Nanometric Hydroxyapatite Particles as Active Ingredient for Bioinks: A Review
Abstract
:1. Introduction
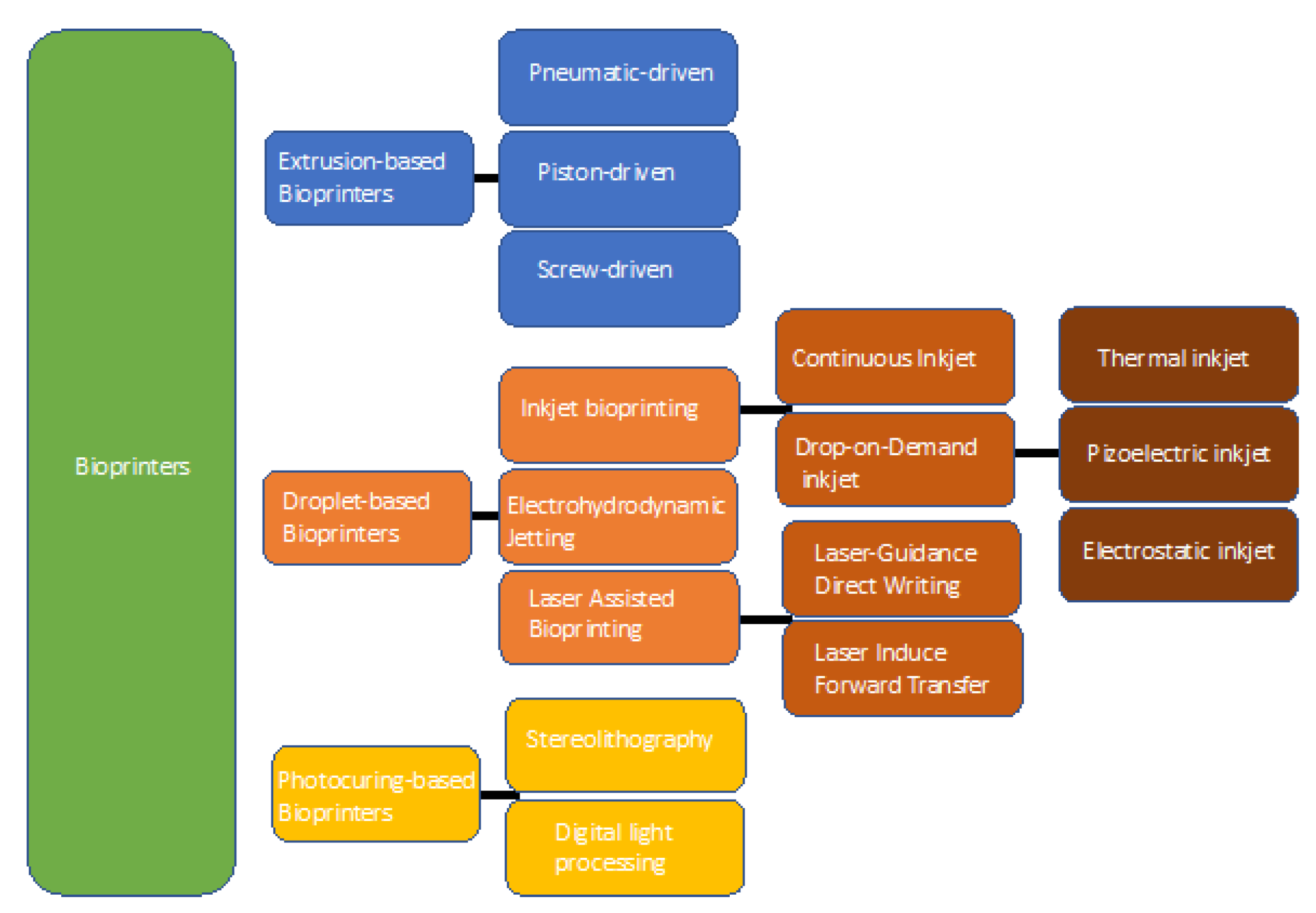
2. Bioprinting: Technology and Suitable Biomaterials
2.1. Extrusion-Based Bioprinting
2.2. Droplet-Based Bioprinting
2.3. Photocuring-Based Bioprinting
2.4. Biomaterials for 3D Bioprinting
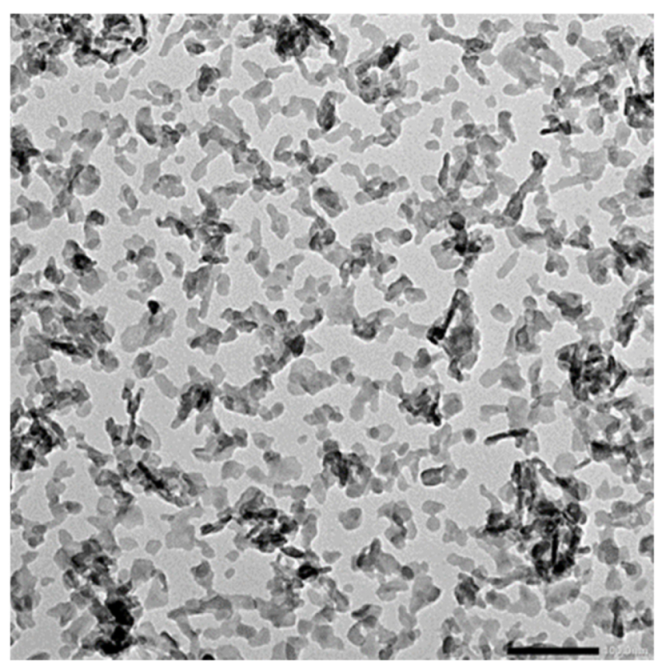
3. Nanohydroxyapatite for Bioprinting
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Szcześ, A.; Hołysz, L.; Chibowski, E. Synthesis of hydroxyapatite for biomedical applications. Adv. Colloid Interface Sci. 2017, 249, 321–330. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef]
- Malmberg, P.; Nygren, H. Methods for the analysis of the composition of bone tissue, with a focus on imaging mass spectrometry (TOF-SIMS). Proteomics 2008, 8, 3755–3762. [Google Scholar] [CrossRef] [PubMed]
- Petit, R. The use of hydroxyapatite in orthopaedic surgery: A ten-year review. Eur. J. Orthop. Surg. Traumatol. 1999, 9, 71–74. [Google Scholar] [CrossRef]
- Balhuc, S.; Campian, R.; Labunet, A.; Negucioiu, M.; Buduru, S.; Kui, A. Dental Applications of Systems Based on Hydroxyapatite. Crystals 2021, 11, 674. [Google Scholar] [CrossRef]
- Mondal, S.; Dorozhkin, S.V.; Pal, U. Recent progress on fabrication and drug delivery applications of nanostructured hydroxyapatite. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2018, 10, e1504. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Imura, M.; Nemoto, Y.; Cheng, C.H.; Yamauchi, Y. Block-copolymer-assisted synthesis of hydroxyapatite nanoparticles with high surface area and uniform size. Sci. Technol. Adv. Mater. 2011, 12, 045005. [Google Scholar] [CrossRef]
- Zhou, M.; Geng, Y.M.; Li, S.Y.; Yang, X.B.; Che, Y.J.; Pathak, J.L.; Wu, G. Nanocrystalline hydroxyapatite-based scaffold adsorbs and gives sustained release of osteoinductive growth factor and facilitates bone regeneration in mice ectopic model. J. Nanomater. 2019, 2019, 1202159. [Google Scholar] [CrossRef] [Green Version]
- Suchanek, W.; Yashima, M.; Kakihana, M.; Yoshimura, M. Processing and mechanical properties of hydroxyapatite reinforced with hydroxyapatite whiskers. Biomaterials 1996, 17, 1715–1723. [Google Scholar] [CrossRef]
- Lodoso-Torrecilla, I.; Klein Gunnewiek, R.; Grosfeld, E.C.; De Vries, R.B.M.; Habibović, P.; Jansen, J.A.; Van Den Beucken, J.J.J.P. Bioinorganic supplementation of calcium phosphate-based bone substitutes to improve: In vivo performance: A systematic review and meta-analysis of animal studies. Biomater. Sci. 2020, 8, 4792–4809. [Google Scholar] [CrossRef] [PubMed]
- Lara-Ochoa, S.; Ortega-Lara, W.; Guerrero-Beltrán, C.E. Hydroxyapatite nanoparticles in drug delivery: Physicochemistry and applications. Pharmaceutics 2021, 13, 1642. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Nanosized and nanocrystalline calcium orthophosphates. Acta Biomater. 2010, 6, 715–734. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, L.; Guo, S. Characterization of biodegradable and cytocompatible nano-hydroxyapatite/polycaprolactone porous scaffolds in degradation in vitro. Polym. Degrad. Stab. 2010, 95, 207–213. [Google Scholar] [CrossRef]
- Li, B.; Guo, B.; Fan, H.; Zhang, X. Preparation of nano-hydroxyapatite particles with different morphology and their response to highly malignant melanoma cells in vitro. Appl. Surf. Sci. 2008, 255, 357–360. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, Y.; Yan, W.; Hu, Q.; Tao, J.; Zhang, M.; Shi, Z.; Tang, R. Role of hydroxyapatite nanoparticle size in bone cell proliferation. J. Mater. Chem. 2007, 17, 3780–3787. [Google Scholar] [CrossRef]
- Agarwal, S.; Saha, S.; Balla, V.K.; Pal, A.; Barui, A.; Bodhak, S. Current Developments in 3D Bioprinting for Tissue and Organ Regeneration–A Review. Front. Mech. Eng. 2020, 6, 90. [Google Scholar] [CrossRef]
- Adepu, S.; Dhiman, N.; Laha, A.; Sharma, C.S.; Ramakrishna, S. ScienceDirect Three-dimensional bioprinting for bone tissue regeneration. Curr. Opin. Biomed. Eng. 2017, 2, 22–28. [Google Scholar] [CrossRef]
- Zadpoor, A.A.; Malda, J. Additive Manufacturing of Biomaterials, Tissues, and Organs. Ann. Biomed. Eng. 2017, 45, 1204–1218. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, F.; O’Connell, C.D.; Mladenovska, T.; Dodds, S. Print Me an Organ? Ethical and Regulatory Issues Emerging from 3D Bioprinting in Medicine. Sci. Eng. Ethics 2018, 24, 73–91. [Google Scholar] [CrossRef]
- Kang, H.W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Fu, J.; Lin, H.; He, Y. Development of 3D bioprinting: From printing methods to biomedical applications. Asian J. Pharm. Sci. 2020, 15, 529–557. [Google Scholar] [CrossRef] [PubMed]
- Klebe, R.J. Cytoscribing: A method for micropositioning cells and the construction of two- and three-dimensional synthetic tissues. Exp. Cell Res. 1988, 179, 362–373. [Google Scholar] [CrossRef]
- Landers, R.; Hübner, U.; Schmelzeisen, R.; Mülhaupt, R. Rapid prototyping of scaffolds derived from thermoreversible hydrogels and tailored for applications in tissue engineering. Biomaterials 2002, 23, 4437–4447. [Google Scholar] [CrossRef]
- Kesti, M.; Eberhardt, C.; Pagliccia, G.; Kenkel, D.; Grande, D.; Boss, A.; Zenobi-Wong, M. Bioprinting Complex Cartilaginous Structures with Clinically Compliant Biomaterials. Adv. Funct. Mater. 2015, 25, 7406–7417. [Google Scholar] [CrossRef] [Green Version]
- Tack, P.; Victor, J.; Gemmel, P.; Annemans, L. 3D-printing techniques in a medical setting: A systematic literature review. Biomed. Eng. Online 2016, 15, 115. [Google Scholar] [CrossRef] [Green Version]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, X.; Du, Z.; Jiang, W.; Han, X.; Zhao, D.; Han, D.; Li, Q. Three dimensional printed macroporous polylactic acid/hydroxyapatite composite scaffolds for promoting bone formation in a critical-size rat calvarial defect model. Sci. Technol. Adv. Mater. 2016, 17, 136–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genova, T.; Roato, I.; Carossa, M.; Motta, C.; Cavagnetto, D.; Mussano, F. Advances on bone substitutes through 3d bioprinting. Int. J. Mol. Sci. 2020, 21, 7012. [Google Scholar] [CrossRef]
- Askari, M.; Afzali Naniz, M.; Kouhi, M.; Saberi, A.; Zolfagharian, A.; Bodaghi, M. Recent progress in extrusion 3D bioprinting of hydrogel biomaterials for tissue regeneration: A comprehensive review with focus on advanced fabrication techniques. Biomater. Sci. 2021, 9, 535–573. [Google Scholar] [CrossRef]
- Visser, J.; Peters, B.; Burger, T.J.; Boomstra, J.; Dhert, W.J.A.; Melchels, F.P.W.; Malda, J. Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication 2013, 5, 035007. [Google Scholar] [CrossRef]
- Banović, L.; Vihar, B. Development of an Extruder for Open Source 3D Bioprinting. J. Open Hardw. 2018, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Tong, A.; Pham, Q.L.; Abatemarco, P.; Mathew, A.; Gupta, D.; Iyer, S.; Voronov, R. Review of Low-Cost 3D Bioprinters: State of the Market and Observed Future Trends. SLAS Technol. 2021, 26, 333–366. [Google Scholar] [CrossRef]
- Li, X.; Liu, B.; Pei, B.; Chen, J.; Zhou, D.; Peng, J.; Zhang, X.; Jia, W.; Xu, T. Inkjet Bioprinting of Biomaterials. Chem. Rev. 2020, 120, 10793–10833. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulos, I.; Allenby, M.C.; Lim, M.; Zamorano, M. Engineering inkjet bioprinting processes toward translational therapies. Biotechnol. Bioeng. 2020, 117, 272–284. [Google Scholar] [CrossRef]
- Kumar, P.; Ebbens, S.; Zhao, X. Inkjet printing of mammalian cells—Theory and applications. Bioprinting 2021, 23, e00157. [Google Scholar] [CrossRef]
- Bernasconi, R.; Brovelli, S.; Viviani, P.; Soldo, M.; Giusti, D.; Magagnin, L. Piezoelectric Drop-On-Demand Inkjet Printing of High-Viscosity Inks. Adv. Eng. Mater. 2021, 2100733. [Google Scholar] [CrossRef]
- Solis, L.H.; Ayala, Y.; Portillo, S.; Varela-Ramirez, A.; Aguilera, R.; Boland, T. Thermal inkjet bioprinting triggers the activation of the VEGF pathway in human microvascular endothelial cells in vitro. Biofabrication 2019, 11, 045005. [Google Scholar] [CrossRef]
- Hoehne, J.L.; Carlstron, R.; Dernorwsek, J.; Cristovam, P.C.; Bachiega, H.L.; Abensur, S.I.; Schor, P. Piezoelectric 3D bioprinting for ophthalmological applications: Process development and viability analysis of the technology. Biomed. Phys. Eng. Express 2020, 6, 035021. [Google Scholar] [CrossRef] [PubMed]
- Umezu, S. Precision printing of gelatin utilizing electrostatic inkjet. Jpn. J. Appl. Phys. 2014, 53, 05HC01. [Google Scholar] [CrossRef]
- Jayasinghe, S.N.; Qureshi, A.N.; Eagles, P.A.M. Electrohydrodynamic jet processing: An advanced electric-field-driven jetting phenomenon for processing living cells. Small 2006, 2, 216–219. [Google Scholar] [CrossRef]
- Catros, S.; Fricain, J.C.; Guillotin, B.; Pippenger, B.; Bareille, R.; Remy, M.; Lebraud, E.; Desbat, B.; Amédée, J.; Guillemot, F. Laser-assisted bioprinting for creating on-demand patterns of human osteoprogenitor cells and nano-hydroxyapatite. Biofabrication 2011, 3, 025001. [Google Scholar] [CrossRef]
- Hakobyan, D.; Kerouredan, O.; Remy, M.; Dusserre, N.; Medina, C.; Devillard, R.; Fricain, J.C.; Oliveira, H. Laser-Assisted Bioprinting for Bone Repair. In 3D Bioprinting. Methods in Molecular Biology; Crook, J., Ed.; Humana: New York, NY, USA, 2020; Volume 2140. [Google Scholar]
- Hakobyan, D.; Médina, C.; Dusserre, N.; Stachowicz, M.L.; Handschin, C.; Fricain, J.C.; Guillermet-Guibert, J.; Oliveira, H. Laser-assisted 3D bioprinting of exocrine pancreas spheroid models for cancer initiation study. Biofabrication 2020, 12, 035001. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yu, K.; Nie, J.; Sun, M.; Fu, J.; Wang, H.; He, Y. Modeling the printability of photocuring and strength adjustable hydrogel bioink during projection-based 3D bioprinting. Biofabrication 2021, 13, 035032. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, H.; Wang, P.; Zheng, Y.; Liu, L.; Hu, J.; Liu, Y.; Gao, Q.; He, Y. Lightweight 3D bioprinting with point by point photocuring. Bioact. Mater. 2021, 6, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, H.; Yang, L.; Zhu, H.; Wu, L.; Ji, P.; Yang, J.; Gu, Z. Recent advances and challenges in materials for 3D bioprinting. Prog. Nat. Sci. Mater. Int. 2020, 30, 618–634. [Google Scholar] [CrossRef]
- Mao, H.; Gu, Z. Polymers in 3D Bioprinting: Progress and Challenges. Mater. China 2018, 37, 949. [Google Scholar] [CrossRef]
- Muthukrishnan, L. Imminent antimicrobial bioink deploying cellulose, alginate, EPS and synthetic polymers for 3D bioprinting of tissue constructs. Carbohydr. Polym. 2021, 260, 117774. [Google Scholar] [CrossRef]
- Vanaei, S.; Parizi, M.S.; Vanaei, S.; Salemizadehparizi, F.; Vanaei, H.R. An Overview on Materials and Techniques in 3D Bioprinting Toward Biomedical Application. Eng. Regen. 2021, 2, 1–18. [Google Scholar] [CrossRef]
- Park, T.Y.; Yang, Y.J.; Ha, D.H.; Cho, D.W.; Cha, H.J. Marine-derived natural polymer-based bioprinting ink for biocompatible, durable, and controllable 3D constructs. Biofabrication 2019, 11, 035001. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Q.; Liu, C.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Natural polymers for organ 3D bioprinting. Polymers (Basel). 2018, 10, 1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levato, R.; Webb, W.R.; Otto, I.A.; Mensinga, A.; Zhang, Y.; van Rijen, M.; van Weeren, R.; Khan, I.M.; Malda, J. The bio in the ink: Cartilage regeneration with bioprintable hydrogels and articular cartilage-derived progenitor cells. Acta Biomater. 2017, 61, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Chow, K.L.; Leng, Y. Study of hydroxyapatite osteoinductivity with an osteogenic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2009, 89, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Bal, Z.; Kaito, T.; Korkusuz, F.; Yoshikawa, H. Bone regeneration with hydroxyapatite-based biomaterials. Emergent Mater. 2020, 3, 521–544. [Google Scholar] [CrossRef]
- Heidari, F.; Razavi, M.; Ghaedi, M.; Forooghi, M. Investigation of mechanical properties of natural hydroxyapatite samples prepared by cold isostatic pressing method. J. Alloys Compd. 2017, 693, 1150–1156. [Google Scholar] [CrossRef]
- Vaishya, R.; Chauhan, M.; Vaish, A. Bone cement. J. Clin. Orthop. Trauma 2013, 4, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Caro Aragonés, I. Cementos óseos con antibiótico. Panor. Actual Medicam. 2016, 40, 634–638. [Google Scholar]
- Dewi, A.H.; Ana, I.D. The use of hydroxyapatite bone substitute grafting for alveolar ridge preservation, sinus augmentation, and periodontal bone defect: A systematic review. Heliyon 2018, 4, e00884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Wang, X.; Xu, H.; Abe, H.; Tan, Z.; Zhao, Y.; Guo, J.; Naito, M.; Ichikawa, H.; Fukumori, Y. Towards sustained delivery of small molecular drugs using hydroxyapatite microspheres as the vehicle. Adv. Powder Technol. 2010, 21, 268–272. [Google Scholar] [CrossRef]
- Desai, V.U. and T.A. In Vitro Analysis of Nanoparticulate Hydroxyapatite/Chitosan Composites as Potential Drug Delivery Platforms for the Sustained Release of Antibiotics in the Treatment of Osteomyelitis. J. Pharm. Sci. 2014, 103, 567–579. [Google Scholar] [CrossRef]
- Cabrera, M.A.; Mulinari-Brenner, F. Radiological evaluation of Calcium Hydroxyapatite-based cutaneous fillers. Surg. Cosmet. Dermatology 2011, 3, 203–205. [Google Scholar]
- De Almeida, A.T.; Figueredo, V.; Da Cunha, A.L.G.; Casabona, G.; Costa De Faria, J.R.; Alves, E.V.; Sato, M.; Branco, A.; Guarnieri, C.; Palermo, E. Consensus Recommendations for the Use of Hyperdiluted Calcium Hydroxyapatite (Radiesse) as a Face and Body Biostimulatory Agent. Plast. Reconstr. Surg.-Glob. Open 2019, 7, e2160. [Google Scholar] [CrossRef]
- Zhang, R.; Hu, H.; Liu, Y.; Tan, J.; Chen, W.; Ying, C.; Liu, Q.; Fu, X.; Hu, S.; Wong, C.P. Homogeneously dispersed composites of hydroxyapatite nanorods and poly(lactic acid) and their mechanical properties and crystallization behavior. Compos. Part A Appl. Sci. Manuf. 2020, 132, 105841. [Google Scholar] [CrossRef]
- d’Angelo, M.; Benedetti, E.; Tupone, M.G.; Catanesi, M.; Castelli, V.; Antonosante, A.; Cimini, A. The Role of Stiffness in Cell Reprogramming: A Potential Role for Biomaterials in Inducing Tissue Regeneration. Cells 2019, 8, 1036. [Google Scholar] [CrossRef] [Green Version]
- Yun, H.S.; Kim, S.E.; Park, E.K. Bioactive glass-poly (ε-caprolactone) composite scaffolds with 3 dimensionally hierarchical pore networks. Mater. Sci. Eng. C 2011, 31, 198–205. [Google Scholar] [CrossRef]
- Zhang, J.; Barbieri, D.; Ten Hoopen, H.; De Bruijn, J.D.; Van Blitterswijk, C.A.; Yuan, H. Microporous calcium phosphate ceramics driving osteogenesis through surface architecture. J. Biomed. Mater. Res. Part A 2015, 103, 1188–1199. [Google Scholar] [CrossRef]
- Lan Levengood, S.K.; Polak, S.J.; Wheeler, M.B.; Maki, A.J.; Clark, S.G.; Jamison, R.D.; Wagoner Johnson, A.J. Multiscale osteointegration as a new paradigm for the design of calcium phosphate scaffolds for bone regeneration. Biomaterials 2010, 31, 3552–3563. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cerezo, M.N.; Peña, J.; Ivanovski, S.; Arcos, D.; Vallet-Regí, M.; Vaquette, C. Multiscale porosity in mesoporous bioglass 3D-printed scaffolds for bone regeneration. Mater. Sci. Eng. C 2021, 120, 111706. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojeda, E.; García-Barrientos, Á.; Martínez de Cestafe, N.; Alonso, J.M.; Pérez-González, R.; Sáez-Martínez, V. Nanometric Hydroxyapatite Particles as Active Ingredient for Bioinks: A Review. Macromol 2022, 2, 20-29. https://doi.org/10.3390/macromol2010002
Ojeda E, García-Barrientos Á, Martínez de Cestafe N, Alonso JM, Pérez-González R, Sáez-Martínez V. Nanometric Hydroxyapatite Particles as Active Ingredient for Bioinks: A Review. Macromol. 2022; 2(1):20-29. https://doi.org/10.3390/macromol2010002
Chicago/Turabian StyleOjeda, Edilberto, África García-Barrientos, Nagore Martínez de Cestafe, José María Alonso, Raúl Pérez-González, and Virginia Sáez-Martínez. 2022. "Nanometric Hydroxyapatite Particles as Active Ingredient for Bioinks: A Review" Macromol 2, no. 1: 20-29. https://doi.org/10.3390/macromol2010002





