM-like mutations in Waldenström Macroglobulinaemia. Br. J. Haematol. 2016, 172, 735–744. [Google Scholar] [CrossRef]
- Kaiser, L.M.; Hunter, Z.R.; Treon, S.P.; Buske, C. CXCR4 in Waldenström’s Macroglobulinema: Chances and challenges. Leukemia 2021, 35, 333–345. [Google Scholar] [CrossRef]
- Ansell, S.M.; Kyle, R.A.; Reeder, C.B.; Fonseca, R.; Mikhael, J.R.; Morice, W.G.; Bergsagel, P.L.; Buadi, F.K.; Colgan, J.P.; Dingli, D.; et al. Diagnosis and management of Waldenström macroglobulinemia: Mayo stratification of macroglobulinemia and risk-adapted therapy (mSMART) guidelines. Mayo Clin. Proc. 2010, 85, 824–833. [Google Scholar] [CrossRef]
- Treon, S.P.; Ioakimidis, L.; Soumerai, J.D.; Patterson, C.J.; Sheehy, P.; Nelson, M.; Willen, M.; Matous, J.; Mattern, J., 2nd; Diener, J.G.; et al. Primary therapy of Waldenström macroglobulinemia with bortezomib, dexamethasone, and rituximab: WMCTG clinical trial 05–180. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 3830–3835. [Google Scholar] [CrossRef]
- Paulus, A.; Ailawadhi, S.; Chanan-Khan, A. Novel therapeutic targets in Waldenstrom macroglobulinemia. Best Pract. Res. Clin. Haematol. 2016, 29, 216–228. [Google Scholar] [CrossRef]
- Grunenberg, A.; Buske, C. Monoclonal IgM Gammopathy and Waldenström’s Macroglobulinemia. Dtsch. Arztebl. Int. 2017, 114, 745–751. [Google Scholar] [CrossRef]
- Schuster, S.R.; Rajkumar, S.V.; Dispenzieri, A.; Morice, W.; Aspitia, A.M.; Ansell, S.; Kyle, R.; Mikhael, J. IgM multiple myeloma: Disease definition, prognosis, and differentiation from Waldenstrom’s macroglobulinemia. Am. J. Hematol. 2010, 85, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Kastritis, E.; Ghobrial, I.M. Waldenström’s macroglobulinemia: A clinical perspective in the era of novel therapeutics. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.J.; Treon, S.P. Toward personalized treatment in Waldenström macroglobulinemia. Hematol. Am. Soc. Hematol. Educ. Program 2017, 2017, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulos, K.V.; Vogel, R.; Ziegler, C.; Altan-Bonnet, G.; Velardi, E.; Calafiore, M.; Dogan, A.; Arcila, M.; Patel, M.; Knapp, K.; et al. Clonal B cells in Waldenström’s macroglobulinemia exhibit functional features of chronic active B-cell receptor signaling. Leukemia 2016, 30, 1116–1125. [Google Scholar] [CrossRef][Green Version]
- Treon, S.P.; Meid, K.; Gustine, J.; Yang, G.; Xu, L.; Liu, X.; Patterson, C.J.; Hunter, Z.R.; Branagan, A.R.; Laubach, J.P.; et al. Long-Term Follow-Up of Ibrutinib Monotherapy in Symptomatic, Previously Treated Patients With Waldenström Macroglobulinemia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 565–575. [Google Scholar] [CrossRef]
- Ravi, G.; Kapoor, P. Current approach to Waldenström Macroglobulinemia. Cancer Treat. Res. Commun. 2022, 31, 100527. [Google Scholar] [CrossRef]
- Owen, R.G.; McCarthy, H.; Rule, S.; D’Sa, S.; Thomas, S.K.; Tournilhac, O.; Forconi, F.; Kersten, M.J.; Zinzani, P.L.; Iyengar, S.; et al. Acalabrutinib monotherapy in patients with Waldenström macroglobulinemia: A single-arm, multicentre, phase 2 study. Lancet Haematol. 2020, 7, e112–e121. [Google Scholar] [CrossRef]
- Dimopoulos, M.; Sanz, R.G.; Lee, H.P.; Trneny, M.; Varettoni, M.; Opat, S.; D’Sa, S.; Owen, R.G.; Cull, G.; Mulligan, S.; et al. Zanubrutinib for the treatment of MYD88 wild-type Waldenström macroglobulinemia: A substudy of the phase 3 ASPEN trial. Blood Adv. 2020, 4, 6009–6018. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Salman, Z.; Buske, C. Ibrutinib and Rituximab in Waldenström’s Macroglobulinemia. N. Engl. J. Med. 2018, 379, 1975–1976. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Trotman, J.; Tedeschi, A.; Matous, J.V.; Macdonald, D.; Tam, C.; Tournilhac, O.; Ma, S.; Oriol, A.; Heffner, L.T.; et al. Ibrutinib for patients with rituximab-refractory Waldenström’s macroglobulinaemia (iNNOVATE): An open-label substudy of an international, multicentre, phase 3 trial. Lancet Oncol. 2017, 18, 241–250. [Google Scholar] [CrossRef]
- Buske, C.; Jurczak, W.; Salem, J.E.; Dimopoulos, M.A. Managing Waldenström’s macroglobulinemia with BTK inhibitors. Leukemia 2023, 37, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Buske, C.; Tedeschi, A.; Trotman, J.; García-Sanz, R.; MacDonald, D.; Leblond, V.; Mahe, B.; Herbaux, C.; Matous, J.V.; Tam, C.S.; et al. Ibrutinib Plus Rituximab Versus Placebo Plus Rituximab for Waldenström’s Macroglobulinemia: Final Analysis From the Randomized Phase III iNNOVATE Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Paludo, J.; Abeykoon, J.P.; Shreders, A.; Ansell, S.M.; Kumar, S.; Ailawadhi, S.; King, R.L.; Koehler, A.B.; Reeder, C.B.; Buadi, F.K.; et al. Bendamustine and rituximab (BR) versus dexamethasone, rituximab, and cyclophosphamide (DRC) in patients with Waldenström macroglobulinemia. Ann. Hematol. 2018, 97, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Laribi, K.; Poulain, S.; Willems, L.; Merabet, F.; Le Calloch, R.; Eveillard, J.R.; Herbaux, C.; Roos-Weil, D.; Chaoui, D.; Roussel, X.; et al. Bendamustine plus rituximab in newly-diagnosed Waldenström macroglobulinaemia patients. A study on behalf of the French Innovative Leukaemia Organization (FILO). Br. J. Haematol. 2019, 186, 146–149. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).