Abstract
Tyrosine kinase inhibitors (TKIs) have revolutionized the treatment of chronic myeloid leukemia (CML), granting patients a life expectancy close to that of the normal population and, in a subset of patients, the possibility to discontinue therapy. Nonetheless, for a not negligible minority of patients, TKIs are not able to control CML. Allogeneic hematopoietic cell transplantation (HCT) has long been a pivotal therapy for CML. At present, allogeneic HCT is considered an option in CML patients diagnosed or progressing to blast phase (BP), for those in chronic phase (CP) resistant to multiple lines of TKI therapy or for those experiencing severe toxicity, mostly hematologic, under TKIs. Moving from real-world cases, we reviewed the results of allogeneic HCT in the setting of advanced-phase CML or failure of TKIs, with a focus on the progresses in transplant technology that has extended transplant options in elderly CML patients and in those lacking a sibling donor, and on the post-HCT strategies for prevention and treatment of disease relapse.
1. Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm with an incidence of 1–2 cases per 100,000 adults, and it accounts for approximately 15% of newly diagnosed adult leukemia [1]. The key feature of CML is the fusion of the Abelson murine leukemia (ABL1) gene on chromosome 9 with the breakpoint cluster region (BCR) gene on chromosome 22. This results in the expression of an oncoprotein termed BCR::ABL1, a constitutively active tyrosine kinase (TK) that promotes the growth and survival of CML cells.
At the end of the last century, hematopoietic cell transplantation (HCT) was shown to be the only curative option, and it was performed especially in the chronic phase [2].
Treatment of CML has been revolutionized by the introduction of TK inhibitors (TKIs) in the early 2000s. At present, the availability of imatinib, three second-generation (2G) TKIs (dasatinib, nilotinib and bosutinib) and one third-generation (3G) TKI (ponatinib) have granted CML patients a life expectancy that is close to that of the general population [3].
As described in Figure 1, as a secondary effect of the success of TKI therapy, the latter has significantly reduced the number of CML patients deemed candidates for HCT that, until the widespread availability of TKIs, has been considered the treatment of choice in younger patients. Notably, chronic phase (CP) CML patients were regarded as the ideal candidates for HCT [4], and CML was among the commonest indications for allogeneic transplants.
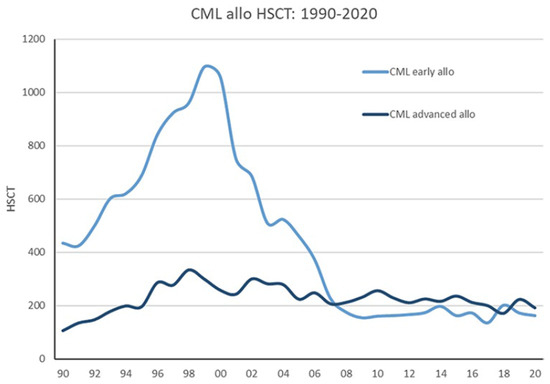
Figure 1.
Development of allogeneic stem cell transplantation for CML in Europe from 1990 to 2020 (EBMT registry) showing CP and AP CML (AP and BC) [5].
Despite remarkable success attained by TKIs, a not negligible proportion of patients will eventually fail drug therapy, either due to progression to accelerated and/or blast phases (ABP), the development of hematologic and/or cytogenetic resistance to multiple TKIs while in CP, or unacceptable toxicities of TKIs. As a matter of fact, allogeneic HCT is still present in the CML armamentarium and remains an indication for selected patients [5].
In the present review, we present some clinical scenarios that have led us to refer CML patients to HCT, discussing the actual indications for transplant, focusing on peculiar features such as donor selection, conditioning regimens and post-HCT strategies.
2. Methods
We present the clinical histories of 3 patients who underwent HCT at the Transplant Center of Udine, Italy, to exemplify some actual indications for HSCT in CML: AP, TKIs toxicity associated with suboptimal response, and failure of multiple lines of TKI therapy. We describe the clinical course of these patients, including treatment before HCT, the HCT procedure and the follow-up after transplant; data are summarized in Table 1. In the Discussion, we moved from the patients’ histories and reviewed the available studies of the following three topics: (1) indication for HCT and TKIs treatment as a “bridge to transplant”; (2) transplant procedure; (3) and follow-up after transplantation, with particular focus on TKI prophylaxis or pre-emptive therapy and the role of donor lymphocytes infusions (DLI).

Table 1.
Clinical features of the patients and the transplants.
3. Clinical Case N.1: Accelerated Phase (AP) CML
A 41-year-old man with no past medical history was referred to our center in February 2013 with fever, fatigue and weight loss. Hepatosplenomegaly was detected by physical examination. Laboratory tests revealed a white blood cell (WBC) count of 200,000 × 109/L with 32% neutrophils, 1% lymphocytes, 12% basophils, 0% eosinophils, 3% monocytes, 20.5% myelocytes, 31.5% blasts and promyelocytes (7.5% and 24%, respectively) and some erythroid precursor observed at the peripheral blood smear. Hemoglobin (Hb) was around 6 g/dL, while platelet (PLT) count was normal. Lactate dehydrogenase (LDH) was 4118 UI/L, and ferritin was 1434 ng/mL. Liver and renal function tests were normal. Bone marrow aspirate showed an excess of immature myeloid precursors and megakaryocyte dysplasia. The quantitative reverse transcription polymerase chain reaction (RT-PCR) test for BCR::ABL1 was positive (transcript type p210), and cytogenetic analysis revealed the canonical t(9;22) (Philadelphia chromosome—Ph+). The patient was diagnosed with CML in AP.
Considering high WBC levels, a cytoreduction with cytosine arabinoside and hydroxyurea was administered; the patient experienced respiratory failure due to leukostasis and concomitant viral infection (influenza type A), needing 9 cycles of non-invasive ventilation in the Intensive Care Unit.
Considering concurrent pneumonia, a first-line treatment with imatinib at a reduced dose of 400 mg/daily was started. The hematologic response lasted 1 month, with a new rise in the WBC count (34,000 × 109/L) and concurrent thrombocytosis (over 1,000,000 × 109/L), and a lowering of hemoglobin levels (Hb 9.6 g/dL). After a second cycle of cytoreduction, in April 2013, the patient started dasatinib 100 mg/die, then increased to 140 mg/day after one month. He obtained a complete hematological response (CHR) with a molecular response (BCR::ABL1 0.6%—MR2) after 4.5 months of dasatinib.
Since he was a young patient with AP CML and a partial response to two lines of TKI therapy, HCT was considered. In September 2013, 6 months after TKI start, the patient underwent HCT from a matched (10/10) unrelated AB0 matched donor (10/10) with peripheral blood stem cells (PBSC). The conditioning regimen included busulfan (12.8 mg/kg) and cyclophosphamide (120 mg/kg) while methotrexate, ATG thymoglobulin (7.5 mg/kg) and cyclosporine were administered as graft-versus-host disease (GVHD) prophylaxis.
The course of transplantation was complicated by grade II skin acute GvHD and posterior reversible encephalopathy syndrome (PRES). Acute GVHD was treated with steroid 2 mg/kg/day with complete response, while blood pressure, electrolytes and cyclosporine blood levels adjustment aided in resolving PRES. One month after HCT, the patient obtained a deep molecular response (DMR) that was confirmed at the last follow-up, 9 years after HCT, without signs of chronic GVHD and good quality of life.
4. Clinical Case N.2: Intolerance to TKIs
A 38-year-old woman was diagnosed with CML in CP in January 2014 with a WBC of 275 × 109/L, Hb 8.5 g/dL and PLT count of 525 × 109/L. Sokal and EUTOS risk scores were intermediate and low, respectively. Bone marrow cytogenetic analysis revealed Ph+ in 100% metaphase cells with no additional abnormalities. RT-PCR indicated that the patient expressed the p210 BCR::ABL1 rearrangement, e14a2 type transcript.
She initially received hydroxyurea, and in February 2014, treatment with nilotinib 600 mg daily was started, obtaining a CHR. In April, nilotinib therapy was suspended due to grade III/IV neutropenia and thrombocytopenia that just slightly improved following granulocyte growth factor administration. After a 4-week break from nilotinib therapy, the patient started to receive nilotinib again but at a lower dose (300 mg daily); however, at the end of May, nilotinib therapy was permanently discontinued due to recurrence of severe thrombocytopenia.
At three months after starting nilotinib therapy, bone marrow aspirate evaluation was consistent with CP CML with trilineage hypoplasia, mainly affecting the megakaryocytopoiesis. Cytogenetic and molecular analyses only showed no cytogenetic (95% of Ph+) nor molecular (BCR::ABL1 transcript 20%) responses. Because of severe hematologic toxicity and suboptimal response to first-line 2G-TKI therapy, the patient was assessed for HCT, and a donor search was initiated. In the meantime, the patient was treated with increasing doses (up to 300 mg per day) of imatinib that was stopped before transplantation three months later, when bone marrow aspirate was consistent with CP CML, but when karyotyping revealed a minor cytogenetic response and a clonal cytogenetic evolution with gain of chromosome 8 in 69% of Ph-negative metaphases; the pre-transplant BCR::ABL1 transcript level on International scale (IS) was 9%.
Considering no available sibling or unrelated matched donor, a mismatched (7/8 alleles) unrelated donor peripheral blood HCT was performed in October 2014. The conditioning regimen included busulphan and cyclophosphamide. Hematologic recovery of donor cells took place by day +15 and +11 post-transplant for absolute neutrophil count >500 × 109/L and platelet count > 20.000 × 109/L, respectively.
At 30 days post-transplant full donor chimerism was documented either in BM and PB, and BM aspirate showed cytologic characteristics of complete remission. The patient achieved a complete cytogenetic response (CCyR) and a DMR. During subsequent months no signs of acute or chronic GVHD were evident, and DMR/undetectable BCR::ABL1 transcript was maintained. In February 2017, the BCR::ABL1 transcript level rose to 0.01%, and the patient started imatinib 400 mg daily, rapidly attaining a new deepening of molecular response. In May 2018 (15 months after initiation of post-transplant imatinib), BCR::ABL1 transcripts became undetectable and so persisted until the last molecular analysis in September 2022.
5. Clinical Case N.3: Resistance to Multiple TKIs
In December 2018, a 63-year-old lady was referred to our Hematology Department due to hyperleukocytosis (WBC 206,000 × 109/L), thrombocytosis (1,098,000 × 109/L) and anemia (Hb 9.6 g/dL). The patient reported a 2-month history of fatigue, weight loss and left hypochondriac pain. The physical examination showed splenomegaly (5 × 6 × 5 cm below the costal margin) without hepatomegaly or lymphadenopathy. A PB smear revealed 12% promyelocytes, 3% basophils, 3% blasts and other circulating precursor cells. BM evaluation was consistent with CML with 0.8% myeloid blasts. PCR analysis was positive for BCR::ABL1 p210 transcript and cytogenetic analysis for an atypical t(9;22;12)(q34,q11,q11) translocation.
Considering the diagnosis of CML in CP with a Sokal score high after a brief cytoreductive therapy with hydroxyurea, a treatment with nilotinib 300 mg twice daily within the SUSTRENIM Study—GIMEMA CML 1415 trial (ClinicalTrials.gov Identifier: NCT02602314), was started. Treatment was complicated by grade 3–4 neutropenia, requiring multiple drug suspensions and dose reduction. The evaluation after 3 months of nilotinib, performed at the end of March 2019, showed a CHR without cytogenetic and molecular remission (persistence of atypical translocation t(9;22;12)(q34,q11,q11) in 20/20 metaphases and BCR::ABL1 7.8%—MR1, respectively). Therefore, in May 2019, second-line treatment with ponatinib 45 mg daily began, and a donor search for an allogenic HCT was started. Pancytopenia was the main adverse event and lasted until the transplant, despite a ponatinib dose reduction to 30 mg in June 2019 and to 15 mg in August 2019 and several drug suspensions.
After 1 year of ponatinib therapy, the patient achieved CCyR without a major molecular remission (BCR::ABL1 0.3%—MR2).
In June 2020, after a myeloablative conditioning regimen with busulfan and fludarabine, a full-matched-unrelated HCT was performed with PBSC. The Hematopoietic Cell Transplantation—specific Comorbidity Index (HCT-CI) was 0. GVHD Prophylaxis consisted of ATG Thymoglobuline, cyclosporine and metotrexate. No acute GVHD was recorded.
The post-transplant phase was complicated in November 2020 by severe neutropenia and moderate chronic GVHD involving skin and oral mucosa that had developed after cyclosporin was withdrawn. She was hospitalized and treated with prednisone 1 mg/kg/day with complete resolution and slow steroid tapering. In April 2021, after steroids suspension, our patient complained of severe fatigue: the laboratory results showed severe anemia (Hb 5.9 g/dL), haptoglobin < 10 mg/dL, LDH 2448 U/L, total bilirubin 8.6 mg/dL, and indirect bilirubin 1.08 mg/dL with autoantibody anti-IgG and anti-C3 at the indirect antiglobulin test. A diagnosis of autoimmune hemolytic anemia (AIHA) was performed, and steroid therapy with 6-methylprednisolone 2 mg/kg/day was started, with a rapid recovery of anemia. A DMR, assessed as soon as 30 days after HCT, was sustained until the last follow-up, 2 years after HCT. The patient had no sign of chronic GVHD and stopped prednisone after a slow decalage.
6. Discussion
6.1. Indication for HCT and TKIs Treatment as “Bridge to Transplant”
Herein, we presented three patients who performed HCT from unrelated donors between 2013 and 2018. These cases exemplified actual indications for HCT, which remains an important option for the minority of patients who still progress to ABP and for CML patients resistant or intolerant to two or more TKIs [5,6,7].
CML AP occurs in a minority of patients at diagnosis (overall incidence 3.5% [8]), with worse outcomes compared to patients diagnosed in CP as, despite the improvement attained with TKIs, lasting remissions are often lacking [9]. Eligibility for HCT in this subset of patients has not changed much over the last 10 years. In 2013, when our clinical case #1 occurred, eligibility criteria for HCT included losing a response previously achieved with TKIs or persistence of molecular disease after second-line therap [10]. At present, both ELN 2020 recommendations and the latest NCCN guidelines suggest that a patient diagnosed in AP should be treated with a TKI (generally a second generation one) followed by evaluation of HCT if the response is not optimal, while a disease progressing from CP to AP during TKIs therapy should be immediately considered for HCT [11,12]. Outcomes of HCT in AP CML are generally inferior to those performed in CP [13,14], although Jiang et al. demonstrated an advantage in OS for HCT over TKI in AP-CML patients [15] in a prospective study. Our patient matched the favorable prognostic factors for OS in ABP CML undergoing allogeneic HCT reported in an EBMT study by Radujkovic et al., i.e., a controlled disease at transplant, young age (<45 years), good performance status and a short time from diagnosis to transplant and an HLA-matched donor [16]. These characteristics could explain the favorable outcome of the few short-term complications; the lack of long-term complications; and the long-lasting, deep molecular response, even without maintenance treatment.
Clinical cases #2 and #3 exemplify the role that HCT still holds in the management of CML patients in first CP failing 2G or 3G TKIs or experiencing severe hematologic toxicity to one or multiple TKIs, which frequently leads to a suboptimal response due to inability to treat patients with effective doses and/or duration of TKIs [17].
Case #2 showed the appearance of clonal chromosomal aberrations (CCA) other than loss of Y chromosome in the Ph-negative clone, which has been associated with reduced survival in CP CML patients treated with various TKIs [18]. Indeed, in some cases, Ph-negative clones with additional CCA are associated with myelodysplastic syndrome/acute myeloid leukemia, mainly in patients with chromosome 7 abnormalities but also in patients with isolated trisomy 8. These cases should be monitored very carefully and may become eligible for other treatments [6] and for quick referral to transplantation. Both cases raise the question about the impact of prior TKI therapy on HCT feasibility and post-transplant complications. In fact, both patients received two lines of TKI treatment (nilotinib and imatinib in case #2, nilotinib and ponatinib in case #3) before HCT. A prospective study performed by EBMT demonstrated the feasibility of HCT in patients previously treated with 2G-TKIs (dasatinib, nilotinib and bosutinib) with engraftment and a post-transplant immunological complications rates comparable to that of TKI-naïve or imatinib-treated patients [19]. In another large series of patients, the favorable outcome seems to be due to a deepening of molecular response before HCT under newer TKIs, without an increase in non-relapse mortality (NRM) [20]. Thus, while 2G-TKIs seem to have no impact on the outcome after transplant, little is known about the effect of prior use of the 3G-TKI ponatinib. A retrospective study performed by Chaladon et al. [21] described 44 patients treated with ponatinib and HCT, showing no differences in OS, progression-free survival (PFS), relapse incidence or NRM when compared to other TKIs. In conclusion, the patients’ histories and the available clinical studies confirm that the administration of TKIs favorably impacts subsequent HCT [21,22], independently from the TKI generation and from the number of lines.
Asciminib, a first-in-class allosteric inhibitor of BCR-ABL1 kinase activity, has now been approved by FDA and EMA for the treatment of patients who failed two lines of therapy or in patients with T315I mutation, and it may represent a strategy that our clinical cases could not benefit from and a possible alternative to HCT. In the ASCEMBL study [22], asciminib demonstrated superior efficacy vs. bosutinib and an improved safety profile with a low rate of treatment discontinuation (5.8% vs. 21.1%, respectively). However, as described by Yeung et al. [23], the relevant comparator of asciminib is ponatinib, and a randomized trial will be needed to clarify the optimal treatment pathway. Considering the current data, asciminib represents the optimal strategy in patients with intolerance to previous TKI therapy, and it could have been a great therapeutic option for clinical case #2.
6.2. The Transplant Procedure
Optimal conditioning intensity for HCT in the TKIs era is crucial. A large retrospective study performed by Chhabra et al. [24] evaluated 1395 CML patients aged between 18 and 60 years who underwent HCT. Of them, myeloablative (MAC) and reduced intensity conditioning regimen (RIC) were performed in 1204 and 191 patients, respectively. Multivariable analysis showed no significant difference in OS between MAC and RIC groups. In addition, leukemia-free survival and NRM did not differ significantly between the two groups. Compared with MAC, the RIC group had a higher risk of early relapse after HCT (hazard ratio (HR), 1.85; p = 0.001). The cumulative incidence of chronic GVHD was lower with RIC than with MAC (HR, 0.77; p = 0.02). Clinical cases #1 and #2 were transplanted around the age of forty years after MAC regimens, with a follow-up after transplantation free from complications. The third patient, who received the transplant at 63 years, experienced chronic GVHD and other immunological complications such as immune-mediated neutropenia and severe AIHA requiring repeated and prolonged steroid treatment. Even if conditioning at reduced toxicity has allowed for performing HCT, it is a matter of fact that chronic GVHD and the prolonged immunosuppression required by GVHD can cause significant morbidity, especially in elderly patients. New strategies for GVHD prevention and treatment, such as post-transplant cyclophosphamide and JAK2 inhibitors, may reduce the burden of mortality and morbidity linked to GVHD also in elderly patients in the near future.
6.3. Follow-up after Transplantation: TKIs Treatment and Donor Lymphocytes Infusions (DLI)
One important issue linked to the follow-up after transplantation is the use of TKIs as a maintenance or pre-emptive strategy. Larger analyses of TKI maintenance tried to assess the effectiveness of this approach in setting Philadelphia chromosome-positive leukemias. In acute lymphoblastic leukemia (ALL), two retrospective studies from the EBMT Acute Leukemia Working Party [20] and MD Anderso [25], respectively, suggest that the use of TKIs after transplant is associated with improved outcomes and is recommended in Ph+ ALL.
In CML, TKIs or/and DLI can be administered after HCT as pre-emptive treatment in patients transplanted in APB or driven by minimal residual disease (MRD) recurrence in patients in the first CP at transplant. No recommendations or prospective studies were reported, and all the available data come from retrospective analyses, with larger experience with imatinib [26] compared to 2G- or 3G-TKIs. As expected, responses were deeper and more prolonged in molecular relapses, and no clear advantage between TKIs and DLI could be detected [27]. A recent study from the Center for International Blood and Marrow Transplant Research tried to answer whether the administration of TKIs after HCT is associated with improved outcomes for patients with CML [28]. A total of 390 adult patients who underwent transplantation between 2007 and 2014 and received maintenance TKI following HCT (n = 89) compared with no TKI maintenance (n = 301) were analyzed. All patients had received TKI therapy before HCT. The majority of patients had a disease status of first CP at HCT (n = 240; 62%). After a median follow-up of 61 months (range: 7–97 months) in the maintenance TKI group and of 68 months (range: 2–98 months) in the no maintenance group, the adjusted estimates for 5-year relapse (maintenance 35% versus no maintenance 26%; p = 0.11), leukemia-free survival (maintenance 42% versus no maintenance 44%; p = 0.65) or OS (maintenance 61% versus no maintenance 57%; p = 0.61) did not differ significantly between the two groups.
The strategy adopted by most transplant centers, including our team [29], is to use the TKI that was best tolerated and that had achieved the deepest response before HCT and to strictly monitor molecular MRD: if MRD negativity/undetectable disease is obtained, TKI therapy is continued until progression or adverse effects; in the case of disease persistence, DLIs can be associated and/or switch to a different TKI may be considered.
7. Conclusions
We feel that there is still room for an effective and well-tolerated HCT procedure in selected CML patients after a TKI treatment aimed at achieving deep responses before transplant. Areas of further investigation should be focused on the improvement of conditioning and GVHD prophylaxis in elderly patients and toward standardization of post-transplant treatment with TKIs and/or DLI.
Author Contributions
M.T. and F.P. designed and wrote the review; G.P., M.P. and G.B. collected the data and wrote the paper; M.L.B., M.C., A.G., G.F., U.P., D.D. and R.F. participated in data interpretation and writing of the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
No new data were created and patients’ personal information are unavailable due to privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jabbour, E.; Kantarjian, H. Chronic myeloid leukemia: 2022 update on diagnosis, therapy, and monitoring. Am. J. Hematol. 2022, 97, 1236–1256. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.M.; Majhail, N.S.; Klein, J.P.; Wang, Z.; Sobocinski, K.A.; Arora, M.; Horowitz, M.M.; Rizzo, J.D. Relapse and late mortality in 5-year survivors of myeloablative allogeneic hematopoietic cell transplantation for chronic myeloid leukemia in first chronic phase. J. Clin. Oncol. 2010, 28, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Bower, H.; Björkholm, M.; Dickman, P.W.; Höglund, M.; Lambert, P.C.; Andersson, T.M.L. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J. Clin. Oncol. 2016, 34, 2851–2857. [Google Scholar] [CrossRef]
- Gratwohl, A. Biological differences in the three major leukaemias. Lancet 1988, 332, 403. [Google Scholar] [CrossRef]
- Niederwieser, C.; Kröger, N. Transplantation in CML in the TKI era: Who, when, and how? Hematol. Am. Soc. Hematol. Educ. Progr. 2022, 2022, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Baccarani, M.; Saglio, G.; Goldman, J.; Hochhaus, A.; Simonsson, B.; Appelbaum, F.; Apperley, J.; Cervantes, F.; Cortes, J.; Deininger, M.; et al. Evolving concepts in the management of chronic myeloid leukemia: Recommendations from an expert panel on behalf of the European LeukemiaNet. Blood 2006, 108, 1809–1820. [Google Scholar] [CrossRef]
- Snowden, J.A.; Sánchez-ortega, I.; Corbacioglu, S.; Basak, G.W.; Chabannon, C.; Camara, R.; Dolstra, H.; Duarte, R.F.; Glass, B.; Greco, R.; et al. Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: Current practice in Europe, 2022. Bone Marrow Transplant. 2022, 57, 1217–1239. [Google Scholar] [CrossRef]
- Hoffmann, V.S.; Baccarani, M.; Hasford, J.; Lindoerfer, D.; Burgstaller, S.; Sertic, D.; Costeas, P.; Mayer, J.; Indrak, K.; Everaus, H.; et al. The EUTOS population-based registry: Incidence and clinical characteristics of 2904 CML patients in 20 European Countries. Leukemia 2015, 29, 1336–1343. [Google Scholar] [CrossRef]
- How, J.; Venkataraman, V.; Hobbs, G.S. Blast and accel er ated phase CML: Room for improvement. Hematol. Am. Soc. Hematol. Educ. Progr. 2021, 2021, 122–128. [Google Scholar] [CrossRef]
- Baccarani, M.; Cortes, J.; Pane, F.; Niederwieser, D.; Saglio, G.; Apperley, J.; Cervantes, F.; Deininger, M.; Gratwohl, A.; Guilhot, F.; et al. Chronic myeloid leukemia: An update of concepts and management recommendations of European LeukemiaNet. J. Clin. Oncol. 2009, 27, 6041–6051. [Google Scholar] [CrossRef]
- Hochhaus, A.; Baccarani, M.; Silver, R.T.; Schiffer, C.; Apperley, J.; Cervantes, F. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia 2020, 34, 966–984. [Google Scholar] [CrossRef] [PubMed]
- Deininger, M.W.; Shah, N.P.; Altman, J.K.; Berman, E.; Bhatia, R.; Bhatnagar, B.; Deangelo, D.J.; Gotlib, J.; Hobbs, G.; Maness, L.; et al. Chronic Myeloid Leukemia, Version 2.2021. J. Natl. Compr. Cancer Netw. 2020, 18, 1385–1415. [Google Scholar] [CrossRef]
- Niederwieser, C.; Morozova, E.; Zubarovskaya, L.; Zabelina, T.; Klyuchnikov, E.; Janson, D.; Wolschke, C.; Christopeit, M.; Ayuk, F.; Moiseev, I.; et al. Risk factors for outcome after allogeneic stem cell transplantation in patients with advanced phase CML. Bone Marrow Transplant. 2021, 56, 2834–2841. [Google Scholar] [CrossRef] [PubMed]
- Gratwohl, A.; Brand, R.; Apperley, J.; Crawley, C.; Ruutu, T.; Corradini, P.; Carreras, E.; Devergie, A.; Guglielmi, C.; Kolb, H.-J.; et al. Allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia in Europe 2006: Transplant activity, long-term data and current results. An analysis by the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Haematologica 2006, 91, 513–521. [Google Scholar] [PubMed]
- Jiang, Q.; Xu, L.; Liu, D.; Liu, K.; Chen, S.; Jiang, B.; Jiang, H.; Chen, H.; Chen, Y.; Han, W.; et al. Imatinib mesylate versus allogeneic hematopoietic stem cell transplantation for patients with chronic myelogenous leukemia in the accelerated phase. Blood 2011, 117, 3032–3040. [Google Scholar] [CrossRef] [PubMed]
- Radujkovic, A.; Dietrich, S.; Blok, H.; Nagler, A.; Ayuk, F.; Passweg, J.; Maertens, J.; Byrne, J.L.; Jindra, P.; Veelken, J.H.; et al. Biology of Blood and Marrow Transplantation Allogeneic Stem Cell Transplantation for Blast Crisis Chronic Myeloid Leukemia in the Era of Tyrosine Kinase Inhibitors: A Retrospective Study by the EBMT Chronic Malignancies Working Party. Biol. Blood Marrow Transplant. 2019, 25, 2008–2016. [Google Scholar] [CrossRef]
- Radich, J. When to Consider Allogeneic Transplantation in CML. Clin. Lymphoma Myeloma Leuk. 2016, 16, S93–S95. [Google Scholar] [CrossRef] [PubMed]
- Issa, G.C.; Kantarjian, H.M.; Gonzalez, G.N.; Borthakur, G.; Tang, G.; Wierda, W.; Sasaki, K.; Short, N.J.; Ravandi, F.; Kadia, T.; et al. Clonal chromosomal abnormalities appearing in Philadelphia chromosome—Negative metaphases during CML treatment. Blood 2017, 130, 2084–2091. [Google Scholar] [CrossRef] [PubMed]
- Masouridi-levrat, S.; Olavarria, E.; Iacobelli, S.; Aljurf, M.; Morozova, E.; Niittyvuopio, R.; Sengeloev, H.; Reményi, P.; Helbig, G.; Browne, P.; et al. Outcomes and toxicity of allogeneic hematopoietic cell transplantation in chronic myeloid leukemia patients previously treated with second-generation tyrosine kinase inhibitors: A prospective non-interventional study from the Chronic Malignancy Working Party of the EBMT. Bone Marrow Transplant. 2022, 57, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, Y.; Murata, M.; Kondo, T.; Minami, Y.; Tachibana, T.; Doki, N.; Uchida, N.; Ozawa, Y.; Yano, S.; Fukuda, T.; et al. The new generation tyrosine kinase inhibitor improves the survival of chronic myeloid leukemia patients after allogeneic stem cell transplantation. Haematologica 2022, 40, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Chalandon, Y.; Apperley, J.; Sbianchi, G.; Gras, L.; Koster, L.; Byrne, J.; Salmenniemi, U.; Sengeloev, H.; Kinsella, F.; Choi, G.; et al. Allogeneic hematopoietic cell transplantation in patients with chronic phase chronic myeloid leukemia in the era of third generation tyrosine kinase inhibitors: A retrospective study by the chronic malignancies working party of the EBMT. Am. J. Hematol. 2023, 98, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Réa, D.; Mauro, M.J.; Boquimpani, C.; Minami, Y.; Lomaia, E.; Voloshin, S.; Turkina, A.; Kim, D.W.; Apperley, J.F.; Abdo, A.; et al. A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, vs bosutinib in CML after 2 or more prior TKIs. Blood 2021, 138, 2031–2041. [Google Scholar] [CrossRef]
- Yeung, D.T.; Shanmuganathan, N.; Hughes, T.P. Asciminib: A new therapeutic option in chronic-phase CML with treatment failure. Blood 2022, 139, 3474–3479. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, S.; Ahn, K.W.; Hu, Z.H.; Jain, S.; Assal, A.; Cerny, J.; Copelan, E.A.; Daly, A.; DeFilipp, Z.; Gadalla, S.M.; et al. Myeloablative vs reduced-intensity conditioning allogeneic hematopoietic cell transplantation for chronic myeloid leukemia. Blood Adv. 2018, 2, 2922–2936. [Google Scholar] [CrossRef] [PubMed]
- Saini, N.; Marin, D.; Ledesma, C.; Delgado, R.; Rondon, G.; Popat, U.R.; Bashir, Q.; Hosing, C.M.; Nieto, Y.; Alousi, A.M.; et al. Impact of TKIs post-allogeneic hematopoietic cell transplantation in Philadelphia chromosome-positive ALL. Blood 2020, 136, 1786–1789. [Google Scholar] [CrossRef] [PubMed]
- Olavarria, E.; Ottmann, O.G.; Deininger, M.; Clark, R.E.; Bandini, G.; Byrne, J.; Lipton, J.; Vitek, A. Response to imatinib in patients who relapse after allogeneic stem cell transplantation for chronic myeloid leukemia. Leukemia 2003, 17, 1707–1712. [Google Scholar] [CrossRef]
- Shanavas, M.; Messner, H.A.; Kamel-reid, S.; Atenafu, E.G.; Gupta, V.; Kuruvilla, J.; Dong, D.; Kim, H.; Uhm, J.; Lambie, A.; et al. A Comparison of Long-Term Outcomes of Donor Lymphocyte Infusions and Tyrosine Kinase Inhibitors in Patients With Relapsed CML after Allogeneic Hematopoietic Cell Transplantation. Clin. Lymphoma Myeloma Leuk. 2014, 14, 87–92. [Google Scholar] [CrossRef][Green Version]
- DeFilipp, Z.; Ancheta, R.; Liu, Y.; Hu, Z.H.; Gale, R.P.; Snyder, D.; Schouten, H.C.; Kalaycio, M.; Hildebrandt, G.C.; Ustun, C.; et al. Maintenance Tyrosine Kinase Inhibitors Following Allogeneic Hematopoietic Stem Cell Transplantation for Chronic Myelogenous Leukemia: A Center for International Blood and Marrow Transplant Research Study. Biol. Blood Marrow Transplant. 2020, 26, 472–479. [Google Scholar] [CrossRef]
- Patriarca, F.; Giaccone, L.; Onida, F.; Castagna, L.; Sarina, B.; Montefusco, V.; Mussetti, A.; Mordini, N.; Greco, R.; Peccatori, J.; et al. New drugs and allogeneic hematopoietic cell transplantation for hematological malignancies: Do they have a role in bridging, consolidating or conditioning transplantation treatment? Expert Opin. Biol. Ther. 2017, 17, 821–836. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).