Exopolysaccharide (EPS) Produced by Leuconostoc mesenteroides SJC113: Characterization of Functional and Technological Properties and Application in Fat-Free Cheese
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacteria
2.3. Production and Purification of EPS
2.4. Characterization of EPS
2.4.1. Monosaccharide Composition and Linkage Analysis
2.4.2. Dextranase Resistance
2.4.3. Protein Content
2.4.4. EPS Production in Milk Whey and Skim Milk
2.5. Functional and Technological Properties of Ln. mesenteroides SJC113
2.5.1. β-Galactosidase Activity
2.5.2. Cholesterol-Reducing Ability
2.5.3. DPPH Scavenging Activity
2.5.4. Hydroxyl Radical Scavenging Assay
2.5.5. Tolerance to Sodium Chloride (NaCl)
2.6. In Vitro Safety Evaluation of Ln. mesenteroides SJC113
2.6.1. Antibiotic Susceptibility
2.6.2. Virulence Genes
2.7. Application of Ln. mesenteroides SJC113 in Fat-Free Fresh Cheese
2.7.1. Effect of Temperature on EPS Production in Skim Milk
2.7.2. Cheese Making
2.7.3. Cheese Composition
2.7.4. Weight Loss
2.7.5. Water-Holding Capacity
2.7.6. Rheological Parameters
2.7.7. Determination of EPS Content in Fresh Cheese
2.7.8. Microbial Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. EPS Yield and Characterization
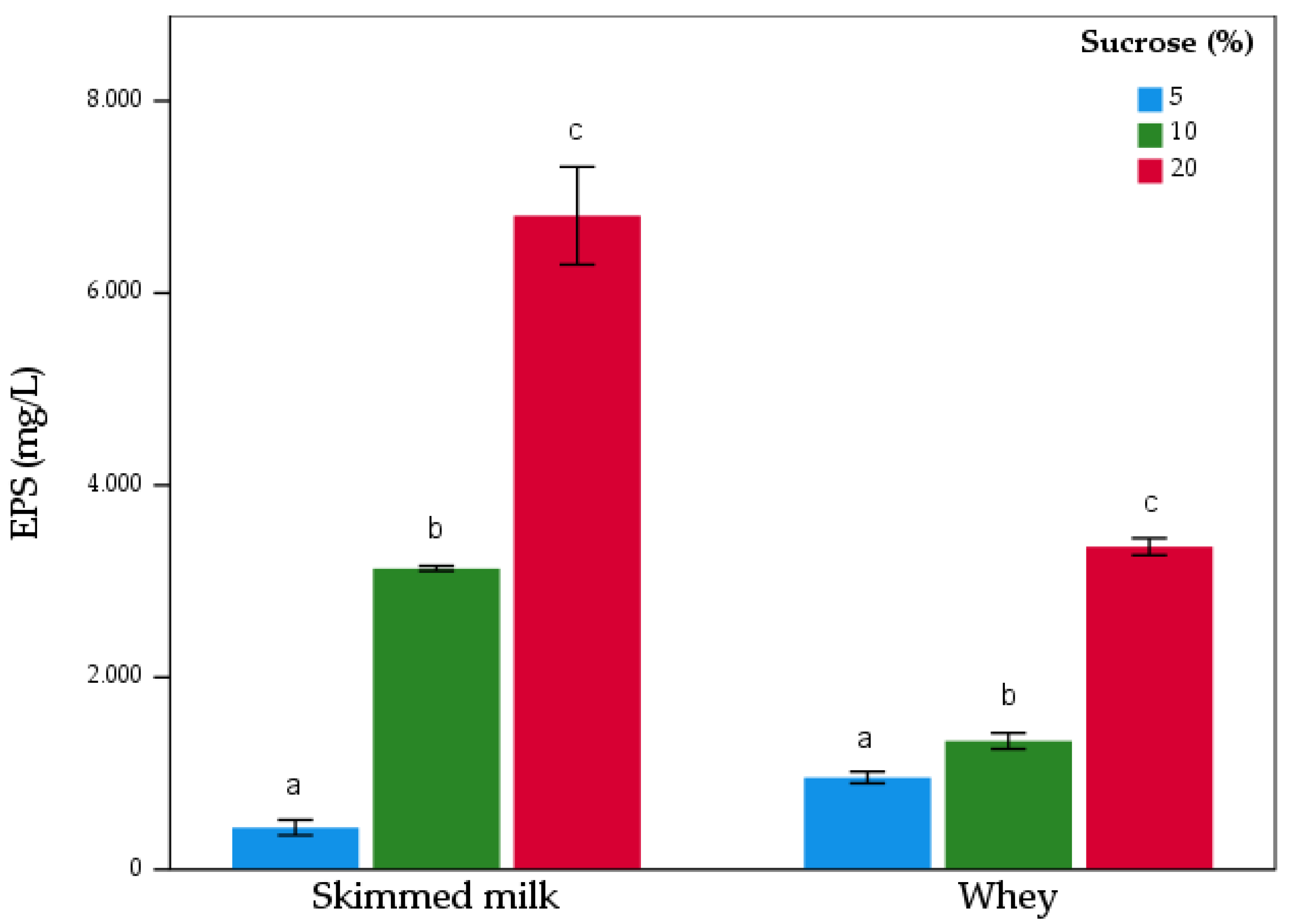
3.2. EPS Production in Milk Whey and Skim Milk
3.3. Functional and Technological Properties of Ln. mesenteroides SJC113
3.4. Antibiotic Susceptibility and Virulence Genes
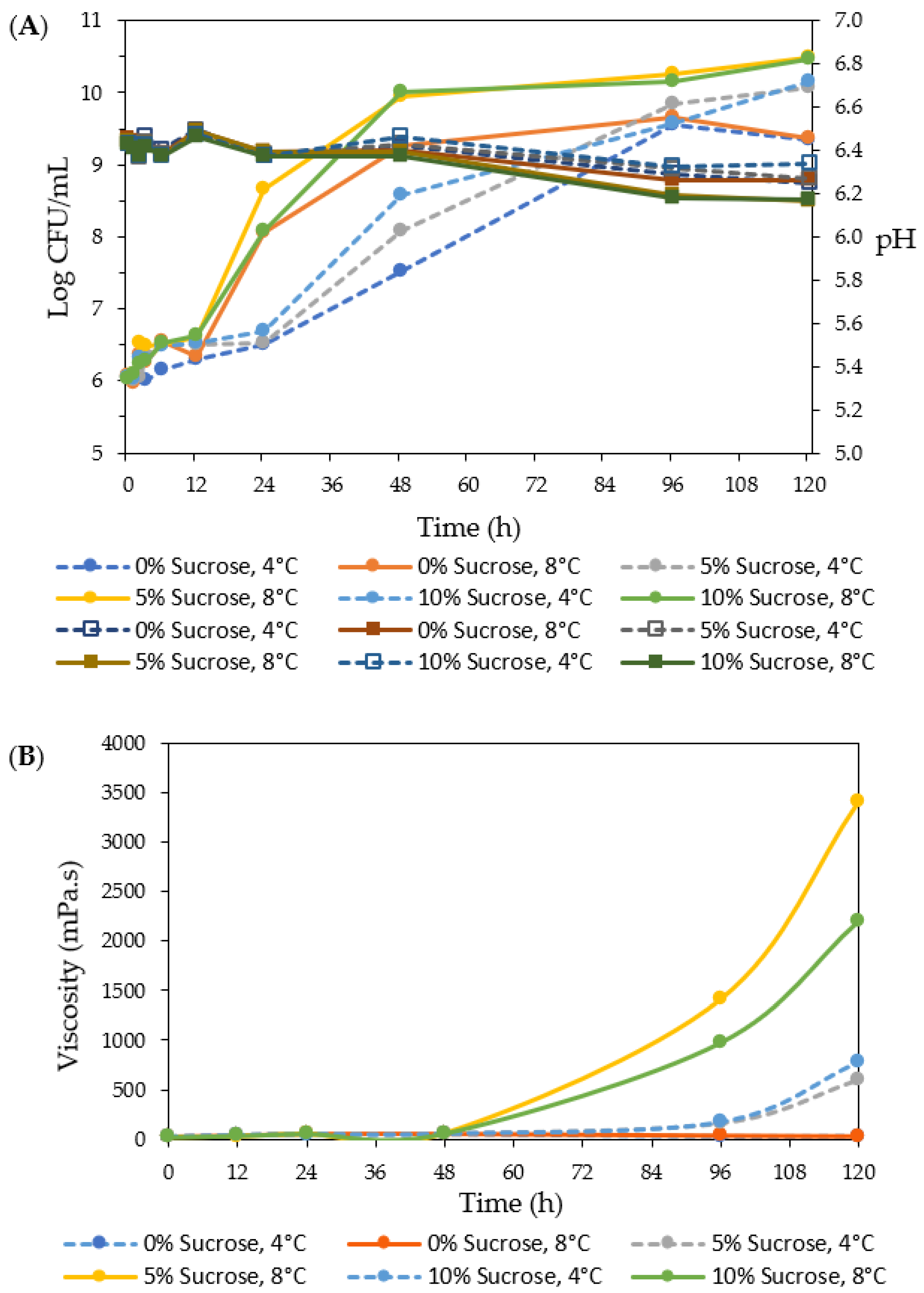
3.5. EPS Production in Skim Milk
3.6. Application of Ln. mesenteroides SJC113 in Fat-Free Fresh Cheese
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banwo, K.; Sanni, A.; Tan, H. Functional properties of Pediococcus species isolated from traditional fermented cereal gruel and milk in Nigeria. Food Biotechnol. 2013, 27, 14–38. [Google Scholar] [CrossRef]
- Aarti, C.; Khusro, A. Functional and technological properties of exopolysaccharide producing autochthonous Lactobacillus plantarum strain AAS3 from dry fish based fermented food. LWT 2019, 114, 108387. [Google Scholar] [CrossRef]
- Mende, S.; Rohm, H.; Jaros, D. Influence of exopolysaccharides on the structure, texture, stability and sensory properties of yoghurt and related products. Int. Dairy J. 2016, 52, 57–71. [Google Scholar] [CrossRef]
- Fontana, C.; Li, S.; Yang, Z.; Widmalm, G. Structural studies of the exopolysaccharide from Lactobacillus plantarum C88 using NMR spectroscopy and the program CASPER. Carbohydr. Res. 2015, 402, 87–94. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of lactic acid bacteria: Structure, bioactivity and associations: A review. Carbohydr. Polym. 2019, 207, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Jurášková, D.; Ribeiro, S.C.; Silva, C.C. Exopolysaccharides produced by lactic acid bacteria: From biosynthesis to health-promoting properties. Foods 2022, 11, 156. [Google Scholar] [CrossRef]
- Naessens, M.; Cerdobbel, A.; Soetaert, W.; Vandamme, E.J. Leuconostoc dextransucrase and dextran: Production, properties and applications. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2005, 80, 845–860. [Google Scholar] [CrossRef]
- Taylan, O.; Yilmaz, M.T.; Dertli, E. Partial characterization of a levan type exopolysaccharide (EPS) produced by Leuconostoc mesenteroides showing immunostimulatory and antioxidant activities. Int. J. Biol. Macromol. 2019, 136, 436–444. [Google Scholar] [CrossRef]
- Yilmaz, M.T.; İspirli, H.; Taylan, O.; Taşdemir, V.; Sagdic, O.; Dertli, E. Characterisation and functional roles of a highly branched dextran produced by a bee pollen isolate Leuconostoc mesenteroides BI-20. Food Biosci. 2022, 45, 101330. [Google Scholar] [CrossRef]
- Montersino, S.; Prieto, A.; Muñoz, R.; De Las Rivas, B. Evaluation of exopolysaccharide production by Leuconostoc mesenteroides strains isolated from wine. J. Food Sci. 2008, 73, M196–M199. [Google Scholar] [CrossRef]
- Abid, Y.; Azabou, S.; Blecker, C.; Gharsallaoui, A.; Corsaro, M.M.; Besbes, S.; Attia, H. Rheological and emulsifying properties of an exopolysaccharide produced by potential probiotic Leuconostoc citreum-BMS strain. Carbohydr. Polym. 2021, 256, 117523. [Google Scholar] [CrossRef] [PubMed]
- Ramos, I.M.; Seseña, S.; Poveda, J.M.; Palop, M.L. Screening of lactic acid bacteria strains to improve the properties of non-fat set yogurt by in situ EPS production. Food Bioprocess Technol. 2023, 16, 2541–2558. [Google Scholar] [CrossRef]
- Tabibloghmany, F.S.; Ehsandoost, E. An overview of healthy and functionality of exopolysaccharides produced by lactic acid bacteria in the dairy industry. Eur. J. Nutr. Food Saf. 2014, 4, 63–86. [Google Scholar] [CrossRef]
- Caggianiello, G.; Kleerebezem, M.; Spano, G. Exopolysaccharides produced by lactic acid bacteria: From health-promoting benefits to stress tolerance mechanisms. Appl. Microbiol. Biotechnol. 2016, 100, 3877–3886. [Google Scholar] [CrossRef]
- Galle, S.; Schwab, C.; Dal Bello, F.; Coffey, A.; Ganzle, M.G.; Arendt, E.K. Influence of in-situ synthesized exopolysaccharides on the quality of gluten-free sorghum sourdough bread. Int. J. Food Microbiol. 2012, 155, 105–112. [Google Scholar] [CrossRef]
- Kumar, A.S.; Mody, K.; Jha, B. Bacterial exopolysaccharides—A perception. J. Basic Microbiol. 2007, 47, 103–117. [Google Scholar] [CrossRef]
- Lynch, K.M.; Zannini, E.; Coffey, A.; Arendt, E.K. Lactic acid bacteria exopolysaccharides in foods and beverages: Isolation, properties, characterization, and health benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Prete, R.; Alam, M.K.; Perpetuini, G.; Perla, C.; Pittia, P.; Corsetti, A. Lactic Acid bacteria exopolysaccharides producers: A sustainable tool for functional foods. Foods 2021, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.M.; Ross, R.P.; Fitzgerald, G.F.; Caplice, N.M.; Stanton, C. Sugar-coated: Exopolysaccharide producing lactic acid bacteria for food and human health applications. Food Funct. 2015, 6, 679–693. [Google Scholar] [CrossRef]
- Bolha, A.; Blaznik, U.; Korošec, M. Influence of intrinsic and extrinsic food attributes on consumers’ acceptance of reformulated food products: A systematic review. Slov. J. Public Health 2020, 60, 72–78. [Google Scholar] [CrossRef]
- Brodock, J.L.; Hayes, J.E.; Masterson, T.D.; Hopfer, H. Differences in preferred fat level, sweetener type, and amount of added sugar in chocolate milk in a choice task relate to physical activity and orthorexia. Appetite 2021, 163, 105214. [Google Scholar] [CrossRef] [PubMed]
- Soltani, M.; Saremnezhad, S.; Faraji, A.; Hayaloglu, A. Perspectives and recent innovations on white cheese produced by conventional methods or ultrafiltration technique. Int. Dairy J. 2022, 125, 105232. [Google Scholar] [CrossRef]
- Khanal, B.K.S.; Bhandari, B.; Prakash, S.; Bansal, N. Simulated oral processing, in vitro digestibility and sensory perception of low fat Cheddar cheese containing sodium alginate. J. Food Eng. 2020, 270, 109749. [Google Scholar] [CrossRef]
- Zhao, Y.; Khalesi, H.; He, J.; Fang, Y. Application of different hydrocolloids as fat replacer in low-fat dairy products: Ice cream, yogurt and cheese. Food Hydrocoll. 2023, 138, 108493. [Google Scholar] [CrossRef]
- Nazari, S.M.; Mortazavi, A.; Hesari, J.; Tabatabaei Yazdi, F. Proteolysis and textural properties of low-fat ultrafiltered Feta cheese as influenced by maltodextrin. Int. J. Dairy Technol. 2020, 73, 244–254. [Google Scholar] [CrossRef]
- Awad, S.; Hassan, A.; Muthukumarappan, K. Application of exopolysaccharide-producing cultures in reduced-fat Cheddar cheese: Texture and melting properties. J. Dairy Sci. 2005, 88, 4204–4213. [Google Scholar] [CrossRef]
- Kondyli, E.; Pappa, E.C.; Kremmyda, A.; Arapoglou, D.; Metafa, M.; Eliopoulos, C.; Israilides, C. Manufacture of reduced fat white-brined cheese with the addition of β-glucans biobased polysaccharides as textural properties improvements. Polymers 2020, 12, 2647. [Google Scholar] [CrossRef]
- Lluis-Arroyo, D.; Flores-Nájera, A.; Cruz-Guerrero, A.; Gallardo-Escamilla, F.; Lobato-Calleros, C.; Jiménez-Guzmán, J.; García-Garibay, M. Effect of an exopolysaccharide-producing strain of Streptococcus thermophilus on the yield and texture of Mexican Manchego-type cheese. Int. J. Food Prop. 2014, 17, 1680–1693. [Google Scholar] [CrossRef]
- Nepomuceno, R.S.A.C.; Junior, L.C.G.C.; Costa, R.G.B. Exopolysaccharide-producing culture in the manufacture of Prato cheese. LWT-Food Sci. Technol. 2016, 72, 383–389. [Google Scholar] [CrossRef]
- Di Cagno, R.; De Pasquale, I.; De Angelis, M.; Buchin, S.; Rizzello, C.G.; Gobbetti, M. Use of microparticulated whey protein concentrate, exopolysaccharide-producing Streptococcus thermophilus, and adjunct cultures for making low-fat Italian Caciotta-type cheese. J. Dairy Sci. 2014, 97, 72–84. [Google Scholar] [CrossRef]
- Şanli, T.; Gursel, A.; Şanli, E.; Acar, E.; Benli, M. The effect of using an exopolysaccharide-producing culture on the physicochemical properties of low-fat and reduced-fat Kasar cheeses. Int. J. Dairy Technol. 2013, 66, 535–542. [Google Scholar] [CrossRef]
- Domingos-Lopes, M.F.P.; Lamosa, P.; Stanton, C.; Ross, R.P.; Silva, C.C.G. Isolation and characterization of an exopolysaccharide-producing Leuconostoc citreum strain from artisanal cheese. Lett. Appl. Microbiol. 2018, 67, 570–578. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Nunes, C.; Silva, L.; Fernandes, A.P.; Guiné, R.P.F.; Domingues, M.R.M.; Coimbra, M.A. Occurrence of cellobiose residues directly linked to galacturonic acid in pectic polysaccharides. Carbohydr. Polym. 2012, 87, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Coimbra, M.A.; Delgadillo, I.; Waldron, K.W.; Selvendran, R.R. Isolation and analysis of cell wall polymers from olive pulp. In Plant Cell Wall Analysis; Linskens, H.F., Jackson, J.F., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 19–44. [Google Scholar]
- Ciucanu, I.; Kerek, F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 1984, 131, 209–217. [Google Scholar] [CrossRef]
- Concórdio-Reis, P.; Ferreira, S.S.; Alves, V.D.; Moppert, X.; Guézennec, J.; Coimbra, M.A.; Reis, M.A.; Freitas, F. Rheological characterization of the exopolysaccharide produced by Alteromonas macleodii Mo 169. Int. J. Biol. Macromol. 2023, 227, 619–629. [Google Scholar] [CrossRef]
- Bastos, R.; Marín-Montesinos, I.; Ferreira, S.S.; Mentink-Vigier, F.; Sardo, M.; Mafra, L.; Coimbra, M.A.; Coelho, E. Covalent connectivity of glycogen in brewer’s spent yeast cell walls revealed by enzymatic approaches and dynamic nuclear polarization NMR. Carbohydr. Polym. 2024, 324, 121475. [Google Scholar] [CrossRef]
- Miller, J. Experiments in Molecular Biology; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1972. [Google Scholar]
- Li, W.; Zhao, X.; Zou, S.; Ma, Y.; Zhang, K.; Zhang, M. Scanning assay of β-galactosidase activity. Appl. Biochem. Microbiol. 2012, 48, 603–607. [Google Scholar] [CrossRef]
- Domingos-Lopes, M.; Stanton, C.; Ross, R.; Silva, C. Histamine and cholesterol lowering abilities of lactic acid bacteria isolated from artisanal Pico cheese. J. Appl. Microbiol. 2020, 129, 1428–1440. [Google Scholar] [CrossRef]
- He, Z.; Luo, H.; Cao, C.; Cui, Z. Photometric determination of hydroxyl free radical in Fenton system by brilliant green. Am. J. Chin. Clin. Med. 2004, 6, 236–243. [Google Scholar]
- Weinstein, M.P. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2021. [Google Scholar]
- Ribeiro, S.; Coelho, M.; Todorov, S.D.; Franco, B.D.G.d.M.; Dapkevicius, M.; Silva, C. Technological properties of bacteriocin-producing lactic acid bacteria isolated from Pico cheese an artisanal cow’s milk cheese. J. Appl. Microbiol. 2014, 116, 573–585. [Google Scholar] [CrossRef]
- Coelho, M.C.; Silva, C.C.G.; Ribeiro, S.C.; Dapkevicius, M.L.N.E.; Rosa, H.J.D. Control of Listeria monocytogenes in fresh cheese using protective lactic acid bacteria. Int. J. Food Microbiol. 2014, 191, 53–59. [Google Scholar] [CrossRef] [PubMed]
- ISO-5534; Cheese and Processed Cheese—Determination of the Total Solids Content (Reference Method). ISO: Geneva, Switzerland, 2004.
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; Method 945.46; AOAC: Arling, VA, USA, 2020. [Google Scholar]
- ISO-3433; Cheese—Determination of Fat Content—Van Gulik method. ISO: Geneva, Switzerland, 2008.
- ISO-8968-3; Milk—Determination of Nitrogen Conten. ISO: Geneva, Switzerland, 2004.
- Linares, D.M.; O’Callaghan, T.F.; O’Connor, P.M.; Ross, R.P.; Stanton, C. Streptococcus thermophilus APC151 strain is suitable for the manufacture of naturally GABA-enriched bioactive yogurt. Front. Microbiol. 2016, 7, 1876. [Google Scholar] [CrossRef] [PubMed]
- Kearney, N.; Stack, H.M.; Tobin, J.T.; Chaurin, V.; Fenelon, M.A.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Lactobacillus paracasei NFBC 338 producing recombinant beta-glucan positively influences the functional properties of yoghurt. Int. Dairy J. 2011, 21, 561–567. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, F.; Zhou, Q.; Zhao, F.; Du, R.; Zhou, Z.; Han, Y. Isolation, purification and characterization of exopolysaccharide produced by Leuconostoc pseudomesenteroides YF32 from soybean paste. Int. J. Biol. Macromol. 2018, 114, 529–535. [Google Scholar] [CrossRef]
- Nemati, V.; Mozafarpour, R. Exopolysaccharides isolated from fermented milk-associated lactic acid bacteria and applied to produce functional value-added probiotic yogurt. LWT 2024, 199, 116116. [Google Scholar] [CrossRef]
- Xing, H.; Du, R.; Zhao, F.; Han, Y.; Xiao, H.; Zhou, Z. Optimization, chain conformation and characterization of exopolysaccharide isolated from Leuconostoc mesenteroides DRP105. Int. J. Biol. Macromol. 2018, 112, 1208–1216. [Google Scholar] [CrossRef]
- London, L.E.E.; Kumar, A.H.S.; Wall, R.; Casey, P.G.; O’Sullivan, O.; Shanahan, F.; Hill, C.; Cotter, P.D.; Fitzgerald, G.F.; Ross, R.P.; et al. Exopolysaccharide-producing probiotic lactobacilli reduce serum cholesterol and modify enteric microbiota in ApoE-deficient mice. J. Nutr. 2014, 144, 1956–1962. [Google Scholar] [CrossRef]
- Bounaix, M.-S.; Gabriel, V.; Morel, S.; Robert, H.; Rabier, P.; Remaud-Simeon, M.; Gabriel, B.; Fontagne-Faucher, C. Biodiversity of exopolysaccharides produced from sucrose by sourdough lactic acid bacteria. J. Agric. Food Chem. 2009, 57, 10889–10897. [Google Scholar] [CrossRef]
- Miao, M.; Bai, A.; Jiang, B.; Song, Y.; Cui, S.W.; Zhang, T. Characterisation of a novel water-soluble polysaccharide from Leuconostoc citreum SK24.002. Food Hydrocoll. 2014, 36, 265–272. [Google Scholar] [CrossRef]
- Leathers, T.D.; Côté, G.L. Biofilm formation by exopolysaccharide mutants of Leuconostoc mesenteroides strain NRRL B-1355. Appl. Microbiol. Biotechnol. 2008, 78, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.Y.M.; Reehana, N.; Jayaraj, K.A.; Ahamed, A.A.P.; Dhanasekaran, D.; Thajuddin, N.; Alharbi, N.S.; Muralitharan, G. Statistical optimization of exopolysaccharide production by Lactobacillus plantarum NTMI05 and NTMI20. Int. J. Biol. Macromol. 2016, 93, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Oleksy-Sobczak, M.; Klewicka, E. Optimization of media composition to maximize the yield of exopolysaccharides production by Lactobacillus rhamnosus strains. Probiotics Antimicrob. Proteins 2020, 12, 774–783. [Google Scholar] [CrossRef]
- Menconi, A.; Kallapura, G.; Latorre, J.D.; Morgan, M.J.; Pumford, N.R.; Hargis, B.M.; Tellez, G. Identification and characterization of lactic acid bacteria in a commercial probiotic culture. Biosci. Microbiota Food Health 2014, 33, 25–30. [Google Scholar] [CrossRef]
- Patil, A.; Disouza, J.; Pawar, S. Shelf life stability of encapsulated lactic acid bacteria isolated from sheep milk thrived in different milk as natural media. Small Rumin. Res. 2019, 170, 19–25. [Google Scholar] [CrossRef]
- Todorov, S.D.; Stojanovski, S.; Iliev, I.; Moncheva, P.; Nero, L.A.; Ivanova, I.V. Technology and safety assessment for lactic acid bacteria isolated from traditional Bulgarian fermented meat product “lukanka”. Braz. J. Microbiol. 2017, 48, 576–586. [Google Scholar] [CrossRef]
- Alliet, P.; Scholtens, P.; Raes, M.; Hensen, K.; Jongen, H.; Rummens, J.-L.; Boehm, G.; Vandenplas, Y. Effect of prebiotic galacto-oligosaccharide, long-chain fructo-oligosaccharide infant formula on serum cholesterol and triacylglycerol levels. Nutrition 2007, 23, 719–723. [Google Scholar] [CrossRef]
- Zaman, U.; Rehman, K.u.; Khan, S.U.; Refat, M.S.; Badshah, S.; Hajira, B.; Iqbal, A.; Khan, W.U.; Alsuhaibani, A.M. Identification, kinetics and thermodynamic analysis of novel β-galactosidase from Convolvulus arvensis seeds: An efficient agent for delactosed milk activity. Int. J. Biol. Macromol. 2022, 220, 1545–1555. [Google Scholar] [CrossRef]
- Ayivi, R.D.; Ibrahim, S.A. Lactic acid bacteria: An essential probiotic and starter culture for the production of yoghurt. Int. J. Food Sci. Technol. 2022, 57, 7008–7025. [Google Scholar] [CrossRef]
- Son, S.-H.; Jeon, H.-L.; Jeon, E.B.; Lee, N.-K.; Park, Y.-S.; Kang, D.-K.; Paik, H.-D. Potential probiotic Lactobacillus plantarum Ln4 from kimchi: Evaluation of β-galactosidase and antioxidant activities. LWT-Food Sci. Technol. 2017, 85, 181–186. [Google Scholar] [CrossRef]
- de Lima, C.P.; Dias, G.M.P.; Soares, M.T.C.V.; Bruno, L.M.; Porto, A.L.F. Coalho cheese as source of probiotic lactic acid bacteria. Res. Soc. Dev. 2020, 9, e266984958. [Google Scholar] [CrossRef]
- Shukla, R.; Iliev, I.; Goyal, A. Leuconostoc mesenteroides NRRL B-1149 as probiotic and its dextran with anticancer properties. J. BioScience Biotechnol. 2014, 3, 79–87. [Google Scholar]
- Pires, V.; Ribeiro, S.C.; Silva, S.P.M.; Juraskova, D.; Silva, C.C.G. Probiotic, technological, and health-related characteristics of lactobacilli isolated from breast milk. J. Appl. Microbiol. 2023, 134, lxad122. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.; Silva, S.; Domingos-Lopes, M.; Bessa, R.; Prates, J.; Rosa, H.; Silva, C. Production of low-cholesterol butter with Lacticaseibacillus paracasei immobilized in calcium-alginate beads. Food Chem. 2022, 393, 133419. [Google Scholar] [CrossRef]
- Wang, H.; Li, L. Comprehensive evaluation of probiotic property, hypoglycemic ability and antioxidant activity of lactic acid bacteria. Foods 2022, 11, 1363. [Google Scholar] [CrossRef]
- Shi, Y.; Cui, X.; Gu, S.; Yan, X.; Li, R.; Xia, S.; Chen, H.; Ge, J. Antioxidative and probiotic activities of lactic acid bacteria isolated from traditional artisanal milk cheese from Northeast China. Probiotics Antimicrob. Proteins 2019, 11, 1086–1099. [Google Scholar] [CrossRef]
- Liu, N.; Miao, S.; Qin, L. Screening and application of lactic acid bacteria and yeasts with l-lactic acid-producing and antioxidant capacity in traditional fermented rice acid. Food Sci. Nutr. 2020, 8, 6095–6111. [Google Scholar] [CrossRef]
- Dlamini, Z.C.; Langa, R.L.S.; Aiyegoro, O.A.; Okoh, A.I. Safety evaluation and colonisation abilities of four lactic acid bacteria as future probiotics. Probiotics Antimicrob. Proteins 2019, 11, 397–402. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Cui, H.; Li, Y.; Sun, Y.; Qiu, H.-J. Characterization of lactic acid bacteria isolated from the gastrointestinal tract of a wild boar as potential probiotics. Front. Vet. Sci. 2020, 7, 49. [Google Scholar] [CrossRef]
- Borges, D.M.; Ribeiro, S.C.; Silva, S.P.; Silva, C.C. Dried algae as potential functional ingredient in fresh cheese. Food Bioeng. 2024, 3, 65–72. [Google Scholar] [CrossRef]
- Batt, C.; Tortorello, M. Encyclopedia of Food Microbiology, 2nd ed.; Academic Press: London, UK, 2014. [Google Scholar]
- Esmaeilnejad-Moghadam, B.; Mokarram, R.R.; Hejazi, M.A.; Khiabani, M.S.; Keivaninahr, F. Low molecular weight dextran production by Leuconostoc mesenteroides strains: Optimization of a new culture medium and the rheological assessments. Bioact. Carbohydr. Diet. Fibre 2019, 18, 100181. [Google Scholar] [CrossRef]
- Zisu, B.; Shah, N. Effects of pH, temperature, supplementation with whey protein concentrate, and adjunct cultures on the production of exopolysaccharides by Streptococcus thermophilus 1275. J. Dairy Sci. 2003, 86, 3405–3415. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.P.; Ribeiro, S.C.; Teixeira, J.A.; Silva, C.C. Application of an alginate-based edible coating with bacteriocin-producing Lactococcus strains in fresh cheese preservation. LWT 2022, 153, 112486. [Google Scholar] [CrossRef]
- Rofeal, M.; Abdelmalek, F. Evaluation of bioactive multilayer and edible gelatin/xanthan gels crosslinked with Echinacea purpurea for Ricotta cheese preservation. Sustain. Chem. Pharm. 2023, 36, 101322. [Google Scholar] [CrossRef]
| Carbohydrate (mg/g) | 352.2 ± 47.9 |
| Protein (mg/g) Dextranase resistance (%) | 415.2 ± 10.4 52.1 ± 2.3 |
| Monosaccharide | Mol% |
| Mannose (Man) | 2 |
| Glucose (Glc) | 92 |
| Uronic acids (UA) | 5 |
| Glycosidic linkage | Mol% |
| t-Manp | 1.3 |
| 2-Manp | 0.3 |
| 2,6-Manp | 0.7 |
| 3,6-Manp | 0.7 |
| Total Man | 3 |
| t-Glcp | 6.3 |
| 4-Glcp | 0.7 |
| 6-Glcp | 84.5 |
| 3,6-Glcp | 5.6 |
| Total Glc | 97 |
| β-galactosidase activity (Miller units) | 2368.4 ± 24.4 |
| Cholesterol-reducing ability (%) | 14.8 ± 4.1 |
| Radical scavenging activity—DPPH (%) | 11.7 ± 0.7 |
| Hydroxyl scavenging activity (%) | 15.7 ± 0.4 |
| Hydroxyl scavenging activity (mg/mL BHT Eq.) | 1.08 ± 0.04 |
| Antibiotics 1 | Virulence Genes 2 | ||||
|---|---|---|---|---|---|
| Ampicillin | S | gelE | - | vanA | - |
| Oxacillin | S | hyl | - | vanB | - |
| Kanamycin | S | asa1 | - | hdc1 | - |
| Gentamicin | S | esp | - | hdc2 | - |
| Streptomycin | S | cylA | - | tdc | - |
| Vancomycin | S | efaA | - | odc | - |
| Tetracycline | S | ace | - | ||
| Erythromycin | S | ||||
| Chloramphenicol | S | ||||
| Parameters | FF | NF | NFLn0 | NFLn5 |
|---|---|---|---|---|
| Moisture (%) | 78.3 ± 1.3 a | 85.8 ± 0.2 b | 85.2 ± 0.6 b | 87.0 ± 0.4 b |
| Crude fat (%) | 11.4 ± 0.2 | ND 1 | ND 1 | ND 1 |
| Crude protein (%) | 7.5 ± 0.8 ab | 9.7 ± 0.4 bc | 10.3 ± 0.6 c | 6.4 ± 0.1 a |
| Crude ash (%) | 1.98 ± 0.06 b | 2.23 ± 0.03 b | 2.26 ± 0.10 b | 1.43 ± 0.08 a |
| Carbohydrates (%) | 0.86 ± 0.44 a | 2.24 ± 0.29 b | 2.25 ± 0.14 b | 5.18 ± 0.26 c |
| Parameters | Day 1 | Day 7 | ||
|---|---|---|---|---|
| NFLn0 | NFLn5 | NFLn0 | NFLn5 | |
| MRS Log (CFU/mL) | 6.09 ± 0.24 a | 7.77 ± 0.15 b | 6.53 ± 0.03 a | 7.96 ± 0.01 b |
| PCA Log (CFU/mL) | 6.06 ± 0.23 a | 7.74 ± 0.13 b | 6.50 ± 003 a | 7.88 ± 0.02 b |
| EPS (mg/g) | ND 1 | 0.44 ± 0.02 A | ND 1 | 1.76 ± 0.28 B |
| Cheeses | Day 1 | Day 7 | ||||||
|---|---|---|---|---|---|---|---|---|
| FF | NF | NFLn0 | NFLn5 | FF | NF | NFLn0 | NFLn5 | |
| pH | 6.58 ± 0.03 a | 6.55 ± 0.02 ab | 6.48 ± 0.01 bc | 6.46 ± 0.02 c | 6.57 ± 0.03 a | 6.57 ± 0.03 a | 6.45 ± 0.02 ab | 6.36 ± 0.04 b |
| WHC (%) | 57.78 ± 5.94 a | 39.59 ± 3.34 b | 41.58 ± 3.89 ab | 57.48 ± 0.76 a | 60.63 ± 1.07 a | 42.35 ± 3.32 bc | 39.95 ± 4.91 c | 55.41 ± 1.13 ab |
| Weight loss (%) | 2.85 ± 0.33 a | 2.91 ± 0.17 a | 1.75 ± 0.39 ab | 0.71 ± 0.04 b | 10.80 ± 0.62 ab | 12.75 ± 0.48 a | 7.60 ± 1.28 b | 3.75 ± 0.61 c |
| Viscosity (Pa.s) | 15.06 ± 2.22 ab | 18.14 ± 2.58 b | 14.89 ± 3.05 ab | 5.67 ± 1.60 a | 13.42 ± 1.75 b | 19.64 ± 2.39 b | 12.38 ± 1.39 b | 3.33 ± 1.16 a |
| Hardness (N) | 0.24 ± 0.03 a | 0.52 ± 0.06 b | 0.26 ± 0.02 a | 0.13 ± 0.04 a | 0.39 ± 0.08 b | 0.62 ± 0.03 c | 0.36 ± 0.03 b | 0.11 ± 0.03 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurášková, D.; Ribeiro, S.C.; Bastos, R.; Coelho, E.; Coimbra, M.A.; Silva, C.C.G. Exopolysaccharide (EPS) Produced by Leuconostoc mesenteroides SJC113: Characterization of Functional and Technological Properties and Application in Fat-Free Cheese. Macromol 2024, 4, 680-696. https://doi.org/10.3390/macromol4030040
Jurášková D, Ribeiro SC, Bastos R, Coelho E, Coimbra MA, Silva CCG. Exopolysaccharide (EPS) Produced by Leuconostoc mesenteroides SJC113: Characterization of Functional and Technological Properties and Application in Fat-Free Cheese. Macromol. 2024; 4(3):680-696. https://doi.org/10.3390/macromol4030040
Chicago/Turabian StyleJurášková, Dominika, Susana C. Ribeiro, Rita Bastos, Elisabete Coelho, Manuel A. Coimbra, and Célia C. G. Silva. 2024. "Exopolysaccharide (EPS) Produced by Leuconostoc mesenteroides SJC113: Characterization of Functional and Technological Properties and Application in Fat-Free Cheese" Macromol 4, no. 3: 680-696. https://doi.org/10.3390/macromol4030040
APA StyleJurášková, D., Ribeiro, S. C., Bastos, R., Coelho, E., Coimbra, M. A., & Silva, C. C. G. (2024). Exopolysaccharide (EPS) Produced by Leuconostoc mesenteroides SJC113: Characterization of Functional and Technological Properties and Application in Fat-Free Cheese. Macromol, 4(3), 680-696. https://doi.org/10.3390/macromol4030040









