State of the Art and Recent Advances on Ester and Ether Derivatives of Polysaccharides from Lignocellulose: Production and Technological Applications
Abstract
1. Introduction
2. Cellulose Esters
2.1. Cellulose Acetate Propionate (CAP)
2.2. Cellulose Sulfate (CS)
2.3. Cellulose Phosphate (CP)
2.4. Cellulose Acetate (CA)
2.5. Cellulose Nitrate (CN)
2.6. Cellulose Benzoate (CB)
2.7. Cellulose Acetate Butyrate (CAB)
2.8. Cellulose Propionate (CP)
3. Cellulose Ethers
3.1. Methyl Cellulose (MC)
3.2. Ethyl Cellulose (EC)
3.3. Hydroxyethyl Cellulose (HEC)
3.4. Hydroxypropyl Cellulose (HPC)
3.5. Hydroxypropyl Methylcellulose (HPMC)
3.6. Carboxymethylcellulose (CMC)
3.7. Benzyl Cellulose (BC)
3.8. Cyanoethyl Cellulose (CEC)
4. Hemicellulose Esters
4.1. Hemicellulose Acetate (HCA)
4.2. Hemicellulose Propionate (HCP)
4.3. Hemicellulose Sulfate (HCS)
5. Hemicellulose Ethers
5.1. Carboxymethyl Hemicellulose (CMH)
5.2. Methyl Hemicellulose (MHC)
5.3. Cyanoethyl Hemicellulose (CEHC)
6. Literature Related to Cellulose Derivatives
7. Conclusions and Research Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muqeet, M.; Mahar, R.B.; Gadhi, T.A.; Ben Halima, N. Insight into Cellulose-Based-Nanomaterials—A Pursuit of Environmental Remedies. Int. J. Biol. Macromol. 2020, 163, 1480–1486. [Google Scholar] [CrossRef]
- Abik, F.; Palasingh, C.; Bhattarai, M.; Leivers, S.; Ström, A.; Westereng, B.; Mikkonen, K.S.; Nypelö, T. Potential of Wood Hemicelluloses and Their Derivates as Food Ingredients. J. Agric. Food Chem. 2023, 71, 2667–2683. [Google Scholar] [CrossRef]
- Arca, H.C.; Mosquera-Giraldo, L.I.; Bi, V.; Xu, D.; Taylor, L.S.; Edgar, K.J. Pharmaceutical Applications of Cellulose Ethers and Cellulose Ether Esters. Biomacromolecules 2018, 19, 2351–2376. [Google Scholar] [CrossRef]
- Nahak, B.K.; Preetam, S.; Sharma, D.; Shukla, S.K.; Syväjärvi, M.; Toncu, D.-C.; Tiwari, A. Advancements in Net-Zero Pertinency of Lignocellulosic Biomass for Climate Neutral Energy Production. Renew. Sustain. Energy Rev. 2022, 161, 112393. [Google Scholar] [CrossRef]
- Kumar, S.; Paritosh, K.; Pareek, N.; Chawade, A.; Vivekanand, V. De-Construction of Major Indian Cereal Crop Residues through Chemical Pretreatment for Improved Biogas Production: An Overview. Renew. Sustain. Energy Rev. 2018, 90, 160–170. [Google Scholar] [CrossRef]
- Rao, J.; Lv, Z.; Chen, G.; Peng, F. Hemicellulose: Structure, Chemical Modification, and Application. Prog. Polym. Sci. 2023, 140, 101675. [Google Scholar] [CrossRef]
- Chen, X.; Shen, M.; Yu, Q.; Chen, Y.; Xie, J. Recent Advance in Chemistry Modified Methods of Natural Polysaccharides and Their Applications. Trends Food Sci. Technol. 2024, 144, 104317. [Google Scholar] [CrossRef]
- Gautam, D.; Rana, V.; Sharma, S.; Kumar Walia, Y.; Kumar, K.; Umar, A.; Ibrahim, A.A.; Baskoutas, S. Hemicelluloses: A Review on Extraction and Modification for Various Applications. ChemistrySelect 2025, 10, e06050. [Google Scholar] [CrossRef]
- Cellulose Esters and Ethers Market Size & Outlook. Available online: https://www.grandviewresearch.com/horizon/outlook/cellulose-esters-and-ethers-market-size/global (accessed on 9 August 2025).
- Moura, H.O.M.A.; Campos, L.M.A.; da Silva, V.L.; de Andrade, J.C.F.; de Assumpção, S.M.N.; Pontes, L.A.M.; de Carvalho, L.S. Investigating Acid/Peroxide-Alkali Pretreatment of Sugarcane Bagasse to Isolate High Accessibility Cellulose Applied in Acetylation Reactions. Cellulose 2018, 25, 5669–5685. [Google Scholar] [CrossRef]
- de Souza, E.C.; Moura, H.O.M.A.; Pereira, A.V.S.; Costa, J.L.B.; Rodríguez-Castellón, E.; Ballesteros-Plata, D.; de Carvalho, L.S. One-Pot Production of Carboxymethyl Holocelluloses from Mango and Pineapple Wastes Optimized via Design of Experiments. Carbohydr. Polym. Technol. Appl. 2025, 10, 100822. [Google Scholar] [CrossRef]
- Abo, B.O.; Gao, M.; Wang, Y.; Wu, C.; Ma, H.; Wang, Q. Lignocellulosic Biomass for Bioethanol: An Overview on Pretreatment, Hydrolysis and Fermentation Processes. Rev. Environ. Health 2019, 34, 57–68. [Google Scholar] [CrossRef]
- Moura, H.O.M.A.; de Souza, E.C.; da Silva, B.R.; Pereira, E.S.; Bicudo, T.d.C.; Rodríguez-Castellón, E.; de Carvalho, L.S. Optimization of Synthesis Method for Carboxymethylcellulose (CMC) from Agro-Food Wastes by Response Surface Methodology (RSM) Using D-Optimal Algorithm. Ind. Crops Prod. 2024, 220, 119413. [Google Scholar] [CrossRef]
- Islam, M.; Sinha, A.S.K.; Prasad, K. Organosolv Delignification of Rice Straw Cellulose Fiber for Functional Food Packaging. Cellulose 2024, 31, 9191–9214. [Google Scholar] [CrossRef]
- Alizadeh, H.-R.; Kansedo, J.; Tan, I.S.; Tan, Y.H.; Suali, E.; Dini, A. Recent Advances on Two-Step and Combined Multi-Step Pretreatment of Lignocellulosic Biomass for Cellulose Extraction. Bioresour. Technol. Rep. 2025, 31, 102243. [Google Scholar] [CrossRef]
- Arce, C.; Kratky, L. Mechanical Pretreatment of Lignocellulosic Biomass toward Enzymatic/Fermentative Valorization. iScience 2022, 25, 104610. [Google Scholar] [CrossRef] [PubMed]
- Banu Jamaldheen, S.; Kurade, M.B.; Basak, B.; Yoo, C.G.; Oh, K.K.; Jeon, B.-H.; Kim, T.H. A Review on Physico-Chemical Delignification as a Pretreatment of Lignocellulosic Biomass for Enhanced Bioconversion. Bioresour. Technol. 2022, 346, 126591. [Google Scholar] [CrossRef]
- Lee, K.M.; Quek, J.D.; Tey, W.Y.; Lim, S.; Kang, H.-S.; Quen, L.K.; Mahmood, W.A.W.; Jamaludin, S.I.S.; Teng, K.H.; Khoo, K.S. Biomass Valorization by Integrating Ultrasonication and Deep Eutectic Solvents: Delignification, Cellulose Digestibility and Solvent Reuse. Biochem. Eng. J. 2022, 187, 108587. [Google Scholar] [CrossRef]
- Al Kamzari, S.M.A.; Nageswara Rao, L.; Lakavat, M.; Gandi, S.; Reddy, P.S.; Kavitha Sri, G. Extraction and Characterization of Cellulose from Agricultural Waste Materials. Mater. Today Proc. 2023, 80, 2740–2743. [Google Scholar] [CrossRef]
- Das, P.; Baruah, J.; Kalita, E. Recent Developments in the Enzymatic and Biocatalytic Pretreatment of Microalgae for Efficient Biofuel Production. In Micro-Algae: Next-Generation Feedstock for Biorefineries; Springer: Singapore, 2022; pp. 193–210. [Google Scholar]
- Kumar, R.; Kim, T.H.; Basak, B.; Patil, S.M.; Kim, H.H.; Ahn, Y.; Yadav, K.K.; Cabral-Pinto, M.M.S.; Jeon, B.-H. Emerging Approaches in Lignocellulosic Biomass Pretreatment and Anaerobic Bioprocesses for Sustainable Biofuels Production. J. Clean. Prod. 2022, 333, 130180. [Google Scholar] [CrossRef]
- Kant Bhatia, S.; Ahuja, V.; Chandel, N.; Gurav, R.; Kant Bhatia, R.; Govarthanan, M.; Kumar Tyagi, V.; Kumar, V.; Pugazendhi, A.; Rajesh Banu, J.; et al. Advances in Algal Biomass Pretreatment and Its Valorisation into Biochemical and Bioenergy by the Microbial Processes. Bioresour. Technol. 2022, 358, 127437. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, J.; Ren, X.; Lau, A.; Rezaei, H.; Takada, M.; Bi, X.; Sokhansanj, S. Steam Explosion of Lignocellulosic Biomass for Multiple Advanced Bioenergy Processes: A Review. Renew. Sustain. Energy Rev. 2022, 154, 111871. [Google Scholar] [CrossRef]
- Zhang, J.; Qi, Y.; Shen, Y.; Li, H. Research Progress on Chemical Modification and Application of Cellulose: A Review. Mater. Sci. 2022, 28, 60–67. [Google Scholar] [CrossRef]
- Lu, Y.; He, Q.; Fan, G.; Cheng, Q.; Song, G. Extraction and Modification of Hemicellulose from Lignocellulosic Biomass: A Review. Green Process. Synth. 2021, 10, 779–804. [Google Scholar] [CrossRef]
- Najjoum, N.; Grimi, N.; Benali, M.; Chadni, M.; Castignolles, P. Extraction and Chemical Features of Wood Hemicelluloses: A Review. Int. J. Biol. Macromol. 2025, 311, 143681. [Google Scholar] [CrossRef]
- Gao, C.; Li, X.; Wei, G.; Wang, S.; Zhao, X.; Kong, F. Cellulose Acetate Propionate Incorporated PVDF-HFP Based Polymer Electrolyte Membrane for Lithium Batteries. Compos. Commun. 2022, 33, 101226. [Google Scholar] [CrossRef]
- Gao, C.; Li, X.; Wei, G.; Wang, S.; Zhao, X.; Kong, F. In-Situ Construction of Cellulose Acetate Propionate-Based Hybrid Gel Polymer Electrolyte for High-Performance Lithium Metal Batteries. Ind. Crops Prod. 2023, 204, 117395. [Google Scholar] [CrossRef]
- Martín-Alfonso, M.A.; Rubio-Valle, J.F.; Hinestroza, J.P.; Martín-Alfonso, J.E.; Franco, J.M. Environmentally Friendly Tailor-Made Oleo-Dispersions of Electrospun Cellulose Acetate Propionate Nanostructures in Castor Oil for Lubricant Applications. Nano Mater. Sci. 2025, 7, 90–104. [Google Scholar] [CrossRef]
- Liu, X.; Xie, J.; Jacquet, N.; Blecker, C. Valorization of Grain and Oil By-Products with Special Focus on Hemicellulose Modification. Polymers 2024, 16, 1750. [Google Scholar] [CrossRef]
- Ebringerová, A. Structural Diversity and Application Potential of Hemicelluloses. Macromol. Symp. 2005, 232, 1–12. [Google Scholar] [CrossRef]
- Heinze, T.; Koschella, A. Solvents Applied in the Field of Cellulose Chemistry: A Mini Review. Polímeros 2005, 15, 84–90. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, K.; Yang, Y.; Kim, M.-S.; Lee, C.-H.; Zhang, R.; Xu, T.; Choi, S.-E.; Si, C. Hemicellulose-Based Hydrogels for Advanced Applications. Front. Bioeng. Biotechnol. 2023, 10, 1110004. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, H.; Yang, B.; Weng, Y. Hemicellulose-Based Film: Potential Green Films for Food Packaging. Polymers 2020, 12, 1775. [Google Scholar] [CrossRef]
- Nechita, P.; Roman, M.; Năstac, S.M. Green Approaches on Modification of Xylan Hemicellulose to Enhance the Functional Properties for Food Packaging Materials—A Review. Polymers 2023, 15, 2088. [Google Scholar] [CrossRef]
- Keldibekova, R.; Suleimenova, S.; Nurgozhina, G.; Kopishev, E. Interpolymer Complexes Based on Cellulose Ethers: Application. Polymers 2023, 15, 3326. [Google Scholar] [CrossRef]
- Wang, C.; Tallian, C.; Su, J.; Vielnascher, R.; Silva, C.; Cavaco-Paulo, A.; Guebitz, G.M.; Fu, J. Ultrasound-Assisted Extraction of Hemicellulose and Phenolic Compounds from Bamboo Bast Fiber Powder. PLoS ONE 2018, 13, e0197537. [Google Scholar] [CrossRef]
- Rhein, F.; Sehn, T.; Meier, M.A.R. Efficient and Accurate Determination of the Degree of Substitution of Cellulose Acetate Using ATR-FTIR Spectroscopy and Machine Learning. Sci. Rep. 2025, 15, 2904. [Google Scholar] [CrossRef]
- Heinze, T.; Liebert, T.F.; Pfeiffer, K.S.; Hussain, M.A. Unconventional Cellulose Esters: Synthesis, Characterization and Structure-Property Relations. Cellulose 2003, 10, 283–296. [Google Scholar] [CrossRef]
- von Schantz, L.; Schagerlöf, H.; Karlsson, E.N.; Ohlin, M. Characterization of the Substitution Pattern of Cellulose Derivatives Using Carbohydrate-Binding Modules. BMC Biotechnol. 2014, 14, 113. [Google Scholar] [CrossRef]
- Yokota, S.; Nishimoto, A.; Kondo, T. Alkali-Activation of Cellulose Nanofibrils to Facilitate Surface Chemical Modification under Aqueous Conditions. J. Wood Sci. 2022, 68, 14. [Google Scholar] [CrossRef]
- Azrak, S.M.E.A.; Gohl, J.A.; Moon, R.J.; Schueneman, G.T.; Davis, C.S.; Youngblood, J.P. Controlled Dispersion and Setting of Cellulose Nanofibril—Carboxymethyl Cellulose Pastes. Cellulose 2021, 28, 9149–9168. [Google Scholar] [CrossRef]
- Verdía Barbará, P.; Choudhary, H.; Nakasu, P.S.; Al-Ghatta, A.; Han, Y.; Hopson, C.; Aravena, R.I.; Mishra, D.K.; Ovejero-Pérez, A.; Simmons, B.A.; et al. Recent Advances in the Use of Ionic Liquids and Deep Eutectic Solvents for Lignocellulosic Biorefineries and Biobased Chemical and Material Production. Chem. Rev. 2025, 125, 5461–5583. [Google Scholar] [CrossRef]
- Yu, J.; Liu, X.; Xu, S.; Shao, P.; Li, J.; Chen, Z.; Wang, X.; Lin, Y.; Renard, C.M.G.C. Advances in Green Solvents for Production of Polysaccharide-based Packaging Films: Insights of Ionic Liquids and Deep Eutectic Solvents. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1030–1057. [Google Scholar] [CrossRef]
- Szabó, L.; Milotskyi, R.; Sharma, G.; Takahashi, K. Cellulose Processing in Ionic Liquids from a Materials Science Perspective: Turning a Versatile Biopolymer into the Cornerstone of Our Sustainable Future. Green. Chem. 2023, 25, 5338–5389. [Google Scholar] [CrossRef]
- Ajayi, S.M.; Olusanya, S.O.; Abimbade, S.F.; Didunyemi, A.E.; Atunde, M.O.; Fapojuwo, D.P.; Olumayede, E.G.; Lawal, O.S.; Akintayo, C.O.; Malomo, D. Preparation and Characterization of Acetate Cellulose Laurate Ester in Sodium Acetate/Zinc Chloride Systems. Biomater. Connect 2024, 1, 1. [Google Scholar] [CrossRef]
- Kostag, M.; Jedvert, K.; Achtel, C.; Heinze, T.; El Seoud, O.A. Recent Advances in Solvents for the Dissolution, Shaping and Derivatization of Cellulose: Quaternary Ammonium Electrolytes and Their Solutions in Water and Molecular Solvents. Molecules 2018, 23, 511. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Lin, Y.; Huang, Y.; Fang, Y.; Xiong, X. Research Progress of the Preparation of Cellulose Ethers and Their Applications: A Short Review. Molecules 2025, 30, 1610. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Shuai, J.; Wang, Y.; Zhou, Y.; Wang, X. Progress on Chemical Modification of Cellulose in “Green” Solvents. Polym. Chem. 2022, 13, 359–372. [Google Scholar] [CrossRef]
- Cellulose Acetate Propionate Market Size, Market Outlook & Forecast. Available online: https://www.verifiedmarketreports.com/product/cellulose-acetate-propionate-market/ (accessed on 21 August 2025).
- Wen, Y.; Zhang, H.; Li, J.; An, S.; Chen, W.; Song, Y.F. Core-Shell Assembly of Heteropolyacids and Polymer: Efficient Preparation of Cellulose Acetate Propionate and Its Processed Products. ACS Sustain. Chem. Eng. 2021, 9, 5179–5186. [Google Scholar] [CrossRef]
- Cellulose Acetate Propionate, Eastman—ChemPoint. Available online: https://www.chempoint.com/products/eastman/eastman-cellulose-esters/cellulose-acetate-propionate (accessed on 10 August 2025).
- Géis Superabsorventes de Propionato Acetato de Celulose e Acetato de Celulose: Síntese, Caracterização e Liberação Controlada de Pesticida. Available online: https://repositorio.ufscar.br/items/a9ff097b-8b2b-4abd-b9b9-a2f956f2cd27 (accessed on 10 August 2025).
- Hussain, S.M.S.; Adewunmi, A.A.; Alade, O.S.; Murtaza, M.; Mahboob, A.; Khan, H.J.; Mahmoud, M.; Kamal, M.S. A Review of Ionic Liquids: Recent Synthetic Advances and Oilfield Applications. J. Taiwan Inst. Chem. Eng. 2023, 153, 105195. [Google Scholar] [CrossRef]
- Gao, G.; Cai, L.; Fan, Y.; Aroche Ginarte, R.; Li, Y.; Sun, W.; Jiang, X.; Li, X.; Pi, Y. Effects of Different Hemicellulose Components on Fermentation Kinetics and Microbial Composition in Fecal Inoculum from Suckling Piglets In Vitro. ACS Omega 2025, 15, 45. [Google Scholar] [CrossRef]
- Morozova, O.V.; Vasil’eva, I.S.; Shumakovich, G.P.; Zaitseva, E.A.; Yaropolov, A.I. Deep Eutectic Solvents for Biotechnology Applications. Biochemistry 2023, 88, S150–S175. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, J.; Zhang, Z.; Zhu, S.; Li, J.; Zhang, W.; Zhang, F.; Chen, K. Controlled Preparation of Cellulose Acetate by Deep Eutectic Solvent Homogeneous Catalysis. Carbohydr. Polym. 2025, 349, 122964. [Google Scholar] [CrossRef]
- Tian, C.; Duan, C.; Bie, Y.; Liu, X.; Zhou, B.; Ma, R.; Fan, Q.; Xie, Z.; Ni, Y. A Deep Eutectic Solvent with Bifunctional Acid Sites Treatment to Upgrade a Bamboo Kraft Pulp into a Cellulose-Acetate Grade Dissolving Pulp. Carbohydr. Polym. 2025, 348, 122942. [Google Scholar] [CrossRef]
- Yahaya, S.H.; Muhammad, C.; Zauro, S.A.; Magami, I.M. An In-Depth Review of Sustainable and Environmentally Friendly Pretreatment Techniques for Cellulose Extraction from Lignocellulosic Biomass and Their Uses. Am. J. Appl. Ind. Chem. 2025, 9, 13–33. [Google Scholar] [CrossRef]
- Gao, C.; Li, X.; Song, C.; Wei, G.; Zhao, X.; Wang, S.; Kong, F. Electrospun Polyimide/Cellulose Acetate Propionate Nanofiber Membrane-Based Gel Polymer Electrolyte with Fast Lithium-Ion Transport and High Interface Stability for Lithium Metal Batteries. Cellulose 2023, 30, 9113–9126. [Google Scholar] [CrossRef]
- Kaur, M.; Pal, J. Evaluation of Efficiency of Wheat Straw Nanocellulose as Nanoadsorbent for the Removal of Divalent Copper, Lead and Zinc from Aqueous Solution. Carbohydr. Polym. Technol. Appl. 2023, 6, 100350. [Google Scholar] [CrossRef]
- Zhang, T.; Yuan, Y.; Wu, X.; Yu, P.; Ji, J.; Chai, J.; Kumar Saini, R.; Liu, J.; Shang, X. The Level of Sulfate Substitution of Polysaccharide Regulates Thermal-Induced Egg White Protein Gel Properties: The Characterization of Gel Structure and Intermolecular Forces. Food Res. Int. 2023, 173, 113349. [Google Scholar] [CrossRef]
- Montenegro, R.; Rincón, E.; Rodríguez, A.; González, Z. Manufacturing Sulfated Cellulose Nanofibers Using a Unique Combined DES-Based Pretreatment-Functionalization Protocol for Metal Ion Decontamination through Porous Adsorbents. Carbohydr. Polym. 2025, 349, 122974. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Fang, H.; Yang, H.; Zou, F.; Li, G.; Wang, L.; Liao, H.; Guan, W.; Hu, X. Cellulose Sulfate Lithium as a Conductive Binder for LiFePO4 Cathode with Long Cycle Life. Carbohydr. Polym. 2023, 313, 120848. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.I.; Borges, W.; Sharma, P.R.; Sharma, S.K.; Chang, H.Y.; Abou-Krisha, M.M.; Alhamzani, A.G.; Hsiao, B.S. Cellulose Sulfate Nanofibers for Enhanced Ammonium Removal. Nanomaterials 2024, 14, 507. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Tae, G. Cellular Infiltration in an Injectable Sulfated Cellulose Nanocrystal Hydrogel and Efficient Angiogenesis by VEGF Loading. Biomater. Res. 2023, 27, 28. [Google Scholar] [CrossRef]
- Normakhamatov, N.; Mischnick, P.; Muhitdinov, B.; Mukhamedov, I.; Turaev, A. Sodium Cellulose Sulfate and Its Antimicrobial Activity. React. Funct. Polym. 2023, 191, 105672. [Google Scholar] [CrossRef]
- Long, Y.; Dimde, M.; Adler, J.M.; Vidal, R.M.; Povolotsky, T.L.; Nickl, P.; Achazi, K.; Trimpert, J.; Kaufer, B.B.; Haag, R.; et al. Sulfated Cellulose Nanofiber Hydrogel with Mucus-Like Activities for Virus Inhibition. ACS Appl. Mater. Interfaces 2024, 16, 67504–67513. [Google Scholar] [CrossRef]
- Silva, F.A.G.S.; Branco, S.; Dourado, F.; Neto, B.; Gama, M. Life Cycle Assessment of Bacterial Cellulose and Comparison to Other Cellulosic Sources. J. Clean. Prod. 2025, 493, 144876. [Google Scholar] [CrossRef]
- Wang, X.; Feng, X.; Chen, G.; Lin, B.; Qi, H. Influence of Sulfation Pretreatment on the Structure and Properties of Cellulose Nanofibrils. Ind. Crops Prod. 2022, 187, 115391. [Google Scholar] [CrossRef]
- Tang, C.; Zhao, Z.; Yang, M.; Lu, X.; Fu, L.; Jiang, G. Preparation and Characterization of Sodium Cellulose Sulfate/Chitosan Composite Films Loaded with Curcumin for Monitoring Pork Freshness. Curr. Res. Food Sci. 2022, 5, 1475–1483. [Google Scholar] [CrossRef]
- Almeida, R.; Ramos, A.; Håkonsen, V.; Maloney, T.; Gamelas, J. Functionalized Cellulose Nanofiber Films as Potential Substitutes for Japanese Paper. Carbohydr. Polym. Technol. Appl. 2024, 8, 100573. [Google Scholar] [CrossRef]
- Fernandes, M.; Alves, C.; Melro, L.; Fernandes, R.D.V.; Padrão, J.; Nio, A.; Salgado, J.; Zille, A. Modification of Nanocellulose. In Handbook of Biomass; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Jokar, M.; Bidhendi, G.N.; Naeimi, H. Catalytic Chemical Reduction of Cr(VI) from Contaminated Waters by the Production of Hydrogen Radical on the Cellulose Sulfate Microfibers Coated with Palladium Nanocatalyst. Desalination Water Treat 2022, 248, 124–133. [Google Scholar] [CrossRef]
- Li, W.; Wang, Z.; Li, M.; Normakhamatov, N. Cellulose Sulfate/EMIMAc Solution: Rheological Properties and Shaping into Polyelectrolyte Complexes for Protein Adsorption. Cellulose 2021, 28, 2849–2861. [Google Scholar] [CrossRef]
- Romanchenko, A.S.; Levdansky, A.V.; Levdansky, V.A.; Kuznetsov, B.N. Study of Cellulose Sulfates by X-Ray Photoelectron Spectroscopy. Russ. J. Bioorg Chem. 2015, 41, 719–724. [Google Scholar] [CrossRef]
- Wu, Q.X.; Guan, Y.X.; Yao, S.J. Sodium Cellulose Sulfate: A Promising Biomaterial Used for Microcarriers’ Designing. Front. Chem. Sci. Eng. 2019, 13, 46–58. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, Z.; Chen, J.; Yang, G.; He, M. Facile Sulfation of Cellulose via Recyclable Ternary Deep Eutectic Solvents for Low-Cost Cellulose Nanofibril Preparation. Nanoscale Adv. 2023, 5, 356–360. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Pan, Y.; Dong, C.; Huang, X.; Zhang, G.; Zhang, K. Sulfated Cellulose Nanocrystal Isolated from Waste Cotton Fabrics by Deep Eutectic Solvent and Its Application in Polymer Nanocomposite Films. J. Vinyl Addit. Technol. 2025, 31, 47–58. [Google Scholar] [CrossRef]
- Live Cell Encapsulation Market Report|Global Forecast from 2025 to 2033. Available online: https://dataintelo.com/report/global-live-cell-encapsulation-market (accessed on 21 August 2025).
- Tang, C.; Zhao, B.; Zhu, J.; Lu, X.; Jiang, G. Preparation and Characterization of Chitosan/Sodium Cellulose Sulfate/Silver Nanoparticles Composite Films for Wound Dressing. Mater. Today Commun. 2022, 33, 104192. [Google Scholar] [CrossRef]
- Li, X.; Ding, W.; Wang, S.; Yang, L.; Yu, Q.; Xiao, C.; Chen, G.; Zhang, L.; Guan, S.; Sun, D. Three-Dimensional Sulfated Bacterial Cellulose/Gelatin Composite Scaffolds for Culturing Hepatocytes. Cyborg Bionic Syst. 2023, 4, 21. [Google Scholar] [CrossRef]
- Li, Z.; Yan, M.; Su, X.; Lei, W.; Han, J.; Huang, Q.; Liao, H.; Hu, X. A Multifunctional Polyelectrolyte Binder to Improve Fast Charging Capability of Graphite Anode in Lithium-Ion Batteries. J. Mater. Sci. Mater. Electron. 2025, 36, 1024. [Google Scholar] [CrossRef]
- Yan, W.; Xian, J.; Huang, S.; Leng, Y.; Liu, Q.; Xiao, T.; Zhao, Y.; Yang, P.; Wu, Y. Scalable and Sustainable Sulfonated Cellulose Separators toward Practical Ah-Level Aqueous Batteries. Energy Storage Mater 2025, 76, 104150. [Google Scholar] [CrossRef]
- Schimper, C.B.; Pachschwöll, P.; Maitz, M.F.; Werner, C.; Rosenau, T.; Liebner, F. Hemocompatibility of Cellulose Phosphate Aerogel Membranes with Potential Use in Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2023, 11, 1152577. [Google Scholar] [CrossRef] [PubMed]
- Suneetha, M.; Kim, H.; Han, S.S. Bone-like Apatite Formation in Biocompatible Phosphate-Crosslinked Bacterial Cellulose-Based Hydrogels for Bone Tissue Engineering Applications. Int. J. Biol. Macromol. 2024, 256, 128364. [Google Scholar] [CrossRef]
- Jeong, D.I.; Kim, S.; Kim, M.-H.; Yoon, I.-S.; Lee, S.-H.; Kim, D.-D.; Cho, H.-J. Donepezil Hydrochloride-Reinforced Cellulose Nanocrystal-Aggregated Gel Structure for Long-Acting Drug Delivery. Carbohydr. Polym. 2022, 296, 119887. [Google Scholar] [CrossRef]
- Garg, T.; Arora, S.; Pahwa, R. Cellulose and Its Derivatives: Structure, Modification, and Application in Controlled Drug Delivery. Future J. Pharm. Sci. 2025, 11, 76. [Google Scholar] [CrossRef]
- Gong, J.; Hou, L.; Ching, Y.C.; Ching, K.Y.; Hai, N.D.; Chuah, C.H. A Review of Recent Advances of Cellulose-Based Intelligent-Responsive Hydrogels as Vehicles for Controllable Drug Delivery System. Int. J. Biol. Macromol. 2024, 264, 130525. [Google Scholar] [CrossRef]
- Hu, T.; Fang, J.; Shen, Y.; Li, M.; Wang, B.; Xu, Z.; Hu, W. Advances of Naturally Derived Biomedical Polymers in Tissue Engineering. Front. Chem. 2024, 12, 1469183. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Cellulose-Based Composites as Scaffolds for Tissue Engineering: Recent Advances. Molecules 2022, 27, 8830. [Google Scholar] [CrossRef]
- Li, Z.; Qian, P.; Li, H.; Xiao, H.; Chen, J.; Li, G. Phosphorylated Cellulose Nanofibers Establishing Reliable Ion-Sieving Barriers for Durable Lithium-Sulfur Batteries. J. Energy Chem. 2024, 92, 619–628. [Google Scholar] [CrossRef]
- Aziam, H.; Ouarga, A.; Ettalibi, O.; Shanmukaraj, D.; Noukrati, H.; Sehaqui, H.; Saadoune, I.; Barroug, A.; Ben Youcef, H. Phosphorylated Cellulose Nanofiber as Sustainable Organic Filler and Potential Flame-Retardant for All-Solid-State Lithium Batteries. J. Energy Storage 2023, 62, 106838. [Google Scholar] [CrossRef]
- Rohaizu, R.; Wanrosli, W.D. Production of Cellulose Phosphate from Oil Palm Empty Fruit Bunch: Effect of Chemical Ratio. J. Phys. Conf. Ser. 2015, 622, 012021. [Google Scholar] [CrossRef]
- Abdulhameed, A.; Mbuvi, H.; Changamu, E.; Githinji, I.; Maingi, F. Synthesis and Characterization of Cellulose Phosphate-Based Superabsorbent Hydrogels from Rice Husk under Microwave Heating. Next Mater. 2025, 6, 100400. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, L.; Liu, Y.; Dong, C.; Zhang, K. Production of Flame-Retardant Phosphorylated Cellulose Nanofibrils by Choline Chloride Based Reactive Deep Eutectic Solvent. Carbohydr. Polym. 2025, 348, 122931. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, J.; Han, S.; Wu, G.; Wu, Q.; Wang, J.; Fu, J.; Shen, S.; Li, Q. One-Step Green Synthesis Durable Flame-Retardant, Antibacterial and Dyeable Cellulose Fabrics with a Recyclable Deep Eutectic Solvent. Int. J. Biol. Macromol. 2025, 299, 140201. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, L.; Cui, M.; Qi, W.; Lam, H.L.; Huang, R.; Su, R. Integrating Solvent-Free Mechanochemistry and Heat Curing for the Green Production of Highly Charged and Highly Crystalline Phosphorylated Cellulose Nanocrystals. Chem. Eng. J. 2025, 511, 162260. [Google Scholar] [CrossRef]
- Functionalized Cellulose Materials; Springer: Berlin/Heidelberg, Germany, 2025. [CrossRef]
- Catalyzing Commercialization: Converting CO2 into Cellulose with Cell-Free Biocatalysis|AIChE. Available online: https://www.aiche.org/resources/publications/cep/2025/august/catalyzing-commercialization-converting-co2-cellulose-cell-free-biocatalysis (accessed on 9 August 2025).
- Zhao, S.; Li, J.; Wu, L.; Hua, M.; Jiang, C.; Pan, Y.; Yao, L.; Xu, S.; Ge, J.; Pan, G. Synthesis and Characterization of Cellulose Diacetate-Graft-Polylactide via Solvent-Free Melt Ring-Opening Graft Copolymerization. Polymers 2022, 15, 143. [Google Scholar] [CrossRef]
- Raza, A.; Farrukh, S.; Hussain, A.; Khan, I.; Othman, M.H.D.; Ahsan, M. Performance Analysis of Blended Membranes of Cellulose Acetate with Variable Degree of Acetylation for CO2/CH4 Separation. Membranes 2021, 11, 245. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Recent Advances in Composites Based on Cellulose Derivatives for Biomedical Applications. Carbohydr. Polym. 2020, 247, 116683. [Google Scholar] [CrossRef] [PubMed]
- Wsoo, M.A.; Shahir, S.; Mohd Bohari, S.P.; Nayan, N.H.M.; Razak, S.I.A. A Review on the Properties of Electrospun Cellulose Acetate and Its Application in Drug Delivery Systems: A New Perspective. Carbohydr. Res. 2020, 491, 107978. [Google Scholar] [CrossRef] [PubMed]
- Ashter, S.A. Chemistry of Cellulosic Polymers. In Technology and Applications of Polymers Derived from Biomass; Elsevier: Amsterdam, The Netherlands, 2018; pp. 57–74. [Google Scholar]
- Bakri, M.K.B.; Rahman, M.R. Extraction, Types, and Classification of Cellulose. In Fundamentals and Recent Advances in Nanocomposites Based on Polymers and Nanocellulose; Elsevier: Amsterdam, The Netherlands, 2022; pp. 19–40. [Google Scholar]
- Cellulose Diacetate Market Size, Consumer Behavior Insights & Forecast. Available online: https://www.verifiedmarketreports.com/product/cellulose-diacetate-market/ (accessed on 22 July 2025).
- Cellulose Triacetate Market Size, Evaluation, Outlook & Forecast. Available online: https://www.verifiedmarketreports.com/product/cellulose-triacetate-market/ (accessed on 22 July 2025).
- Das, A.M.; Ali, A.A.; Hazarika, M.P. Synthesis and Characterization of Cellulose Acetate from Rice Husk: Eco-Friendly Condition. Carbohydr. Polym. 2014, 112, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, H.M.; Anis, A.; Poulose, A.M.; Al-Zahrani, S.M.; Madhar, N.A.; Alhamidi, A.; Aldeligan, S.H.; Alsubaie, F.S. Synthesis and Characterization of Cellulose Triacetate Obtained from Date Palm (Phoenix dactylifera L.) Trunk Mesh-Derived Cellulose. Molecules 2022, 27, 1434. [Google Scholar] [CrossRef]
- Bamba, M.; Assanvo, E.F.; Kouassi, E.K.A.; Soro, D.; Ouattara, L.Y.; Yao, K.B.; Drogui, A.P.; Tyagi, D.R. Preparation and Characterization of Cellulose Triacetate from Cocoa Pod Husk. Bioresources 2023, 18, 1684–1698. [Google Scholar] [CrossRef]
- Esmaeilzadeh, M.; Kaschuk, J.; Nguyen, H.M.; Palo, E.; Al Haj, Y.; Vapaavuori, J.; Miettunen, K. Nano-Imprinted Cellulose Acetate Structures for Light Management of Dye-Sensitized Solar Cells. Front. Mater. Sci. 2025, 19, 250725. [Google Scholar] [CrossRef]
- de Freitas, R.R.M.; Senna, A.M.; Botaro, V.R. Influence of Degree of Substitution on Thermal Dynamic Mechanical and Physicochemical Properties of Cellulose Acetate. Ind. Crops Prod. 2017, 109, 452–458. [Google Scholar] [CrossRef]
- Stiriba, S.-E. Recent Advances in Cellulose Chemistry. Int. J. Mol. Sci. 2024, 25, 8977. [Google Scholar] [CrossRef]
- Wu, C.-S. Mechanical Properties, Biocompatibility, and Biodegradation of Cross-Linked Cellulose Acetate-Reinforced Polyester Composites. Carbohydr. Polym. 2014, 105, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mandal, T.; Goswami, S. Fabrication of Cellulose Acetate Nanocomposite Films with Lignocelluosic Nanofiber Filler for Superior Effect on Thermal, Mechanical and Optical Properties. Nano-Struct. Nano-Objects 2021, 25, 100642. [Google Scholar] [CrossRef]
- Bashir, Z.; Lock, S.S.M.; e Hira, N.; Ilyas, S.U.; Lim, L.G.; Lock, I.S.M.; Yiin, C.L.; Darban, M.A. A Review on Recent Advances of Cellulose Acetate Membranes for Gas Separation. RSC Adv. 2024, 14, 19560–19580. [Google Scholar] [CrossRef]
- Sakellariou, P.; Rowe, R.C.; White, E.F.T. The Solubility Parameters of Some Cellulose Derivatives and Polyethylene Glycols Used in Tablet Film Coating. Int. J. Pharm. 1986, 31, 175–177. [Google Scholar] [CrossRef]
- Bastida, G.A.; Aguado, R.J.; Galván, M.V.; Zanuttini, M.Á.; Delgado-Aguilar, M.; Tarrés, Q. Impact of Cellulose Nanofibers on Cellulose Acetate Membrane Performance. Cellulose 2024, 31, 2221–2238. [Google Scholar] [CrossRef]
- Wang, J.; Abbas, S.C.; Li, L.; Walker, C.C.; Ni, Y.; Cai, Z. Cellulose Membranes: Synthesis and Applications for Water and Gas Separation and Purification. Membranes 2024, 14, 148. [Google Scholar] [CrossRef]
- Rodríguez-Liébana, J.A.; Robles-Solano, E.; Jurado-Contreras, S.; Morillas-Gutiérrez, F.; Moya, A.J.; Mateo, S.; Navas-Martos, F.J.; La Rubia, M.D. Production and Characterization of Cellulose Acetate Using Olive Tree Pruning Biomass as Feedstock. Biofuels Bioprod. Biorefining 2024, 18, 865–882. [Google Scholar] [CrossRef]
- Anwar, M.; Suwanto, A.; Wahono, S.K.; Prasetyo, D.J.; Sugiharto, S.P.; Maryana, R.; Setiyoko, A. Synthesis and Characterization of Cellulose Acetate from Fiber Waste of Sugar Palm Stem (Arenga pinnata sp.). AIP Conf. Proc. 2024, 2957, 060024. [Google Scholar] [CrossRef]
- Jia, X.; Guo, D.; Yan, Q.; Yu, H.; Lyu, Q.; Han, L.; Zhou, C.; Xiao, W. Synthesis and Characterization of Corn Stover-Based Cellulose Triacetate Catalyzed by Ionic Liquid Phosphotungstate. Int. J. Mol. Sci. 2022, 23, 6783. [Google Scholar] [CrossRef] [PubMed]
- Sezali, N.A.A.; Ong, H.L.; Mohd Pisal, M.H.; Jullok, N.; Manzano, M.C.; Villagracia, A.R.; Doong, R.A. Preparation and Characterization of Cellulose Acetate from Rice Straw. In Green Energy and Technology; Springer: Singapore, 2023; pp. 429–436. [Google Scholar] [CrossRef]
- Bae, S.H.; Lee, G.; You, J.B.; Yoo, Y. Fabrication of Cellulose Acetate Membrane Using Deep Eutectic Solvent for Water/Isopropyl Alcohol Pervaporation. Sep. Purif. Technol. 2025, 364, 132406. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Ain, Q.U.; Tong, Z. Efficient Synthesis of Cellulose Acetate through One-Step Homogeneous Acetylation of Cotton Cellulose in Binary Ionic Liquids. Int. J. Biol. Macromol. 2024, 281, 136306. [Google Scholar] [CrossRef]
- Mofokeng, L.E.; Hlekelele, L.; Moma, J.; Tetana, Z.N.; Chauke, V.P. Energy-Efficient CuO/TiO2@GCN Cellulose Acetate-Based Membrane for Concurrent Filtration and Photodegradation of Ketoprofen in Drinking and Groundwater. Appl. Sci. 2022, 12, 1649. [Google Scholar] [CrossRef]
- An, Y.; Li, F.; Di, Y.; Zhang, X.; Lu, J.; Wang, L.; Yan, Z.; Wang, W.; Liu, M.; Fei, P. Hydrophobic Modification of Cellulose Acetate and Its Application in the Field of Water Treatment: A Review. Molecules 2024, 29, 5127. [Google Scholar] [CrossRef]
- Mottola, S.; Viscusi, G.; Tohamy, H.-A.S.; El-Sakhawy, M.; Gorrasi, G.; De Marco, I. Application of Electrospun N-Doped Carbon Dots Loaded Cellulose Acetate Membranes as Cationic Dyes Adsorbent. J. Environ. Manag. 2024, 370, 122714. [Google Scholar] [CrossRef] [PubMed]
- Ahangari, H.; Ebrahimi, A.; Ehsani, A.; Amjadi, S. Multipurpose Packaging System Based on Intelligent Carboxymethyl Cellulose Film and Activated Cellulose Acetate Electrospun Nanofibers for Seafoods. Int. J. Biol. Macromol. 2025, 298, 140115. [Google Scholar] [CrossRef]
- Marques, C.S.; Silva, R.R.A.; Arruda, T.R.; Ferreira, A.L.V.; de Oliveira, T.V.; Moraes, A.R.F.; Dias, M.V.; Vanetti, M.C.D.; Soares, N.d.F.F. Development and Investigation of Zein and Cellulose Acetate Polymer Blends Incorporated with Garlic Essential Oil and β-Cyclodextrin for Potential Food Packaging Application. Polysaccharides 2022, 3, 277–291. [Google Scholar] [CrossRef]
- Irimia, A.; Grigoraș, V.C.; Popescu, C.M. Active Cellulose-Based Food Packaging and Its Use on Foodstuff. Polymers 2024, 16, 389. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, Q.; Zhang, R.; Ma, W.; Pan, S.; Zhao, Y.; Wang, Q.; Fang, P. Piezoelectric Nanogenerator Based on Electrospinning PVDF/Cellulose Acetate Composite Membranes for Energy Harvesting. Materials 2022, 15, 7026. [Google Scholar] [CrossRef]
- Sharma, M.; Das, P.P.; Sood, T.; Chakraborty, A.; Purkait, M.K. Reduced Graphene Oxide Incorporated Polyvinylidene Fluoride/Cellulose Acetate Proton Exchange Membrane for Energy Extraction Using Microbial Fuel Cells. J. Electroanal. Chem. 2022, 907, 115890. [Google Scholar] [CrossRef]
- Luo, R.; Zhang, F.; Yang, J.H.; Zhang, N.; Wang, Y. Synchronously Enhanced Breakdown Strength and Energy Storage Ability of Cellulose Acetate Flexible Films via Introducing Ultra-Low Content of Carbonized Polymer Dots. Carbohydr. Polym. 2025, 347, 122752. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tian, X.; Li, X.; Liu, J.; Li, C.; Feng, X.; Shu, C.; Yu, Z.Z. An Environmental Energy-Enhanced Solar Steam Evaporator Derived from MXene-Decorated Cellulose Acetate Cigarette Filter with Ultrahigh Solar Steam Generation Efficiency. J. Colloid. Interface Sci. 2022, 606, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Varghese, H.; Hakkeem, H.M.A.; Chauhan, K.; Thouti, E.; Pillai, S.; Chandran, A. A High-Performance Flexible Triboelectric Nanogenerator Based on Cellulose Acetate Nanofibers and Micropatterned PDMS Films as Mechanical Energy Harvester and Self-Powered Vibrational Sensor. Nano Energy 2022, 98, 107339. [Google Scholar] [CrossRef]
- Wang, M.; Huang, C.; Chen, Y.; Ji, Y.; Yu, D.G.; Bligh, S.W.A. Medicated Tri-Layer Fibers Based on Cellulose Acetate and Polyvinylpyrrolidone for Enhanced Antibacterial and Wound Healing Properties. Carbohydr. Polym. 2025, 348, 122856. [Google Scholar] [CrossRef]
- Ndlovu, S.P.; Alven, S.; Hlalisa, K.; Aderibigbe, B.A. Cellulose Acetate-Based Wound Dressings Loaded with Bioactive Agents: Potential Scaffolds for Wound Dressing and Skin Regeneration. Curr. Drug Deliv. 2023, 21, 1226–1240. [Google Scholar] [CrossRef]
- Lan, X.; Wang, Y.; Yin, M. Enhancing Periodontal Ligament Regeneration via PDLSC Delivery Using Electrospun PCL/Collagen/Cellulose Acetate Scaffolds and Collagen Hydrogel Incorporated with Curcumin-Loaded ZIF-8 Nanoparticles. Int. J. Nanomed. 2025, 20, 887–906. [Google Scholar] [CrossRef]
- Mahalakshmi, M.; Selvanayagam, S.; Selvasekarapandian, S.; Chandra, M.V.L.; Sangeetha, P.; Manjuladevi, R. Magnesium Ion-Conducting Solid Polymer Electrolyte Based on Cellulose Acetate with Magnesium Nitrate (Mg(NO3)2·6H2O) for Electrochemical Studies. Ionics 2020, 26, 4553–4565. [Google Scholar] [CrossRef]
- Mattar, H.; Baz, Z.; Saleh, A.; Shalaby, A.S.; Elsayed Azzazy, A.; Salah, H.; Ismail, I. Nitrocellulose: Structure, Synthesis, Characterization, and Applications. Water Energy Food Environ. J. Int. J. 2020, 1, 1–15. [Google Scholar] [CrossRef]
- Abdul Rahim, K.S.; Samsuri, A.; Jamal, S.H.; Mohd Nor, S.A.; Rusly, S.N.A.; Ariff, H.; Abdul Latif, N.S. Key Attributes of Nitrocellulose-Based Energetic Materials and Recent Developments. Heliyon 2025, 11, e41282. [Google Scholar] [CrossRef]
- Morris, E.; Pulham, C.R.; Morrison, C.A. Structure and Properties of Nitrocellulose: Approaching 200 Years of Research. RSC Adv. 2023, 13, 32321–32333. [Google Scholar] [CrossRef]
- Li, L.; Frey, M. Preparation and Characterization of Cellulose Nitrate-Acetate Mixed Ester Fibers. Polymer 2010, 51, 3774–3783. [Google Scholar] [CrossRef]
- Jamal, S.H.; Roslan, N.J.; Shah, N.A.A.; Noor, S.A.M.; Ong, K.K.; Yunus, W.M.Z.W. Preparation and Characterization of Nitrocellulose from Bacterial Cellulose for Propellant Uses. Mater. Today Proc. 2020, 29, 185–189. [Google Scholar] [CrossRef]
- Sakovich, G.V.; Mikhailov, Y.M.; Budaeva, V.V.; Korchagina, A.A.; Gismatulina, Y.A.; Kozyrev, N.V. Cellulose Nitrates from Unconventional Feedstocks. Dokl. Chem. 2018, 483, 287–291. [Google Scholar] [CrossRef]
- Kashcheyeva, E.I.; Korchagina, A.A.; Gismatulina, Y.A.; Gladysheva, E.K.; Budaeva, V.V.; Sakovich, G.V. Simultaneous Production of Cellulose Nitrates and Bacterial Cellulose from Lignocellulose of Energy Crop. Polymers 2024, 16, 42. [Google Scholar] [CrossRef]
- Muvhiiwa, R.; Mawere, E.; Moyo, L.B.; Tshuma, L. Utilization of Cellulose in Tobacco (Nicotiana tobacum) Stalks for Nitrocellulose Production. Heliyon 2021, 7, e07598. [Google Scholar] [CrossRef] [PubMed]
- binti Abdul Rahim, K.S.; binti Samsuri, A.; binti Jamal, S.H.; binti Mohd Nor, S.A.; binti Rusly, S.N.A.; binti Ariff, H.; binti Abdul Latif, N.S. Redefining Biofuels: Investigating Oil Palm Biomass as a Promising Cellulose Feedstock for Nitrocellulose-Based Propellant Production. Def. Technol. 2024, 37, 111–132. [Google Scholar] [CrossRef]
- Gismatulina, Y.A.; Budaeva, V.V. Cellulose Nitrates-Blended Composites from Bacterial and Plant-Based Celluloses. Polymers 2024, 16, 1183. [Google Scholar] [CrossRef] [PubMed]
- Gismatulina, Y.A.; Budaeva, V.V.; Sakovich, G.V. Nitrocellulose Synthesis from Miscanthus Cellulose. Propellants Explos. Pyrotech. 2018, 43, 96–100. [Google Scholar] [CrossRef]
- Duan, X.; Li, Z.; Wu, B.; Shen, J.; Pei, C. Preparation of Nitrocellulose by Homogeneous Esterification of Cellulose Based on Ionic Liquids. Propellants Explos. Pyrotech. 2023, 48, e202200186. [Google Scholar] [CrossRef]
- Santos, D.; Iop, G.D.; Bizzi, C.A.; Mello, P.A.; Mesko, M.F.; Balbinot, F.P.; Flores, E.M.M. A Single Step Ultrasound-Assisted Nitrocellulose Synthesis from Microcrystalline Cellulose. Ultrason. Sonochem. 2021, 72, 105453. [Google Scholar] [CrossRef] [PubMed]
- Gashtroudkhani, A.K.; Dahmardeh Ghalehno, M.; Abadi, S.S.; Pouyani, M. A Novel, Low-Cost, and High-Efficiency Method for Nitrocellulose Synthesis from Plasma-Modified Cellulose. Sci. Rep. 2025, 15, 6281. [Google Scholar] [CrossRef]
- Han, J.-H.; Wang, M.; Bai, P.; Brushett, F.R.; Bazant, M.Z. Dendrite Suppression by Shock Electrodeposition in Charged Porous Media. Sci. Rep. 2016, 6, 28054. [Google Scholar] [CrossRef]
- Abdelaziz, A.; Trache, D.; Tarchoun, A.F.; Boukeciat, H.; Pal, Y.; Thakur, S.; Pang, W.; Klapötke, T.M. Synergistic Effect of Nitrocellulose Coating on Structural and Reactivity Stabilization of Ammonium Nitrate Oxidizer. Def. Technol. 2025, 43, 35–43. [Google Scholar] [CrossRef]
- Hu, C.S.; Sun, K.; Zhang, Y. Preparation of Nitrocellulose Microspheres Based on Low-Cost High-Throughput Microfluidic Technology. Microfluid. Nanofluid. 2024, 28, 65. [Google Scholar] [CrossRef]
- Dourari, M.; Tarchoun, A.F.; Trache, D.; Abdelaziz, A.; Barkat, T.; Tiliouine, R.; Bekhouche, S.; Bessa, W. Elucidating the Effect of Nitrocellulose-Encapsulated MgAl–CuO on the Thermal Behavior of Double Base Propellant Based on Nitrocellulose and Diethylene Glycol Dinitrate. React. Kinet. Mech. Catal. 2023, 136, 2309–2325. [Google Scholar] [CrossRef]
- Trisnawati, E.W.; Suryanti, V.; Pramono, E. Fabrication and Evaluation of PVDF Membranes Modified with Cellulose and Cellulose Esters from Peanut (Arachis hypogea L.) Shell for Application in Methylene Blue Filtration. JCIS Open 2024, 16, 100123. [Google Scholar] [CrossRef]
- Takao, S.; Rajabzadeh, S.; Shibata, M.; Otsubo, C.; Hamada, T.; Kato, N.; Nakagawa, K.; Kitagawa, T.; Matsuyama, H.; Yoshioka, T. Preparation of Chemically Resistant Cellulose Benzoate Hollow Fiber Membrane via Thermally Induced Phase Separation Method. Membranes 2022, 12, 1199. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Chen, Z.; Ma, M.; Xie, X.; Weng, H.; Zhang, Y.; Chen, J.; Xiao, A. Synthesis, Characterization, Antibacterial and Emulsifying Properties of Agar Benzoate. Int. J. Biol. Macromol. 2023, 239, 124254. [Google Scholar] [CrossRef] [PubMed]
- Edgar, K.J.; Buchanan, C.M.; Debenham, J.S.; Rundquist, P.A.; Seiler, B.D.; Shelton, M.C.; Tindall, D. Advances in Cellulose Ester Performance and Application. Prog. Polym. Sci. 2001, 26, 1605–1688. [Google Scholar] [CrossRef]
- Trisnawati, E.W.; Cahyani, I.S.; Safriyani, D.; Pramono, E.; Suryanti, V. Cellulose, Cellulose Benzoate and Cellulose Citrate from Screw Pine (Pandanus tectorius) Leaves as PVDF Filler for Improved Permeability and Anti-Fouling Properties. Period. Polytech. Chem. Eng. 2023, 67, 504–515. [Google Scholar] [CrossRef]
- Ci, Y.; Yang, X.; Ma, Y.; Xu, F.; Tang, Y. Cellulose Benzoate Synthesis via Homogeneous Transesterification Catalyzed by Superbase-Derived Ionic Liquids for Advanced Applications. Green Chem. 2025, 27, 3764–3776. [Google Scholar] [CrossRef]
- Liu, S.; Wang, J. Exploring the Potential of Cellulose Benzoate Adsorbents Modified with Carbon Nanotubes and Magnetic Carbon Nanotubes for Microplastic Removal from Water. Chem. Eng. J. 2023, 469, 143910. [Google Scholar] [CrossRef]
- Chen, M.-J.; Li, R.-M.; Zhang, X.-Q.; Feng, J.; Feng, J.; Liu, C.-F.; Shi, Q.-S. Homogeneous Transesterification of Sugar Cane Bagasse toward Sustainable Plastics. ACS Sustain. Chem. Eng. 2017, 5, 360–366. [Google Scholar] [CrossRef]
- Teixeira Polez, R.; Voltarelli Ferracini, T.; Cardoso de Paula, S.F.; Passos de Oliveira Santos, R.; Porto, A.L.M.; Frollini, E. Synthesis of Cellulose Hexanoate, Benzoate, and Mixed Esters: Exploring Their Potential as Enzyme Immobilization Platforms. Macromol. Biosci. 2025, e00221. [Google Scholar] [CrossRef] [PubMed]
- Cellulose Acetate Butyrate: Characteristics, Applications and Selection Guide-Schem.Net. Available online: https://www.schem.net/blog/cellulose-acetate-butyrate-characteristics-applications-and-selection-guide_b143 (accessed on 10 August 2025).
- Cellulose Acetate Butyrate Market Size & Forecast to 2030. Available online: https://www.researchandmarkets.com/report/cellulose-acetate-butyrate?srsltid=AfmBOoqry-QLUHg4s-LXxDidwrtyYwqPeEgtjp-BG78vF6JkkAlaU_Vr (accessed on 21 August 2025).
- Kuang, J.; Wang, J.H.; Bai, Y.; Li, Y. Effects and Mechanism of Cellulose Acetate Butyrate on the Crystallization of Polylactic Acid. Eur. Polym. J. 2019, 121, 109286. [Google Scholar] [CrossRef]
- Ioelovich, M. Adjustment of Hydrophobic Properties of Cellulose Materials. Polymers 2021, 13, 1241. [Google Scholar] [CrossRef]
- Lau, C.C.; Bayazit, M.K.; Knowles, J.C.; Tang, J. Tailoring Degree of Esterification and Branching of Poly(Glycerol Sebacate) by Energy Efficient Microwave Irradiation. Polym. Chem. 2017, 8, 3937–3947. [Google Scholar] [CrossRef]
- Fischer, J.; Thümmler, K.; Zlotnikov, I.; Mikhailova, D.; Fischer, S. Synthesis of Cellulose Acetate Butyrate Microspheres as Precursor for Hard Carbon-Based Electrodes in Symmetric Supercapacitors. Polymers 2024, 16, 2176. [Google Scholar] [CrossRef]
- Huang, A.; Wei, L.; Zhao, Z.; Wei, G.; Zhang, Y.; Huang, Z.; Li, X.; Hu, H.; Qin, X.; Yang, M. A Comparative Analysis of the Preparation of Cellulose Acetate Butyrate and the Characteristics of Applying in Pearlescent Coating Film. Polym. Bull. 2020, 77, 2873–2887. [Google Scholar] [CrossRef]
- Huang, K.; Wang, B.; Cao, Y.; Li, H.; Wang, J.; Lin, W.; Mu, C.; Liao, D. Homogeneous Preparation of Cellulose Acetate Propionate (CAP) and Cellulose Acetate Butyrate (CAB) from Sugarcane Bagasse Cellulose in Ionic Liquid. J. Agric. Food Chem. 2011, 59, 5376–5381. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Zhang, J.; He, J. 1-Allyl-3-Methylimidazolium Chloride Room Temperature Ionic Liquid: A New and Powerful Nonderivatizing Solvent for Cellulose. Macromolecules 2005, 38, 8272–8277. [Google Scholar] [CrossRef]
- Cao, Y.; Li, H.; Zhang, J. Homogeneous Synthesis and Characterization of Cellulose Acetate Butyrate (CAB) in 1-Allyl-3-Methylimidazolium Chloride (AmimCl) Ionic Liquid. Ind. Eng. Chem. Res. 2011, 50, 7808–7814. [Google Scholar] [CrossRef]
- Abarkan, A.; Achalhi, N.; El Yousfi, R.; El Idrissi, A.; El Barkany, S.; Aqil, M. “Greener” Homogeneous Esterification of Cellulose Isolated from Stipa Tenacissima Plant Located in the Eastern Region of Morocco Using Ionic Liquids as Reaction Medium. Polym. Bull. 2024, 81, 5375–5402. [Google Scholar] [CrossRef]
- Huang, A.; Li, X.; Liang, X.; Zhang, Y.; Hu, H.; Yin, Y.; Huang, Z. Solid-Phase Synthesis of Cellulose Acetate Butyrate as Microsphere Wall Materials for Sustained Release of Emamectin Benzoate. Polymers 2018, 10, 1381. [Google Scholar] [CrossRef]
- Ran, S.; Xue, L.; Wei, X.; Huang, J.; Yan, X.; He, T.-C.; Tang, Z.; Zhang, H.; Gu, M. Recent Advances in Injectable Hydrogel Therapies for Periodontitis. J. Mater. Chem. B 2024, 12, 6005–6032. [Google Scholar] [CrossRef] [PubMed]
- Edgar, K.J. Cellulose Esters in Drug Delivery. Cellulose 2006, 14, 49–64. [Google Scholar] [CrossRef]
- Nejström, M.; Andreasson, B.; Sjölund, J.; Eivazi, A.; Svanedal, I.; Edlund, H.; Norgren, M. On Structural and Molecular Order in Cellulose Acetate Butyrate Films. Polymers 2023, 15, 2205. [Google Scholar] [CrossRef]
- Milotskyi, R.; Serizawa, R.; Yanagisawa, K.; Sharma, G.; Ito, E.R.D.; Fujie, T.; Wada, N.; Takahashi, K. Composite of Cellulose-Nanofiber-Reinforced Cellulose Acetate Butyrate: Improvement of Mechanical Strength by Cross-Linking of Hydroxyl Groups. J. Compos. Sci. 2023, 7, 130. [Google Scholar] [CrossRef]
- Khaing, E.M.; Lertsuphotvanit, N.; Thammasut, W.; Rojviriya, C.; Chansatidkosol, S.; Phattarateera, S.; Pichayakorn, W.; Phaechamud, T. Cellulose Acetate Butyrate-Based In Situ Gel Comprising Doxycycline Hyclate and Metronidazole. Polymers 2024, 16, 3477. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Kai, D.; Pasbakhsh, P.; Teow, S.Y.; Lim, Y.Y.; Pushpamalar, J. Electrospun Cellulose Acetate Butyrate/Polyethylene Glycol (CAB/PEG) Composite Nanofibers: A Potential Scaffold for Tissue Engineering. Colloids Surf. B Biointerfaces 2020, 188, 110713. [Google Scholar] [CrossRef] [PubMed]
- Tanvir, A.; Ting, V.P.; Eichhorn, S.J. Nanoporous Electrospun Cellulose Acetate Butyrate Nanofibres for Oil Sorption. Mater. Lett. 2020, 261, 127116. [Google Scholar] [CrossRef]
- Dehmen, O.G.; Onen, H.A.; Yildiz, Z.; Gungor, A. Chemical, Mechanical, and Thermal Properties of UV-Curable Cellulose Acetate Butyrate-Based Oligomers and Their Electrospun Fibrous Mats. J. Coat. Technol. Res. 2020, 17, 1043–1052. [Google Scholar] [CrossRef]
- El Nemr, A.; Eleryan, A.; Mashaly, M.; Khaled, A. Rapid Synthesis of Cellulose Propionate and Its Conversion to Cellulose Nitrate Propionate. Polym. Bull. 2021, 78, 4149–4182. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Bednarek, P.S.; Kowalczuk, P.B. Cellulose and Its Derivatives as a Sustainable Reagent in Mineral Flotation: Mechanisms and Applications. Sep. Purif. Technol. 2025, 378, 134690. [Google Scholar] [CrossRef]
- Properties and Applications of Cellulose Propionate (CP). Available online: https://www.azom.com/article.aspx?ArticleID=389 (accessed on 10 August 2025).
- Xu, Z.-M.; Luo, J.-Y.; Huang, Y.-B. Recent Advances in the Chemical Valorization of Cellulose and Its Derivatives into Ester Compounds. Green. Chem. 2022, 24, 3895–3921. [Google Scholar] [CrossRef]
- El Nemr, A.; Eleryan, A.; Mashaly, M.; Khaled, A. Comparative Study of Synthesis of Cellulose Propionate from Different Sources Using NIS as a New Catalyst. Polym. Bull. 2021, 78, 4369–4386. [Google Scholar] [CrossRef]
- Furlan Sandrini, D.M.; Morgado, D.L.; de Oliveira, A.J.A.; de Moraes, D.A.; Varanda, L.C.; Frollini, E. Cellulose Esters: Synthesis for Further Formation of Films with Magnetite Nanoparticles Incorporated. Int. J. Biol. Macromol. 2024, 264, 130594. [Google Scholar] [CrossRef]
- Utrera-Barrios, S.; Pinho Lopes, O.; Mas-Giner, I.; Verdejo, R.; López-Manchado, M.A.; Hernández Santana, M. Sustainable Composites with Self-healing Capability: Epoxidized Natural Rubber and Cellulose Propionate Reinforced with Cellulose Fibers. Polym. Compos. 2024, 45, 7918–7931. [Google Scholar] [CrossRef]
- Lee, C.; Kang, S.W. Derivation of Porous Cellulose Propionate Using Hydrated Hydroxyl Groups and Hydraulic Pressure. Int. J. Biol. Macromol. 2024, 262, 130240. [Google Scholar] [CrossRef] [PubMed]
- Cellulose Propionate Market Size, Industry Outlook & Forecast. Available online: https://www.verifiedmarketreports.com/product/cellulose-propionate-market/ (accessed on 1 October 2025).
- Nasatto, P.L.; Pignon, F.; Silveira, J.L.M.; Duarte, M.E.R.; Noseda, M.D.; Rinaudo, M. Methylcellulose, a Cellulose Derivative with Original Physical Properties and Extended Applications. Polymers 2015, 7, 777–803. [Google Scholar] [CrossRef]
- Xiong, J.; Feng, L.; Liu, B.; Wang, X. Research Progress of Methylcellulose-Based Thermosensitive Hydrogels Applied in Biomedical Field. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2024, 41, 199–204. [Google Scholar] [CrossRef]
- Ye, D.; Farriol, X. A Facile Method to Prepare Methylcellulose from Annual Plants and Wood Using Iodomethane. e-Polymers 2005, 5, 040. [Google Scholar] [CrossRef]
- Dimethyl Sulfate|CASRN 77-78-1|DTXSID5024055|IRIS|US EPA, ORD. Available online: https://iris.epa.gov/ChemicalLanding/&substance_nmbr%3D365 (accessed on 21 August 2025).
- Methyl Iodide|CASRN 74-88-4|DTXSID0024187|IRIS|US EPA, ORD. Available online: https://iris.epa.gov/ChemicalLanding/&substance_nmbr%3D650 (accessed on 21 August 2025).
- Oliveira, R.L.; Vieira, J.G.; Barud, H.S.; Assunção, R.M.N.; Rodrigues Filho, G.; Ribeiro, S.J.L.; Messadeqq, Y. Synthesis and Characterization of Methylcellulose Produced from Bacterial Cellulose under Heterogeneous Condition. J. Braz. Chem. Soc. 2015, 26, 1861–1870. [Google Scholar] [CrossRef]
- Viera, R.G.P.; Filho, G.R.; de Assunção, R.M.N.; da Carla, C.; Vieira, J.G.; de Oliveira, G.S. Synthesis and Characterization of Methylcellulose from Sugar Cane Bagasse Cellulose. Carbohydr. Polym. 2007, 67, 182–189. [Google Scholar] [CrossRef]
- Vieira, J.G.; Filho, G.R.; Meireles, C.D.S.; Faria, F.A.C.; Gomide, D.D.; Pasquini, D.; Cruz, S.F.D.; De Assunção, R.M.N.; Motta, L.A.D.C. Synthesis and Characterization of Methylcellulose from Cellulose Extracted from Mango Seeds for Use as a Mortar Additive. Polímeros 2012, 22, 80–87. [Google Scholar] [CrossRef]
- dos Santos, M.A.; Grenha, A. Polysaccharide Nanoparticles for Protein and Peptide Delivery: Exploring Less-Known Materials. Adv. Protein Chem. Struct. Biol. 2015, 98, 223–261. [Google Scholar] [CrossRef]
- Ghorbani, F.; Ghalandari, B.; Liu, Z.; Li, D.; Yu, B. Injectable Light-Assisted Thermo-Responsive Methylcellulose-Sodium Humate Hydrogel Proposed for Photothermal Ablation and Localized Delivery of Cisplatin. Front. Bioeng. Biotechnol. 2022, 10, 967438. [Google Scholar] [CrossRef]
- Ahlfeld, T.; Guduric, V.; Duin, S.; Akkineni, A.R.; Schütz, K.; Kilian, D.; Emmermacher, J.; Cubo-Mateo, N.; Dani, S.; Witzleben, M.V.; et al. Methylcellulose—A Versatile Printing Material That Enables Biofabrication of Tissue Equivalents with High Shape Fidelity. Biomater. Sci. 2020, 8, 2102–2110. [Google Scholar] [CrossRef] [PubMed]
- Methylcellulose Market Report|Global Forecast from 2025 to 2033. Available online: https://dataintelo.com/report/global-methylcellulose-market (accessed on 11 August 2025).
- Nagel, M.C.V.; Koschella, A.; Voiges, K.; Mischnick, P.; Heinze, T. Homogeneous Methylation of Wood Pulp Cellulose Dissolved in LiOH/Urea/H2O. Eur. Polym. J. 2010, 46, 1726–1735. [Google Scholar] [CrossRef]
- Pirsa, S.; Hafezi, K. Hydrocolloids: Structure, Preparation Method, and Application in Food Industry. Food Chem. 2023, 399, 133967. [Google Scholar] [CrossRef]
- Davidovich-Pinhas, M.; Barbut, S.; Marangoni, A.G. The Role of Surfactants on Ethylcellulose Oleogel Structure and Mechanical Properties. Carbohydr. Polym. 2015, 127, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Pratama, J.H.; Lestari, W.W.; Rofida, A.; Putri, A.K.; Widian, R.N.; Gunawan, T.; Hastuti, D.S.; Sulistiono, D.O.; Sari, K.P. Novel Polymer Composite Coated with Ethylcellulose Nanoparticle from Waste Paper as an Alternative Material to Extracorporeal Oxygenation Membrane. J. Polym. Res. 2023, 30, 220. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, Y.; Tan, Z.; Zhou, T. Cellulose Extraction from Rice Straw Waste for Biodegradable Ethyl Cellulose Films Preparation Using Green Chemical Technology. J. Clean. Prod. 2024, 439, 140839. [Google Scholar] [CrossRef]
- Yavuzturk Gul, B.; Pekgenc, E.; Vatanpour, V.; Koyuncu, I. A Review of Cellulose-Based Derivatives Polymers in Fabrication of Gas Separation Membranes: Recent Developments and Challenges. Carbohydr. Polym. 2023, 321, 121296. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-G.; Kresse, I.; Xu, Z.-K.; Springer, J. Effect of Temperature and Pressure on Gas Transport in Ethyl Cellulose Membrane. Polymer 2001, 42, 6801–6810. [Google Scholar] [CrossRef]
- Gorji, N.; Jahanshahi, M.; Shahavi, M.H.; Ayrilmis, N. Ethylcellulose Microparticles as Green Encapsulation for Slow Release of Microspherical Abamectin Pesticide for Agricultural Applications: Improvement of Process Parameters. Int. J. Biol. Macromol. 2025, 321, 146336. [Google Scholar] [CrossRef]
- Xu, P.; Yu, D.; Wang, S.; Shi, W.; Xing, G.; Wang, A.; Teng, Z.; Hao, D. Thiamethoxam-Loaded Ethyl Cellulose Microspheres for Extending the Efficacy Duration and Reducing the Toxicity on the Growth of Maize (Zea mays L.). Langmuir 2024, 40, 27270–27278. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Li, X.; Huang, B.; Li, N.; Wang, A.; An, C.; Jiang, J.; Shen, Y.; Wang, C.; Zhan, S.; et al. Construction and Characterization of Ethyl Cellulose-Based Nano-Delivery System for Phenamacril. Int. J. Biol. Macromol. 2022, 221, 1251–1258. [Google Scholar] [CrossRef]
- Ji, Y.; Zhao, H.; Liu, H.; Zhao, P.; Yu, D.-G. Electrosprayed Stearic-Acid-Coated Ethylcellulose Microparticles for an Improved Sustained Release of Anticancer Drug. Gels 2023, 9, 700. [Google Scholar] [CrossRef]
- Wildy, M.; Hao, Q.; Wei, W.; Nguyen, D.H.; Xu, K.; Schossig, J.; Hu, X.; Salas-de la Cruz, D.; Hyun, D.C.; Wang, Z.; et al. Tunable Chemotherapy Release Using Biocompatible Fatty Acid-Modified Ethyl Cellulose Nanofibers. Carbohydr. Polym. Technol. Appl. 2025, 9, 100670. [Google Scholar] [CrossRef]
- Li, Z.; Tan, X.; Yarmolenko, M.A.; Keneshbekova, A.; Wang, A.; Liu, X.; Jiang, X. Deposition of Ethyl Cellulose-Based Drug-Carrying Coating by Low-Energy Electron Beam Dispersion and Its Antifungal Properties. Vacuum 2025, 236, 114136. [Google Scholar] [CrossRef]
- Mohamed, R.; Chou, S.F. Physicomechanical Characterizations and in Vitro Release Studies of Electrospun Ethyl Cellulose Fibers, Solvent Cast Carboxymethyl Cellulose Films, and Their Composites. Int. J. Biol. Macromol. 2024, 267, 131374. [Google Scholar] [CrossRef] [PubMed]
- Aboelazayem, S.; Nasra, M.; Ebada, H.; Abdallah, O. Ethyl-Cellulose Nanosponges for Topical Delivery of Simvastatin with Preferential Skin Retention for Wound Healing in a Full-Thickness Wound Rat Model. AAPS PharmSciTech 2025, 26, 126. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zhi, H.; Shi, Q.; Zhang, Y.; Feng, J.; Liu, J.; Huang, H.; Xie, X. Tannic Acid Interfacial Modification of Prochloraz Ethyl Cellulose Nanoparticles for Enhancing the Antimicrobial Effect and Biosafety of Fungicides. ACS Appl. Mater. Interfaces 2023, 15, 41324–41336. [Google Scholar] [CrossRef] [PubMed]
- Soleimanian, Y.; Ghazani, S.M.; Marangoni, A.G. Ethylcellulose Oleogels of Oil Glycerolysis Products as Functional Adipose Tissue Mimetics. Food Hydrocoll. 2024, 151, 109756. [Google Scholar] [CrossRef]
- Horvat, G.; Rožanc, J.; Maver, U.; Finšgar, M.; Knez, Ž.; Novak, Z. Reinforcing Ethyl Cellulose Aerogels with Poly(Lactic Acid) for Enhanced Bone Regeneration. Cellulose 2024, 31, 4421–4439. [Google Scholar] [CrossRef]
- Ghorbani, M.; Ramezani, S.; Rashidi, M.R. Fabrication of Honey-Loaded Ethylcellulose/Gum Tragacanth Nanofibers as an Effective Antibacterial Wound Dressing. Colloids Surf. A Physicochem. Eng. Asp. 2021, 621, 126615. [Google Scholar] [CrossRef]
- Zhang, Z.; Xia, Y.; Gong, W.; Zhou, J.; Yu, D.G.; Xie, Y. feng Electrospun Chitosan//Ethylcellulose-Vitamin E//Ethylcellulose-Curcumin Tri-Chamber Eccentric Janus Nanofibers for a Joint Antibacterial and Antioxidant Performance. Int. J. Biol. Macromol. 2024, 281, 135753. [Google Scholar] [CrossRef]
- Cadena, I.A.; Adhikari, G.; Almer, A.; Jenne, M.; Obasi, N.; Soria Zurita, N.F.; Rochefort, W.E.; Mueller, J.L.; Fogg, K.C.; Robinson, J.; et al. Development of a 3D in Vitro Human-Sized Model of Cervical Dysplasia to Evaluate the Delivery of Ethyl Cellulose-Ethanol Injection. Front. Biomater. Sci. 2024, 3, 1365781. [Google Scholar] [CrossRef]
- Quang, T.T.; Yang, J.; Kaluzienski, M.L.; Parrish, A.; Farooqui, A.; Katz, D.; Crouch, B.; Ramanujam, N.; Mueller, J.L. In Vivo Evaluation of Safety and Efficacy of Ethyl Cellulose-Ethanol Tissue Ablation in a Swine Cervix Model. Bioengineering 2023, 10, 1246. [Google Scholar] [CrossRef]
- Shan, P.; Wang, K.; Sun, F.; Li, Y.; Sun, L.; Li, H.; Peng, L. Humidity-Adjustable Functional Gelatin Hydrogel/Ethyl Cellulose Bilayer Films for Active Food Packaging Application. Food Chem. 2024, 439, 138202. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Chen, J.; Tan, K.B. Ethyl Cellulose Matrixed Poly(Sulfur-Co-Sorbic Acid) Composite Films: Regulation of Properties and Application for Food Preservation. Int. J. Biol. Macromol. 2024, 279, 135183. [Google Scholar] [CrossRef]
- Ghiasi, F.; Golmakani, M.T. Fabrication and Characterization of a Novel Biphasic System Based on Starch and Ethylcellulose as an Alternative Fat Replacer in a Model Food System. Innov. Food Sci. Emerg. Technol. 2022, 78, 103028. [Google Scholar] [CrossRef]
- Coelho, A.L.K.; de Andrade Barbosa Guilherme, M.; de Freitas, R.A.; Mafra, M.R.; Mafra, L.I. Tunable Oleogels from Sunflower Oil, Ethylcellulose, and Quillaja Saponin: Synergistic Interactions, Structural Properties, and Oxidative Stability. Food Hydrocoll. 2026, 170, 111744. [Google Scholar] [CrossRef]
- Kanmaz, N.; Buğdaycı, M.; Demirçivi, P. Solvent-Free Mechanochemical Synthesis of TiO2-Ethyl Cellulose Biocomposite for Adsorption of Tetracycline and Organic Dyes. J. Mol. Liq. 2023, 378, 121643. [Google Scholar] [CrossRef]
- Aghaei, F.; Tangestaninejad, S.; Bahadori, M.; Moghadam, M.; Mirkhani, V.; Mohammadpoor−Baltork, I.; Khalaji, M.; Asadi, V. Green Synthesize of Nano-MOF-Ethylcellulose Composite Fibers for Efficient Adsorption of Congo Red from Water. J. Colloid. Interface Sci. 2023, 648, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Karimian, E.; Moslehi, M.; Tangestaninejad, S.; Moghadam, M.; Malekpour, A.; Mohammadpoor-Baltork, I. Highly Efficient Water Desalination via Electrospun Ethyl Cellulose/Polystyrene Composites Integrated with Metal-Organic Frameworks. Sci. Rep. 2025, 15, 27221. [Google Scholar] [CrossRef]
- Palmieri, E.; Cancelliere, R.; Maita, F.; Micheli, L.; Maiolo, L. An Ethyl Cellulose Novel Biodegradable Flexible Substrate Material for Sustainable Screen-Printing. RSC Adv. 2024, 14, 18103–18108. [Google Scholar] [CrossRef]
- Palmieri, E.; Maiolo, L.; Lucarini, I.; Fattorini, A.D.; Tamburri, E.; Orlanducci, S.; Calarco, R.; Maita, F. Toward Sustainable Electronics: Exploiting the Potential of a Biodegradable Cellulose Blend for Photolithographic Processes and Eco-Friendly Devices. Adv. Mater. Technol. 2024, 9, 2301282. [Google Scholar] [CrossRef]
- Ethyl Cellulose Market 2025—Growth, Outlook and Trends 2034. Available online: https://www.thebusinessresearchcompany.com/report/ethyl-cellulose-global-market-report (accessed on 11 August 2025).
- Noreen, A.; Zia, K.M.; Tabasum, S.; Khalid, S.; Shareef, R. A Review on Grafting of Hydroxyethylcellulose for Versatile Applications. Int. J. Biol. Macromol. 2020, 150, 289–303. [Google Scholar] [CrossRef]
- Zia, F.; Nazli, Z.-H.; Zia, K.M.; Aftab, W.; Tabasum, S.; Asrar, M. Synthesis and Characterization of Hydroxyethyl Cellulose Copolymer Modified Polyurethane Bionanocomposites. Int. J. Biol. Macromol. 2021, 179, 345–352. [Google Scholar] [CrossRef]
- Bajaber, M.A.; Anjum, M.N.; Ibrahim, M.; Farooq, T.; Ahmad, M.N.; ul Abideen, Z. Synthesis and Characterization of Hydroxyethyl Cellulose Grafted with Copolymer of Polyaniline and Polypyrrole Biocomposite for Adsorption of Dyes. Molecules 2022, 27, 8238. [Google Scholar] [CrossRef]
- Orhan, B.; Ziba, C.A.; Morcali, M.H.; Dolaz, M. Synthesis of Hydroxyethyl Cellulose from Industrial Waste Using Microwave Irradiation. Sustain. Environ. Res. 2018, 28, 403–411. [Google Scholar] [CrossRef]
- Yin, H.; Song, P.; Chen, X.; Xiao, M.; Tang, L.; Huang, H. Smart PH-Sensitive Hydrogel Based on the Pineapple Peel-Oxidized Hydroxyethyl Cellulose and the Hericium Erinaceus Residue Carboxymethyl Chitosan for Use in Drug Delivery. Biomacromolecules 2022, 23, 253–264. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Li, M.; Wu, X.; Cheng, G. Homogeneous Hydroxyethylation of Cellulose in NaOH/Urea Aqueous Solution. Polym. Bull. 2005, 53, 243–248. [Google Scholar] [CrossRef]
- Zhou, J.; Qin, Y.; Liu, S.; Zhang, L. Homogenous Synthesis of Hydroxyethylcellulose in NaOH/Urea Aqueous Solution. Macromol. Biosci. 2006, 6, 84–89. [Google Scholar] [CrossRef]
- Köhler, S.; Liebert, T.; Heinze, T.; Vollmer, A.; Mischnick, P.; Möllmann, E.; Becker, W. Interactions of Ionic Liquids with Polysaccharides 9. Hydroxyalkylation of Cellulose without Additional Inorganic Bases. Cellulose 2010, 17, 437–448. [Google Scholar] [CrossRef]
- US8541571B2—Homogeneous Synthesis of Cellulose Ethers in Ionic Liquids—Google Patents. Available online: https://patents.google.com/patent/US8541571B2/en (accessed on 11 August 2025).
- Hydroxyethyl Cellulose Market by Application (Detergents & Cleaners, Oil & Gas, Paint & Coatings), Type (High Molecular Weight, Low Molecular Weight, Medium Molecular Weight), Form, Distribution Channel—Global Forecast 2025–2030. Available online: https://www.researchandmarkets.com/reports/5716427/hydroxyethyl-cellulose-market-by-application (accessed on 11 August 2025).
- Murray, J.C.F. 25-Cellulosics. In Handbook of Hydrocolloids: Second Edition; Elsevier: Amsterdam, The Netherlands, 2009; pp. 710–723. [Google Scholar] [CrossRef]
- Chen, B.J.; Liu, G.G.; Wang, X.; Liu, H.R.; Zhang, Y.; Wang, C.F.; Liu, C.X.; Qiao, Y.J. Development and Characterization of an Antioxidant and Antimicrobial Film Composited by Hydroxyethyl Cellulose and Sulfated Rice Bran Polysaccharides for Food Packaging. Foods 2024, 13, 819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, H.; Luo, W.; Chen, G.; Xiao, N.; Xiao, G.; Liu, C. Development of Functional Hydroxyethyl Cellulose-Based Composite Films for Food Packaging Applications. Front. Bioeng. Biotechnol. 2022, 10, 989893. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jin, T.; Liu, W.; Hao, W.; Yan, L.; Zheng, L. Effects of Hydroxyethyl Cellulose and Sodium Alginate Edible Coating Containing Asparagus Waste Extract on Postharvest Quality of Strawberry Fruit. LWT 2021, 148, 111770. [Google Scholar] [CrossRef]
- McMullen, R.L.; Ozkan, S.; Gillece, T. Physicochemical Properties of Cellulose Ethers. Cosmetics 2022, 9, 52. [Google Scholar] [CrossRef]
- Mohammad, A.F.; Mourad, A.A.H.I.; Al-Marzouqi, A.H.; Galiwango, E.; Lwisa, E.G.; Mustafa, J. Hydroxyethyl Cellulose as a Multifunctional Agent for Integrated Brine Desalination, CO2 Capture, and Enhanced Oil Recovery. Chem. Eng. Process.-Process Intensif. 2025, 216, 110414. [Google Scholar] [CrossRef]
- Abou Taleb, M.F.; Alzidan, K. Multifunctional Applications of Seaweed Extract-Infused Hydroxyethyl Cellulose-Polyvinylpyrrolidone Aerogels: Antibacterial, and Antibiofilm Proficiency for Water Decontamination. Int. J. Biol. Macromol. 2024, 278, 135021. [Google Scholar] [CrossRef]
- Sun, W.; Yue, D.; Wang, S.; Sun, D.; Yin, L.; Wang, Y. Prewetting Induced Underwater Super Oleophobic Hydroxyethyl Cellulose-SiO2-Graphene Microfiltration Membranes for Emulsion Separation. Sep. Purif. Technol. 2025, 358, 130421. [Google Scholar] [CrossRef]
- Khan, F.; Siddique, A.B.; Irfan, M.I.; Hassan, M.N.U.; Sher, M.; Alhazmi, H.A.; Qramish, A.N.; Amin, H.M.A.; Qadir, R.; Abbas, A. Maleated Hydroxyethyl Cellulose for the Efficient Removal of Cd(II) Ions from an Aqueous Solution: Isothermal, Kinetic and Regeneration Studies. Water Air Soil. Pollut. 2024, 235, 536. [Google Scholar] [CrossRef]
- Ho, H.N.; Le, H.H.; Le, T.G.; Duong, T.H.A.; Ngo, V.Q.T.; Dang, C.T.; Nguyen, V.M.; Tran, T.H.; Nguyen, C.N. Formulation and Characterization of Hydroxyethyl Cellulose-Based Gel Containing Metronidazole-Loaded Solid Lipid Nanoparticles for Buccal Mucosal Drug Delivery. Int. J. Biol. Macromol. 2022, 194, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Kafili, G.; Tamjid, E.; Niknejad, H.; Simchi, A. Development of Bioinspired Nanocomposite Bioinks Based on Decellularized Amniotic Membrane and Hydroxyethyl Cellulose for Skin Tissue Engineering. Cellulose 2024, 31, 2989–3013. [Google Scholar] [CrossRef]
- Nam, C.J.; Johari, N.F.I.M.; Khan, S.; Kabeb, S.M.; Zulkifli, F.H. Effect of Crosslinking Agent on the Cellulose Nanocrystals Reinforced Hydroxyethyl Cellulose/Poly(Vinyl Alcohol) Scaffolds. Macromol. Symp. 2025, 414, 2300264. [Google Scholar] [CrossRef]
- Mohammadzadeh, V.; Mahmoudi, E.; Ramezani, S.; Navaeian, M.; Taheri, R.A.; Ghorbani, M. Design of a Novel Tannic Acid Enriched Hemostatic Wound Dressing Based on Electrospun Polyamide-6/Hydroxyethyl Cellulose Nanofibers. J. Drug Deliv. Sci. Technol. 2023, 86, 104625. [Google Scholar] [CrossRef]
- Prasathkumar, M.; George, A.; Sadhasivam, S. Influence of Chitosan and Hydroxyethyl Cellulose Modifications towards the Design of Cross-Linked Double Networks Hydrogel for Diabetic Wound Healing. Int. J. Biol. Macromol. 2024, 265, 130851. [Google Scholar] [CrossRef]
- Fekete, T.; Borsa, J.; Takács, E.; Wojnárovits, L. Synthesis and Characterization of Superabsorbent Hydrogels Based on Hydroxyethylcellulose and Acrylic Acid. Carbohydr. Polym. 2017, 166, 300–308. [Google Scholar] [CrossRef]
- Fratila, D.N.; Virvescu, D.I.; Luchian, I.; Hancianu, M.; Baciu, E.R.; Butnaru, O.; Budala, D.G. Advances and Functional Integration of Hydrogel Composites as Drug Delivery Systems in Contemporary Dentistry. Gels 2024, 10, 661. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.K.; Kumar, V.; Jansson, E.; Huttunen, O.; Yamamoto, A.; Vikman, M.; Khakalo, A.; Hiltunen, J.; Behfar, M.H. Biodegradable Cellulose Nanocomposite Substrate for Recyclable Flexible Printed Electronics. Adv. Electron. Mater. 2023, 9, 2201094. [Google Scholar] [CrossRef]
- Wang, Q.; Pan, X.; Guo, J.; Huang, L.; Chen, L.; Ma, X.; Cao, S.; Ni, Y. Lignin and Cellulose Derivatives-Induced Hydrogel with Asymmetrical Adhesion, Strength, and Electriferous Properties for Wearable Bioelectrodes and Self-Powered Sensors. Chem. Eng. J. 2021, 414, 128903. [Google Scholar] [CrossRef]
- Weißenborn, E.; Braunschweig, B. Hydroxypropyl Cellulose as a Green Polymer for Thermo-Responsive Aqueous Foams. Soft Matter 2019, 15, 2876–2883. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cheng, X.; Ma, T.; Wang, S.; Yang, S.; Zhu, W.; Song, J.; Han, J.; Jin, Y.; Guo, J. High-Substituted Hydroxypropyl Cellulose Prepared by Homogeneous Method and Its Clouding and Self-Assembly Behaviors. Carbohydr. Polym. 2024, 330, 121822. [Google Scholar] [CrossRef]
- Joshi, G.; Rana, V.; Naithani, S.; Varshney, V.K.; Sharma, A.; Rawat, J.S. Chemical Modification of Waste Paper: An Optimization towards Hydroxypropyl Cellulose Synthesis. Carbohydr. Polym. 2019, 223, 115082. [Google Scholar] [CrossRef]
- Zhong, S.; Xue, Y.; Wang, K.; Wang, L.; Jiang, T. A Sustainable Utilization Approach of Waste Biomass Resources to Smart Materials for Buildings. Mater. Today Commun. 2024, 40, 109506. [Google Scholar] [CrossRef]
- Hydroxypropyl Cellulose Market Size and Statistics—2035. Available online: https://www.factmr.com/report/hydroxypropyl-cellulose-market (accessed on 21 August 2025).
- Okubo, M.; Iohara, D.; Anraku, M.; Higashi, T.; Uekama, K.; Hirayama, F. A Thermoresponsive Hydrophobically Modified Hydroxypropylmethylcellulose/Cyclodextrin Injectable Hydrogel for the Sustained Release of Drugs. Int. J. Pharm. 2020, 575, 118845. [Google Scholar] [CrossRef]
- Xue, H.; Zhu, C.; Wang, Y.; Gu, Q.; Shao, Y.; Jin, A.; Zhang, X.; Lei, L.; Li, Y. Stimulus-Responsive Cellulose Hydrogels in Biomedical Applications and Challenges. Mater. Today Bio 2025, 32, 101814. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Akhter, T.; Faheem, M.; Mahmood, A.; Al-Masry, W.; Nadeem, S.; Hassan, S.U.; Park, C.H. Metal-Free, Visible Light-Mediated Atom Transfer Radical Polymerization of Hydroxypropyl Cellulose-Graft-Poly(Methyl Methacrylate)s: Effect of Polymer Side Chains on Thermo-Responsive Behavior of Hydroxypropyl Cellulose. Cellulose 2023, 30, 7519–7533. [Google Scholar] [CrossRef]
- Kawasaki, R.; Yamana, K.; Shimada, R.; Sugikawa, K.; Ikeda, A. Water Solubilization and Thermal Stimuli-Triggered Release of Porphyrin Derivatives Using Thermoresponsive Polysaccharide Hydroxypropyl Cellulose for Mitochondria-Targeted Photodynamic Therapy. ACS Omega 2021, 6, 3209–3217. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhai, Z.; Yao, Y.; Stant, J.C.; Landrum, S.L.; Bortner, M.J.; Frazier, C.E.; Edgar, K.J. Oxidized Hydroxypropyl Cellulose/Carboxymethyl Chitosan Hydrogels Permit PH-Responsive, Targeted Drug Release. Carbohydr. Polym. 2023, 300, 120213. [Google Scholar] [CrossRef]
- Gunaki, M.N.; Masti, S.P.; D’souza, O.J.; Eelager, M.P.; Kurabetta, L.K.; Chougale, R.B.; Kadapure, A.J.; Praveen Kumar, S.K. Fabrication of CuO Nanoparticles Embedded Novel Chitosan/Hydroxypropyl Cellulose Bio-Nanocomposites for Active Packaging of Jamun Fruit. Food Hydrocoll. 2024, 152, 109937. [Google Scholar] [CrossRef]
- Wardana, A.A.; Wigati, L.P.; Tanaka, F.; Tanaka, F. Functional Enhancement of Hydroxypropyl Cellulose-based Bionanocomposite Films Incorporating Chitosan Nanoparticles. Int. J. Food Sci. Technol. 2023, 58, 907–920. [Google Scholar] [CrossRef]
- Yan, K.; Zhang, X.; Liu, Y.; Cheng, J.; Zhai, C.; Shen, K.; Liang, W.; Fan, W. 3D-Bioprinted Silk Fibroin-Hydroxypropyl Cellulose Methacrylate Porous Scaffold with Optimized Performance for Repairing Articular Cartilage Defects. Mater. Des. 2023, 225, 111531. [Google Scholar] [CrossRef]
- Filip, D.; Macocinschi, D.; Zaltariov, M.-F.; Ciubotaru, B.-I.; Bargan, A.; Varganici, C.-D.; Vasiliu, A.-L.; Peptanariu, D.; Balan-Porcarasu, M.; Timofte-Zorila, M.-M. Hydroxypropyl Cellulose/Pluronic-Based Composite Hydrogels as Biodegradable Mucoadhesive Scaffolds for Tissue Engineering. Gels 2022, 8, 519. [Google Scholar] [CrossRef] [PubMed]
- Tialiou, A.; Athab, Z.H.; Woodward, R.T.; Biegler, V.; Keppler, B.K.; Halbus, A.F.; Reithofer, M.R.; Chin, J.M. Fabrication of Graded Porous Structure of Hydroxypropyl Cellulose Hydrogels via Temperature-Induced Phase Separation. Carbohydr. Polym. 2023, 315, 120984. [Google Scholar] [CrossRef]
- Mohebian, Z.; Tajmohammadi, I.; Yavari Maroufi, L.; Ramezani, S.; Ghorbani, M. A Novel Aloe Vera-Loaded Ethylcellulose/Hydroxypropyl Methylcellulose Nanofibrous Mat Designed for Wound Healing Application. J. Polym. Environ. 2022, 30, 867–877. [Google Scholar] [CrossRef]
- Ding, L.; Qi, Q.; Zhang, S.; Ren, C.; Deng, M.; Sun, Z.; Zhang, R.; Liu, Q.; Duan, S.; Wang, X.; et al. Hydroxypropyl Methylcellulose Reinforced Collagen/PVA Composite Hydrogel Wound Dressing with Self-Adaptive, Hemostasis and Antibacterial Ability for Wound Healing. Int. J. Biol. Macromol. 2025, 304, 140811. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, Y.; Wei, H.; Nie, L.; Ding, P.; Sun, H.; Guo, Y.; Chen, T.; Okoro, O.V.; Shavandi, A.; et al. Injectable Hydrogels Based on Silk Fibroin Peptide Grafted Hydroxypropyl Chitosan and Oxidized Microcrystalline Cellulose for Scarless Wound Healing. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129062. [Google Scholar] [CrossRef]
- Yan, H.; Gu, H.; Lu, S.; Meng, F.; Ma, Q.; Xing, X.; Pan, S.; Che, Y. Bioinspired Multifunctional Conductive Hydrogel Based on Hydroxypropyl Methyl Cellulose for Flexible Sensors. Carbohydr. Polym. 2025, 368, 124192. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Yuan, W. Tunable Thermoresponsive and Stretchable Hydrogel Sensor Based on Hydroxypropyl Cellulose for Human Motion/Health Detection, Visual Signal Transmission and Information Encryption. Carbohydr. Polym. 2024, 343, 122497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.; Lv, X.; Xie, T.; Chen, J.; Fang, D.; Yi, S. Construction of Mechanically Robust and Recyclable Catalytic Hydrogel Based on Hydroxypropyl Cellulose-Supported by Montmorillonite/Ionic Liquid with Different Anions for Pollutants(4-NP, MB, CR, Rhb) Degradation. Int. J. Biol. Macromol. 2024, 273, 132788. [Google Scholar] [CrossRef]
- Ledwon, P.; Andrade, J.R.; Lapkowski, M.; Pawlicka, A. Hydroxypropyl Cellulose-Based Gel Electrolyte for Electrochromic Devices. Electrochim. Acta 2015, 159, 227–233. [Google Scholar] [CrossRef]
- Shi, H.; Deng, Y.; Shi, Y. Cellulose-Based Stimuli-Responsive Anisotropic Hydrogel for Sensor Applications. ACS Appl. Nano Mater. 2023, 6, 11524–11530. [Google Scholar] [CrossRef]
- Timmins, P.; Pygall, S.R.; Melia, C.D. Hydrophilic Matrix Dosage Forms: Definitions, General Attributes, and the Evolution of Clinical Utilization. AAPS Adv. Pharm. Sci. Ser. 2014, 16, 1–15. [Google Scholar] [CrossRef]
- Meng, Z.; Qi, K.; Guo, Y.; Wang, Y.; Liu, Y. Macro-Micro Structure Characterization and Molecular Properties of Emulsion-Templated Polysaccharide Oleogels. Food Hydrocoll. 2018, 77, 17–29. [Google Scholar] [CrossRef]
- Sharma, S.; Ansari, T.N.; Handa, S. HPMC: A Biomass-Based Semisynthetic Sustainable Additive Enabling Clean and Fast Chemistry in Water. ACS Sustain. Chem. Eng. 2021, 9, 12719–12728. [Google Scholar] [CrossRef]
- Bahrami, A.; Rezaei Mokarram, R.; Sowti Khiabani, M.; Ghanbarzadeh, B.; Salehi, R. Physico-Mechanical and Antimicrobial Properties of Tragacanth/Hydroxypropyl Methylcellulose/Beeswax Edible Films Reinforced with Silver Nanoparticles. Int. J. Biol. Macromol. 2019, 129, 1103–1112. [Google Scholar] [CrossRef]
- Klangmuang, P.; Sothornvit, R. Barrier Properties, Mechanical Properties and Antimicrobial Activity of Hydroxypropyl Methylcellulose-Based Nanocomposite Films Incorporated with Thai Essential Oils. Food Hydrocoll. 2016, 61, 609–616. [Google Scholar] [CrossRef]
- Larsson, M.; Viridén, A.; Stading, M.; Larsson, A. The Influence of HPMC Substitution Pattern on Solid-State Properties. Carbohydr. Polym. 2010, 82, 1074–1081. [Google Scholar] [CrossRef]
- Jin, C.; Wu, F.; Hong, Y.; Shen, L.; Lin, X.; Zhao, L.; Feng, Y. Updates on Applications of Low-Viscosity Grade Hydroxypropyl Methylcellulose in Coprocessing for Improvement of Physical Properties of Pharmaceutical Powders. Carbohydr. Polym. 2023, 311, 120731. [Google Scholar] [CrossRef]
- Khiste, R.; Bhapkar, N.; Kulkarni, N. A Review on Applications of Hydroxy Propyl Methyl Cellulose and Natural Polymers for the Development of Modified Release Drug Delivery Systems. Res. J. Pharm. Technol. 2021, 14, 1163–1170. [Google Scholar] [CrossRef]
- Ngatirah, N.; Ruswanto, A.; Sunardi, S. Effect of Hydroxypropyl Methylcellulose from Oil Palm Empty Fruit Bunch on Oil Uptake and Physical Properties of French Fries. Food Sci. Technol. 2022, 42, e110421. [Google Scholar] [CrossRef]
- Akbar, M.H.; Harmita; Suryadi, H. Preparation and Characterization of Hydroxypropyl Methylcellulose Produced from A-Cellulose Betung Bamboo (Dendrocalamus asper) and It’s Evaluation on Gel Formulation. Int. J. Pharm. Pharm. Sci. 2020, 12, 156–165. [Google Scholar] [CrossRef]
- Vlad, R.-A.; Pintea, A.; Pintea, C.; Rédai, E.-M.; Antonoaea, P.; Bîrsan, M.; Ciurba, A. Hydroxypropyl Methylcellulose—A Key Excipient in Pharmaceutical Drug Delivery Systems. Pharmaceutics 2025, 17, 784. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, J.; Tan, K.B.; Chen, M.; Zhu, Y. Development of Hydroxypropyl Methylcellulose Film with Xanthan Gum and Its Application as an Excellent Food Packaging Bio-Material in Enhancing the Shelf Life of Banana. Food Chem. 2022, 374, 131794. [Google Scholar] [CrossRef]
- Tundisi, L.L.; Mostaço, G.B.; Carricondo, P.C.; Petri, D.F.S. Hydroxypropyl Methylcellulose: Physicochemical Properties and Ocular Drug Delivery Formulations. Eur. J. Pharm. Sci. 2021, 159, 105736. [Google Scholar] [CrossRef]
- Mady, O.Y.; Dewedar, O.; Abdine, N.; Zaytoon, H.; Haggag, Y. Bioadhesive Behaviors of HPMC E5: Comparative Analysis of Various Techniques, Histological and Human Radiological Evidence. Sci. Rep. 2024, 14, 1–14. [Google Scholar] [CrossRef]
- Zhang, W.; Piao, S.; Lin, L.; Yin, Y.; Guo, J.; Jiang, Z.; Cho, Y.; Li, R.; Gao, J.; Pang, H.; et al. Wearable and Antibacterial HPMC-Anchored Conductive Polymer Composite Strain Sensor with High Gauge Factors under Small Strains. Chem. Eng. J. 2022, 435, 135068. [Google Scholar] [CrossRef]
- Hidroxipropílico Mercado de Metilcelulose, Tamanho e Tendência 2025–2035. Available online: https://www.metatechinsights.com/pt/industry-insights/hydroxypropyl-methylcellulose-market-2507 (accessed on 21 August 2025).
- Ambjörnsson, H.A.; Schenzel, K.; Germgård, U. Carboxymethyl Cellulose Produced at Different Mercerization Conditions and Characterized by NIR FT Raman Spectroscopy in Combination with Multivariate Analytical Methods. Bioresources 2013, 8, 1918–1932. [Google Scholar] [CrossRef]
- Kontogiorgos, V. Stabilisers. In Encyclopedia of Dairy Sciences; Elsevier: Amsterdam, The Netherlands, 2022; pp. 689–694. [Google Scholar]
- Arif, M.B.; Ndruru, S.T.C.L.; Ghozali, M. A Review on the Preparation of Carboxymethylcellulose-Based Membrane as Polymer Electrolyte for Energy Devices. Biomass Bioenergy 2025, 193, 107542. [Google Scholar] [CrossRef]
- Akhlaq, M.; Uroos, M. Tetrabutylphosphonium Hydroxide Ionic Liquid-Assisted Highly Efficient Green Synthesis of Carboxymethyl Cellulose. J. Mol. Struct. 2025, 1327, 141221. [Google Scholar] [CrossRef]
- Pinto, E.; Aggrey, W.N.; Boakye, P.; Amenuvor, G.; Sokama-Neuyam, Y.A.; Fokuo, M.K.; Karimaie, H.; Sarkodie, K.; Adenutsi, C.D.; Erzuah, S.; et al. Cellulose Processing from Biomass and Its Derivatization into Carboxymethylcellulose: A Review. Sci. Afr. 2022, 15, e01078. [Google Scholar] [CrossRef]
- Carboxymethyl Cellulose Market Size, Forecast Report, Analysis 2025—2030. Available online: https://www.mordorintelligence.com/industry-reports/carboxymethyl-cellulose-cmc-market (accessed on 21 August 2025).
- Chen, Y.; Yu, H.-Y.; Li, Y. Highly Efficient and Superfast Cellulose Dissolution by Green Chloride Salts and Its Dissolution Mechanism. ACS Sustain. Chem. Eng. 2020, 8, 18446–18454. [Google Scholar] [CrossRef]
- Moussa, I.; Khiari, R.; Moussa, A.; Belgacem, M.N.; Mhenni, M.F. Preparation and Characterization of Carboxymethyl Cellulose with a High Degree of Substitution from Agricultural Wastes. Fibers Polym. 2019, 20, 933–943. [Google Scholar] [CrossRef]
- Zininga, J.T.; Puri, A.K.; Dlangamandla, N.; Wang, Z.; Singh, S.; Permaul, K. Integrated Biorefinery of Mucor circinelloides Biomass and Sugarcane Bagasse for Application of High-Value Biopolymers. Biomass Convers. Biorefin 2024, 14, 17863–17874. [Google Scholar] [CrossRef]
- Ndruru, S.T.C.L.; Syamsaizar, A.D.; Hermanto, S.; Sitanggang, B.C.; Tawa, B.D.; Kareem, A.A.; Hayati, A.T.; Ramadhoni, B.F.; Sofyan, M.I.; Annas, D.; et al. Synthesis of Carboxymethyl Cellulose from Coconut Fibers and Its Application as Solid Polymer Electrolyte Membranes. J Appl. Polym. Sci. 2024, 141, e55629. [Google Scholar] [CrossRef]
- Churam, T.; Usubharatana, P.; Phungrassami, H. Sustainable Production of Carboxymethyl Cellulose: A Biopolymer Alternative from Sugarcane (Saccharum officinarum L.) Leaves. Sustainability 2024, 16, 2352. [Google Scholar] [CrossRef]
- Vieira, F.; Santana, H.E.P.; Nunes, M.M.O.; Silva, I.P.; Silva, D.P.; Ruzene, D.S. Sequential Alkaline-Organosolv Pretreatment of Coconut Mesocarp Biomass: A Sustainable Strategy for Enhanced Carboxymethylcellulose Production. Cellulose 2025, 35, 7553–7572. [Google Scholar] [CrossRef]
- Kaur, P.; Bohidar, H.B.; Williams, R.; Pfeffer, F.M.; Agrawal, R. Turning Trash into Treasure: Conversion of Agroresidue Rice Straw into Carboxymethylcellulose Biopolymer. Biofuels Bioprod. Biorefining 2025, 19, 139–150. [Google Scholar] [CrossRef]
- Tuan Mohamood, N.F.A.Z.; Abdul Halim, A.H.; Zainuddin, N. Carboxymethyl Cellulose Hydrogel from Biomass Waste of Oil Palm Empty Fruit Bunch Using Calcium Chloride as Crosslinking Agent. Polymers 2021, 13, 4056. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Fang, C.; Cheng, Y.; Tan, T.; Han, H. Synthesis and Structure of Carboxymethylcellulose with a High Degree of Substitution Derived from Waste Disposable Paper Cups. Carbohydr. Polym. 2020, 237, 116040. [Google Scholar] [CrossRef]
- Gargey, I.A.; Indira, D.; Jayabalan, R.; Balasubramanian, P. Optimization of Etherification Reactions for Recycling of Tea Fungal Biomass Waste into Carboxymethylcellulose. In Green Buildings and Sustainable Engineering; Springer: Singapore, 2019; pp. 337–346. [Google Scholar] [CrossRef]
- Kukrety, A.; Singh, R.K.; Singh, P.; Ray, S.S. Comprehension on the Synthesis of Carboxymethylcellulose (CMC) Utilizing Various Cellulose Rich Waste Biomass Resources. Waste Biomass Valorization 2018, 9, 1587–1595. [Google Scholar] [CrossRef]
- Fischer, S.; Thümmler, K.; Pfeiffer, K.; Liebert, T.; Heinze, T. Evaluation of Molten Inorganic Salt Hydrates as Reaction Medium for the Derivatization of Cellulose. Cellulose 2002, 9, 293–300. [Google Scholar] [CrossRef]
- Heinze, T.; Pfeiffer, K. Studies on the Synthesis and Characterization of Carboxymethylcellulose. Die Angew. Makromol. Chem. 1999, 266, 37–45. [Google Scholar] [CrossRef]
- Ramos, L.A.; Frollini, E.; Heinze, T. Carboxymethylation of Cellulose in the New Solvent Dimethyl Sulfoxide/Tetrabutylammonium Fluoride. Carbohydr. Polym. 2005, 60, 259–267. [Google Scholar] [CrossRef]
- Heinze, T.; Schwikal, K.; Barthel, S. Ionic Liquids as Reaction Medium in Cellulose Functionalization. Macromol. Biosci. 2005, 5, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Casarano, R.; Pires, P.A.R.; Borin, A.C.; Seoud, O.A.E. Novel Solvents for Cellulose: Use of Dibenzyldimethylammonium Fluoride/Dimethyl Sulfoxide (DMSO) as Solvent for the Etherification of the Biopolymer and Comparison with Tetra(1-Butyl)Ammonium Fluoride/DMSO. Ind. Crops Prod. 2014, 54, 185–191. [Google Scholar] [CrossRef]
- Berikbol, N.; Klivenko, A.; Markin, V.; Orazzhanova, L.; Yelemessova, G.; Kassymova, Z. Development of Interpolyelectrolyte Complex Based on Chitosan and Carboxymethylcellulose for Stabilizing Sandy Soil and Stimulating Vegetation of Scots Pine (Pinus sylvestris L.). Polymers 2024, 16, 2373. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, M.; Chen, H.; Chen, Y.; Wang, L.; Wang, R. Fertigation and Carboxymethyl Cellulose Applications Enhance Water-Use Efficiency, Improving Soil Available Nutrients and Maize Yield in Salt-Affected Soil. Sustainability 2023, 15, 9602. [Google Scholar] [CrossRef]
- Vo, P.P.; Thi Ngoc Le, H.; Thi Truc Tran, M.; Truong-Lam, H.S. Modification of Carboxymethylcellulose Materials for Use in the Treatment of Aquaculture Water for Fluoroquinolone Antimicrobials. Desalination Water Treat. 2025, 323, 101274. [Google Scholar] [CrossRef]
- Cui, C.; Li, D.; Wang, L.J.; Wang, Y. Curdlan/Sodium Carboxymethylcellulose Composite Adsorbents: A Biodegradable Solution for Organic Dye Removal from Water. Carbohydr. Polym. 2024, 328, 121737. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Cavaco-Paulo, A.; Wang, H.; Su, J. Double-Defense Barrier Design for Anti-Fouling Separation Membrane with the Biological Catalysis and Hydrogel Coating for Dye/Salt Separation. Sep. Purif. Technol. 2025, 359, 130575. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, P.; Huang, Z.; Zhang, L.; Xie, S.; Qi, Z. Carboxymethylcellulose Sodium-Derived Carbon Aerogels for Solar-Driven Water Purification. Chemosphere 2024, 358, 142109. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, J.; Wang, A.; Yuan, H.; Chi, Y. Adsorption Behavior and Mechanism of Cu(II) by Sodium Alginate/Carboxymethylcellulose/Magnesium Hydroxide (SC-MH) Hydrogel. Int. J. Biol. Macromol. 2024, 277, 134046. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Li, D.; Chen, S.; Zhang, F.; Zhang, Z.; Tan, H.; Yang, L.; Hou, J.; Tan, R.; et al. Carboxymethylcellulose-Based Zwitterionic Cryogels for Efficient U(VI) Extraction from Water. Sep. Purif. Technol. 2024, 346, 127485. [Google Scholar] [CrossRef]
- Chand, R.; Janarthanan, G.; Elkhoury, K.; Vijayavenkataraman, S. Digital Light Processing 3D Bioprinting of Biomimetic Corneal Stroma Equivalent Using Gelatin Methacryloyl and Oxidized Carboxymethylcellulose Interpenetrating Network Hydrogel. Biofabrication 2025, 17, 025011. [Google Scholar] [CrossRef]
- Seiti, M.; Mazzoldi, E.L.; Pandini, S.; Giliani, S.; Ferraris, E.; Ginestra, P.S.; Ceretti, E. FRESH 3D Bioprinting of Alginate—Cellulose—Gelatin Constructs for Soft Tissue Biofabrication. Procedia CIRP 2024, 125, 42–47. [Google Scholar] [CrossRef]
- Budharaju, H.; Chandrababu, H.; Zennifer, A.; Chellappan, D.; Sethuraman, S.; Sundaramurthi, D. Tuning Thermoresponsive Properties of Carboxymethyl Cellulose (CMC)–Agarose Composite Bioinks to Fabricate Complex 3D Constructs for Regenerative Medicine. Int. J. Biol. Macromol. 2024, 260, 129443. [Google Scholar] [CrossRef]
- Le, M.A.T.; Duong-Huu, L.T.; Luong, T.D.; Vu, B.T.; Tang, T.N.; Le, K.M.; Pham, D.K.; Doan, H.N.; Van, T.V.; Nguyen, T.H. Investigation of Fast in Situ Injectable Carboxymethylcellulose-Xanthan Gum Hydrogel via Acyl Hydrazone Linkages for Tissue Regeneration. Cellulose 2025, 32, 4873–4896. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, S.; Niu, W.; Wei, L.; Liu, Y.; Wang, H.; Niu, B.; Li, W. Carboxymethylcellulose Hydrogel Beads Containing Quercetin-Embedded Porous Starch: An Intestinal Tract Drug Delivery System. Int. J. Biol. Macromol. 2025, 304, 140927. [Google Scholar] [CrossRef]
- Silva, N.d.C.; Silva, C.J.T.; Gonçalves, M.P.; Borsagli, F.G.L.M. Carboxymethyl-Cellulose-Based Hydrogels Incorporated with Cellulose Nanocrystals Loaded with Vitamin D for Controlled Drug Delivery. Processes 2024, 12, 1437. [Google Scholar] [CrossRef]
- Alruwaili, N.K.; Ahmad, N.; Alzarea, A.I.; Alomar, F.A.; Alquraini, A.; Akhtar, S.; Shahari, M.S.B.; Zafar, A.; Elmowafy, M.; Elkomy, M.H.; et al. Arabinoxylan-Carboxymethylcellulose Composite Films for Antibiotic Delivery to Infected Wounds. Polymers 2022, 14, 1769. [Google Scholar] [CrossRef] [PubMed]
- Thambirajoo, M.; Md Fadilah, N.I.; Maarof, M.; Lokanathan, Y.; Mohamed, M.A.; Zakaria, S.; Bt Hj Idrus, R.; Fauzi, M.B. Functionalised Sodium–Carboxymethylcellulose–Collagen Bioactive Bilayer as an Acellular Skin Substitute for Future Use in Diabetic Wound Management: The Evaluation of Physicochemical, Cell Viability, and Antibacterial Effects. Polymers 2024, 16, 2252. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, Z.; Baharifar, H.; Najmoddin, N.; Khoshnevisan, K. Evaluation of Carboxymethyl Cellulose/Gelatin Hydrogel-Based Dressing Containing Cefdinir for Wound Healing Promotion in Animal Model. Gels 2025, 11, 38. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Santiago-Aliste, A.; Correa-Guimarães, A.; Martín-Gil, J.; Gavara-Clemente, R.J.; Martín-Ramos, P. Carvacrol Encapsulation in Chitosan–Carboxymethylcellulose–Alginate Nanocarriers for Postharvest Tomato Protection. Int. J. Mol. Sci. 2024, 25, 1104. [Google Scholar] [CrossRef]
- Elmehbad, N.Y.; Mohamed, N.A.; El-Ghany, N.A.A. Synthesis of Novel Antimicrobial and Food-Preserving Hydrogel Nanocomposite Films Based on Carboxymethylcellulose. Starch-Stärke 2024, 76, 2300258. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Haider, A.; ur Rehman, F.; Cui, G.; Waseem, M.; Iqbal, M.; Mousavi Khaneghah, A. Application of Carboxymethylcellulose in Combination with Essential Oils Nano-Emulsions Edible Coating for the Preservation of Kiwifruit. Int. J. Biol. Macromol. 2024, 261, 129947. [Google Scholar] [CrossRef]
- Vargas-Torrico, M.F.; Aguilar-Méndez, M.A.; Ronquillo-de Jesús, E.; Jaime-Fonseca, M.R.; von Borries-Medrano, E. Preparation and Characterization of Gelatin-Carboxymethylcellulose Active Film Incorporated with Pomegranate (Punica granatum L.) Peel Extract for the Preservation of Raspberry Fruit. Food Hydrocoll. 2024, 150, 109677. [Google Scholar] [CrossRef]
- Ragab, H.M.; Diab, N.S.; Elneim, E.A.A.; El Fewaty, N.H.; Al-Hakimi, A.N.; Farea, M.O. Exploring the Optical Properties of CuCo2O4-Doped Polyethylene Oxide and Carboxymethylcellulose for Optoelectronic Application. Opt. Quantum Electron. 2024, 56, 323. [Google Scholar] [CrossRef]
- Zheng, N.; Pan, H.; Chai, Z.; Liu, Z.; Gao, F.; Wang, G.; Huang, X. Anisotropic Rotunda-Shaped Carboxymethylcellulose/Carbon Nanotube Aerogels Supported Phase Change Materials for Efficient Solar-Thermal Energy Conversion. ChemSusChem 2024, 17, e202301971. [Google Scholar] [CrossRef]
- Tang, B.; Jin, J.; Han, K.; Li, T.; Zhang, H.; Zhang, X.; Molokeev, M.; Wang, Y.; Xia, Z.; Lei, B. In Situ Crystallization of Copper(I)-Based Hybrid Halides Assisted by Carboxymethylcellulose Sodium for a Large-Area Scintillation Imaging Screen. Adv. Funct. Mater. 2025, 35, 2500806. [Google Scholar] [CrossRef]
- Arif, M.B.; Yulianti, E.; Sabrina, Q.; Sudaryanto, S.; Ndruru, S.T.C.L.; Ghozali, M. Preparation of Bio-Polyelectrolyte Complex Membrane from Carboxymethylcellulose and Chitosan as a Selective Alternative Zinc-Ion Battery Separator. Polymer 2025, 323, 128181. [Google Scholar] [CrossRef]
- Bo, S.; Yu, L.; Zhang, L.; Saeed, G.; Kim, M.; Lee, J.W.; Kwon, S.H.; Park, W.I.; Kim, K.H. Engineering FeSe2 Encapsulated within Carboxymethylcellulose-Derived Porous Carbon for Enhanced Surface Reaction Kinetics in Li-Ion Half/Full Battery Anodes. Surf. Interfaces 2024, 51, 104696. [Google Scholar] [CrossRef]
- Luo, X.; Wang, S.; Wu, N.; Zhang, L.; He, Q.; Ren, L.; Song, W. Dual-Network Conductive Hydrogels Based on Carboxymethylcellulose/ Aminated Carbon Nanotube Reinforcement: Monitoring Human Micro-Expressions and Signal Changes. Int. J. Biol. Macromol. 2025, 320, 146012. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Xu, Y.; Maimaitiyiming, X. Egg White/Gelatin/Carboxymethylcellulose Superbly Bonded and Biocompatible Flexible Self-Adhesive Multifunctional Sensor. Cellulose 2024, 31, 6779–6795. [Google Scholar] [CrossRef]
- Shivananda, C.S.; Pavan Kumar, G.; Vivek, M.V.; Madhu, S.; Lakshmeesha Rao, B. Silver Nanoparticles Reinforced on Silk Fibroin/Carboxymethylcellulose Composite Films for Electrical Applications. Mater. Sci. Eng. B 2024, 304, 117392. [Google Scholar] [CrossRef]
- Anupama Devi, V.K.; Shyam, R.; Palaniappan, A.; Jaiswal, A.K.; Oh, T.H.; Nathanael, A.J. Self-Healing Hydrogels: Preparation, Mechanism and Advancement in Biomedical Applications. Polymers 2021, 13, 3782. [Google Scholar] [CrossRef]
- Arumughan, V.; Nypelö, T.; Hasani, M.; Larsson, A. Calcium Ion-Induced Structural Changes in Carboxymethylcellulose Solutions and Their Effects on Adsorption on Cellulose Surfaces. Biomacromolecules 2022, 23, 47–56. [Google Scholar] [CrossRef]
- Dueramae, I.; Okhawilai, M.; Kasemsiri, P.; Uyama, H. High Electrochemical and Mechanical Performance of Zinc Conducting-Based Gel Polymer Electrolytes. Sci. Rep. 2021, 11, 13268. [Google Scholar] [CrossRef]
- Ramos, L.d.A.; Frollini, E.; Heinze, T. Benzylation of Cellulose in the New Solvent Dimethyl Sulfoxide/Tetrabutylammonium Fluoride Trihydrate. Anais da ABPol; São Carlos, Brazil, 2004. Available online: https://www.eucalyptus.com.br/artigos/2004_Eigth+Symposium+Lignins+Wood+Components_Arquivo+80.pdf (accessed on 6 October 2025).
- Trivedi, P.; Fardim, P. Recent Advances in Cellulose Chemistry and Potential Applications. In Production of Materials from Sustainable Biomass Resources; Springer: Singapore, 2019; pp. 99–115. [Google Scholar] [CrossRef]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R.; et al. A Review on the Modification of Cellulose and Its Applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef] [PubMed]
- Shokri, M.; Moradi, S.; Amini, S.; Shahlaei, M.; Seidi, F.; Saedi, S. A Novel Amino Cellulose Derivative Using ATRP Method: Preparation, Characterization, and Investigation of Its Antibacterial Activity. Bioorg Chem. 2021, 106, 104355. [Google Scholar] [CrossRef] [PubMed]
- Rohleder, E.; Heinze, T. Comparison of Benzyl Celluloses Synthesized in Aqueous NaOH and Dimethyl Sulfoxide/Tetrabutylammonium Fluoride. Macromol. Symp. 2010, 294, 107–116. [Google Scholar] [CrossRef]
- Yusoff, N.A.; Shahib, N.M.; Zainol, N.A.; Sohaimi, K.S.A.; Rohaizad, N.M.; Wikurendra, E.A.; Andini, A.; Syafiuddin, A. Microwave-Assisted Synthesis and Characterization of Polyacrylamide Grafted Cellulose Derived from Waste Newspaper for Surface Water Treatment. Desalination Water Treat. 2022, 259, 90–97. [Google Scholar] [CrossRef]
- Ramos, L.A.; Frollini, E.; Koschella, A.; Heinze, T. Benzylation of Cellulose in the Solvent Dimethylsulfoxide/Tetrabutylammonium Fluoride Trihydrate. Cellulose 2005, 12, 607–619. [Google Scholar] [CrossRef]
- Abe, M.; Sugimura, K.; Nishiyama, Y.; Nishio, Y. Rapid Benzylation of Cellulose in Tetra-n-Butylphosphonium Hydroxide Aqueous Solution at Room Temperature. ACS Sustain. Chem. Eng. 2017, 5, 4505–4510. [Google Scholar] [CrossRef]
- Saliu, O.D.; Olatunji, G.A.; Yakubu, A.; Arowona, M.T.; Mohammed, A.A. Catalytic Crosslinking of a Regenerated Hydrophobic Benzylated Cellulose and Nano TiO2 Composite for Enhanced Oil Absorbency. e-Polymers 2017, 17, 295–302. [Google Scholar] [CrossRef]
- Shibano, M.; Ochiai, H.; Suzuki, K.; Kamitakahara, H.; Kaji, H.; Takano, T. Thermally Activated Delayed Fluorescence Benzyl Cellulose Derivatives for Nondoped Organic Light-Emitting Diodes. Macromolecules 2020, 53, 2864–2873. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Q.; Song, Y.; Zhang, L.; Lin, X. A Facile Method for the Homogeneous Synthesis of Cyanoethyl Cellulose in NaOH/Urea Aqueous Solutions. Polym. Chem. 2010, 1, 1662–1668. [Google Scholar] [CrossRef]
- Wang, B.; Kang, H.; Yang, H.; Xie, J.; Liu, R. Preparation and Dielectric Properties of Porous Cyanoethyl Cellulose Membranes. Cellulose 2019, 26, 1261–1275. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, X.; Yang, R.; Wang, J.; Lu, C.; Guo, K.; Zhu, N.; Hu, X. Itaconic Anhydride Functionalized Cyanoethyl Cellulose with Crosslinked Structure Enabled Improved Dielectric Properties. Polym. Int. 2024, 73, 1022–1029. [Google Scholar] [CrossRef]
- Joshi, G.; Naithani, S.; Varshney, V.K.; Bisht, S.S.; Rana, V. Potential Use of Waste Paper for the Synthesis of Cyanoethyl Cellulose: A Cleaner Production Approach towards Sustainable Environment Management. J. Clean. Prod. 2017, 142, 3759–3768. [Google Scholar] [CrossRef]
- Ansari, T.; Chandra, G.; Gupta, P.K.; Joshi, G.; Rana, V. Synthesis of Pine Needle Cyanoethyl Cellulose Using Taguchi L25 Orthogonal Array. Ind. Crops Prod. 2023, 191, 115973. [Google Scholar] [CrossRef]
- Wang, H.Q.; Shao, Z.Q.; Lv, S.Y. New Facile Method for the Hetergeneous Synthesis of Cyanoethyl Cellulose- Solvent Method, and Comparing with Traditional Method. Adv. Mat. Res. 2011, 239–242, 2084–2090. [Google Scholar] [CrossRef]
- Mi, S.; Yao, Z.; Liu, F.; Li, Y.; Wang, J.; Na, H.; Zhu, J. Homogeneous Cyanoethylation of Cellulose with Acrylonitrile in a CO2 Switchable Solvent. Green. Chem. 2022, 24, 8677–8684. [Google Scholar] [CrossRef]
- Wu, L.; Zhao, J.; Li, Z.; Zhai, Y.; Zhang, Y.; Zhen, Q.; Cheng, Y.; Ding, X.; Li, P.; Liu, J.; et al. Flexible Cyanoethyl Cellulose-Based Nanocomposites with Superior Energy Storage Capability. J. Mater. Chem. C Mater. 2022, 10, 15416–15423. [Google Scholar] [CrossRef]
- Chen, L.; Sun, H.; Guo, J.; Liu, S.; Guo, Y.; Zhou, G.; Yuan, D.; Tang, B.; Lai, J. Novel Cyanoethyl Cellulose-Based Bilayer Materials for Electrowetting Displays at Low Voltage. ACS Appl. Mater. Interfaces 2025, 17, 17509–17520. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, W.; Li, Z.; Wang, S.; Suwardi, A.; Ye, E.; Li, B.; Liu, Y.; Wu, Z.; Dong, Y.; et al. Dual-Electric-Polarity Augmented Cyanoethyl Cellulose-Based Triboelectric Nanogenerator with Ultra-High Triboelectric Charge Density and Enhanced Electrical Output Property at High Humidity. Nano Energy 2022, 103, 107748. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, L.; Du, X.; Zhang, J.; Tian, S.; Liu, T.; Zhang, J.; Hu, S.; Song, W.; Zhou, X.; et al. Cyanoethyl Cellulose-Based Eutectogel Electrolyte Enabling High-Voltage-Tolerant and Ion-Conductive Solid-State Lithium Metal Batteries. Carbon. Energy 2022, 4, 1093–1106. [Google Scholar] [CrossRef]
- Dacrory, S.; Hammad, A.B.A.; El Nahrawy, A.M.; Abou-Yousef, H.; Kamel, S. Cyanoethyl Cellulose/BaTiO3/GO Flexible Films with Electroconductive Properties. ECS J. Solid. State Sci. Technol. 2021, 10, 083004. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Wei, Y.; Zhang, A.; Liu, C. Homogeneous Esterification Mechanism of Bagasse Modified with Phthalic Anhydride in Ionic Liquid. Part 2: Reactive Behavior of Hemicelluloses. Carbohydr. Polym. 2017, 157, 1365–1373. [Google Scholar] [CrossRef]
- Akkus, M.; Ozkan, N.; Bakir, U. Efficient Acetylation of Xylans by Exploiting the Potassium Acetate Formed During the Alkaline Extraction. J. Polym. Environ. 2018, 26, 3397–3403. [Google Scholar] [CrossRef]
- Martins, J.R.; Llanos, J.H.R.; Botaro, V.; Gonçalves, A.R.; Brienzo, M. Hemicellulose Biomass Degree of Acetylation (Natural Versus Chemical Acetylation) as a Strategy for Based Packaging Materials. Bioenergy Res. 2024, 17, 877–896. [Google Scholar] [CrossRef]
- Sun, L.; Lee, J.W.; Yook, S.; Lane, S.; Sun, Z.; Kim, S.R.; Jin, Y.S. Complete and Efficient Conversion of Plant Cell Wall Hemicellulose into High-Value Bioproducts by Engineered Yeast. Nat. Commun. 2021, 12, 4975. [Google Scholar] [CrossRef]
- Berglund, J.; Mikkelsen, D.; Flanagan, B.M.; Dhital, S.; Gaunitz, S.; Henriksson, G.; Lindström, M.E.; Yakubov, G.E.; Gidley, M.J.; Vilaplana, F. Wood Hemicelluloses Exert Distinct Biomechanical Contributions to Cellulose Fibrillar Networks. Nat. Commun. 2020, 11, 4692. [Google Scholar] [CrossRef]
- Wang, S.; Gao, W.; Wang, Y.; Song, T.; Qi, H.; Xiang, Z. Emulsifying Properties of Naturally Acetylated Xylans and Their Application in Lutein Delivery Emulsion. Carbohydr. Polym. 2022, 296, 119927. [Google Scholar] [CrossRef]
- Fuso, A.; Rosso, F.; Rosso, G.; Risso, D.; Manera, I.; Caligiani, A. Production of Xylo-Oligosaccharides (XOS) of Tailored Degree of Polymerization from Acetylated Xylans through Modelling of Enzymatic Hydrolysis. Food Res. Int. 2022, 162, 112019. [Google Scholar] [CrossRef]
- Mugwagwa, L.R.; Chimphango, A.F.A. Enhancing the Functional Properties of Acetylated Hemicellulose Films for Active Food Packaging Using Acetylated Nanocellulose Reinforcement and Polycaprolactone Coating. Food Packag. Shelf Life 2020, 24, 100481. [Google Scholar] [CrossRef]
- Yong, Q.; Xu, J.; Wang, L.; Tirri, T.; Gao, H.; Liao, Y.; Toivakka, M.; Xu, C. Synthesis of Galactoglucomannan-Based Latex via Emulsion Polymerization. Carbohydr. Polym. 2022, 291, 119565. [Google Scholar] [CrossRef]
- Nizam, P.A.; Hu, L.; Hemmimg, J.; Rosqvist, E.; Peltonen, J.; Toivakka, M.; Xu, C. Dual Function Galactoglucomannan Derivative for Emulsion Polymerization towards Barrier Coating Applications. Chem. Eng. J. 2025, 511, 162183. [Google Scholar] [CrossRef]
- Martins, J.R.; Llanos, J.H.R.; Abe, M.M.; Costa, M.L.; Brienzo, M. New Blend of Renewable Bioplastic Based on Starch and Acetylated Xylan with High Resistance to Oil and Water Vapor. Carbohydr. Res. 2024, 537, 109068. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Q.; Qi, H.; Xiang, Z. Significantly Improve Film Formability of Acetylated Xylans by Structure Optimization and Solvent Screening. Int. J. Biol. Macromol. 2024, 256, 128523. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, W.; Koppolu, R.; van Bochove, B.; Seppälä, J.; Hupa, L.; Willför, S.; Xu, C.; Wang, X. Injectable Thiol-Ene Hydrogel of Galactoglucomannan and Cellulose Nanocrystals in Delivery of Therapeutic Inorganic Ions with Embedded Bioactive Glass Nanoparticles. Carbohydr. Polym. 2022, 276, 118780. [Google Scholar] [CrossRef]
- Wang, S.; Xiang, Z. Highly Stable Pickering Emulsions with Xylan Hydrate Nanocrystals. Nanomaterials 2021, 11, 2558. [Google Scholar] [CrossRef] [PubMed]
- de Paula Castanheira, J.; Llanos, J.H.R.; Martins, J.R.; Costa, M.L.; Brienzo, M. Acetylated Xylan from Sugarcane Bagasse: Advancing Bioplastic Formation with Enhanced Water Resistance. Polym. Bull. 2025, 82, 1705–1722. [Google Scholar] [CrossRef]
- FI20206137A1—Method for the Production of Esterified Cellulose and/or Hemicellulose—Google Patents. Available online: https://patents.google.com/patent/FI20206137A1/en (accessed on 22 August 2025).
- Wang, Z.M.; Li, L.; Zheng, B.S.; Normakhamatov, N.; Guo, S.Y. Preparation and Anticoagulation Activity of Sodium Cellulose Sulfate. Int. J. Biol. Macromol. 2007, 41, 376–382. [Google Scholar] [CrossRef]
- Ragab, T.I.M.; Amer, H.; Mossa, A.T.; Emam, M.; Hasaballah, A.A.; Helmy, W.A. Anticoagulation, Fibrinolytic and the Cytotoxic Activities of Sulfated Hemicellulose Extracted from Rice Straw and Husk. Biocatal. Agric. Biotechnol. 2018, 15, 86–91. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, X.; Shan, J.; Tang, C.; Hu, R.; Shen, T.; Qiao, H.; Li, M.; Zhuang, W.; Zhu, C.; et al. Flow Synthesis, Characterization, Anticoagulant Activity of Xylan Sulfate from Sugarcane Bagasse. Int. J. Biol. Macromol. 2020, 155, 1460–1467. [Google Scholar] [CrossRef]
- Fröhlich, A.C.; Bazzo, G.C.; Stulzer, H.K.; Parize, A.L. Synthesis and Physico-Chemical Characterization of Quaternized and Sulfated Xylan-Derivates with Enhanced Microbiological and Antioxidant Properties. Biocatal. Agric. Biotechnol. 2022, 43, 102416. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Chen, G.; Liu, B. Cellulose Sulfate Based Film with Slow-Release Antimicrobial Properties Prepared by Incorporation of Mustard Essential Oil and β-Cyclodextrin. Food Hydrocoll. 2016, 55, 100–107. [Google Scholar] [CrossRef]
- Pires, C.; Régnier, B.M.; dos Santos, M.J.R.; Alves de Freitas, R. Effect of Sulfate-Ester Content and Nanocellulose Allomorph on Stability of Amylopectin-Xyloglucan Water-in-Water Emulsions. Food Hydrocoll. 2023, 141, 108700. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mathew, A.P. Cellulose-Based Materials for Water Remediation: Adsorption, Catalysis, and Antifouling. Front. Chem. Eng. 2021, 3, 790314. [Google Scholar] [CrossRef]
- Kaya, E.C.; Yucel, U.; Kaya, E.C.; Yucel, U. Advances in Cellulose-Based Packaging Films for Food Products. In Cellulose—Fundamentals and Conversion Into Biofuel and Useful Chemicals; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Berezhnaya, Y.D.; Kazachenko, A.S.; Kazachenko, A.S.; Malyar, Y.N.; Borovkova, V.S. Sulfation of Various Polysaccharide Structures: Different Methods and Perspectives. Chemistry 2024, 6, 640–665. [Google Scholar] [CrossRef]
- Laribi, N.; Maatoug, S.; Jebali, Z.; Zouari, R.; Majdoub, H.; Cheikhrouhou, M. Low-Cost Carboxymethyl Holocellulose And Carboxymethyl Cellulose from Wheat Straw. Cellul. Chem. Technol. 2020, 54, 225–236. [Google Scholar] [CrossRef]
- Peng, X.-W.; Ren, J.-L.; Zhong, L.-X.; Cao, X.-F.; Sun, R.-C. Microwave-Induced Synthesis of Carboxymethyl Hemicelluloses and Their Rheological Properties. Biomacromolecules 2010, 11, 3519–3524. [Google Scholar] [CrossRef]
- Tohamy, H.A.S. Carboxymethyl Hemicellulose Hydrogel as a Fluorescent Biosensor for Bacterial and Fungal Detection with DFT and Molecular Docking Studies. Sci. Rep. 2025, 15, 741. [Google Scholar] [CrossRef]
- Bai, X.; Chen, T.; Li, M.; Yang, W.; Xu, W.; Zheng, X.; Zhang, W.; Tang, Y. Extraction and Carboxymethylation of Hemicelluloses from Moso Bamboo. Chung-Kuo Tsao Chih/China Pulp Pap. 2023, 42, 68–77. [Google Scholar] [CrossRef]
- Liu, H.; Chen, T.; Dong, C.; Pan, X. Biomedical Applications of Hemicellulose-Based Hydrogels. Curr. Med. Chem. 2020, 27, 4647–4659. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.-L.; Sun, R.-C.; Peng, F. Carboxymethylation of Hemicelluloses Isolated from Sugarcane Bagasse. Polym. Degrad. Stab. 2008, 93, 786–793. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Sun, R.C.; Tomkinson, J.; Wang, Y.X.; Xiao, B. Physico-Chemical and Structural Characterization of Hemicelluloses from Wheat Straw by Alkaline Peroxide Extraction. Polymer 2000, 41, 2647–2656. [Google Scholar] [CrossRef]
- Liu, M.; Imiete, I.E.; Staropoli, M.; Steiner, P.; Duez, B.; Lenoble, D.; Scolan, E.; Thomann, J.-S. Hydrophobized MFC as Reinforcing Additive in Industrial Silica/SBR Tire Tread Compound. Polymers 2023, 15, 3937. [Google Scholar] [CrossRef]
- He, Y.; Liu, Y.; Zhang, M. Hemicellulose and Unlocking Potential for Sustainable Applications in Biomedical, Packaging, and Material Sciences: A Narrative Review. Int. J. Biol. Macromol. 2024, 280, 135657. [Google Scholar] [CrossRef]
- Abou-Zeid, N.Y.; Waly, A.I.; Kandile, N.G.; Rushdy, A.A.; El-Sheikh, M.A.; Ibrahim, H.M. Preparation, Characterization and Antibacterial Properties of Cyanoethylchitosan/Cellulose Acetate Polymer Blended Films. Carbohydr. Polym. 2011, 84, 223–230. [Google Scholar] [CrossRef]
- Fiege, K.; Lünsdorf, H.; Atarijabarzadeh, S.; Mischnick, P. Cyanoethylation of the Glucans Dextran and Pullulan: Substitution Pattern and Formation of Nanostructures and Entrapment of Magnetic Nanoparticles. Beilstein J. Org. Chem. 2012, 8, 551–566. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Li, W.; Zhang, L.; Wan, C.; Gao, S. Synthesis of Cyanoethyl Konjac Glucomannan and Its Liquid Crystalline Behavior in an Ionic Liquid. J. Polym. Res. 2012, 19, 9758. [Google Scholar] [CrossRef]
- Cao, X.; Sun, S.; Peng, X.; Zhong, L.; Sun, R. Synthesis and Characterization of Cyanoethyl Hemicelluloses and Their Hydrated Products. Cellulose 2013, 20, 291–301. [Google Scholar] [CrossRef]
- D’Urso, F.; Iaccarino, P.; Giordano, M.; Oliviero, M.; Di Maio, E.; Sansone, L. A Preliminary Study on 3D Printing Feedstock Derived from Cellulose Recovered from Cigarette Butts. Cellulose 2024, 31, 5097–5114. [Google Scholar] [CrossRef]
- Tiller, P.; Park, S.; Sanders, J.; Treasure, T.; Park, S. Evaluating the Quality and Processability of Cotton Linter-Derived Cellulose Acetate by Characterization of Native and Artificial Fines. Cellulose 2025, 32, 2989–3005. [Google Scholar] [CrossRef]
- Fernandes, M.A.M.; Nörnberg, L.V.; Acosta, A.P.; Barbosa, K.T.; Cardoso, G.V. Green Chemistry in the Production of Cellulose Acetate: The Use of a New Low-Cost, Highly Available Waste Product. Biomass Convers. Biorefin. 2025, 15, 12515–12523. [Google Scholar] [CrossRef]
- Uzoh, R.D.; Jildawa, D. Production Of Biodegradable Plastic, Cellulose Acetate from Agricultural Biomass for Biomedical and Packaging Uses. Int. J. Health Res. Phys. Study 2024, 4, 183–189. [Google Scholar]
- da Fonseca Antunes, B.; Santana, L.R.; Oliveira, R.M.; Valério Filho, A.; Carreno, N.L.V.; Wolke, S.I.; da Silva, R.; Fajardo, A.R.; Dias, A.R.G.; Rosa Zavareze, E.d. Cellulose, Cellulose Nanofibers, and Cellulose Acetate from Butia Fruits (Butia odorata): Chemical, Morphological, Structural, and Thermal Properties. Int. J. Biol. Macromol. 2024, 281, 136151. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.E.; Elwardany, R.E.; Mustafa, A.A.; Shokry, H. Remediation of Contaminated Water Using Cellulose Acetate Membrane Hybrid by Sunflower Seed Shell–Activated Carbon. Biomass Convers. Biorefin. 2025, 15, 5701–5717. [Google Scholar] [CrossRef]
- Arif, Z.; Fendy; Akhmad Aghzath, A.; Saprudin, D.; Rohaeti, E. Simple Synthesis of Cellulose Triacetate from HVS Paper Waste and Its Application for Optode. Indones. J. Chem. Stud. 2024, 3, 16–21. [Google Scholar] [CrossRef]
- Shakhabutdinov, S.S.; Yugay, S.M.; Ashurov, N.S.; Ergashev, D.J.; Atakhanov, A.A.; Rashidova, S.S. Characterization Electrospun Nanofibers Based on Cellulose Triacetate Synthesized from Licorice Root Cellulose. Eurasian J. Chem. 2024, 29, 21–31. [Google Scholar] [CrossRef]
- Dong, H.; Wang, D.; Wang, C.; Qi, S.; Shi, M.; Tong, G.; Wang, J.; Bian, B. Solid Superacid SO42−/TiO2/Al2O3 with Unique “Ecological Infiltration System” for Efficient Catalytic Synthesis of Cellulose Triacetate. Cellulose 2025, 32, 7029–7044. [Google Scholar] [CrossRef]
- Hindi, S.S.Z.; Abohassan, R.A. Cellulose Triacetate Synthesis from Cellulosic Wastes by Heterogeneous Reactions. Bioresources 2015, 10, 5030–5048. [Google Scholar] [CrossRef]
- Alessandro, L.; Chan, E.W.C.; Jaafar, J.; Beardall, J.; Soo, M.O.Y. Characterization of Porous Cellulose Triacetate Derived from Kapok Fibres (Ceiba pentandra) as a Tool to Enhance Crude Oil Absorption. Discov. Mater. 2025, 5, 39. [Google Scholar] [CrossRef]
- de Oliveira Fadul, J.A.G.; Souza, V. da S. de; Souza, D. Electrospinning of Cellulose Acetate Propionate: Optimization of Processing Parameters for Advanced Applications. Mater. Res. 2025, 28, e20250232. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Ferri, M.; Degli Esposti, M.; Richert, A.; Fabbri, P. Innovative Biobased Active Composites of Cellulose Acetate Propionate with Tween 80 and Cinnamic Acid for Blueberry Preservation. Polymers 2025, 17, 2072. [Google Scholar] [CrossRef]
- Najaflou, S.; Rad, M.F.; Baghdadi, M.; Nabi Bidhendi, G.R. Removal of Pb(II) from Contaminated Waters Using Cellulose Sulfate/Chitosan Aerogel: Equilibrium, Kinetics, and Thermodynamic Studies. J. Environ. Manag. 2021, 286, 112167. [Google Scholar] [CrossRef] [PubMed]
- Rol, F.; Sillard, C.; Bardet, M.; Yarava, J.R.; Emsley, L.; Gablin, C.; Léonard, D.; Belgacem, N.; Bras, J. Cellulose Phosphorylation Comparison and Analysis of Phosphorate Position on Cellulose Fibers. Carbohydr. Polym. 2020, 229, 115294. [Google Scholar] [CrossRef] [PubMed]
- Etale, A.; Onyianta, A.J.; Turner, S.R.; Eichhorn, S.J. Cellulose: A Review of Water Interactions, Applications in Composites, and Water Treatment. Chem. Rev. 2023, 123, 2016–2048. [Google Scholar] [CrossRef]
- Ranjan, R.; Mone, V.P.; Rai, R.; Kant, C.; Dhar, P. All-Biomass-Derived Cellulose Phosphate-Based Heat-Sealable Films and Thermally Stable Antifizzing Cups with Improved Recyclability. ACS Sustain. Chem. Eng. 2025, 13, 6074–6089. [Google Scholar] [CrossRef]
- Cao, R.; Long, Y.; Li, T.; Lv, W.; Wu, H.; Wang, B.; Song, Y.; Gao, H.; Nie, Y. Swelling and Dissolution Behaviors of Cellulose in Phosphate-Based Ionic Liquids-H2O Binary Systems: In-Situ Observation and Molecular Dynamics Simulation. J. Mol. Liq. 2024, 415, 126315. [Google Scholar] [CrossRef]
- Korchagin, A.A. Synthesis of Cellulose Nitrates from Miscanthus × Giganteus Var. KAMIS Cellulose Obtained under Pilot Production Conditions. Proc. Univ. Appl. Chem. Biotechnol. 2023, 13, 392–401. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Abdelaziz, A.; Bekhouche, S.; Boukeciat, H. Exploration of Palm Fronds as a Prominent Alternative Resource for the Production of Energetic Cellulose-Rich Biopolymers. Mater. Today Proc. 2022, 53, 31–35. [Google Scholar] [CrossRef]
- Elmaghraby, N.A.; Omer, A.M.; Kenawy, E.R.; Gaber, M.; El Nemr, A. Fabrication of Cellulose Acetate/Cellulose Nitrate/Carbon Black Nanofiber Composite for Oil Spill Treatment. Biomass Convers. Biorefin. 2024, 14, 27575–27593. [Google Scholar] [CrossRef]
- Bekhouche, S.; Trache, D.; Abdelaziz, A.; Tarchoun, A.F.; Chelouche, S.; Boudjellal, A.; Mezroua, A. Preparation and Characterization of MgAl-CuO Ternary Nanothermite System by Arrested Reactive Milling and Its Effect on the Thermocatalytic Decomposition of Cellulose Nitrate. Chem. Eng. J. 2023, 453, 139845. [Google Scholar] [CrossRef]
- Dallocchio, R.; Dessì, A.; Sechi, B.; Peluso, P. Molecular Dynamics Simulations of Amylose- and Cellulose-Based Selectors and Related Enantioseparations in Liquid Phase Chromatography. Molecules 2023, 28, 7419. [Google Scholar] [CrossRef]
- Elsayed, K.A.; Mahmoud, K.H.; Haladu, S.A.; Magami, S.M.; Manda, A.A.; Kayed, T.S.; Baroot, A.; Khan, M.Y.; Cevik, E.; Drmosh, Q.A.; et al. Thermal, Dielectric and Optical Studies on Cellulose Acetate Butyrate-Gold Nanocomposite Films Prepared by Laser Ablation. J. Mater. Res. Technol. 2023, 23, 419–437. [Google Scholar] [CrossRef]
- Xiao, B.; Qian, Y.; Li, X.; Tao, Y.; Yi, Z.; Jiang, Q.; Luo, Y.; Yang, J. Enhancing the Stability of Planar Perovskite Solar Cells by Green and Inexpensive Cellulose Acetate Butyrate. J. Energy Chem. 2023, 76, 259–265. [Google Scholar] [CrossRef]
- Lee, J.; Kang, S.W. Porous Cellulose Propionate Induced by Mobile Phase for Specific Channels. Korean J. Chem. Eng. 2023, 40, 2997–3002. [Google Scholar] [CrossRef]
- Kung, D.C.N.; Moon, J.; Cho, Y.; Kang, H.; Kang, S.W. Enhancing Barrier Properties of Cellulose Propionate Films through the Integration of Ionic Liquid: A Study on Water Pressure Resistance. Int. J. Biol. Macromol. 2024, 282, 136680. [Google Scholar] [CrossRef]
- Khan, S.; Ghosh, A.K.; Ramachandhran, V.; Bellare, J.; Hanra, M.S.; Trivedi, M.K.; Misra, B.M. Synthesis and Characterization of Low Molecular Weight Cut off Ultrafiltration Membranes from Cellulose Propionate Polymer. Desalination 2000, 128, 57–66. [Google Scholar] [CrossRef]
- Haladu, S.A.; Elsayed, K.A.; Ercan, İ.; Ercan, F.; Kayed, T.S.; Demirci, T.; Yildiz, M.; Magami, S.M.; Manda, A.A. Investigation on Structural, Optical, Thermal, and Dielectric Properties of Cellulose Propionate/Styrene-Maleic Anhydride Copolymer/Molybdenum Nanocomposite Prepared by Pulsed Laser Ablation. J. Mol. Struct. 2024, 1310, 138262. [Google Scholar] [CrossRef]
- Belmokaddem, F.-Z.; Pinel, C.; Huber, P.; Petit-Conil, M.; Da Silva Perez, D. Green Synthesis of Xylan Hemicellulose Esters. Carbohydr. Res. 2011, 346, 2896–2904. [Google Scholar] [CrossRef]
- Jincy, E.M.; Femina, K.S. Heteropolymer in Biomass: Hemicellulose Extraction and Modifications. In Handbook of Biomass; Springer: Singapore, 2024; pp. 665–696. [Google Scholar]
- Suzuki, S.; Hamano, Y.; Wada, N.; Takahashi, K. Controlled Allocation of Aromatic/Aliphatic Substituents to Polysaccharides and Lignin in Sugarcane Bagasse via Successive Homogeneous Transesterification Using Ionic Liquid. ACS Omega 2023, 8, 18582–18590. [Google Scholar] [CrossRef]
- Liu, K.; Li, Y.; Xu, L.; Zhu, F.; Zhang, Y.; Meng, Y.; Xia, X. Preparation of Ethyl Cellulose Microencapsulated Ammonium Polyphosphate and Its Application in Flame Retardant Cellulose Paper. Ind. Crops. Prod. 2024, 210, 118132. [Google Scholar] [CrossRef]
- Naeli, M.H.; Milani, J.M.; Farmani, J.; Zargaraan, A. Developing and Optimizing Low-Saturated Oleogel Shortening Based on Ethyl Cellulose and Hydroxypropyl Methyl Cellulose Biopolymers. Food Chem. 2022, 369, 130963. [Google Scholar] [CrossRef] [PubMed]
- Song, X.C.; Yu, Y.L.; Yang, G.Y.; Jiang, A.L.; Ruan, Y.J.; Fan, S.H. One-Step Emulsification for Controllable Preparation of Ethyl Cellulose Microcapsules and Their Sustained Release Performance. Colloids Surf. B Biointerfaces 2022, 216, 112560. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Liao, J.H.; Hu, T.G.; Zong, M.H.; Wen, P.; Wu, H. Fabrication of Multifunctional Ethyl Cellulose/Gelatin-Based Composite Nanofilm for the Pork Preservation and Freshness Monitoring. Int. J. Biol. Macromol. 2024, 265, 130813. [Google Scholar] [CrossRef]
- Cao, Q.; Kang, S.; Lu, C.; Sun, D.; Li, J.; Chen, H.; Li, X. Properties and Corrosion Resistance Mechanism of a Self-Healing Octadecyl Amine Loaded Ethyl Cellulose Microcapsule Coatings Loaded with Epoxy Resin. Sci. Rep. 2025, 15, 1386. [Google Scholar] [CrossRef]
- Achalhi, N.; El Ouardi, Y.; El Yousfi, R.; Moumnassi, S.; El Barkany, S.; Asehraou, A.; El Idrissi, A. Single-Step Synthesis and Comprehensive Characterization of Hydroxyethyl Cellulose Grafted with (3-Aminopropyl)Triethoxysilane: Mechanistic Insights and Evaluation of Antimicrobial Properties. Carbohydr. Polym. 2025, 360, 123613. [Google Scholar] [CrossRef]
- Gadhave, R.V. Polyvinyl Acetate Wood Adhesive Stabilized with Hydroxyethyl Cellulose: Synthesis and Characterizations. J. Adhes. Sci. Technol. 2025, 39, 498–522. [Google Scholar] [CrossRef]
- Nono-Tagne, S.; Heinze, T.; Gericke, M.; Otsuka, I. Electrospinning of Cellulose Benzyl Carbamates for Enantioselective Membrane Filtration. Macromol. Biosci. 2025, 25, 2400415. [Google Scholar] [CrossRef]
- Sundman, O.; Gillgren, T.; Broström, M. Homogenous Benzylation of Cellulose Impact of Different Methods on Product Properties. Cellul. Chem. Technol. 2015, 49, 745–755. [Google Scholar]
- de Oliveira, R.J.V.; Sousa, F.L.N.; Freitas, D.V.; Silva, F.A.C.; de Almeida, T.S.; Aguilera, P.; Machado, G.; Araújo, B.G.P. Synthesis of Silver Nanoparticles Co-Stabilized by Carboxymethylcellulose Using a Sugarcane Endophytic Aspergillus Brasiliensis. Microbe 2025, 6, 100223. [Google Scholar] [CrossRef]
- Rezanezhad, S.; Nazarnezhad, N.; Resalati, H.; Majid Zabihzadeh, S. Investigating of the Use of Carboxymethylcellulose-Based Magnetic Biocomposite in Paper Coating. Res. Artic. Iran. J. Wood Pap. Sci. Res. 2025, 40, 54–66. [Google Scholar] [CrossRef]
- Florencio, C.; Brondi, M.G.; Silva, M.J.; Bondancia, T.J.; Elias, A.M.; Martins, M.A.; Farinas, C.S.; Ribeiro, C.; Mattoso, L.H.C. Carboxymethylcellulose Production from Sugarcane Bagasse: A New Approach in Biorefinery Concept. Int. J. Biol. Macromol. 2024, 282, 136998. [Google Scholar] [CrossRef] [PubMed]
- Suchaiya, V.; Choochouy, N.; Chokboribal, J.; Khammee, T.; Nueangnun, K.; Jaroennon, P. Effects of Reaction Time on Degree of Substitution, Yield and Morphology of Carboxymethyl Cellulose from Banana Peel. J. Phys. Conf. Ser. 2022, 2175, 012033. [Google Scholar]
- Joyline, G.; Gachoki, K.P.; Ngure, G.A.; Nyambura, N.C.; Shigwenya, M.E. High Swelling Carboxymethyl Cellulose Synthesized from Coconut Fibers. J. Nat. Fibers 2023, 20, 2283549. [Google Scholar] [CrossRef]
- Nguyen, T.D.P.; Le, N.T.; Vu, T.M.; Pham, T.S.; Phan, T.D.; Pham, N.L.; Phan, T.T.M. Synthesis and Characterization of Carboxymethyl Cellulose with High Degree Substitution from Vietnamese Pineapple Leaf Waste. Minist. Sci. Technol. Vietnam. 2022, 64, 13–18. [Google Scholar] [CrossRef]
- Yeasmin, M.S.; Mondal, M.I.H. Synthesis of Highly Substituted Carboxymethyl Cellulose Depending on Cellulose Particle Size. Int. J. Biol. Macromol. 2015, 80, 725–731. [Google Scholar] [CrossRef]
- Sun, R.; Fanga, J.M.; Tomkinson, J.; Hill, C.A.S. Esterification of Hemicelluloses from Poplar Chips in Homogenous Solution of N, N—Dimethylformamide/Lithium Chloride. J. Wood Chem. Technol. 1999, 19, 287–306. [Google Scholar] [CrossRef]
- In, S.; Khunnonkwao, P.; Wong, N.; Phosiran, C.; Jantama, S.S.; Jantama, K. Combining Metabolic Engineering and Evolutionary Adaptation in Klebsiella Oxytoca KMS004 to Significantly Improve Optically Pure D-(−)-Lactic Acid Yield and Specific Productivity in Low Nutrient Medium. Appl. Microbiol. Biotechnol. 2020, 104, 9565–9579. [Google Scholar] [CrossRef]
- Wu, H.; Qiao, Z.; Zhang, H.; Zhou, J. Lyotropic Liquid Crystal Behavior of Cyanoethyl Cellulose in Dichloroacetic Acid/Water Systems. Cellulose 2025, 32, 5465–5477. [Google Scholar] [CrossRef]
- Sharmoukh, W.; Tohamy, H.A.S. The Dielectric Properties of Conducting Films Derived from Sugarcane Bagasse: Implications for Cyanoethyl Cellulose/Cellulose Acetate Synthesis. Waste Biomass Valorization 2025, 15, 30235. [Google Scholar] [CrossRef]
- Luo, D.; Xia, Y.; Ye, X.; Cheng, D.; Zhang, Q.; Wang, C. Functionalized Cellulose-Based Binders for Lithium Cobalt Oxide Cathodes: Improving Stability and Lithium-Ion Transport Under High Voltage. Macromol. Rapid Commun. 2025, 46, 2500074. [Google Scholar] [CrossRef]
- Li, C.; Luo, X. Cyanoethyl Cellulose-Enabled Cathodes with Interpenetrating Electron Transport and Ion Transport Paths via Phase Inversion Method for High Energy Density Lithium Metal Batteries. Chem. Eng. J. 2024, 502, 158073. [Google Scholar] [CrossRef]
- You, Z.; Liu, S.; Kuang, B.; Shao, Z.; Wang, C.; Jin, H.; Li, J. Reduced Graphene Oxide/Cyanoethyl Cellulose Composite: An Example of Dielectric Tailoring Design for Efficient Microwave Absorption Materials. Compos. Sci. Technol. 2024, 248, 110441. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, Y.; Zhang, X.; Zhang, G.; Fang, K.; Peng, Q.; Zhang, X.; Sun, X.; Wang, K.; Ma, Y. Tailoring Highly Ion-Conductive and Stabled PVDF-Based Solid Electrolyte via Surface Coordination Chemistry. Adv. Funct. Mater. 2025, 35, 202422461. [Google Scholar] [CrossRef]
- Silva, P.M.; Prieto, C.; Lagarón, J.M.; Pastrana, L.M.; Coimbra, M.A.; Vicente, A.A.; Cerqueira, M.A. Food-Grade Hydroxypropyl Methylcellulose-Based Formulations for Electrohydrodynamic Processing: Part I—Role of Solution Parameters on Fibre and Particle Production. Food Hydrocoll. 2021, 118, 106761. [Google Scholar] [CrossRef]
- Aghajani, F.; Rafati, H.; Aliahmadi, A.; Moghimi, R. Novel Nanoemulsion-Loaded Hydroxyl Propyl Methyl Cellulose Films as Bioactive Food Packaging Materials Containing Satureja Khuzestanica Essential Oil. Carbohydr. Polym. Technol. Appl. 2024, 8, 100544. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, J.; Song, Y.; Zhang, M.; Qu, M.; Yang, F.; Bai, R.; Chi, H.; Wang, J.; Ma, J. Sodium Alginate and Hydroxypropyl Methylcellulose Hierarchical Control of Surface Tension on Silk Fabrics for Clean and Efficient Inkjet Printing. Int. J. Biol. Macromol. 2025, 304, 140981. [Google Scholar] [CrossRef]
- Knarr, M.; Rogers, T.L.; Petermann, O.; Adden, R. Investigation and Rank-Ordering of Hydroxypropyl Methylcellulose (HPMC) Properties Impacting Controlled Release Performance. J. Drug Deliv. Sci. Technol. 2025, 104, 106425. [Google Scholar] [CrossRef]
- Lino, R.C.; de Carvalho, S.M.; Noronha, C.M.; Sganzerla, W.G.; da Rosa, C.G.; Nunes, M.R.; D’Avila, R.F.; Zambiazi, R.C.; Barreto, P.L.M. Production of Methylcellulose Films Functionalized with Poly-ε-Caprolactone Nanocapsules Entrapped β-Carotene for Food Packaging Application. Food Res. Int. 2022, 160, 111750. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sun, H.; Lv, J.; Wang, Y.; Zhang, Y.; Wang, F. Effects of Polysaccharide Thickening Agent on the Preparation of Walnut Oil Oleogels Based on Methylcellulose: Characterization and Delivery of Curcumin. Int. J. Biol. Macromol. 2023, 232, 123291. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhao, N.; Pan, Z.; Han, M.; Hao, F.; Yuan, J.; Mei, S.; Pan, M. Functionalized Hydroxypropyl Methylcellulose Suspension Copolymerization Vinyl Chloride towards Preparation of Long-Term Stable Hydrophilic and Antifouling Ultrafiltration Membrane. Sep. Purif. Technol. 2025, 364, 132468. [Google Scholar] [CrossRef]
- Ko, K.; Park, S.; Kim, S.; Cheon, J.; Kim, T.-I.; Ryu, K. Synthesis and Characterization of Polyethyleneimine Modified Methylcellulose Nanocarrier for Doxorubicin Delivery. Macromol. Res. 2025, 33, 1059–1068. [Google Scholar] [CrossRef]
- Shi, Z.; Li, Y.; Duan, H.; Wang, Y.; Zhang, X.; Cao, D.; Wang, S.; Yan, X. Interfacial Hydrogen Bonding Reorganization-Assisted Aqueous Assembly of Hydroxypropyl Cellulose for Robust Construction of Hollow Nanocapsules. Int. J. Biol. Macromol. 2025, 318, 145223. [Google Scholar] [CrossRef]
- Pomon, B.; Madler, A.; Jiang, X.; Abbaspourrad, A. Fabrication of Ethyl Cellulose and Hydroxypropyl Cellulose Composite Microcapsules via Solid-Oil-Oil Emulsion for Encapsulation of l-Tryptophan. Food Bioproc. Tech. 2025, 18, 7618–7632. [Google Scholar] [CrossRef]
- Kožák, J.; Bühlbecker, P.; Rautenberg, A.; Chrétien, C.; Pellequer, Y.; Stoyanov, E.; Lamprecht, A. Hydroxypropyl Cellulose-Based Lyospheres Accelerate Dissolution of Aprepitant Combined with Its Supersaturation for Oral Delivery. Eur. J. Pharm. Sci. 2025, 213, 107220. [Google Scholar] [CrossRef]
- Sharma, D.; Harte, F.M.; Ziegler, G.R. Fabrication and Physicomechanical Performance of Casein-Hydroxypropyl Methylcellulose Nanofibers. J. Colloid Interface Sci. 2025, 693, 137601. [Google Scholar] [CrossRef]
- Wang, X.; Sun, H. Effect of Hydroxypropyl Methylcellulose (HPMC) Modified Microbial Induced Carbonate Precipitation on Strength and Water Stability of Loess. Bull. Eng. Geol. Environ. 2025, 84, 183. [Google Scholar] [CrossRef]
- Zheng, Z.; Chen, T.; Zhao, M.; Wang, Z.; Zhao, X. Engineering Escherichia Coli for Succinate Production from Hemicellulose via Consolidated Bioprocessing. Microb. Cell Factories 2012, 11, 37. [Google Scholar] [CrossRef] [PubMed]

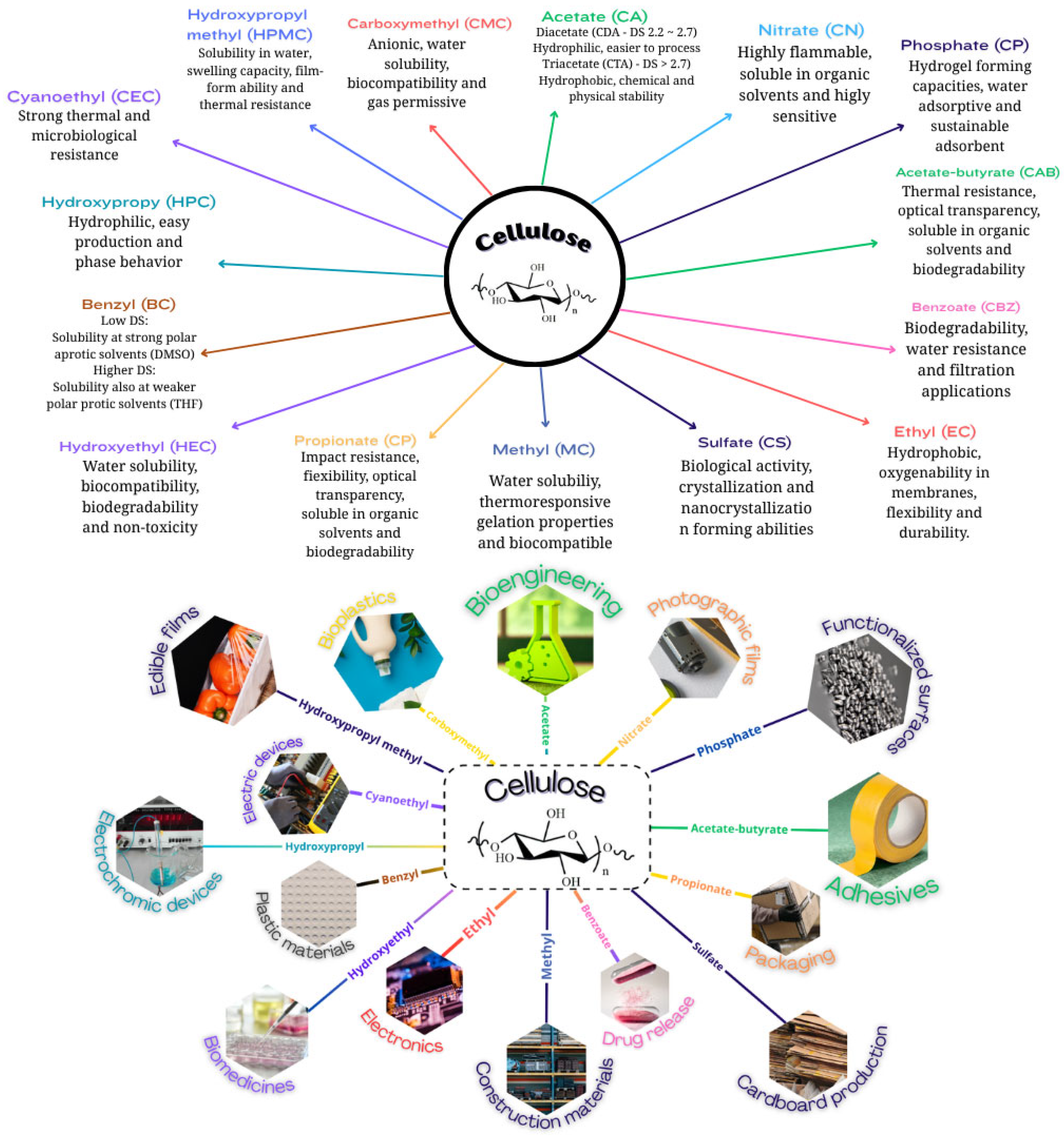

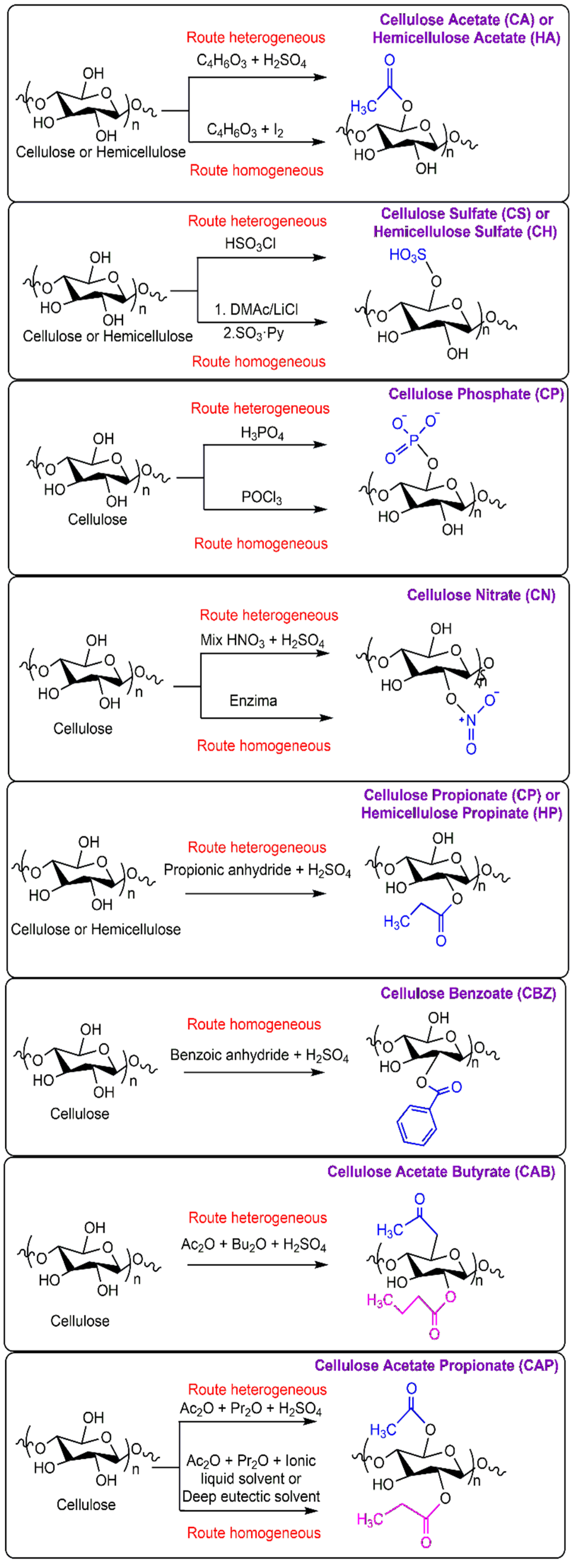

| Derivative | Biomass Feedstock | Extraction Method | Yield (w/w%) | DS | Application | Ref. |
|---|---|---|---|---|---|---|
| Cellulose Acetate | Olive tree pruning | Acid hydrolysis | 43.6 | 2.4 | Dye adsorption | [121] |
| Cigarette butts | Soxhlet (acetone/ethanol), alkaline hydrolysis | 32.1 | 2.28 | Membrane for water filtration | [426] | |
| Cotton linters | Acetylation with acetic anhydride and catalyst | 85.6 | 2.45 | Biodegradable packaging films | [427] | |
| Eucalyptus bark | Alkaline + bleaching + acetylation | 48.2 | 2.56 | Bioplastics | [428] | |
| Sugarcane bagasse | Alkaline, bleaching, acetylation | 54.0 | 2.34 | Drug delivery systems | [429] | |
| Butia odorata fruit | Alkaline + NaClO2 bleaching + acetylation | 49.4 | - | Reinforcement in polymer matrices | [430] | |
| Commercial CA (39.7% acetyl, Mw ~50,000, Sigma-Aldrich, St. Louis, MI, USA) | Dissolution in ternary solvent system (acetone/DMF/water, 3:2:1) followed by electrospinning | – | 39.7% acetyl content | Membranes for water treatment, biomedical uses, filtration, and as support for CNF reinforcement | [119] | |
| Bleached eucalyptus pulp (Suzano, São Paulo, Brazil) | Mechanical refining (PFI mill, 10,000 rev.) + chemical pretreatment with 50% oxalic acid (1 h, 90 °C) + high-pressure homogenization (300 bar, 5 passes) + centrifugation for nano/microfraction separation | 48.9% nanofibrillation yield | – | Reinforcement in CA membranes; improved tensile strength (~383%), higher Young’s modulus (up to 580 MPa), enhanced hydrophobicity and functionalization (Eu3+ luminescence) | ||
| Sunflower seed shell | Phase inversion + AC hybrid with CA | - | - | Hybrid membranes for dye removal | [431] | |
| Cellulose Triacetate | Corn Stover | Ionic liquid | 82.8 | 2.8 | Synthesis of cellulose triacetate | [123] |
| Cocoa Pod Husk | Acid hydrolysis | 31.8 | - | Nanoparticles applied to chitosan films | [111] | |
| Sugarcane bagasse | Organosolv + acetylation (acetic anhydride) | 62.5 | 2.8–2.9 | Food packaging films | [432] | |
| Licorice root waste | Alkaline + peroxide bleaching, acetylation | - | 2.9 | Electrospun nanofibers for filtration | [433] | |
| Eucalyptus wood | Acid hydrolysis + acetylation | - | 2.9 | Barrier coatings in multilayer films | [29] | |
| Corn husk | Alkaline treatment + acetylation | 78.6 | 2.85 | Biodegradable composite films | [434] | |
| Cotton fibers (CF) | Heterogeneous acetylation with glacial acetic acid, acetic anhydride, H2SO4; isolation by differential solubility | AP: 112%/CTA: 87% | 2.86 | CTA soluble in chloroform and CHCl3/MeOH (9:1); pore diameter ~256 nm | [435] | |
| Recycled writing paper (RWP) | Same as above | AP: 94%/CTA: 80% | 2.84 | CTA soluble in chloroform; pore diameter ~83 nm | [435] | |
| Recycled newspaper (RN) | Same as above | AP: 84%/CTA: 68% | 2.85 | CTA soluble in chloroform; pore diameter ~142 nm | [435] | |
| Macerated woody fibers (MWFL) | Delignification (H2O2 + AcOH, 60 °C, 48 h) → heterogeneous acetylation (Ac2O + H2SO4 in AcOH) → isolation by solubility | AP: 73%/CTA: 55% | 2.89 | CTA soluble in chloroform; pore diameter ~108 nm | [435] | |
| Kapok fibers | Alkaline + bleaching + acetylation | - | 2.9 | Oil-absorbing membranes (PVDF/CTA blend) | [436] | |
| Cellulose Acetate Propionate | Commercial Cellulose | Chemical | 71.5–88.4 | 1.3–3.0 | Cellulose propionate production | [189] |
| Commercial Cellulose | Alkaline and bleaching | 120.0 | 2.8 | Transparent and flexible films | [51] | |
| Commercial cellulose | Alkaline | 24.3 | 0.4–0.2 | Sustainable packaging | [27] | |
| Commercial CAP | Solubilization in acetone/DMF + electrospinning | - | - | Nanofibrous membranes for filtration and wound dressing | [437] | |
| Corncob cellulose | Alkaline delignification + acetylation/propionylation | 58.3 | 2.4–2.7 | Bioplastic films for food packaging | [438] | |
| Bagasse cellulose | Steam explosion + chemical modification | 65 | 2.6 | Coating material for controlled drug delivery | [29] | |
| Cellulose Sulfate | Commercial cellulose | Alkaline and bleaching | 75.3 | 2.4 | Adsorption of dyes | [74] |
| Raw jute fiber | Sulfonation with chlorosulfonic acid (CSA) in DMF | 54.1–44.2 | - | Ammonium removal | [439] | |
| Cotton, microcrystalline cellulose | Sulfation with H2SO4/Alc or ClSO3H/pyridine | - | 0.58–2.98 | Antibacterial agent | [67] | |
| Cotton (sulfonated) | Sulfonation with chlorosulfonic acid (CSA) in DMF | - | - | Pb(II) removal from water | [440] | |
| Cellulose Phosphate | Commercial CMC | Alkaline | 88.1 | 0.8 | Binding agent for drugs | [94] |
| Bleached softwood pulp | Phosphoric acid + urea (thermal), comparison of methods | - | 0.18–0.20 P/AGU (via 31P NMR) | Fiber modification and structural analysis | [441] | |
| CNC from cotton | Phosphoric acid + urea (solid-state reaction) | - | High phosphate incorporation | Enhanced dispersion, electrochemical sensors | [442] | |
| Rice straw | Delignification + phosphorylation (DAHP/urea) | 43–51 | Charge density: 1488–2199 mmol/kg | Heat-sealable films, antifizzing cups | [443] | |
| Wood pulp, MCC | Phosphate-based ILs with H2O (e.g., [Emim]DEP) | - | High swelling ratio 40.1% (no DS reported) | Pretreatment for dissolution | [444] | |
| Cellulose Nitrate | Unconventional feedstocks | Alkaline | Flaxseed: 8.5 Oats: 13.4 Gram: 10.2 | Flaxseed: 0.2 Oats: 0.6 Gram: 0.4 | Biodegradable packaging | [147] |
| Miscanthus × giganteus | Alkaline and acid hydrolysis, nitration | 116–131 | 11.35–11.83% N | Energetic materials, biosensors | [148] | |
| Miscanthus × giganteus (KAMIS) | Industrial nitric acid method | 150 | 11.18% N | Inks, lacquers, adhesives | [445] | |
| Date palm fronds (DPF) | Sulfonitric nitration | - | OCN: 12.59%, MCCN: 13.17% | Solid propellants | [446] | |
| Commercial CA and CN | Electrospinning, carbon black doping | - | 12.1% (CN) | Oil spill adsorption | [447] | |
| Cellulose nitrate (12.6% N) | Acetone-based mixing, milling with ARM | - | 12.6% N (in NC) | Combustion enhancement, propellants | [448] | |
| Cellulose Benzoate | Screw pine | Ionic liquid | - | 0.1 | PVDF filler for membranes | [164] |
| Regenerated cellulose | Benzoylation in DMAc/LiCl | - | ~2.4 | Transparent, moisture-resistant films | [161] | |
| Cotton-based cellulose | Benzoylation in pyridine | ~89 | 2.40–2.45 | UV-shielding, biodegradable films | [165] | |
| Microcrystalline cellulose | Esterification in AmimCl ionic liquid | - | - | Microplastic removal from water | [166] | |
| Various cellulose sources | Benzoylation via deep eutectic solvents (DES) | - | DS up to 3.0 | Green solvent chemistry, functional films | [49] | |
| Polysaccharide derivatives | Theoretical modeling of benzoyl/phenyl substitution | - | Substitution at 2,3,6 | Chiral selectors in chromatography | [449] | |
| Cellulose Acetate Butyrate | Commercial Cellulose | Solid-phase | - | - | Microsphere material | [180] |
| Cellulose (plant-based) | Acetate method (emulsion-solvent evaporation) | - | DS ~2.9 (CAB100: DSAc 0.0; DSBu ~2.9) | Supercapacitor electrodes | [174] | |
| CAB + CuO nanoparticles | Pulsed laser ablation in liquid (PLAL) | - | - | 2-Nitrophenol reduction in wastewater treatment | [450] | |
| Commercial CAB (various grades) | Solvent casting (acetone) | - | DS range: DSBu 0.76–2.54; DSAc 0.16–2.03 | Film development for packaging/coating | [183] | |
| - | Drop casting on the perovskite layer | - | - | Improves perovskite solar cell stability | [451] | |
| Cellulose Propionate | CP/LA porous film | Solvent casting + lactic acid + water pressure | - | - | Battery separator with straight pores | [452] |
| CP/[N4444][SS] film | Solvent casting with ionic liquid | - | - | Barrier film (water/pressure resistance) | [453] | |
| Cotton linters (>95% cellulose) | Direct propylation with propionic anhydride using N-iodosuccinimide (NIS) as catalyst under solvent-free conditions | 61.42–94.19% | 1.32–2.85 | Industrial use in plastics, coatings, films, optical materials, and pollutant removal | [194] | |
| Rice husk | Alkali treatment (NaOH), bleaching (NaOCl, H2O2), then propylation with propionic anhydride using NIS catalyst | 59.59–86.44% | 1.76–3.00 | Same as above; higher DS makes it suitable for high-value industrial esters | [194] | |
| Wheat straw | Alkali treatment (NaOH), bleaching (NaOCl, H2O2), then propylation with propionic anhydride using NIS catalyst | 48.92–69.46% | 1.60–3.00 | Used in plastics, fibers, membranes, coatings; lower yield but high DS achievable | [194] | |
| Purchased polymer | Dissolution in DMAc, casting with additives (methanol, oxalic acid, maleic acid, etc.), phase inversion in water | Not reported as w/w% (evaluated by flux/retention) | DS fixed at 2.66 (supplied polymer) | Ultrafiltration membranes: dye removal, salt separation, oil-water separation, protein filtration | [454] | |
| CP/SMAC/Mo nanocomposite | Pulsed laser ablation (with SMAC) | ~91 total mass loss | - | Dielectric and optoelectronic devices | [455] | |
| Hemicellulose acetate | Birchwood xylan | Alkaline aqueous (DMF or solvent-free) | Up to 85 | 0.9–2.0 | Bioplastics, coatings, bioactive polymers | [456] |
| Switchgrass | Alkaline extraction with NaOH | 27 | Up to 2.9 | Hydrophobic films (brittle) | [457] | |
| Sugarcane bagasse | One-pot IL-based transesterification | - | ~2.0 (estimated) | Thermoplastics with good flow & strength | [372] | |
| Lignocellulose | Direct functionalization in ILs | - | ~1–3 | Functional polymers, recyclable plastics | [458] | |
| Ethyl and hydroxyethyl cellulose | Rice straw cellulose (RSC) | Acid–base extraction of rice straw → ethylation (ethyl bromide, toluene) | 79.60 | 2.0–2.5 | Biodegradable films (EC–ethanol films) | [372] |
| Commercial ethyl cellulose (purchased) | Microencapsulation of APP with EC (in-pulp addition; coating) | - | - | Flame-retardant cellulose paper | [459] | |
| Commercial EC + HPMC | Dissolve EC in oil (heat) ± mix with HPMC (oleogelation) | - | - | Low-saturated oleogel shortening (food) | [460] | |
| Commercial EC | One-step emulsification (microfluidic O/W template) + solvent evaporation | - | - | Microcapsules for sustained curcumin release (drug delivery) | [461] | |
| Commercial EC | Casting with polysulfide (inverse vulcanization composite) | - | - | Food preservation films; antibacterial/packaging | [234] | |
| Commercial EC + gelatin | Electrospinning (EC/gelatin matrix with anthocyanin, ε-polylysine) | - | - | Intelligent packaging/pork freshness monitoring | [462] | |
| Commercial EC | Microencapsulation (EC shells) loaded with octadecyl amine; added to epoxy coatings | - | - | Self-healing/anti-corrosion coatings | [463] | |
| Hydroxyethyl cellulose (HEC) | Single-step grafting with (3-aminopropyl)triethoxysilane (APTES) | - | - | Antimicrobial/modified HEC materials | [464] | |
| Air particle vacuum dust (APVD) | NaOH and H2O2 | - | 1.1 | - | [246] | |
| HEC | Emulsion polymerization/grafting to PVAc (SLS emulsifier) | - | - | PVAc wood adhesive stabilizer/improved wet strength | [465] | |
| Benzyl Cellulose | Commercial cellulose | Modular synthesis from cellulose phenyl carbonate with benzyl amine | - | 2.45 | Enantioselective membrane filtration | [466] |
| Various lignocellulosic sources | Homogeneous benzylation in ionic liquids | >90 | 2.0–2.5 | Optical and thermal materials | [370] | |
| Microcrystalline cellulose | Solvent-based benzylation using benzyl chloride | 85–95 | 1.8–2.2 | Hydrophobic coating materials | [467] | |
| Wood pulp | Heterogeneous benzylation | ~80 | 1.5–2.0 | Compatibilizer in polymer composites | [367] | |
| Carboxymethylcellulose | Coconut mesocarp | Sequential alkaline extraction + organosolv pretreatment | 22 | NR | CMC production; thickener/emulsifier | [321] |
| Commercial/not specified | Added as a co-stabilizer in AgNP synthesis | - | NR | AgNP stabilizer; antibacterial formulations | [468] | |
| CMC (substrate) | In situ co-precipitation of Fe salts on CMC (pH 11) | - | NR | Paper coating (magnetic); improved barrier | [469] | |
| Sugarcane bagasse | Hydrothermal pretreatment + alkaline extraction + MCA etherification | - | 0.44 | Encapsulation; packaging/coating | [470] | |
| Waste disposable paper cups | Alkali extraction of fiber + MCA etherification | - | 1.21 (max.) | Composites; flexible materials | [324] | |
| Banana peel | Delignification + bleaching + hydrolysis; MCA etherification | 152.65 | 0.61 | Binder/thickener; valorize agro-waste | [471] | |
| Coconut fibers | Alcohol medium carboxymethylation (alkaline + MCA) | 9.45 g (reported; not given as %) | 1.82 | Superabsorbent; emulsifiers | [472] | |
| Pineapple leaf cellulose | NaOH swelling + MCA (isopropanol) etherification | 136.6 | 2.3 | High-solubility CMC; functional materials | [473] | |
| Corn (corn husk) | Heterogeneous carboxymethylation; optimized particle size | 240 | 2.41 | High-DS CMC for advanced applications | [474] | |
| Sugarcane bagasse (hemicellulose) | Microwave-assisted carboxymethylation; N-CDs embedding | - | NR | Fluorescent hydrogel biosensor; antibacterial/antifungal | [414] | |
| Hemicellulose propionate | Suckling Piglets | - | - | - | Bioengineering | [401] |
| Poplar chips | Delignification with NaClO2 (75 °C, pH 3.2, 4 h) → extraction with 8% NaOH + 1% Na2B4O7·10H2O (16 h) | 32.9–80.2% | 0.32–1.51 (propionate typically ~1.1–1.2) | Biodegradable plastics, resins, films, food coatings, hydrophobic materials | [475] | |
| Hemicellulose hydrolysate succinate | Cassava Starch | Bioconversion | 98.0 | - | Pure D-(-)-lactic acid production | [476] |
| Wheat straw | Extraction with aqueous NaOH, followed by purification and precipitation | ~65–85% | 0.9–1.3 | Biodegradable plastics, films, resins, and compatibilization with hydrophobic polymers | [477] | |
| Cyanoethyl cellulose | Cotton linter pulp | Homogeneous NaOH/urea dissolution → reaction with acrylonitrile (freeze–thaw then AN add, neutralize, lyophilize) | - | 1.64–2.12 | Lyotropic liquid-crystal studies/optics | [478] |
| Sugarcane bagasse (bagasse → HEC → CEC) | Mercerization/HEC formation then cyanoethylation with acrylonitrile in alcoholic NaOH (room temp, 2 h) | - | 1.64 | Dielectric conducting films, optoelectronics, thermoelectrics | [479] | |
| Natural cellulose (unspecified source) | Michael addition (AN)/etherification-controlled reaction time; homogeneous route (NaOH/urea style) | - | DS 2.19–2.62 (samples CEC-0.5h, CEC-1.5h, CEC-3h) | Battery binders (LCO cathodes), enhanced adhesion/ion transport | [480] | |
| Cotton linter pulp (α-cellulose >95%) | Homogeneous synthesis in 7 wt% NaOH/12 wt% urea (pre-chill), then AN addition; phase-inversion to make CEC-based cathode | - | 0.42 | Phase-inversion cathodes—interpenetrating ion/electron paths (Li batteries) | [481] | |
| Commercial CEC | Commercial CEC (DS specified by supplier) blended with rGO by solution blending | - | 2.55–2.80 | rGO/CEC composites for microwave absorption (electromagnetic wave absorbers) | [482] | |
| Cellulose (used to functionalize garnet filler in PVDF electrolyte) | In situ polymerization/coordination of C≡N groups on garnet surface → ultrathin PCEC layer on LLZTO | - | - | Interface modification for PVDF/LLZTO composite solid electrolyte (solid-state batteries) | [483] | |
| Sugarcane bagasse | Synthesis and film casting | - | - | Conducting films | [481] | |
| Cotton linter pulp | Homogeneous cyanoethylation (NaOH/urea), then phase inversion to form a porous electrode | - | 0.42 | High-energy lithium metal cathodes (improved porosity, Li-ion diffusion) | [205] | |
| Methylcellulose | Sugarcane bagasse | - | - | 0.7–1.2 | - | [206] |
| Mango seed fibers | Delignification with nitric acid/ethanol | - | 1.3–0.5 | Mortar additive | [484] | |
| Commercial cellulose | Etherification | - | Hydroxypropyl & methyl substitution (varies by MW) | Electrospun fibers or electrosprayed particles for food/nano use | [485] | |
| Cellulose + Satureja khuzestanica oil | Emulsification and casting | - | - | Antimicrobial edible films for food packaging | [482] | |
| HPMC + Sodium alginate | Surface pretreatment on silk | - | - | Textile printing (enhanced dye uniformity & efficiency) | [486] | |
| Commercial cellulose | Etherification | - | %Me and %HP (variable) | Controlled drug release tablets | [487] | |
| Cellulose + PCL + β-carotene | Nanoprecipitation and casting | - | - | Active packaging with antioxidant release | [488] | |
| Methylcellulose + walnut oil + κ-carrageenan | Emulsion-templated method | 97.37 | - | Delivery of curcumin | [489] | |
| HPMC + vinyl chloride | Suspension copolymerization | - | Double-bond functionalization | Antifouling ultrafiltration membranes | [490] | |
| Methylcellulose + polyethyleneimine | Chemical conjugation | - | Varies by PEI molecular weight | Doxorubicin drug delivery | [491] | |
| Hydroxypropyl cellulose | Commercial cellulose | Etherification with propylene oxide | - | 53.4–80.5 | Hollow nanocapsules for drug/antioxidant delivery | [492] |
| Commercial cellulose | Etherification, then S–O–O emulsion + evaporation | 66–81 | - | Microcapsules for tryptophan taste-masking | [493] | |
| Commercial cellulose | Spray freeze drying with DMSO | - | - | Amorphous solid dispersion for aprepitant | [494] | |
| Hydroxypropyl methylcellulose (HPMC) | Commercial HPMC | (Study of commercial product) | - | - | Controlled-release tablets | [487] |
| HPMC, Carvedilol | Freeze-drying and inkjet printing | - | - | Poorly soluble drug delivery | [48] | |
| HPMC, Casein | Electrospinning | - | - | Biodegradable nanofibers | [116] | |
| HPMC, shellac, CNTs, ZnO-NPs | Crosslinking and loading | - | - | Antimicrobial packaging films | [224] | |
| Hemicellulose succinate | Hemicellulose (oil-palm fruit bunch) | Metabolic engineering of E. coli | - | - | Production of succinic acid | [476] |
| Hemicellulose (xylan) | Consolidated bioprocessing (E. coli) | - | - | Production of succinate | [495] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moura, H.O.M.A.; Pereira, A.V.S.; de Souza, E.C.; Freitas, A.M.N.; do Nascimento, D.N.R.; Kramer, C.A.C.; Matos, J.S.; Costa, J.L.B.; Nobre, D.Q.; Campos, L.M.A.; et al. State of the Art and Recent Advances on Ester and Ether Derivatives of Polysaccharides from Lignocellulose: Production and Technological Applications. Macromol 2025, 5, 47. https://doi.org/10.3390/macromol5040047
Moura HOMA, Pereira AVS, de Souza EC, Freitas AMN, do Nascimento DNR, Kramer CAC, Matos JS, Costa JLB, Nobre DQ, Campos LMA, et al. State of the Art and Recent Advances on Ester and Ether Derivatives of Polysaccharides from Lignocellulose: Production and Technological Applications. Macromol. 2025; 5(4):47. https://doi.org/10.3390/macromol5040047
Chicago/Turabian StyleMoura, Heloise O. M. A., Aisha V. S. Pereira, Elaine C. de Souza, Adriano M. N. Freitas, Daniella N. R. do Nascimento, Carlos A. C. Kramer, Janaína S. Matos, Jordanna L. B. Costa, Daniel Q. Nobre, Leila M. A. Campos, and et al. 2025. "State of the Art and Recent Advances on Ester and Ether Derivatives of Polysaccharides from Lignocellulose: Production and Technological Applications" Macromol 5, no. 4: 47. https://doi.org/10.3390/macromol5040047
APA StyleMoura, H. O. M. A., Pereira, A. V. S., de Souza, E. C., Freitas, A. M. N., do Nascimento, D. N. R., Kramer, C. A. C., Matos, J. S., Costa, J. L. B., Nobre, D. Q., Campos, L. M. A., Silva, K. K. O. S., & de Carvalho, L. S. (2025). State of the Art and Recent Advances on Ester and Ether Derivatives of Polysaccharides from Lignocellulose: Production and Technological Applications. Macromol, 5(4), 47. https://doi.org/10.3390/macromol5040047










