Abstract
Given the fossil fuel crisis and the steady consumption of finite resources, the use of green polymers is becoming necessary. However, the term “green” describes materials that present green properties (such as biological origin and/or biodegradability) and are produced via sustainable processes conducted under mild conditions and not requiring the use of chemical catalysts, toxic solvents or reagents. Truly green materials must combine these characteristics; consequently, enzymatically synthesized bio-based and/or biodegradable polymers can be characterized as truly green. The present review focuses on the most promising, commercially available aliphatic and alipharomatic polyesters that can be synthesized enzymatically. In particular, the recent developments in the enzymatic polymerization of PLA and PBS and alipharomatic furan-based polyesters (e.g., PBF) are herein analyzed. Based on this analysis, it can be concluded that important steps have been taken toward synthesizing sustainably green polymers. Still, it is necessary to evaluate the applied methods regarding their capability to be used on an industrial scale.
1. Introduction
Even though most plastics are still fossil-based, the fossil fuel crisis has become more and more evident, especially since the late 1990s [1]. Given their steady consumption and the fact that fossil fuels are finite resources, their depletion seems unavoidable. As a result, greener materials, e.g., bio-based and/or biodegradable polymers and greener processes, e.g., enzymatic polymerization, are becoming necessary.
Bio-based polymers are defined as materials obtained from renewable resources [2]. Bio-based polymers include a. natural polymers obtained from biomass (e.g., agropolymers originated from agroresources such as starch and cellulose), b. polymers synthesized from microorganisms (e.g., PHAs) and c. polymers originated from bio-based monomers and synthesized in laboratories or by industry (e.g., PLA, PBS, PEF) [3]. These polymers could probably replace conventional, fossil-based polymers in case they are also economically competitive [4]. It is of high essence that there is an increasing interest in bio-based materials in the open literature [5,6,7].
Biodegradable polymers are polymeric materials that can be degraded in the environment via the enzymatic action of microorganisms, which can be measured by standardized tests in a specific time interval reflecting available disposal conditions [8]. At the end of biodegradation, they are meant to be broken down into biomass, carbon dioxide and water [9]. A subset of biodegradable polymers may also be compostable with specific reference to their biodegradation in a compost system. These must be capable of undergoing biological decomposition in a compost site in a way in which the plastic is not visually distinguishable and breaks down to carbon dioxide, etc., at a rate consistent with known compostable materials (e.g., cellulose) [8]. The use of biodegradable polymers, however, is not a “panacea”, given that when they are left to degrade on their own in anaerobic landfill leachate or marine conditions, the degradation kinetics are much slower and significantly higher times are required to reach a high degree of disintegration compared to industrial composting [10,11]. On that basis, biodegradable polymers seem to be a promising alternative to nondegradable fossil-based polymers, providing their end-of-life management options are further studied, especially when they are to be released into the environment [12].
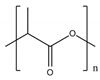
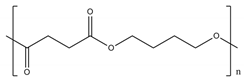
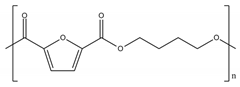
Among bio-based and/or biodegradable polymers, the most promising ones are the already commercially available aliphatic and alipharomatic polyesters. This review will focus on the enzymatic polymerization of the aliphatic PLA and PBS, as well as on a novel category of alipharomatic furan-based polyesters (e.g., PBF) (Table 1). Even though furan-based polyesters are not biodegradable, their bio-based character, along with their excellent properties, make them promising sustainable candidates for future applications, especially when synthesized via a sustainable method such as enzymatic polymerization.

Table 1.
Structures of the selected aliphatic and alipharomatic polyesters.
In particular, poly(lactic acid) (PLA) is a bio-based, biodegradable and biocompatible polyester, able to be used for bioapplications and pharmaceutical applications (e.g., sutures, scaffolds and drug delivery systems) [4,13], as well as in packaging, as it is a promising alternative to polystyrene (PS) and poly(ethylene terephthalate) (PET) [14]. PLA is becoming more and more popular as a packaging material, providing consumers with extra end-use benefits such as avoiding paying the “green tax” in Germany or meeting environmental regulations in Japan [15]. However, when PLA is conventionally produced—via direct condensation polymerization, azeotropic dehydration condensation or lactide ring-opening polymerization (ROP) [16]—either impurities or catalyst residues (organometallic, cationic, organic or bifunctional [17]) unavoidably remain in the final product, making it inappropriate to be used for food contact applications. Indicatively, according to the standards for compostable and biodegradable polymers packaging, the level of heavy metals should not exceed the maximum allowable level, which is 150 mg kg−1 on dry substance [18]. The most commonly used industrial production method for PLA is ring-opening polymerization (ROP) of the lactone monomers and tin(II) octoate (stannous bis(2-ethylhexanoate): Sn(Oct)2) is the most popular catalyst [19]. Sn(Oct)2 is biologically safe and approved by the FDA (the US Food and Drug Administration) for use in both food and medical applications [19]. However, the approval is dependent on empirical safety data and studies with long-term liberation and physiological effects of tin-ions from large tissue-embedded scaffold-matrices have not yet been conducted and may impose a different level of risk [20]. It is thus clear that there is a need to establish another PLA production method, one not strictly requiring the removal of the used catalysts’ residues, such as enzymatic polymerization.
As a bio-based, biodegradable and biocompatible polyester, poly(butylene succinate) (PBS) can be used in the biomedical field, especially in bone tissue engineering, as well as in the field of food packaging. More precisely, PBS is superior to PLA regarding human mesenchymal stem cell attachment, proliferation and osteogenesis [21]. Furthermore, PBS presents excellent hydrolytic degradability, hydrophilicity and good processability, thus being an alternative novel biomaterial for soft tissue repair [22]. PBS’s biodegradability makes it ideal to be used for single-use food packaging, too, as it can degrade at high rates over short periods. PBS is already available in direct food contact grades [23] and it was recently introduced to the active food packaging sector; for instance, PBS films modified with quercetin [24], a natural polyphenolic antioxidant, have already been tested as antimicrobial and antioxidant food packaging materials. Moreover, PBS films filled with kesum [25], a new antimicrobial active agent, have been submitted to in vivo direct food contact analysis in a chicken fillet package. The results of both these studies are more than encouraging. Nevertheless, metal-based transesterification catalysts, such as titanium (IV) tert-butoxide, tin (II) 2-ethylhexanoate, antimony (III) oxide, titanium (IV) isopropoxide, titanium (IV) isobutoxide, zirconium (IV) n-butoxide, antinomy (III) n-butoxide, hafnium (IV) n-butoxide, bismuth (III) neo-decanoate, germanium (IV) oxide, as well as high reaction temperatures, are required for conventional PBS synthesis [26]. The use of this kind of catalyst, along with the high reaction temperatures, usually cause the final product’s yellowing—as well as side reactions (e.g., the dehydration of 1,4-butanediol (BDO) to THF) [27,28]. As a result, the removal of catalyst residues is unavoidable, especially when PBS is to be used for biomedical and food packaging applications. On the other hand, another approach is PBS enzymatic polymerization, where non-toxic enzymes are used as catalysts that do not have to be completely removed from the final product, making it appropriate for use in biomedical and (active) food packaging applications.
Poly(ethylene furanoate) (PEF) is a bio-based alternative to PET [29] specifically for packaging applications, presenting superior barrier properties compared to it (19 times decreased CO2 permeability, 11 times decreased O2 permeability, 31 times diffusivity) [30]. To date, PEF is produced via a two-stage melt polymerization method carried out under harsh conditions (e.g., high temperature of 230 °C), in the presence of metal catalysts (Sb, Ti, Ge and Sn) [30,31]. However, this route has several negative effects on the final product’s properties, including coloration, thermal instability and decreased electrical performance. Additionally, potential environmental and health problems may be caused mainly due to the high applied temperatures and the metal catalysts’ residues that are hard to remove [31]. It is of high essence, though, that to obtain a sustainable polymer, the bio-based character of the final product is not sufficient; a sustainable synthetic route, conducted under mild conditions, not requiring the use of metal catalysts (e.g., enzymatic polymerization), can be established and followed for the production of a truly green product.
In light of the above, it is important to focus on sustainable polymerization methods, such as enzymatic polymerization, that can be used to produce sustainable polymers. Indeed, there is an increasing interest in synthesizing bio-based and/or biodegradable polyesters (PLA, PBS, PBF) via enzymatic polymerization. Indicatively, a literature survey was conducted for the period 2008–2021 using the Scopus database (November 2021) and “enzymatic polymerization” as a keyword provided us with 4062 documents. The results were subsequently refined using the keyword “polyesters”, following which the number of documents was reduced to 739. The results were further refined with the use of the keywords “bio-based” and “biodegradable”, after which one hundred and ten (110) documents were retrieved—their histogram appears in Figure 1. Among them almost 17% of papers dealt with the enzymatic synthesis of PLA-, PBS- and furan-based polymers. In 2018, a review paper of the author’s group was published, focusing on the enzymatic polymerization of polycondensation polymers, including polyesters obtained by polycondensation [32]. However, PLA and furan-based polyesters were not included in the analysis, given that their enzymatic synthesis has been developed and presented in literature very recently.
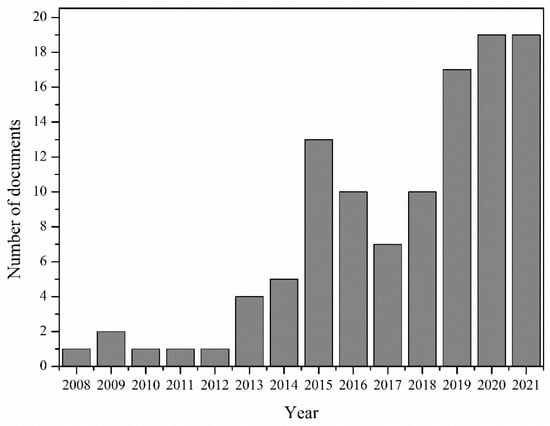
Figure 1.
Published papers at Scopus database for the period 2008–2021 (November).
This paper aspires to present the most recent developments in the enzymatic polymerization of some of the most popular sustainable polymers (PLA, PBS, furan-based polyesters), having a great impact on society, both scientifically and industrially. In this context, the appropriate enzymes to produce each polymer and their main characteristics are presented at the beginning of each chapter. The key experimental conditions of each applied synthesis method are subsequently discussed. The applied synthetic methods are critically evaluated regarding their environmental impact and capability to be used on an industrial scale. Finally, the most crucial monitoring variables of each synthesized polymer are analyzed.
2. Enzymatic Polymerization of Aliphatic Polyesters
2.1. Type and Characteristics of the Used Enzyme for PLA Enzymatic Polymerization
PLA can be enzymatically synthesized via either ROP of lactides or direct polycondensation of lactic acid (Figure 2). ROP is a propagation process of cyclic monomers initiated by different ions; for PLA production, the used cyclic monomer is a lactide, which is the cyclic dimer of lactic acid. Polycondensation of lactic acid occurs by connecting carboxyl and hydroxyl groups, producing water as a by-product [33].
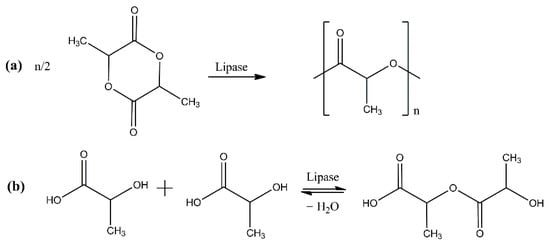
Figure 2.
Enzymatic ROP of lactide (eROP) (a) and polycondensation of lactic acid (b).
In most of the recently published works on the enzymatic polymerization of PLA, eROP is the preferred synthesis method [13,14,34,35,36,37,38,39,40,41,42,43]. The same trend has been observed for the conventional synthesis of PLA, probably due to the wider range of molecular weights that can be achieved via ROP by controlling the purity of lactide. Polycondensation is mostly used to produce PLA with low molecular weight using basic equipment and processes [33]. Given that the enzymatically synthesized polymers usually present low molecular weights, the eROP route seems to be more appropriate for the enzymatic synthesis of PLA. The crucial process parameters will be discussed below.
Hydrolases and, more specifically, lipases are effective catalysts in the enzymatic polymerization of aliphatic polyesters [44]. The most widely used enzyme for the synthesis of PLA—via either eROP of lactones (cyclic esters) or direct polycondensation of lactic acid—is Novozym 435 (N435) [13,14,37,38,40,41,42,43,45], consisting of immobilized Candida antarctica lipase B (CALB) physically adsorbed within the macro-porous resin Lewatit VPOC 1600 (activity 10,000 PLU/g, optimum temperature 30–60 °C) (Table 2). Other commercially available enzymes that have been used for PLA enzymatic polymerization are Candida rugosa lipase (LCR) [34,46] and the lipase from P. fluorescens (lipase AK), as well as lipases from Burkholderia cepacia (LBC) and porcine pancreas (PPL) [36,38,39]. Another approach is to isolate, after screening, a strain of a microorganism that produces the desired enzyme and then isolate it in a crude form in the lab. In this context, Panyachanakul et al. [47] isolated a solvent-tolerant and thermostable lipase-producing actinomycete strain (A3301), identified as Streptomyces sp. The obtained lipase’s activity, optimum temperature and thermostability were 108 U/mL, 60 °C and 45–55 °C, respectively, and it was able to tolerate 10–20% (v/v) hexane and toluene.

Table 2.
Literature data on the enzymatic polymerization of PLA.
The mechanism of the lipases acting during the eROP of lactones has been studied (Figure 3). In brief, the active site of CALB contains the amino acids Ser105–His224–Asp187, with Ser being the nucleophile, His the basic residue and Asp the acidic residue. Thus, a hydrogen-bonding network (serine–histidine and histidine–aspartic acid) is formed. An acyl-enzyme intermediate is formed by the reaction of the serine residue with the lactone, rendering the carbonyl group more prone to nucleophilic attack. The next step, initiation, includes the deacylation of the acyl-enzyme intermediate by an appropriate nucleophile such as water, producing the corresponding ω-hydroxycarboxylic acid/ester. When the nucleophile is an alcohol or amine, the product contains the corresponding ester or amide as an end-group. Finally, chain growth or propagation occurs by deacylation of the acyl-enzyme intermediate by the terminal hydroxyl group of the growing polymer chain to produce a polymer chain elongated by one monomer unit [48]. The mechanism of other types of reactions for the preparation of PLA—including (direct) polycondensation of lactic acid—is similar to the one described above [49], starting with a stage of formation of an enzyme-activated monomer, which further participates in the stages of chain initiation and growth. The nucleophilic attack of the carbonyl carbon atom of the enzyme-activated monomer with the terminal hydroxyl group of the chain leads to the elongation of the polymer chain by one monomeric unit.
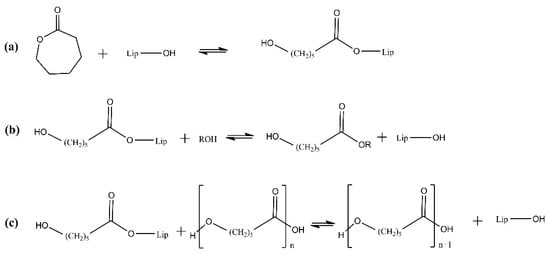
Figure 3.
Mechanism of the lipase-catalyzed ROP of lactones. (a) Formation of the acyl-enzyme intermediate. (b) Initiation. (c) Propagation.
However, eROP of lactones kinetics is usually slow and only some monomers are polymerized [38]. The type of enzyme obviously affects the polymerization rate, given that each enzyme presents specific stability and catalytic activity under the applied experimental conditions. Regarding lactide polymerization, N435 is the most efficient enzyme, presenting the highest activity. Duchiron et al. [38] determined the optimal activity temperature for four different lipases, including N435, by performing a simple esterification reaction and defined the initial reaction rate at different temperatures. As was expected, N435 presented (four times) higher activity compared to the other tested lipases (LBC, LCR, PPL) even at lower temperatures (e.g., 30 °C, 50 °C, 70 °C). The effect of the type of the enzyme, which is strongly related to its activity, can be explained by the fact that the formation of the acyl–enzyme intermediate is the rate-determining step of the enzymatic polymerization [48]. Duchiron et al. [38] conducted eROP of lactide stereoisomers with the use of N435 and Burkholderia cepacia to synthesize PLA. The authors stated that the reaction kinetics were slow compared to metal-based catalysts or organocatalysts, as expected, since 80% monomer conversion was achieved after 24 and 48 h for N435 and LBC, respectively. N435 being more effective compared to LBC resulted in a significant yield (90%) but slow kinetics and generally low molecular mass (less than 4000 g/mol), probably attributed to a weak affinity between lipases binding site and lactide.
Even though in most of the published works on the enzymatic polymerization of PLA, the enzyme is not dried prior use, the water content in the enzyme has been found to play a crucial role in the polymerization process. There are two types of water molecules associated with enzymes; the “essential” or “free” or “hydration” water and the “bond” or “structural” water. Essential water is found at the surface layer of the enzyme, while bond water is embedded inside the enzymes’ molecules [41]. Zhao et al. [41] measured the ‘free water’ content in N435, which was found in the range 0.77–1.97 wt%. The conducted eROP of L-lactide was catalyzed by different batches of N435. The researchers found that the molecular weight of PLA decreased with the increase in initial water content in the immobilized lipase. Only in a few studies is there a reference to the drying process of the enzyme. Indicatively, N435 has undergone drying for one or two days under vacuum at room temperature [13,36] or for 2 h in a high vacuum pump (Vacuubrand, Germany) [43]. Otherwise, it has been stored under vacuum in a desiccator at 4 °C [37]. On the other hand, using high vacuum for the enzyme’s drying may be a significant obstacle to possible process scale-up. Another drying option found in the open literature is freeze-drying [40,42]. Certain enzymes (e.g., Candida rugosa) are commercially available in the form of freeze-dried powders [38]. In this context, Duchiron et al. [38] attempted to increase the PLA chain length and improve reaction kinetics via two different approaches: (a) freeze-drying of the enzyme to significantly reduce the hydration water level without having a negative impact on the structural water content and (b) addition of an amino base, TEA (anhydrous triethyl amine), as a co-solvent to toluene, which was used as solvent for the lactide’s eROP. The first approach aimed at controlling the relative rate of the chain coupling (kcc) reaction concerning the water reaction rate (kw) and the water content in the medium (Figure 4). The second approach was used to increase the relative constant of coupling chain reaction (kcc/kcc′); by adding TEA, the lactide solubility was enhanced and the chain-ends of the formed oligomers were activated (Figure 5a). Due to its slight basic character and nucleophilicity, TEA probably increased the reaction rate by activating the monomer for its ring-opening (Figure 5b). The enzymes’ freeze-drying (approach a) resulted in an increase of 17 and 40% in the for N435 and LBC, respectively, while the TEA addition (approach b) permitted a significant kinetic improvement (reaction was five times faster for N435) and an increase in the molar mass (17% for N435).
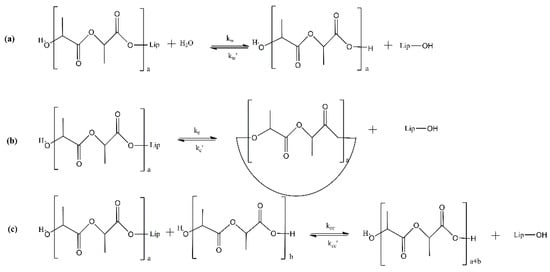
Figure 4.
Reaction pathways for enzymatic ring-opening polymerization of lactide. (a) Water reaction. (b) Cyclisation reaction. (c) Chain coupling reaction.
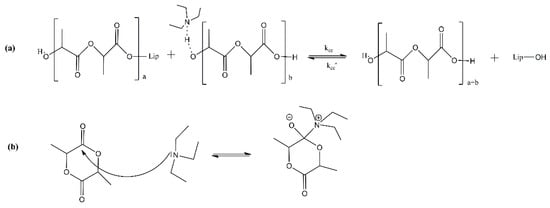
Figure 5.
Oligomer chain-end activation (a) and nucleophilic activation of lactide (b).
Other approaches aiming at the improvement of enzymatic polymerization kinetics include the immobilization of the enzymes. Immobilization of an enzyme aims at avoiding the aggregation of the hydrophilic enzyme molecules, recycling the enzyme to reduce processing costs and preventing the contamination of the product by the enzyme protein [50,51]. Additionally, immobilization enhances the enzyme properties by increasing the enzyme rigidity and heat tolerance, which is caused by low conformational flexibility of the enzyme and is generally indicated by an increase in the optimum temperature and stability against inactivation [52]. When a non-immobilized lipase is submitted to heating, thermal deactivation occurs. The protein unfolding is considered a major cause of this kind of deactivation. However, R. miehei lipase’s thermal deactivation, which was studied by Noel and Combes [53] was attributed predominantly to the formation of aggregates rather than to protein unfolding. Several commercially available enzymes, such as the N435, are adsorbed within resins, improving their stability and efficiency. Düskünkorura et al. [37] conducted eROP of D-, L- and D,L-lactide isomers while comparing free Candida antarctica lipase B (CALB) and its clay- and acrylic resin-immobilized forms. It was concluded that CALB immobilized on neat and organo-modified montmorilonite used for the D-lactide polymerization allowed 70% monomer conversion to be reached after four days but led to small oligomers. This slower polymerization kinetics compared with free CALB was partially attributed to hydroxyl groups at the clay surface that could be involved as co-initiators during polymerization.
Since N435 is immobilized, it can be reused up to ten times for numerous reactions [54]. Loeker et al. [55] studied the eROP of poly(ε-caprolactone) (PCL) in supercritical carbon dioxide; it was found that reusing N435 led to the formation of high molecular weight values ( 34,000–37,000 g/mol), even after the enzyme’s cleaning. On the other hand, the achieved mass yields were lower after the first recycling step. This was attributed to either leaching of the lipase B from the immobilization matrix or denaturation leading to a partial loss in overall catalyst activity. Poojari et al. [56] reported the N435 recovery and reuse for up to ten reaction cycles during PCL synthesis in toluene at 70 °C. High molecular weights ( 50,000 g/mol) were constantly achieved. It was also stated that the multiple reuse cycles of N435 would be unattainable if leaching of CALB occurred due to physical desorption during polymer synthesis or if it was submitted to high mechanical shear (e.g., intense mechanical stirring). Very recently, Adhami et al. [57] conducted eROP of lactones to produce PCL and poly(δ-valerolactone) (PVL) using N435 in a flow tubular reactor. Due to the decreased reaction time of each cycle, the researchers synthesized PCL in toluene at 70 °C while reusing the enzyme more than ten times. More precisely, high efficiency of the enzyme was proved, as it was used about twenty times in a flow tubular micro-reactor without the need for any extra step. The achieved molecular weight and conversion values were slightly lower compared to the fresh enzyme after the eighth run.
2.2. PLA Enzymatic Polymerization Key Process Parameters
The selected enzyme’s concentration is one of the most important parameters of the enzymatic polymerization process. First, it has a considerable impact on the method’s economical sustainability. As already discussed, immobilization aims at the enzymes’ recycling; however, it is crucial to use as little enzyme quantity as possible considering their high current cost [58,59]. In most of the published works N435 and lipases produced by Burkholderia cepacia and Streptomyces sp. A3301 are used, and the selected average concentration is 10% relative to the monomer’s mass [14,36,37,38,39,40,47]. Of course, there are some exceptions; 2 and 3% of Candida rugosa lipase has also been used for eROP and direct polycondensation of lactic acid [34,46], while 20% of N435 (0.5 g of lactide, 100 mg of N435) has been used by Zhao et al. [41] for eROP.
Second, the selected enzyme’s concentration has a significant effect on the obtained product’s properties. Zhao et al. [41] used 2% free CALB (0.5 g of lactide, 10 mg of free CALB) for the eROP of the L-lactide and obtained a product of 2800 g/mol. When the enzyme’s quantity increased to 10% (0.5 g of lactide, 50 mg of free CALB), the achieved of the product was 17,500 g/mol under the same experimental conditions.
Another crucial parameter of the enzymatic polymerization is the type of solvent used when solution polymerization is selected. The most commonly used solvent for both eROP of the lactides and direct polycondensation of lactic acid is toluene [13,35,37,38,42,47]. In the literature, toluene has been evaluated, along with other conventional solvents, based on its environmental risk. Toluene’s environmental risk ranking is 0.7344, while water’s is 1.000 and benzene’s is 0.6098. Generally, toluene is characterized as usable but problematic [60], so not fully aligning with the principles of green polymerization. Toluene is a volatile, non-polar, aromatic hydrocarbon and, along with hexane and benzene, it has been identified as a hazardous air pollutant, carcinogen, mutagen and reprotoxin [61]. Especially in the case of toluene, the primary target organ for chronic exposure is the central nervous system (CNS), gastrointestinal (GI) tract and cardiovascular system, along with the hepatic, renal, hematological and dermal systems [62]. The replacement of toluene with a green solvent could be a solution to this problem. Gu and Jérôme in their review paper [63] formulated the twelve criteria that a green solvent needs to meet: availability, price, recyclability, grade (technical grade solvents are preferred compared to highly pure solvents), synthesis, toxicity, biodegradability, performance, stability, flammability, storage and renewability. It is stated there without being proven that MeTHF, a commercially available solvent produced from renewable resources (lignocellulosic biomass) resembling toluene in many ways in terms of physical properties, could theoretically replace it. However, it should always be considered that there is no absolutely green solvent, as it is a relative term—always depending on the context of each application. The only way to safely evaluate a solvent’s level of greenness is to conduct an LCA [64].
Apart from the sustainability issue, the use of low-polarity toluene is not ideal for eROP since lactides present high polarity and low solubility [43]. In this context, other alternative approaches, including ionic liquids (ILs) and supercritical fluids (SCFs), have been developed. ILs are considered green alternatives as they have a negligible vapor pressure and, as such, do not contribute to the problematic volatile organic compounds [64]. The term ILs includes chemically different solvents and liquid groups with different characteristics—except that they are all salts and liquids at low to moderate temperatures. Probably the most popular IL group contains N,N′-diacyl substituted imidazolium cations combined with a convenient counterion. The cations’ synthesis, however, involves non-green reactions and there is no enzyme known that could cleave the N–C bonds. As a result, these cations are not biodegradable. Two other IL groups are considered greener; the first includes ILs consisting of cations found in the metabolism of living species or derived from them. Such cations can be based on choline, betaine, carnitine or amino acids. The second group includes ILs containing ethylene oxide groups. In contrast to other ILs, where the bulky cations energetically hinder crystallization, their low melting temperature is the consequence of high entropy contributions caused by introducing several ethylene oxide groups. Due to these structural features, these ions show high flexibility and favor the liquid state [64].
However, ILs have attracted great attention in the 21st century due to their ability to replace conventional solvents [60]. Most of the ILs that have been tested for ROP were inappropriate, except for 1-butyl-3-methylimidazoliumhexa-fluorophosphate ([BMIM] [PF6]) [41]. The efficiency of [BMIM] [PF6] could be attributed to the relatively high miscibility of PLA with [BMIM] [PF6] (up to 10% by mass) and its low tendency to decompose the polymer matrix during melt-mixing, as was found by Gardella et al. [65]. [BMIM] [PF6] has been used to some extent in eROP of lactides [40,41]. On the other hand, ILs present certain drawbacks that should be considered. Often their synthesis is not green at all and many of them are not very biodegradable and show significant toxicity. Additionally, their price is relatively high, especially for reasonably pure ILs, as they often present high to very high viscosity that necessitates much effort to synthesize [64].
SCFs have also been used in the eROP of lactides, probably due to the enhanced solubility of the monomer and the growing polyester chains in this kind of media [14,39]. Generally, eROP is conducted in a biphasic media system where the supercritical phase (e.g., supercritical carbon dioxide) coexists with a liquid organic phase mainly composed of melted monomer, wherein the formed PLA chains are soluble [14]. The polarity and hydrophobicity of SCFs such as 1,1,1,2-tetrafluoroethane scR134a contribute to this enhanced solubility of the monomer and subsequent polymeric products [39]. The use of SCFs may, however, influence enzyme activity by a direct pressure effect, which may lead to denaturation, and by the indirect effects of pressure on enzymatic activity and selectivity; when supercritical CO2 is used, lower catalytic activities may be observed due to the formation of carbonic acid [66].
Another approach to developing a green process for the enzymatic synthesis of PLA is the combination of ILs and SCFs. According to Mena et al. [43], the use of a homogenous medium composed of the ionic liquid [C4MIM] [PF6] with miscible compressed 1,1,1,2-tetrafluoroethane (R134a) above the critical points of the mixture led to the successful enzymatic syntheses of linear and hyperbranched PLLA. The molecular weight of the obtained PLLA products were up to three-fold higher than those obtained with the ionic liquid medium (up to 28,000 g/mol).
It is always preferable to avoid using a solvent when it is not necessary. Bulk systems have also been studied in the context of eROP of lactides [36,41,67]. Mälberg et al. [36] observed that lipase from B. cepacia was effective in both bulk and in mini-emulsion eROP, while Zhao et al. [41] who used N435 for the L-lactide, stated that most of the tested organic solvents led to much lower molecular weights and yields compared to the bulk system, with the exception of xylene and DMA at a low concentration. This behavior could be attributed to the fact that the oligo(lactide) solidifies easily in bulk systems, creating a mass transfer barrier for continuing the enzymatic reaction. Additionally, the water content in solvents may lead to unfavorable side reactions, including polyester hydrolysis. On the other hand, the use of a solvent at a low concentration (e.g., 0.25 mL for 0.5 g L-lactide) may be beneficial to produce higher molecular weight PLA. Yoshizawa-Fujita et al. [67], who conducted eROP of the L-lactide, concluded that the achieved monomer conversions and molecular weights for certain ILs were better than the bulk and solution methods’ and they attributed this to the fact that the anion of ILs has a molecular interaction during the polymerization. According to the researchers, the activity of lipases is strongly affected by the anion species of the IL; ILs having [BF4], [PF6] and [NTf2] (from the IL 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)amide or [C4MIM] [NTf2]) anions induce the polymerization activity of lipases. However, chloride and [N(CN)2] anions have exhibited lipase deactivation due to the strong interaction of the lipase with the [N(CN)2] anion. Additionally, stability studies have indicated that lipases exhibit greater stability in ionic liquids than in organic solvents, including hexane [68]. However, the polymer yields were relatively lower than those of bulk polymerization, because PLLAs present great solubility in ILs and it is thus difficult to extract at the end of the process.
The reaction temperature, time and pressure, are also crucial factors for the properties of the product to be obtained. In general, the enzymatic polymerization reaction rate increases with temperature. The melting temperature of the L-lactide is 93 °C, so when eROP takes place in solventless systems, the first step is usually the lactide’s heating until it melts. In light of the above, Rahmayetty et al. [34] tested different temperatures in the range of 70–130 °C for eROP of L-lactide in the presence of Candida rugosa lipase (CRL). They concluded that the highest CRL activity was obtained at 90 °C for 72 h. The researchers noticed that above 90 °C, the lipase underwent discoloration from yellowish-white to dark brown and confirmed that high temperatures caused the denaturation of the proteins of enzymes. Mälberg et al. [36] conducted bulk eROP for PLA synthesis at 100 and 125 °C in the presence of lipase from B. cepacia for seven (7) days. The bulk direct polycondensation method, which was conducted by Rahmayetty et al. [46] in the presence of Candida rugosa lipase, presents great interest regarding the applied experimental conditions; the mixture of the monomer and the enzyme was heated at different temperatures (60–120 °C) and a vacuum pressure of 0.1 bar for 72 h. The researchers found out that the rising temperature causes the rising of viscosity and density of PLA solution. They also confirmed that PLA’s yield is affected by temperature.
The applied reaction temperatures were slightly lower (60–90 °C) when enzymatic polymerization was conducted in toluene [13,35,37,38,42,47]. This could be attributed to toluene’s relatively low boiling point (110 °C). Indicatively, Takwa et al. [35] conducted eROP in the presence of wild type and redesigned CALB at 60 °C for 48 h in D8-toluene, while Panyachanakul et al. [47] conducted polycondensation in the presence of a lipase produced by Streptomyces sp. A3301 at 60 °C for 8 h in toluene under nitrogen atmosphere. Higher temperatures are sometimes applied when ILs are used. For example, Zhao et al. [41] noticed that a temperature of 130 °C led to higher molecular weights and conversions than lower temperatures (80–110 °C) in [BMIM] [PF6], so 130 °C was the selected reaction for the polymerization process that was conducted for 7 days.
2.3. PLA Enzymatic Polymerization Monitoring Variables
The most important parameter to evaluate the level of success of an applied polymerization process is the molecular weight (MW) of the obtained product. Other important parameters include the thermal properties, glass transition, melting temperature, crystallinity and process yield.
The MW of the obtained PLA grades of each study could be classified into three categories; oligomers or low-molecular-weight (<5000 g/mol) PLA grades, moderate-(5000–15,000 g/mol) and high-(>15,000 g/mol) molecular-weight PLA grades.
The lowest obtained number-average molecular weight () of the enzymatically synthesized PLA grades was 525 g/mol, synthesized via polycondensation in the presence of a lipase from Streptomyces sp. (A3301), at 60 °C in toluene by Panyachanakul et al. [47]. Based on their poor properties, the produced short oligomers would not be able to be used for any application. Still, as was suggested by the researchers, they could be applied as prepolymers for PDLLA synthesis in other experiments and applications. Similarly, Rahmayetty et al. [46], who applied direct polycondensation of lactic acid in the presence of Candida rugosa lipase, obtained low-molecular-weight products in the range 1400–1500 g/mol. It could be very difficult to study and suggest a scaling up of this process, given that the use of a relatively high vacuum (0.1 bar) is required. In general, the low molecular weights obtained in these works could be attributed to the applied direct polycondensation, which is known to produce low molecular weight products even when conventional catalysts are used [33,69]. However, it is valuable that new enzymes were isolated and tested for the enzymatic polymerization of PLA and the academic community can undoubtedly make good use of these data.
Lassalle and Ferreira [45] used immobilized CALB in isopropyl ether for 96 h to conduct direct polycondensation. The obtained products were solids, presenting values of between 400 and 2400 g/mol and LA conversion between 22 and 96%. The percentage of the solid PLA was in the range of 2–55%. Since a polymer of these properties can be used for a limited number of applications, a novel thermal treatment consisting of heating at 190 °C, under vacuum, for 24 h was proposed to further increase the , giving promising results.
As regards eROP, Takwa et al. [35] synthesized PLA of 780 g/mol, corresponding to a degree of polymerization (DP) of 4.5 lactide units at 60 °C in toluene. They used the D-lactide and compared the efficiency of the wild type and several mutants of Candida antarctica of lipase B as biocatalysts. They found out that two mutants presented 90-fold increased activity compared to the wild type and the achieved conversion was 89%. Based on a molecular dynamics simulation, the researchers concluded that the larger space created by a specific mutation altered the orientation of the dilactyl moiety in the tetrahedral intermediate, thus improving the deacylation step of the propagation in the ROP reaction drastically. On the other hand, the relevant low molecular weight that was obtained is not in complete accordance with the literature, as it is suggested that CALB has a good selectivity towards D-lactide (better than L-isomer) [35,38].
Rahmayetty et al. [34] performed eROP of L-lactide in the presence of Candida rugosa lipase at 90 °C. The resulting PLA presented of 2854 g/mol at a yield of 93%. The crystallinity of the enzymatically synthesized PLA was 31% and its melting point () 120 °C. It is known that PLA is a semicrystalline polyester with a melting temperature between 170 and 180 °C [70]. The lower melting point of the obtained PLA grade is attributed to its low molecular weight. This product was recommended for biomedical applications.
eROP of D- and L-lactides was conducted by Duchiron et al. and Düskünkorur et al. [37,38]. Both research groups used N435 and selected reaction temperatures of 90 and 115 °C, respectively. Duchiron et al. [38] obtained oligomers of ranging from 500 to 4900 g/mol and confirmed the selectivity of N435 towards D-lactide and Burkholderia cepacia towards L-lactide. The most important finding of this group was the activating role of TEA which, as an aprotic amino base, permitted a significant kinetic improvement (five times faster enzymatic reactions) and an increase in the molar mass of the final product (17%). This approach could reveal new opportunities for lipases’ reverse catalysis and make eROP a competitive method, comparable with classical organometallic catalysis. Düskünkorur et al. [37] achieved the complete conversion of D-lactide to PDLA with of 2600 g/mol.
Moderate-molecular-weight PLA grades were synthesized via eROP by Guzmán-Lagunes et al., García-Arrazola et al. and Hans et al. [13,14,39]. Guzmán-Lagunes et al. [39] and García-Arrazola et al. [14] used the L-lactide and the enzymes Burkholderia cepacia lipase and N435, respectively. It is of great interest that both these groups used SCFs (scR134a and scCO2) to synthesize PLA of 14,000 g/mol (PDI < 2) and up to 12,900 g/mol, respectively. It is of note that the grade of 14,000 g/mol presented crystallinity of a maximum of 35% and melting temperature in the range of 170–180 °C, in agreement with the reported values of PLA [70].
Hans et al. [13] obtained via eROP of D-lactide a narrowly distributed polymer (PDI 1.1) with of 12,000 g/mol. The reaction temperature was 70 °C and the researchers used a monomer/toluene ratio of 1:2 (g:mL). They concluded that upon careful adjustment of the reaction conditions, it is possible to obtain PDLA with reasonable molecular weights in high yields enzymatically with the use of the biocatalyst Novozyme 435.
Zhao et al. [41] conducted eROP at 130 °C with the use of IL [BMIM][PF6] to produce PLA grades of around 20,000 g/mol or around 10,000 g/mol (for an average PDI of 2), meaning medium-molecular-weight polymers. The researchers stated that this type of polyester could be used as the soft block of thermoplastic elastomers or carriers for controlled drug delivery and release.
It is interesting to observe that several high molecular weight PLA grades have been synthesized enzymatically [36,40,42,43]. The highest molecular weight of enzymatically synthesized PLA grades ( 78,100 g/mol) was achieved by Mälberg et al. [36] with the use of a lipase from Burkholderia cepacia at 125 °C for seven days in bulk. Chanfreau et al. [40] used the IL [BMIM] [PF6] to obtain PLLA of 37,800 g/mol. The measured PLLA yield was 63% and it was attained at 90 °C. Omay et al. [42] and Mena et al. [43] synthesized PDLA and PLLA grades of of 26,000 g/mol and 28,000 g/mol, respectively. In both works, the used enzyme was N435, but PDLA was synthesized in toluene at 80 °C, while PLLA was synthesized in a homogenous media composed of the ionic liquid [C4MIM] [PF6] in combination with miscible compressed 1,1,1,2-tetrafluoroethane (R134a). This is very promising, given that the PDIs of the polymers were in the range of 1.1–1.7 (indicating narrow distribution), the percentages of crystallinity of the PLLAs were in the range 43–50% (which is expected for semicrystalline polymers), the Tg of the polymers were in the range of 48.8–62 °C (in agreement with published reports), the melting temperatures ranged between 109 and 138 °C and the degradation temperatures of the polymers were between 155 and 122 °C.
2.4. Type and Characteristics of the Used Enzyme for PBS Enzymatic Polymerization
Since the 1990s, PBS and its copolymers have been highlighted in polymer science, mainly due to their ability to be used in biomedical and food packaging applications. PBS’s biodegradability makes it an appropriate material for soft-tissue repair and tissue engineering, as well as in the single-use packaging sector. However, properties such as crystallinity play a major role in the degradation process and may limit its suitability for soft-tissue applications, mainly due to the relatively slow degradation and resorption rate [71]. Additionally, crystallinity degree and crystalline morphology have significant effects on the gas barrier properties of a packaging material [72]. It is thus important to fine-tune the production process of PBS to obtain residues grades that are free of catalyst, which is appropriate to be used in biomedical and packaging applications while presenting controlled properties such as crystallinity. The most important parameters of the recently conducted enzymatic polymerizations of PBS will be discussed in the following.
N435 has been used for every conducted enzymatic PBS polymerization for the last 20 years (Table 3). There is only one work—published in 1998—where the researchers used the lipase from Rhizomucor miehei to produce biodegradable aliphatic polyesters, including PBS [73]. Even though the results were satisfying and the lipase Rhizomucor miehei generally seems adequate for esterification reactions due to its high stability in anhydrous media and good esterification activity [74], it has not been selected as a biocatalyst for the most recently conducted enzymatic polymerizations on which we will mainly focus.

Table 3.
Literature data on the enzymatic polymerization of PBS.
Enzymatic polycondensation and ring-opening polymerization that have been analyzed above for PLA occur through similar mechanisms and reaction intermediates (acyl-enzyme intermediates) [85]. The lipase mechanism during polycondensation has been described in detail by Hevilla et al. [85] and is depicted in Figure 6. In the first step, the primary alcohol from the nucleophilic serine (–CH2OH) interacts with the carbonyl group of a substrate molecule (carboxylic acid or its esters) RC(=O)–OR′, forming a tetrahedral intermediate (* I). This intermediate gets stabilized through three hydrogen bonds from glutamine (Gln 106, 1 hydrogen bond) and threonine (Thr40, 2 hydrogen bonds). Meanwhile, the imidazole group of His224 residue pulls the proton from Ser105. As a result, the nucleophilicity of the oxygen is increased enough to attack the carbonyl carbon of the substrate. In parallel, the carboxylate group of Asp187 helps the His224 residue to pull the proton. Consequently, the acyl-enzyme complex (AEC, * II), also known as enzyme-activated monomer specie (EAM), is formed while liberating R′O–H (acylating step), which is removed from His224. In the deacylating step, a nucleophile, e.g., a primary alcohol from a diol (R″O–H), reacts with the AEC carbonyl carbon, forming a new tetrahedral intermediate (* III) that is stabilized by the active site. Finally, the enzyme is deacylated and regenerated after releasing the product (ester, polyester).
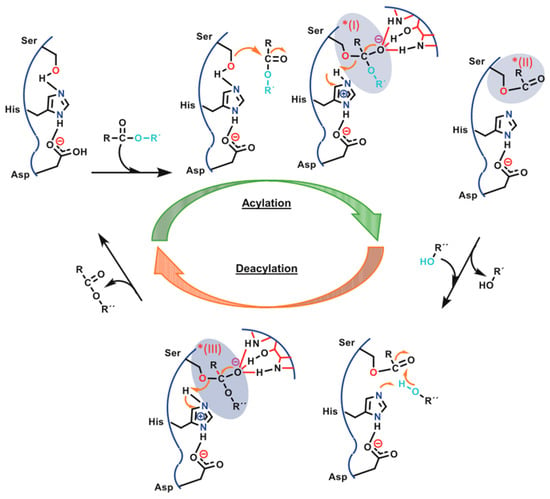
Figure 6.
Enzymatic polycondensation in the presence of CALB. * (I) Tetrahedral intermediate, * (II) acyl–enzyme complex (AEC), * (III) tetrahedral intermediate [85].
It has been observed that OH-rich PBS prepolymers are synthesized via enzymatic transesterification, probably attributed to increased diol concentration in the enzyme’s active site due to sterical factors, given that the BDO carbon content is lower compared to DES [44].
To the best of our knowledge, there are not any completed published studies on the kinetics of PBS enzymatic polymerization. The only exception is a reference to El Fray and Gradzik’s work [77]. In this work, PBS was synthesized via enzymatic polymerization under varying pressures (0.3, 1 and 2 mmHg) in diphenyl ether at 80 °C. The researchers observed that when the pressure of the polycondensation stage changed, the conversion of the reaction corresponding to the kinetics of polycondensation was altered. The highest molecular weight was obtained when the lowest pressure (0.3 mmHg) was applied and the achieved yield under these conditions was 37%. The relevant low yield was attributed to the limited reaction time (9 h) and it was stated that it probably should be extended.
Sonseca et al. [86] synthesized poly(butylene succinate-dilinoleic succinate) (PBS-DLS), a biodegradable copolymer that may be used for applications requiring contact with the human body. The researchers evaluated the kinetics of enzymatic transesterification with the use of variable amounts of hard/soft segments (70 to 50 wt% butylene succinate hard segments and 30 to 50 wt% dilinoleic succinate soft segments). Based on the 1HNMR analysis, it was found that copolymer oligomers had been formed after 3 h of reaction (under atmospheric pressure and N2). Additionally, the end groups of BDO, dimer linoleic diol (DLAOH) and DES were also identified; after 24 h of total reaction and the application of slight vacuum (600 Torr), the end groups from DLAOH disappeared and the proton signals from ester formation increased, indicating that DLAOH was incorporated into the chains of the oligomers. At the end of this low-vacuum stage, the BDO and DES end groups also decreased, indicating the conversion of the initially formed products into higher molecular weight oligomers. In the next step, the vacuum pressure increased and a more than two-fold increase in of all copolymers was observed from 21 to 48 h reaction time. It was concluded that the DLAOH had a significant effect on the molecular weights of the copolyesters; the high incorporation of DLAOH into the PBS backbone led to the formation of copolyesters of increasing molecular weights with an increasing amount of soft segments. It was also found that the efficacy of the DLAOH incorporation was limited by the catalytic pocket size of the enzyme and its hydrophobic nature, favoring the formation of long PBS sequences—especially at the early synthesis stages.
For N435 and PBS, it has been confirmed that the enzymes’ drying before use is crucial for the polymerization process, as four modes of reversible reactions may occur during the lipase-catalyzed polyester synthesis, inducing hydrolysis, esterification, transesterification (alcoholysis and acidolysis) and interesterification [30]. On the other hand, even though a dry enzyme is usually more efficient and better resistant against microbiological degradation or chemical inactivation [87], the use of vacuum for the enzyme’s drying is a limiting factor to a polymerization process scaling-up. In addition to that, the use of a solid, dried enzyme presents more drawbacks. Considering the cost aspect, it is quite expensive to evaporate water to obtain the enzyme in the solid form. Additionally, the enzyme could be inactivated by the drying process. Thus, liquid enzyme formulations are an alternative to the use of solid enzymes. The choice of solvent system of the liquid formulation is very important. Even though water is the common solvent for enzymes, co-solvents are often added to improve the stability of the native protein. They are often added in high concentrations and can either be other solvents (e.g., glycerol) or solids (e.g., sucrose), aiming to reduce water activity [87].
However, the usage of dried enzymes in enzymatic polymerization of PBS is widespread; Pellis et al. [75] dried N435 under vacuum for 96 h at 25 °C and stored it in a desiccator before use, while Jiang et al. [79] placed CALB into a 25 mL flask and stored it in a desiccator with phosphorus pentoxide at room temperature under high vacuum for 16 h. P2O5 was also used by Sugihara et al. [84] for the enzyme drying under vacuum at 25 °C for 2 h. Azim et al. [80] and El Fray et al. [77] used N435 predried under 0.1 mmHg vacuum at 25 °C for 24 h
Enzymatic polymerization’s kinetics are usually slow and thus immobilization is an approach aiming at its improvement. Interestingly, the activity of polymer-immobilized CALB (N435) may depend on the enzyme storage time, as was stated very recently by Pospiech et al. [81]. The researchers used lipase CALB for melt polycondensation of aliphatic polyesters including PBS and observed that the enzymatic activity of immobilized CALB, which influenced the molar mass of the obtained products, was affected by the storage time. The activity of CALB at 50 °C stored for 2 years was found to be 1400 ± 100 U/g while fresh CALB’s was 2900 ± 700 U/g. PBS grades of 11,000 g/mol and 2900 g/mol were synthesized using fresh and 2 year-stored CALB, respectively. Even though the measurements’ standard deviations were significant (7–24%), the enzyme’s degradation could probably be attributed to inappropriate storage conditions, e.g., temperature above 25 °C.
The reusability of N435 during transesterification reactions has been studied by Nasr et al. [88]. The researchers used 1,6-hexanediol and diethyl adipate in bulk and solvent (diphenyl ether) systems to produce enzymatically poly(hexylene adipate). Constant values of the polyester were achieved over three consecutive cycles in bulk, even at relatively high temperatures (100 °C), while a 17% decrease in the values was observed in the solution system. The decreased N435 activity was attributed to the better heat transfer in the solution system compared to the bulk, rendering the enzyme more prone to elevated temperatures—leading to degradation or leaching. Additionally, given that N435 is prepared via the interfacial activation of lipases to support hydrophobic surfaces, it becomes more susceptible to release in the presence of organic solvents such as diphenyl ether.
2.5. PBS Enzymatic Polymerization Key Process Parameters
PBS has been synthesized enzymatically via esterification or transesterification of succinic acid or diethyl succinate, respectively. In contrast to the conventional PBS synthesis, which usually includes esterification, transesterification is the most commonly applied enzymatic polymerization route. This is attributed to the fact that lipases, including N435, present higher specificity to esters and alcohols than acid substrates.
Typical monomers that have been used for PBS enzymatic polymerization via transesterification are BDO and diethyl succinate (DES) or dimethyl succinate (DMS) [75,77,78,79,80,82,84]. A commercial-scale process to produce bio-based BDO (from sugar) has already been developed and published [89], while bio-based DES has already been used for PBS enzymatic co-polymerization with dilinoleic succinate [90]. The use of these bio-based monomers is completely in line with the principles of green polymerization.
The selected enzyme’s concentration for PBS enzymatic polymerizations is 10–40% relative to monomers [75,77,78,79,80,82,83,84]. Most researchers, especially in recent studies, have used only 10% wt relative to monomers. Considering the principles of the circular economy, the use of a small quantity of reusable materials, including enzymes, is always desirable. On the other hand, using large quantities of enzymes would significantly increase the cost of the whole process. Moreover, it is well known that small quantities of an enzyme compared to their substrate are sufficient to lower the activation energy of the reaction during catalytic action [91].
A crucial parameter for equilibrium reactions is removing the polycondensation by-product. The use of diesters such as DES or DMS instead of dicarboxylic acids can shift the equilibrium towards polymerization, as it results in more volatile by-products (alcohols) compared to water. In this sense, in most of the conducted PBS enzymatic polymerizations, two-stage polymerization routes with increasing vacuum/temperature are applied to achieve higher-molecular-weight polymers.
Pellis et al. [75] conducted two-step enzymatic polymerizations; the first stage was conducted at 85 °C for 5 h under atmospheric pressure. Subsequently, a vacuum of 20 mbar was applied for 18 more hours while maintaining the reaction temperature at 85 °C. At the end of the first stage, oligomerization was achieved, while during the second stage, the vacuum effectively removed the alcohol by-product and enabled the elongation of the polymeric chain. Kanelli et al. [78] conducted PBS enzymatic polymerization through a two-stage route. The first was applied at 75 °C for 2 h under nitrogen atmosphere considering BDO high volatility and the second at 75 °C for 2 h under a vacuum of 20 mbar. The researchers also observed that the product’s morphology was improved when prolonging both stages (first stage: 5 h and second stage: 24 h). In the work of Azim et al. [80], the polycondensation reaction took place for 21 h and then the polymerization temperature increased from 80 to 95 °C. During the second stage, the polymer remained soluble so that the reaction proceeded in a single liquid phase that facilitated a further molecular weight increase. Sugihara et al. [84] applied direct polycondensation of BDO with dimethyl succinate at 100 °C for 24 h. They concluded that the ring-opening polymerization of lactones is probably a better method when aiming at high-molecular-weight polyesters, including PBS. The achieved molecular weight was low and it was attributed to the reverse reaction and transesterification. A two-stage method was also selected by el Fray et al. [77]. The reaction occurred at 80 °C for 2 h under atmospheric pressure, which was subsequently reduced to 0.03 mmHg. As a result, the obtained polyesters’ molecular weight and melting points increased while the number of –OH groups decreased.
The selected technique is solution polymerization in most of these works and the most commonly used solvent is diphenyl ether [73,77,78,79,80]. The extensive use of diphenyl ether could be attributed to its high boiling point, permitting the application of a high vacuum to remove by-products. On the other hand, the use of various solvents (e.g., diphenyl ether for solution polymerization, chloroform for filtration, methanol for precipitation) and the requirement of high vacuum for by-product removal are drawbacks that may impede the processes’ scaling up [92]. For instance, Azim et al. [80] used diphenyl ether as a solvent, while An et al. [82] applied a very high vacuum (10 mmHg)—both at relatively high reaction temperatures (>80 °C). The authors’ group recently developed a PBS enzymatic prepolymerization route that can be realistically scaled up [44].This is attributed to the use of low-boiling point solvents (isooctane or toluene) that are easy to remove by the system (Tb = 99 °C, Tb = 110.6 °C, respectively), require milder reaction conditions (40–60 °C, atmospheric pressure) and do not demand the use of many other solvents for the product’s isolation, in contrast to the high-boiling-point diphenyl ether.
2.6. PBS Enzymatic Polymerization Monitoring Variables
The achieved molecular weight of an enzymatically synthesized polymer is one of the most important parameters to evaluate the efficiency of the selected enzyme and the applied process. The MW of the synthesized PBS grades will be classified into three categories; oligomers or low-molecular-weight (<10,000 g/mol) PBS grades, moderate- (10,000–80,000 g/mol) and high- (>80,000 g/mol) molecular-weight PBS grades.
The enzymatically synthesized PBS grade with the lowest weight-average molecular weight (1094 g/mol) was obtained in a bulk system kept at 85 °C for 24 h (under atmospheric pressure the first 6 h and 20 mbar for 18 h) [75].The researchers tested different diesters and concluded that there is a strong effect of the selected alkyl group of the diester (dimethyl, diethyl and dibutyl) for all polyesters; more precisely, lower molecular weights and monomer conversions were obtained using dibutyl esters since it was more difficult to remove the butanol by-product during the reaction due to its higher boiling point (relative to methanol and ethanol). Uyama et al. [76] synthesized low molecular-weight PBS 2550 g/mol) in a bulk system at 60 °C. The researchers confirmed that the methylene length of the monomers strongly affected the polymerization characteristics. Other low-molecular weight PBS grades ( 2500–7000 g/mol) were also obtained through solution polymerizations; diphenyl ether was used [77,78]. El Fray et al. [77] obtained PBS of 2500 g/mol and Tm 110 °C at a reaction temperature of 80 °C and pressure of 0.3 mmHg for 9 h with the use of diphenyl ether. They confirmed that the decreased pressure during the polycondensation reaction increased the molecular weight, reduced the number of –OH groups and increased the melting point of the products. However, the achieved process yield (app. 40%) and the molecular weight were low, indicating that the reaction time should be extended. Kanelli et al. [78] synthesized a sticky PBS grade of 3900 g/mol, which was subsequently upgraded via solid-state post-polymerization. The SSP process was examined under reduced pressure for 24 h at 84 °C and a simultaneous increase in Tm and ΔH was observed, implying a crystal reorganization/perfectioning. Consequently, a two-step SSP process was conducted for 36 h; the first step, which served as a pre-crystallization step, was conducted at 77 °C for 24 h while the second was conducted at 90 °C for 12 h. The molecular weight and Tm were significantly enhanced ( with simultaneous improvement of prepolymer physical characteristics as the stickiness disappeared. Gkountela et al. [44] recently synthesized PBS of 2000 g/mol and Tm 78 °C, in isooctane at 50 °C. This prepolymer was subsequently submitted to a post-polymerization stage, including a first step of heat treatment at 80 °C for 2 h and then the second step at 90 °C for 8 h to upgrade its quality in terms of molecular weight and thermal properties. The final product presented an increase of 26 °C in the Tm and 126% in the .
Concerning the moderate-molecular-weight grades, Jiang et al. [79] synthesized fully bio-based poly(butylene succinate) and poly(butylene succinate-co-itaconate) given that all used building blocks and catalysts were generated from renewable resources. The synthesized PBS grade presented a of 11,500 g/mol and a Tm of 112.9 °C, which is very close to a commercial PBS Tm of 75,000 g/mol (ca. 114 °C [93]). The applied process included a first step at 80 °C for 2–24 h under atmospheric pressure and a nitrogen atmosphere. A second step under very low pressure (2–40 mmHg) was applied for another 94 h at the same temperature. Azim et al. [80] synthesized PBS of 38,000 g/mol. However, the researchers used diphenyl ether as the solvent and applied a very high vacuum; the process was carried out first at 80 °C for 5–21 h at 1.8–2.2 mmHg to form PBS oligomers and subsequently the reaction temperature was raised to 95 °C at 1.8–2.2 mmHg. An et al. [82] also applied a very high vacuum of 10 mmHg at 95 °C for 25 h to produce PBS with a molecular weight of 44,000 g/mol. Ren et al. [83] applied a two-step route for the synthesis of bio-based PBS using succinic anhydride and BDO. In the first step, the polycondensation reaction took place for 12 h at 95 °C in the presence of succinic acid. The nucleophilic attack to succinic anhydride by BDO facilitated the polycondensation via esterification between di-acidic and diol units. A linear oligomer was formed, enabling the solvation of monomers and paving the way for the ensuing lipase-catalyzed polymerization in the presence of N435 in toluene. The researchers did not apply vacuum to remove the by-product but a hydrous azeotrope (water with toluene) was formed, facilitating the reuse of both the lipase and the solvent while minimizing energy consumption. The obtained PBS grade presented 73,000 g/mol. It is of high essence that the recovered lipase catalyst was tested and presented a similar performance after six cycles.
An interesting approach to synthesize high-molecular-weight PBS ( 130,000 g/mol) was suggested by Sugihara et al. [84]. The researchers developed a new strategy for the green production of bio-based plastics, including PBS. In the first step, a cyclic oligomer was produced by the lipase-catalyzed condensation of dimethyl succinate and BDO in a dilute toluene solution. In the next step, ring-opening polymerization of the cyclic oligomer took place in a more concentrated solution or in bulk with the same lipase. The conventional, direct polycondensation route was compared to this new strategy. In this context, PBS was synthesized enzymatically through polycondensation of BDO and dimethyl succinate. During polycondensation, methanol was removed using molecular sieves 4Å and the obtained polyester presented a of 45,000. However, it was stated that lipase itself contains water and thus complete removal of the small molecules, such as methanol and water, is difficult. Therefore, the researchers concluded that to obtain a higher molecular weight polyester the ring-opening polymerization of cyclic oligomers is the most effective route for such a lipase-catalyzed polymerization.
3. Enzymatic Polymerization of Alipharomatic Polyesters
3.1. Type and Characteristics of the Used Enzyme for Furan-Based Polyesters Enzymatic Polymerization
The chemical 2,5-Furandicarboxylic acid (FDCA) is a bio-based compound consisting of two carboxylic acid groups attached to a furan ring. FDCA’s chemical structure, along with its thermal stability and aromatic nature, make it capable of replacing terephthalic acid, which is used to produce polymers such as PET and poly(butylene terephthalate) (PBT). FDCA is one of the monomers for poly(ethylene 2,5-furandicarboxylate) (PEF) and poly(butylene 2,5-furandicarboxylate) (PBF) [94]. Avantium catalytically converts plant-based sugars into FDCA to produce 100% plant-based, 100% recyclable and degradable PEF, which presents superior performance, including barrier properties, compared to PET. According to Gert-Jan M. Gruter and Thomas B. van Aken from Avantium [95], “a strong barrier preventing oxygen from entering the bottle (relevant for juice and beer) and CO2 leaving the bottle (relevant for any carbonated beverage) was an unmet need in the market”. As a result, special coatings, additives (scavengers) or nylon layers were used for PET bottles to improve this barrier. This practice increased the process and final product’s cost and made PET’s recycling more difficult. PEF monolayer was a great step toward solving this unmet market need.
Furan-based polyesters have been gaining popularity very recently and their conventional synthesis has started to be studied. Papadopoulos et al. [96] synthesized PEF from FDCA and ethylene glycol (EG) and tested two different antimony catalysts (antimony oxide and antimony acetate) and different reaction temperatures while applying a two-step polycondensation method. The authors concluded that for the first step of the polymerization, i.e., the esterification of FDCA with EG to afford PEF oligomers, Sb2O3 was more active than Sb(CH3COO)3 at lower temperatures (160 °C), while in the second step of the polymerization, Sb2O3 exhibited the highest activity. Zhu et al. [97] synthesized PBF with the use of titanium tetraisopropoxide (Ti[OCH(CH3)2]4) as a catalyst at temperatures in the range 150–200 °C by melt condensation polymerization. It was concluded that the synthesized PBF presented excellent thermal stability, strength and ductility and could probably replace PBT. Poulopoulou et al. [98] synthesized bio-based PBF with the use of tetrabutyltitanate (TBT) by melt polycondensation. The researchers also prepared blends with its terephthalate counterpart, PBT, and studied the thermal properties of the homopolymers and the blends by employing both conventional and fast scanning calorimetry. Thus, amorphous samples were obtained to reveal the glass transitions of the polymers and improve the blends’ miscibility.
It is important, though, to investigate all the alternative furan-based polyesters’ synthetic routes that could provide us with truly green products—in terms of performance properties and synthesis processes. In that sense, even though furan-based polyesters’ enzymatic synthesis has not been studied greatly, the most important parameters of the synthesis processes will be discussed to provide valuable guidance on their enzymatic synthesis in the coming years.
The only enzyme that has been used for the synthesis of the furan-based polyesters so far is Novozyme 435 (N435) (Table 4). Even though numerous aliphatic polyesters have already been successfully synthesized via N435-catalyzed polymerization, the number of enzymatically synthesized alipharomatic and aromatic polyesters is limited. This is attributed to the high melting temperature (Tm) of alipharomatic and aromatic polyesters and their low solubility in the reaction media, as well as the lack of reactivity of aromatic monomers in enzymatic polyesterification [30].

Table 4.
Literature data on the enzymatic polymerization of furan-based polymers.
It can be assumed that the enzymatic production of furan-based polyesters in the presence of lipase N435 occurs through the already-described enzymatic polycondensation mechanism and reaction intermediates. Maniar et al. [100], who used dimethyl 2,5-furandicarboxylate (DMFDCA), 2,5-bis(hydroxymethyl)furan (BHMF), aliphatic linear diols and diacid ethyl esters to synthesize furan-based copolyesters, studied the synthetic mechanism of the copolymerization in detail, firstly by varying the aliphatic linear monomers: the methylene units of the tested diols were 4, 6, 8, 10 and 12. The results indicated that Candida antarctica lipase B (CALB) prefers longer linear diols (n = 8, 10 and 12) compared to shorter linear ones (n = 4 and 6). The copolyester P(FMF-co-OF), synthesized with the use of 1,8-ODO, presented the highest degrees of polymerization. The researchers observed that when the chain length was decreased to 6 and 4 (1,6 hexanediol (or 1,6-HDO) and 1,4-BDO), the and of furan-based copolyesters significantly decreased. A second approach of the researchers to evaluate the synthetic mechanism was by changing the aliphatic monomers from diols to diacid ethyl esters. Based on the obtained results, a significant decrease in the molecular weight was observed. In light of the above, the researchers presented the proposed copolymerization mechanism, depicted in Figure 7. The polymerization starts with the formation of the acyl–enzyme complex and continues with polycondensation. During the polycondensation, an intermediate product (b) that can inhibit the polymerization is formed. Steric hindrance of (b) creates structure incompatibility with the enzyme active site and consequently the polymer growth is terminated. When diacid ethyl ester is used, the OH functionality in (b) may also transform into BHMF ether, eventually terminating the copolyester chain elongation.
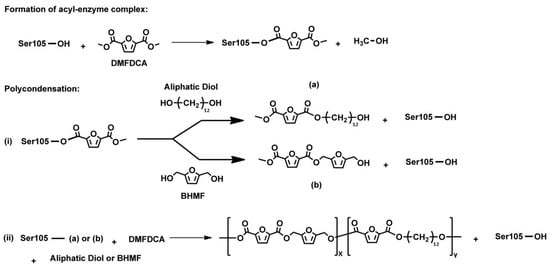
Figure 7.
Proposed copolymerization mechanism of CALB-catalyzed formation of P(FMF-co-DOF) [100].
The enzymatic polymerization kinetics of furan-based polyesters has not been studied yet. However, Jiang et al. [102] studied the kinetics of the enzymatic synthesis of 2,5-furandicarboxylic acid-based alipharomatic polyamides. The relevant conclusions could be valuable information for a future furan-based polyester enzymatic polymerization kinetics study. The researchers produced a series of FDCA-based alipharomatic polyamides using N435, bio-based DMFDCA and aliphatic diamines differing in chain length (C4–C12).
A one-stage method (90 °C in toluene) was applied and the researchers found that N435 presented the highest selectivity towards 1,8-octanediamine (1,8-ODA) among the tested aliphatic diamines. Based on the results of the enzymatic polymerization kinetics study, increases significantly with the increase in polymerization time. Additionally, phase separation of the FDCA-based oligoamides/polyamides occurs in the early stage of polymerization and the resulting product undergoes a subsequent enzymatic solid-state polymerization. However, due to the phase separation and the low efficiency of the enzymatic solid-state polymerization, a large proportion of the resulting polyamides possess low molecular weights. As a result, less than 50% of the products can be obtained after purification.
The water content seems to be the most important factor for the enzymes’ efficiency in the polymerization processes. Jiang et al. [99], who produced furanic-aliphatic polyesters including PBF, used CALB, which was predried overnight in the presence of phosphorus pentoxide (P2O5) at room temperature under high vacuum. Comerford et al. [92], who produced oligoesters such as PBF, used CALB, which was submitted to drying under vacuum for 48 h at 25 °C and stored in a desiccator prior to use. Maniar et al. [100], who synthesized furan-based copolyesters and furanic-aliphatic poly(ester amide)s, used CALB predried in the presence of P2O5 in a desiccator at room temperature under high vacuum for 16 h. In addition to eliminating the enzyme’s water content, selecting an immobilized enzyme such as N435 aims to improve the enzymatic polymerization kinetics, which is expected to be slow.
3.2. Furan-Based Polyesters Enzymatic Polymerization Key Process Parameters
DMFDCA is a bio-based diester that is used as a monomer in all the furan-based enzymatic polymerizations [92,99,100,101]. This is attributed to its better solubility in the reaction medium (e.g., diphenyl ether) under mild conditions and the lower melting temperature of DMFDCA (Tm = 112 °C) than FDCA (Tm = 342 °C) [99]. It is also known that lipases including N435 present higher specificity to esters and alcohols than acid substrates. Various potentially renewable aliphatic diols, BHMF and diamines or amino alcohols are used too as monomers/comonomers.
There does not seem to be a specific trend regarding the most appropriate enzyme concentration. For PBF synthesis, Jiang et al. [99] used 0.4 g of CALB with 5.4304 mmol (0.999 g) of DMFDCA corresponding to 40 wt% of the monomer, while Comerford et al. [92] used 10% of the total amount of the monomers. Maniar et al. [100,101] used 20 wt% and 15 wt% in relation to the total amount of the monomer for the synthesis of furan-based copolyesters and furanic-aliphatic poly(ester amide)s, respectively.
The by-product removal, strongly affecting polycondensation reactions’ equilibrium, is achieved firstly with the use of diesters, resulting in many volatile by-products (alcohols) compared to water from dicarboxylic acids, and secondly with the application of vacuum, usually via a two-stage synthesis route. Jiang et al. [99] conducted enzymatic polymerization of DMFDCA and different aliphatic diols in diphenyl ether and tested two different synthetic routes. The first route included a first stage at 80 °C for 2 h under an atmospheric nitrogen environment and a second at 80 °C under reduced pressure (2 mmHg) for 72 h. The second tested route was a temperature-varied two-stage method; the first stage was performed at 80 °C for 2 h under a nitrogen atmosphere. In the second stage, the pressure was reduced to 2 mmHg while maintaining the reaction temperature at 80 °C for the first 24 h. The temperature was subsequently increased to 95 °C for another 24 h. Finally, the reaction temperature was regulated at 95, 120 or 140 °C for the last 24 h. Based on the enzymatic synthesis process that was applied by Comerford et al. [92], the reaction occurred at 50 °C for 6 h at a pressure of 1000 mbar. A vacuum of 20 mbar was subsequently applied for an additional 18 h, maintaining the reaction temperature at 50 °C. The total reaction time was 24 h. According to Maniar et al. [100], in the first step of the reaction, the monomers mixture (e.g., DMFDCA, BHMF, diethyl succinate) in the presence of diphenyl ether was heated to 80 °C for 2 h under a nitrogen atmosphere. In the second stage, the pressure was reduced stepwise to 2 mmHg while maintaining the reaction temperature at 80 °C for the first 48 h. Finally, the reaction temperature was increased to 95 °C under full vacuum for the last 24 h. Maniar et al. [101] produced furanic-aliphatic poly(ester amide)s via a one-stage method; the reaction took place at 90 °C in the presence of toluene under a nitrogen atmosphere for 72 h.
The main disadvantage of the applied enzymatic polymerization/copolymerization methods is using the high-boiling point diphenyl ether as already discussed, along with the high vacuum requirement, impeding a process scale-up. Lower-boiling point toluene for the furanic-aliphatic poly(ester amide)s synthesis seems more promising; however, toluene is still not a green solvent. In this context, Maniar et al. [101] performed the polymerization to produce furanic-aliphatic poly(ester amide)s in an ionic liquid ([BMIM] [PF6] and [EMIM] [BF4] or 1-ethyl-3-methylimidazolium tetrafluoroborate) as the reaction solvent.
3.3. Furan-Based Polyesters Enzymatic Polymerization Monitoring Variables
The enzymatically synthesized PBF grades presented weight-average molecular weight values of 600–5500 g/mol. Comerford et al. [92] synthesized the lowest molecular weight PBF grade ( 600 g/mol, 500 g/mol) and the polymerization was conducted in a solventless system. The achieved low molecular weight could probably be attributed to the selected polymerization method. More precisely, higher molecular weight polymers are usually synthesized enzymatically via solution polymerization, given that the solution reaction system presents lower viscosity, decreasing diffusion constrictions among polymer chain reactants, monomers and oligomers. On the other hand, in bulk, the reaction medium has a higher viscosity, reducing the diffusion of the reactants [103].To upgrade the enzymatically synthesized PBF oligoester, the researchers subsequently performed a thermal, catalyst-free treatment. A 3.3 times higher than the initial oligomer was achieved. Jiang et al. [99] synthesized via a two-stage method (1st stage: 80 °C, 2 h, N2 atmosphere and 2nd stage: 80 °C, 72 h, 2 mmHg) a PBF grade of 1700 g/mol and 1200 g/mol. Considering that the major obstacle for enzymatic synthesis of furanic–aliphatic polyesters at mild temperatures is the phase separation, mainly caused by the high Tm and the low solubility of the final products, the researchers performed the enzymatic polymerization at higher reaction temperatures. Thus, they applied a temperature-varied two-stage method (1st stage: 80 °C, 2 h, N2 atmosphere and 2nd stage: (a) 80 °C, 24 h, 2 mmHg, (b) 95 °C, 24 h, (c) 95, 120 or 140 °C, 24 h). When the reaction temperature of the last 24 h of the 2nd stage (c) was increased from 80 °C to 140 °C, the corresponding and values increased from 1700 and 1200 g/mol to 5500 and 1600 g/mol, respectively. The significant increase in molecular weights with reaction temperature is first attributed to the fact that phase separation was delayed at higher reaction temperatures. It can also be attributed to the fact that eliminating alcohol by-products and the residual water is easier at higher temperatures, thus improving the enzymatic polycondensation efficiency. Finally, the mobility of the amorphous phase of furanic-aliphatic polyesters, which is enhanced at higher temperatures, facilitates the CALB-catalyzed solid-state polymerization. Concerning the melting point of the synthesized PBF grade, it increased from 145 to 168 °C when its increased from 1700 to 5500 g/mol. This indicates that the chain growth of PBF was limited at the tested temperatures. As a result, the molecular weights of the obtained PBF increased with reaction temperature but could not reach higher values.
Maniar et al. [100] used DMFDCA, BHMF and different aliphatic linear diols to synthesize various furan-based copolyesters with up to 35,000 g/mol. More precisely, when 1,8-ODO was used, P(FMF-co-OF) with and 122 and 269 was formed, which was the highest amongst the tested aliphatic diols.
The first novel FDCA-based poly(ester amide)s were enzymatically synthesized by Maniar et al. [101] and poly(dodecamethylene furanoate-co-dodecamethylene furanamide) (PEAF12) presented the highest (21,000 g/mol). This material presented a Tm of 92 °C and a Td of 395 °C, comparable to PEF, which presents a Tm of 211 °C and a Td of around 389 °C [103].
4. Conclusions
The enzymatic polymerization of bio-based and/or biodegradable polymers has become more and more popular. This is attributed to the fact that bio-based and biodegradable materials are not sufficient; sustainable methods such as enzymatic polymerization must be applied to obtain truly sustainable products.
As regards the aliphatic polyesters PLA and PBS, their enzymatic synthesis has been studied to a sufficient extent. Ring-opening polymerization (eROP) and polycondensation are the most popular synthetic methods for the enzymatic synthesis of PLA and PBS, respectively. As regards eROP, the slow rate of enzymatic polymerization and the use of solvents such as toluene are the main drawbacks of the applied processes. The use of green solvents such as ionic liquids (IL) has been tested and seems promising to provide high molecular weight PLA grades. This is attributed to the increased stability of lipases in ionic liquids. However, the most commonly used ILs’ synthesis is not entirely green and also comes with the drawbacks of decreased biodegradability and high price. With respect to PBS enzymatic polycondensation, one of the most crucial parameters of the applied processes is by-product removal, which strongly affects the reaction equilibrium. High-boiling point diphenyl ether is the most commonly used solvent, permitting the application of a high vacuum to remove polycondensation by-products. The use of low-boiling point solvents, which are easy to remove from the system, is a promising new approach.
Furan-based polyesters are novel materials and their enzymatic polycondensation has not been studied in detail. However, PBF has been synthesized enzymatically both in bulk and solvent (diphenyl ether) systems with the use of N435. Other conducted approaches include the synthesis of furan-based copolyesters and furanic-aliphatic poly(ester amide)s with the use of toluene and ionic liquids, respectively. The obtained results indicate that the furan-based polyester enzymatic synthesis needs to be studied in more detail.
Author Contributions
Conceptualization, C.I.G. and S.N.V.; investigation, C.I.G.; writing—original draft preparation, C.I.G.; writing—review and editing, C.I.G. and S.N.V.; supervision, S.N.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Glossary
| BDO | 1,4-butanediol |
| BHMF | 2,5- bis(hydroxymethyl)furan |
| [BMIM][PF6] | 1-butyl-3-methylimidazoliumhexa-fluorophosphate |
| CALB | Candida antarctica lipase B |
| [C4MIM][NTf2] | 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)amide |
| [C4MIM][PF6] | 1-butyl-3-methylimidazolium hexafluorophosphate |
| CNS | central nervous system |
| DES | diethyl succinate |
| DLAOH | dimer linoleic diol |
| DMA | N,N-Dimethylacetamide |
| DMFDCA | dimethyl 2,5-furandicarboxylate |
| DMS | dimethyl succinate |
| number-average degree of polymerization | |
| weight-average degree of polymerization | |
| EG | ethylene glycol |
| [EMIM][BF4] | 1-ethyl-3-methylimidazolium tetrafluoroborate |
| eROP | enzymatic ring opening polymerization |
| FDCA | 2,5-furandicarboxylic acid |
| GI | gastrointestinal |
| 1,6-HDO | 1,6 hexanediol |
| [HMIM][PF6] | 1-hexyl-3-methylimidazolium hexafluorophosphate |
| ILs | ionic liquids |
| LBC | lipase from Burkholderia cepacia |
| LCR | lipase from Candida rugosa |
| number-average molecular weight | |
| weight-average molecular weight | |
| MW | molecular weight |
| N435 | novozym 435 |
| 1,8-ODA | 1,8-octanediamine |
| PBF | poly(butylene 2,5-furandicarboxylate) |
| PBS | poly(butylene succinate) |
| PBT | poly(butylene terephthalate) |
| PCL | poly(ε-caprolactone) |
| PDLA | Poly(D-lactic acid) |
| PEAF12 | poly(dodecamethylene furanoate-co-dodecamethylene furanamide) |
| PEF | Poly(ethylene furanoate |
| PET | poly(ethylene terephthalate) |
| P(FMF-co-OF) | poly(2,5-furandimethylene furanoate-co-octamethylene furanoate) |
| PLA | poly(lactic acid) |
| PLLA | poly(L-lactic acid) |
| PPL | lipase from porcine pancreas |
| PS | polystyrene |
| PVL | poly(δ-valerolactone) |
| R134a | 1,1,1,2-tetrafluoroethane |
| ROP | ring-opening polymerization |
| scCO2 | supercritical carbon dioxide |
| SCFs | supercritical fluids |
| Tb | boiling temperature |
| TEA | triethyl amine |
| Tg | glass transition temperature |
| THF | tetrahydrofuran |
| Tm | melting temperature |
References
- Nakajima, H.; Dijkstra, P.; Loos, K. The Recent Developments in Biobased Polymers toward General and Engineering Applications: Polymers That Are Upgraded from Biodegradable Polymers, Analogous to Petroleum-Derived Polymers, and Newly Developed. Polymers 2017, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- Babu, R.P.; O’Connor, K.; Seeram, R. Current Progress on Bio-Based Polymers and Their Future Trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilyas, R.A.; Sapuan, S.M.; Kadier, A.; Kalil, M.S.; Ibrahim, R.; Atikah, M.S.N.; Nurazzi, N.M.; Nazrin, A.; Lee, C.H.; Faiz Norrrahim, M.N.; et al. Properties and Characterization of PLA, PHA, and Other Types of Biopolymer Composites; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128196618. [Google Scholar]
- Savioli Lopes, M.; Jardini, A.L.; Maciel Filho, R. Poly(Lactic Acid) Production for Tissue Engineering Applications. Procedia Eng. 2012, 42, 1402–1413. [Google Scholar] [CrossRef] [Green Version]
- Rehman, T.U.; Shah, L.A.; Khan, M.; Irfan, M.; Khattak, N.S. Zwitterionic Superabsorbent Polymer Hydrogels for Efficient and Selective Removal of Organic Dyes. RSC Adv. 2019, 9, 18565–18577. [Google Scholar] [CrossRef] [Green Version]
- Aziz, T.; Fan, H.; Khan, F.U.; Ullah, R.; Haq, F.; Iqbal, M.; Ullah, A. Synthesis of Carboxymethyl Starch-Bio-Based Epoxy Resin and Their Impact on Mechanical Properties. Z. Phys. Chem. 2020, 234, 1759–1769. [Google Scholar] [CrossRef]
- Li, C.; Fan, H.; Aziz, T.; Bittencourt, C.; Wu, L.; Wang, D.Y.; Dubois, P. Biobased Epoxy Resin with Low Electrical Permissivity and Flame Retardancy: From Environmental Friendly High-Throughput Synthesis to Properties. ACS Sustain. Chem. Eng. 2018, 6, 8856–8867. [Google Scholar] [CrossRef]
- Song, J.H.; Murphy, R.J.; Narayan, R.; Davies, G.B.H. Biodegradable and Compostable Alternatives to Conventional Plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2127–2139. [Google Scholar] [CrossRef]
- Luyt, A.S.; Malik, S.S. Can Biodegradable Plastics Solve Plastic Solid Waste Accumulation? In Plastics to Energy; Al-Salem, S.M., Ed.; Plastics Design Library; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 403–423. ISBN 978-0-12-813140-4. [Google Scholar]
- Rudnik, E.; Briassoulis, D. Comparative Biodegradation in Soil Behaviour of Two Biodegradable Polymers Based on Renewable Resources. J. Polym. Environ. 2011, 19, 18–39. [Google Scholar] [CrossRef]
- Gallet, G.; Lempiäinen, R.; Karlsson, S. Characterization by Solid Phase Microextraction-Gas Chromatography-Mass Spectrometry of Matrix Changes of Poly(L-Lactide) Exposed to Outdoor Soil Environment. Polym. Degrad. Stab. 2000, 71, 147–151. [Google Scholar] [CrossRef]
- Narancic, T.; Verstichel, S.; Reddy Chaganti, S.; Morales-Gamez, L.; Kenny, S.T.; de Wilde, B.; Babu Padamati, R.; O’Connor, K.E. Biodegradable Plastic Blends Create New Possibilities for End-of-Life Management of Plastics but They Are Not a Panacea for Plastic Pollution. Environ. Sci. Technol. 2018, 52, 10441–10452. [Google Scholar] [CrossRef]
- Hans, M.; Keul, H.; Moeller, M. Ring-Opening Polymerization of DD-Lactide Catalyzed by Novozyme 435. Macromol. Biosci. 2009, 9, 239–247. [Google Scholar] [CrossRef]
- García-Arrazola, R.; López-Guerrero, D.A.; Gimeno, M.; Bárzana, E. Lipase-Catalyzed Synthesis of Poly-l-Lactide Using Supercritical Carbon Dioxide. Polym. Adv. Technol. 2008, 19, 1396–1400. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An Overview of Polylactides as Packaging Materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Li, G.; Zhao, M.; Xu, F.; Yang, B.; Li, X.; Meng, X.; Teng, L.; Sun, F.; Li, Y. Synthesis and Biological Application of Polylactic Acid. Molecules 2020, 25, 5023. [Google Scholar] [CrossRef]
- Montané, X.; Montornes, J.M.; Nogalska, A.; Olkiewicz, M.; Giamberini, M.; Garcia-Valls, R.; Badia-Fabregat, M.; Jubany, I.; Tylkowski, B. Synthesis and Synthetic Mechanism of Polylactic Acid. Phys. Sci. Rev. 2020, 5, 20190102. [Google Scholar] [CrossRef]
- Bahramian, B.; Ma, Y.; Rohanizadeh, R.; Chrzanowski, W.; Dehghani, F. A New Solution for Removing Metal-Based Catalyst Residues from a Biodegradable Polymer. Green Chem. 2016, 18, 3740–3748. [Google Scholar] [CrossRef]
- Masutani, K.; Kimura, Y. PLA Synthesis and Polymerization. In Poly(Lactic Acid) Science and Technology: Processing, Properties, Additives and Applications; Jiménez, A., Peltzer, M., Ruseckaite, R., Eds.; Royal Society of Chemistry: Cambridge, UK, 2014; pp. 3–36. ISBN 9781782624806. [Google Scholar]
- Hege, C.S.; Schiller, S.M. Non-Toxic Catalysts for Ring-Opening Polymerizations of Biodegradable Polymers at Room Temperature for Biohybrid Materials. Green Chem. 2014, 16, 1410–1416. [Google Scholar] [CrossRef]
- Ojansivu, M.; Johansson, L.; Vanhatupa, S.; Tamminen, I.; Hannula, M.; Hyttinen, J.; Kellomäki, M.; Miettinen, S. Knitted 3D Scaffolds of Polybutylene Succinate Support Human Mesenchymal Stem Cell Growth and Osteogenesis. Stem Cells Int. 2018, 2018, 5928935. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Chang, J.; Cao, A.; Wang, J. In Vitro Evaluation of Biodegradable Poly(Butylene Succinate) as a Novel Biomaterial. Macromol. Biosci. 2005, 5, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Rafiqah, S.A.; Khalina, A.; Harmaen, A.S.; Tawakkal, I.A.; Zaman, K.; Asim, M.; Nurrazi, M.N.; Lee, C.H. A Review on Properties and Application of Bio-based Poly(Butylene Succinate). Polymers 2021, 13, 1436. [Google Scholar] [CrossRef] [PubMed]
- Łopusiewicz, Ł.; Zdanowicz, M.; Macieja, S.; Kowalczyk, K.; Bartkowiak, A. Development and Characterization of Bioactive Poly(Butylene-Succinate) Films Modified with Quercetin for Food Packaging Applications. Polymers 2021, 13, 1798. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.; Mazlan, M.M.; Tawakkal, I.S.M.A.; Talib, R.A.; Kian, L.K.; Jawaid, M. Characterization of Active Polybutylene Succinate Films Filled Essential Oils for Food Packaging Application. J. Polym. Environ. 2021, 1–12. [Google Scholar] [CrossRef]
- Jacquel, N.; Freyermouth, F.; Fenouillot, F.; Rousseau, A.; Pascault, J.P.; Fuertes, P.; Saint-Loup, R. Synthesis and Properties of Poly(Butylene Succinate): Efficiency of Different Transesterification Catalysts. J. Polym. Sci. Part A Polym. Chem. 2011, 48, 5301–5312. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Achilias, D.S. Synthesis of Poly(Alkylene Succinate) Biodegradable Polyesters, Part II: Mathematical Modelling of the Polycondensation Reaction. Polymer 2008, 49, 3677–3685. [Google Scholar] [CrossRef]
- Papaspyrides, C.D.; Vouyiouka, S.; Georgousopoulou, I.N.; Marinkovic, S.; Estrine, B.; Joly, C.; Dole, P. Feasibility of Solid-State Postpolymerization on Fossil- and Bio-Based Poly(Butylene Succinate) Including Polymer Upcycling Routes. Ind. Eng. Chem. Res. 2016, 55, 5832–5842. [Google Scholar] [CrossRef]
- Pellis, A.; Malinconico, M.; Guarneri, A.; Gardossi, L. Renewable Polymers and Plastics: Performance beyond the Green. New Biotechnol. 2021, 60, 146–158. [Google Scholar] [CrossRef]
- Jiang, Y.; Loos, K. Enzymatic Synthesis of Biobased Polyesters and Polyamides. Polymers 2016, 8, 243. [Google Scholar] [CrossRef] [Green Version]
- Loos, K.; Zhang, R.; Pereira, I.; Agostinho, B.; Hu, H.; Maniar, D. A Perspective on PEF Synthesis, Properties, and End-Life. Front. Chem. 2020, 8, 585. [Google Scholar] [CrossRef]
- Douka, A.; Vouyiouka, S.; Papaspyridi, L.M.; Papaspyrides, C.D. A Review on Enzymatic Polymerization to Produce Polycondensation Polymers: The Case of Aliphatic Polyesters, Polyamides and Polyesteramides. Prog. Polym. Sci. 2018, 79, 1–25. [Google Scholar] [CrossRef]
- Hu, Y.; Daoud, W.A.; Cheuk, K.K.L.; Lin, C.S.K. Newly Developed Techniques on Polycondensation, Ring-Opening Polymerization and Polymer Modification: Focus on Poly(Lactic Acid). Materials 2016, 9, 133. [Google Scholar] [CrossRef] [Green Version]
- Rahmayetty; Whulanza, Y.; Sukirno; Rahman, S.F.; Suyono, E.A.; Yohda, M.; Gozan, M. Use of Candida Rugosa Lipase as a Biocatalyst for L-Lactide Ring-Opening Polymerization and Polylactic Acid Production. Biocatal. Agric. Biotechnol. 2018, 16, 683–691. [Google Scholar] [CrossRef]
- Takwa, M.; Larsen, M.W.; Hult, K.; Martinelle, M. Rational Redesign of Candida Antarctica Lipase B for the Ring Opening Polymerization of d,d-Lactide. Chem. Commun. 2011, 47, 7392–7394. [Google Scholar] [CrossRef] [PubMed]
- Målberg, S.; Finne-Wistrand, A.; Albertsson, A.C. The Environmental Influence in Enzymatic Polymerization of Aliphatic Polyesters in Bulk and Aqueous Mini-Emulsion. Polymer 2010, 51, 5318–5322. [Google Scholar] [CrossRef]
- Düşkünkorur, H.Ö.; Bégué, A.; Pollet, E.; Phalip, V.; Güvenilir, Y.; Avérous, L. Enzymatic Ring-Opening (Co)Polymerization of Lactide Stereoisomers Catalyzed by Lipases. Toward the in Situ Synthesis of Organic/Inorganic Nanohybrids. J. Mol. Catal. B Enzym. 2015, 115, 20–28. [Google Scholar] [CrossRef]
- Duchiron, S.W.; Pollet, E.; Givry, S.; Avérous, L. Mixed Systems to Assist Enzymatic Ring Opening Polymerization of Lactide Stereoisomers. RSC Adv. 2015, 5, 84627–84635. [Google Scholar] [CrossRef]
- Guzmán-Lagunes, F.; López-Luna, A.; Gimeno, M.; Bárzana, E. Enzymatic Synthesis of Poly-l-Lactide in Supercritical R134a. J. Supercrit. Fluids 2012, 72, 186–190. [Google Scholar] [CrossRef]
- Chanfreau, S.; Mena, M.; Porras-Domínguez, J.R.; Ramírez-Gilly, M.; Gimeno, M.; Roquero, P.; Tecante, A.; Bárzana, E. Enzymatic Synthesis of Poly-L-Lactide and Poly-L-Lactide-Co-Glycolide in an Ionic Liquid. Bioprocess Biosyst. Eng. 2010, 33, 629–638. [Google Scholar] [CrossRef]
- Zhao, H.; Nathaniel, G.A.; Merenini, P.C. Enzymatic Ring-Opening Polymerization (ROP) of Lactides and Lactone in Ionic Liquids and Organic Solvents: Digging the Controlling Factors. RSC Adv. 2017, 7, 48639–48648. [Google Scholar] [CrossRef] [Green Version]
- Omay, D.; Guvenilir, Y. Synthesis and Characterization of Poly(d,l-Lactic Acid) via Enzymatic Ring Opening Polymerization by Using Free and Immobilized Lipase. Biocatal. Biotransform. 2013, 31, 132–140. [Google Scholar] [CrossRef]
- Mena, M.; Shirai, K.; Tecante, A.; Bárzana, E.; Gimeno, M. Enzymatic Syntheses of Linear and Hyperbranched Poly-L-Lactide Using Compressed R134a–Ionic Liquid Media. J. Supercrit. Fluids 2015, 103, 77–82. [Google Scholar] [CrossRef]
- Gkountela, C.; Rigopoulou, M.; Barampouti, E.M.; Vouyiouka, S. Enzymatic Prepolymerization Combined with Bulk Post-Polymerization towards the Production of Bio-Based Polyesters: The Case of Poly(Butylene Succinate). Eur. Polym. J. 2021, 143, 110197. [Google Scholar] [CrossRef]
- Lassalle, V.; Ferreira, M. Lipase-Catalyzed Synthesis of Polylactic Acid: An Overview of the Experimental Aspects. J. Chem. Technol. Biotechnol. 2008, 83, 1493–1502. [Google Scholar] [CrossRef]
- Rahmayetty; Barleany, D.R.; Suhendi, E.; Prasetya, B.; Andiyani, T. Polylactic Acid Synthesis via Direct Polycondensation Method Using Candida Rugosa Lipase Catalyst. World Chem. Eng. J. 2017, 1, 70–74. [Google Scholar]
- Panyachanakul, T.; Lomthong, T.; Lorliam, W.; Prajanbarn, J.; Tokuyama, S.; Kitpreechavanich, V.; Krajangsang, S. New Insight into Thermo-Solvent Tolerant Lipase Produced by Streptomyces Sp. A3301 for Re-Polymerization of Poly(DL-Lactic Acid). Polymer 2020, 204, 122812. [Google Scholar] [CrossRef]
- Yeniad, B.; Naik, H.; Heise, A. Lipases in Polymer Chemistry. Adv. Biochem. Eng. Biotechnol. 2011, 125, 69–95. [Google Scholar] [CrossRef]
- Nikulin, M.; Švedas, V. Prospects of Using Biocatalysis for the Synthesis and Modification of Polymers. Molecules 2021, 26, 2750. [Google Scholar] [CrossRef]
- Pellis, A.; Herrero Acero, E.; Ferrario, V.; Ribitsch, D.; Guebitz, G.M.; Gardossi, L. The Closure of the Cycle: Enzymatic Synthesis and Functionalization of Bio-Based Polyesters. Trends Biotechnol. 2016, 34, 316–328. [Google Scholar] [CrossRef]
- Filho, D.G.; Silva, A.G.; Guidini, C.Z. Lipases: Sources, Immobilization Methods, and Industrial Applications. Appl. Microbiol. Biotechnol. 2019, 103, 7399–7423. [Google Scholar] [CrossRef]
- Mokhtar, N.F.; Noor, R.; Raja, Z.; Rahman, A.; Dina, N.; Noor, M. The Immobilization of Lipases on Porous Support by Adsorption and Hydrophobic Interaction Method. Catalysts 2020, 10, 744. [Google Scholar] [CrossRef]
- Noel, M.; Combes, D. Effects of Temperature and Pressure on Rhizomucor Miehei Lipase Stability. J. Biotechnol. 2003, 102, 23–32. [Google Scholar] [CrossRef]
- Kundys, A.; Białecka-Florjańczyk, E.; Fabiszewska, A.; Małajowicz, J. Candida Antarctica Lipase B as Catalyst for Cyclic Esters Synthesis, Their Polymerization and Degradation of Aliphatic Polyesters. J. Polym. Environ. 2018, 26, 396–407. [Google Scholar] [CrossRef]
- Loeker, F.C.; Duxbury, C.J.; Kumar, R.; Gao, W.; Gross, R.A.; Howdle, S.M. Enzyme-Catalyzed Ring-Opening Polymerization of ε-Caprolactone in Supercritical Carbon Dioxide. Macromolecules 2004, 37, 2450–2453. [Google Scholar] [CrossRef]
- Poojari, Y.; Beemat, J.S.; Clarson, S.J. Enzymatic Synthesis of Poly(ε-Caprolactone): Thermal Properties, Recovery, and Reuse of Lipase B from Candida Antarctica Immobilized on Macroporous Acrylic Resin Particles. Polym. Bull. 2013, 70, 1543–1552. [Google Scholar] [CrossRef]
- Adhami, W.; Bakkour, Y.; Rolando, C. Polylactones Synthesis by Enzymatic Ring Opening Polymerization in Flow. Polymer 2021, 230, 124040. [Google Scholar] [CrossRef]
- Luna, C.; Luna, D.; Bautista, F.M.; Estevez, R.; Calero, J.; Posadillo, A.; Romero, A.A.; Sancho, E.D. Application of Enzymatic Extracts from a CALB Standard Strain as Biocatalyst within the Context of Conventional Biodiesel Production Optimization. Molecules 2017, 22, 2025. [Google Scholar] [CrossRef] [Green Version]
- Pedro, K.C.N.R.; Parreira, J.M.; Correia, I.N.; Henriques, C.A.; Langone, M.A.P. Enzymatic Biodiesel Synthesis from Acid Oil Using a Lipase Mixture. Quim. Nova 2018, 41, 284–291. [Google Scholar] [CrossRef]
- Yilmaz, E.; Soylak, M. Type of Green Solvents Used in Separation and Preconcentration Methods; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128185698. [Google Scholar]
- Pellis, A.; Byrne, F.P.; Sherwood, J.; Vastano, M.; Comerford, J.W.; Farmer, T.J. Safer Bio-Based Solvents to Replace Toluene and Tetrahydrofuran for the Biocatalyzed Synthesis of Polyesters. Green Chem. 2019, 21, 1686–1694. [Google Scholar] [CrossRef] [Green Version]
- Joshi, D.R.; Adhikari, N. An Overview on Common Organic Solvents and Their Toxicity. J. Pharm. Res. Int. 2019, 28, 1–18. [Google Scholar] [CrossRef]
- Gu, Y.; Jérôme, F. Bio-Based Solvents: An Emerging Generation of Fluids for the Design of Eco-Efficient Processes in Catalysis and Organic Chemistry. Chem. Soc. Rev. 2013, 42, 9550–9570. [Google Scholar] [CrossRef]
- Häckl, K.; Kunz, W. Some Aspects of Green Solvents. Comptes Rendus Chim. 2018, 21, 572–580. [Google Scholar] [CrossRef]
- Gardella, L.; Furaro, D.; Galimberti, M.; Monticelli, O. On the Development of a Facile Approach Based on the Use of Ionic Liquids: Preparation of PLLA (Sc-PLA)/High Surface Area Nano-Graphite Systems. Green Chem. 2015, 17, 4082–4088. [Google Scholar] [CrossRef]
- Knez; Markočič, E.; Leitgeb, M.; Primožič, M.; Knez Hrnčič, M.; Škerget, M. Industrial Applications of Supercritical Fluids: A Review. Energy 2014, 77, 235–243. [Google Scholar] [CrossRef]
- Yoshizawa-Fujita, M.; Saito, C.; Takeoka, Y.; Rikukawa, M. Lipase-Catalyzed Polymerization of L-Lactide in Ionic Liquids. Polym. Adv. Technol. 2008, 19, 1396–1400. [Google Scholar] [CrossRef]
- Kaar, J.L.; Jesionowski, A.M.; Berberich, J.A.; Moulton, R.; Russell, A.J. Impact of Ionic Liquid Physical Properties on Lipase Activity and Stability. J. Am. Chem. Soc. 2003, 125, 4125–4131. [Google Scholar] [CrossRef]
- Horváth, T.; Marossy, K.; Szabó, T.J. Ring-Opening Polymerization and Plasticization of Poly(L-Lactic)Acid by Adding of Glycerol-Dioleate. J. Therm. Anal. Calorim. 2021, 147, 2221–2227. [Google Scholar] [CrossRef]
- Vouyiouka, S.N.; Papaspyrides, C.D. Mechanistic Aspects of Solid-State Polycondensation. Polym. Sci. A Compr. Ref. 2012, 4, 857–874. [Google Scholar] [CrossRef]
- Wcislek, A.; Olalla, A.S.; McClain, A.; Piegat, A.; Sobolewski, P.; Puskas, J.; Fray, M. el Enzymatic Degradation of Poly(Butylene Succinate) Copolyesters Synthesized with the Use of Candida Antarctica Lipase, B. Polymers 2018, 10, 688. [Google Scholar] [CrossRef] [Green Version]
- Guinault, A.; Sollogoub, C.; Domenek, S.; Grandmontagne, A.; Ducruet, V. Influence of Crystallinity on Gas Barrier and Mechanical Properties of Pla Food Packaging Films. Int. J. Mater. Form. 2010, 3, 603–606. [Google Scholar] [CrossRef]
- Linko, Y.Y.; Lämsä, M.; Wu, X.; Uosukainen, E.; Seppälä, J.; Linko, P. Biodegradable Products by Lipase Biocatalysis. J. Biotechnol. 1998, 66, 41–50. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Fernandez-Lafuente, R. Lipase from Rhizomucor Miehei as an Industrial Biocatalyst in Chemical Process. J. Mol. Catal. B Enzym. 2010, 64, 1–22. [Google Scholar] [CrossRef]
- Pellis, A.; Comerford, J.W.; Maneffa, A.J.; Sipponen, M.H.; Clark, J.H.; Farmer, T.J. Elucidating Enzymatic Polymerisations: Chain-Length Selectivity of Candida Antarctica Lipase B towards Various Aliphatic Diols and Dicarboxylic Acid Diesters. Eur. Polym. J. 2018, 106, 79–84. [Google Scholar] [CrossRef]
- Uyama, H.; Inada, K.; Kobayashi, S. Lipase-Catalyzed Synthesis of Aliphatic Polyesters by Polycondensation of Dicarboxylic Acids and Glycols in Solvent-Free System. Polym. J. 2000, 32, 440–443. [Google Scholar] [CrossRef] [Green Version]
- El Fray, M.; Gradzik, B. Synteza Enzymatyczna Poli(Bursztynianu Butylenu) (Pbs) Katalizowana Lipazą B Ze Szczepu Candida Obiecujący Materiał Dla Enzymatic Synthesis of Poly(Butylene Succinate) (Pbs) Catalyzed By Lipase B From Candida Antarctica: A New Promising Material. Eng. Biomater. 2012, 115, 26–31. [Google Scholar]
- Kanelli, M.; Douka, A.; Vouyiouka, S.; Papaspyrides, C.D.; Topakas, E.; Papaspyridi, L.M.; Christakopoulos, P. Production of Biodegradable Polyesters via Enzymatic Polymerization and Solid State Finishing. J. Appl. Polym. Sci. 2014, 131, 2–9. [Google Scholar] [CrossRef]
- Jiang, Y.; Woortman, A.J.J.; Alberda van Ekenstein, G.O.R.; Loos, K. Enzyme-Catalyzed Synthesis of Unsaturated Aliphatic Polyesters Based on Green Monomers from Renewable Resources. Biomolecules 2013, 3, 461–480. [Google Scholar] [CrossRef] [PubMed]
- Azim, H.; Dekhterman, A.; Jiang, Z.; Gross, R.A. Candida Antarctica Lipase B Catalyzed Synthesis of Poly(Butylene Succinate): Shorter Chain Building Blocks Also Work. Biomacromolecules 2006, 7, 3093–3097. [Google Scholar] [CrossRef]
- Pospiech, D.; Choińska, R.; Flugrat, D.; Sahre, K.; Jehnichen, D.; Korwitz, A.; Friedel, P.; Werner, A.; Voit, B. Enzymatic Synthesis of Poly(Alkylene Succinate)s: Influence of Reaction Conditions. Processes 2021, 9, 411. [Google Scholar] [CrossRef]
- An, S.; Zhu, J.; Lu, D.; Liu, Z. Lipase-Catalyzed Synthesis and Characterization of High-Molecular-Weight PBS. CIESC J. 2013, 64, 1855–1861. [Google Scholar]
- Ren, L.; Wang, Y.; Ge, J.; Lu, D.; Liu, Z. Enzymatic Synthesis of High-Molecular-Weight Poly(Butylene Succinate) and Its Copolymers. Macromol. Chem. Phys. 2015, 216, 636–640. [Google Scholar] [CrossRef]
- Sugihara, S.; Toshima, K.; Matsumura, S. New Strategy for Enzymatic Synthesis of High-Molecular-Weight Poly(Butylene Succinate) via Cyclic Oligomers. Macromol. Rapid Commun. 2006, 27, 203–207. [Google Scholar] [CrossRef]
- Hevilla, V.; Sonseca, A.; Echeverría, C.; Muñoz-Bonilla, A.; Fernández-García, M. Enzymatic Synthesis of Polyesters and Their Bioapplications: Recent Advances and Perspectives. Macromol. Biosci. 2021, 21, 2100156. [Google Scholar] [CrossRef] [PubMed]
- Sonseca, A.; McClain, A.; Puskas, J.E.; el Fray, M. Kinetic Studies of Biocatalyzed Copolyesters of Poly(Butylene Succinate)(PBS)Containing Fully Bio-Based Dilinoleic Diol. Eur. Polym. J. 2019, 116, 515–525. [Google Scholar] [CrossRef]
- Misset, O.; van Dijk, A. Diagnosing the Inactivating Process of Enzymes. In Stability and Stabilization of Biocatalysts; Ballesteros, A., Plou, F.J., Iborra, J.L., Halling, P.J., Eds.; Progress in Biotechnology; Elsevier: Amsterdam, The Netherlands, 1998; Volume 15, pp. 3–18. [Google Scholar]
- Nasr, K.; Meimoun, J.; Favrelle-Huret, A.; de Winter, J.; Raquez, J.M.; Zinck, P. Enzymatic Polycondensation of 1,6-Hexanediol and Diethyl Adipate: A Statistical Approach Predicting the Key-Parameters in Solution and in Bulk. Polymers 2020, 12, 1907. [Google Scholar] [CrossRef] [PubMed]
- Burgard, A.; Burk, M.J.; Osterhout, R.; van Dien, S.; Yim, H. Development of a Commercial Scale Process for Production of 1,4-Butanediol from Sugar. Curr. Opin. Biotechnol. 2016, 42, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Sokołowska, M.; Fray, M.E. “Green” Poly(Butylene Succinate-Co-Dilinoleic Succinate) Copolymers Synthesized Using Candida Antarctica Lipase B (CAL-B) as Biocatalyst. Proceedings 2020, 69, 33. [Google Scholar] [CrossRef]
- Bhatia, S. Introduction to Enzymes and Their Applications. In Introduction to Pharmaceutical Biotechnology, Volume 2: Enzymes, Proteins and Bioinformatics; IOP SCIENCE: Bristol, UK, 2018; pp. 1–29. ISBN 9780750313025. [Google Scholar]
- Comerford, J.W.; Byrne, F.P.; Weinberger, S.; Farmer, T.J.; Guebitz, G.M.; Gardossi, L.; Pellis, A. Thermal Upgrade of Enzymatically Synthesized Aliphatic and Aromatic Oligoesters. Materials 2020, 13, 368. [Google Scholar] [CrossRef] [Green Version]
- Georgousopoulou, I.N.; Vouyiouka, S.; Dole, P.; Papaspyrides, C.D. Thermo-Mechanical Degradation and Stabilization of Poly(Butylene Succinate). Polym. Degrad. Stab. 2016, 128, 182–192. [Google Scholar] [CrossRef]
- Carlos Morales-Huerta, J.; Martínez De Ilarduya, A.; Muñoz-Guerra, S. Poly(Alkylene 2,5-Furandicarboxylate)s (PEF and PBF) by Ring Opening Polymerization. Polymer 2016, 87, 148–158. [Google Scholar] [CrossRef] [Green Version]
- Gruter, G.-J.M.; van Aken, T.B. Making an Impact: Sustainable Success Stories. In How to Commercialize Chemical Technologies for a Sustainable Future; Clark, T.J., Pasternak, A.S., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021; pp. 263–270. [Google Scholar]
- Papadopoulos, L.; Zamboulis, A.; Kasmi, N.; Wahbi, M.; Nannou, C.; Lambropoulou, D.A.; Kostoglou, M.; Papageorgiou, G.Z.; Bikiaris, D.N. Investigation of the Catalytic Activity and Reaction Kinetic Modeling of Two Antimony Catalysts in the Synthesis of Poly(Ethylene Furanoate). Green Chem. 2021, 23, 2507–2524. [Google Scholar] [CrossRef]
- Zhu, J.; Cai, J.; Xie, W.; Chen, P.H.; Gazzano, M.; Scandola, M.; Gross, R.A. Poly(Butylene 2,5-Furan Dicarboxylate), a Biobased Alternative to PBT: Synthesis, Physical Properties, and Crystal Structure. Macromolecules 2013, 46, 796–804. [Google Scholar] [CrossRef]
- Poulopoulou, N.; Guigo, N.; Sbirrazzuoli, N.; Papageorgiou, D.G.; Bikiaris, D.N.; Nikolaidis, G.N.; Papageorgiou, G.Z. Towards Increased Sustainability for Aromatic Polyesters: Poly(Butylene 2,5-Furandicarboxylate) and Its Blends with Poly(Butylene Terephthalate). Polymer 2021, 212, 123157. [Google Scholar] [CrossRef]
- Jiang, Y.; Woortman, A.J.J.; Alberda Van Ekenstein, G.O.R.; Loos, K. A Biocatalytic Approach towards Sustainable Furanic-Aliphatic Polyesters. Polym. Chem. 2015, 6, 5198–5211. [Google Scholar] [CrossRef] [Green Version]
- Maniar, D.; Jiang, Y.; Woortman, A.J.J.; van Dijken, J.; Loos, K. Furan-Based Copolyesters from Renewable Resources: Enzymatic Synthesis and Properties. ChemSusChem 2019, 12, 990–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maniar, D.; Silvianti, F.; Ospina, V.M.; Woortman, A.J.J.; van Dijken, J.; Loos, K. On the Way to Greener Furanic-Aliphatic Poly(Ester Amide)s: Enzymatic Polymerization in Ionic Liquid. Polymer 2020, 205, 122662. [Google Scholar] [CrossRef]
- Jiang, Y.; Maniar, D.; Woortman, A.J.J.; Loos, K. Enzymatic Synthesis of 2,5-Furandicarboxylic Acid-Based Semi-Aromatic Polyamides: Enzymatic Polymerization Kinetics, Effect of Diamine Chain Length and Thermal Properties. RSC Adv. 2016, 6, 67941–67953. [Google Scholar] [CrossRef] [Green Version]
- Silvianti, F.; Maniar, D.; Boetje, L.; Loos, K. Green Pathways for the Enzymatic Synthesis of Furan-Based Polyesters and Polyamides. ACS Symp. Ser. 2020, 1373, 3–29. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).