Study of Thermoplastic Starch/Poly (Butylene Succinate) Blends: The Effect of Reactive Compatibilizers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Compounding
2.3. Injection Molding
2.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.5. Melt Flow Index (MFI)
2.6. Differential Scanning Calorimetry
2.7. Rheological Test
2.8. Tensile Test
2.9. Fracture Observation
2.10. Statistical Analysis
3. Results and Discussion
3.1. Fourier Transform Infrared Spectroscopy
3.2. Morphological Observation
3.3. Melt Flow Index
3.4. Differential Scanning Calorimetry
3.5. Tensile Result
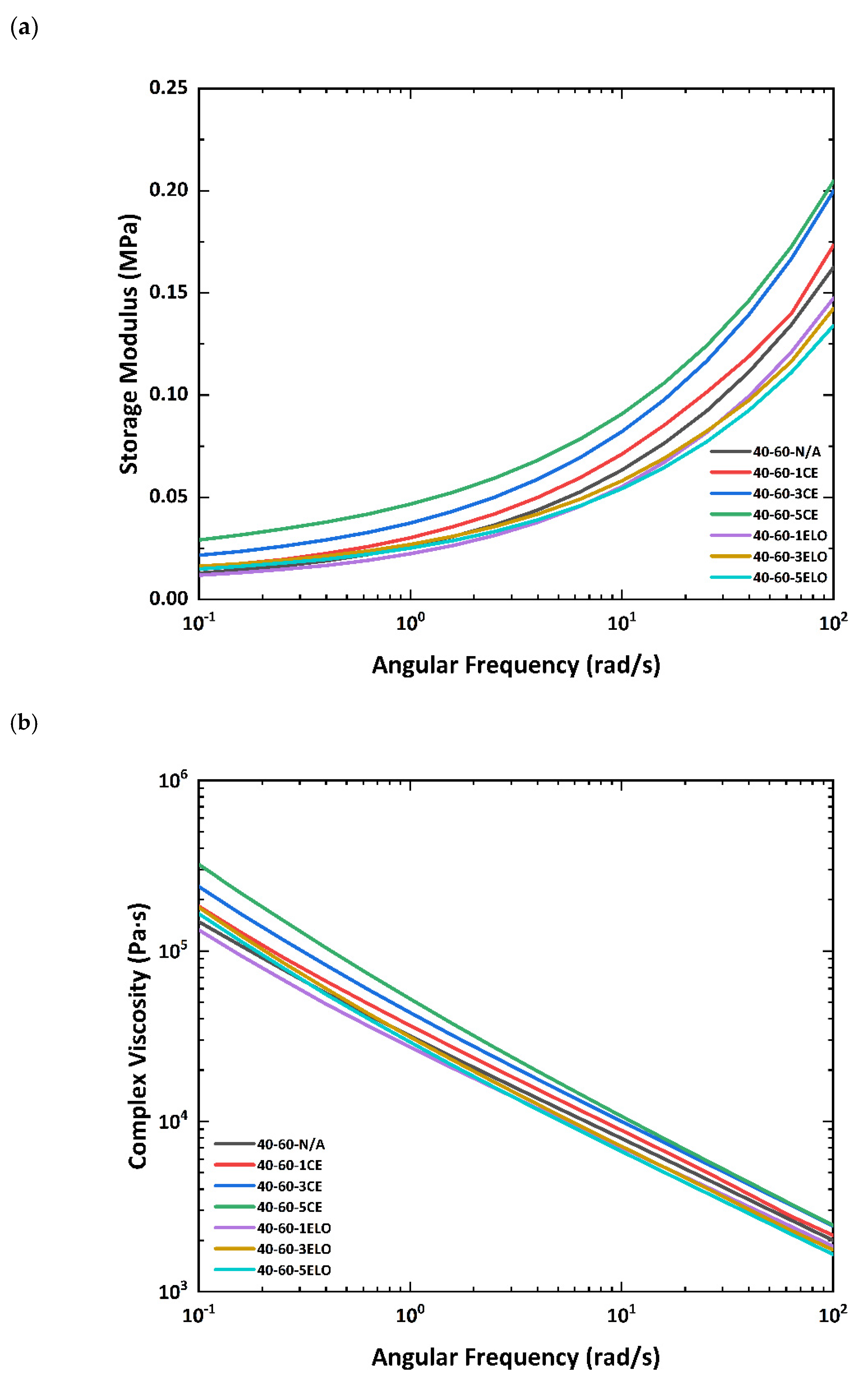
3.6. Rheological Result
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, W.; Ma, P.; Wang, S.; Chen, M.; Cai, X.; Zhang, Y. Effect of partial crosslinking on morphology and properties of the poly(β-hydroxybutyrate)/poly(d,l-lactic acid) blends. Polym. Degrad. Stab. 2013, 98, 1549–1555. [Google Scholar] [CrossRef]
- Kwon, H.J.; Jang, J.; Koh, W.G.; Lee, J.Y.; Hwang, K. Ductile Effect of PGA/PCL Blending Plastics Using a Novel Ionic Chain Extender with Non-Covalent Bonds. Polymers 2023, 15, 3025. [Google Scholar] [CrossRef]
- Cailloux, J.; Santana, O.O.; Franco-Urquiza, E.; Bou, J.J.; Carrasco, F.; Gamez-Perez, J.; Maspoch, M.L. Sheets of branched poly(lactic acid) obtained by one step reactive extrusion calendering process: Melt rheology analysis. Express Polym. Lett. 2013, 7, 304–318. [Google Scholar] [CrossRef]
- Corre, Y.-M.; Duchet, J.; Reignier, J.; Maazouz, A. Melt strengthening of poly (lactic acid) through reactive extrusion with epoxy-functionalized chains. Rheol. Acta 2011, 50, 613–629. [Google Scholar] [CrossRef]
- Rios, L.M.; Moore, C.; Jones, P.R. Persistent organic pollutants carried by synthetic polymers in the ocean environment. Mar. Pollut. Bull. 2007, 54, 1230–1237. [Google Scholar] [CrossRef]
- Balart, J.F.; Fombuena, V.; Fenollar, O.; Boronat, T.; Sánchez-Nacher, L. Processing and characterization of high environmental efficiency composites based on PLA and hazelnut shell flour (HSF) with biobased plasticizers derived from epoxidized linseed oil (ELO). Compos. Part B Eng. 2016, 86, 168–177. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montanes, N.; Sammon, C.; Balart, R.; Torres-Giner, S. Compatibilization of highly sustainable polylactide/almond shell flour composites by reactive extrusion with maleinized linseed oil. Ind. Crops Prod. 2018, 111, 878–888. [Google Scholar] [CrossRef]
- Gonzalez, L.; Agüero, A.; Quiles-Carrillo, L.; Lascano, D.; Montanes, N. Optimization of the loading of an environmentally friendly compatibilizer derived from linseed oil in poly(lactic acid)/diatomaceous earth composites. Materials 2019, 12, 1627. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, Y.; Tian, Y.; Yang, Z.; Zhao, Z.; Du, W.; Zhang, X. Effect of ionic liquid 1-buyl-3-methylimidazolium halide on the structure and tensile property of PBS/corn starch blends. Int. J. Biol. Macromol. 2021, 172, 170–177. [Google Scholar] [CrossRef]
- Tejada-Oliveros, R.; Balart, R.; Ivorra-Martinez, J.; Gomez-Caturla, J.; Montanes, N.; Quiles-Carrillo, L. Improvement of Impact Strength of Polylactide Blends with a Thermoplastic Elastomer Compatibilized with Biobased Maleinized Linseed Oil for Applications in Rigid Packaging. Molecules 2021, 26, 240. [Google Scholar] [CrossRef]
- Liu, D.; Qi, Z.; Zhang, Y.; Xu, J.; Guo, B. Poly(butylene succinate) (PBS)/ionic liquid plasticized starch blends: Preparation, characterization, and properties. Starch/Staerke 2015, 67, 802–809. [Google Scholar] [CrossRef]
- Wang, N.; Yu, J.; Chang, P.R.; Ma, X. Influence of Citric Acid on the Properties of Glycerol-plasticized dry Starch (DTPS) and DTPS/Poly(lactic acid) Blends. Starch/Stärke 2007, 59, 409–417. [Google Scholar] [CrossRef]
- Li, H.; Huneault, M.A. Comparison of sorbitol and glycerol as plasticizers for thermoplastic starch in TPS/PLA blends. J. Appl. Polym. Sci. 2011, 119, 2439–2448. [Google Scholar] [CrossRef]
- Lomelí-Ramírez, M.G.; Barrios-Guzmán, A.J.; García-Enriquez, S.; de Jesús Rivera-Prado, J.; Manríquez-González, R. Chemical and Mechanical Evaluation of Bio-composites Based on Thermoplastic Starch and Wood Particles Prepared by Thermal Compression. Bioresources 2014, 9, 2960–2974. [Google Scholar] [CrossRef]
- Zhang, S.; He, Y.; Yin, Y.; Jiang, G. Fabrication of innovative thermoplastic starch bio-elastomer to achieve high toughness poly(butylene succinate) composites. Carbohydr. Polym. 2019, 206, 827–836. [Google Scholar] [CrossRef]
- Ren, J.; Fu, H.; Ren, T.; Yuan, W. Preparation, characterization and properties of binary and ternary blends with thermoplastic starch, poly(lactic acid) and poly(butylene adipate-co-terephthalate). Carbohydr. Polym. 2009, 77, 576–582. [Google Scholar] [CrossRef]
- Gouveia, T.I.A.; Biernacki, K.; Castro, M.C.R.; Gonçalves, M.P.; Souza, H.K.S. A new approach to develop biodegradable films based on thermoplastic pectin. Food Hydrocoll. 2019, 97, 105175. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Huang, J.; Lin, N.; Ahmad, I.; Mariano, M.; Dufresne, A.; Thomas, S.; Gałęski, A. Recent developments in nanocellulose-based biodegradable polymers, thermoplastic polymers, and porous nanocomposites. Prog. Polym. Sci. 2018, 87, 197–227. [Google Scholar] [CrossRef]
- Gao, H.; Hu, S.; Su, F.; Zhang, J.; Tang, G. Mechanical, thermal, and biodegradability properties of PLA/modified starch blends. Polym. Compos. 2011, 32, 2093–2100. [Google Scholar] [CrossRef]
- Siqueira, L.D.V.; Arias, C.I.L.F.; Maniglia, B.C.; Tadini, C.C. Starch-based biodegradable plastics: Methods of production, challenges and future perspectives. Curr. Opin. Food Sci. 2021, 38, 122–130. [Google Scholar] [CrossRef]
- Santos, B.H.D.; De Souza Do Prado, K.; Jacinto, A.A.; Da Silva Spinacé, M.A. Influence of sugarcane bagasse fiber size on biodegradable composites of thermoplastic starch. J. Renew. Mater. 2018, 6, 176–182. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, X.; Liu, Q.; Hrymak, A. The Effect of Polymeric Chain Extenders on Physical Properties of Thermoplastic Starch and Polylactic Acid Blends. J. Polym. Environ. 2012, 20, 315–325. [Google Scholar] [CrossRef]
- Moghaddam, M.R.A.; Razavi, S.M.A.; Jahani, Y. Effects of Compatibilizer and Thermoplastic Starch (TPS) Concentration on Morphological, Rheological, Tensile, Thermal and Moisture Sorption Properties of Plasticized Polylactic Acid/TPS Blends. J. Polym. Environ. 2018, 26, 3202–3215. [Google Scholar] [CrossRef]
- Martin, O.; Ârous, L.A. Poly(lactic acid): Plasticization and properties of biodegradable multiphase systems. Polymer 2001, 42, 6209–6219. [Google Scholar] [CrossRef]
- Ke, T.; Sun, X. Effects of Moisture Content and Heat Treatment on the Physical Properties of Starch and Poly(lactic acid) Blends. J. Appl. Polym. Sci. 2001, 81, 3069–3082. [Google Scholar] [CrossRef]
- Huneault, M.A.; Li, H. Morphology and properties of compatibilized polylactide/thermoplastic starch blends. Polymer 2007, 48, 270–280. [Google Scholar] [CrossRef]
- Clasen, S.H.; Müller, C.M.O.; Pires, A.T.N. Maleic anhydride as a compatibilizer and plasticizer in TPS/PLA blends. J. Braz. Chem. Soc. 2015, 26, 1583–1590. [Google Scholar] [CrossRef]
- Noivoil, N.; Yoksan, R. Oligo(lactic acid)-grafted starch: A compatibilizer for poly(lactic acid)/thermoplastic starch blend. Int. J. Biol. Macromol. 2020, 160, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Palai, B.; Biswal, M.; Mohanty, S.; Nayak, S.K. In situ reactive compatibilization of polylactic acid (PLA) and thermoplastic starch (TPS) blends; synthesis and evaluation of extrusion blown films thereof. Ind. Crops Prod. 2019, 141, 111748. [Google Scholar] [CrossRef]
- Schwach, E.; Six, J.L.; Avérous, L. Biodegradable blends based on starch and poly(lactic acid): Comparison of different strategies and estimate of compatibilization. J. Polym. Environ. 2008, 16, 286–297. [Google Scholar] [CrossRef]
- Yokesahachart, C.; Yoksan, R. Effect of amphiphilic molecules on characteristics and tensile properties of thermoplastic starch and its blends with poly(lactic acid). Carbohydr. Polym. 2011, 83, 22–31. [Google Scholar] [CrossRef]
- Wootthikanokkhan, J.; Kasemwananimit, P.; Sombatsompop, N.; Kositchaiyong, A.; Ayutthaya, S.I.N.; Kaabbuathong, N. Preparation of modified starch-grafted poly(lactic acid) and a study on compatibilizing efficacy of the copolymers in poly(lactic acid)/thermoplastic starch blends. J. Appl. Polym. Sci. 2012, 126, E389–E396. [Google Scholar] [CrossRef]
- Li, H.; Huneault, M.A. Effect of chain extension on the properties of PLA/TPS blends. J. Appl. Polym. Sci. 2011, 122, 134–141. [Google Scholar] [CrossRef]
- Ferri, J.M.; Garcia-Garcia, D.; Sánchez-Nacher, L.; Fenollar, O.; Balart, R. The effect of maleinized linseed oil (MLO) on mechanical performance of poly(lactic acid)-thermoplastic starch (PLA-TPS) blends. Carbohydr. Polym. 2016, 147, 60–68. [Google Scholar] [CrossRef]
- Turco, R.; Ortega-Toro, R.; Tesser, R.; Mallardo, S.; Collazo-Bigliardi, S.; Boix, A.C.; Malinconico, M.; Rippa, M.; Di Serio, M.; Santagata, G. Poly (lactic acid)/thermoplastic starch films: Effect of cardoon seed epoxidized oil on their chemicophysical, mechanical, and barrier properties. Coatings 2019, 9, 574. [Google Scholar] [CrossRef]
- Ortega-Toro, R.; López-Córdoba, A.; Avalos-Belmontes, F. Epoxidised sesame oil as a biobased coupling agent and plasticiser in polylactic acid/thermoplastic yam starch blends. Heliyon 2021, 7, e06176. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Contreras, P.; Contreras-Camacho, M.; Avalos-Belmontes, F.; Collazo-Bigliardi, S.; Ortega-Toro, R. Physicochemical Properties of Composite Materials Based on Thermoplastic Yam Starch and Polylactic Acid Improved with the Addition of Epoxidized Sesame Oil. J. Polym. Environ. 2021, 29, 3324–3334. [Google Scholar] [CrossRef]
- Monika; Mulchandani, N.; Katiyar, V. Generalized kinetics for thermal degradation and melt rheology for poly (lactic acid)/poly (butylene succinate)/functionalized chitosan based reactive nanobiocomposite. Int. J. Biol. Macromol. 2019, 141, 831–842. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.; Wang, L.; Li, S. Compatibility and mechanical properties of gelatin-filled polybutylene succinate composites. J. Appl. Polym. Sci. 2019, 137, 48881. [Google Scholar] [CrossRef]
- Yin, Q.J.; Chen, F.P.; Zhang, H.; Liu, C.S. Mechanical Properties and Thermal Behavior of TPS/PBS Blends with Maleated PBS as a Compatibilizer. Adv. Mat. Res. 2015, 1119, 306–309. [Google Scholar] [CrossRef]
- Zeng, J.B.; Jiao, L.; Li, Y.D.; Srinivasan, M.; Li, T.; Wang, Y.Z. Bio-based blends of starch and poly(butylene succinate) with improved miscibility, mechanical properties, and reduced water absorption. Carbohydr. Polym. 2011, 83, 762–768. [Google Scholar] [CrossRef]
- Li, J.; Luo, X.; Lin, X.; Zhou, Y. Comparative study on the blends of PBS/thermoplastic starch prepared from waxy and normal corn starches. Starch/Staerke 2013, 65, 831–839. [Google Scholar] [CrossRef]
- Li, J.; Luo, X.; Lin, X.; Huang, Y. Morphology and viscoelastic behavior of PBS/TPS blends containing varying amylose contents. Huagong Xuebao/CIESC J. 2013, 64, 2300–2305. [Google Scholar] [CrossRef]
- Boonprasith, P.; Wootthikanokkhan, J.; Nimitsiriwat, N. Mechanical, thermal, and barrier properties of nanocomposites based on poly(butylene succinate)/thermoplastic starch blends containing different types of clay. J. Appl. Polym. Sci. 2013, 130, 1114–1123. [Google Scholar] [CrossRef]
- Fahrngruber, B.; Fortea-Verdejo, M.; Wimmer, R.; Mundigler, N. Starch/Poly(butylene succinate) Compatibilizers: Effect of Different Reaction-Approaches on the Properties of Thermoplastic Starch-Based Compostable Films. J. Polym. Environ. 2020, 28, 257–270. [Google Scholar] [CrossRef]
- Gong, K.; Lu, Y.; Portela, A.; Taghinezhad, S.F.; Lawlor, D.; Connolly, S.; Hu, M.; Chen, Y.; Collins, M.N. A Comparative Study on the Compatibilization of Thermoplastic Starch/Polybutylene Succinate Blends by Chain Extender and Epoxidized Linseed Oil. Macromol 2025, 5, 24. [Google Scholar] [CrossRef]
- Gong, K.; Lu, Y.; Liu, H.; Portela, A.; de Lima, T.; Xu, H.; Collins, M.N.; Chen, Y. A comparison of granule-based material extrusion and fused filament fabrication in the performances of TPS/PBS blend. J. Mater. Res. Technol. 2025, 37, 5177–5186. [Google Scholar] [CrossRef]
- ASTM D1238-10; Test Method for Melt Flow Rates of Thermoplastics by Extrusion Plastometer. ASTM International: West Conshohocken, PA, USA, 2010. [CrossRef]
- Yan, G.; Cao, Z.; Devine, D.; Penning, M.; Gately, N.M. Physical properties of shellac material used for hot melt extrusion with potential application in the pharmaceutical industry. Polymers 2021, 13, 3723. [Google Scholar] [CrossRef]
- Liu, H.; Gong, K.; Portela, A.; Cao, Z.; Dunbar, R.; Chen, Y. Granule-based material extrusion is comparable to filament-based material extrusion in terms of mechanical performances of printed PLA parts: A comprehensive investigation. Addit. Manuf. 2025, 75, 103744. [Google Scholar] [CrossRef]
- Gigli, M.; Negroni, A.; Zanaroli, G.; Lotti, N.; Fava, F.; Munari, A. Environmentally friendly PBS-based copolyesters containing PEG-like subunit: Effect of block length on solid-state properties and enzymatic degradation. React. Funct. Polym. 2013, 73, 764–771. [Google Scholar] [CrossRef]
- ASTM D638-03; Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2003. [CrossRef]
- Gong, K.; Liu, H.; Huang, C.; Cao, Z.; Fuenmayor, E.; Major, I. Hybrid Manufacturing of Acrylonitrile Butadiene Styrene (ABS) via the Combination of Material Extrusion Additive Manufacturing and Injection Molding. Polymers 2022, 14, 5093. [Google Scholar] [CrossRef]
- Koski, C.; Bose, S. Effects of amylose content on the mechanical properties of starch-hydroxyapatite 3D printed bone scaffolds. Addit. Manuf. 2019, 30, 100817. [Google Scholar] [CrossRef]
- Salazar, M.; Calvache, L.; Zapata, J.; Samuel, H.; Duque, J.F.S. Soil biodegradation of cassava starch and polylactic acid blend. Ing. Investig. 2022, 42, e93710. [Google Scholar] [CrossRef]
- Standau, T.; Nofar, M.; Dörr, D.; Ruckdäschel, H.; Altstädt, V. A Review on Multifunctional Epoxy-Based Joncryl® ADR Chain Extended Thermoplastics. Polym. Rev. 2022, 62, 296–350. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Rheological, morphological, and interfacial properties of compatibilized PLA/PBAT blends. Rheol. Acta 2014, 53, 501–517. [Google Scholar] [CrossRef]
- Yun, I.S.; Hwang, S.W.; Shim, J.K.; Seo, K.H. A study on the thermal and mechanical properties of poly (butylene succinate)/thermoplastic starch binary blends. Int. J. Precis. Eng. Manuf. Technol. 2016, 3, 289–296. [Google Scholar] [CrossRef]
- Pawlak, A.; Mucha, M. Thermogravimetric and FTIR studies of chitosan blends. Thermochim. Acta 2003, 396, 153–166. [Google Scholar] [CrossRef]
- Ferri, J.M.; Garcia-Garcia, D.; Montanes, N.; Fenollar, O.; Balart, R. The effect of maleinized linseed oil as biobased plasticizer in poly(lactic acid)-based formulations. Polym. Int. 2017, 66, 882–891. [Google Scholar] [CrossRef]
- Balsamo, V.; Gouveia, L.M.; Herrera, L.; Laredo, E.; Méndez, B. Miscibilidad en mezclas de poli (estireno-co-anhídrido maleico) y poli (e-caprolactona)(SMA/PCL). Rev. Latinoam. Metal. Y Mater. 2004, 24, 17–30. [Google Scholar]
- Li, X.; Yan, X.; Yang, J.; Pan, H.; Gao, G.; Zhang, H.; Dong, L. Improvement of compatibility and mechanical properties of the poly(lactic acid)/poly(butylene adipate-co-terephthalate) blends and films by reactive extrusion with chain extender. Polym. Eng. Sci. 2018, 58, 1868–1878. [Google Scholar] [CrossRef]
- da Silva, J.M.F.; Soares, B.G. Epoxidized cardanol-based prepolymer as promising biobased compatibilizing agent for PLA/PBAT blends. Polym. Test. 2021, 93, 106889. [Google Scholar] [CrossRef]
- Silverajah, V.S.G.; Ibrahim, N.A.; Zainuddin, N.; Yunus, W.M.Z.W.; Hassan, H.A. Mechanical, thermal and morphological properties of poly(lactic acid)/epoxidized palm olein blend. Molecules 2012, 17, 11729–11747. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Then, Y.Y.; Loo, Y.Y. Epoxidized vegetable oils plasticized poly(lactic acid) biocomposites: Mechanical, thermal and morphology properties. Molecules 2014, 19, 16024–16038. [Google Scholar] [CrossRef]
- Wu, D.D.; Guo, Y.; Huang, A.P.; Xu, R.W.; Liu, P. Effect of the multi-functional epoxides on the thermal, mechanical and rheological properties of poly(butylene adipate-co-terephthalate)/polylactide blends. Polym. Bull. 2021, 78, 5567–5591. [Google Scholar] [CrossRef]
- Zhang, N.; Zeng, C.; Wang, L.; Ren, J. Preparation and Properties of Biodegradable Poly(lactic acid)/Poly(butylene adipate-co-terephthalate) Blend with Epoxy-Functional Styrene Acrylic Copolymer as Reactive Agent. J. Polym. Environ. 2013, 21, 286–292. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Carbonell-Verdu, A.; Arrieta, M.P.; López-Martínez, J.; Samper, M.D. Improvement of PLA film ductility by plasticization with epoxidized karanja oil. Polym. Degrad. Stab. 2020, 179, 109259. [Google Scholar] [CrossRef]
- Agüero, Á.; Garcia-Sanoguera, D.; Lascano, D.; Rojas-Lema, S.; Ivorra-Martinez, J.; Fenollar, O.; Torres-Giner, S. Evaluation of different compatibilization strategies to improve the performance of injection-molded green composite pieces made of polylactide reinforced with short flaxseed fibers. Polymers 2020, 12, 821. [Google Scholar] [CrossRef]
- Niu, Z.; Chen, F.; Zhang, H.; Liu, C. High Content of Thermoplastic Starch, Poly(butylenes adipate-co-terephthalate) and Poly(butylene succinate) Ternary Blends with a Good Balance in Strength and Toughness. Polymers 2023, 15, 2040. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, H.; Jiang, T.; Hu, C.; Zhang, J.; Zheng, H.; Zeng, G. Effect of epoxy chain extenders on molecular structure and properties of polylactic acid. J. Appl. Polym. Sci. 2023, 140, e54379. [Google Scholar] [CrossRef]
- Najafi, N.; Heuzey, M.C.; Carreau, P.J.; Wood-Adams, P.M. Control of thermal degradation of polylactide (PLA)-clay nanocomposites using chain extenders. Polym. Degrad. Stab. 2012, 97, 554–565. [Google Scholar] [CrossRef]
- Kahraman, Y.; Özdemir, B.; Gümüş, B.E.; Nofar, M. Morphological, rheological, and mechanical properties of PLA/TPU/nanoclay blends compatibilized with epoxy-based Joncryl chain extender. Colloid. Polym. Sci. 2023, 301, 51–62. [Google Scholar] [CrossRef]
- Altınbay, A.; Özsaltık, C.; Jahani, D.; Nofar, M. Reactivity of Joncryl chain extender in PLA/PBAT blends: Effects of processing temperature and PBAT aging on blend performance. Int. J. Biol. Macromol. 2025, 303, 140703. [Google Scholar] [CrossRef] [PubMed]
- Ferri, J.M.; Samper, M.D.; García-Sanoguera, D.; Reig, M.J.; Fenollar, O.; Balart, R. Plasticizing effect of biobased epoxidized fatty acid esters on mechanical and thermal properties of poly(lactic acid). J. Mater. Sci. 2016, 51, 5356–5366. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Hussein, M.Z. Plasticized poly(lactic acid) with low molecular weight poly(ethylene glycol): Mechanical, thermal, and morphology properties. J. Appl. Polym. Sci. 2013, 130, 4576–4580. [Google Scholar] [CrossRef]
- Mikus, P.-Y.; Alix, S.; Soulestin, J.; Lacrampe, M.; Krawczak, P.; Coqueret, X.; Dole, P. Deformation mechanisms of plasticized starch materials. Carbohydr. Polym. 2014, 114, 450–457. [Google Scholar] [CrossRef]
- Tian, X.; Liu, T.; Wang, Q.; Dilmurat, A.; Li, D.; Ziegmann, G. Recycling and remanufacturing of 3D printed continuous carbon fiber reinforced PLA composites. J. Clean. Prod. 2017, 142, 1609–1618. [Google Scholar] [CrossRef]
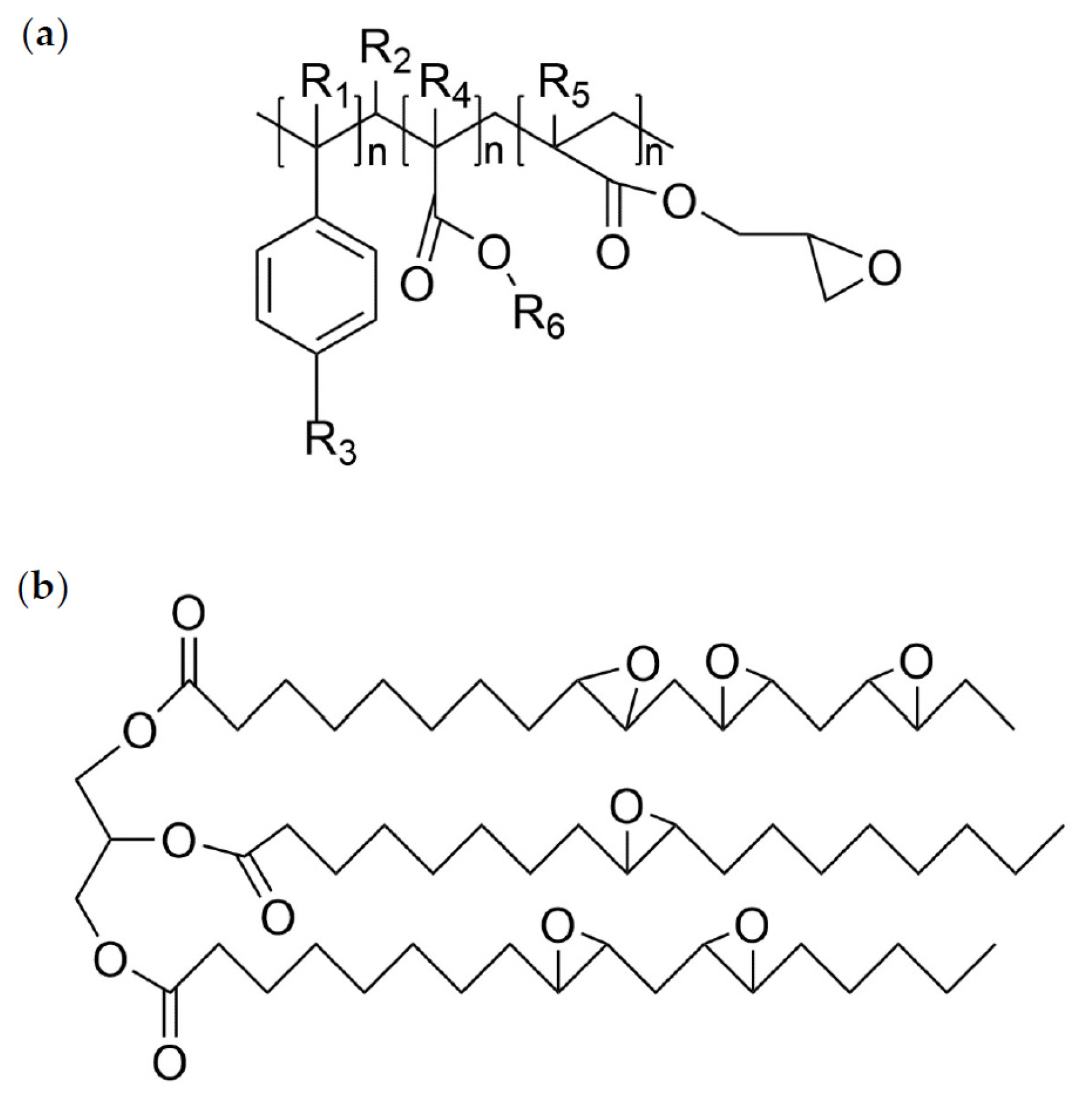
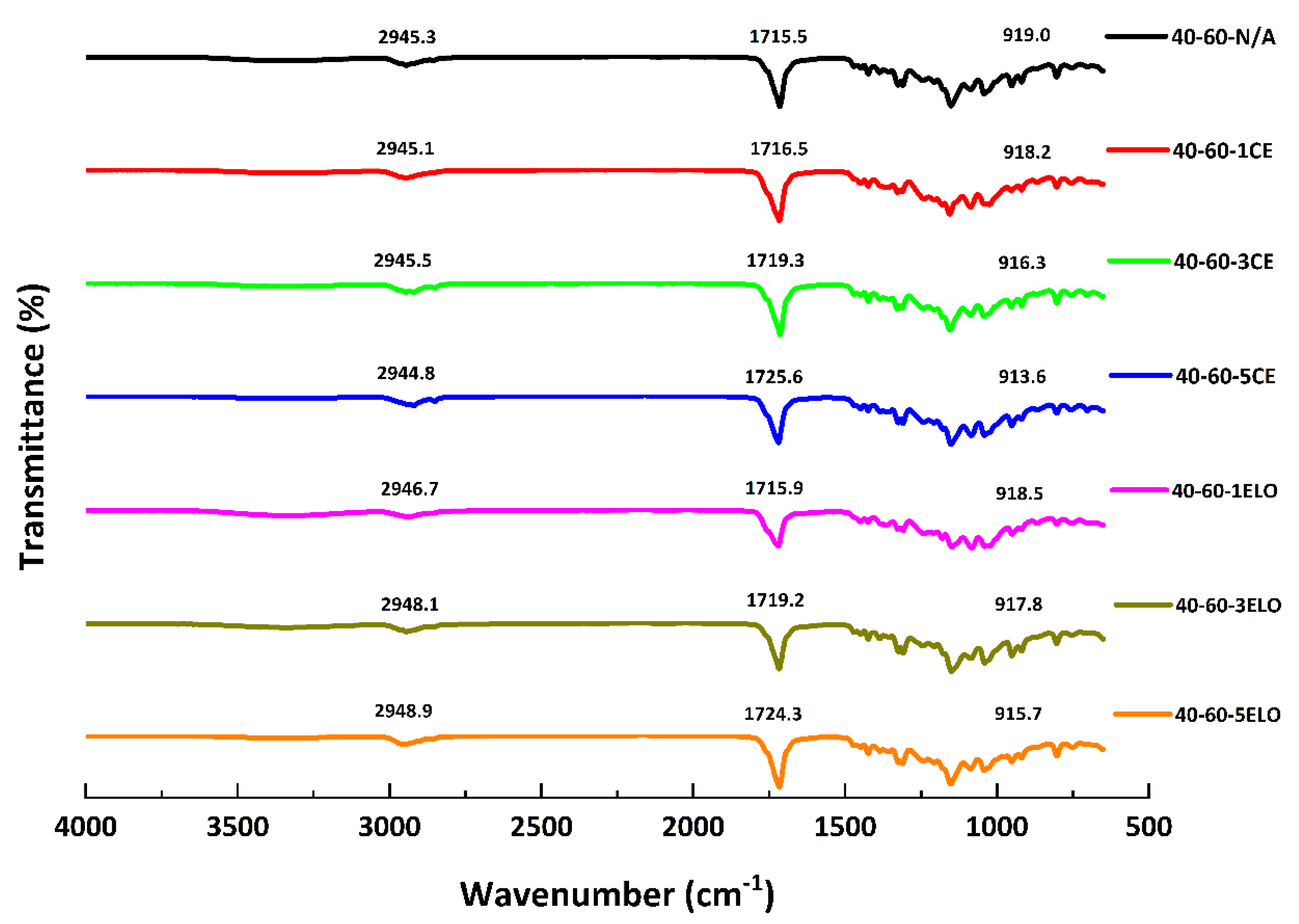
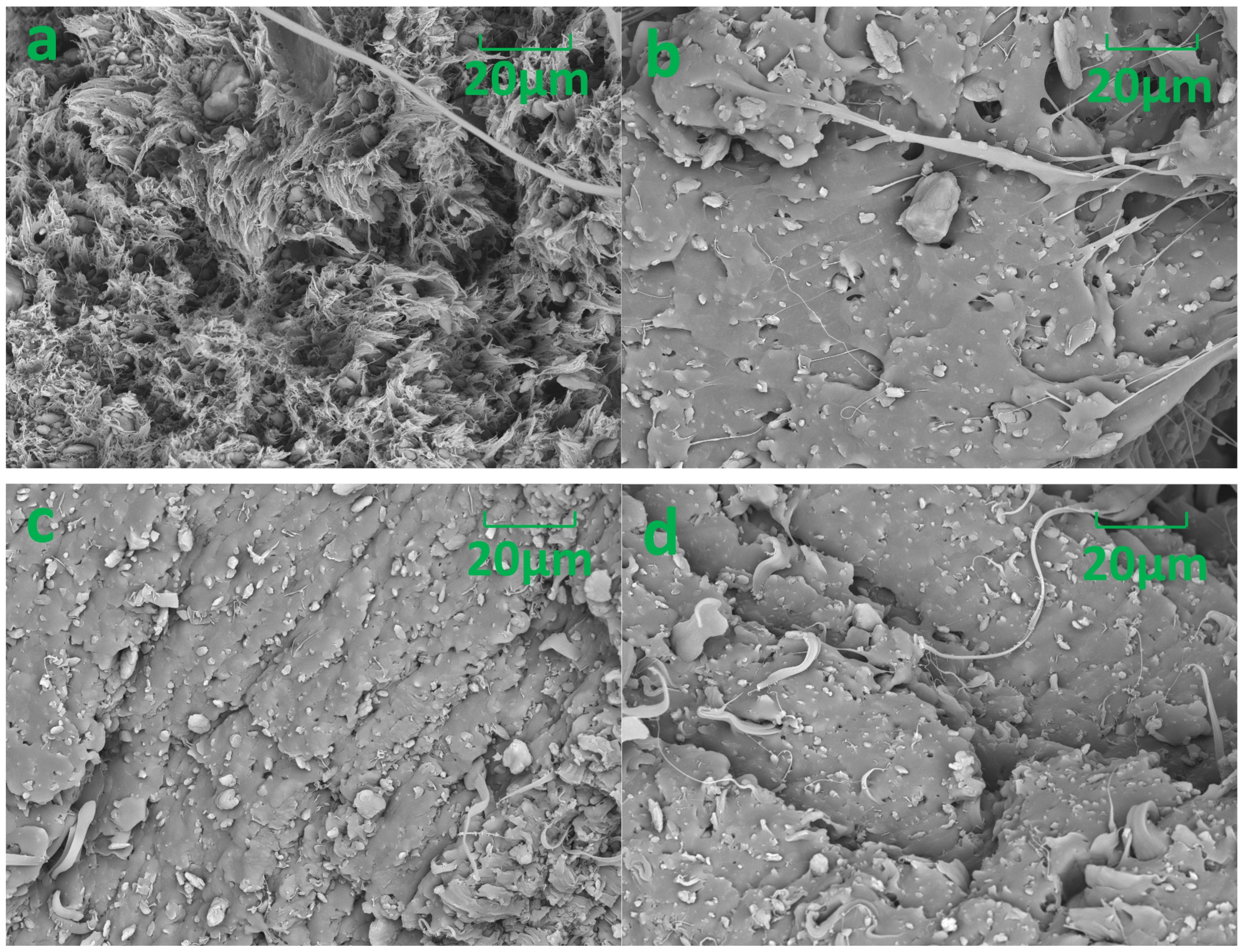
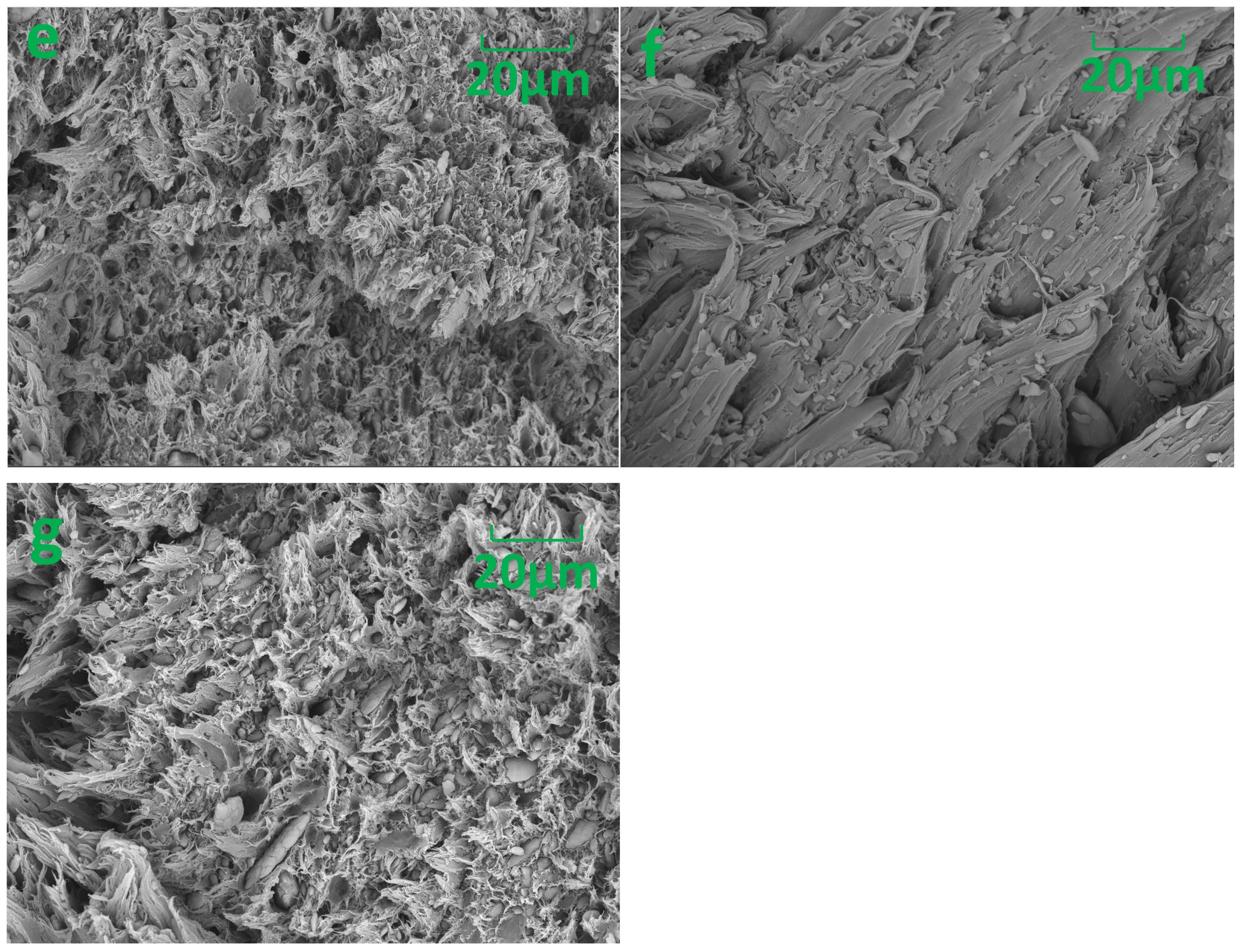

| Characteristics | Values |
|---|---|
| Specific Gravity, 25 °C (g/cm3) | 1.08 |
| MW (Ave) | 7300 |
| Tg (°C) | 59 |
| Non-volatile by GC (%) | >99 |
| Epoxy equivalent weight (g/mol) | 310 |
| Characteristics | Values |
|---|---|
| Melting Point (°C) | −5–1 |
| Viscosity, 25 °C (mPa·s) | 899–1200 |
| Water Solubility, 20 °C (mg/L) | 0.421–1.780 |
| Density, 25 °C (g/cm3) | 1.031–1.038 |
| Vapor Pressure, 25 °C, (Pa) | ≤0.000017 |
| Batch | Component Composition | Compatibilizer | |
|---|---|---|---|
| TPS (%) | PBS (%) | ||
| 40-60-N/A | 40 | 60 | No Compatibilizer |
| 40-60-1CE | 40 | 60 | 1 phr Joncryl® ADR 4468 |
| 40-60-3CE | 40 | 60 | 3 phr Joncryl® ADR 4468 |
| 40-60-5CE | 40 | 60 | 5 phr Joncryl® ADR 4468 |
| 40-60-1ELO | 40 | 60 | 1 phr Epoxidized Linseed Oil |
| 40-60-3ELO | 40 | 60 | 3 phr Epoxidized Linseed Oil |
| 40-60-5ELO | 40 | 60 | 5 phr Epoxidized Linseed Oil |
| Batch | MFI (g/10 min) |
|---|---|
| 40-60-N/A | 2.79 ± 0.25 |
| 40-60-1CE | 1.93 ± 0.05 |
| 40-60-3CE | 1.67 ± 0.12 |
| 40-60-5CE | 0.82 ± 0.03 |
| 40-60-1ELO | 4.15 ± 0.08 |
| 40-60-3ELO | 5.19 ± 0.19 |
| 40-60-5ELO | 7.24 ± 0.46 |
| Batch | Tcc (°C) | Tm (°C) | ΔHm (J/g) | ΔHcc (J/g) | Xc (%) |
|---|---|---|---|---|---|
| 40-60-N/A | 88.1 | 115.1 | 45.58 | 36.13 | 14.28 |
| 40-60-1CE | 88.3 | 115.4 | 46.21 | 36.42 | 14.79 |
| 40-60-3CE | 88.6 | 115.6 | 47.35 | 36.23 | 16.80 |
| 40-60-5CE | 88.7 | 115.7 | 48.41 | 35.24 | 19.90 |
| 40-60-1ELO | 88 | 115 | 43.23 | 32.74 | 15.85 |
| 40-60-3ELO | 87.7 | 114.8 | 47.11 | 33.13 | 21.12 |
| 40-60-5ELO | 87.6 | 114.6 | 51.75 | 35.51 | 24.54 |
| Batch | Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation at Break (%) |
|---|---|---|---|
| 40-60-N/A | 30.6 ± 0.2 | 651.3 ± 27.2 | 68.8 |
| 40-60-1CE | 31.3 ± 0.3 | 688.2 ± 19.3 | 65.8 |
| 40-60-3CE | 32.1 ± 0.1 | 701.2 ± 16.5 | 63.0 |
| 40-60-5CE | 32.6 ± 0.2 | 724.1 ± 14.3 | 61.0 |
| 40-60-1ELO | 29.7 ± 0.4 | 627.6 ± 12.9 | 85.9 |
| 40-60-3ELO | 27.8 ± 0.3 | 584.2 ± 14.2 | 91.4 |
| 40-60-5ELO | 25.5 ± 0.3 | 541.2 ± 11.0 | 78.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, K.; Chen, Y.; Lu, Y.; Zhao, Z.; Portela, A.; Xu, H.; Hu, M.; Liu, H.; Collins, M.N. Study of Thermoplastic Starch/Poly (Butylene Succinate) Blends: The Effect of Reactive Compatibilizers. Macromol 2025, 5, 42. https://doi.org/10.3390/macromol5030042
Gong K, Chen Y, Lu Y, Zhao Z, Portela A, Xu H, Hu M, Liu H, Collins MN. Study of Thermoplastic Starch/Poly (Butylene Succinate) Blends: The Effect of Reactive Compatibilizers. Macromol. 2025; 5(3):42. https://doi.org/10.3390/macromol5030042
Chicago/Turabian StyleGong, Ke, Yuanyuan Chen, Yinshi Lu, Zijian Zhao, Alexandre Portela, Han Xu, Mengli Hu, Handai Liu, and Maurice N. Collins. 2025. "Study of Thermoplastic Starch/Poly (Butylene Succinate) Blends: The Effect of Reactive Compatibilizers" Macromol 5, no. 3: 42. https://doi.org/10.3390/macromol5030042
APA StyleGong, K., Chen, Y., Lu, Y., Zhao, Z., Portela, A., Xu, H., Hu, M., Liu, H., & Collins, M. N. (2025). Study of Thermoplastic Starch/Poly (Butylene Succinate) Blends: The Effect of Reactive Compatibilizers. Macromol, 5(3), 42. https://doi.org/10.3390/macromol5030042







