Highly Efficient Purification of Recombinant VSV-∆G-Spike Vaccine against SARS-CoV-2 by Flow-Through Chromatography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Virus Culture
2.2. Denarase Treatment and Clarification
2.3. Ultrafiltration
2.4. Chromatography Conditions and Resins
2.5. Membrane Adsorbers
2.6. Purification Using CaptoTM Core 700 Resin
2.7. Determination of Binding Capacity of Vero HCP
2.8. Analytical Methods
2.8.1. Infectivity Assay
2.8.2. Host Cell Protein Quantification Assay
2.8.3. Residual DNA Measurement
3. Results
3.1. Clarification and Ultrafiltration
3.2. Virus Stability
3.3. Membrane Adsorbers
3.4. Packed-Bed Ion-Exchange Chromatography
3.5. CaptoTM Core 700
3.5.1. Binding Capacity Determination of Vero HCP
3.5.2. Flow-through Chromatography
3.5.3. Direct Loading of the Clarified Harvest to CaptoTM Core 700
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, F.; Deng, L.; Zhang, L.; Cai, Y.; Cheung, C.W.; Xia, Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19). J. Gen. Intern. Med. 2020, 35, 1545–1549. [Google Scholar] [CrossRef] [Green Version]
- WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 13 September 2021).
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020, 181, 281–292. [Google Scholar] [CrossRef]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef]
- WHO—Draft Landscape of COVID-19 Candidate Vaccines. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 2 September 2021).
- Emergency Use Listing (EUL). Available online: https://www.who.int/news/item/07-05-2021-who-lists-additional-covid-19-vaccine-for-emergency-use-and-issues-interim-policy-recommendations (accessed on 2 September 2021).
- COVID-19 Vaccines. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines (accessed on 2 September 2021).
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; Buonocore, L.; Price, R.; Forman, J.; Rose, J.K. Attenuated vesicular stomatitis viruses as vaccine vectors. J. Virol. 1999, 73, 3723–3732. [Google Scholar] [CrossRef] [Green Version]
- Clarke, D.K.; Nasar, F.; Lee, M.; Johnson, J.E.; Wright, K.; Calderon, P.; Guo, M.; Natuk, R.; Cooper, D.; Hendry, R.M.; et al. Synergistic attenuation of vesicular stomatitis virus by combination of specific G gene truncations and N gene translocations. J. Virol. 2007, 81, 2056–2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, N.F.; Marx, P.A.; Luckay, A.; Nixon, D.F.; Moretto, W.J.; Donahoe, S.M.; Montefiori, D.; Roberts, A.; Buonocore, L.; Rose, J.K. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 2001, 106, 539–549. [Google Scholar] [CrossRef] [Green Version]
- Kretzschmar, E.; Buonocore, L.; Schnell, M.J.; Rose, J.K. High-efficiency incorporation of functional influenza virus glycoproteins into recombinant vesicular stomatitis viruses. J. Virol. 1997, 71, 5982–5989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geisbert, T.W.; Feldmann, H. Recombinant vesicular stomatitis virus–based vaccines against Ebola and Marburg virus infections. J. Infect. Dis. 2011, 204, S1075–S1081. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E. “Make Ebola a thing of the past”: First vaccine against deadly virus approved. Nature 2019, 575, 425–426. [Google Scholar] [CrossRef] [PubMed]
- Ge, P.; Tsao, J.; Schein, S.; Green, T.J.; Luo, M.; Zhou, Z.H. Cryo-EM model of the bullet-shaped vesicular stomatitis virus. Science 2010, 327, 689–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, F.A.; Harrison, A.K. Electron microscopy of the rhabdoviruses of animals. In Rhabdoviruses; CRC Press: Boca Raton, FL, USA, 2018; Volume 1, pp. 65–106. ISBN 9781351084833. [Google Scholar]
- Yahalom-Ronen, Y.; Tamir, H.; Melamed, S.; Politi, B.; Shifman, O.; Achdout, H.; Vitner, E.B.; Israeli, O.; Milrot, E.; Stein, D.; et al. A single dose of recombinant VSV-∆G-spike vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 2020, 11, 6402. [Google Scholar] [CrossRef] [PubMed]
- Evaluate the Safety, Immunogenicity and Potential Efficacy of an rVSV-SARS-CoV-2-S Vaccine. Available online: https://clinicaltrials.gov/ct2/show/NCT04608305 (accessed on 22 August 2021).
- Case, J.B.; Rothlauf, P.W.; Chen, R.E.; Liu, Z.; Zhao, H.; Kim, A.S.; Bloyet, L.-M.; Zeng, Q.; Tahan, S.; Droit, L.; et al. Neutralizing antibody and soluble ACE2 inhibition of a replication-competent VSV-SARS-CoV-2 and a clinical isolate of SARS-CoV-2. Cell Host Microbe 2020, 28, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Sheng-Fowler, L.; Lewis, A.M., Jr.; Peden, K. Issues associated with residual cell-substrate DNA in viral vaccines. Biologicals 2009, 37, 190–195. [Google Scholar] [CrossRef]
- The United States Pharmacopeia. Residual Host Cell Protein Measurement in Biopharmaceuticals; National Formulary; United States Pharmacopeial Convention: Rockville, MD, USA, 2016; Volume 1, p. 1132. [Google Scholar]
- Segura, M.M.; Kamen, A.A.; Garnier, A. Overview of current scalable methods for purification of viral vectors. In Viral Vectors for Gene Therapy; Humana Press Inc.: Totowa, NJ, USA, 2011; pp. 89–116. [Google Scholar]
- Makovitzki, A.; Lerer, E.; Kafri, Y.; Adar, Y.; Cherry, L.; Lupu, E.; Monash, A.; Levy, R.; Israeli, O.; Dor, E.; et al. Evaluation of a Downstream Process for the Recovery and Concentration of a Cell-Culture-Derived rVSV-Spike COVID-19 Vaccine Candidate. 2021. Preprint. [Google Scholar]
- Wolf, M.W.; Reichl, U. Downstream processing of cell culture-derived virus particles. Expert Rev. Vaccines 2011, 10, 1451–1475. [Google Scholar] [CrossRef]
- Kramberger, P.; Urbas, L.; Štrancar, A. Downstream processing and chromatography based analytical methods for production of vaccines, gene therapy vectors, and bacteriophages. Hum. Vaccines Immunother. 2015, 11, 1010–1021. [Google Scholar] [CrossRef]
- Morenweiser, R. Downstream processing of viral vectors and vaccines. Gene Ther. 2005, 12, S103–S110. [Google Scholar] [CrossRef] [PubMed]
- Ausubel, L.J.; Meseck, M.; Derecho, I.; Lopez, P.; Knoblauch, C.; McMahon, R.; Anderson, J.; Dunphy, N.; Quezada, V.; Khan, R. Current good manufacturing practice production of an oncolytic recombinant vesicular stomatitis viral vector for cancer treatment. Hum. Gene Ther. 2011, 22, 489–497. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.; Cutler, M.W.; Ouattara, A.A.; Syvertsen, K.E. Purification Processes for Isolating Purified Vesicular Stomatitis Virus from Cell Culture. U.S. Patent No. 7,875,446, 25 January 2011. [Google Scholar]
- Federspiel, M.J.; Wegman, T.R.; Langfield, K.K.; Walker, H.J.; Stephan, S.A. Rhabdoviridae Virus Preparations. U.S. Patent Application No 2010/0143889 A1, 10 June 2010. [Google Scholar]
- Orr, V.; Zhong, L.; Moo-Young, M.; Chou, C.P. Recent advances in bioprocessing application of membrane chromatography. Biotechnol. Adv. 2013, 31, 450–465. [Google Scholar] [CrossRef]
- Peixoto, C.; Ferreira, T.B.; Sousa, M.F.Q.; Carrondo, M.J.T.; Alves, P.M. Towards purification of adenoviral vectors based on membrane technology. Biotechnol. Prog. 2008, 24, 1290–1296. [Google Scholar] [CrossRef]
- Vicente, T.; Peixoto, C.; Carrondo, M.J.T.; Alves, P.M. Purification of recombinant baculoviruses for gene therapy using membrane processes. Gene Ther. 2009, 16, 766–775. [Google Scholar] [CrossRef]
- McNally, D.J.; Darling, D.; Farzaneh, F.; Levison, P.R.; Slater, N.K.H. Optimised concentration and purification of retroviruses using membrane chromatography. J. Chromatogr. A 2014, 1340, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Kutner, R.H.; Puthli, S.; Marino, M.P.; Reiser, J. Simplified production and concentration of HIV-1-based lentiviral vectors using HYPERFlask vessels and anion exchange membrane chromatography. BMC Biotechnol. 2009, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, K.; Scheibe, O.; Kocourek, A.; Muelich, J.; Jurkiewicz, E.; Pfeifer, A. Highly efficient concentration of lenti-and retroviral vector preparations by membrane adsorbers and ultrafiltration. BMC Biotechnol. 2011, 11, 55. [Google Scholar] [CrossRef] [Green Version]
- Grein, T.A.; Michalsky, R.; López, M.V.; Czermak, P. Purification of a recombinant baculovirus of Autographa californica M nucleopolyhedrovirus by ion exchange membrane chromatography. J. Virol. Methods 2012, 183, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Kalbfuss, B.; Wolff, M.; Geisler, L.; Tappe, A.; Wickramasinghe, R.; Thom, V.; Reichl, U. Direct capture of influenza A virus from cell culture supernatant with Sartobind anion-exchange membrane adsorbers. J. Membr. Sci. 2007, 299, 251–260. [Google Scholar] [CrossRef]
- Okada, T.; Nonaka-Sarukawa, M.; Uchibori, R.; Kinoshita, K.; Hayashita-Kinoh, H.; Nitahara-Kasahara, Y.; Takeda, S.; Ozawa, K. Scalable purification of adeno-associated virus serotype 1 (AAV1) and AAV8 vectors, using dual ion-exchange adsorptive membranes. Hum. Gene Ther. 2009, 20, 1013–1021. [Google Scholar] [CrossRef]
- Vicente, T.; Sousa, M.F.Q.; Peixoto, C.; Mota, J.P.B.; Alves, P.M.; Carrondo, M.J.T. Anion-exchange membrane chromatography for purification of rotavirus-like particles. J. Membr. Sci. 2008, 311, 270–283. [Google Scholar] [CrossRef]
- Wolff, M.W.; Siewert, C.; Lehmann, S.; Hansen, S.P.; Djurup, R.; Faber, R.; Reichl, U. Capturing of cell culture-derived modified Vaccinia Ankara virus by ion exchange and pseudo-affinity membrane adsorbers. Biotechnol. Bioeng. 2010, 105, 761–769. [Google Scholar] [CrossRef]
- Guiochon, G. Monolithic columns in high-performance liquid chromatography. J. Chromatogr. A 2007, 1168, 101–168. [Google Scholar] [CrossRef]
- Oksanen, H.M.; Domanska, A.; Bamford, D.H. Monolithic ion exchange chromatographic methods for virus purification. Virology 2012, 434, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Krajacic, M.; Ravnikar, M.; Štrancar, A.; Gutiérrez-Aguirre, I. Application of monolithic chromatographic supports in virus research. Electrophoresis 2017, 38, 2827–2836. [Google Scholar] [CrossRef]
- Gerster, P.; Kopecky, E.-M.; Hammerschmidt, N.; Klausberger, M.; Krammer, F.; Grabherr, R.; Mersich, C.; Urbas, L.; Kramberger, P.; Paril, T.; et al. Purification of infective baculoviruses by monoliths. J. Chromatogr. A 2013, 1290, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Banjac, M.; Roethl, E.; Gelhart, F.; Kramberger, P.; Jarc, B.L.; Jarc, M.; Štrancar, A.; Muster, T.; Peterka, M. Purification of Vero cell derived live replication deficient influenza A and B virus by ion exchange monolith chromatography. Vaccine 2014, 32, 2487–2492. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, P.P.; Reiter, K.; Wetter, V.; Steppert, P.; Maresch, D.; Ling, W.L.; Satzer, P.; Jungbauer, A. Capture and purification of Human Immunodeficiency Virus-1 virus-like particles: Convective media vs porous beads. J. Chromatogr. A 2020, 1627, 461378. [Google Scholar] [CrossRef] [PubMed]
- Vincent, D.; Kramberger, P.; Hudej, R.; Štrancar, A.; Wang, Y.; Zhou, Y.; Velayudhan, A. The development of a monolith-based purification process for Orthopoxvirus vaccinia virus Lister strain. J. Chromatogr. A 2017, 1524, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liu, X. Mixed-mode chromatography in pharmaceutical and biopharmaceutical applications. J. Pharm. Biomed. Anal. 2016, 128, 73–88. [Google Scholar] [CrossRef] [Green Version]
- Weigel, T.; Solomaier, T.; Peuker, A.; Pathapati, T.; Wolff, M.W.; Reichl, U. A flow-through chromatography process for influenza A and B virus purification. J. Virol. Methods 2014, 207, 45–53. [Google Scholar] [CrossRef]
- Blom, H.; Åkerblom, A.; Kon, T.; Shaker, S.; van der Pol, L.; Lundgren, M. Efficient chromatographic reduction of ovalbumin for egg-based influenza virus purification. Vaccine 2014, 32, 3721–3724. [Google Scholar] [CrossRef]
- James, K.T.; Cooney, B.; Agopsowicz, K.; Trevors, M.A.; Mohamed, A.; Stoltz, D.; Hitt, M.; Shmulevitz, M. Novel high-throughput approach for purification of infectious virions. Sci. Rep. 2016, 6, 36826. [Google Scholar] [CrossRef] [Green Version]
- Lothert, K.; Offersgaard, A.F.; Pihl, A.F.; Mathiesen, C.K.; Jensen, T.B.; Alzua, G.P.; Fahnøe, U.; Bukh, J.; Gottwein, J.M.; Wolff, M.W. Development of a downstream process for the production of an inactivated whole hepatitis C virus vaccine. Sci. Rep. 2020, 10, 16261. [Google Scholar] [CrossRef]
- Mundle, S.T.; Kishko, M.; Groppo, R.; DiNapoli, J.; Hamberger, J.; McNeil, B.; Kleanthous, H.; Parrington, M.; Zhang, L.; Anderson, S.F. Core bead chromatography for preparation of highly pure, infectious respiratory syncytial virus in the negative purification mode. Vaccine 2016, 34, 3690–3696. [Google Scholar] [CrossRef]
- Barrett, P.N.; Mundt, W.; Kistner, O.; Howard, M.K. Vero cell platform in vaccine production: Moving towards cell culture-based viral vaccines. Expert Rev. Vaccines 2009, 8, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, S.; Wang, Y.J. Trehalose: Current use and future applications. J. Pharm. Sci. 2011, 100, 2020–2053. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.; Carvalho, A.; Carmo, M.; Carrondo, M.J.T.; Alves, P.M.; Cruz, P.E. Scaleable purification process for gene therapy retroviral vectors. J. Gene Med. A Cross-Discip. J. Res. Sci. Gene Transf. Its Clin. Appl. 2007, 9, 233–243. [Google Scholar] [CrossRef]
- Gélinas, J.-F.; Azizi, H.; Kiesslich, S.; Lanthier, S.; Perdersen, J.; Chahal, P.S.; Ansorge, S.; Kobinger, G.; Gilbert, R.; Kamen, A.A. Production of rVSV-ZEBOV in serum-free suspension culture of HEK 293SF cells. Vaccine 2019, 37, 6624–6632. [Google Scholar] [CrossRef] [PubMed]
- Burova, E.; Ioffe, E. Chromatographic purification of recombinant adenoviral and adeno-associated viral vectors: Methods and implications. Gene Ther. 2005, 12, S5–S17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trilisky, E.I.; Lenhoff, A.M. Sorption processes in ion-exchange chromatography of viruses. J. Chromatogr. A 2007, 1142, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Iyer, G.; Ramaswamy, S.; Asher, D.; Mehta, U.; Leahy, A.; Chung, F.; Cheng, K.-S. Reduced surface area chromatography for flow-through purification of viruses and virus like particles. J. Chromatogr. A 2011, 1218, 3973–3981. [Google Scholar] [CrossRef] [PubMed]
- Kalbfuss, B.; Wolff, M.; Morenweiser, R.; Reichl, U. Purification of cell culture-derived human influenza A virus by size-exclusion and anion-exchange chromatography. Biotechnol. Bioeng. 2007, 96, 932–944. [Google Scholar] [CrossRef] [PubMed]
- Junter, G.-A.; Lebrun, L. Polysaccharide-based chromatographic adsorbents for virus purification and viral clearance. J. Pharm. Anal. 2020, 10, 291–312. [Google Scholar] [CrossRef] [PubMed]
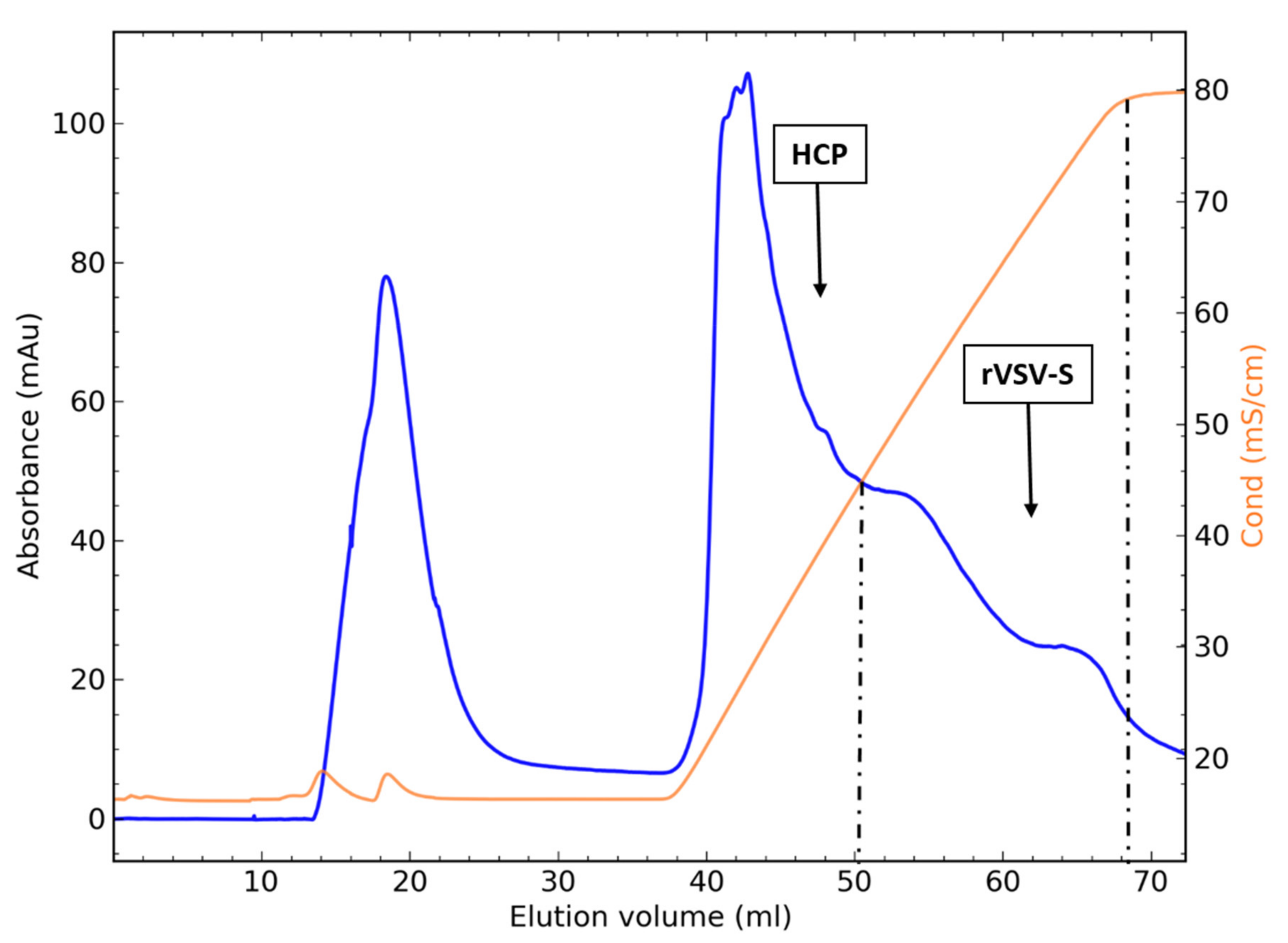
| Chromatography Method | Mode | Column/Membrane | NaCl Concentration (mM) | Recovery (%) |
|---|---|---|---|---|
| Membrane adsorbers | Strong anion exchange | Mustang® Q | 150 | 13 |
| Natrix® Q | 150 | 14 | ||
| QF5 | 150 | 3 | ||
| Weak anion exchange | DF5 | 150 | 2 | |
| Packed-bed | Strong anion exchange | HiTrap Q XL | 100 | 32 |
| HiTrap Q XL | 150 | 33 | ||
| Fractogel® TMEA | 150 | 18 | ||
| Weak anion exchange | Fractogel® DMEA | 50 | 27 | |
| Fractogel® DMEA | 100 | 26 | ||
| Fractogel® DMEA | 150 | 26 | ||
| Mixed-mode | Size-exclusion and anion-exchange | CaptoTM Core 700 | 150 | 85 |
| Sample | Description | Total rVSV-S (PFU) | Recovery (%) | Total HCP (µg) |
|---|---|---|---|---|
| 1 | Source | 4.0 × 108 | - | 694 |
| 2 | Flow-through | 4.6 × 106 | 1 | 256 |
| 3 | Elution 16–45 ms/cm | 4.4 × 107 | 11 | 241 |
| 4 | Elution 46–60 ms/cm | 4.2 × 107 | 11 | 3.97 |
| 5 | Elution 61–80 ms/cm | 8.4 × 107 | 21 | 9.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lerer, E.; Oren, Z.; Kafri, Y.; Adar, Y.; Toister, E.; Cherry, L.; Lupu, E.; Monash, A.; Levy, R.; Dor, E.; et al. Highly Efficient Purification of Recombinant VSV-∆G-Spike Vaccine against SARS-CoV-2 by Flow-Through Chromatography. BioTech 2021, 10, 22. https://doi.org/10.3390/biotech10040022
Lerer E, Oren Z, Kafri Y, Adar Y, Toister E, Cherry L, Lupu E, Monash A, Levy R, Dor E, et al. Highly Efficient Purification of Recombinant VSV-∆G-Spike Vaccine against SARS-CoV-2 by Flow-Through Chromatography. BioTech. 2021; 10(4):22. https://doi.org/10.3390/biotech10040022
Chicago/Turabian StyleLerer, Elad, Ziv Oren, Yaron Kafri, Yaakov Adar, Einat Toister, Lilach Cherry, Edith Lupu, Arik Monash, Rona Levy, Eyal Dor, and et al. 2021. "Highly Efficient Purification of Recombinant VSV-∆G-Spike Vaccine against SARS-CoV-2 by Flow-Through Chromatography" BioTech 10, no. 4: 22. https://doi.org/10.3390/biotech10040022
APA StyleLerer, E., Oren, Z., Kafri, Y., Adar, Y., Toister, E., Cherry, L., Lupu, E., Monash, A., Levy, R., Dor, E., Epstein, E., Levin, L., Girshengorn, M., Natan, N., Zichel, R., & Makovitzki, A. (2021). Highly Efficient Purification of Recombinant VSV-∆G-Spike Vaccine against SARS-CoV-2 by Flow-Through Chromatography. BioTech, 10(4), 22. https://doi.org/10.3390/biotech10040022





