Study of Purified Cellulosic Pulp and Lignin Produced by Wheat Straw Biorefinery
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Wheat Straw Composition Determination
2.3. Pre Extraction Process
2.4. Dilute Acid Hydrolysis
2.5. Isolation of Organosolv Lignin
2.6. Extraction of Silica from Wheat Straw Cellulosic Pulp
2.7. Bleaching of Cellulosic Pulp
2.8. Physicochemical and Spectroscopic Analysis of Lignin, Cellulose, and Silica
2.8.1. High-Performance Liquid Chromatography Analysis of Carbohydrates, Furfural, and 5-Hydroxymethyl Furfural
2.8.2. Nuclear Magnetic Resonance Characterization of the Wheat Straw Organosolv Lignin and Cellulose
2.8.3. Fourier-Transform Infrared Spectroscopy
2.8.4. Gel Permeation Chromatography of Organosolv Lignin Samples
2.8.5. X-ray Diffractometry
2.8.6. Viscometry Measurements of Polymer Properties of Bleached Cellulosic Pulp
2.8.7. X-ray Fluorescence (XRF) Analysis of Wheat Straw Ashes
2.9. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of Biomass Composition throughout Different Stages of the Biorefinery Process
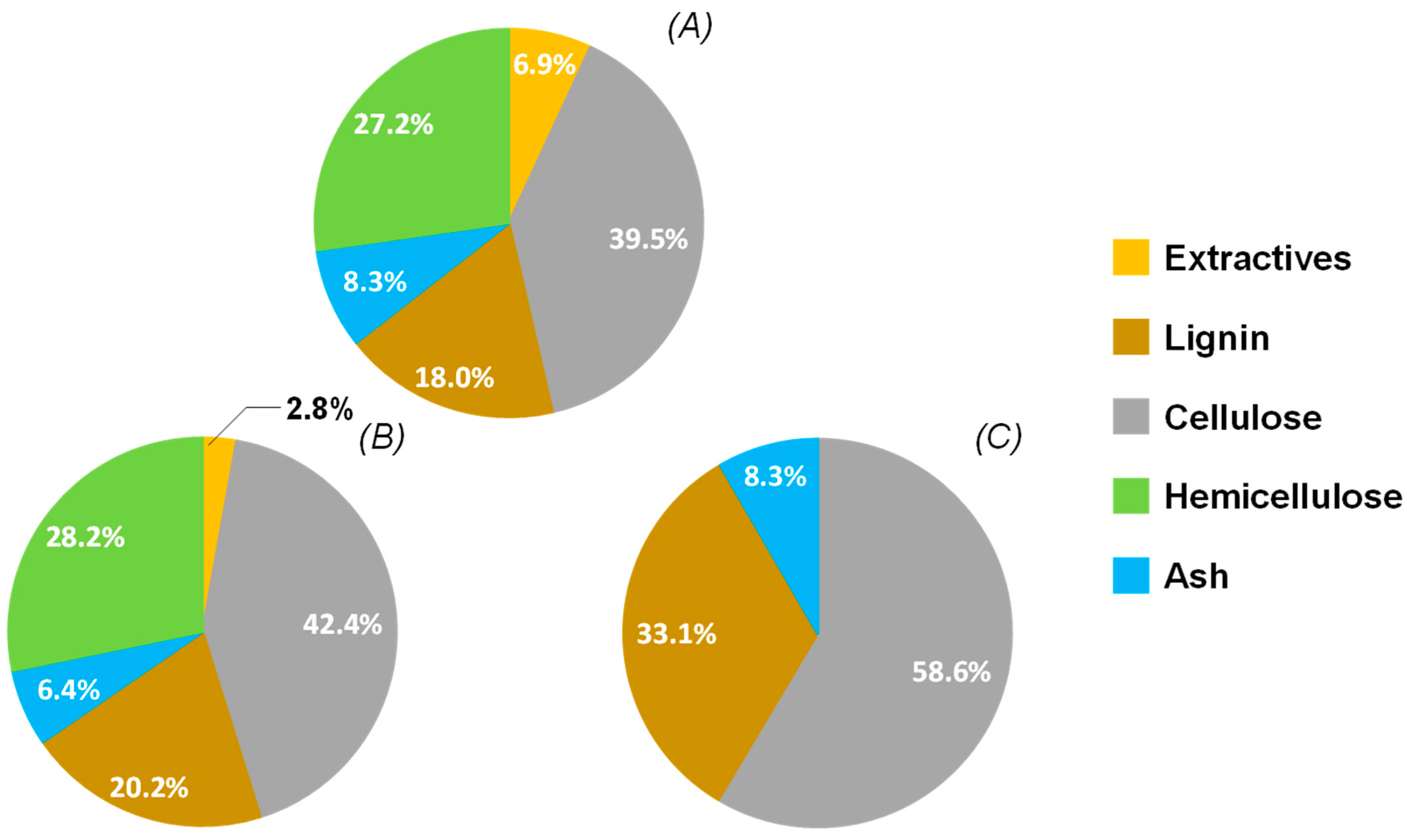
3.1.1. Composition of the Original Raw Wheat Straw
3.1.2. Pre-Extraction Step with Ethanol:Water (1:1, v/v) of Wheat Straw
3.1.3. Wheat Straw Hydrolysis
3.2. Adapting the Organosolv Process to Wheat Straw for High Lignin Recovery
3.2.1. Effect of the Catalyst Concentration
3.2.2. Effect of Wheat Straw:Solvent Ratio on the Organosolv Lignin Recovery from Pre-Hydrolyzed Wheat Straw
3.2.3. Effect of Pulping Time on the Organosolv Lignin Recovery
3.2.4. Effect of Pulping Temperature on the Wheat Straw Organosolv Lignin Recovery
3.2.5. Effect of Ethanol:Water Ratio on the Organosolv Lignin Recovery from Pre-Hydrolyzed Wheat Straw
3.2.6. Effect of Pre-Hydrolysis on the Organosolv Lignin Recovery Rate from Wheat Straw
3.3. Wheat Straw Organosolv Lignin Characterization
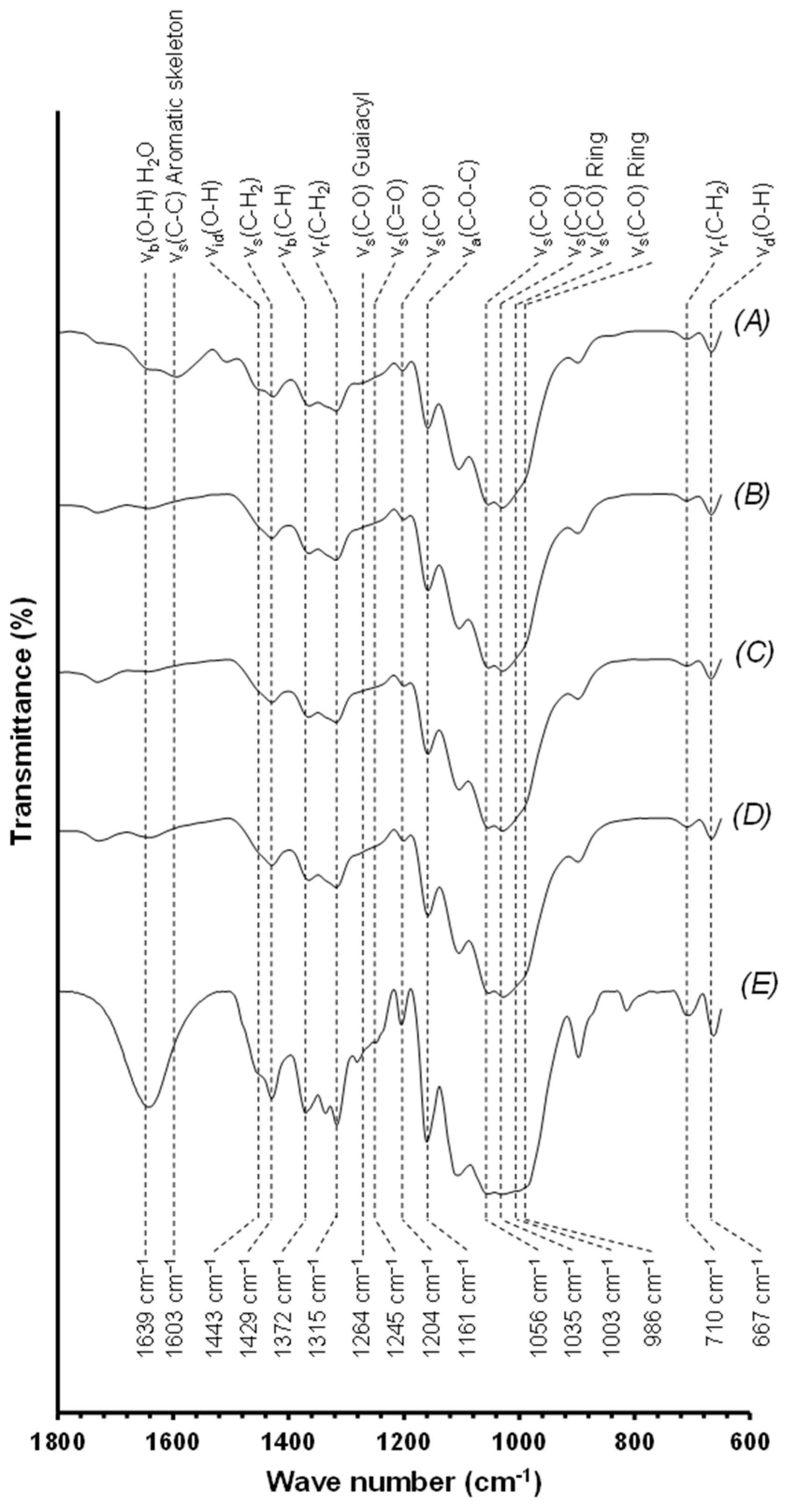
3.3.1. Fourier-Transform Infrared Spectroscopy of Lignin Samples
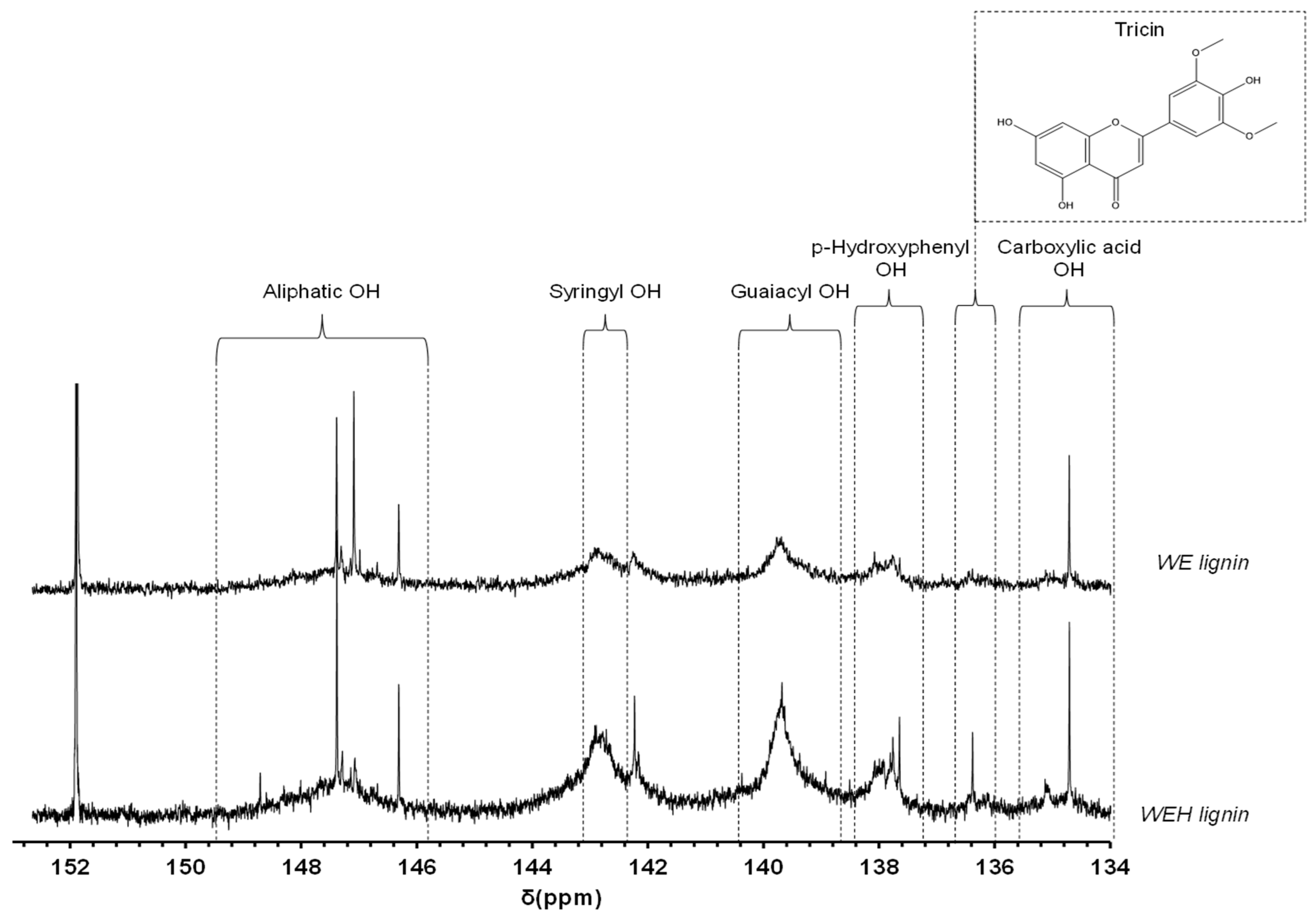
3.3.2. 31P Nuclear Magnetic Resonance Analysis of Organosolv Lignins
3.3.3. Heteronuclear Single Quantum Coherence Spectra of Wheat Straw Lignin Samples
3.3.4. Polymer Properties of Wheat Straw Organosolv Lignins
3.4. Improvement of the Silica Extraction Process from Wheat Straw Cellulosic Pulp
3.4.1. Effect of Na2CO3 Concentration on the Silica Removal Efficiency from Cellulosic Pulp Obtained from Hydrolyzed and Extractive-Free Wheat Straw (WEH)
3.4.2. Effect of Wheat Straw Cellulosic Pulp:Solvent Ratio on the Silica Removal Efficiency
3.4.3. Effect of Extraction Time on the Silica Removal from Wheat Straw Cellulosic Pulp
3.4.4. Effect of Temperature on the Silica Removal from Wheat Straw Cellulosic Pulp Samples
3.5. Characterization of the Silica-Free Wheat Straw Cellulosic Pulp
3.5.1. Fourier-Transform Infrared Spectroscopy Performed on the Bleached and Unbleached Cellulosic Samples
3.5.2. 13C Nuclear Magnetic Resonance Analyses of the Bleached Wheat STRAW Cellulose Samples
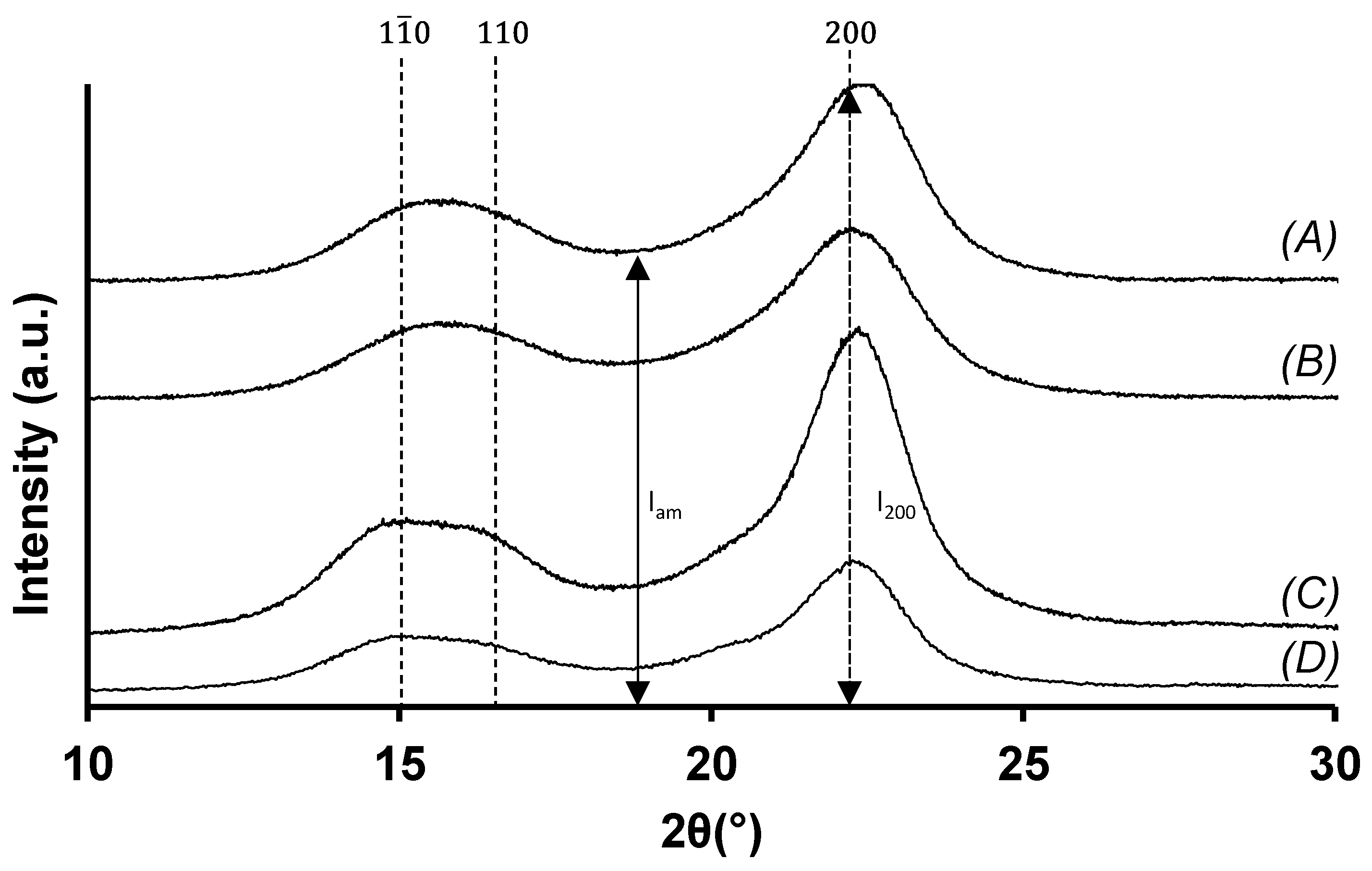
3.5.3. X-ray Diffraction Analyses of the Bleached Wheat STRAW Cellulose Samples
3.5.4. Viscometry Measurement Performed on the Bleached Cellulosic Samples
3.5.5. Fourier-Transform Infrared Spectroscopy Performed on the Silica Samples Isolated Following the Sodium Silicate Extraction of the Wheat Straw Cellulosic Pulp
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaigbe-Togbe, V.; Lina, B.; Gu, D.; Spoorenberg, T.; Lubov, Z. World Population Prospects 2022: Summary of Results; United Nations Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2022; p. 52. [Google Scholar]
- van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A Meta-Analysis of Projected Global Food Demand and Population at Risk of Hunger for the Period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Available online: http://www.fao.org/faostat/fr/#data/QC/visualize (accessed on 23 March 2021).
- Awika, J.M. Major Cereal Grains Production and Use around the World. In Advances in Cereal Science: Implications to Food Processing and Health Promotion; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2011; Volume 1089, pp. 1–13. ISBN 978-0-8412-2636-4. [Google Scholar]
- Cobreros, C.; Espinosa, D.; Hidalgo, F.; Manzano-Ramírez, A.; Reyes, J.L. Cereal Straw Production Analysis, Availability of Materials, and Provincial Map for Manufacturing of Sustainable Prefabricated Panels. BioResources 2014, 9, 786–800. [Google Scholar]
- Talebnia, F.; Karakashev, D.; Angelidaki, I. Production of Bioethanol from Wheat Straw: An Overview on Pretreatment, Hydrolysis and Fermentation. Bioresour. Technol. 2010, 101, 4744–4753. [Google Scholar] [CrossRef]
- Jiang, D.; An, P.; Cui, S.; Sun, S.; Zhang, J.; Tuo, T. Effect of Modification Methods of Wheat Straw Fibers on Water Absorbency and Mechanical Properties of Wheat Straw Fiber Cement-Based Composites. Adv. Mater. Sci. Eng. 2020, 2020, e5031025. [Google Scholar] [CrossRef]
- Sun, M.; Xu, X.; Wang, C.; Bai, Y.; Fu, C.; Zhang, L.; Fu, R.; Wang, Y. Environmental Burdens of the Comprehensive Utilization of Straw: Wheat Straw Utilization from a Life-Cycle Perspective. J. Clean. Prod. 2020, 259, 120702. [Google Scholar] [CrossRef]
- Govumoni, S.P.; Koti, S.; Kothagouni, S.Y.; Venkateshwar, S.; Linga, V.R. Evaluation of Pretreatment Methods for Enzymatic Saccharification of Wheat Straw for Bioethanol Production. Carbohydr. Polym. 2013, 91, 646–650. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of Process Parameters on Production of Biochar from Biomass Waste through Pyrolysis: A Review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar]
- Tufail, T.; Saeed, F.; Afzaal, M.; Ain, H.B.U.; Gilani, S.A.; Hussain, M.; Anjum, F.M. Wheat Straw: A Natural Remedy against Different Maladies. Food Sci. Nutr. 2021, 9, 2335–2344. [Google Scholar] [CrossRef]
- Constant, S.; Barakat, A.; Robitzer, M.; Di Renzo, F.; Dumas, C.; Quignard, F. Composition, Texture and Methane Potential of Cellulosic Residues from Lewis Acids Organosolv Pulping of Wheat Straw. Bioresour. Technol. 2016, 216, 737–743. [Google Scholar] [CrossRef]
- Del Río, J.C.; Rencoret, J.; Prinsen, P.; Martínez, Á.T.; Ralph, J.; Gutiérrez, A. Structural Characterization of Wheat Straw Lignin as Revealed by Analytical Pyrolysis, 2D-NMR, and Reductive Cleavage Methods. J. Agric. Food Chem. 2012, 60, 5922–5935. [Google Scholar] [CrossRef]
- Biricik, H.; Aköz, F.; Lhan Berktay, I.; Tulgar, A.N. Study of Pozzolanic Properties of Wheat Straw Ash. Cem. Concr. Res. 1999, 29, 637–643. [Google Scholar] [CrossRef]
- Liu, R.; Yu, H.; Huang, Y. Structure and Morphology of Cellulose in Wheat Straw. Cellulose 2005, 12, 25–34. [Google Scholar] [CrossRef]
- Sun, R.C.; Tompkinson, J. Comparative Study of Organic Solvent and Water-Soluble Lipophilic Extractives from Wheat Straw I: Yield and Chemical Composition. J. Wood Sci. 2003, 49, 0047–0052. [Google Scholar] [CrossRef]
- Luan, P.; Zhao, X.; Copenhaver, K.; Ozcan, S.; Zhu, H. Turning Natural Herbaceous Fibers into Advanced Materials for Sustainability. Adv. Fiber Mater. 2022, 4, 736–757. [Google Scholar] [CrossRef]
- Buranov, A.U.; Mazza, G. Lignin in Straw of Herbaceous Crops. Ind. Crops Prod. 2008, 28, 237–259. [Google Scholar] [CrossRef]
- Kasangana, P.; Auclair, N.; Daassi, R.; Durand, K.; Rodrigue, D.; Stevanovic, T. Impact of Pre-Extraction on Xylose Recovery from Two Lignocellulosic Agro-Wastes. BioResources 2022, 17, 6131–6147. [Google Scholar] [CrossRef]
- Durand, K.; Daassi, R.; Rodrigue, D.; Stevanovic, T. Study of Biopolymers and Silica Recovery from Pre-Hydrolyzed Rice Husks. Biomass Conv. Bioref. 2024. [Google Scholar] [CrossRef]
- Koumba-Yoya, G.; Stevanovic, T. New Biorefinery Strategy for High Purity Lignin Production. ChemistrySelect 2016, 1, 6562–6570. [Google Scholar] [CrossRef]
- Rodrigues Gurgel da Silva, A.; Errico, M.; Rong, B.-G. Techno-Economic Analysis of Organosolv Pretreatment Process from Lignocellulosic Biomass. Clean Techn Environ. Policy 2018, 20, 1401–1412. [Google Scholar] [CrossRef]
- Thoresen, P.P.; Matsakas, L.; Rova, U.; Christakopoulos, P. Recent Advances in Organosolv Fractionation: Towards Biomass Fractionation Technology of the Future. Bioresour. Technol. 2020, 306, 123189. [Google Scholar] [CrossRef]
- Rabelo, S.C.; Nakasu, P.Y.S.; Scopel, E.; Araújo, M.F.; Cardoso, L.H.; da Costa, A.C. Organosolv Pretreatment for Biorefineries: Current Status, Perspectives, and Challenges. Bioresour. Technol. 2023, 369, 128331. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Yuan, H.; Lyu, G.; Xie, J. FeCl3-Catalyzed Ethanol Pretreatment of Sugarcane Bagasse Boosts Sugar Yields with Low Enzyme Loadings and Short Hydrolysis Time. Bioresour. Technol. 2018, 249, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, R.; Fu, S. FeCl3 Pretreatment of Three Lignocellulosic Biomass for Ethanol Production. ACS Sustain. Chem. Eng. 2015, 3, 1794–1800. [Google Scholar] [CrossRef]
- Zhang, H.; Ye, G.; Wei, Y.; Li, X.; Zhang, A.; Xie, J. Enhanced Enzymatic Hydrolysis of Sugarcane Bagasse with Ferric Chloride Pretreatment and Surfactant. Bioresour. Technol. 2017, 229, 96–103. [Google Scholar] [CrossRef]
- Parot, M.; Rodrigue, D.; Stevanovic, T. High Purity Softwood Lignin Obtained by an Eco-Friendly Organosolv Process. Bioresour. Technol. Rep. 2022, 17, 100880. [Google Scholar] [CrossRef]
- D07 Committee Standard Test Method for Ethanol-Toluene Solubility of Wood. Available online: https://www.astm.org/d1107-96r13.html (accessed on 7 November 2022).
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass—NREL/TP-510-42618. Lab. Anal. Proced. (LAP) 2008. [Google Scholar]
- D07 Committee Standard Test Method for Ash in Wood. Available online: https://standards.globalspec.com/std/14376482/astm-d1102-84-2021 (accessed on 7 November 2022).
- Hamilton, L.A.; Kennedy, J.F. Sourcebook of Methods of Analysis for Biomass and Biomass Conversion Processes. Carbohydr. Polym. 1992, 19, 230. [Google Scholar] [CrossRef]
- Felissia, F.; Vallejos, M.; Area, M. Lignin Recovery from Spent Liquors from Ethanolwater Fractionation of Sugar Cane Bagasse. Cellul. Chem. Technol. 2010, 44, 311–318. [Google Scholar]
- Pekarovic, J.; Pekarovicova, A.; Joyce, T.W. Desilication of Agricultural Residues—The First Step Prior to Pulping. Appita Technol. Innov. Manuf. Environ. 2020, 58, 130–134. [Google Scholar]
- McCluer, R.H. Methods in Carbohydrate Chemistry. Volume 1, Analysis and Preparation of Sugars (Whistler, Roy L.; Wolfrom, M.L.; Ed.s). J. Chem. Educ. 1963, 40, A394. [Google Scholar] [CrossRef]
- Meng, X.; Crestini, C.; Ben, H.; Hao, N.; Pu, Y.; Ragauskas, A.J.; Argyropoulos, D.S. Determination of Hydroxyl Groups in Biorefinery Resources via Quantitative 31P NMR Spectroscopy. Nat. Protoc. 2019, 14, 2627–2647. [Google Scholar] [CrossRef] [PubMed]
- Atalla, R.H.; Vanderhart, D.L. The Role of Solid State 13C NMR Spectroscopy in Studies of the Nature of Native Celluloses. Solid State Nucl. Magn. Reson. 1999, 15, 1–19. [Google Scholar] [CrossRef]
- Imai, T.; Sugiyama, J.; Itoh, T.; Horii, F. Almost Pure I(Alpha) Cellulose in the Cell Wall of Glaucocystis. J. Struct. Biol. 1999, 127, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.-J.; Eom, S.H.; Wada, M. Thermal Decomposition of Native Cellulose: Influence on Crystallite Size. Polym. Degrad. Stab. 2010, 95, 778–781. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 26 July 2024).
- Dodson, J. Wheat Straw Ash and Its Use as a Silica Source. Ph.D. Thesis, University of York, Heslington, UK, 2011. [Google Scholar]
- Kapoor, M.; Panwar, D.; Kaira, G.S. Chapter 3—Bioprocesses for Enzyme Production Using Agro-Industrial Wastes: Technical Challenges and Commercialization Potential. In Agro-Industrial Wastes as Feedstock for Enzyme Production; Dhillon, G.S., Kaur, S., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 61–93. ISBN 978-0-12-802392-1. [Google Scholar]
- Chen, H.; Wang, F.; Zhang, C.; Shi, Y.; Jin, G.; Yuan, S. Preparation of Nano-Silica Materials: The Concept from Wheat Straw. J. Non-Cryst. Solids 2010, 356, 2781–2785. [Google Scholar] [CrossRef]
- Kumar, S.; Milstein, Y.; Brami, Y.; Elbaum, M.; Elbaum, R. Mechanism of Silica Deposition in Sorghum Silica Cells. New Phytol. 2017, 213, 791–798. [Google Scholar] [CrossRef]
- Guerriero, G.; Hausman, J.-F.; Legay, S. Silicon and the Plant Extracellular Matrix. Front. Plant Sci. 2016, 7, 463. [Google Scholar] [CrossRef]
- Van Loo, S.; Koppejan, J. The Handbook of Biomass Combustion and Co-Firing; Earthscan: London, UK, 2008; pp. 249–372. [Google Scholar]
- Giraldo, L.F.; López, B.L.; Pérez, L.; Urrego, S.; Sierra, L.; Mesa, M. Mesoporous Silica Applications. Macromol. Symp. 2007, 258, 129–141. [Google Scholar] [CrossRef]
- Singh, L.P.; Bhattacharyya, S.K.; Kumar, R.; Mishra, G.; Sharma, U.; Singh, G.; Ahalawat, S. Sol-Gel Processing of Silica Nanoparticles and Their Applications. Adv. Colloid Interface Sci. 2014, 214, 17–37. [Google Scholar] [CrossRef]
- Jeelani, P.G.; Mulay, P.; Venkat, R.; Ramalingam, C. Multifaceted Application of Silica Nanoparticles. A Review. Silicon 2020, 12, 1337–1354. [Google Scholar] [CrossRef]
- Bartůněk, V.; Sedmidubský, D.; Bouša, D.; Jankovský, O. Production of Pure Amorphous Silica from Wheat Straw Ash. Green Mater. 2018, 6, 1–5. [Google Scholar] [CrossRef]
- Mishra, A. Impact of Silica Mining on Environment. JGRP 2015, 8, 150–156. [Google Scholar] [CrossRef]
- Atanacković, N.; Dragišić, V.; Stojković, J.; Papić, P.; Živanović, V. Hydrochemical Characteristics of Mine Waters from Abandoned Mining Sites in Serbia and Their Impact on Surface Water Quality. Environ. Sci. Pollut. Res. 2013, 20, 7615–7626. [Google Scholar] [CrossRef]
- Wu, S.; Chen, J.; Peng, D.; Wu, Z.; Li, Q.; Huang, T. Effects of Water Leaching on the Ash Sintering Problems of Wheat Straw. Energies 2019, 12, 387. [Google Scholar] [CrossRef]
- Shimada, K.; Hosoya, S.; Ikeda, T. Condensation Reactions of Softwood and Hardwood Lignin Model Compounds Under Organic Acid Cooking Conditions. J. Wood Chem. Technol. 1997, 17, 57–72. [Google Scholar] [CrossRef]
- Mcdonough, T.J. The Chemistry of Organosolv Delignification; Tappi Press: Atlanta, GA, USA, 1992; pp. 241–248. [Google Scholar]
- Hagel, S.; Schütt, F. Reinforcement Fiber Production from Wheat Straw for Wastepaper-Based Packaging Using Steam Refining with Sodium Carbonate. Clean Technol. 2024, 6, 322–338. [Google Scholar] [CrossRef]
- Wildschut, J.; Smit, A.T.; Reith, J.H.; Huijgen, W.J.J. Ethanol-Based Organosolv Fractionation of Wheat Straw for the Production of Lignin and Enzymatically Digestible Cellulose. Bioresour. Technol. 2013, 135, 58–66. [Google Scholar] [CrossRef]
- Song, J.; Fan, H.; Ma, J.; Han, B. Conversion of Glucose and Cellulose into Value-Added Products in Water and Ionic Liquids. Green Chem. 2013, 15, 2619–2635. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial Application of Cellulose Nano-Composites—A Review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef]
- Yu, O.; Kim, K.H. Lignin to Materials: A Focused Review on Recent Novel Lignin Applications. Appl. Sci. 2020, 10, 4626. [Google Scholar] [CrossRef]
- Liao, J.J.; Latif, N.H.A.; Trache, D.; Brosse, N.; Hussin, M.H. Current Advancement on the Isolation, Characterization and Application of Lignin. Int. J. Biol. Macromol. 2020, 162, 985–1024. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D. Lignin as a Base Material for Materials Applications: Chemistry, Application and Economics. Ind. Crops Prod. 2008, 27, 202–207. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Yuan, T.-Q.; Sun, R.-C. Lignin-Phenol-Formaldehyde Resin Adhesives Prepared with Biorefinery Technical Lignins. J. Appl. Polym. Sci. 2015, 132, 42493. [Google Scholar] [CrossRef]
- Pang, B.; Lam, S.S.; Shen, X.; Cao, X.; Liu, S.; Yuan, T.; Sun, R. Valorization of Technical Lignin for the Production of Desirable Resins with High Substitution Rate and Controllable Viscosity. ChemSusChem 2020, 13, 4446–4454. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Ruzene, D.S.; Silva, D.P.; da Silva, F.F.M.; Vicente, A.A.; Teixeira, J.A. Development and Characterization of an Environmentally Friendly Process Sequence (Autohydrolysis and Organosolv) for Wheat Straw Delignification. Appl. Biochem. Biotechnol. 2011, 164, 629–641. [Google Scholar] [CrossRef]
- Bouasker, M.; Belayachi, N.; Hoxha, D.; Al-Mukhtar, M. Physical Characterization of Natural Straw Fibers as Aggregates for Construction Materials Applications. Materials 2014, 7, 3034–3048. [Google Scholar] [CrossRef]
- Xu, F.; Sun, J.-X.; Sun, R.; Fowler, P.; Baird, M.S. Comparative Study of Organosolv Lignins from Wheat Straw. Ind. Crops Prod. 2006, 23, 180–193. [Google Scholar] [CrossRef]
- Salapa, I.; Katsimpouras, C.; Topakas, E.; Sidiras, D. Organosolv Pretreatment of Wheat Straw for Efficient Ethanol Production Using Various Solvents. Biomass Bioenergy 2017, 100, 10–16. [Google Scholar] [CrossRef]
- Wang, K.; Yang, H.; Yao, X.; Xu, F.; Sun, R. Structural Transformation of Hemicelluloses and Lignin from Triploid Poplar during Acid-Pretreatment Based Biorefinery Process. Bioresour. Technol. 2012, 116, 99–106. [Google Scholar] [CrossRef]
- Liu, L.; Sun, J.; Li, M.; Wang, S.; Pei, H.; Zhang, J. Enhanced Enzymatic Hydrolysis and Structural Features of Corn Stover by FeCl3 Pretreatment. Bioresour. Technol. 2009, 100, 5853–5858. [Google Scholar] [CrossRef] [PubMed]
- Minu, K.; Jiby, K.K.; Kishore, V.V.N. Isolation and Purification of Lignin and Silica from the Black Liquor Generated during the Production of Bioethanol from Rice Straw. Biomass Bioenergy 2012, 39, 210–217. [Google Scholar] [CrossRef]
- Li, M.; Pu, Y.; Yoo, C.G.; Ragauskas, A.J. The Occurrence of Tricin and Its Derivatives in Plants. Green Chem. 2016, 18, 1439–1454. [Google Scholar] [CrossRef]
- Xie, M.; Chen, Z.; Xia, Y.; Lin, M.; Li, J.; Lan, W.; Zhang, L.; Yue, F. Influence of the Lignin Extraction Methods on the Content of Tricin in Grass Lignins. Front. Energy Res. 2021, 9, 756285. [Google Scholar] [CrossRef]
- Li, M.; Pu, Y.; Tschaplinski, T.J.; Ragauskas, A.J. 31P NMR Characterization of Tricin and Its Structurally Similar Flavonoids. ChemistrySelect 2017, 2, 3557–3561. [Google Scholar] [CrossRef]
- Del Río, J.C.; Rencoret, J.; Gutiérrez, A.; Elder, T.; Kim, H.; Ralph, J. Lignin Monomers from beyond the Canonical Monolignol Biosynthetic Pathway: Another Brick in the Wall. ACS Sustain. Chem. Eng. 2020, 8, 4997–5012. [Google Scholar] [CrossRef]
- Zeng, J.; Helms, G.L.; Gao, X.; Chen, S. Quantification of Wheat Straw Lignin Structure by Comprehensive NMR Analysis. J. Agric. Food Chem. 2013, 61, 10848–10857. [Google Scholar] [CrossRef]
- van Erven, G.; Nayan, N.; Sonnenberg, A.S.M.; Hendriks, W.H.; Cone, J.W.; Kabel, M.A. Mechanistic Insight in the Selective Delignification of Wheat Straw by Three White-Rot Fungal Species through Quantitative 13C-IS Py-GC-MS and Whole Cell Wall HSQC NMR. Biotechnol. Biofuels 2018, 11, 262. [Google Scholar] [CrossRef]
- Wilkie, K.C.B. The Hemicelluloses of Grasses and Cereals. In Advances in Carbohydrate Chemistry and Biochemistry; Tipson, R.S., Horton, D., Eds.; Academic Press: Cambridge, MA, USA, 1979; Volume 36, pp. 215–264. [Google Scholar]
- Tarasov, D.; Leitch, M.; Fatehi, P. Lignin–Carbohydrate Complexes: Properties, Applications, Analyses, and Methods of Extraction: A Review. Biotechnol. Biofuels 2018, 11, 269. [Google Scholar] [CrossRef]
- Constant, S.; Basset, C.; Dumas, C.; Di Renzo, F.; Robitzer, M.; Barakat, A.; Quignard, F. Reactive Organosolv Lignin Extraction from Wheat Straw: Influence of Lewis Acid Catalysts on Structural and Chemical Properties of Lignins. Ind. Crops Prod. 2015, 65, 180–189. [Google Scholar] [CrossRef]
- Lange, H.; Schiffels, P.; Sette, M.; Sevastyanova, O.; Crestini, C. Fractional Precipitation of Wheat Straw Organosolv Lignin: Macroscopic Properties and Structural Insights. ACS Sustain. Chem. Eng. 2016, 4, 5136–5151. [Google Scholar] [CrossRef]
- Labauze, H.; Cachet, N.; Benjelloun-Mlayah, B. Acid-Based Organosolv Lignin Extraction from Wheat Straw: Kinetic and Structural Analysis. Ind. Crops Prod. 2022, 187, 115328. [Google Scholar] [CrossRef]
- Rusănescu, C.O.; Ciobanu, M.; Rusănescu, M.; Dinculoiu, R.L. Pretreatments Applied to Wheat Straw to Obtain Bioethanol. Appl. Sci. 2024, 14, 1612. [Google Scholar] [CrossRef]
- Yuan, Z.; Wen, Y.; Kapu, N.S.; Beatson, R. Evaluation of an Organosolv-Based Biorefinery Process to Fractionate Wheat Straw into Ethanol and Co-Products. Ind. Crops Prod. 2018, 121, 294–302. [Google Scholar] [CrossRef]
- Crundwell, F.K. On the Mechanism of the Dissolution of Quartz and Silica in Aqueous Solutions. ACS Omega 2017, 2, 1116–1127. [Google Scholar] [CrossRef]
- Kim, T.; Olek, J. Chemical Sequence and Kinetics of Alkali-Silica Reaction Part I. Experiments. J. Am. Ceram. Soc. 2014, 97, 2195–2203. [Google Scholar] [CrossRef]
- Abidi, N.; Cabrales, L.; Haigler, C.H. Changes in the Cell Wall and Cellulose Content of Developing Cotton Fibers Investigated by FTIR Spectroscopy. Carbohydr. Polym. 2014, 100, 9–16. [Google Scholar] [CrossRef]
- Festucci-Buselli, R.A.; Otoni, W.C.; Joshi, C.P. Structure, Organization, and Functions of Cellulose Synthase Complexes in Higher Plants. Braz. J. Plant Physiol. 2007, 19, 1–13. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, C.M.; Kafle, K. Characterization of Crystalline Cellulose in Biomass: Basic Principles, Applications, and Limitations of XRD, NMR, IR, Raman, and SFG. Korean J. Chem. Eng. 2013, 30, 2127–2141. [Google Scholar] [CrossRef]
- Gümüşkaya, E.; Usta, M. Crystalline Structure Properties of Bleached and Unbleached Wheat Straw (Triticum aestivum L.) Soda-Oxygen Pulp. Turk. J. Agric. For. 2002, 26, 247–252. [Google Scholar]
- Chen, Y.; Wang, Y.; Wan, J.; Ma, Y. Crystal and Pore Structure of Wheat Straw Cellulose Fiber during Recycling. Cellulose 2010, 17, 329–338. [Google Scholar] [CrossRef]
- Molina-Guerrero, C.E.; de la Rosa, G.; Castillo-Michel, H.; Sánchez, A.; García-Castañeda, C.; Hernández-Rayas, A.; Valdez-Vazquez, I.; Suarez-Vázquez, S. Physicochemical Characterization of Wheat Straw during a Continuous Pretreatment Process. Chem. Eng. Technol. 2018, 41, 1350. [Google Scholar] [CrossRef]
- French, A.D. Increment in Evolution of Cellulose Crystallinity Analysis. Cellulose 2020, 27, 5445–5448. [Google Scholar] [CrossRef]
- Nam, S.; French, A.D.; Condon, B.D.; Concha, M. Segal Crystallinity Index Revisited by the Simulation of X-ray Diffraction Patterns of Cotton Cellulose Iβ and Cellulose II. Carbohydr. Polym. 2016, 135, 1–9. [Google Scholar] [CrossRef]
- Park, S.; Johnson, D.K.; Ishizawa, C.I.; Parilla, P.A.; Davis, M.F. Measuring the Crystallinity Index of Cellulose by Solid State 13C Nuclear Magnetic Resonance. Cellulose 2009, 16, 641–647. [Google Scholar] [CrossRef]
- Dinand, E.; Vignon, M.; Chanzy, H.; Heux, L. Mercerization of Primary Wall Cellulose and Its Implication for the Conversion of Cellulose I→cellulose II. Cellulose 2002, 9, 7–18. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Debnath, B.; Haldar, D.; Purkait, M.K. A Critical Review on the Techniques Used for the Synthesis and Applications of Crystalline Cellulose Derived from Agricultural Wastes and Forest Residues. Carbohydr. Polym. 2021, 273, 118537. [Google Scholar] [CrossRef]
- Daassi, R.; Durand, K.; Rodrigue, D.; Stevanovic, T. Optimization of the Electrospray Process to Produce Lignin Nanoparticles for PLA-Based Food Packaging. Polymers 2023, 15, 2973. [Google Scholar] [CrossRef]



| Characterization | Organosolv Lignin from Extractive-Free Hydrolyzed Wheat Straw (WEH) | Organosolv Lignin from Extractive-Free Wheat Straw (WE) | |
|---|---|---|---|
| Klason lignin (insoluble) | 95.9 ± 0.1% | 94.4 ± 0.6% | |
| Acid-soluble lignin | 0.266 ± 0.004% | 0.260 ± 0.001% | |
| Hydrolysate composition | 3.8% | 5.3% | |
| Furfural | 45 ± 2% | 71 ± 3% | |
| 5-HMF | 37 ± 2% | 20 ± 1% | |
| Other | 17 ± 2% | 9 ± 2% | |
| Ash content Ash composition | 0.39 ± 0.02% | 0.53 ± 0.03% | |
| Fe2O3 | 26.3% | 72.6% | |
| SiO2 | 67.6% | 14.7% | |
| Other (CaO, P2O5, SO3, K2O, …) | 6.1% | 12.7% | |
| Lignin polymer properties | |||
| Molecular weight (Mn) | 470 ± 5 Da | 538 ± 12 Da | |
| Molecular weight (Mw) | 1033 ± 43 Da | 1297 ± 139 Da | |
| Free hydroxyl groups in organosolv lignins (31P NMR) | S | 0.70 mmol/g | 0.73 mmol/g |
| G | 1.13 mmol/g | 1.17 mmol/g | |
| H | 0.44 mmol/g | 0.44 mmol/g | |
| Aliphatic | 0.70 mmol/g | 0.64 mmol/g | |
| Carboxylic | 0.173 mmol/g | 0.170 mmol/g | |
| S/G | 0.62 | 0.62 | |
| Lignin aromatic units (HSQC NMR) | S | 23.8% | 25.5% |
| G | 53.1% | 51.8% | |
| H | 23.1% | 22.8% | |
| S/G | 44.8% | 49.2% | |
| Lignin interunit linkages (HSQC NMR) | β-O-4′ | 33.8% | 50.5% |
| β-5′ | 17.6% | 10.4% | |
| β-β | 20.0% | 8.3% | |
| Characterization | Silica-Free Cellulosic Pulp from Extractive-Free Hydrolyzed Wheat Straw | Silica-Free Cellulosic Pulp from Extractive-Free Wheat Straw | |
|---|---|---|---|
| Klason lignin (insoluble) | 11.2 ± 0.4% | 3.2 ± 0.2% | |
| Acid-soluble lignin | 0.047 ± 0.001% | 0.0589 ± 0.0003% | |
| Cellulose | 86.8% | 94.4% | |
| Ash | 2.0 ± 0.2% | 2.32 ± 0.2% | |
| Average composition | Na2O | 22.2% | 4.6% |
| CaO | 27.0% | 17.6% | |
| SiO2 | 30.3% | 48.8% | |
| Fe2O3 | 10.0% | 24.4% | |
| Other (Fe2O3, MgO, SO3…) | 10.4% | 4.6% | |
| Characterization | Bleached silica-free cellulosic pulp from extractive-free hydrolyzed wheat straw | Bleached silica-free cellulosic pulp from extractive-free wheat straw | |
| DP | 89.09 ± 0.07 | 648 ± 13 | |
| Mw | 14446 ± 12 Da | 105141 ± 2051 Da | |
| Crystallinity (13C) | 45 ± 5% | 30 ± 3% | |
| Crystallinity (XRD) | 87% | 81% | |
| Crystallite size | 32 ± 2 Å | 29 ± 1 Å | |
| Iα (FTIR) | - | - | |
| Iβ (FTIR) | 100% | 100% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durand, K.; Daassi, R.; Rodrigue, D.; Stevanovic, T. Study of Purified Cellulosic Pulp and Lignin Produced by Wheat Straw Biorefinery. Macromol 2024, 4, 650-679. https://doi.org/10.3390/macromol4030039
Durand K, Daassi R, Rodrigue D, Stevanovic T. Study of Purified Cellulosic Pulp and Lignin Produced by Wheat Straw Biorefinery. Macromol. 2024; 4(3):650-679. https://doi.org/10.3390/macromol4030039
Chicago/Turabian StyleDurand, Kalvin, Rodrigue Daassi, Denis Rodrigue, and Tatjana Stevanovic. 2024. "Study of Purified Cellulosic Pulp and Lignin Produced by Wheat Straw Biorefinery" Macromol 4, no. 3: 650-679. https://doi.org/10.3390/macromol4030039
APA StyleDurand, K., Daassi, R., Rodrigue, D., & Stevanovic, T. (2024). Study of Purified Cellulosic Pulp and Lignin Produced by Wheat Straw Biorefinery. Macromol, 4(3), 650-679. https://doi.org/10.3390/macromol4030039









