Starch Science Advancement: Isolation Techniques, Modification Strategies, and Multifaceted Applications
Abstract
1. Introduction
2. Materials and Methods
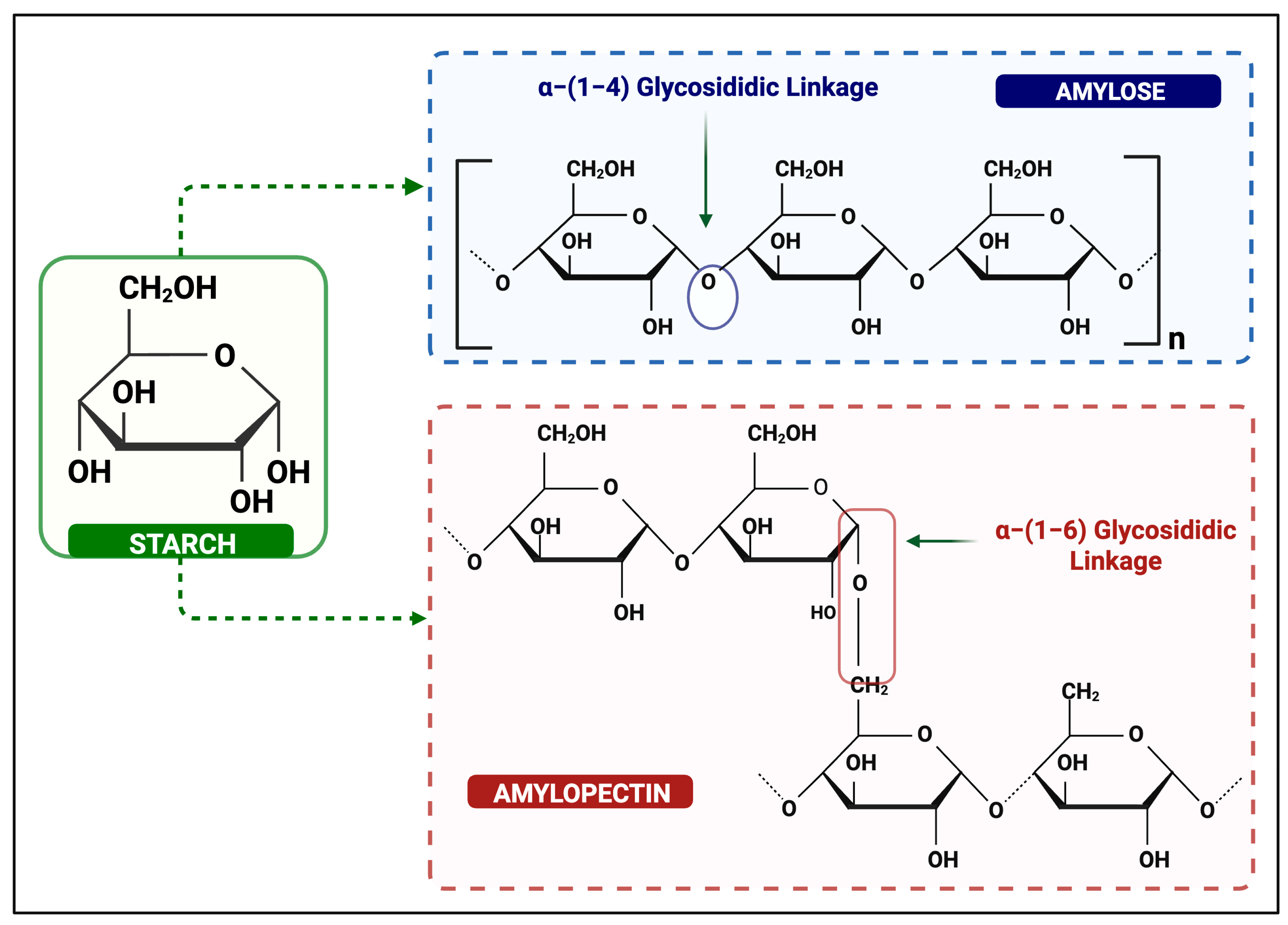
3. Importance of Starch as a Polysaccharide
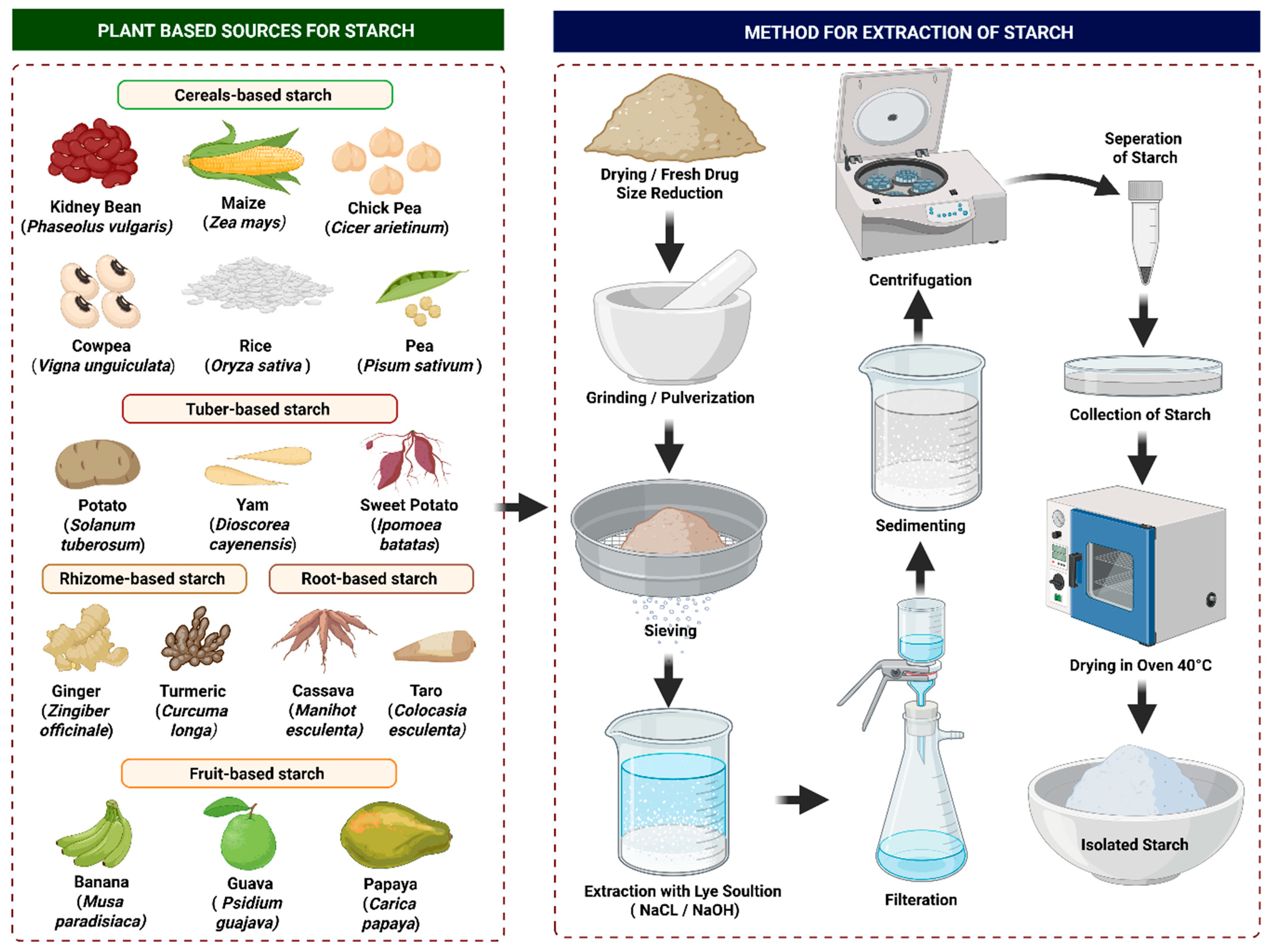
4. Sources of Starch
| Examples | Characteristics | Amylose Content | Amylopectin Content | Moisture Content | Shape\ Size (µm) | Reference |
|---|---|---|---|---|---|---|
| Corn, wheat, rice, barley | High gelatinization temperature, moderate water absorption | 20–30% | 70–80% | 10–14% | Polygonal/spherical (2–35) | [22,23] |
| Potatoes, cassava, sweet potatoes | High water-binding capacity, low gelatinization temperature | 18–22% | 78–82% | 12–20% | Oval/rounded (5–100) | [24,25] |
| Banana, green plantain | High-resistant starch, suitable for dietary and functional foods | 17–21% | 79–83% | 11–15% | Elongated/oval (10–50) | [26,27] |
| Beans, garden peas, chickpeas | Resistant starch, good nutritional properties | 25–30% | 70–75% | 8–13% | Oval/irregular (10–60) | [28] |
| Sago Palm, Arrowroot | Fine texture, easily digestible | 20–25% | 75–80% | 10–15% | Smooth/rounded (10–45) | [29] |
| Yam, taro, pumpkin | High water-binding capacity, excellent viscosity, and gelling | 20–25% | 75–80% | 60–80% | Oval/rounded (5–80) | [30,31,32] |
| Amaranth, quinoa (pseudo cereals) | Gluten-free, high gelling and stability properties for bioplastics and food use | 15–25% | 75–85% | 30% | Small/polygonal (1–3) | [33,34] |
| Ginger (rhizomes) | High starch content, used for medicinal and food purposes | 15–20% | 75–80% | 8–12% | Irregular/fibrous (3–20) | [35] |
| Avocado pits | Starch found in avocado seeds has potential in food processing and biodegradable films | 18–22% | 75–80% | 41% | Round/irregular (3–15) | [36] |
| Duckweed, water chestnuts (aquatic), | High starch yield, promising for biofuel production | 15–25% | 75–85% | 8–12% | Rounded/smooth (3–12) | [37] |
| Chestnuts, lotus seeds (nuts and seeds) | High amylose, potential for functional food applications | 22–28% | 72–78% | 6–10% | Irregular/spherical (8–25) | [38] |
| Red algae, brown algae (seaweed-derived) | Polysaccharides with unique gelatinizing and stabilizing properties | 10% | 80–85% | 5–8% | Fibrous/irregular (2–10) | [39] |
| Date palm seeds, oil palm trunk | Rich in non-conventional starch, useful in biodegradable plastics | 20–25% | 75–80% | 7–9% | Rounded/granular (5–20) | [40] |
| Water hyacinth, kudzu | High starch content, eco-friendly solution for invasive species | 20–25% | 75–80% | 85–95% | Irregular/granular (5–15) | [41] |
| Bamboo shoots, sorghum | Abundant cellulose and starch, potential for biofuel application | 18–22% | 78–82% | 30–50% | Fibrous/polygonal (5–50) | [42] |
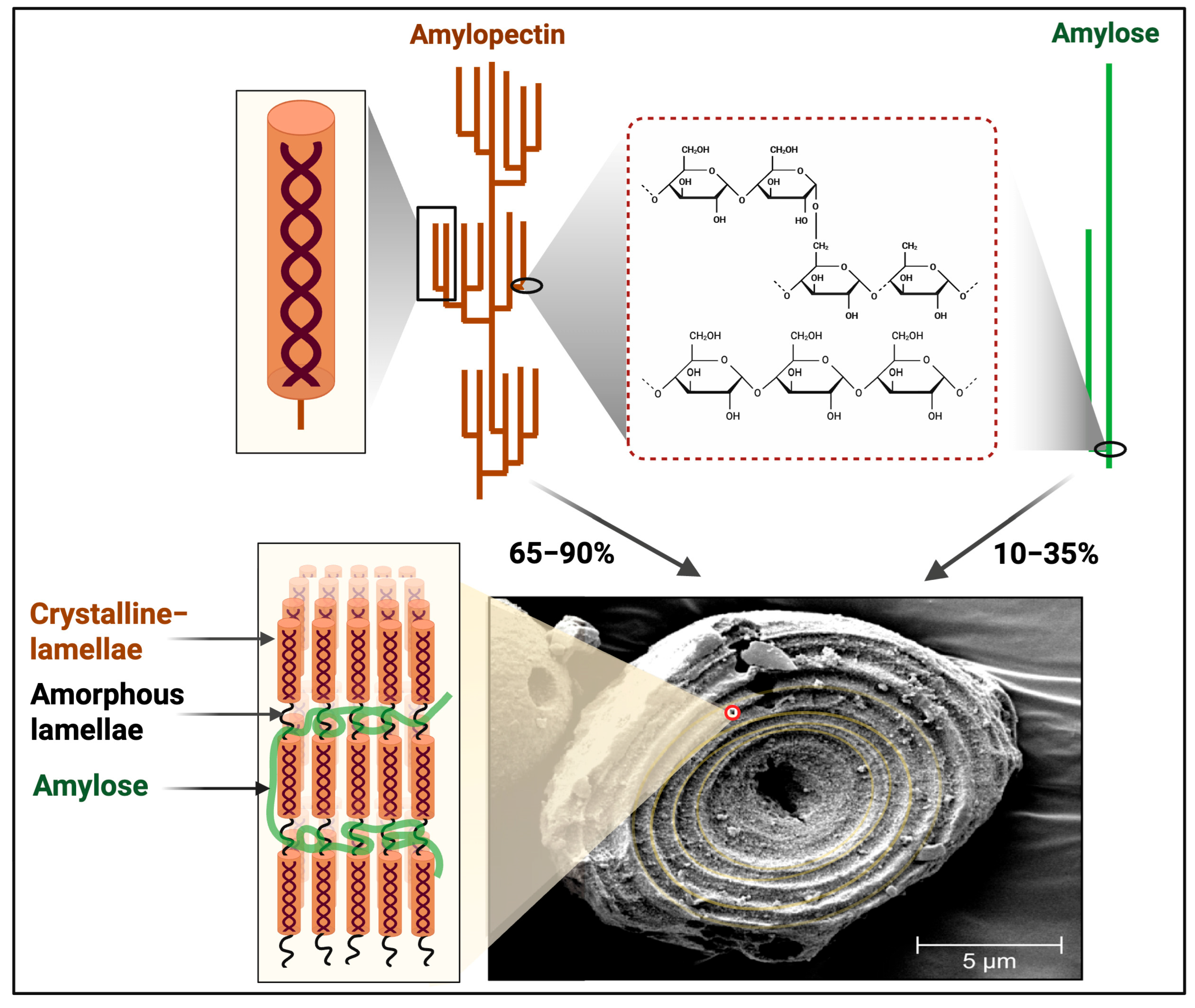
5. Classification of Starch-Based Polymorphic Forms
6. Starch Importance, Isolation, and Modification
6.1. Industrial and Scientific Importance of Starch
6.2. Isolation of Starch from Different Sources
6.2.1. General Isolation Process
6.2.2. Isolation from Various Sources
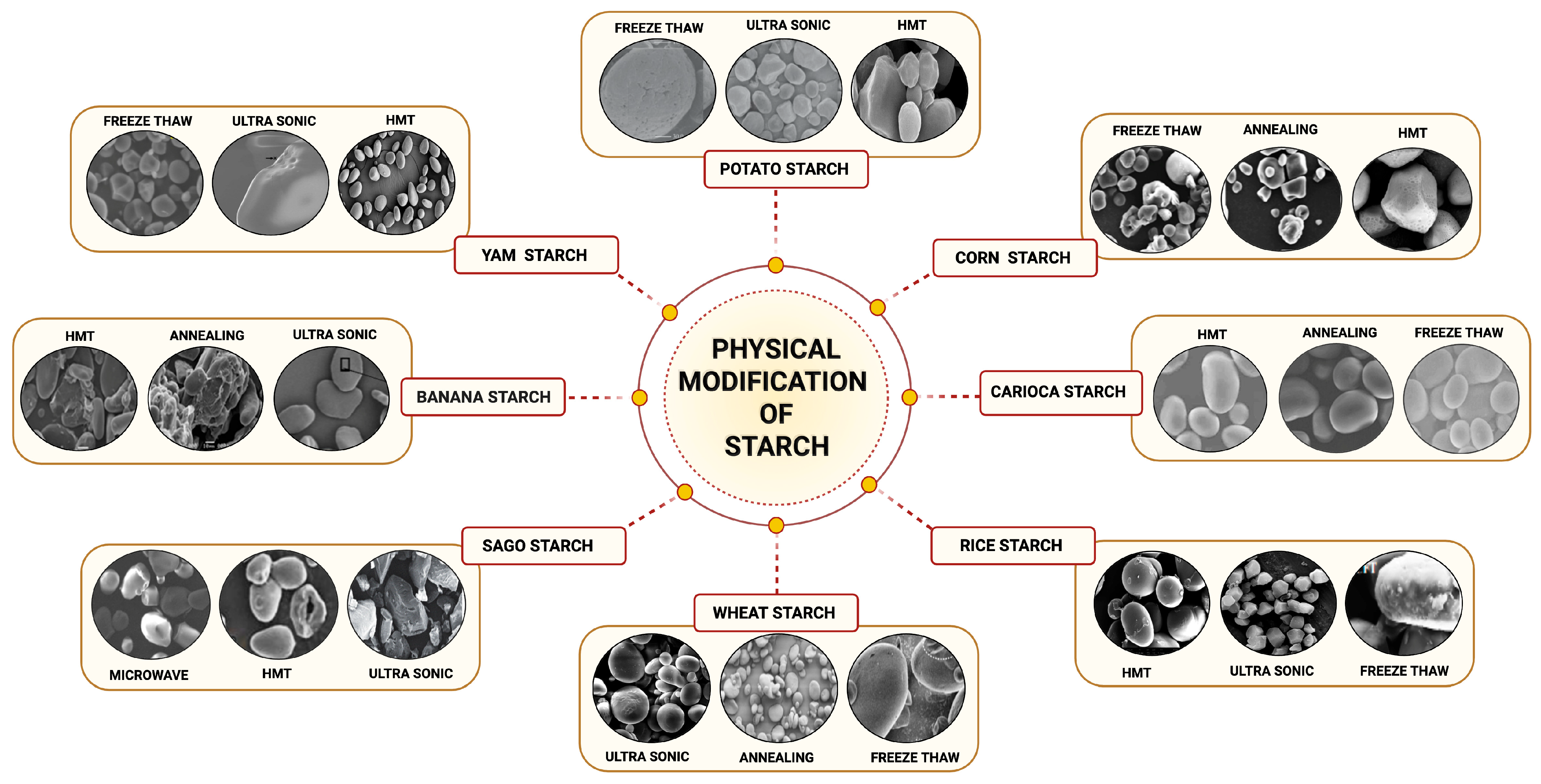
6.2.3. Modification of Starch
Physical Modifications
Chemical Modifications
Dual Modifications
Enzymatic Modifications
Biotechnological Modifications
7. Application of Modified Starch
7.1. Starch-Based Nanomaterials for Wastewater Treatment
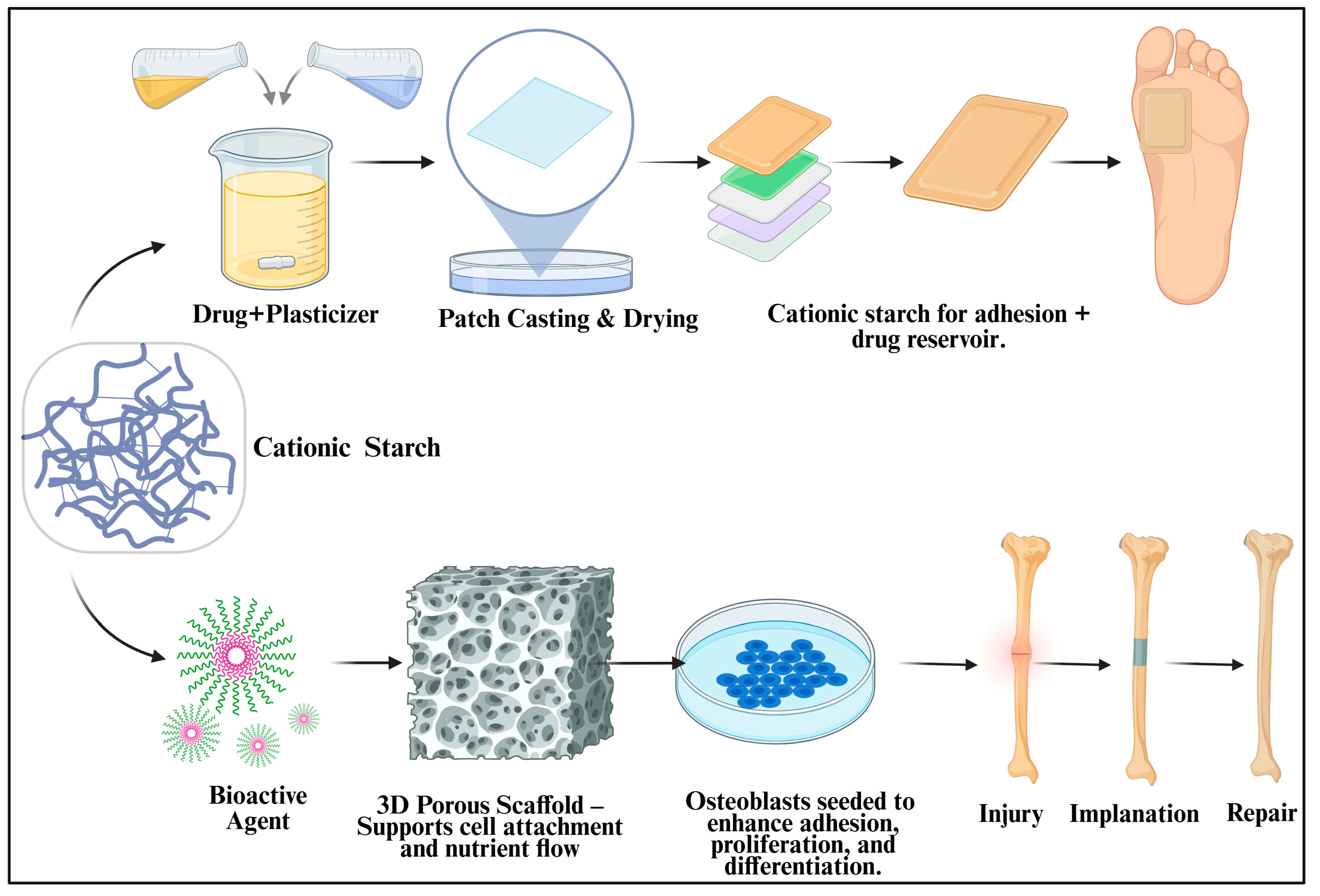
7.2. Biomedical Potential of Cationic Starch-Based Advanced Drug Delivery Systems
7.3. Sustainable Applications of Modified Starch: From Bio-Ethanol Production to Biodegradable Composites
8. Patent Information
9. Challenges, Limitations, Innovative Trends, and Future Prospects
9.1. Challenges in Starch Extraction and Modification
9.2. Limitation
9.3. Innovative Trends and Future Prospects
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Suri, S.; Singh, A. Modification of starch by novel and traditional ways: Influence on the structure and functional properties. Sustain. Food Technol. 2023, 1, 348–362. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.; Hu, Y.; Zhou, Z.; Olsen, J.W.; Guan, Y. Ancient Starch Remains Reveal the Vegetal Diet of the Neolithic Late Dawenkou Culture in Jiangsu, East China. Front. Ecol. Evol. 2021, 9, 722103. [Google Scholar] [CrossRef]
- Abelti, A.L.; Teka, T.A.; Bultosa, G. Structural and physicochemical characterization of starch from water lily (Nymphaea lotus) for food and non-food applications. Carbohydr. Polym. Technol. Appl. 2024, 7, 100458. [Google Scholar] [CrossRef]
- Bojarczuk, A.; Skąpska, S.; Mousavi Khaneghah, A.; Marszałek, K. Health benefits of resistant starch: A review of the literature. J. Funct. Foods 2022, 93, 105094. [Google Scholar] [CrossRef]
- Bertoft, E. Understanding Starch Structure: Recent Progress. Agronomy 2017, 7, 56. [Google Scholar] [CrossRef]
- Case, S.; Capitani, T.; Whaley, J.; Shi, Y.; Trzasko, P.; Jeffcoat, R.; Goldfarb, H. Physical properties and gelation behavior of a low-amylopectin maize starch and other high-amylose maize starches. J. Cereal Sci. 1998, 27, 301–314. [Google Scholar] [CrossRef]
- Fredriksson, H.; Silverio, J.; Andersson, R.; Eliasson, A.-C.; Åman, P. The influence of amylose and amylopectin characteristics on gelatinization and retrogradation properties of different starches. Carbohydr. Polym. 1998, 35, 119–134. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, X.; Al-Maqtari, Q.A.; He, H.-J.; Othman, N. Evaluation of amylose content: Structural and functional properties, analytical techniques, and future prospects. Food Chem. 2024, 24, 101830. [Google Scholar] [CrossRef]
- Sharath Kumar, N.; Sunil, C.K.; Verma, M.K.; Palanimuthu, V. Banana starch: Modification methods and their effect on starch properties–a recent review. Food Humanit. 2025, 4, 100543. [Google Scholar] [CrossRef]
- El Farkhani, M.; Dadou, S.; El Miz, Y.; Elyoussfi, A.; El Miz, M.; Salhi, A.; Koudad, M.; Benchat, N. A review of the chemical modification and applications of starch. BIO Web Conf. 2024, 109, 01020. [Google Scholar] [CrossRef]
- Berski, W.; Ptaszek, A.; Ptaszek, P.; Ziobro, R.; Kowalski, G.; Grzesik, M.; Achremowicz, B. Pasting and rheological properties of oat starch and its derivatives. Carbohydr. Polym. 2011, 83, 665–671. [Google Scholar] [CrossRef]
- Srichuwong, S.; Sunarti, T.C.; Mishima, T.; Isono, N.; Hisamatsu, M. Starches from different botanical sources I: Contribution of amylopectin fine structure to thermal properties and enzyme digestibility. Carbohydr. Polym. 2005, 60, 529–538. [Google Scholar] [CrossRef]
- Salimi, M.; Channab, B.; El Idrissi, A.; Zahouily, M.; Motamedi, E. A comprehensive review on starch: Structure, modification, and applications in slow/controlled-release fertilizers in agriculture. Carbohydr. Polym. 2023, 322, 121326. [Google Scholar] [CrossRef]
- Shoukat, R.; Cappai, M.; Pilia, L.; Pia, G. Rice Starch Chemistry, Functional Properties, and Industrial Applications: A Review. Polymers 2025, 17, 110. [Google Scholar] [CrossRef]
- Song, J.H.; Murphy, R.; Narayan, R.; Davies, G. Biodegradable and compostable alternatives to conventional plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2127–2139. [Google Scholar] [CrossRef]
- Kringel, D.H.; Dias, Á.R.G.; Zavareze, E.d.R.; Gandra, E.Á. Fruit Wastes as Promising Sources of Starch: Extraction, Properties, and Applications. Starch—Stärke 2020, 72, 1900200. [Google Scholar] [CrossRef]
- Adjei, F.K.; Osei, Y.A.; Kuntworbe, N.; Ofori-Kwakye, K. Evaluation of the Disintegrant Properties of Native Starches of Five New Cassava Varieties in Paracetamol Tablet Formulations. J. Pharm. 2017, 2017, 2326912. [Google Scholar] [CrossRef] [PubMed]
- Velde, F.v.d.; Riel, J.v.; Tromp, R.H. Visualisation of Starch Granule Morphologies Using Confocal Scanning Laser Microscopy (CSLM). J. Sci. Food Agric. 2002, 82, 1528–1536. [Google Scholar] [CrossRef]
- Bangar, S.P.; Dhull, S.B.; Manzoor, M.; Chandak, A.; Esua, O.J. Functionality and Applications of Non-Conventional Starches From Different Sources. Starch—Stärke 2023, 76, 2300073. [Google Scholar] [CrossRef]
- Šárka, E.; Sinica, A.; Smrčková, P.; Sluková, M. Non-Traditional Starches, Their Properties, and Applications. Foods 2023, 12, 3794. [Google Scholar] [CrossRef]
- Santana, Á.L.; Meireles, M.Â.A. New Starches Are the Trend for Industry Applications: A Review. Food Public Health 2014, 4, 229–241. [Google Scholar] [CrossRef]
- Chakraborty, I.; N, P.; Mal, S.S.; Paul, U.C.; Rahman, M.H.; Mazumder, N. An Insight into the Gelatinization Properties Influencing the Modified Starches Used in Food Industry: A review. Food Bioprocess Technol. 2022, 15, 1195–1223. [Google Scholar] [CrossRef]
- Carvalho, H.J.; Barcia, M.T.; Schmiele, M. Non-Conventional Starches: Properties and Potential Applications in Food and Non-Food Products. Macromol 2024, 4, 886–909. [Google Scholar] [CrossRef]
- Mariappan Kadarkarainadar, M.; Jawaid, M.; Asim, M. Corn and Rice Starch-Based Bio-Plastics as Alternative Packaging Materials. Fibers 2019, 7, 32. [Google Scholar] [CrossRef]
- Perez, S.; Bertoft, E. The molecular structures of starch components and their contribution to the architecture of starch granules: A comprehensive review. Starch—Stärke 2010, 62, 389–420. [Google Scholar] [CrossRef]
- Zhang, P.; Whistler, R.L.; BeMiller, J.N.; Hamaker, B.R. Banana starch: Production, physicochemical properties, and digestibility—A review. Carbohydr. Polym. 2005, 59, 443–458. [Google Scholar] [CrossRef]
- Abhijeet, V.P.; Vrushali, N.G. Isolation, Characterization of Banana Starch and its Evaluation as a Disintegrating Agent in Dispersible Lornoxicam Tablet. Drug Deliv. Lett. 2022, 12, 276–286. [Google Scholar] [CrossRef]
- Gao, L.; Wu, Y.; Wan, C.; Wang, P.; Yang, P.; Gao, X.; Eeckhout, M.; Gao, J. Structural and physicochemical properties of pea starch affected by germination treatment. Food Hydrocoll. 2022, 124, 107303. [Google Scholar] [CrossRef]
- Du, C.; Jiang, F.; Jiang, W.; Ge, W.; Du, S. Physicochemical and structural properties of sago starch. Int. J. Biol. Macromol. 2020, 164, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.R.; Chaves Ribeiro, A.E.; Gondim, Í.C.; Alves dos Santos, E.; Resende de Oliveira, É.; Mendes Coutinho, G.S.; Soares Júnior, M.S.; Caliari, M. Isolation and characterization of yam (Dioscorea alata L.) starch from Brazil. LWT-Food Sci. Technol. 2021, 149, 111843. [Google Scholar] [CrossRef]
- Huang, G.; Wang, F.; Yang, R.; Wang, Z.-C.; Fang, Z.; Lin, Y.; Zhu, Y.; Bai, L. Characterization of the physicochemical properties of Lipu Colocasia esculenta (L.) Schott starch: A potential new food ingredient. Int. J. Biol. Macromol. 2024, 254, 127803. [Google Scholar] [CrossRef]
- Yin, L.; Wang, C. Morphological, Thermal and Physicochemical Properties of Starches from Squash (Cucurbita maxima) and Pumpkin (Cucurbita moschata). J. Hortic. 2016, 3, 187. [Google Scholar] [CrossRef]
- Olagunju, A.I.; Omoba, O.S. Chapter 10—Amaranth starch: Physicochemical, functional, and nutritional properties. In Non-Conventional Starch Sources; Lorenzo, J.M., Bangar, S.P., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 281–313. [Google Scholar] [CrossRef]
- Li, G.; Zhu, F. Quinoa starch: Structure, properties, and applications. Carbohydr. Polym. 2018, 181, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Reyes, F.; D’Appolonia, B.; Ciacco, C.; Montgomery, M. Characterization of Starch from Ginger Root (Zingiber officinale). Starch-Starke 1982, 34, 40–44. [Google Scholar] [CrossRef]
- Martins, S.H.F.; Pontes, K.V.; Fialho, R.L.; Fakhouri, F.M. Extraction and characterization of the starch present in the avocado seed (Persea americana mill) for future applications. J. Agric. Food Res. 2022, 8, 100303. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, F.; Daroch, M.; Tang, J. Positive effects of duckweed polycultures on starch and protein accumulation. Biosci. Rep. 2016, 36, e00380. [Google Scholar] [CrossRef]
- Zhu, F. Structures, properties, and applications of lotus starches. Food Hydrocoll. 2017, 63, 332–348. [Google Scholar] [CrossRef]
- Prabhu, M.; Chemodanov, A.; Gottlieb, R.; Kazir, M.; Nahor, O.; Gozin, M.; Israel, A.; Livney, Y.D.; Golberg, A. Starch from the sea: The green macroalga Ulva ohnoi as a potential source for sustainable starch production in the marine biorefinery. Algal Res. 2019, 37, 215–227. [Google Scholar] [CrossRef]
- Sobini, N.; Darsiga, S.; Kananke, T.C.; Srivijeindran, S. Characterization of modified palmyrah tuber starch by pre-gelatinization, acid and dextrinization processes and its applicability. Food Chem. Adv. 2022, 1, 100143. [Google Scholar] [CrossRef]
- Li, M.; Miao, M.; Sun, J.; Fang, H.; Liu, L.; Xu, X.; Zheng, Y.; Lai, Q.; Tang, Y.; Liu, X.; et al. Structure and physicochemical properties of starches from six accessions of the genus Pueraria in China. Int. J. Biol. Macromol. 2024, 279, 135508. [Google Scholar] [CrossRef]
- Felisberto, M.H.F.; Beraldo, A.L.; Costa, M.S.; Boas, F.V.; Franco, C.M.L.; Clerici, M.T.P.S. Physicochemical and structural properties of starch from young bamboo culm of Bambusa tuldoides. Food Hydrocoll. 2019, 87, 101–107. [Google Scholar] [CrossRef]
- Katsumi, N.; Okazaki, M.; Yonebayashi, K.; Kawashima, F.; Nishiyama, S.; Nishi, T. New proposal for “crystalline index” of starch. Sago Palm 2014, 22, 25–30. [Google Scholar] [CrossRef]
- Dome, K.; Podgorbunskikh, E.; Bychkov, A.; Lomovsky, O. Changes in the Crystallinity Degree of Starch Having Different Types of Crystal Structure after Mechanical Pretreatment. Polymers 2020, 12, 641. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, M.E.; Hernandez-Landaverde, M.A.; Delgado, J.M.; Ramirez-Gutierrez, C.F.; Ramirez-Cardona, M.; Millan-Malo, B.M.; Londoño-Restrepo, S.M. Crystalline structures of the main components of starch. Curr. Opin. Food Sci. 2021, 37, 107–111. [Google Scholar] [CrossRef]
- Lourdin, D.; Putaux, J.-L.; Potocki-Véronèse, G.; Chevigny, C.; Rolland-Sabaté, A.; Buléon, A. Crystalline Structure in Starch. In Starch: Metabolism and Structure; Nakamura, Y., Ed.; Springer: Tokyo, Japan, 2015; pp. 61–90. [Google Scholar]
- Vilivalam, V.D.; Illum, L.; Iqbal, K. Starch capsules: An alternative system for oral drug delivery. Pharm. Sci. Technol. Today 2000, 3, 64–69. [Google Scholar] [CrossRef]
- Arias, L.V.A.; Silva, V.d.S.; Vieira, J.M.M.; Fakhouri, F.M.; de Oliveira, R.A. Plant-Based Films for Food Packaging as a Plastic Waste Management Alternative: Potato and Cassava Starch Case. Polymers 2024, 16, 2390. [Google Scholar] [CrossRef] [PubMed]
- Le Thanh-Blicharz, J.; Lewandowicz, J. Functionality of Native Starches in Food Systems: Cluster Analysis Grouping of Rheological Properties in Different Product Matrices. Foods 2020, 9, 1073. [Google Scholar] [CrossRef] [PubMed]
- Chemelli, A.; Gomernik, F.; Thaler, F.; Huber, A.; Hirn, U.; Bauer, W.; Spirk, S. Cationic starches in paper-based applications—A review on analytical methods. Carbohydr. Polym. 2020, 235, 115964. [Google Scholar] [CrossRef]
- Admase, A.T.; Mersha, D.A.; Kebede, A.Y. Cassava starch-based hot melt adhesive for textile industries. Sci. Rep. 2024, 14, 20927. [Google Scholar] [CrossRef] [PubMed]
- Karow, M.F.; Santos, F.N.d.; Biduski, B.; Krolow, A.C.R.; Silva, F.T.d.; El Halal, S.L.M.; Macagnan, K.L.; Zavareze, E.d.R.; Dias, A.R.G.; Diaz, P.S. Natural fermentation of potato (Solanum tuberosum L.) starch: Effect of cultivar, amylose content, and drying method on expansion, chemical and morphological properties. Int. J. Biol. Macromol. 2024, 261, 129608. [Google Scholar] [CrossRef]
- Roberts, S.A.; Cameron, R.E. The effects of concentration and sodium hydroxide on the rheological properties of potato starch gelatinisation. Carbohydr. Polym. 2002, 50, 133–143. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, H.; Dai, Y.; Hou, H.; Dong, H. Effect of Alkali Treatment on Structure and Properties of High Amylose Corn Starch Film. Materials 2019, 12, 1705. [Google Scholar] [CrossRef]
- Makroo, H.A.; Naqash, S.; Saxena, J.; Sharma, S.; Majid, D.; Dar, B.N. Recovery and characteristics of starches from unconventional sources and their potential applications: A review. Appl. Food Res. 2021, 1, 100001. [Google Scholar] [CrossRef]
- Heena; Kumar, N.; Singh, R.; Upadhyay, A.; Giri, B.S. Application and functional properties of millet starch: Wet milling extraction process and different modification approaches. Heliyon 2024, 10, e25330. [Google Scholar] [CrossRef]
- Sridhar, A.; Vaishampayan, V.; Senthil Kumar, P.; Ponnuchamy, M.; Kapoor, A. Extraction techniques in food industry: Insights into process parameters and their optimization. Food Chem. Toxicol. 2022, 166, 113207. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, R.K.; Srivastav, P.P. Exploring novel frontiers of advancements in purple yam (Dioscorea alata L.) starch extraction, modification, characterization, applications in food and other industries. Meas. Food 2024, 15, 100196. [Google Scholar] [CrossRef]
- Silva, G.M.d.S.; Veloso, C.M.; Santos, L.S.; Melo Neto, B.A.d.; Fontan, R.d.C.I.; Bonomo, R.C.F. Extraction and characterization of native starch obtained from the inhambu tuber. J. Food Sci. Technol. 2020, 57, 1830–1839. [Google Scholar] [CrossRef]
- Haq, N.; Rashem, W.; Mubashir, N.; Dure, S. Physical and Chemical Modifications in Starch Structure and Reactivity. In Chemical Properties of Starch; Martins, E., Ed.; IntechOpen: Rijeka, Croatia, 2020; Chapter 2. [Google Scholar]
- Navaf, M.; Sunooj, K.V. Physical Modifications of Starch. In Advanced Research in Starch; Mazumder, N., Rahman, M.H., Eds.; Springer Nature: Singapore, 2024; pp. 1–45. [Google Scholar]
- Katopo, H.; Song, Y.; Jane, J. Effect and mechanism of ultrahigh hydrostatic pressure on the structure and properties of starches. Carbohydr. Polym. 2002, 47, 233–244. [Google Scholar] [CrossRef]
- Ye, S.-J.; Baik, M.-Y. Characteristics of physically modified starches. Food Sci. Biotechnol. 2023, 32, 875–883. [Google Scholar] [CrossRef]
- Fonseca, L.M.; Halal, S.L.M.E.; Dias, A.R.G.; Zavareze, E.d.R. Physical modification of starch by heat-moisture treatment and annealing and their applications: A review. Carbohydr. Polym. 2021, 274, 118665. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Singh, A.; Ashogbon, A.O.; Bobade, H. Ball-milling: A sustainable and green approach for starch modification. Int. J. Biol. Macromol. 2023, 237, 124069. [Google Scholar] [CrossRef]
- Hong, Y.; Liu, X. Pre-gelatinized Modification of Starch. In Physical Modifications of Starch; Sui, Z., Kong, X., Eds.; Springer: Singapore, 2018; pp. 51–61. [Google Scholar]
- Adawiyah, D.R.; Akuzawa, S.; Sasaki, T.; Kohyama, K. A comparison of the effects of heat moisture treatment (HMT) on rheological properties and amylopectin structure in sago (Metroxylon sago) and arenga (Arenga pinnata) starches. J. Food Sci. Technol. 2017, 54, 3404–3410. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh, M.; Karim, R.; Sultan, M.T.; Paykary, M.; Johnson, S.K.; Shekarforoush, E. Comparison of starch films and effect of different rice starch-based coating formulations on physical properties of walnut during storage time at accelerated temperature. J. Food Process Eng. 2018, 41, e12607. [Google Scholar] [CrossRef]
- Li, L.; He, S.; Lin, Y.; Zheng, B.; Zhang, Y.; Zeng, H. A novel lotus seed cross-linked resistant starch: Structural, physicochemical and digestive properties. Front. Nutr. 2022, 9, 989042. [Google Scholar] [CrossRef]
- Liu, Y.; Jinmei, G.; Duo, F.; Jiangyan, Z.; Yu, G.; Jian, Z.; Wenhao, L.; Yan, W. Modification of structural and physicochemical properties of repeated freeze-thawed cycle maize starch. Int. J. Food Prop. 2020, 23, 1597–1610. [Google Scholar] [CrossRef]
- Kiseleva, V.I.; Krivandin, A.V.; Fornal, J.; Błaszczak, W.; Jeliński, T.; Yuryev, V.P. Annealing of normal and mutant wheat starches. LM, SEM, DSC, and SAXS studies. Carbohydr. Res. 2005, 340, 75–83. [Google Scholar] [CrossRef]
- Sujka, M.; Jamroz, J. Ultrasound-treated starch: SEM and TEM imaging, and functional behaviour. Food Hydrocoll. 2013, 31, 413–419. [Google Scholar] [CrossRef]
- Braşoveanu, M.; Nemţanu, M.R. Behaviour of starch exposed to microwave radiation treatment. Starch—Stärke 2014, 66, 3–14. [Google Scholar] [CrossRef]
- Wang, S.; Hu, X.; Wang, Z.; Bao, Q.; Zhou, B.; Li, T.; Li, S. Preparation and characterization of highly lipophilic modified potato starch by ultrasound and freeze-thaw treatments. Ultrason. Sonochem. 2020, 64, 105054. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Melnyk, O.; Marenkova, T.; Luo, Y. Modification in Physicochemical, Structural and Digestive Properties of Potato Starch During Heat-Moisture Treatment Combined with Microwave Pre- and Post-Treatment. Pol. J. Food Nutr. Sci. 2022, 72, 249–261. [Google Scholar] [CrossRef]
- Bonto, A.P.; Tiozon, R.N.; Sreenivasulu, N.; Camacho, D.H. Impact of ultrasonic treatment on rice starch and grain functional properties: A review. Ultrason. Sonochem. 2021, 71, 105383. [Google Scholar] [CrossRef]
- Subroto, E.; Mahani; Indiarto, R.; Yarlina, V.P.; Izzati, A.N. A Mini Review of Physicochemical Properties of Starch and Flour by Using Hydrothermal Treatment. Polymers 2022, 14, 5447. [Google Scholar] [CrossRef] [PubMed]
- Suriya, M.; Reddy, C.K.; Haripriya, S. Functional and thermal behaviors of heat-moisture treated elephant foot yam starch. Int. J. Biol. Macromol. 2019, 137, 783–789. [Google Scholar] [CrossRef]
- Dewi, A.M.; Santoso, U.; Pranoto, Y.; Marseno, D.W. Dual Modification of Sago Starch via Heat Moisture Treatment and Octenyl Succinylation to Improve Starch Hydrophobicity. Polymers 2022, 14, 1086. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Q.; Zhao, Y.; Qin, Z.; Zheng, M.; Liu, H.; Liu, J. Preparation and characterization of low oil absorption corn starch by ultrasonic combined with freeze–thaw treatment. Food Chem. 2022, 15, 100410. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Hu, X.; Zhang, F.; Fang, C.; Liu, C.; Luo, S. Freeze-thaw stability of rice starch modified by Improved Extrusion Cooking Technology. Carbohydr. Polym. 2016, 151, 113–118. [Google Scholar] [CrossRef]
- Zailani, M.A.; Hanisah, K.; Ahmad, H.; Sarbini, S.R. Physicochemical properties of microwave heated sago (Metroxylon sagu) starch. CyTA-J. Food 2021, 19, 596–605. [Google Scholar] [CrossRef]
- Zanella Pinto, V.; Goncalves Deon, V.; Moomand, K.; Levien Vanier, N.; Pilatti-Riccio, D.; da Rosa Zavareze, E.; Lim, L.-T.; Guerra Dias, A.R. Characteristics of Modified Carioca Bean Starch upon Single and Dual Annealing, Heat-Moisture-Treatment, and Sonication. Starch—Stärke 2019, 71, 1800173. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Y.; Zheng, L.; Zheng, X.; Xiao, D.; Wang, S.; Zhang, Z.; Ai, B.; Sheng, Z. Physicochemical, Structural, and Digestive Properties of Banana Starch Modified by Ultrasound and Resveratrol Treatments. Foods 2022, 11, 3741. [Google Scholar] [CrossRef]
- Yang, Y.; Fu, J.; Duan, Q.; Xie, H.; Dong, X.; Yu, L. Strategies and Methodologies for Improving Toughness of Starch Films. Foods 2024, 13, 4036. [Google Scholar] [CrossRef]
- Masina, N.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Govender, M.; Indermun, S.; Pillay, V. A review of the chemical modification techniques of starch. Carbohydr. Polym. 2017, 157, 1226–1236. [Google Scholar] [CrossRef]
- Kuakpetoon, D.; Wang, Y.-J. Characterization of Different Starches Oxidized by Hypochlorite. Starch—Stärke 2001, 53, 211–218. [Google Scholar] [CrossRef]
- Gong, Y.; Xiao, S.; Yao, Z.; Deng, H.; Chen, X.; Yang, T. Factors and modification techniques enhancing starch gel structure and their applications in foods:A review. Food Chem. 2024, 24, 102045. [Google Scholar] [CrossRef]
- Zarski, A.; Kapusniak, K.; Ptak, S.; Rudlicka, M.; Coseri, S.; Kapusniak, J. Functionalization Methods of Starch and Its Derivatives: From Old Limitations to New Possibilities. Polymers 2024, 16, 597. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, Y.; Qiao, Q.; Tao, X.; Liu, P.; Xie, F. Comparison of the structure and properties of hydroxypropylated acid-hydrolysed maize starches with different amylose/amylopectin contents. Food Hydrocoll. 2021, 110, 106134. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, N.; Xu, Y.; Huang, J.; Yuan, M.a.; Wu, D.; Shu, X. Physicochemical properties of hydroxypropylated and cross-linked rice starches differential in amylose content. Int. J. Biol. Macromol. 2019, 128, 775–781. [Google Scholar] [CrossRef]
- Hirsch, J.B.; Kokini, J.L. Understanding the mechanism of cross-linking agents (POCl3, STMP, and EPI) through swelling bebavior and pasting properties of cross-linked waxy maize starches. Cereal Chem. 2002, 79, 102–107. [Google Scholar] [CrossRef]
- Seker, M.; Hanna, M.A. Cross-linking starch at various moisture contents by phosphate substitution in an extruder. Carbohydr. Polym. 2005, 59, 541–544. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, Y.; Zheng, X.; Wang, H.; Wang, N. Determination of optimum experimental conditions for preparation and functional properties of hydroxypropylated, phosphorylated and hydroxypropyl-phosphorylated glutinous rice starch. Int. J. Biol. Macromol. 2017, 105, 317–327. [Google Scholar] [CrossRef]
- Singh, V.; Ali, S.Z. Acid degradation of starch. The effect of acid and starch type. Carbohydr. Polym. 2000, 41, 191–195. [Google Scholar] [CrossRef]
- Puri, A.; Syukri, D.M.; Silvia, E.; Ladyani, F.; Mohite, P.; Ade, N.; Munde, S.; Chidrawar, V.R.; Singh, S.; Shafi, S. Waste-to-Value-Added Customized Cationic Banana Starch for Potential Flocculant Application. J. Polym. Environ. 2024, 32, 6096–6113. [Google Scholar] [CrossRef]
- Radosta, S.; Vorwerg, W.; Ebert, A.; Begli, A.H.; Grülc, D.; Wastyn, M. Properties of Low-substituted Cationic Starch Derivatives Prepared by Different Derivatisation Processes. Starch—Stärke 2004, 56, 277–287. [Google Scholar] [CrossRef]
- Su, Y.; Du, H.; Huo, Y.; Xu, Y.; Wang, J.; Wang, L.; Zhao, S.; Xiong, S. Characterization of cationic starch flocculants synthesized by dry process with ball milling activating method. Int. J. Biol. Macromol. 2016, 87, 34–40. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, H.; Wang, l.; Abdin, Z.; Chen, Y.; Wang, J.; Zhou, W.; Yang, X.; Khan, R.U.; Zhang, H.; et al. Recent progress in chemical modification of starch and its applications. RSC Adv. 2015, 5, 67459–67474. [Google Scholar] [CrossRef]
- Minimol, P.F.; Paul, W.; Sharma, C.P. PEGylated starch acetate nanoparticles and its potential use for oral insulin delivery. Carbohydr. Polym. 2013, 95, 1–8. [Google Scholar] [CrossRef]
- Chávez-Murillo, C.E.; Wang, Y.-J.; Bello-Pérez, L.A. Morphological, Physicochemical and Structural Characteristics of Oxidized Barley and Corn Starches. Starch—Stärke 2008, 60, 634–645. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.; Wang, Y.; Xiong, G.; Mei, X.; Wu, W.; Ding, A.; Li, X.; Qiao, Y.; Liao, L. Effects of fatty acid chain length on properties of potato starch–fatty acid complexes under partially gelatinization. Int. J. Food Prop. 2018, 21, 2121–2134. [Google Scholar] [CrossRef]
- Xiao, H.-X.; Lin, Q.-L.; Liu, G.-Q.; Yu, F.-X. A Comparative Study of the Characteristics of Cross-Linked, Oxidized and Dual-Modified Rice Starches. Molecules 2012, 17, 10946–10957. [Google Scholar] [CrossRef]
- Sun, F.; Liu, J.; Liu, X.; Wang, Y.; Li, K.; Chang, J.; Yang, G.; He, G. Effect of the phytate and hydrogen peroxide chemical modifications on the physicochemical and functional properties of wheat starch. Food Res. Int. 2017, 100, 180–192. [Google Scholar] [CrossRef]
- Pornsuksomboon, K.; Szécsényi, K.M.; Holló, B.; Kaewtatip, K. Preparation of native cassava starch and cross-linked starch blended foams. Starch—Stärke 2014, 66, 818–823. [Google Scholar] [CrossRef]
- Kim, N.-H.; Kim, J.-H.; Lee, S.; Lee, H.; Yoon, J.-W.; Wang, R.; Yoo, S.-H. Combined effect of autoclaving-cooling and cross-linking treatments of normal corn starch on the resistant starch formation and physicochemical properties. Starch—Stärke 2010, 62, 358–363. [Google Scholar] [CrossRef]
- Sangseethong, K.; Termvejsayanon, N.; Sriroth, K. Characterization of physicochemical properties of hypochlorite- and peroxide-oxidized cassava starches. Carbohydr. Polym. 2010, 82, 446–453. [Google Scholar] [CrossRef]
- Utrilla-Coello, R.G.; Hernández-Jaimes, C.; Carrillo-Navas, H.; González, F.; Rodríguez, E.; Bello-Pérez, L.A.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Acid hydrolysis of native corn starch: Morphology, crystallinity, rheological and thermal properties. Carbohydr. Polym. 2014, 103, 596–602. [Google Scholar] [CrossRef]
- Ulbrich, M.; Natan, C.; Flöter, E. Acid modification of wheat, potato, and pea starch applying gentle conditions—Impacts on starch properties. Starch—Stärke 2014, 66, 903–913. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, C.; Wang, R.; Yang, L.; Du, S.; Wang, F.; Ruan, H.; He, G. Lipase-coupling esterification of starch with octenyl succinic anhydride. Carbohydr. Polym. 2012, 87, 2137–2144. [Google Scholar] [CrossRef]
- Wang, R.; Li, M.; Liu, J.; Wang, F.; Wang, J.; Zhou, Z. Dual modification manipulates rice starch characteristics following debranching and propionate esterification. Food Hydrocoll. 2021, 119, 106833. [Google Scholar] [CrossRef]
- Zarski, A.; Ptak, S.; Siemion, P.; Kapusniak, J. Esterification of potato starch by a biocatalysed reaction in an ionic liquid. Carbohydr. Polym. 2016, 137, 657–663. [Google Scholar] [CrossRef]
- Abedi, E.; Pourmohammadi, K.; Abbasi, S. Dual-frequency ultrasound for ultrasonic-assisted esterification. Food Sci. Nutr. 2019, 7, 2613–2624. [Google Scholar] [CrossRef]
- Isotton, F.S.; Bernardo, G.L.; Baldasso, C.; Rosa, L.M.; Zeni, M. The plasticizer effect on preparation and properties of etherified corn starchs films. Ind. Crops Prod. 2015, 76, 717–724. [Google Scholar] [CrossRef]
- Clasen, S.H.; Müller, C.M.O.; Parize, A.L.; Pires, A.T.N. Synthesis and characterization of cassava starch with maleic acid derivatives by etherification reaction. Carbohydr. Polym. 2018, 180, 348–353. [Google Scholar] [CrossRef]
- Bakouri, H.; Guemra, K. Etherification and cross-linking effect on physicochemical properties of Zea mays starch executed at different sequences in 1-butyl-3-methylimidazolium chloride [BMIM]Cl ionic liquid media. Int. J. Biol. Macromol. 2019, 125, 1118–1127. [Google Scholar] [CrossRef]
- Sumardiono, S.; Riska, L.; Jos, B.; Pudjiastuti, I. Effect of Esterification on Cassava Starch: Physicochemical Properties and Expansion Ability. Reaktor 2019, 19, 34–41. [Google Scholar] [CrossRef]
- Rajan, A.; Sudha, J.D.; Abraham, T.E. Enzymatic modification of cassava starch by fungal lipase. Ind. Crops Prod. 2008, 27, 50–59. [Google Scholar] [CrossRef]
- Jeon, Y.-S.; Lowell, A.V.; Gross, R.A. Studies of Starch Esterification: Reactions with Alkenylsuccinates in Aqueous Slurry Systems. Starch—Stärke 1999, 51, 90–93. [Google Scholar] [CrossRef]
- Lee, H.S.; Jeong, G.A.; Lim, S.; Lee, C.J. Impact of Esterification with Octenyl Succinic Anhydride on the Structural Characteristics and Glucose Response in Mice of Wheat Starch. Foods 2024, 13, 1395. [Google Scholar] [CrossRef]
- Naknaen, P. Physicochemical, thermal, pasting and microstructure properties of hydroxypropylated jackfruit seed starch prepared by etherification with propylene oxide. Food Biophys. 2014, 9, 249–259. [Google Scholar] [CrossRef]
- Aminian, M.; Nafchi, A.M.; Bolandi, M.; Alias, A.K. Preparation and characterization of high degree substituted sago (Metroxylon sagu) starch with propylene oxide. Starch—Stärke 2013, 65, 686–693. [Google Scholar] [CrossRef]
- Schmitz, C.S.; De Simas, K.N.; Santos, K.; Joao, J.J.; de Mello Castanho Amboni, R.D.; Amante, E.R. Cassava starch functional properties by etherification–hydroxypropylation. Int. J. Food Sci. Technol. 2006, 41, 681–687. [Google Scholar] [CrossRef]
- Ačkar, Đ.; Babić, J.; Šubarić, D.; Kopjar, M.; Miličević, B. Isolation of starch from two wheat varieties and their modification with epichlorohydrin. Carbohydr. Polym. 2010, 81, 76–82. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Siroha, A.K.; Punia, S.; Sangwan, L.; Nehra, M.; Purewal, S.S. Effect of degree of cross linking on physicochemical, rheological and morphological properties of Sorghum starch. Carbohydr. Polym. Technol. Appl. 2021, 2, 100073. [Google Scholar] [CrossRef]
- Kavaliauskaite, R.; Klimaviciute, R.; Zemaitaitis, A. Factors influencing production of cationic starches. Carbohydr. Polym. 2008, 73, 665–675. [Google Scholar] [CrossRef]
- Jyothi, A.N.; Moorthy, S.N.; Rajasekharan, K.N. Effect of cross-linking with epichlorohydrin on the properties of cassava (Manihot esculenta Crantz) starch. Starch—Stärke 2006, 58, 292–299. [Google Scholar] [CrossRef]
- Zhou, F.; Liu, Q.; Zhang, H.; Chen, Q.; Kong, B. Potato starch oxidation induced by sodium hypochlorite and its effect on functional properties and digestibility. Int. J. Biol. Macromol. 2016, 84, 410–417. [Google Scholar] [CrossRef]
- Garrido, L.H.; Schnitzler, E.; Zortéa, M.E.B.; de Souza Rocha, T.; Demiate, I.M. Physicochemical properties of cassava starch oxidized by sodium hypochlorite. J. Food Sci. Technol. 2014, 51, 2640–2647. [Google Scholar] [CrossRef]
- Emam, H.E.; Zahran, M.K.; Ahmed, H.B. Generation of biocompatible nanogold using H2O2–starch and their catalytic/antimicrobial activities. Eur. Polym. J. 2017, 90, 354–367. [Google Scholar] [CrossRef]
- Dias, A.R.G.; da Rosa Zavareze, E.; Helbig, E.; de Moura, F.A.; Vargas, C.G.; Ciacco, C.F. Oxidation of fermented cassava starch using hydrogen peroxide. Carbohydr. Polym. 2011, 86, 185–191. [Google Scholar] [CrossRef]
- Pereira, J.; Evangelho, J.; Moura, F.; Gutkoski, L.; Zavareze, E.; Dias, A. Crystallinity, thermal and gel properties of oat starch oxidized using hydrogen peroxide. Int. Food Res. J. 2017, 24, 1545–1552. [Google Scholar]
- Hwang, D.-K.; Kim, B.-Y.; Baik, M.-Y. Physicochemical Properties of Non-thermally Cross-linked Corn Starch with Phosphorus Oxychloride using Ultra High Pressure (UHP). Starch—Stärke 2009, 61, 438–447. [Google Scholar] [CrossRef]
- Lohmar, R.; Sloan, J.W.; Rist, C.E. Phosphorylation of Starch. J. Am. Chem. Soc. 1950, 72, 5717–5720. [Google Scholar] [CrossRef]
- Kim, H.-S.; Hwang, D.-K.; Kim, B.-Y.; Baik, M.-Y. Cross-linking of corn starch with phosphorus oxychloride under ultra high pressure. Food Chem. 2012, 130, 977–980. [Google Scholar] [CrossRef]
- Khatijah, I. Effects of reaction pH and concentration of phosphorus oxychloride on cross-linking of tapioca starch. J. Trop. Agric. Food Sci. 2000, 28, 95–100. [Google Scholar]
- Kulicke, W.M.; Aggour, Y.A.; Elsabee, M.Z. Preparation, Characterisation, and Rheological Behaviour of Starch-Sodium Trimetaphosphate Hydrogels. Starch—Stärke 1990, 42, 134–141. [Google Scholar] [CrossRef]
- Muhammad, K.; Hussin, F.; Man, Y.C.; Ghazali, H.M.; Kennedy, J.F. Effect of pH on phosphorylation of sago starch. Carbohydr. Polym. 2000, 42, 85–90. [Google Scholar] [CrossRef]
- Woo, K.; Seib, P.A. Cross-linking of wheat starch and hydroxypropylated wheat starch in alkaline slurry with sodium trimetaphosphate. Carbohydr. Polym. 1997, 33, 263–271. [Google Scholar] [CrossRef]
- Mao, G.-J.; Wang, P.; Meng, X.-S.; Zhang, X.; Zheng, T. Crosslinking of corn starch with sodium trimetaphosphate in solid state by microwave irradiation. J. Appl. Polym. Sci. 2006, 102, 5854–5860. [Google Scholar] [CrossRef]
- Cordoba, L.; Ribeiro, L.; Colman, T.; Oliveira, C.; Andrade, M.; Costa, F.; Schnitzler, E. Effect of hydrochloric acid in different concentrations and temperatures up to some properties of organic cassava starch. Braz. J. Therm. Anal 2013, 2, 6–11. [Google Scholar] [CrossRef][Green Version]
- de Andrade de Siqueira, G.L.; Hornung, P.S.; da Silveira, A.C.; da Silveira Lazzarotto, S.R.; do Prado Cordoba, L.; Schnitzler, E.; Lazzarotto, M. Impact of treatment with HCl/alcoholic in the modification of corn starch. J. Therm. Anal. Calorim. 2017, 129, 1705–1713. [Google Scholar] [CrossRef]
- Chung, Y.-L.; Lai, H.-M. Molecular and granular characteristics of corn starch modified by HCl-methanol at different temperatures. Carbohydr. Polym. 2006, 63, 527–534. [Google Scholar] [CrossRef]
- Hoover, R. Acid-Treated Starches. Food Rev. Int. 2000, 16, 369–392. [Google Scholar] [CrossRef]
- Ashogbon, A.O. Dual modification of various starches: Synthesis, properties and applications. Food Chem. 2021, 342, 128325. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Liu, X.; Wang, J.; Guo, P.; Wang, S. Preparation and characterization of chemically modified tapioca starch-ionic liquid antibacterial films. Carbohydr. Polym. 2024, 324, 121519. [Google Scholar] [CrossRef]
- Hazarika, B.J.; Sit, N. Effect of dual modification with hydroxypropylation and cross-linking on physicochemical properties of taro starch. Carbohydr. Polym. 2016, 140, 269–278. [Google Scholar] [CrossRef]
- Babu, A.S.; Mohan, R.J.; Parimalavalli, R. Effect of single and dual-modifications on stability and structural characteristics of foxtail millet starch. Food Chem. 2019, 271, 457–465. [Google Scholar] [CrossRef]
- Khurshida, S.; Das, M.J.; Deka, S.C.; Sit, N. Effect of dual modification sequence on physicochemical, pasting, rheological and digestibility properties of cassava starch modified by acetic acid and ultrasound. Int. J. Biol. Macromol. 2021, 188, 649–656. [Google Scholar] [CrossRef]
- Sriprablom, J.; Tatikunakorn, P.; Lerdpriyanun, P.; Suphantharika, M.; Wongsagonsup, R. Effect of single and dual modifications with cross-linking and octenylsuccinylation on physicochemical, in-vitro digestibility, and emulsifying properties of cassava starch. Food Res. Int. 2023, 163, 112304. [Google Scholar] [CrossRef]
- Das, A.B.; Singh, G.; Singh, S.; Riar, C.S. Effect of acetylation and dual modification on physico-chemical, rheological and morphological characteristics of sweet potato (Ipomoea batatas) starch. Carbohydr. Polym. 2010, 80, 725–732. [Google Scholar] [CrossRef]
- Raina, C.S.; Singh, S.; Bawa, A.S.; Saxena, D.C. Some characteristics of acetylated, cross-linked and dual modified Indian rice starches. Eur. Food Res. Technol. 2006, 223, 561–570. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Ashogbon, A.O.; Singh, A.; Chaudhary, V.; Whiteside, W.S. Enzymatic modification of starch: A green approach for starch applications. Carbohydr. Polym. 2022, 287, 119265. [Google Scholar] [CrossRef]
- Kazimierczak, J.; Ciechanska, D.; Wawro, D.; Guzinska, K. Enzymatic modification of potato starch. Fibres Text. East. Eur. 2007, 15, 100. [Google Scholar]
- Guo, W.; Yang, L.; Shi, X.; Cong, X.; Cheng, S.; Li, L.; Cheng, H. Effects of color protection and enzymatic hydrolysis on the microstructure, digestibility, solubility and swelling degree of chestnut flour. Food Chem. 2024, 23, 101770. [Google Scholar] [CrossRef]
- Walker, G.J.; Hope, P.M. The action of some α-amylases on starch granules. Biochem. J. 1963, 86, 452. [Google Scholar] [CrossRef]
- Dinges, J.R.; Colleoni, C.; James, M.G.; Myers, A.M. Mutational analysis of the pullulanase-type debranching enzyme of maize indicates multiple functions in starch metabolism. Plant Cell 2003, 15, 666–680. [Google Scholar] [CrossRef]
- Ulbrich, M.; Asiri, S.A.; Bussert, R.; Flöter, E. Enzymatic Modification of Granular Potato Starch Using Isoamylase Investigation of Morphological, Physicochemical, Molecular, and Techno-Functional Properties. Starch—Stärke 2021, 73, 2000080. [Google Scholar] [CrossRef]
- Lin, Q.; Huang, B.; Zhang, M.; Zhang, X.; Rivenbark, J.; Lappe, R.L.; James, M.G.; Myers, A.M.; Hennen-Bierwagen, T.A. Functional interactions between starch synthase III and isoamylase-type starch-debranching enzyme in maize endosperm. Plant Physiol. 2012, 158, 679–692. [Google Scholar] [CrossRef]
- Kubo, A.; Fujita, N.; Harada, K.; Matsuda, T.; Satoh, H.; Nakamura, Y. The starch-debranching enzymes isoamylase and pullulanase are both involved in amylopectin biosynthesis in rice endosperm. Plant Physiol. 1999, 121, 399–410. [Google Scholar] [CrossRef]
- Regina, A.; Li, Z.; Morell, M.K.; Jobling, S.A. Chapter 2—Genetically Modified Starch: State of Art and Perspectives. In Starch Polymers; Halley, P.J., Avérous, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 13–29. [Google Scholar] [CrossRef]
- Jin, B.; van Leeuwen, H.J.; Patel, B.; Yu, Q. Utilisation of starch processing wastewater for production of microbial biomass protein and fungal α-amylase by Aspergillus oryzae. Bioresour. Technol. 1998, 66, 201–206. [Google Scholar] [CrossRef]
- Leemhuis, H.; Kelly, R.M.; Dijkhuizen, L. Engineering of cyclodextrin glucanotransferases and the impact for biotechnological applications. Appl. Microbiol. Biotechnol. 2010, 85, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Radwan, E.K.; El-Naggar, M.E.; Abdel-Karim, A.; Wassel, A.R. Multifunctional 3D cationic starch/nanofibrillated cellulose/silver nanoparticles nanocomposite cryogel: Synthesis, adsorption, and antibacterial characteristics. Int. J. Biol. Macromol. 2021, 189, 420–431. [Google Scholar] [CrossRef]
- Almonaitytė, K.; Bendoraitienė, J.; Rutkaitė, R. Optimization of synthesis of cationic starches for wastewater sludge and microalgae separation. Int. J. Biol. Macromol. 2024, 282, 136834. [Google Scholar] [CrossRef]
- Mohseni, A.; Fan, L.; Roddick, F.; Li, H.; Gao, Y.; Liu, Z. Cationic starch: An effective flocculant for separating algal biomass from wastewater RO concentrate treated by microalgae. J. Appl. Phycol. 2021, 33, 917–928. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Cao, S.; Ding, Y.; Wang, H.; Chang, N. Preparation of Crosslinking-Grafting Cationic Starch Flocculant and Its Study on Textile Dyes Removal. J. Polym. Environ. 2024, 32, 3407–3421. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, G.; Zhai, B.; Zhang, H.; Liang, Y.; Liang, H. Cationic starch-grafted-cationic polyacrylamide based graphene oxide ternary composite flocculant for the enhanced flocculation of oil sludge suspension. Compos. Part B Eng. 2019, 177, 107416. [Google Scholar] [CrossRef]
- Chittapun, S.; Jangyubol, K.; Charoenrat, T.; Piyapittayanun, C.; Kasemwong, K. Cationic cassava starch and its composite as flocculants for microalgal biomass separation. Int. J. Biol. Macromol. 2020, 161, 917–926. [Google Scholar] [CrossRef]
- Abd El-Ghany, N.A.; Elella, M.H.A.; Abdallah, H.M.; Mostafa, M.S.; Samy, M. Recent Advances in Various Starch Formulation for Wastewater Purification via Adsorption Technique: A Review. J. Polym. Environ. 2023, 31, 2792–2825. [Google Scholar] [CrossRef]
- Santander-Ortega, M.J.; Stauner, T.; Loretz, B.; Ortega-Vinuesa, J.L.; Bastos-González, D.; Wenz, G.; Schaefer, U.F.; Lehr, C.M. Nanoparticles made from novel starch derivatives for transdermal drug delivery. J. Control. Release 2010, 141, 85–92. [Google Scholar] [CrossRef]
- Xu, K.; Sun, X.; Chong, C.; Ren, L.; Tan, L.; Sun, H.; Wang, X.; Li, L.; Xia, J.; Zhang, R.; et al. Green Starch-Based Hydrogels with Excellent Injectability, Self-Healing, Adhesion, Photothermal Effect, and Antibacterial Activity for Promoting Wound Healing. ACS Appl. Mater. Interfaces 2024, 16, 2027–2040. [Google Scholar] [CrossRef]
- Savekar, P.L.; Nadaf, S.J.; Killedar, S.G.; Kumbar, V.M.; Hoskeri, J.H.; Bhagwat, D.A.; Gurav, S.S. Citric acid cross-linked pomegranate peel extract-loaded pH-responsive β-cyclodextrin/carboxymethyl tapioca starch hydrogel film for diabetic wound healing. Int. J. Biol. Macromol. 2024, 274, 133366. [Google Scholar] [CrossRef]
- Wu, X.; Yan, X.; Zhang, B.; Zhang, Q.; Zhang, X.; Zhang, J.; Wu, X. Effect of strengthening agents on properties of dual-modified cassava starch-based degradable films. Int. J. Biol. Macromol. 2025, 291, 139142. [Google Scholar] [CrossRef]
- Sá-Lima, H.; Caridade, S.G.; Mano, J.F.; Reis, R.L. Stimuli-responsive chitosan-starch injectable hydrogels combined with encapsulated adipose-derived stromal cells for articular cartilage regeneration. Soft Matter 2010, 6, 5184–5195. [Google Scholar] [CrossRef]
- Alkhursani, S.A.; Ghobashy, M.M.; Al-Gahtany, S.A.; Meganid, A.S.; Abd El-Halim, S.M.; Ahmad, Z.; Khan, F.S.; Atia, G.A.N.; Cavalu, S. Application of nano-inspired scaffolds-based biopolymer hydrogel for bone and periodontal tissue regeneration. Polymers 2022, 14, 3791. [Google Scholar] [CrossRef] [PubMed]
- Rashwan, A.K.; Younis, H.A.; Abdelshafy, A.M.; Osman, A.I.; Eletmany, M.R.; Hafouda, M.A.; Chen, W. Plant starch extraction, modification, and green applications: A review. Environ. Chem. Lett. 2024, 22, 2483–2530. [Google Scholar] [CrossRef]
- Krajang, M.; Malairuang, K.; Sukna, J.; Rattanapradit, K.; Chamsart, S. Single-step ethanol production from raw cassava starch using a combination of raw starch hydrolysis and fermentation, scale-up from 5-L laboratory and 200-L pilot plant to 3000-L industrial fermenters. Biotechnol. Biofuels 2021, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Monroy, Y.; Rivero, S.; García, M.A. Sustainable panels design based on modified cassava starch bioadhesives and wood processing byproducts. Ind. Crops Prod. 2019, 137, 171–179. [Google Scholar] [CrossRef]
- Pimpisai, T.; Maneerattanarungroj, C.; Kingkaew, E.; Ochaikul, D. Bioethanol production from cassava starch using co-culture of saccharolytic molds with Saccharomyces cerevisiae TISTR 5088. ScienceAsia 2024, 50, 1–8. [Google Scholar] [CrossRef]
- Chamorro, A.F.; Palencia, M.; Arrieta, Á.A. Development of High-Efficiency Fertilizer by Hydrogels Obtained from Cassava Starch and Citric Acid for Slow Release of Ammonium and Potassium. Gels 2024, 10, 434. [Google Scholar] [CrossRef]
- Zhao, C.; Peng, D.; Huang, Z.; Wu, Z.; Huang, T. Magnesium-modified starch cryogels for the recovery of nitrogen and phosphorus from wastewater and its potential as a fertilizer substitute: A novel nutrient recovery approach. J. Environ. Chem. Eng. 2024, 12, 114044. [Google Scholar] [CrossRef]
- Luo, J.; Wang, L.; Lai, S.; Sun, Z.; Guo, H.; Lai, Y.; Zhu, L.; Liu, P. Enzymatic preparation of rice-starch-based surface-modified disordered carbon as an anode for lithium-ion batteries. Ionics 2021, 27, 59–64. [Google Scholar] [CrossRef]
- Lodi, L.A.; Borges, R.; Lopes, M.M.; Graciano, V.A.; Bortoletto-Santos, R.; Barud, H.S.; Oliveira-Paiva, C.A.d.; Ribeiro, C.; Farinas, C.S. Spray-Drying Microencapsulation of Bacillus megaterium in PVA/Cationic Starch/Zinc Oxide for Promoting Growth and Zinc Availability in Soybean Plants. ACS Agric. Sci. Technol. 2024, 4, 1271–1283. [Google Scholar] [CrossRef]
- Foshan Guonong Starch Co., Ltd. A Cross-Linked Emulsified Modified Starch and Its Preparation Method and Application. CN118530378A, 23 August 2024. [Google Scholar]
- Minle Fengyuan Potato Starch Co., Ltd. Potato Starch Vacuum Sterilization Device and Application Method Thereof. China Patent CN117441845A, 26 January 2024. [Google Scholar]
- Linyi Jiangyuan Decoration Materials Co., Ltd. Aldehyde-Free Impregnated Paper Adhesive and Preparation Method Thereof. China Patent CN117757384A, 26 March 2024. [Google Scholar]
- Alcohol International Biotechnology Beijing Co., Ltd. Cleansing Cream with Moisturizing and Skin Care Effects and Preparation Method Thereof. China Patent CN118078729A, 28 May 2024. [Google Scholar]
- Jilin Province Boguan Modified Starch Co., Ltd. Mixed Wastewater Treatment Process of Wet Modified Starch and Hydroxypropyl Modified Starch. CN116282640A, 23 June 2023. [Google Scholar]
- South China University of Technology (SCUT). Curcumin-Loaded Composite Gel Microsphere Based on Cross-Linked Corn Porous Starch, and Preparation Method Therefor. WO2023151350A1, 17 August 2023.
- Roquette Freres, SA. Low Acetylated Pea Starch as Egg White Replacer. EP3987941A1, 27 April 2022. [Google Scholar]
- Marc Lemieu, Bradut MITRASCA, Sonia Gervais, Damon SmithAltus Formulation Inc. Starch-Based Release Modifying Excipients and Pharmaceutical Compositions Derived Therefrom. US20210299259A1, 20 December 2022.
- Sinopec Dalian Petrochemical Research Institute Co., Ltd. China Petroleum and Chemical Corp. Method for Preparing Nano-Scale Crosslinked Starch Microspheres. CN113121852A, 16 July 2021. [Google Scholar]
- Hainan University. A Kind of Starch Cross-Linked Tea Polyphenol Antibacterial and Degradable Food Packaging Material and Preparation Method Thereof. CN111171385A, 19 May 2020.
- Shandong Zhonggu Starch Sugar Co., Ltd. A System for Clean Utilization of Waste Heat in Corn Starch Production. CN210229574U, 3 April 2020. [Google Scholar]
- Сергей Евгеньевич Лаевский. High-Efficient for Modified Starch Making Beating Device. CN210206558U, 31 March 2020.
- Laevsky, S.E. Medicament Exhibiting Wound Healing Action. RU2712088C1, 24 January 2020. [Google Scholar]
- Huazhong Agricultural University. A Kind of Liquid Foundation Containing Compound Modified Starch and Preparation Method Thereof. CN110141529A, 20 August 2019.
- Bemiller, J.N. Starch Modification: Challenges and Prospects. Starch—Stärke 1997, 49, 127–131. [Google Scholar] [CrossRef]




| Source | Extraction Methods | Advantages | Limitations | Reference |
|---|---|---|---|---|
| Legumes (chickpeas, lentils) | Dry milling: grinding seeds into flour and sieving. Alkaline extraction: soaking in NaOH to dissolve proteins. Enzymatic extraction: enzymes break down non-starch components. | Dry milling is cost-effective for small-scale operations. Alkaline methods handle high protein content well. Enzymatic extraction yields high purity. | Lower purity with dry milling. Alkaline methods produce wastewater requiring treatment. Enzymes are costly. | [55] |
| Cereals (maize, rice, wheat) | Wet milling: soaking grains, grinding, and centrifugation to isolate starch. Fermentation: microbial action dissolves non-starch components. Alkaline extraction: dissolves proteins. | Wet milling is widely used industrially for high yields. Fermentation improves purity and enhances starch properties. | Wet milling requires significant water and energy. Fermentation is time-intensive and less scalable for the industry. | [56] |
| Fruits (Banana, breadfruit) | Pulping and sieving: mashing pulp, sieving starch granules. Microwave-assisted extraction: heating disrupts cell structures for starch release. | Simple and cost-effective pulping method. Microwave methods are fast, eco-friendly, and reduce water use. | High moisture and inefficient large-scale extraction. Microwave methods are fast, eco-friendly, and reduce water use. | [57] |
| Roots (arrowroot, taro) | Sedimentation: grating roots, washing, and natural settling. Ultrasonic extraction: ultrasound breaks cell walls to release starch. | Sedimentation is low-cost and traditional. Ultrasonic techniques are efficient, eco-friendly, and reduce time. | Sedimentation is labor-intensive. Ultrasonic extraction requires expensive equipment. | [58] |
| Tubers (potato, cassava) | Wet milling: crushing, creating slurry, filtering, and sedimentation. Enzymatic extraction: amylase breaks down cell walls. | High yield and purity. Wet milling is suitable for industrial-scale production | Large amounts of water are required in wet milling. Sedimentation is labor-intensive and time-consuming. | [59] |
| Sources | Type | Parameters | Concentration | Properties | References |
|---|---|---|---|---|---|
| Corn, potato, mung bean, rice | Heat–moisture treatment | Temperature: 80–120 °C | Moisture content: 10% at 100 °C for 16 h | Improves resistant starch, enhances digestibility | [64,67,68] |
| Wheat, corn | Dry heating | Temperature: 120–150 °C | Dry heat for 20 h at 140 °C | Resistant starch alters gelatinization | [64] |
| Lotus, maize, wheat, potato | Freeze–thawing | Freezing cycles: 3–5 | 4 cycles at −20 °C for 24 h | Increases crystallinity, Improves textural properties | [69,70] |
| Corn, yam, banana | Annealing | Temperature: 40–60 °C | Water content: 40% for 24 h | Enhances thermal stability and gelatinization temperature | [71] |
| Rice, sago, wheat, banana | Ultrasonic treatment | Frequency: 20–40 kHz | Sonication for 15 min at 25 °C | Reduces particle size, modifies viscosity | [72] |
| Sago potato | Microwave treatment | Power: 500–1000 W, Time: 1–10 min | Microwave exposure for 5 min at 600 W | Alters crystallinity, improves solubility | [73] |
| Sources | Type | Reagent | Parameters | Properties | References |
|---|---|---|---|---|---|
| Maize cassava | Esterification | Acetic anhydride | 5–8% (w/w) starch with 0.1% sodium hydroxide as a catalyst at 27 °C pH 8.5–9.0 | Enhances solubility, viscosity | [118,119] |
| Corn starch | Succinic anhydride | 6% solution, reacted for 1–2 h at 60 °C pH 8.5–9.0 | Improves hydrophilicity | [110,120] | |
| Jackfruit, sago, cassava | Etherification | Propylene oxide | 8–10% (v/v) solution, reaction at 45 °C for 5 h | Increases freeze–thaw stability | [121,122,123] |
| Cassava, wheat Maize, corn | Epichlorohydrin | 0.05–0.1% (w/w) solution of starch reacts at pH 10 | Improves gel strength, cross-linking | [124,125,126,127] | |
| Potato, cassava, mug bean | Oxidation | Sodium hypochlorite | 0.5–2% (v/v) for 30–60 min at pH 8 | Reduces viscosity, enhances whiteness | [87,128,129] |
| Potato, cassava, wheat | Hydrogen peroxide | 2% (v/v) solution pH 6 at 25 °C | Produces low-viscosity starch | [104,130,131,132] | |
| Corn, tapioca Sago | Cross-linking | Phosphorus oxychloride | 0.03–0.1% (w/w), reaction at pH 10, for 5 h at 40 °C | Improves shear and thermal stability | [133,134,135,136] |
| Corn, wheat, tapioca | Sodium Tri-metaphosphate | 5% (w/w) starch solution, reaction at pH 11 for 2 h at 40 °C | Stabilizes against mechanical forces | [137,138,139,140] | |
| Corn, potato, maize | Acid hydrolysis | Hydrochloric acid | 1% HCl, stirred for 6 h at 40 °C | Reduces molecular weight, improves digestibility | [141,142,143] |
| Corn starch, cassava | Sulfuric acid | 1.5% solution, reaction for 4 h at 50 °C | Enhances the breakdown of amylose | [108,144] |
| Starch Source | Dual Modification | Properties | References |
|---|---|---|---|
| Tapioca starch | Crosslinking with esterification | Composite film preparation, improving hydrophobicity | [146] |
| Taro starch | Hydroxypropylation with crosslinking | Improving physicochemical properties, enhancing stability | [147] |
| Foxtail millet starch | Single and dual modifications | Stability, structural characteristics improvement | [148] |
| Cassava starch | Acetic acid with ultrasound | Pasting, rheological, and digestibility properties | [149] |
| Cassava starch | Crosslinking with octenyl succinylation | Physicochemical properties, in-vitro digestibility, and emulsifying properties | [150] |
| Sweet potato starch | Acetylation with dual modification | Physicochemical, rheological, and morphological characteristics | [151] |
| Indian rice starch | Acetylation with crosslinking | Improving physicochemical characteristics | [152] |
| Enzyme | Sources | Mechanism | Conditions | Function | References |
|---|---|---|---|---|---|
| Amylase (α-Amylase) | Maize | Hydrolyzes α-1,4 glycosidic bonds | Enzyme concentration: 0.1–0.5% (w/v) Temp: 60–70 °C Time: 30–60 min. pH: 4.5–6.5 | Breakdown of starch into dextrins or maltodextrins | [156] |
| Pullulanase | Potato sorghum rice | Hydrolyzes α-1,6 glycosidic bonds | Enzyme concentration: 0.2–1.0% (w/v) Temp: 50–60 °C Time: 10–30 min pH: 4.5–5.5 | Debranching amylopectin | [154,157] |
| Isoamylase | Maize rice | Cleaves α-1,6 glycosidic bonds in amylopectin | Enzyme concentration: 0.2–0.8% (w/v) Temp: 50–60 °C Time: 30–60 min pH:4.5–6 | Debranching of starch molecules | [158,159,160] |
| Modification | Parameters | Mechanism | Application | References |
| Genetic modification | Gene editing using CRISPR or RNAi | Alters starch biosynthesis genes to modify amylose/amylopectin ratio | Development of genetically modified starch with desirable traits | [161] |
| Fungal enzyme treatment | Enzyme conc.: 0.5–1.0% (w/v) Temp: 50 °C pH: 5–9 | Use of fungal α-amylase and glucoamylase to break down starch | Enhanced hydrolysis for unique starch structures | [162] |
| Bacterial enzyme treatment | Enzyme conc.: 0.2–0.8% (w/v) Temp: 40–50 °C pH: 6.5–7.5 | Bacterial pullulanase or isoamylase for debranching; bacterial cyclodextrin glycosyltransferase (CGTase) for cyclodextrins | Introduction of novel enzymes for branching/debranching starch | [163] |
| Title | Use | Year | Patent ID | Ref. |
|---|---|---|---|---|
| Cross-linked emulsified modified starch, as well as preparation method and application thereof | Emulsifier | 2024 | CN118530378A | [185] |
| Potato starch vacuum sterilization device and use method thereof | conveyors and a spiral roller for automated, uniform sterilization, improving efficiency and safety while reducing costs. | 2024 | CN117441845A | [186] |
| Formaldehyde-Free Impregnated Paper Adhesive and Preparation Method Thereof | Paper adhesives, eco-friendly adhesives, wood, and the paper industry | 2024 | CN117757384A | [187] |
| Makeup removal cleaning cream with moisturizing and skin care effects | Moisturizing, makeup removal | 2024 | CN118078729A | [188] |
| Wet modified starch and hydroxypropyl-modified starch mixed sewage treatment process | ability to improve the sedimentation and removal of suspended solids and other impurities | 2023 | CN116282640A | [189] |
| Curcumin-Loaded Composite Gel Microsphere Based on Cross-Linked Corn Porous Starch, And Preparation Method Therefore | Paper adhesives, eco-friendly adhesives, wood, and the paper industry | 2023 | WO2023151350A1 | [190] |
| Low Acetylated Pea Starch as Egg White Replacer | Replacement of egg proteins | 2022 | EP 3 987 941 A1 | [191] |
| Starch-based release-modifying excipients and pharmaceutical | Used as a release excipient in controlled release, to improve release profiles and overcome issues with conventional systems like Contramid® | 2022 | (US20210299259A1) | [192] |
| Method for preparing nanoscale cross-linked starch microspheres | Microsphere | 2021 | CN113121852A | [193] |
| Starch cross-linked tea polyphenol antibacterial degradable food packaging material and preparation method thereof | Antibacterial properties as a barrier for food packaging | 2021 | CN111171385A | [194] |
| Waste heat clean utilization system in corn starch production | Heat exchangers remove harmful gases like SO2 using a washing tower, which helps reduce corrosion, steam consumption, and production costs. | 2020 | CN210229574U | [195] |
| Efficient pulping device for modified starch | Quick and stable pulping of modified starch with water | 2020 | CN210206558U | [196] |
| Medicament Exhibiting Wound-Healing Action | Effective for wound healing | 2020 | RU2712088C1 | [197] |
| A kind of liquid foundation containing a compound modified starch and a preparation method thereof | Used in liquid foundation enhances the skin | 2019 | CN110141529A | [198] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puri, A.; Mohite, P.; Ramole, A.; Verma, S.; Kamble, M.; Ranch, K.; Singh, S. Starch Science Advancement: Isolation Techniques, Modification Strategies, and Multifaceted Applications. Macromol 2025, 5, 40. https://doi.org/10.3390/macromol5030040
Puri A, Mohite P, Ramole A, Verma S, Kamble M, Ranch K, Singh S. Starch Science Advancement: Isolation Techniques, Modification Strategies, and Multifaceted Applications. Macromol. 2025; 5(3):40. https://doi.org/10.3390/macromol5030040
Chicago/Turabian StylePuri, Abhijeet, Popat Mohite, Aakansha Ramole, Sonali Verma, Milind Kamble, Ketan Ranch, and Sudarshan Singh. 2025. "Starch Science Advancement: Isolation Techniques, Modification Strategies, and Multifaceted Applications" Macromol 5, no. 3: 40. https://doi.org/10.3390/macromol5030040
APA StylePuri, A., Mohite, P., Ramole, A., Verma, S., Kamble, M., Ranch, K., & Singh, S. (2025). Starch Science Advancement: Isolation Techniques, Modification Strategies, and Multifaceted Applications. Macromol, 5(3), 40. https://doi.org/10.3390/macromol5030040










