Chronic Myelomonocytic Leukemia Gold Jubilee
Abstract
1. Introduction
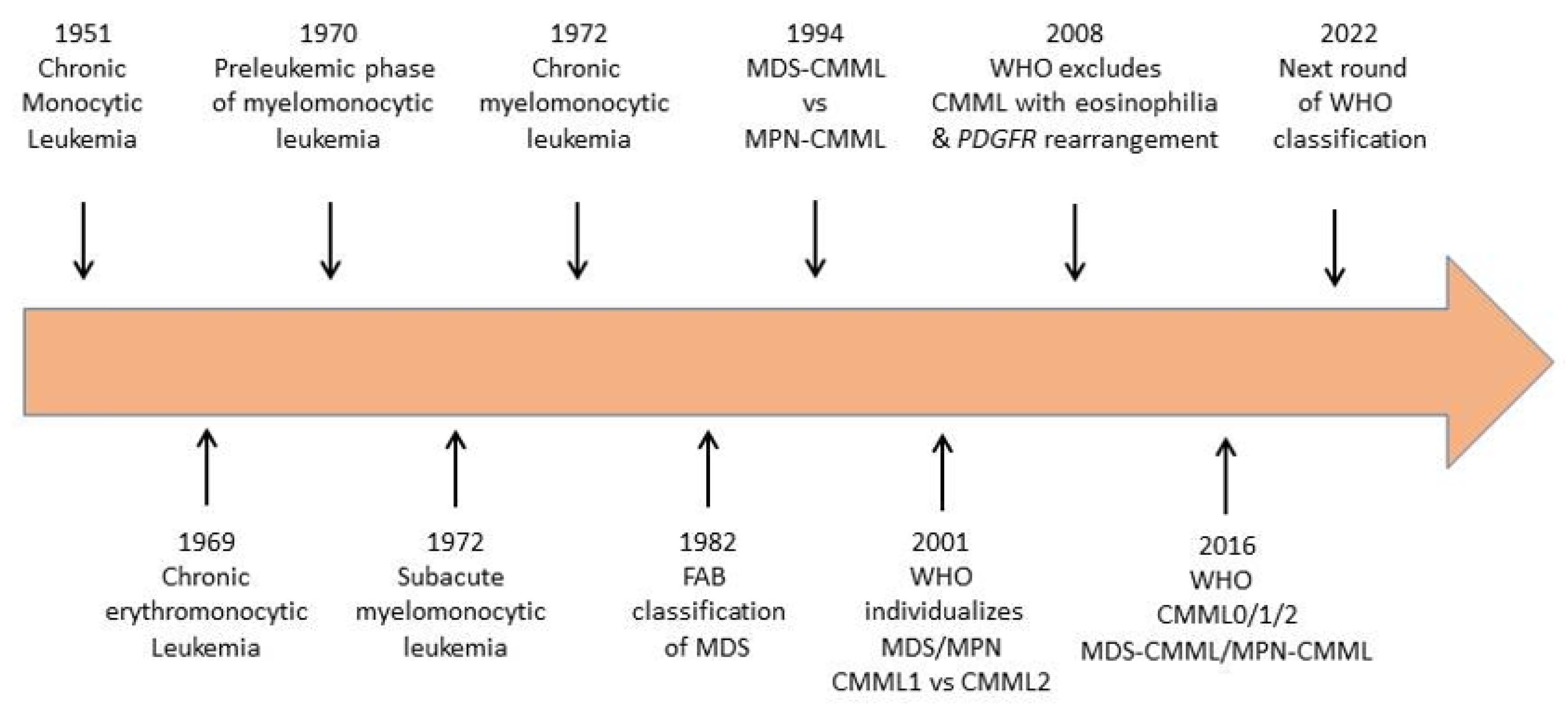
2. From Disease Identification to Current Definition
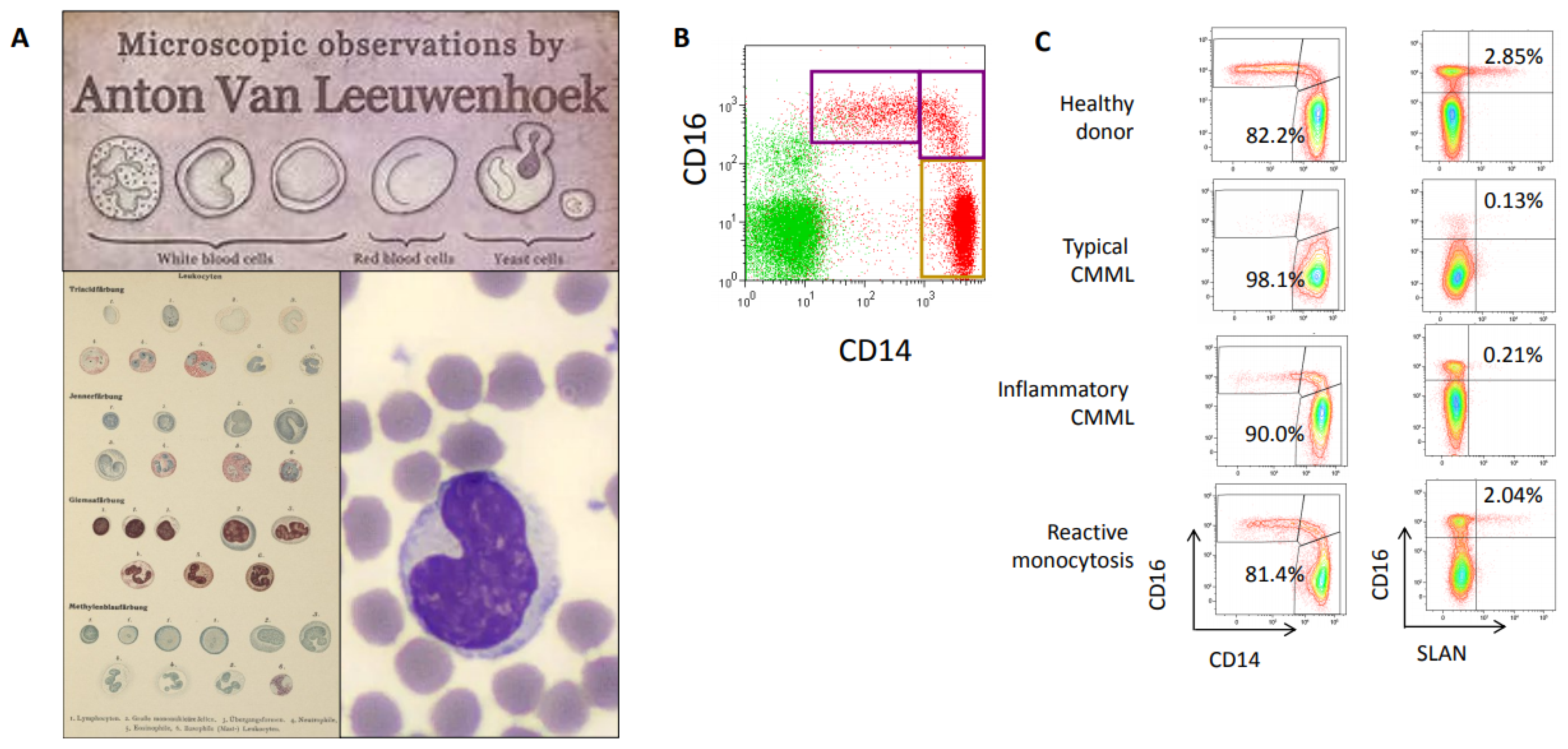
3. Flow Cytometry Improvement of CMML Recognition
4. Incorporating Genetic Analyses in CMML Diagnosis
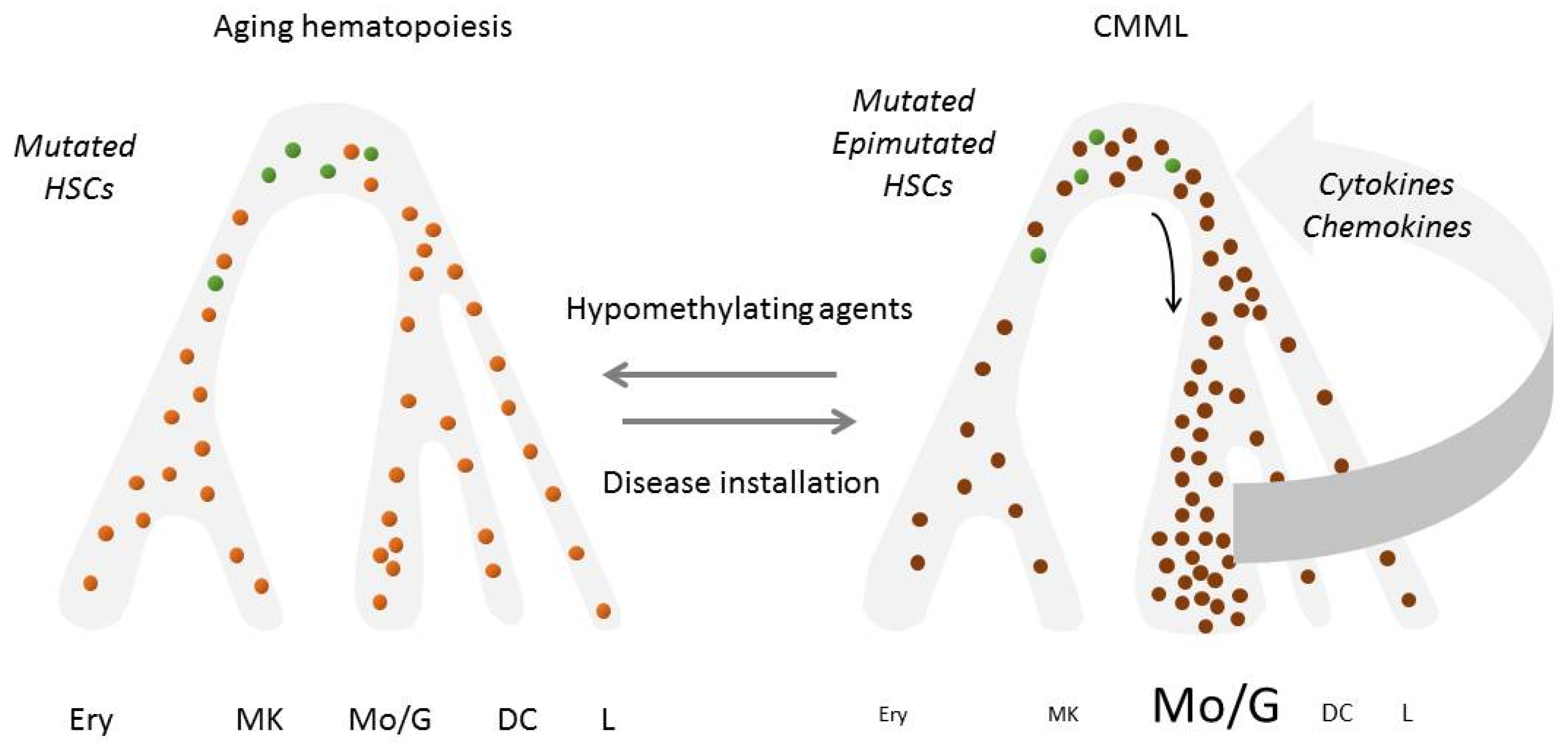
5. Depicting the Role of Epigenetics in CMML Phenotype
6. Dissecting the Role of the Inflammatory Climate
7. Exploring the Role of the Micro-Environment
8. Generating Experimental Model Systems to Explore CMML
9. Depicting the Diversity of CMML Clinical Expression
10. Looking for a Performant Prognostication Method
11. Defining Appropriate Therapeutic Response Criteria
12. Looking for Better Therapeutic Approaches
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hurdle, A.D.; Garson, O.M.; Buist, D.G. Clinical and cytogenetic studies in chronic myelomonocytic leukaemia. Br. J. Haematol. 1972, 22, 773–782. [Google Scholar]
- Miescher, P.A.; Farguet, J.J. Chronic myelomonocytic leukemia in adults. Semin. Hematol. 1974, 11, 129–139. [Google Scholar]
- Beattie, J.W.; Seal, R.M.; Crowther, K.V. Chronic monocytic leukaemia. Q J. Med. 1951, 20, 131–139. [Google Scholar] [PubMed]
- Broun, G.O., Jr. Chronic erythromonocytic leukemia. Am. J. Med. 1969, 47, 785–796. [Google Scholar] [CrossRef]
- Zittoun, R.; Bernadou, A.; Bilski-Pasquier, G.; Bousser, J. Subacute myelo-monocytic leukemia. Study of 27 cases and review of the literature. Sem. Hop. 1972, 48, 1943–1956. [Google Scholar] [PubMed]
- Sexauer, J.; Kass, L.; Schnitzer, B. Subacute myelomonocytic leukemia. Clinical, morphologic and ultrastructural studies of 10 cases. Am. J. Med. 1974, 57, 853–861. [Google Scholar] [CrossRef]
- Linman, J.W. Myelomonocytic leukemia and its preleukemic phase. J. Chronic Dis. 1970, 22, 713–716. [Google Scholar] [CrossRef]
- Saarni, M.I.; Linman, J.W. Preleukemia. The hematologic syndrome preceding acute leukemia. Am. J. Med. 1973, 55, 38–48. [Google Scholar] [CrossRef]
- Geary, C.G.; Catovsky, D.; Wiltshaw, E.; Milner, G.R.; Scholes, M.C.; Van Noorden, S.; Wadsworth, L.D.; Muldal, S.; MacIver, J.E.; Galton, D.A. Chronic myelomonocytic leukaemia. Br. J. Haematol. 1975, 30, 289–302. [Google Scholar] [CrossRef]
- Bennett, J.M.; Catovsky, D.; Daniel, M.T.; Flandrin, G.; Galton, D.A.; Gralnick, H.R.; Sultan, C. Proposals for the classification of the myelodysplastic syndromes. Br. J. Haematol. 1982, 51, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.M.; Catovsky, D.; Daniel, M.T.; Flandrin, G.; Galton, D.A.; Gralnick, H.; Sultan, C.; Cox, C. The chronic myeloid leukaemias: Guidelines for distinguishing chronic granulocytic, atypical chronic myeloid, and chronic myelomonocytic leukaemia. Proposals by the French-AmericanBritish cooperative leukaemia group. Br. J. Haematol. 1994, 87, 746–754. [Google Scholar] [CrossRef]
- Greenberg, P.; Cox, C.; LeBeau, M.M.; Fenaux, P.; Morel, P.; Sanz, G.; Sanz, M.; Vallespi, T.; Hamblin, T.; Oscier, D.; et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997, 89, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, E.S.; Harris, N.E.; Stein, H.; Vardiman, J.W. (Eds.) Pathology and Genetics: Tumors of haematolopoietic and Lymphoid Tissues; IARC Press: Lyon, France, 2001. [Google Scholar]
- Germing, U.; Strupp, C.; Knipp, S.; Kuendgen, A.; Giagounidis, A.; Hildebrandt, B.; Aul, C.; Haas, R.; Gattermann, N.; Bennett, J.M. Chronic myelomonocytic leukemia in the light of the WHO proposals. Haematologica 2007, 92, 974–977. [Google Scholar] [CrossRef]
- Vardiman, J.W.; Thiele, J.; Arber, D.A.; Brunning, R.D.; Borowitz, M.J.; Porwit, A.; Harris, N.L.; Le Beau, M.M.; Hellström-Lindberg, E.; Tefferi, A.; et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood 2009, 114, 937–951. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Lane, N. The unseen world: Reflections on Leeuwenhoek (1677) ‘Concerning little animals’. Philos. Trans. R Soc. Lond. B Biol. Sci. 2015, 370, 20140344. [Google Scholar] [CrossRef]
- Ehrlich, P. Methodologische beiträge zur physiologie und pathologie der verschiedenen formen der leukocyten. In The Collected Papers of Paul Ehrlich; Himmelweit, F., Ed.; Pergamon: Oxford, UK, 1956; pp. 553–560. [Google Scholar]
- Pappenheim, A.; Ferrata, A. Uber die verschiedenen lymphoiden Zellformen des normalen und pathologischen Blutes Folia Haematol. Klinkhardt 1910, 10, 72–208. [Google Scholar]
- Chang, Z.L. Recent development of the mononuclear phagocyte system: In memory of Metchnikoff and Ehrlich on the 100th Anniversary of the 1908 Nobel Prize in Physiology or Medicine. Biol. Cell 2009, 101, 709–721. [Google Scholar] [CrossRef]
- Guilliams, M.; Mildner, A.; Yona, S. Developmental and Functional Heterogeneity of Monocytes. Immunity 2018, 49, 595–613. [Google Scholar] [CrossRef]
- Passlick, B.; Flieger, D.; Ziegler-Heitbrock, H.W. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 1989, 74, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.; Liu, Y.J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef]
- Wong, K.L.; Tai, J.J.; Wong, W.C.; Han, H.; Sem, X.; Yeap, W.H.; Kourilsky, P.; Wong, S.C. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood 2011, 118, e16–e31. [Google Scholar] [CrossRef]
- Villani, A.C.; Satija, R.; Reynolds, G.; Sarkizova, S.; Shekhar, K.; Fletcher, J.; Griesbeck, M.; Butler, A.; Zheng, S.; Lazo, S.; et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 2017, 356, eaah4573. [Google Scholar] [CrossRef] [PubMed]
- Selimoglu-Buet, D.; Wagner-Ballon, O.; Saada, V.; Bardet, V.; Itzykson, R.; Bencheikh, L.; Morabito, M.; Met, E.; Debord, C.; Benayoun, E.; et al. Francophone Myelodysplasia Group. Characteristic repartition of monocyte subsets as a diagnostic signature of chronic myelomonocytic leukemia. Blood 2015, 125, 3618–3626. [Google Scholar] [CrossRef]
- Lacronique-Gazaille, C.; Chaury, M.P.; Le Guyader, A.; Faucher, J.L.; Bordessoule, D.; Feuillard, J. A simple method for detection of major phenotypic abnormalities in myelodysplastic syndromes: Expression of CD56 in CMML. Haematologica 2007, 92, 859–860. [Google Scholar] [CrossRef]
- Sojitra, P.; Gandhi, P.; Fitting, P.; Kini, A.R.; Alkan, S.; Velankar, M.M.; Venkataraman, G. Chronic myelomonocytic leukemia monocytes uniformly display a population of monocytes with CD11c underexpression. Am. J. Clin. Pathol. 2013, 140, 686–692. [Google Scholar] [CrossRef]
- Cargo, C.; Cullen, M.; Taylor, J.; Short, M.; Glover, P.; Van Hoppe, S.; Smith, A.; Evans, P.; Crouch, S. The use of targeted sequencing and flow cytometry to identify patients with a clinically significant monocytosis. Blood 2019, 133, 1325–1334. [Google Scholar] [CrossRef]
- Talati, C.; Zhang, L.; Shaheen, G.; Kuykendall, A.; Ball, M.; Zhang, Q.; Lancet, J.E.; Zuckerman, K.S.; List, A.F.; Komrokji, R.; et al. Monocyte subset analysis accurately distinguishes CMML from MDS and is associated with a favorable MDS prognosis. Blood 2017, 129, 1881–1883. [Google Scholar] [CrossRef]
- Greenberg, P.L. The classical nature of distinctive CMML monocytes. Blood 2017, 129, 1745–1746. [Google Scholar] [CrossRef][Green Version]
- Patnaik, M.M.; Timm, M.M.; Vallapureddy, R.; Lasho, T.L.; Ketterling, R.P.; Gangat, N.; Shi, M.; Tefferi, A.; Solary, E.; Reichard, K.K.; et al. Flow cytometry based monocyte subset analysis accurately distinguishes chronic myelomonocytic leukemia from myeloproliferative neoplasms with associated monocytosis. Blood Cancer J. 2017, 7, e584. [Google Scholar] [CrossRef]
- Picot, T.; Aanei, C.M.; Flandrin Gresta, P.; Noyel, P.; Tondeur, S.; Tavernier Tardy, E.; Guyotat, D.; Campos Catafal, L. Evaluation by flow cytometry of mature monocyte subpopulations for the diagnosis and follow-up of chronic myelomonocytic leukemia. Front. Oncol. 2018, 8, 109. [Google Scholar] [CrossRef]
- Hudson, C.A.; Burack, W.R.; Leary, P.C.; Bennett, J.M. Clinical utility of classical and nonclassical monocyte percentage in the diagnosis of chronic myelomonocytic leukemia. Am. J. Clin. Pathol. 2018, 150, 293–302. [Google Scholar] [CrossRef]
- Itzykson, R.; Fenaux, P.; Bowen, D.; Cross, N.C.P.; Cortes, J.; De Witte, T.; Germing, U.; Onida, F.; Padron, E.; Platzbecker, U.; et al. Diagnosis and treatment of chronic myelomonocytic leukemias in adults: Recommendations from the european hematology association and the european leukemianet. Hemasphere 2018, 2, e150. [Google Scholar] [CrossRef]
- Vazquez, R.; Roussel, M.; Badaoui, B.; Freynet, N.; Tarfi, S.; Solary, E.; Selimoglu-Buet, D.; Wagner-Ballon, O.; Groupe Francophone des Myélodysplasies (GFM). High sensitivity of the Hematoflow™ solution for chronic myelomonocytic leukemia screening. Cytom. B Clin. Cytom. 2018, 94, 658–661. [Google Scholar] [CrossRef]
- Tarfi, S.; Badaoui, B.; Freynet, N.; Morabito, M.; Lafosse, J.; Toma, A.; Etienne, G.; Micol, J.B.; Sloma, I.; Fenaux, P.; et al. Disappearance of slan-positive non-classical monocytes for diagnosis of chronic myelomonocytic leukemia with associated inflammatory state. Haematologica 2019, 2019, 219782. [Google Scholar] [CrossRef]
- Jestin, M.; Tarfi, S.; Duchmann, M.; Badaoui, B.; Freynet, N.; Tran Quang, V.; Sloma, I.; Droin, N.; Morabito, M.; Leclerc, M.; et al. Prognostic value of monocyte subset distribution in chronic myelomonocytic leukemia: Results of a multicenter study. Leukemia 2021, 35, 893–896. [Google Scholar] [CrossRef]
- Cheng, C.K.; Chan, J.; Cembrowski, G.S.; van Assendelft, O.W. Complete blood count reference interval diagrams derived from NHANES III: Stratification by age, sex, and race. Lab. Hematol. 2004, 10, 42–53. [Google Scholar] [CrossRef]
- Wakeman, L.; Al-Ismail, S.; Benton, A.; Beddall, A.; Gibbs, A.; Hartnell, S.; Morris, K.; Munro, R. Robust, routine haematology reference ranges for healthy adults. Int. J. Lab. Hematol. 2007, 29, 279–283. [Google Scholar] [CrossRef]
- Lichtman, M.A. Oligomonocytic chronic myelomonocytic leukemia. Thoughts Suggest. Blood Cells Mol. Dis. 2021, 88, 102546. [Google Scholar] [CrossRef]
- Geyer, J.T.; Tam, W.; Liu, Y.C.; Chen, Z.; Wang, S.A.; Bueso-Ramos, C.; Oak, J.; Arber, D.A.; Hsi, E.; Rogers, H.J.; et al. Oligomonocytic chronic myelomonocytic leukemia (chronic myelomonocytic leukemia without absolute monocytosis) displays a similar clinicopathologic and mutational profile to classical chronic myelomonocytic leukemia. Mod. Pathol. 2017, 30, 1213–1222. [Google Scholar] [CrossRef]
- Calvo, X.; Garcia-Gisbert, N.; Parraga, I.; Gibert, J.; Florensa, L.; Andrade-Campos, M.; Merchan, B.; Garcia-Avila, S.; Montesdeoca, S.; Fernández-Rodríguez, C.; et al. Oligomonocytic and overt chronic myelomonocytic leukemia show similar clinical, genomic, and immunophenotypic features. Blood Adv. 2020, 4, 5285–5296. [Google Scholar] [CrossRef]
- Montalban-Bravo, G.; Kanagal-Shamanna, R.; Guerra, V.; Ramos-Perez, J.; Hammond, D.; Shilpa, P.; Naqvi, K.; Sasaki, K.; Jabbour, E.; DiNardo, C.; et al. Clinical outcomes and influence of mutation clonal dominance in oligomonocytic and classical chronic myelomonocytic leukemia. Am. J. Hematol. 2021, 96, E50–E53. [Google Scholar] [CrossRef] [PubMed]
- Rigolin, G.M.; Cuneo, A.; Roberti, M.G.; Bardi, A.; Castoldi, G. Myelodysplastic syndromes with monocytic component: Hematologic and cytogenetic characterization. Haematologica 1997, 82, 25–30. [Google Scholar] [PubMed]
- Selimoglu-Buet, D.; Badaoui, B.; Benayoun, E.; Toma, A.; Fenaux, P.; Quesnel, B.; Etienne, G.; Braun, T.; Abermil, N.; Morabito, M.; et al. Accumulation of classical monocytes defines a subgroup of MDS that frequently evolves into CMML. Blood 2017, 130, 832–835. [Google Scholar] [CrossRef]
- Cytogenetics of chronic myelomonocytic leukemia. Cancer Genet. Cytogenet. 1986, 21, 11–30. [CrossRef]
- Onida, F.; Kantarjian, H.M.; Smith, T.L.; Ball, G.; Keating, M.J.; Estey, E.H.; Glassman, A.B.; Albitar, M.; Kwari, M.I.; Beran, M. Prognostic factors and scoring systems in chronic myelomonocytic leukemia: A retrospective analysis of 213 patients. Blood 2002, 99, 840–849. [Google Scholar] [CrossRef]
- Such, E.; Cervera, J.; Costa, D.; Solé, F.; Vallespí, T.; Luño, E.; Collado, R.; Calasanz, M.J.; Hernández-Rivas, J.M.; Cigudosa, J.C.; et al. Cytogenetic risk stratification in chronic myelomonocytic leukemia. Haematologica 2011, 96, 375–383. [Google Scholar] [CrossRef]
- Such, E.; Germing, U.; Malcovati, L.; Cervera, J.; Kuendgen, A.; Della Porta, M.G.; Nomdedeu, B.; Arenillas, L.; Luño, E.; Xicoy, B.; et al. Development and validation of a prognostic scoring system for patients with chronic myelomonocytic leukemia. Blood 2013, 121, 3005–3015. [Google Scholar] [CrossRef]
- Elena, C.; Gallì, A.; Such, E.; Meggendorfer, M.; Germing, U.; Rizzo, E.; Cervera, J.; Molteni, E.; Fasan, A.; Schuler, E.; et al. Integrating clinical features and genetic lesions in the risk assessment of patients with chronic myelomonocytic leukemia. Blood 2016, 128, 1408–1417. [Google Scholar] [CrossRef]
- Wassie, E.A.; Itzykson, R.; Lasho, T.L.; Kosmider, O.; Finke, C.M.; Hanson, C.A.; Ketterling, R.P.; Solary, E.; Tefferi, A.; Patnaik, M.M. Molecular and prognostic correlates of cytogenetic abnormalities in chronic myelomonocytic leukemia: A Mayo Clinic-French Consortium Study. Am. J. Hematol. 2014, 89, 1111–1115. [Google Scholar] [CrossRef]
- Patnaik, M.M.; Tefferi, A. Cytogenetic and molecular abnormalities in chronic myelomonocytic leukemia. Blood Cancer J. 2016, 6, e393. [Google Scholar] [CrossRef] [PubMed]
- Reiter, A.; Gotlib, J. Myeloid neoplasms with eosinophilia. Blood 2017, 129, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Cheah, C.Y.; Burbury, K.; Apperley, J.F.; Huguet, F.; Pitini, V.; Gardembas, M.; Ross, D.M.; Forrest, D.; Genet, P.; Rousselot, P.; et al. Patients with myeloid malignancies bearing PDGFRB fusion genes achieve durable long-term remissions with imatinib. Blood 2014, 123, 3574–3577. [Google Scholar] [CrossRef] [PubMed]
- Schwaab, J.; Naumann, N.; Luebke, J.; Jawhar, M.; Somervaille, T.C.P.; Williams, M.S.; Frewin, R.; Jost, P.J.; Lichtenegger, F.S.; La Rosée, P.; et al. Response to tyrosine kinase inhibitors in myeloid neoplasms associated with PCM1-JAK2, BCR-JAK2 and ETV6-ABL1 fusion genes. Am. J. Hematol. 2020, 95, 824–833. [Google Scholar] [CrossRef]
- Bell, G.C.; Padron, E. Detection of a PDGFRB fusion in refractory CMML without eosinophilia: A case for broad spectrum tumor profiling. Leuk. Res. Rep. 2015, 4, 70–71. [Google Scholar] [CrossRef][Green Version]
- Jaiswal, S.; Ebert, B.L. Clonal hematopoiesis in human aging and disease. Science 2019, 366, eaan4673. [Google Scholar] [CrossRef]
- Mason, C.C.; Khorashad, J.S.; Tantravahi, S.K.; Kelley, T.W.; Zabriskie, M.S.; Yan, D.; Pomicter, A.D.; Reynolds, K.R.; Eiring, A.M.; Kronenberg, Z.; et al. Age-related mutations and chronic myelomonocytic leukemia. Leukemia 2016, 30, 906–913. [Google Scholar] [CrossRef]
- Itzykson, R.; Kosmider, O.; Renneville, A.; Gelsi-Boyer, V.; Meggendorfer, M.; Morabito, M.; Berthon, C.; Adès, L.; Fenaux, P.; Beyne-Rauzy, O.; et al. Prognostic score including gene mutations in chronic myelomonocytic leukemia. J. Clin. Oncol. 2013, 31, 2428–2436. [Google Scholar] [CrossRef]
- Malcovati, L.; Papaemmanuil, E.; Ambaglio, I.; Elena, C.; Gallì, A.; Della Porta, M.G.; Travaglino, E.; Pietra, D.; Pascutto, C.; Ubezio, M.; et al. Driver somatic mutations identify distinct disease entities within myeloid neoplasms with myelodysplasia. Blood 2014, 124, 1513–1521. [Google Scholar] [CrossRef]
- Merlevede, J.; Droin, N.; Qin, T.; Meldi, K.; Yoshida, K.; Morabito, M.; Chautard, E.; Auboeuf, D.; Fenaux, P.; Braun, T.; et al. Mutation allele burden remains unchanged in chronic myelomonocytic leukaemia responding to hypomethylating agents. Nat. Commun. 2016, 7, 10767. [Google Scholar] [CrossRef]
- Sakr, R.; Renneville, A.; Saada, V.; Cotteret, S.; Martin, J.E.; Droin, N.; Selimoglu-Buet, D.; Besse, B.; Hollebecque, A.; Marzac, C.; et al. Next-generation sequencing discriminates myelodysplastic/myeloproliferative neoplasms from paraneoplastic leukemoid reaction in cancer patients with hyperleukocytosis. Leuk. Lymphoma 2018, 59, 1742–1745. [Google Scholar] [CrossRef]
- Palomo, L.; Meggendorfer, M.; Hutter, S.; Twardziok, S.; Ademà, V.; Fuhrmann, I.; Fuster-Tormo, F.; Xicoy, B.; Zamora, L.; Acha, P.; et al. Molecular landscape and clonal architecture of adult myelodysplastic/myeloproliferative neoplasms. Blood 2020, 136, 1851–1862. [Google Scholar] [CrossRef]
- Itzykson, R.; Kosmider, O.; Renneville, A.; Morabito, M.; Preudhomme, C.; Berthon, C.; Adès, L.; Fenaux, P.; Platzbecker, U.; Gagey, O.; et al. Clonal architecture of chronic myelomonocytic leukemias. Blood 2013, 121, 2186–2198. [Google Scholar] [CrossRef] [PubMed]
- Carr, R.M.; Vorobyev, D.; Lasho, T.; Marks, D.L.; Tolosa, E.J.; Vedder, A. RAS mutations drive proliferative chronic myelomonocytic leukemia via a novel KMT2A-PLK1 axis. Nat. Commun. 2021, 356, eaah4573. [Google Scholar]
- Cazzola, M. Clonal monocytosis of clinical significance. Blood 2019, 133, 1271–1272. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Orazi, A.; Savona, M.R.; Patnaik, M.M.; Onida, F.; van de Loosdrecht, A.A.; Haase, D.; Haferlach, T.; Elena, C.; Pleyer, L.; et al. Proposed diagnostic criteria for classical chronic myelomonocytic leukemia (CMML), CMML variants and pre-CMML conditions. Haematologica 2019, 104, 1935–1949. [Google Scholar] [CrossRef] [PubMed]
- Wlodarski, M.W.; Collin, M.; Horwitz, M.S. GATA2 deficiency and related myeloid neoplasms. Semin. Hematol. 2017, 54, 81–86. [Google Scholar] [CrossRef]
- Antony-Debré, I.; Bluteau, D.; Itzykson, R.; Baccini, V.; Renneville, A.; Boehlen, F.; Morabito, M.; Droin, N.; Deswarte, C.; Chang, Y.; et al. MYH10 protein expression in platelets as a biomarker of RUNX1 and FLI1 alterations. Blood 2012, 120, 2719–2722. [Google Scholar] [CrossRef] [PubMed]
- Shiba, N.; Hasegawa, D.; Park, M.J.; Murata, C.; Sato-Otsubo, A.; Ogawa, C.; Manabe, A.; Arakawa, H.; Ogawa, S.; Hayashi, Y. CBL mutation in chronic myelomonocytic leukemia secondary to familial platelet disorder with propensity to develop acute myeloid leukemia (FPD/AML). Blood 2012, 119, 2612–2614. [Google Scholar] [CrossRef]
- Bluteau, D.; Balduini, A.; Balayn, N.; Currao, M.; Nurden, P.; Deswarte, C.; Leverger, G.; Noris, P.; Perrotta, S.; Solary, E.; et al. Thrombocytopenia-associated mutations in the ANKRD26 regulatory region induce MAPK hyperactivation. J. Clin. Investig. 2014, 124, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Perez Botero, J.; Oliveira, J.L.; Chen, D.; Reichard, K.K.; Viswanatha, D.S.; Nguyen, P.L.; Pruthi, R.K.; Majerus, J.; Gada, P.; Gangat, N.; et al. ASXL1 mutated chronic myelomonocytic leukemia in a patient with familial thrombocytopenia secondary to germline mutation in ANKRD26. Blood Cancer J. 2015, 5, e315. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Churpek, J.E.; Keel, S.B.; Walsh, T.; Lee, M.K.; Loeb, K.R.; Gulsuner, S.; Pritchard, C.C.; Sanchez-Bonilla, M.; Delrow, J.J.; et al. Germline ETV6 mutations in familial thrombocytopenia and hematologic malignancy. Nat. Genet. 2015, 47, 180–185. [Google Scholar] [CrossRef]
- Polprasert, C.; Schulze, I.; Sekeres, M.A.; Makishima, H.; Przychodzen, B.; Hosono, N.; Singh, J.; Padgett, R.A.; Gu, X.; Phillips, J.G.; et al. Inherited and Somatic Defects in DDX41 in Myeloid Neoplasms. Cancer Cell 2015, 27, 658–670. [Google Scholar] [CrossRef]
- Sébert, M.; Passet, M.; Raimbault, A.; Rahmé, R.; Raffoux, E.; Sicre de Fontbrune, F.; Cerrano, M.; Quentin, S.; Vasquez, N.; Da Costa, M.; et al. Germline DDX41 mutations define a significant entity within adult MDS/AML patients. Blood 2019, 134, 1441–1444. [Google Scholar] [CrossRef]
- Nucera, S.; Fazio, G.; Piazza, R.; Rigamonti, S.; Fontana, D.; Gambacorti Passerini, C.; Maitz, S.; Rovelli, A.; Biondi, A.; Cazzaniga, G.; et al. Germ-Line TP53 Mutation in an Adolescent With CMML/Atypical CML and Familiar Cancer Predisposition. Hemasphere 2020, 4, e460. [Google Scholar] [CrossRef] [PubMed]
- Saliba, J.; Saint-Martin, C.; Di Stefano, A.; Lenglet, G.; Marty, C.; Keren, B.; Pasquier, F.; Valle, V.D.; Secardin, L.; Leroy, G.; et al. Germline duplication of ATG2B and GSKIP predisposes to familial myeloid malignancies. Nat. Genet. 2015, 47, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Delhommeau, F.; Dupont, S.; Della Valle, V.; James, C.; Trannoy, S.; Massé, A.; Kosmider, O.; Le Couedic, J.P.; Robert, F.; Alberdi, A.; et al. Mutation in TET2 in myeloid cancers. N. Engl. J. Med. 2009, 360, 2289–2301. [Google Scholar] [CrossRef]
- Abdel-Wahab, O.; Mullally, A.; Hedvat, C.; Garcia-Manero, G.; Patel, J.; Wadleigh, M.; Malinge, S.; Yao, J.; Kilpivaara, O.; Bhat, R.; et al. Genetic characterization of TET1, TET2, and TET3 alterations in myeloid malignancies. Blood 2009, 114, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, A.M.; Szpurka, H.; Tiu, R.V.; Makishima, H.; Afable, M.; Huh, J.; O’Keefe, C.L.; Ganetzky, R.; McDevitt, M.A.; Maciejewski, J.P. Loss of heterozygosity 4q24 and TET2 mutations associated with myelodysplastic/myeloproliferative neoplasms. Blood 2009, 113, 6403–6410. [Google Scholar] [CrossRef]
- Tefferi, A.; Lim, K.H.; Abdel-Wahab, O.; Lasho, T.L.; Patel, J.; Patnaik, M.M.; Hanson, C.A.; Pardanani, A.; Gilliland, D.G.; Levine, R.L. Detection of mutant TET2 in myeloid malignancies other than myeloproliferative neoplasms: CMML, MDS, MDS/MPN and AML. Leukemia 2009, 23, 1343–1345. [Google Scholar] [CrossRef]
- Gelsi-Boyer, V.; Trouplin, V.; Adélaïde, J.; Bonansea, J.; Cervera, N.; Carbuccia, N.; Lagarde, A.; Prebet, T.; Nezri, M.; Sainty, D.; et al. Mutations of polycomb-associated gene ASXL1 in myelodysplastic syndromes and chronic myelomonocytic leukaemia. Br. J. Haematol. 2009, 145, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.; Huang, Y.; Jankowska, A.M.; Pape, U.J.; Tahiliani, M.; Bandukwala, H.S.; An, J.; Lamperti, E.D.; Koh, K.P.; Ganetzky, R.; et al. Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature 2010, 468, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Pronier, E.; Almire, C.; Mokrani, H.; Vasanthakumar, A.; Simon, A.; da Costa Reis Monte Mor, B.; Massé, A.; Le Couédic, J.P.; Pendino, F.; Carbonne, B.; et al. Inhibition of TET2-mediated conversion of 5-methylcytosine to 5-hydroxymethylcytosine disturbs erythroid and granulomonocytic differentiation of human hematopoietic progenitors. Blood 2011, 118, 2551–2555. [Google Scholar] [CrossRef] [PubMed]
- Pérez, C.; Martínez-Calle, N.; Martín-Subero, J.I.; Segura, V.; Delabesse, E.; Fernandez-Mercado, M.; Garate, L.; Alvarez, S.; Rifon, J.; Varea, S.; et al. TET2 mutations are associated with specific 5-methylcytosine and 5-hydroxymethylcytosine profiles in patients with chronic myelomonocytic leukemia. PLoS ONE 2012, 7, e31605. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, J.; Taby, R.; Vasanthakumar, A.; Macrae, T.; Ostler, K.R.; Shen, L.; Kantarjian, H.M.; Estecio, M.R.; Jelinek, J.; Godley, L.A.; et al. Effects of TET2 mutations on DNA methylation in chronic myelomonocytic leukemia. Epigenetics 2012, 7, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, O.; Adli, M.; LaFave, L.M.; Gao, J.; Hricik, T.; Shih, A.H.; Pandey, S.; Patel, J.P.; Chung, Y.R.; Koche, R.; et al. ASXL1 mutations promote myeloid transformation through loss of PRC2-mediated gene repression. Cancer Cell 2012, 22, 180–193. [Google Scholar] [CrossRef]
- Stephenson, J.; Akdag, R.; Ozbek, N.; Mufti, G.J. Methylation status within exon 3 of the c-myc gene as a prognostic marker in myeloma and leukaemia. Leuk. Res. 1993, 17, 291–293. [Google Scholar] [CrossRef]
- Tessema, M.; Länger, F.; Dingemann, J.; Ganser, A.; Kreipe, H.; Lehmann, U. Aberrant methylation and impaired expression of the p15(INK4b) cell cycle regulatory gene in chronic myelomonocytic leukemia (CMML). Leukemia 2003, 17, 910–918. [Google Scholar] [CrossRef][Green Version]
- Kantarjian, H.; Oki, Y.; Garcia-Manero, G.; Huang, X.; O’Brien, S.; Cortes, J.; Faderl, S.; Bueso-Ramos, C.; Ravandi, F.; Estrov, Z.; et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood 2007, 109, 52–57. [Google Scholar] [CrossRef]
- Jiang, Y.; Dunbar, A.; Gondek, L.P.; Mohan, S.; Rataul, M.; O’Keefe, C.; Sekeres, M.; Saunthararajah, Y.; Maciejewski, J.P. Aberrant DNA methylation is a dominant mechanism in MDS progression to AML. Blood 2009, 113, 1315–1325. [Google Scholar] [CrossRef]
- Unnikrishnan, A.; Papaemmanuil, E.; Beck, D.; Deshpande, N.P.; Verma, A.; Kumari, A.; Woll, P.S.; Richards, L.A.; Knezevic, K.; Chandrakanthan, V.; et al. Integrative Genomics Identifies the Molecular Basis of Resistance to Azacitidine Therapy in Myelodysplastic Syndromes. Cell Rep. 2017, 20, 572–585. [Google Scholar] [CrossRef]
- Ali, A.; Penneroux, J.; Dal Bello RJr Massé, A.; Quentin, S.; Unnikrishnan, A.; Hernandez, L.; Raffoux, E.; Ben Abdelali, R.; Renneville, A.; Preudhomme, C.; et al. Granulomonocytic progenitors are key target cells of azacytidine in higher risk myelodysplastic syndromes and acute myeloid leukemia. Leukemia 2018, 32, 1856–1860. [Google Scholar] [CrossRef]
- Aucagne, R.; Droin, N.; Paggetti, J.; Lagrange, B.; Largeot, A.; Hammann, A.; Bataille, A.; Martin, L.; Yan, K.P.; Fenaux, P.; et al. Transcription intermediary factor 1γ is a tumor suppressor in mouse and human chronic myelomonocytic leukemia. J. Clin. Investig. 2011, 121, 2361–2370. [Google Scholar] [CrossRef]
- Patel, A.A.; Zhang, Y.; Fullerton, J.N.; Boelen, L.; Rongvaux, A.; Maini, A.A.; Bigley, V.; Flavell, R.A.; Gilroy, D.W.; Asquith, B.; et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J. Exp. Med. 2017, 214, 1913–1923. [Google Scholar] [CrossRef]
- Selimoglu-Buet, D.; Rivière, J.; Ghamlouch, H.; Bencheikh, L.; Lacout, C.; Morabito, M.; Diop, M.; Meurice, G.; Breckler, M.; Chauveau, A.; et al. A miR-150/TET3 pathway regulates the generation of mouse and human non-classical monocyte subset. Nat. Commun. 2018, 9, 5455. [Google Scholar] [CrossRef]
- Berg, J.L.; Perfler, B.; Hatzl, S.; Mayer, M.C.; Wurm, S.; Uhl, B.; Reinisch, A.; Klymiuk, I.; Tierling, S.; Pregartner, G.; et al. Micro-RNA-125a mediates the effects of hypomethylating agents in chronic myelomonocytic leukemia. Clin. Epigenetics 2021, 13, 1. [Google Scholar] [CrossRef]
- Tobiasson, M.; Abdulkadir, H.; Lennartsson, A.; Katayama, S.; Marabita, F.; De Paepe, A.; Karimi, M.; Krjutskov, K.; Einarsdottir, E.; Grövdal, M.; et al. Comprehensive mapping of the effects of azacitidine on DNA methylation, repressive/permissive histone marks and gene expression in primary cells from patients with MDS and MDS-related disease. Oncotarget 2017, 8, 28812–28825. [Google Scholar] [CrossRef]
- Meldi, K.; Qin, T.; Buchi, F.; Droin, N.; Sotzen, J.; Micol, J.B.; Selimoglu-Buet, D.; Masala, E.; Allione, B.; Gioia, D.; et al. Specific molecular signatures predict decitabine response in chronic myelomonocytic leukemia. J. Clin. Investig. 2015, 125, 1857–1872. [Google Scholar] [CrossRef]
- Duchmann, M.; Yalniz, F.F.; Sanna, A.; Sallman, D.; Coombs, C.C.; Renneville, A.; Kosmider, O.; Braun, T.; Platzbecker, U.; Willems, L.; et al. Prognostic Role of Gene Mutations in Chronic Myelomonocytic Leukemia Patients Treated with Hypomethylating Agents. EBioMedicine 2018, 31, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.; Vallania, F.; Warsinske, H.C.; Donato, M.; Schaffert, S.; Chang, S.E.; Dvorak, M.; Dekker, C.L.; Davis, M.M.; Utz, P.J.; et al. Single-cell chromatin modification profiling reveals increased epigenetic variations with aging. Cell 2018, 173, 1385–1397.e14. [Google Scholar] [CrossRef]
- Sagaster, V.; Ohler, L.; Berer, A.; Kabrna, E.; Ofner, P.; Lechner, K.; Geissler, K. High spontaneous colony growth in chronic myelomonocytic leukemia correlates with increased disease activity and is a novel prognostic factor for predicting short survival. Ann. Hematol. 2004, 83, 9–13. [Google Scholar] [CrossRef]
- Everson, M.P.; Brown, C.B.; Lilly, M.B. Interleukin-6 and granulocyte-macrophage colony-stimulating factor are candidate growth factors for chronic myelomonocytic leukemia cells. Blood 1989, 74, 1472–1476. [Google Scholar] [CrossRef] [PubMed]
- Geissler, K.; Jäger, E.; Barna, A.; Gurbisz, M.; Graf, T.; Graf, E.; Nösslinger, T.; Pfeilstöcker, M.; Tüchler, H.; Sliwa, T.; et al. Correlation of RAS-Pathway Mutations and Spontaneous Myeloid Colony Growth with Progression and Transformation in Chronic Myelomonocytic Leukemia-A Retrospective Analysis in 337 Patients. Int. J. Mol. Sci. 2020, 21, 3025. [Google Scholar] [CrossRef]
- Ramshaw, H.S.; Bardy, P.G.; Lee, M.A.; Lopez, A.F. Chronic myelomonocytic leukemia requires granulocyte-macrophage colony-stimulating factor for growth in vitro and in vivo. Exp. Hematol. 2002, 30, 1124–1131. [Google Scholar] [CrossRef]
- Zhang, Y.; He, L.; Selimoglu-Buet, D.; Jego, C.; Morabito, M.; Willekens, C.; Diop, M.K.; Gonin, P.; Lapierre, V.; Droin, N.; et al. Engraftment of chronic myelomonocytic leukemia cells in immunocompromised mice supports disease dependency on cytokines. Blood Adv. 2017, 1, 972–979. [Google Scholar] [CrossRef]
- Akashi, K.; Shibuya, T.; Harada, M.; Takamatsu, Y.; Uike, N.; Eto, T.; Niho, Y. Interleukin 4 suppresses the spontaneous growth of chronic myelomonocytic leukemia cells. J. Clin. Investig. 1991, 88, 223–230. [Google Scholar] [CrossRef][Green Version]
- Yanagisawa, K.; Hatta, N.; Watanabe, I.; Horiuchi, T.; Hasegawa, H.; Fujita, S. IL-4 stimulates the growth of chronic myelomonocytic leukemia (CMMoL) once leukemic transformation has occurred. Leukemia 1995, 9, 1056–1059. [Google Scholar]
- Iversen, P.O.; Hart, P.H.; Bonder, C.S.; Lopez, A.F. Interleukin (IL)-10, but not IL-4 or IL-13, inhibits cytokine production and growth in juvenile myelomonocytic leukemia cells. Cancer Res. 1997, 57, 476–480. [Google Scholar]
- de Waal Malefyt, R.; Abrams, J.; Bennett, B.; Figdor, C.G.; de Vries, J.E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: An autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 1991, 174, 1209–1220. [Google Scholar] [CrossRef]
- Geissler, K.; Ohler, L.; Födinger, M.; Virgolini, I.; Leimer, M.; Kabrna, E.; Kollars, M.; Skoupy, S.; Bohle, B.; Rogy, M.; et al. Interleukin 10 inhibits growth and granulocyte/macrophage colony-stimulating factor production in chronic myelomonocytic leukemia cells. J. Exp. Med. 1996, 184, 1377–1384. [Google Scholar] [CrossRef]
- Pöchlauer, S.; Jäger, E.; Jäger, U.; Geissler, K. Recombinant human interleukin-10 in patients with chronic myelomonocytic leukemia. Ann. Hematol. 2014, 93, 1775–1776. [Google Scholar] [CrossRef]
- Niyongere, S.; Lucas, N.; Zhou, J.M.; Sansil, S.; Pomicter, A.D.; Balasis, M.E.; Robinson, J.; Kroeger, J.; Zhang, Q.; Zhao, Y.L.; et al. Heterogeneous expression of cytokines accounts for clinical diversity and refines prognostication in CMML. Leukemia 2019, 33, 205–216. [Google Scholar] [CrossRef]
- Emanuel, P.D.; Bates, L.J.; Zhu, S.W.; Castleberry, R.P.; Gualtieri, R.J.; Zuckerman, K.S. The role of monocyte-derived hemopoietic growth factors in the regulation of myeloproliferation in juvenile chronic myelogenous leukemia. Exp. Hematol. 1991, 19, 1017–1024. [Google Scholar]
- Birnbaum, R.A.; O’Marcaigh, A.; Wardak, Z.; Zhang, Y.Y.; Dranoff, G.; Jacks, T.; Clapp, D.W.; Shannon, K.M. Nf1 and Gmcsf interact in myeloid leukemogenesis. Mol. Cell 2000, 5, 189–195. [Google Scholar] [CrossRef]
- Kotecha, N.; Flores, N.J.; Irish, J.M.; Simonds, E.F.; Sakai, D.S.; Archambeault, S.; Diaz-Flores, E.; Coram, M.; Shannon, K.M.; Nolan, G.P.; et al. Single-cell profiling identifies aberrant STAT5 activation in myeloid malignancies with specific clinical and biologic correlates. Cancer Cell 2008, 14, 335–343. [Google Scholar] [CrossRef]
- Reynaud, D.; Pietras, E.; Barry-Holson, K.; Mir, A.; Binnewies, M.; Jeanne, M.; Sala-Torra, O.; Radich, J.P.; Passegué, E. IL-6 controls leukemic multipotent progenitor cell fate and contributes to chronic myelogenous leukemia development. Cancer Cell 2011, 20, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Welner, R.S.; Amabile, G.; Bararia, D.; Czibere, A.; Yang, H.; Zhang, H.; Pontes, L.L.; Ye, M.; Levantini, E.; Di Ruscio, A.; et al. Treatment of chronic myelogenous leukemia by blocking cytokine alterations found in normal stem and progenitor cells. Cancer Cell 2015, 27, 671–681. [Google Scholar] [CrossRef]
- Padron, E.; Painter, J.S.; Kunigal, S.; Mailloux, A.W.; McGraw, K.; McDaniel, J.M.; Kim, E.; Bebbington, C.; Baer, M.; Yarranton, G.; et al. GM-CSF-dependent pSTAT5 sensitivity is a feature with therapeutic potential in chronic myelomonocytic leukemia. Blood 2013, 121, 5068–5077. [Google Scholar] [CrossRef]
- Solary, E. Unplugging JAK/STAT in Chronic Myelomonocytic Leukemia. Clin. Cancer Res. 2016, 22, 3707–3709. [Google Scholar] [CrossRef]
- Patnaik, M.M.; Sallman, D.A.; Mangaonkar, A.A.; Heuer, R.; Hirvela, J.; Zblewski, D.; Al-Kali, A.; Binder, M.; Balasis, M.E.; Newman, H.; et al. Phase 1 study of lenzilumab, a recombinant anti-human GM-CSF antibody, for chronic myelomonocytic leukemia. Blood 2020, 136, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Alexandrakis, M.; Coulocheri, S.; Xylouri, I.; Ganotakis, E.; Eliakis, P.; Karkavitsas, N.; Eliopoulos, G.D. Elevated serum TNF-alpha concentrations are predictive of shortened survival in patients with high-risk myelodysplastic syndromes. Haematologia 1998, 29, 13–24. [Google Scholar] [PubMed]
- Verhoef, G.E.; De Schouwer, P.; Ceuppens, J.L.; Van Damme, J.; Goossens, W.; Boogaerts, M.A. Measurement of serum cytokine levels in patients with myelodysplastic syndromes. Leukemia 1992, 6, 1268–1272. [Google Scholar]
- Wiseman, D.H.; Baker, S.M.; Dongre, A.V.; Gurashi, K.; Storer, J.A.; Somervaille, T.C.; Batta, K. Chronic myelomonocytic leukaemia stem cell transcriptomes anticipate disease morphology and outcome. EBioMedicine 2020, 58, 102904. [Google Scholar] [CrossRef]
- Franzini, A.; Pomicter, A.D.; Yan, D.; Khorashad, J.S.; Tantravahi, S.K.; Than, H.; Ahmann, J.M.; O’Hare, T.; Deininger, M.W. The transcriptome of CMML monocytes is highly inflammatory and reflects leukemia-specific and age-related alterations. Blood Adv. 2019, 3, 2949–2961. [Google Scholar] [CrossRef]
- Montagner, S.; Leoni, C.; Emming, S.; Della Chiara, G.; Balestrieri, C.; Barozzi, I.; Piccolo, V.; Togher, S.; Ko, M.; Rao, A.; et al. TET2 regulates mast cell differentiation and proliferation through catalytic and non-catalytic activities. Cell Rep. 2016, 15, 1566–1579. [Google Scholar] [CrossRef] [PubMed]
- Costa, Y.; Ding, J.; Theunissen, T.W.; Faiola, F.; Hore, T.A.; Shliaha, P.V.; Fidalgo, M.; Saunders, A.; Lawrence, M.; Dietmann, S.; et al. NANOG-dependent function of TET1 and TET2 in establishment of pluripotency. Nature 2013, 495, 370–374. [Google Scholar] [CrossRef]
- de la Rica, L.; Rodríguez-Ubreva, J.; García, M.; Islam, A.B.; Urquiza, J.M.; Hernando, H.; Christensen, J.; Helin, K.; Gómez-Vaquero, C.; Ballestar, E. PU.1 target genes undergo Tet2-coupled demethylation and DNMT3b-mediated methylation in monocyte-to-osteoclast differentiation. Genome Biol. 2013, 14, R99. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, M.; Chen, X.; Chen, L.; Xu, Y.; Lv, L.; Wang, P.; Yang, H.; Ma, S.; Lin, H.; et al. WT1 recruits TET2 to regulate its target gene expression and suppress leukemia cell proliferation. Mol. Cell 2015, 57, 662–673. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, Y.; Bian, C.; Fujiki, R.; Yu, X. TET2 promotes histone O-GlcNAcylation during gene transcription. Nature 2013, 493, 561–564. [Google Scholar] [CrossRef]
- Deplus, R.; Delatte, B.; Schwinn, M.K.; Defrance, M.; Méndez, J.; Murphy, N.; Dawson, M.A.; Volkmar, M.; Putmans, P.; Calonne, E.; et al. TET2 and TET3 regulate GlcNAcylation and H3K4 methylation through OGT and SET1/COMPASS. EMBO J. 2013, 32, 645–655. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, K.; Shen, Q.; Han, Y.; Gu, Y.; Li, X.; Zhao, D.; Liu, Y.; Wang, C.; Zhang, X.; et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature 2015, 525, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Jimenez, A.; Deniz, Ö.; Niklison-Chirou, M.V.; Ruiz, R.; Bezerra-Salomão, K.; Stratoulias, V.; Amouroux, R.; Yip, P.K.; Vilalta, A.; Cheray, M.; et al. TET2 Regulates the Neuroinflammatory Response in Microglia. Cell Rep. 2019, 29, 697–713. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Natarajan, P.; Silver, A.J.; Gibson, C.J.; Bick, A.G.; Shvartz, E.; McConkey, M.; Gupta, N.; Gabriel, S.; Ardissino, D.; et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 111–121. [Google Scholar] [CrossRef]
- Fuster, J.J.; MacLauchlan, S.; Zuriaga, M.A.; Polackal, M.N.; Ostriker, A.C.; Chakraborty, R.; Wu, C.L.; Sano, S.; Muralidharan, S.; Rius, C.; et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 2017, 355, 842–847. [Google Scholar] [CrossRef]
- Sano, S.; Oshima, K.; Wang, Y.; MacLauchlan, S.; Katanasaka, Y.; Sano, M.; Zuriaga, M.A.; Yoshiyama, M.; Goukassian, D.; Cooper, M.A.; et al. Tet2-Mediated Clonal Hematopoiesis Accelerates Heart Failure Through a Mechanism Involving the IL-1β/NLRP3 Inflammasome. J. Am. Coll. Cardiol. 2018, 71, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Coltro, G.; Mangaonkar, A.A.; Lasho, T.L.; Finke, C.M.; Pophali, P.; Carr, R.; Gangat, N.; Binder, M.; Pardanani, A.; Fernandez-Zapico, M.; et al. Clinical, molecular, and prognostic correlates of number, type, and functional localization of TET2 mutations in chronic myelomonocytic leukemia (CMML)-a study of 1084 patients. Leukemia 2019, 34, 1407–1421. [Google Scholar] [CrossRef]
- Pronk, E.; Raaijmakers, M.H.G.P. The mesenchymal niche in MDS. Blood 2019, 133, 1031–1038. [Google Scholar] [CrossRef]
- Marcondes, A.M.; Mhyre, A.J.; Stirewalt, D.L.; Kim, S.H.; Dinarello, C.A.; Deeg, H.J. Dysregulation of IL-32 in myelodysplastic syndrome and chronic myelomonocytic leukemia modulates apoptosis and impairs NK function. Proc. Natl. Acad. Sci. USA 2008, 105, 2865–2870. [Google Scholar] [CrossRef]
- Zannoni, J.; Mauz, N.; Seyve, L.; Meunier, M.; Pernet-Gallay, K.; Brault, J.; Jouzier, C.; Laurin, D.; Pezet, M.; Pernollet, M.; et al. Tumor microenvironment and clonal monocytes from chronic myelomonocytic leukemia induce a procoagulant climate. Blood Adv. 2019, 3, 1868–1880. [Google Scholar] [CrossRef]
- Kramann, R.; Schneider, R.K. The identification of fibrosis-driving myofibroblast precursors reveals new therapeutic avenues in myelofibrosis. Blood 2018, 131, 2111–2119. [Google Scholar] [CrossRef]
- Leimkühler, N.B.; Gleitz, H.F.E.; Ronghui, L.; Snoeren, I.A.M.; Fuchs, S.N.R.; Nagai, J.S.; Banjanin, B.; Lam, K.H.; Vogl, T.; Kuppe, C.; et al. Heterogeneous bone-marrow stromal progenitors drive myelofibrosis via a druggable alarmin axis. Cell Stem Cell 2020, 28, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Aurelius, J.; Hallner, A.; Werlenius, O.; Riise, R.; Möllgård, L.; Brune, M.; Hansson, M.; Martner, A.; Thorén, F.B.; Hellstrand, K. NOX2-dependent immunosuppression in chronic myelomonocytic leukemia. J. Leukoc. Biol. 2017, 102, 459–466. [Google Scholar] [CrossRef]
- Sevin, M.; Debeurme, F.; Laplane, L.; Badel, S.; Morabito, M.; Newman, H.L.; Torres-Martin, M.; Yang, Q.; Badaoui, B.; Wagner-Ballon, O.; et al. Cytokine-like protein 1-induced survival of monocytes suggests a combined strategy targeting MCL1 and MAPK in CMML. Blood 2021, 137, 1628–1640. [Google Scholar] [CrossRef] [PubMed]
- Droin, N.; Lucas, N.; Parinet, V.; Selimoglu-Buet, D.; Humbert, M.; Saada, V.; Lambotte, O.; Solary, E.; Noël, N. Eosinophil-rich tissue infiltrates in chronic myelomonocytic leukemia patients. Leuk. Lymphoma 2017, 58, 2875–2879. [Google Scholar] [CrossRef]
- Lucas, N.; Duchmann, M.; Rameau, P.; Noël, F.; Michea, P.; Saada, V.; Kosmider, O.; Pierron, G.; Fernandez-Zapico, M.E.; Howard, M.T.; et al. Biology and prognostic impact of clonal plasmacytoid dendritic cells in chronic myelomonocytic leukemia. Leukemia 2019, 33, 2466–2480. [Google Scholar] [CrossRef]
- Yang, H.; Bueso-Ramos, C.; DiNardo, C.; Estecio, M.R.; Davanlou, M.; Geng, Q.R.; Fang, Z.; Nguyen, M.; Pierce, S.; Wei, Y.; et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia 2014, 28, 1280–1288. [Google Scholar] [CrossRef]
- Chien, K.S.; Class, C.A.; Montalban-Bravo, G.; Wei, Y.; Sasaki, K.; Naqvi, K.; Ganan-Gomez, I.; Yang, H.; Soltysiak, K.A.; Kanagal-Shamanna, R.; et al. LILRB4 expression in chronic myelomonocytic leukemia and myelodysplastic syndrome based on response to hypomethylating agents. Leuk. Lymphoma 2020, 61, 1493–1499. [Google Scholar] [CrossRef]
- 150 Quivoron, C.; Couronné, L.; Della Valle, V.; Lopez, C.K.; Plo, I.; Wagner-Ballon, O.; Do Cruzeiro, M.; Delhommeau, F.; Arnulf, B.; Stern, M.H.; et al. TET2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer Cell 2011, 20, 25–38. [Google Scholar] [CrossRef]
- Kim, E.; Ilagan, J.O.; Liang, Y.; Daubner, G.M.; Lee, S.C.; Ramakrishnan, A.; Li, Y.; Chung, Y.R.; Micol, J.B.; Murphy, M.E.; et al. SRSF2 Mutations Contribute to Myelodysplasia by Mutant-Specific Effects on Exon Recognition. Cancer Cell 2015, 27, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zheng, H.; Bao, N.; Jiang, S.; Bueso-Ramos, C.E.; Khoury, J.; Class, C.; Lu, Y.; Lin, K.; Yang, H.; et al. KDM6B overexpression activates innate immune signaling and impairs hematopoiesis in mice. Blood Adv. 2018, 2, 2491–2504. [Google Scholar] [CrossRef] [PubMed]
- Kunimoto, H.; Meydan, C.; Nazir, A.; Whitfield, J.; Shank, K.; Rapaport, F.; Maher, R.; Pronier, E.; Meyer, S.C.; Garrett-Bakelman, F.E.; et al. Cooperative Epigenetic Remodeling by TET2 Loss and NRAS Mutation Drives Myeloid Transformation and MEK Inhibitor Sensitivity. Cancer Cell 2018, 33, 44–59.e8. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Qin, T.; Zhao, M.; Bailey, N.; Liu, L.; Yang, K.; Ng, V.; Higashimoto, T.; Coolon, R.; Ney, G.; et al. Oncogenic N-Ras and Tet2 haploinsufficiency collaborate to dysregulate hematopoietic stem and progenitor cells. Blood Adv. 2018, 2, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Hasegawa, A.; Hirano, I.; Yamamoto, M.; Shimizu, R. GATA2 hypomorphism induces chronic myelomonocytic leukemia in mice. Cancer Sci. 2019, 110, 1183–1193. [Google Scholar] [CrossRef]
- Nakata, Y.; Ueda, T.; Nagamachi, A.; Yamasaki, N.; Ikeda, K.I.; Sera, Y.; Takubo, K.; Kanai, A.; Oda, H.; Sanada, M.; et al. Acquired expression of Cbl(Q367P) in mice induces dysplastic myelopoiesis mimicking chronic myelomonocytic leukemia. Blood 2017, 129, 2148–2160. [Google Scholar] [CrossRef] [PubMed]
- Zinkel, S.S.; Ong, C.C.; Ferguson, D.O.; Iwasaki, H.; Akashi, K.; Bronson, R.T.; Kutok, J.L.; Alt, F.W.; Korsmeyer, S.J. Proapoptotic BID is required for myeloid homeostasis and tumor suppression. Genes Dev. 2003, 17, 229–239. [Google Scholar] [CrossRef][Green Version]
- Yasuda, T.; Shirakata, M.; Iwama, A.; Ishii, A.; Ebihara, Y.; Osawa, M.; Honda, K.; Shinohara, H.; Sudo, K.; Tsuji, K.; et al. Dok-2 in myeloid homeostasis suppression of leukemia. J. Exp. Med. 2004, 200, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Lamothe, B.; Lai, Y.; Hur, L.; Orozco, N.M.; Wang, J.; Campos, A.D.; Xie, M.; Schneider, M.D.; Lockworth, C.R.; Jakacky, J.; et al. Deletion of TAK1 in the myeloid lineage results in the spontaneous development of myelomonocytic leukemia in mice. PLoS ONE 2012, 7, e51228. [Google Scholar]
- Yoshimi, A.; Balasis, M.E.; Vedder, A.; Feldman, K.; Ma, Y.; Zhang, H.; Lee, S.C.; Letson, C.; Niyongere, S.; Lu, S.X.; et al. Robust patient-derived xenografts of MDS/MPN overlap syndromes capture the unique characteristics of CMML and JMML. Blood 2017, 130, 397–407. [Google Scholar] [CrossRef]
- Eisenwort, G.; Sadovnik, I.; Keller, A.; Ivanov, D.; Peter, B.; Berger, D.; Stefanzl, G.; Bauer, K.; Slavnitsch, K.; Greiner, G.; et al. Phenotypic characterization of leukemia-initiating stem cells in chronic myelomonocytic leukemia. Leukemia 2021. [Google Scholar] [CrossRef]
- Zhang, Q.; Ball, M.C.; Zhao, Y.; Balasis, M.; Letson, C.; Vedder, A.; List, A.F.; Epling-Burnette, P.K.; Komrokji, R.S.; Padron, E. Intrapatient functional clonality deconvoluted by coupling intracellular flow cytometry and next-generation sequencing in human leukemia. Leukemia 2018, 32, 532–538. [Google Scholar] [CrossRef]
- Kloos, A.; Mintzas, K.; Winckler, L.; Gabdoulline, R.; Alwie, Y.; Jyotsana, N.; Kattre, N.; Schottmann, R.; Scherr, M.; Gupta, C.; et al. Effective drug treatment identified by in vivo screening in a transplantable patient-derived xenograft model of chronic myelomonocytic leukemia. Leukemia 2020, 34, 2951–2963. [Google Scholar] [CrossRef]
- Taoka, K.; Arai, S.; Kataoka, K.; Hosoi, M.; Miyauchi, M.; Yamazaki, S.; Honda, A.; Aixinjueluo, W.; Kobayashi, T.; Kumano, K.; et al. Using patient-derived iPSCs to develop humanized mouse models for chronic myelomonocytic leukemia and therapeutic drug identification, including liposomal clodronate. Sci. Rep. 2018, 8, 15855. [Google Scholar] [CrossRef]
- Beke, A.; Laplane, L.; Riviere, J.; Yang, Q.; Torres-Martin, M.; Dayris, T.; Rameau, P.; Saada, V.; Bilhou-Nabera, C.; Hurtado, A.; et al. Multilayer intraclonal heterogeneity in chronic myelomonocytic leukemia. Haematologica 2020, 105, 112–123. [Google Scholar] [CrossRef]
- Chang, C.J.; Kotini, A.G.; Olszewska, M.; Georgomanoli, M.; Teruya-Feldstein, J.; Sperber, H.; Sanchez, R.; DeVita, R.; Martins, T.J.; Abdel-Wahab, O.; et al. Dissecting the Contributions of Cooperating Gene Mutations to Cancer Phenotypes and Drug Responses with Patient-Derived iPSCs. Stem Cell Rep. 2018, 10, 1610–1624. [Google Scholar] [CrossRef]
- Laplane, L.; Beke, A.; Vainchenker, W.; Solary, E. Concise Review: Induced Pluripotent Stem Cells as New Model Systems in Oncology. Stem Cells 2015, 33, 2887–2892. [Google Scholar] [CrossRef][Green Version]
- Ball, M.; List, A.F.; Padron, E. When clinical heterogeneity exceeds genetic heterogeneity: Thinking outside the genomic box in chronic myelomonocytic leukemia. Blood 2016, 128, 2381–2387. [Google Scholar] [CrossRef]
- Moreno Berggren, D.; Kjellander, M.; Backlund, E.; Engvall, M.; Garelius, H.; Lorenz, F.; Nilsson, L.; Rasmussen, B.; Lehmann, S.; Hellström-Lindberg, E.; et al. Prognostic scoring systems and comorbidities in chronic myelomonocytic leukaemia: A nationwide population-based study. Br. J. Haematol. 2021, 192, 474–483. [Google Scholar] [CrossRef]
- Roupie, A.L.; Guedon, A.; Terrier, B.; Lahuna, C.; Jachiet, V.; Regent, A.; de Boysson, H.; Carrat, F.; Seguier, J.; Terriou, L.; et al. Vasculitis associated with myelodysplastic syndrome and chronic myelomonocytic leukemia: French multicenter case-control study. Semin. Arthritis Rheum. 2020, 50, 879–884. [Google Scholar] [CrossRef]
- Ambinder, A.J.; Miller, J.; DeZern, A.E. Autoimmune disease in CMML-the chicken or the egg? Best Pract. Res. Clin. Haematol. 2020, 33, 101136. [Google Scholar] [CrossRef]
- Oh, Y.J.; Shin, D.Y.; Hwang, S.M.; Kim, S.M.; Im, K.; Park, H.S.; Kim, J.A.; Song, Y.W.; Márquez, A.; Martín, J.; et al. Mutation of ten-eleven translocation-2 is associated with increased risk of autoimmune disease in patients with myelodysplastic syndrome. Korean J. Intern. Med. 2020, 35, 457–464. [Google Scholar] [CrossRef]
- Zhao, L.P.; Boy, M.; Azoulay, C.; Clappier, E.; Sébert, M.; Amable, L.; Klibi, J.; Benlagha, K.; Espéli, M.; Balabanian, K.; et al. Genomic landscape of MDS/CMML associated with systemic inflammatory and autoimmune disease. Leukemia 2021, 23. [Google Scholar] [CrossRef]
- Patnaik, M.M.; Rangit Vallapureddy Lasho, T.L.; Hoversten, K.P.; Finke, C.M.; Ketterling, R.P.; Hanson, C.A.; Gangat, N.; Tefferi, A.; Pardanani, A. A comparison of clinical and molecular characteristics of patients with systemic mastocytosis with chronic myelomonocytic leukemia to CMML alone. Leukemia 2018, 32, 1850–1856. [Google Scholar] [CrossRef]
- Gur, H.D.; Loghavi, S.; Garcia-Manero, G.; Routbort, M.; Kanagal-Shamanna, R.; Quesada, A.; Khogeer, H.; Pierce, S.; Medeiros, L.J.; Kantarjian, H.; et al. Chronic Myelomonocytic Leukemia with Fibrosis Is a Distinct Disease Subset with Myeloproliferative Features and Frequent JAK2 p.V617F Mutations. Am. J. Surg. Pathol. 2018, 42, 799–806. [Google Scholar] [CrossRef]
- Sotlar, K.; Colak, S.; Bache, A.; Berezowska, S.; Krokowski, M.; Bültmann, B.; Valent, P.; Horny, H.P. Variable presence of KITD816V in clonal haematological non-mast cell lineage diseases associated with systemic mastocytosis (SM-AHNMD). J. Pathol. 2010, 220, 586–595. [Google Scholar] [CrossRef]
- Deininger, M.W.N.; Tyner, J.W.; Solary, E. Turning the tide in myelodysplastic/myeloproliferative neoplasms. Nat. Rev. Cancer 2017, 17, 425–440. [Google Scholar] [CrossRef]
- Solal-Celigny, P.; Desaint, B.; Herrera, A.; Chastang, C.; Amar, M.; Vroclans, M.; Brousse, N.; Mancilla, F.; Renoux, M.; Bernard, J.F.; et al. Chronic myelomonocytic leukemia according to FAB classification: Analysis of 35 cases. Blood 1984, 63, 634–638. [Google Scholar] [CrossRef]
- Mufti, G.J.; Stevens, J.R.; Oscier, D.G.; Hamblin, T.J.; Machin, D. Myelodysplastic syndromes: A scoring system with prognostic significance. Br. J. Haematol. 1985, 59, 425–433. [Google Scholar] [CrossRef]
- Sanz, G.F.; Sanz, M.A.; Vallespí, T.; Cañizo, M.C.; Torrabadella, M.; García, S.; Irriguible, D.; San Miguel, J.F. Two regression models and a scoring system for predicting survival and planning treatment in myelodysplastic syndromes: A multivariate analysis of prognostic factors in 370 patients. Blood 1989, 74, 395–408. [Google Scholar] [CrossRef]
- Aul, C.; Gattermann, N.; Heyll, A.; Germing, U.; Derigs, G.; Schneider, W. Primary myelodysplastic syndromes: Analysis of prognostic factors in 235 patients and proposals for an improved scoring system. Leukemia 1992, 6, 52–59. [Google Scholar]
- Morel, P.; Hebbar, M.; Lai, J.L.; Duhamel, A.; Preudhomme, C.; Wattel, E.; Bauters, F.; Fenaux, P. Cytogenetic analysis has strong independent prognostic value in de novo myelodysplastic syndromes and can be incorporated in a new scoring system: A report on 408 cases. Leukemia 1993, 7, 1315–1323. [Google Scholar]
- Kantarjian, H.; O’Brien, S.; Ravandi, F.; Cortes, J.; Shan, J.; Bennett, J.M.; List, A.; Fenaux, P.; Sanz, G.; Issa, J.P.; et al. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer 2008, 113, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Solé, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef]
- Germing, U.; Strupp, C.; Aivado, M.; Gattermann, N. New prognostic parameters for chronic myelomonocytic leukemia. Blood 2002, 100, 731–732. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, M.M.; Padron, E.; LaBorde, R.R.; Lasho, T.L.; Finke, C.M.; Hanson, C.A.; Hodnefield, J.M.; Knudson, R.A.; Ketterling, R.P.; Al-kali, A.; et al. Mayo prognostic model for WHO-defined chronic myelomonocytic leukemia: ASXL1 and spliceosome component mutations and outcomes. Leukemia 2013, 27, 1504–1510. [Google Scholar] [CrossRef]
- Padron, E.; Garcia-Manero, G.; Patnaik, M.M.; Itzykson, R.; Lasho, T.; Nazha, A.; Rampal, R.K.; Sanchez, M.E.; Jabbour, E.; Al Ali, N.H.; et al. An international data set for CMML validates prognostic scoring systems and demonstrates a need for novel prognostication strategies. Blood Cancer J. 2015, 5, e333. [Google Scholar] [CrossRef]
- Duchmann, M.; Braun, T.; Micol, J.B.; Platzbecker, U.; Park, S.; Pilorge, S.; Beyne-Rauzy, O.; Vey, N.; Sébert, M.; Gruson, B.; et al. Validation of response assessment according to international consortium for MDS/MPN criteria in chronic myelomonocytic leukemia treated with hypomethylating agents. Blood Cancer J. 2017, 7, e562. [Google Scholar] [CrossRef]
- Cheson, B.D.; Bennett, J.M.; Kantarjian, H.; Pinto, A.; Schiffer, C.A.; Nimer, S.D.; Löwenberg, B.; Beran, M.; de Witte, T.M.; Stone, R.M.; et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood 2000, 96, 3671–3674. [Google Scholar]
- Cheson, B.D.; Greenberg, P.L.; Bennett, J.M.; Lowenberg, B.; Wijermans, P.W.; Nimer, S.D.; Pinto, A.; Beran, M.; de Witte, T.M.; Stone, R.M.; et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 2006, 108, 419–425. [Google Scholar] [CrossRef]
- Sekeres, M.A.; Othus, M.; List, A.F.; Odenike, O.; Stone, R.M.; Gore, S.D.; Litzow, M.R.; Buckstein, R.; Fang, M.; Roulston, D.; et al. Randomized Phase II Study of Azacitidine Alone or in Combination with Lenalidomide or with Vorinostat in Higher-Risk Myelodysplastic Syndromes and Chronic Myelomonocytic Leukemia: North American Intergroup Study SWOG S1117. J. Clin. Oncol. 2017, 35, 2745–2753. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Itzykson, R.; Renneville, A.; de Renzis, B.; Dreyfus, F.; Laribi, K.; Bouabdallah, K.; Vey, N.; Toma, A.; Recher, C.; et al. Molecular predictors of response to decitabine in advanced chronic myelomonocytic leukemia: A phase 2 trial. Blood 2011, 118, 3824–3831. [Google Scholar] [CrossRef]
- Padron, E.; Dezern, A.; Andrade-Campos, M.; Vaddi, K.; Scherle, P.; Zhang, Q.; Ma, Y.; Balasis, M.E.; Tinsley, S.; Ramadan, H.; et al. A Multi-Institution Phase I Trial of Ruxolitinib in Patients with Chronic Myelomonocytic Leukemia (CMML). Clin. Cancer Res. 2016, 22, 3746–3754. [Google Scholar] [CrossRef] [PubMed]
- Assi, R.; Kantarjian, H.M.; Garcia-Manero, G.; Cortes, J.E.; Pemmaraju, N.; Wang, X. A phase II trial of ruxolitinib in combination with azacytidine in myelodysplastic syndrome/myeloproliferative neoplasms. Am. J. Hematol. 2018, 93, 277–285. [Google Scholar] [CrossRef]
- Savona, M.R.; Malcovati, L.; Komokji, R.; Tiu, R.V.; Mughal, T.I.; Orazi, A.; Kiladjian, J.J.; Padron, E.; Solary, E.; Tibes, R.; et al. MDS/MPN International Working Group. An international consortium proposal of uniform response criteria for myelodyplastic / myeloproliferative neoplasms (MDS/MPN) in adults. Blood 2015, 125, 1857–1865. [Google Scholar] [CrossRef]
- Emanuel, R.M.; Dueck, A.C.; Geyer, H.L.; Kiladjian, J.J.; Slot, S.; Zweegman, S.; te Boekhorst, P.A.; Commandeur, S.; Schouten, H.C.; Sackmann, F.; et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: Prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J. Clin. Oncol. 2012, 30, 4098–4103. [Google Scholar] [CrossRef]
- Solary, E.; Itzykson, R. How I treat chronic myelomonocytic leukemia. Blood 2017, 130, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Xicoy, B.; Germing, U.; Jimenez, M.J.; Garcia, O.; Garcia, R.; Schemenau, J.; Pedro, C.; Luño, E.; Bernal, T.; González, B.; et al. Response to erythropoietic-stimulating agents in patients with chronic myelomonocytic leukemia. Eur. J. Haematol. 2016, 97, 33–38. [Google Scholar] [CrossRef]
- Fenaux, P.; Platzbecker, U.; Mufti, G.J.; Garcia-Manero, G.; Buckstein, R.; Santini, V.; Díez-Campelo, M.; Finelli, C.; Cazzola, M.; Ilhan, O.; et al. Luspatercept in Patients with Lower-Risk Myelodysplastic Syndromes. N. Engl. J. Med. 2020, 382, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Komrokji, R.; Garcia-Manero, G.; Ades, L.; Prebet, T.; Steensma, D.P.; Jurcic, J.G.; Sekeres, M.A.; Berdeja, J.; Savona, M.R.; Beyne-Rauzy, O.; et al. Sotatercept with long-term extension for the treatment of anaemia in patients with lower-risk myelodysplastic syndromes: A phase 2, dose-ranging trial. Lancet Haematol. 2018, 5, e63–e72. [Google Scholar] [CrossRef]
- Rabian, F.; Lambert, J.; Barbieri, D.; Gruson, B.; Thepot, S.; Braun, T.; Vey, N.; Delaunay, J.; Legros, L.; Lejeune, J.; et al. Eltrombopag in Chronic Myelomonocytic Leukemia (CMML) with Severe Thrombocytopenia: Final Results of a Multicenter Phase II Study. Blood 2020, 136 (Suppl. 1), 15–16. [Google Scholar] [CrossRef]
- Wattel, E.; Guerci, A.; Hecquet, B.; Economopoulos, T.; Copplestone, A.; Mahé, B.; Couteaux, M.E.; Resegotti, L.; Voglova, V.; Foussard, C.; et al. A randomized trial of hydroxyurea versus VP16 in adult chronic myelomonocytic leukemia. Groupe Français des Myélodysplasies and European CMML Group. Blood 1996, 88, 2480–2487. [Google Scholar] [CrossRef]
- Catalano, L.; Majolino, I.; Musto, P.; Fragrasso, A.; Molica, S.; Cirincione, S.; Selleri, C.; Luciano, L.; De Renzo, A.; Vecchione, R.; et al. Alpha interferon in the treatment of chronic myelomonocytic leukemia. Haematologica 1989, 74, 577–581. [Google Scholar]
- Cambier, N.; Wattel, E.; Menot, M.L.; Guerci, A.; Chomienne, C.; Fenaux, P. All-trans retinoic acid in adult chronic myelomonocytic leukemia: Results of a pilot study. Leukemia 1996, 10, 1164–1167. [Google Scholar]
- de Witte, T.; Bowen, D.; Robin, M.; Malcovati, L.; Niederwieser, D.; Yakoub-Agha, I.; Mufti, G.J.; Fenaux, P.; Sanz, G.; Martino, R.; et al. Allogeneic hematopoietic stem cell transplantation for MDS and CMML: Recommendations from an international expert panel. Blood 2017, 129, 1753–1762. [Google Scholar] [CrossRef]
- Robin, M.; Itzykson, R. Contemporary treatment approaches to CMML—Is allogeneic HCT the only cure? Best Pract. Res. Clin. Haematol. 2020, 33, 101138. [Google Scholar] [CrossRef] [PubMed]
- Gagelmann, N.; Badbaran, A.; Beelen, D.W.; Salit, R.B.; Stölzel, F.; Rautenberg, C.; Becker, H.; Radujkovic, A.; Panagiota, V.; Bogdanov, R.; et al. A prognostic score including mutation profile and clinical features for patients with CMML undergoing stem cell transplantation. Blood Adv. 2021, 5, 1760–1769. [Google Scholar] [CrossRef]
- Pophali, P.; Matin, A.; Mangaonkar, A.A.; Carr, R.; Binder, M.; Al-Kali, A.; Begna, K.H.; Reichard, K.K.; Alkhateeb, H.; Shah, M.V.; et al. Prognostic impact and timing considerations for allogeneic hematopoietic stem cell transplantation in chronic myelomonocytic leukemia. Blood Cancer J. 2020, 10, 121. [Google Scholar] [CrossRef]
- Duchmann, M.; Itzykson, R. Clinical update on hypomethylating agents. Int. J. Hematol. 2019, 110, 161–169. [Google Scholar] [CrossRef]
- Silverman, L.R.; Demakos, E.P.; Peterson, B.L.; Kornblith, A.B.; Holland, J.C.; Odchimar-Reissig, R.; Stone, R.M.; Nelson, D.; Powell, B.L.; DeCastro, C.M.; et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: A study of the cancer and leukemia group B. J. Clin. Oncol. 2002, 20, 2429–2440. [Google Scholar] [CrossRef]
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Finelli, C.; Giagounidis, A.; Schoch, R.; Gattermann, N.; Sanz, G.; List, A.; et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009, 10, 223–232. [Google Scholar] [CrossRef]
- Pleyer, L.; Germing, U.; Sperr, W.R.; Linkesch, W.; Burgstaller, S.; Stauder, R.; Girschikofsky, M.; Schreder, M.; Pfeilstocker, M.; Lang, A.; et al. Azacitidine in CMML: Matched-pair analyses of daily-life patients reveal modest effects on clinical course and survival. Leuk. Res. 2014, 38, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Wijermans, P.W.; Rüter, B.; Baer, M.R.; Slack, J.L.; Saba, H.I.; Lübbert, M. Efficacy of decitabine in the treatment of patients with chronic myelomonocytic leukemia (CMML). Leuk. Res. 2008, 32, 587–591. [Google Scholar] [CrossRef]
- Santini, V.; Allione, B.; Zini, G.; Gioia, D.; Lunghi, M.; Poloni, A.; Cilloni, D.; Sanna, A.; Masiera, E.; Ceccarelli, M.; et al. Multicentre trial of decitabine in higher-risk chronic myelomonocytic. Leukemia 2018, 32, 413–418. [Google Scholar] [CrossRef]
- Pleyer, L.; Leisch, M.; Kourakli, A.; Padron, E.; Maciejewski, J.P.; Xicoy Cirici, B.; Kaivers, J.; Ungerstedt, J.; Heibl, S.; Patiou, P.; et al. Outcomes of patients with chronic myelomonocytic leukaemia treated with non-curative therapies: A retrospective cohort study. Lancet Haematol. 2021, 8, e135–e148. [Google Scholar] [CrossRef]
- Itzykson, R.; Santini, V.; Chaffaut, C.; Adès, L.; Thepot, S.; Giagounidis, A. Decitabine versus hydroxyurea for advanced proliferative CMML: Results of the EMSCO randomized phase 3 DACOTA trial. Blood 2020, 136, 53–54. [Google Scholar] [CrossRef]
- Bejanyan, N.; Anasetti, C. First-line hypomethylating agents for patients with high risk chronic myelomonocytic leukaemia. Lancet Haematol. 2021, 8, e99–e101. [Google Scholar] [CrossRef]
- Savona, M.R.; Odenike, O.; Amrein, P.C.; Steensma, D.P.; DeZern, A.E.; Michaelis, L.C.; Faderl, S.; Harb, W.; Kantarjian, H.; Lowder, J.; et al. An oral fixed-dose combination of decitabine and cedazuridine in myelodysplastic syndromes: A multicentre, open-label, dose-escalation, phase 1 study. Lancet Haematol. 2019, 6, e194–e203. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Griffiths, E.A.; Steensma, D.P.; Roboz, G.J.; Wells, R.; McCloskey, J.; Odenike, O.; DeZern, A.E.; Yee, K.; Busque, L.; et al. Oral cedazuridine/decitabine for MDS and CMML: A phase 2 pharmacokinetic/pharmacodynamic randomized crossover study. Blood 2020, 136, 674–683. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Pei, S.; Pollyea, D.A.; Gustafson, A.; Stevens, B.M.; Minhajuddin, M.; Fu, R.; Riemondy, K.A.; Gillen, A.E.; Sheridan, R.M.; Kim, J.; et al. Monocytic Subclones Confer Resistance to Venetoclax-Based Therapy in Patients with Acute Myeloid Leukemia. Cancer Discov. 2020, 10, 536–551. [Google Scholar] [CrossRef]
- Sekeres, M.A.; Watts, J.; Radinoff, A.; Sangerman, M.A.; Cerrano, M.; Lopez, P.F.; Zeidner, J.F.; Campelo, M.D.; Graux, C.; Liesveld, J.; et al. Randomized phase 2 trial of pevonedistat plus azacytidine versus azacitidine for higher-risk MDS/CMML or low-blast AML. Leukemia 2021. [Google Scholar] [CrossRef]
- Wolff, F.; Leisch, M.; Greil, R.; Risch, A.; Pleyer, L. The double-edged sword of (re)expression of genes by hypomethylating agents: From viral mimicry to exploitation as priming agents for targeted immune checkpoint modulation. Cell Commun. Signal. 2017, 15, 13. [Google Scholar] [CrossRef]
- Montalban-Bravo, G.; Darbaniyan, F.; Kanagal-Shamanna, R.; Ganan-Gomez, I.; Class, C.A.; Sasaki, K.; Naqvi, K.; Wei, Y.; Yang, H.; Soltysiak, K.A.; et al. Type I interferon upregulation and deregulation of genes involved in monopoiesis in chronic myelomonocytic leukemia. Leuk. Res. 2021, 101, 106511. [Google Scholar] [CrossRef] [PubMed]
- Crotti, C.; Biggioggero, M.; Becciolini, A.; Agape, E.; Favalli, E.G. Mavrilimumab: A unique insight and update on the current status in the treatment of rheumatoid arthritis. Expert Opin. Investig. Drugs 2019, 28, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Goebel, L.; Müller, M.P.; Goody, R.S.; Rauh, D. KRasG12C inhibitors in clinical trials: A short historical perspective. RSC Med. Chem. 2020, 11, 760–770. [Google Scholar] [CrossRef]
- Samatar, A.A.; Poulikakos, P.I. Targeting RAS-ERK signalling in cancer: Promises and challenges. Nat. Rev. Drug Discov. 2014, 13, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Van den Bossche, J.; Lardon, F.; Deschoolmeester, V.; De Pauw, I.; Vermorken, J.B.; Specenier, P.; Pauwels, P.; Peeters, M.; Wouters, A. Spotlight on Volasertib: Preclinical and Clinical Evaluation of a Promising Plk1 Inhibitor. Med. Res. Rev. 2016, 36, 749–786. [Google Scholar] [CrossRef]
- Chatani, P.D.; Yang, J.C. Mutated RAS: Targeting the “Untargetable” with T Cells. Clin. Cancer Res. 2020, 26, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, G.; Westley, T.; Cappelleri, J.C.; Arondekar, B.; Chan, G.; Bell, T.J.; Briggs, A. Overall survival of glasdegib in combination with low-dose cytarabine, azacitidine, and decitabine among adult patients with previously untreated AML: Comparative effectiveness using simulated treatment comparisons. Clin. Outcomes Res. 2019, 11, 551–565. [Google Scholar] [CrossRef]
- Belizaire, R.; Koochaki, S.H.J.; Udeshi, N.D.; Vedder, A.; Sun, L.; Svinkina, T.; Hartigan, C.; McConkey, M.E.; Kovalcik, V.; Bizuayehu, A.; et al. CBL mutations drive PI3K/AKT signaling via increased interaction with LYN and PIK3R1. Blood 2021, 7, 2209–2220. [Google Scholar] [CrossRef]
- Tefferi, A.; Lasho, T.L.; Begna, K.H.; Patnaik, M.M.; Zblewski, D.L.; Finke, C.M.; Laborde, R.R.; Wassie, E.; Schimek, L.; Hanson, C.A.; et al. A pilot study of the telomerase inhibitor imetelstat for myelofibrosis. N. Engl. J. Med. 2015, 373, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Seiler, M.; Yoshimi, A.; Darman, R.; Chan, B.; Keaney, G.; Thomas, M.; Agrawal, A.A.; Caleb, B.; Csibi, A.; Sean, E.; et al. H3B-8800, an orally available small-molecule splicing modulator, induces lethality in spliceosome-mutant cancers. Nat. Med. 2018, 24, 497–504. [Google Scholar] [CrossRef]
- Steensma, D.P.; Wermke, M.; Klimek, V.M.; Greenberg, P.L.; Font, P.; Komrokji RS et, a.l. Results of a Clinical Trial of H3B-8800, a Splicing Modulator, in Patients with Myelodysplastic Syndromes (MDS), Acute Myeloid Leukemia (AML) or Chronic Myelomonocytic Leukemia (CMML). Blood 2019, 134, 673. [Google Scholar] [CrossRef]
- Pemmaraju, N.; Lane, A.A.; Sweet, K.L.; Stein, A.S.; Vasu, S.; Blum, W.; Rizzieri, D.A.; Wang, E.S.; Duvic, M.; Sloan, J.M.; et al. Tagraxofusp in Blastic Plasmacytoid Dendritic-Cell Neoplasm. N. Engl. J. Med. 2019, 380, 1628–1637. [Google Scholar] [CrossRef]
- Krishnan, A.; Li, B.; Pagane, M.; McGovern, E.; Stone-Molloy, Z.; Chen, J. Evaluation of Combination Tagraxofusp (SL-401) and Hypomethylating Agent (HMA) Therapy for the Treatment of Chronic Myelomonocytic Leukemia (CMML). Blood 2018, 132, 1809. [Google Scholar] [CrossRef]
| Somatic Variants | Germline Predisposition * |
|---|---|
| >10% of patients | ANKRD26 (ANKRD26-RT) |
| ASXL1 | ATG2B/GSKIP |
| CBL | DDX1 |
| KRAS | ETV6 c.1160G > A (p.Arg369Gln) |
| NRAS | GATA-2 (GATA-2 deficiency) |
| RUNX1 | RUNX1 (RUNX1-FTD) |
| SRSF2 | |
| TET2 | Cytogenetic abnormalities ** |
| 5–10% of patients | Low risk |
| BCOR/BCORL | Normal karyotype |
| DNMT3A | Loss of Y |
| EZH2 | High risk |
| JAK2 | Monosomy 7/deletion 7q |
| PHF6 | Trisomy 7 |
| SETBP1 | Complex karyotype |
| SF3B1 | Intermediate risk |
| U2AF1 | Deletion 20q |
| ZRSR2 | Trisomy 21 |
| <5% of patients | Other: 3q-, 5q-, 12q-, 13q-, iso [17], +X etc…. |
| ASXL2 | |
| BRAF | CMML with MPN driver *** |
| CUX1 | PDGFR1 rearrangement |
| FLT3 | PDGFRB rearrangement |
| IDH2 | FGFR1 rearrangement |
| IDH1 | PCM1-JAK2 |
| NF1 | |
| PTPN11 | SM-CMML **** |
| TP53 | KIT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solary, E.; Itzykson, R. Chronic Myelomonocytic Leukemia Gold Jubilee. Hemato 2021, 2, 403-428. https://doi.org/10.3390/hemato2030026
Solary E, Itzykson R. Chronic Myelomonocytic Leukemia Gold Jubilee. Hemato. 2021; 2(3):403-428. https://doi.org/10.3390/hemato2030026
Chicago/Turabian StyleSolary, Eric, and Raphael Itzykson. 2021. "Chronic Myelomonocytic Leukemia Gold Jubilee" Hemato 2, no. 3: 403-428. https://doi.org/10.3390/hemato2030026
APA StyleSolary, E., & Itzykson, R. (2021). Chronic Myelomonocytic Leukemia Gold Jubilee. Hemato, 2(3), 403-428. https://doi.org/10.3390/hemato2030026






