Abstract
Homeobox genes encode transcription factors which control basic processes in development and differentiation. Concerning the sequence conservation in their homeobox, these genes are arranged into particular groups sharing evolutionary ancestry and resembling in function. We have recently described the physiological expression patterns of two homeobox gene groups, NKL and TALE, in early hematopoiesis and subsequent lymphopoiesis. The hematopoietic activities of eleven NKL and nine TALE homeobox genes have been termed as NKL- and TALE-codes, respectively. Due to the developmental impact of homeobox genes, these expression data indicate a key role for their activity in normal hematopoietic differentiation processes, including B-cell development. On the other hand, aberrant expression of NKL- and TALE-code members or ectopic activation of non-code members have been frequently reported in lymphoid malignancies, demonstrating their oncogenic potential in the hematopoietic compartment. Here, we provide an overview of the established NKL- and TALE-codes in normal lymphopoiesis and of deregulated homeobox genes in Hodgkin lymphoma, demonstrating the capability of gene codes to identify homeo-oncogenes in lymphoid malignancies.
1. Hematopoiesis and B-Cell Development
In the course of hematopoiesis, all of the blood and immune cells are produced. Today, developing and mature hematopoietic cells are extensively defined even at the molecular level, allowing for the retracement of underlying mechanisms of cell differentiation [1]. Hematopoietic stem cells (HSCs) are located in the bone marrow and generate common progenitors, which represent the starting points for the myeloid and lymphoid cell lineages. The common lymphoid progenitor (CLP) generates all of the types of lymphocytes, comprising B-cells, T-cells, natural killer (NK)-cells, and innate lymphoid cells (ILC). The process of B-cell development begins with the CLP-derived B-cell progenitor (BCP) and includes the rearrangements of B-cell receptor genes encoding the immunoglobulin chains. BCPs differentiate via the pro-B-cell and pre-B-cell stages into naïve B-cells. Early T-cell progenitors migrate into the thymus to complete their differentiation. In contrast, for the final differentiation steps to memory B-cells and plasma cells via the stage of germinal center (GC) B-cells, naïve B-cells migrate into lymph nodes, the spleen, and other lymphoid tissues. In these compartments, additional molecular alterations occur, such as the somatic hypermutation and class switching of the B-cell receptor genes. These alterations are operated at the DNA level and prone to generate oncogenic gene rearrangements and mutations.
The main steps of lymphopoiesis including B-cell development are controlled at the transcriptional level [2,3]. Accordingly, several transcription factors (TFs), such as BCL6, EBF1, MYB, PAX5, PRDM1, SPIB, and TCF3 are members of a B-cell specific regulatory network, which orchestrates basic differentiation processes [4,5,6,7]. TCF3 plays a prominent role for the development of all types of lymphocytes, while EBF1 and PAX5 are basic factors of the B-cell lineage [3,8]. BCL6 and PRDM1 inhibit each other and are involved in differentiation processes taking place in the GC [9]. Provoked by aberrant chromosomal rearrangements or gene mutations, deregulations of these developmental TFs are reported to contribute to the generation of B-cell malignancies [10,11]. Therefore, the knowledge of physiological activities of developmental TFs supports the understanding of both the normal and abnormal processes in B-cell differentiation.
2. Classification of Homeobox Genes
Homeobox genes encode TFs, which mainly control development and cell/tissue differentiation [12]. They represent the second strongest group of TFs in humans [13]. Their conserved homeobox encodes the homeodomain, which forms a 3D-structure classified as helix–turn–helix. The homeodomain is about 60 amino acid residues long and consists of three helices. Helix 3 shows the strongest sequence conformance, fits into the major groove of the DNA, and performs sequence–specific interactions [14]. Accordingly, helix 3 has been termed as a recognition helix. Moreover, the homeodomain mediates contacts with chromatin and cofactors, thus representing the operating basis of these TFs [12].
With regards to the similarities in their homeobox sequences, these genes are arranged in classes and subclasses. Overall, eleven classes have been recognized, comprising ANTP, CERS, CUT, HNF, LIM, POU, PRD, PROS, SINE, TALE, and ZF [15]. The largest classes are antennapedia (ANTP) and paired (PRD). The ANTP class contains the subclasses HOX-like (HOXL) and NK-like (NKL). This established scheme reflects the evolutionary history of these genes, which have been detected in all of the eukaryotes and expanded in Metazoa [16]. The human genome contains 235 homeobox genes [15]. The process of gene duplication has generated gene clusters, which subsequently diversified and separated, but are still present for the HOX genes and to some extent, for the NKL genes [17]. The human genome contains four HOX gene clusters, called HOXA, HOXB, HOXC, and HOXD, showing an evolutionary conserved arrangement and colinear activities in the embryonal development. Furthermore, the human genome contains 48 NKL subclass members. For instance, NKL homeobox genes TLX3, NKX2-5, and MSX2 are distant neighbors and located at chromosomal position 5q35, displaying their evolutionary history as part of an ancient and more comprehensive NKL gene cluster [15,17].
The basic impact of homeobox genes in developmental processes is evident by nominating several members as master genes. NKL homeobox gene NKX2-3 has been described as a fundamental regulator of spleen development. Knockdown of this gene in embryonal mice results in asplenic animals [18]. Furthermore, NKX2-3 is hematopoietically expressed in human HSCs and silenced in the following stages of differentiation, indicating functional roles at the stem cell level [19]. NKX2-5 is involved in the development of both the spleen and heart [20,21]. Its role in heart development is evolutionary conserved in vertebrates and insects, highlighting the profound developmental impact of homeobox genes. Finally, PAX5 is a member of the PRD class of homeobox genes and controls the differentiation of B-cells [22]. Loss of PAX5 activity in the hematopoietic compartment disturbs the B-cell development in mice [23]. Therefore, these master genes regulate fundamental steps in the differentiation of specific cells, tissues, and organs.
3. Homeobox Gene Signatures: Lymphoid NKL- and TALE-Codes
Homeobox gene codes have been described for the first time for the clustered HOX genes expressed in the developing fruit fly Drosophila and later in vertebrate embryos [24]. Regarding their genomic arrangement, these genes display a colinear anterio–posterio expression pattern in the embryonic head region of mice, which has been called HOX-code [25]. The DLX-code describes a dorso–ventral expression pattern of DLX homeobox genes in the developing pharyngeal region [26]. Therefore, these gene codes represent the expression signatures of related homeobox genes for particular tissues or body regions.
Recently, we have created the NKL-code, which describes the expression of particular NKL homeobox genes in the course of hematopoiesis, including early stem cell stages, lymphopoiesis, myelopoiesis, and mature lymphoid and myeloid cells [19,27,28]. This code encompasses eleven NKL homeobox genes, showing a specific expression pattern in stem and progenitor cells, as well as mature blood and immune cells [29]. The lymphoid NKL-code is depicted in Figure 1. With regards to this code, the expression of NKX2-3 and NANOG is restricted to hematopoietic stem/progenitor cells, while HHEX and HLX are active in developing and mature lymphocytes. The activity of NKL homeobox genes is downregulated in the final differentiation stages of T-cells, ILC1, and ILC2. In B-cell development, HLX is silenced after the stage of B-cell progenitors, while HHEX and NKX6-3 contribute to the differentiation of memory B-cells and plasma cells, respectively.
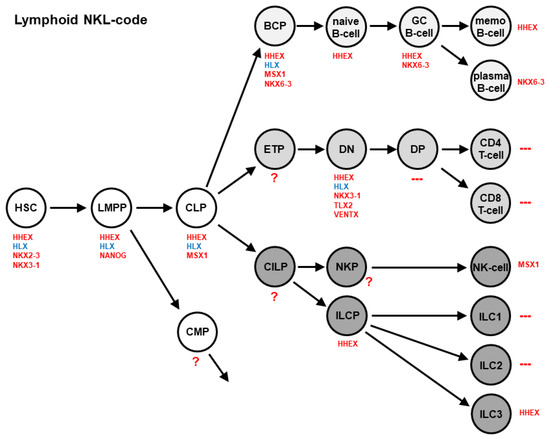
Figure 1.
The lymphoid NKL-code describes activities of NKL homeobox genes (red) in stages and cell types of early hematopoiesis and lymphopoiesis, including developing B-cells, T-cells, NK-cells, and ILCs. NKL homeobox gene HLX is highlighted in blue. BCP: B-cell progenitor; CILP: Common innate lymphoid cell progenitor; CLP: Common lymphoid progenitor; CMP: Common myeloid progenitor; DN: Double negative thymocytes; DP: Double positive thymocytes; ETP: Early T-cell progenitor; GC: Germinal center; HSC: Hematopoietic stem cell; ILC: Innate lymphoid cells; LMPP: Lymphoid and myeloid primed progenitor; memo: Memory; NKP: NK-cell progenitor; ?: Cell types with unknown NKL gene activities; ---: Cell types with absent NKL gene activities.
TALE genes represent a conspicuous homeobox gene class [12,30]. All of the members contain a three amino acid loop extension between helix 1 and 2, abbreviated as TALE. This very ancient group of homeobox genes encodes TFs, which are able to cooperate with other TALE or with particular HOX proteins to regulate target genes. Regarding the generation of the NKL-code, we have created the lymphoid TALE-code [31]. This gene signature includes 11 of the 20 genes of the strong TALE homeobox gene class, expressed in early hematopoiesis and lymphopoiesis (Figure 2).
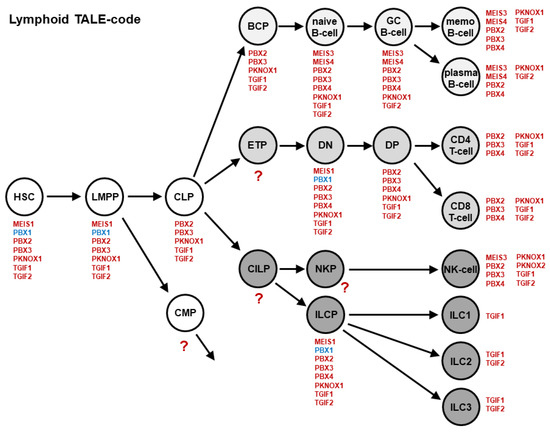
Figure 2.
The lymphoid TALE-code describes the activities for TALE homeobox genes (red) in stages and cell types of early hematopoiesis and lymphopoiesis, including developing B-cells, T-cells, NK-cells, and ILCs. TALE homeobox gene PBX1 is highlighted in blue. BCP: B-cell progenitor; CILP: Common innate lymphoid cell progenitor; CLP: Common lymphoid progenitor; CMP: Common myeloid progenitor; DN: Double negative thymocytes; DP: Double positive thymocytes; ETP: Early T-cell progenitor; GC: Germinal center; HSC: Hematopoietic stem cell; ILC: Innate lymphoid cells; LMPP: Lymphoid and myeloid primed progenitor; memo: Memory; NKP: NK-cell progenitor; ?: Cell types with unknown TALE gene activities.
Each stage in lymphopoiesis expresses between one (ILC1) and eight (for example, naïve B-cells) TALE homeobox genes. TGF1 is expressed in all of the analyzed cell types, while PBX1, for example, is restricted to stem and progenitor cells. Thereafter, PBX1 is silenced in the course of B-cell development [31,32], indicating a suppressive function in lymphoid maturation.
4. Deregulated Homeobox Genes in Hodgkin Lymphoma
4.1. Hodgkin Lymphoma
Hodgkin lymphoma (HL) is a GC-derived B-cell malignancy, although rare cases with T-cell origin have been described [33]. With regards to the histological and phenotypical characteristics, this tumor type is classified into two main groups, classical HL and nodular lymphocyte predominant HL (NLPHL). Classical HL is further divided into the subtypes nodular sclerosis, mixed cellularity, lymphocyte-rich, and lymphocyte-depleted. The typical large tumor cells are called Hodgkin Reed Sternberg (HRS) and lymphocyte predominant (LP) cells, respectively for the classical HL and NLPHL groups, occurring rarely in infiltrated lymph nodes. Therefore, most of the cells of the tumor mass represent reactive lymphocytes, macrophages, dendritic cells, and granulocytes [34].
The specific phenotype and rareness of HRS and LP cells complicate their analysis. However, established bona fide HL cell lines may serve as suitable models to investigate their molecular abnormalities [35]. HL has a long and intense background of causal research revealing several hallmarks, including aberrant receptor-signaling, inhibition of apoptosis, activated NFkB-pathway, loss of B-cell associated TFs, EBV infection, and immune escape [34,36]. HL is one of the most frequent lymphomas in the Western world. In addition, the current chemotherapeutic and radiation protocols have substantially improved the prognosis [34].
In HL, several deregulated homeobox genes have been reported, belonging to different classes/subclasses: ANTP/HOXL (HOXB9, MNX1/HLXB9), ANTP/NKL (DLX1, EMX2, HLX, NKX2-2, NKX3-2, TLX2), POU (POU2AF1/BOB1, POU2F2/OCT2), PRD (HOPX, PAX5, MIXL1, OTX1, OTX2), SINE (SIX1), TALE (IRX3, IRX4, MEIS1, MEIS3, PBX1, PBX4, TGIF1), and ZF (ZHX2) [29,31,37,38,39,40,41,42,43,44,45,46]. Downregulation of B-cell associated homeobox gene activities has been described for PAX5, POU2AF1/BOB1, POU2F2/OCT2, and ZHX2, basically contributing to the generation of this malignancy by disturbed B-cell differentiation [41,46]. The remaining deregulated homeobox genes are aberrantly overexpressed or ectopically activated. Recently, we have systematically analyzed the groups of NKL and TALE homeobox genes in hematopoiesis and revealed aberrant activities of two members in HL, which are detailed in the following section [30,36].
4.2. Deregulated NKL Homeobox Gene HLX in HL
A systematic screening for deregulated NKL homeobox genes in HL revealed six members [27]. A subsequent comparison of lymphoid NKL-code members with HL patient data demonstrated aberrant overexpression of HLX in about 8% of patients [37]. HL cell line L-540 showed conspicuously high HLX expression levels and served as a model to investigate the upstream and downstream factors. Expression profiling analysis, knockdown and inhibitor experiments, reporter gene assays, in addition to chromatin immunoprecipitation data showed that STAT3 directly activates HLX in L-540. Furthermore, analyses of the subcellular localization of STAT3 indicated that deacetylation of STAT3 protein supports nuclear translocation and the subsequent activation of HLX expression [37]. Consistently, Kube et al. have reported that STAT3 represents an aberrantly activated TF in HL [47]. Analyses of HLX target genes revealed the inhibition of differentiation factors BCL11A, MSX1 and SPIB, and of pro-apoptotic factor BCL2L11 [37]. Therefore, HLX impacts the HL hallmark processes B-cell differentiation and apoptosis. The deregulated activity and oncogenic function of HLX in HL cells is summarized in Figure 3.
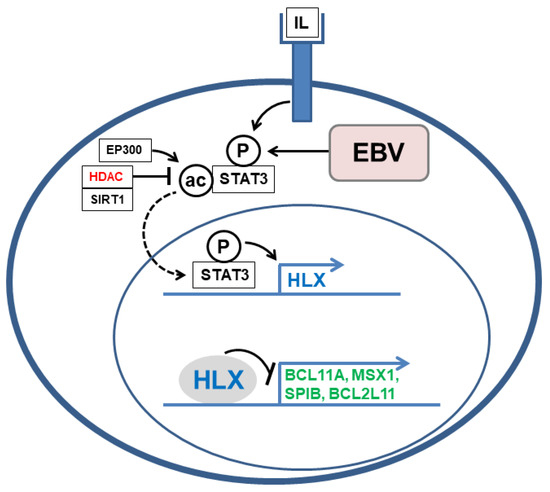
Figure 3.
This diagram shows the regulation and function of aberrantly expressed NKL homeobox gene HLX. STAT3 is activated by interleukin (IL)-receptor-signaling or EBV-infection resulting in phosphorylation (P). The subcellular localization of STAT3 is regulated by acetylation (ac). EP300 mediates acetylation, while SIRT1 and overexpressed histone deacetylases (HDACs, red) perform deacetylation. Phosphorylated and deacetylated STAT3 translocate into the nucleus (dashed arrow) to activate its target gene HLX. HLX can in turn operate as a repressor to inhibit the target genes BCL11A, MSX1, SPIB, and BCL2L11 (green).
The activator role of STAT3 for HLX expression has also been shown in the pathological contexts of anaplastic large cell lymphoma (ALCL) and EBV-positive diffuse large B-cell lymphoma (DLBCL). In ALCL, STAT3 is overexpressed, mutated, and aberrantly activated, representing a hallmark oncogene for this disease [48,49]. Using ALCL cell lines as models, we have demonstrated that STAT3 in addition to STAT3-activators and STAT3-target genes including HLX are overexpressed and targeted by copy number gains. Moreover, the oncogenic fusion protein NPM1–ALK activates STAT3, which can in turn drive HLX expression in ALCL cell lines [50].
Infection with Epstein–Barr virus (EBV) plays a pathognomonic role in B-cell lymphomas, including HL and DLBCL [51]. EBV-encoded proteins deregulate the activity of several signaling pathways, including JAK–STAT [52,53,54]. Accordingly, isolated EBV-positive and EBV-negative cell populations of DLBCL cell line DOHH-2 were used to demonstrate that EBV-factors LMP1 and LMP2A mediate STAT3 activation, which can in turn drive HLX expression in this malignancy [55]. Therefore, aberrant HLX activation by STAT3 is a general oncogenic transaction, playing a role in HL, ALCL, and DLBCL.
4.3. Deregulated TALE Homeobox Gene PBX1 in HL
Screening for deregulated TALE homeobox genes in HL patients revealed seven class members, including PBX1 [31]. The HL cell line SUP-HD1 expressed elevated PBX1 levels and served as a model to analyze upstream and downstream factors of this TALE homeobox gene. Genomic analysis demonstrated a copy number gain for PBX1 at 1q23 in SUP-HD1, which may underlie its upregulation. Target gene analysis revealed that PBX1 activated the differentiation factor NFIB and NKL homeobox gene TLX2. Of note, aberrant overexpression of NFIB in addition to NFIA, NFIC, and NFIX may indicate that this family of developmental TFs plays a substantial role in the pathogenesis of HL [31].
TALE homeodomain factors are described to interact with homeodomain factors of the TALE class or of the HOXL subclass [56]. This feature may represent a very ancient function of TALE factors [30]. Recently, we have found by a combination of PCR-screens the use of degenerate oligonucleotides and expression profiling analysis overexpression of HOXL subclass member HOXB9 in HL cell lines. HOXB9 is normally silent in hematopoietic cells, indicating ectopic activation. Therefore, once again, a copy number gain may underlie elevated HOXB9 expression in HL [39]. Additional analyses of the identified PBX1-target genes demonstrated that TLX2 is coregulated by PBX1 and HOXB9, while NFIB and TNFRSF9 are regulated by PBX1 or HOXB9, respectively. Deregulation of NFIB and TLX2 may impact B-cell differentiation, while TNFRSF9 has been implicated in immune escape [31,57,58]. Furthermore, TLX2 was shown to inhibit apoptosis via TBX15 and BCL2L11 (Figure 4). Therefore, PBX1 and HOXB9 support several oncogenic hallmark processes in HL.
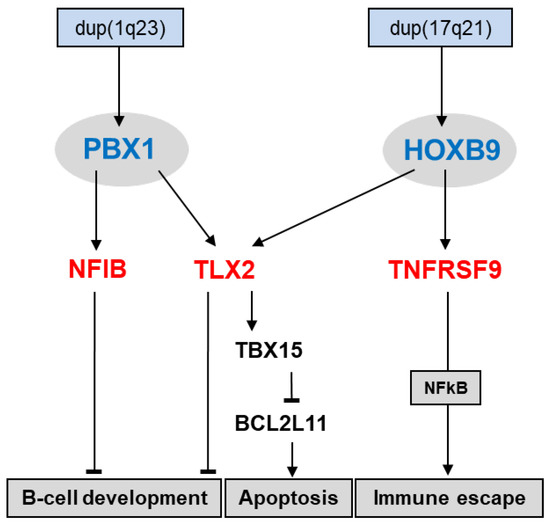
Figure 4.
This diagram shows the activation and function of aberrantly expressed TALE homeobox gene PBX1 and HOXL homeobox gene HOXB9 in HL. Both genes are targeted by genomic copy number gains. PBX1 activates NFIB and TLX2, while HOXB9 activates TLX2 and TNFRSF9. NFIB and TLX2 mediate the inhibition of B-cell development, and TLX2 inhibits apoptosis via TBX15 and BCL2L11. TNFRSF9 supports aberrant immune escape via NFkB-signaling.
5. Conclusions
In conclusion, homeobox genes encode basic TFs with oncogenic functions, in the case that they are deregulated. Homeobox gene codes serve to describe the physiological differentiation processes and to identify and evaluate aberrant homeobox gene activities in coresponding tumor entities. Deregulated NKL- and TALE-code members HLX and PBX1 play important pathogenic roles in HL, and may thus have diagnostic and/or therapeutic potentials.
Funding
This research received no external funding.
Acknowledgments
I would like to thank Corinna Meyer for excellent technical support.
Conflicts of Interest
The author declares no conflict of interest.
References
- Liggett, L.A.; Sankaran, V.G. Unraveling hematopoiesis through the lens of genomics. Cell 2020, 182, 1384–1400. [Google Scholar] [CrossRef] [PubMed]
- Boller, S.; Grosschedl, R. The regulatory network of B-cell differentiation: A focused view of early B-cell factor 1 function. Immunol. Rev. 2014, 261, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, E.V. Transcriptional control of early T and B cell developmental choices. Annu. Rev. Immunol. 2014, 32, 283–321. [Google Scholar] [CrossRef] [PubMed]
- Méndez, A.; Mendoza, L. A network model to describe the terminal differentiation of B cells. PLoS Comput. Biol. 2016, 12, e1004696. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Sigvardsson, M. The roles of transcription factors in B lymphocyte commitment, development, and transformation. J. Leukoc. Biol. 2004, 75, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Nutt, S.L.; Kee, B.L. The transcriptional regulation of B cell lineage commitment. Immunity 2007, 26, 715–725. [Google Scholar] [CrossRef]
- Sokalski, K.M.; Li, S.K.; Welch, I.; Cadieux-Pitre, H.A.; Gruca, M.R.; DeKoter, R.P. Deletion of genes encoding PU.1 and Spi-B in B cells impairs differentiation and induces pre-B cell acute lymphoblastic leukemia. Blood 2011, 118, 2801–2808. [Google Scholar] [CrossRef]
- Kucinski, I.; Wilson, N.K.; Hannah, R.; Kinston, S.J.; Cauchy, P.; Lenaerts, A.; Grosschedl, R.; Göttgens, B. Interactions between lineage-associated transcription factors govern haematopoietic progenitor states. EMBO J. 2020, 39, e104983. [Google Scholar] [CrossRef]
- Shaffer, A.L.; Yu, X.; He, Y.; Boldrick, J.; Chan, E.P.; Staudt, L.M. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity 2000, 13, 199–212. [Google Scholar] [CrossRef]
- Ma, E.S. Recurrent cytogenetic abnormalities in Non-Hodgkin’s lymphoma and chronic lymphocytic leukemia. Methods Mol. Biol. 2017, 1541, 279–293. [Google Scholar]
- Bödör, C.; Reiniger, L. Catalog of genetic progression of human cancers: Non-Hodgkin lymphoma. Cancer Metastasis Rev. 2016, 35, 109–127. [Google Scholar] [CrossRef] [PubMed]
- Bürglin, T.R. Homeodomain subtypes and functional diversity. Subcell Biochem. 2011, 52, 95–122. [Google Scholar] [PubMed]
- Vaquerizas, J.M.; Kummerfeld, S.K.; Teichmann, S.A.; Luscombe, N.M. A census of human transcription factors: Function, expression and evolution. Nat. Rev. Genet. 2009, 10, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Gehring, W.J.; Müller, M.; Affolter, M.; Percival-Smith, A.; Billeter, M.; Qian, Y.Q.; Otting, G.; Wüthrich, K. The structure of the homeodomain and its functional implications. Trends Genet. 1990, 6, 323–329. [Google Scholar] [CrossRef]
- Holland, P.W.; Booth, H.A.; Bruford, E.A. Classification and nomenclature of all human homeobox genes. BMC Biol. 2007, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Weirauch, M.T.; Hughes, T.R. A catalogue of eukaryotic transcription factor types, their evolutionary origin, and species distribution. Subcell Biochem. 2011, 52, 25–73. [Google Scholar]
- Pollard, S.L.; Holland, P.W. Evidence for 14 homeobox gene clusters in human genome ancestry. Curr. Biol. 2000, 10, 1059–1062. [Google Scholar] [CrossRef]
- Pabst, O.; Zweigerdt, R.; Arnold, H.H. Targeted disruption of the homeobox transcription factor Nkx2-3 in mice results in postnatal lethality and abnormal development of small intestine and spleen. Development 1999, 126, 2215–2225. [Google Scholar] [CrossRef]
- Nagel, S.; Pommerenke, C.; Scherr, M.; Meyer, C.; Kaufmann, M.; Battmer, K.; MacLeod, R.A.; Drexler, H.G. NKL homeobox gene activities in hematopoietic stem cells, T-cell development and T-cell leukemia. PLoS ONE 2017, 12, e0171164. [Google Scholar] [CrossRef]
- Lints, T.J.; Parsons, L.M.; Hartley, L.; Lyons, I.; Harvey, R.P. Nkx-2.5: A novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development 1993, 119, 419–431. [Google Scholar] [CrossRef]
- Brendolan, A.; Ferretti, E.; Salsi, V.; Moses, K.; Quaggin, S.; Blasi, F.; Cleary, M.L.; Selleri, L. A Pbx1-dependent genetic and transcriptional network regulates spleen ontogeny. Development 2005, 132, 3113–3126. [Google Scholar] [CrossRef] [PubMed]
- Cobaleda, C.; Schebesta, A.; Delogu, A.; Busslinger, M. Pax5: The guardian of B cell identity and function. Nat. Immunol. 2007, 8, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Urbánek, P.; Wang, Z.Q.; Fetka, I.; Wagner, E.F.; Busslinger, M. Complete block of early B cell differentiation and altered patterning of the posterior midbrain in mice lacking Pax5/BSAP. Cell 1994, 79, 901–912. [Google Scholar] [CrossRef]
- Lewis, E.B. A gene complex controlling segmentation in Drosophila. Nature 1978, 276, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Hunt, P.; Gulisano, M.; Cook, M.; Sham, M.H.; Faiella, A.; Wilkinson, D.; Boncinelli, E.; Krumlauf, R. A distinct Hox code for the branchial region of the vertebrate head. Nature 1991, 353, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Depew, M.J.; Simpson, C.A.; Morasso, M.; Rubenstein, J.L. Reassessing the Dlx code: The genetic regulation of branchial arch skeletal pattern and development. J. Anat. 2005, 207, 501–561. [Google Scholar] [CrossRef]
- Nagel, S.; MacLeod, R.A.F.; Meyer, C.; Kaufmann, M.; Drexler, H.G. NKL homeobox gene activities in B-cell development and lymphomas. PLoS ONE 2018, 13, e0205537. [Google Scholar] [CrossRef]
- Nagel, S.; Scherr, M.; MacLeod, R.A.F.; Pommerenke, C.; Koeppel, M.; Meyer, C.; Kaufmann, M.; Dallmann, I.; Drexler, H.G. NKL homeobox gene activities in normal and malignant myeloid cells. PLoS ONE 2019, 14, e0226212. [Google Scholar] [CrossRef]
- Nagel, S. NKL-Code in normal and aberrant hematopoiesis. Cancers 2021, 13, 1961. [Google Scholar] [CrossRef]
- Mukherjee, K.; Bürglin, T.R. Comprehensive analysis of animal TALE homeobox genes: New conserved motifs and cases of accelerated evolution. J. Mol. Evol. 2007, 65, 137–153. [Google Scholar] [CrossRef]
- Nagel, S.; Pommerenke, C.; Meyer, C.; MacLeod, R.A.F.; Drexler, H.G. Establishment of the TALE-code reveals aberrantly activated homeobox gene PBX1 in Hodgkin lymphoma. PLoS ONE 2021, 16, e0246603. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, M.; Tung, J.W.; Karsunky, H.; Zeng, H.; Selleri, L.; Weissman, I.L.; Herzenberg, L.A.; Cleary, M.L. B-cell development fails in the absence of the Pbx1 proto-oncogene. Blood 2007, 109, 4191–4199. [Google Scholar] [CrossRef] [PubMed]
- Küppers, R. The biology of Hodgkin’s lymphoma. Nat. Rev. Cancer 2009, 9, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Küppers, R.; Engert, A.; Hansmann, M.L. Hodgkin lymphoma. J. Clin. Invest. 2012, 122, 3439–3447. [Google Scholar] [CrossRef] [PubMed]
- Drexler, H.G.; Pommerenke, C.; Eberth, S.; Nagel, S. Hodgkin lymphoma cell lines: To separate the wheat from the chaff. Biol. Chem. 2018, 399, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Weniger, M.A.; Küppers, R. Molecular biology of Hodgkin lymphoma. Leukemia 2021, 35, 968–981. [Google Scholar] [CrossRef] [PubMed]
- Nagel, S.; Pommerenke, C.; Meyer, C.; Kaufmann, M.; MacLeod, R.A.F.; Drexler, H.G. Aberrant expression of NKL homeobox gene HLX in Hodgkin lymphoma. Oncotarget 2018, 9, 14338–14353. [Google Scholar] [CrossRef][Green Version]
- Nagel, S.; MacLeod, R.A.F.; Pommerenke, C.; Meyer, C.; Kaufmann, M.; Drexler, H.G. NKL homeobox gene NKX2-2 is aberrantly expressed in Hodgkin lymphoma. Oncotarget 2018, 9, 37480–37496. [Google Scholar] [CrossRef][Green Version]
- Nagel, S.; Burek, C.; Venturini, L.; Scherr, M.; Quentmeier, H.; Meyer, C.; Rosenwald, A.; Drexler, H.G.; MacLeod, R.A. Comprehensive analysis of homeobox genes in Hodgkin lymphoma cell lines identifies dysregulated expression of HOXB9 mediated via ERK5 signaling and BMI1. Blood 2007, 109, 3015–3023. [Google Scholar] [CrossRef]
- Nagel, S.; Scherr, M.; Quentmeier, H.; Kaufmann, M.; Zaborski, M.; Drexler, H.G.; MacLeod, R.A. HLXB9 activates IL6 in Hodgkin lymphoma cell lines and is regulated by PI3K signalling involving E2F3. Leukemia 2005, 19, 841–846. [Google Scholar] [CrossRef]
- Stein, H.; Marafioti, T.; Foss, H.D.; Laumen, H.; Hummel, M.; Anagnostopoulos, I.; Wirth, T.; Demel, G.; Falini, B. Down-regulation of BOB.1/OBF.1 and Oct2 in classical Hodgkin disease but not in lymphocyte predominant Hodgkin disease correlates with immunoglobulin transcription. Blood 2001, 97, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Krenacs, L.; Himmelmann, A.W.; Quintanilla-Martinez, L.; Fest, T.; Riva, A.; Wellmann, A.; Bagdi, E.; Kehrl, J.H.; Jaffe, E.S.; Raffeld, M. Transcription factor B-cell-specific activator protein (BSAP) is differentially expressed in B cells and in subsets of B-cell lymphomas. Blood 1998, 92, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Drakos, E.; Rassidakis, G.Z.; Leventaki, V.; Guo, W.; Medeiros, L.J.; Nagarajan, L. Differential expression of the human MIXL1 gene product in non-Hodgkin and Hodgkin lymphomas. Hum. Pathol. 2007, 38, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Nagel, S.; Ehrentraut, S.; Meyer, C.; Kaufmann, M.; Drexler, H.G.; MacLeod, R.A. Aberrantly Eexpressed OTX homeobox genes deregulate B-Cell differentiation in Hodgkin lymphoma. PLoS ONE 2015, 10, e0138416. [Google Scholar] [CrossRef]
- Nagel, S.; Meyer, C.; Kaufmann, M.; Drexler, H.G.; MacLeod, R.A. Aberrant expression of homeobox gene SIX1 in Hodgkin lymphoma. Oncotarget 2015, 6, 40112–40126. [Google Scholar] [CrossRef]
- Nagel, S.; Schneider, B.; Rosenwald, A.; Meyer, C.; Kaufmann, M.; Drexler, H.G.; MacLeod, R.A. t(4;8)(q27;q24) in Hodgkin lymphoma cells targets phosphodiesterase PDE5A and homeobox gene ZHX2. Genes Chromosomes Cancer 2011, 50, 996–1009. [Google Scholar] [CrossRef]
- Kube, D.; Holtick, U.; Vockerodt, M.; Ahmadi, T.; Haier, B.; Behrmann, I.; Heinrich, P.C.; Diehl, V.; Tesch, H. STAT3 is constitutively activated in Hodgkin cell lines. Blood 2001, 98, 762–770. [Google Scholar] [CrossRef]
- Zamo, A.; Chiarle, R.; Piva, R.; Howes, J.; Fan, Y.; Chilosi, M.; Levy, D.E.; Inghirami, G. Anaplastic lymphoma kinase (ALK) activates Stat3 and protects hematopoietic cells from cell death. Oncogene 2002, 21, 1038–1047. [Google Scholar] [CrossRef]
- Crescenzo, R.; Abate, F.; Lasorsa, E.; Tabbo, F.; Gaudiano, M.; Chiesa, N.; Di Giacomo, F.; Spaccarotella, E.; Barbarossa, L.; Ercole, E.; et al. Convergent mutations and kinase fusions lead to oncogenic STAT3 activation in anaplastic large cell lymphoma. Cancer Cell. 2015, 27, 516–532. [Google Scholar] [CrossRef]
- Nagel, S.; Pommerenke, C.; MacLeod, R.A.F.; Meyer, C.; Kaufmann, M.; Drexler, H.G. The NKL-code for innate lymphoid cells reveals deregulated expression of NKL homeobox genes HHEX and HLX in anaplastic large cell lymphoma (ALCL). Oncotarget 2020, 11, 3208–3226. [Google Scholar] [CrossRef]
- Shannon-Lowe, C.; Rickinson, A.B.; Bell, A.I. Epstein-Barr virus-associated lymphomas. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160271. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Karube, K.; Yamamoto, K.; Takizawa, J.; Tsuzuki, S.; Yatabe, Y.; Kanda, T.; Katayama, M.; Ozawa, Y.; Ishitsuka, K. Gene expression profiling of Epstein-Barr virus-positive diffuse large B-cell lymphoma of the elderly reveals alterations of characteristic oncogenetic pathways. Cancer Sci. 2014, 105, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.P.; Raab-Traub, N. Epstein-Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor through effects on Bcl-3 and STAT3. J. Virol. 2008, 82, 5486–5493. [Google Scholar] [CrossRef] [PubMed]
- Incrocci, R.; Barse, L.; Stone, A.; Vagvala, S.; Montesano, M.; Subramaniam, V.; Swanson-Mungerson, M. Epstein-Barr Virus Latent Membrane Protein 2A (LMP2A) enhances IL-10 production through the activation of Bruton’s tyrosine kinase and STAT3. Virology 2017, 500, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Nagel, S.; Uphoff, C.C.; Dirks, W.G.; Pommerenke, C.; Meyer, C.; Drexler, H.G. Epstein-Barr virus (EBV) activates NKL homeobox gene HLX in DLBCL. PLoS ONE 2019, 14, e0216898. [Google Scholar] [CrossRef] [PubMed]
- Selleri, L.; Zappavigna, V.; Ferretti, E. “Building a perfect body”: Control of vertebrate organogenesis by PBX-dependent regulatory networks. Genes Dev. 2019, 33, 258–275. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.S.; Lim, J.W.C.; Richards, L.J.; Bunt, J. The convergent roles of the nuclear factor I transcription factors in development and cancer. Cancer Lett. 2017, 410, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.T.; Pang, W.L.; Chong, S.M.; Castella, A.; Al-Salam, S.; Tan, T.E.; Moh, M.C.; Koh, L.K.; Gan, S.U.; Cheng, C.K.; et al. Expression of CD137 on Hodgkin and Reed-Sternberg cells inhibits T-cell activation by eliminating CD137 ligand expression. Cancer Res. 2013, 73, 652–661. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).