Comparison of Hydroxypropylcellulose and Hot-Melt Extrudable Hypromellose in Twin-Screw Melt Granulation of Metformin Hydrochloride: Effect of Rheological Properties of Polymer on Melt Granulation and Granule Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Powder Blending
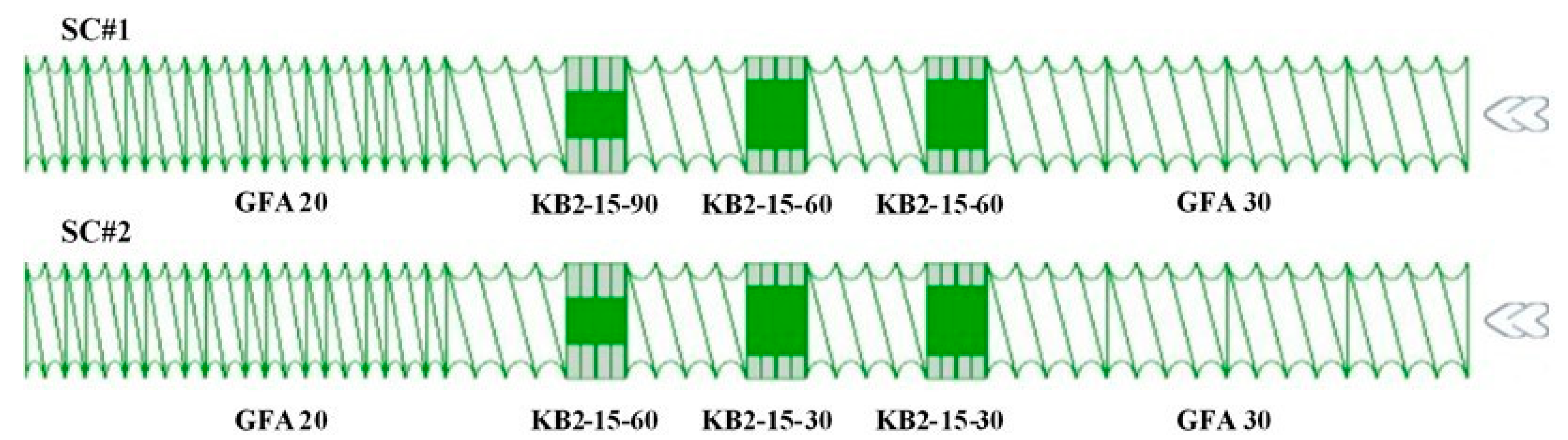
2.2. Twin-Screw Melt Granulation
2.3. Differential Scanning Calorimetry
2.4. Milling
2.5. Granule Size Analysis
2.6. Tablet Compaction
2.7. Dissolution
2.8. Scanning Electron Microscopy (SEM)
2.9. Rotational Melt Rheology
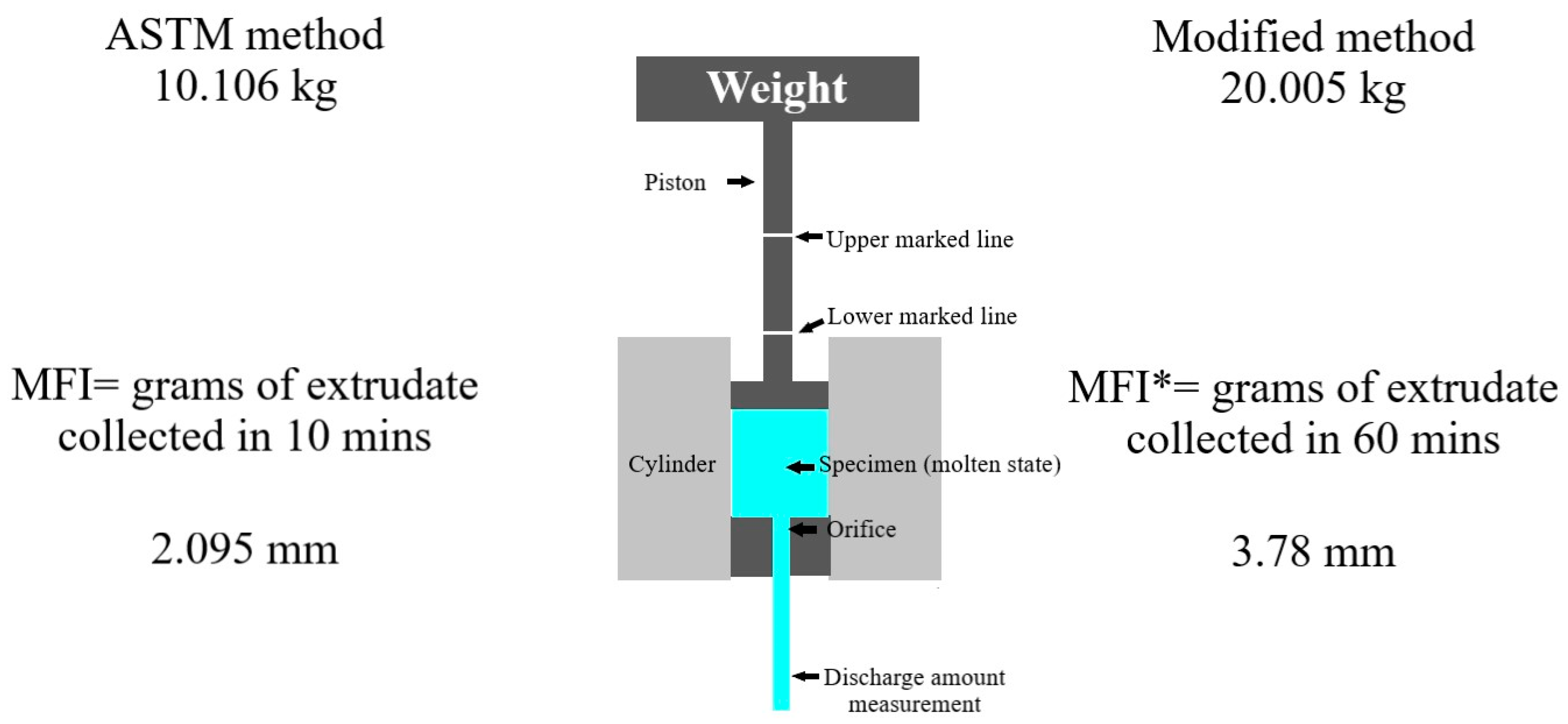
2.10. Melt Flow Index Using Extrusion Plastometer
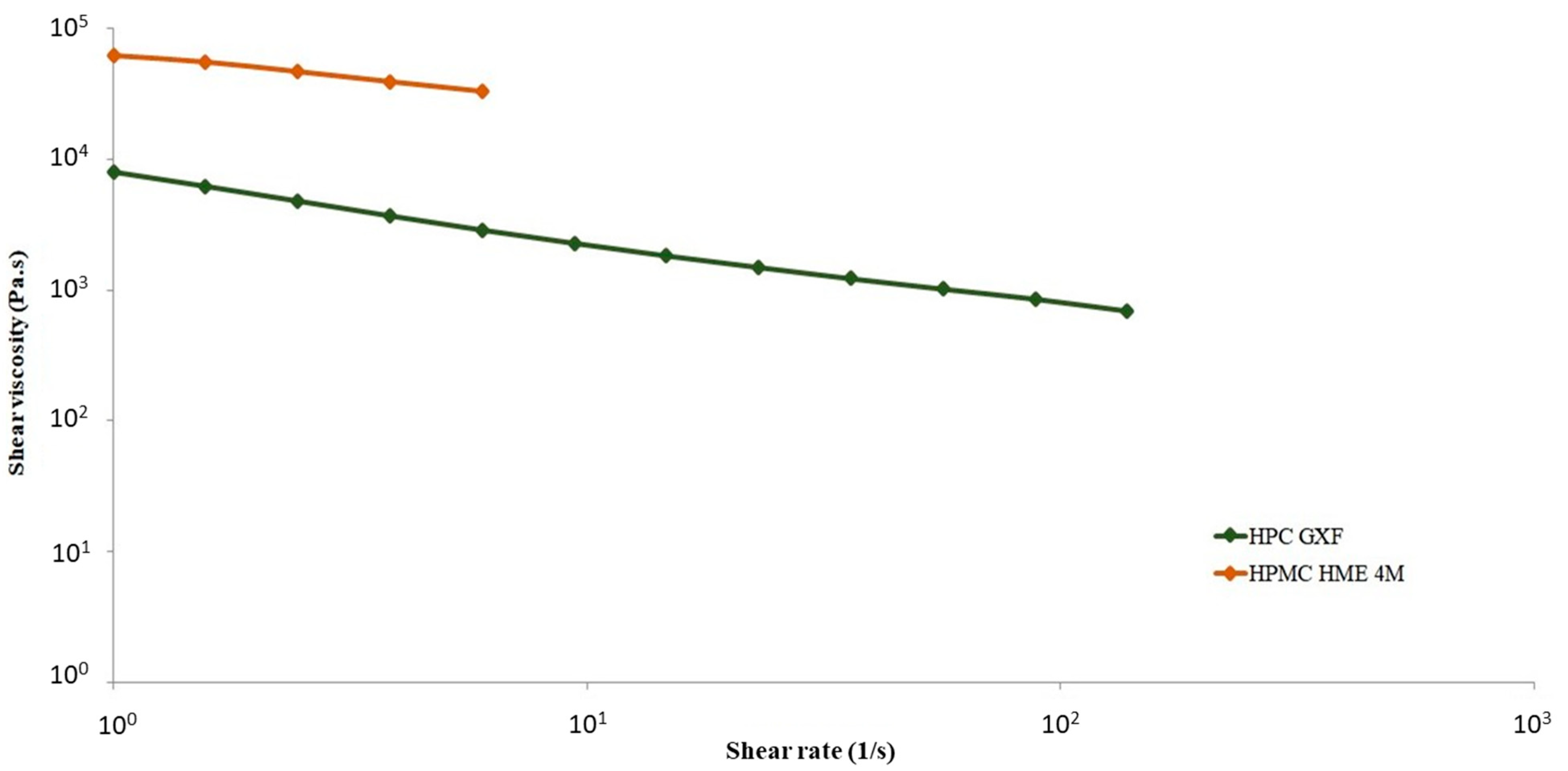
2.11. Steady-State Viscosity Using Capillary Rheometer
3. Results and Discussion
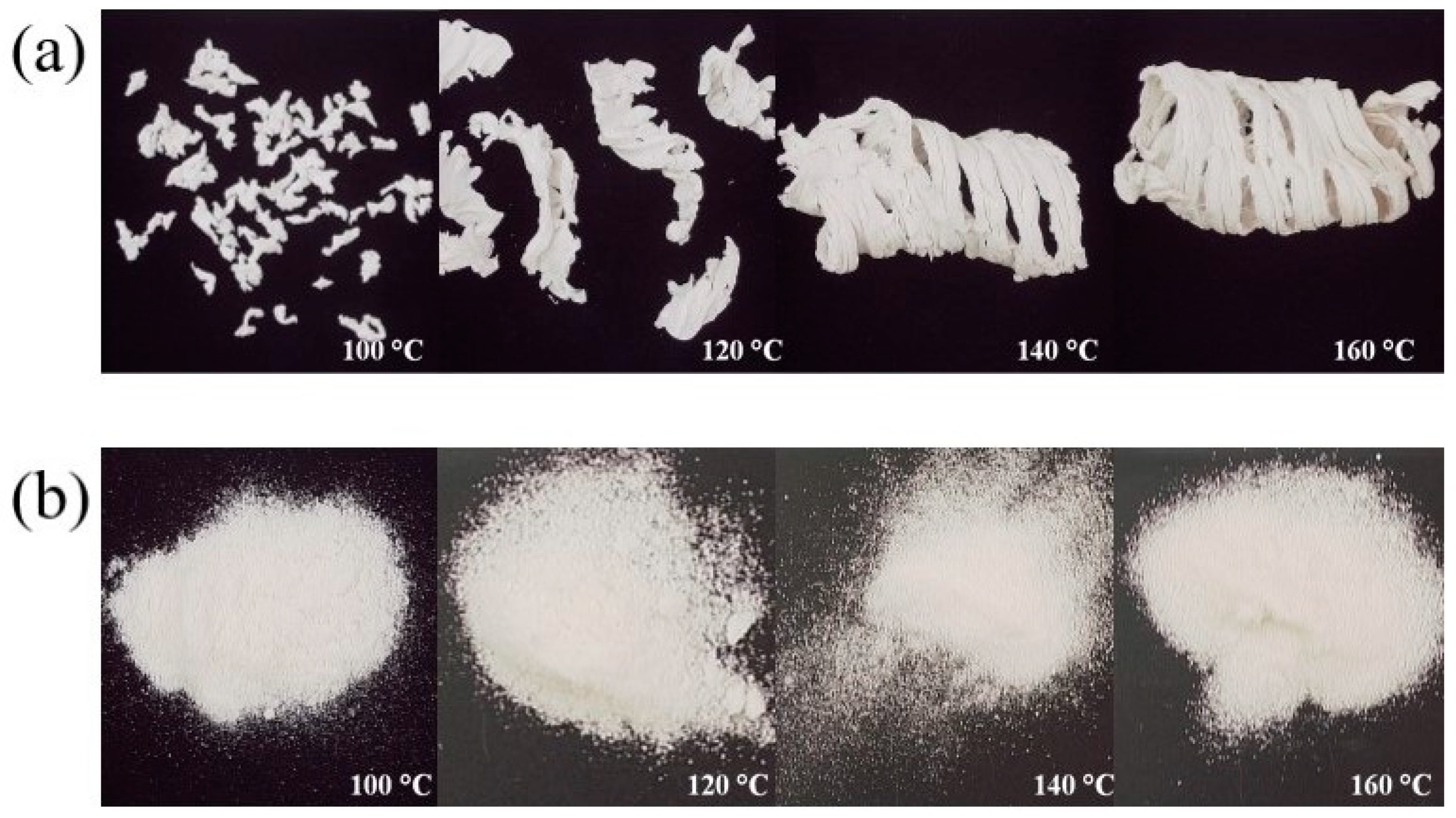
3.1. Processing Conditions for Drug–Polymer Blends
3.2. Granule Properties
3.3. Differential Scanning Calorimetry (DSC)
3.4. Milling and Sieve Analysis
3.5. Tablet Compaction
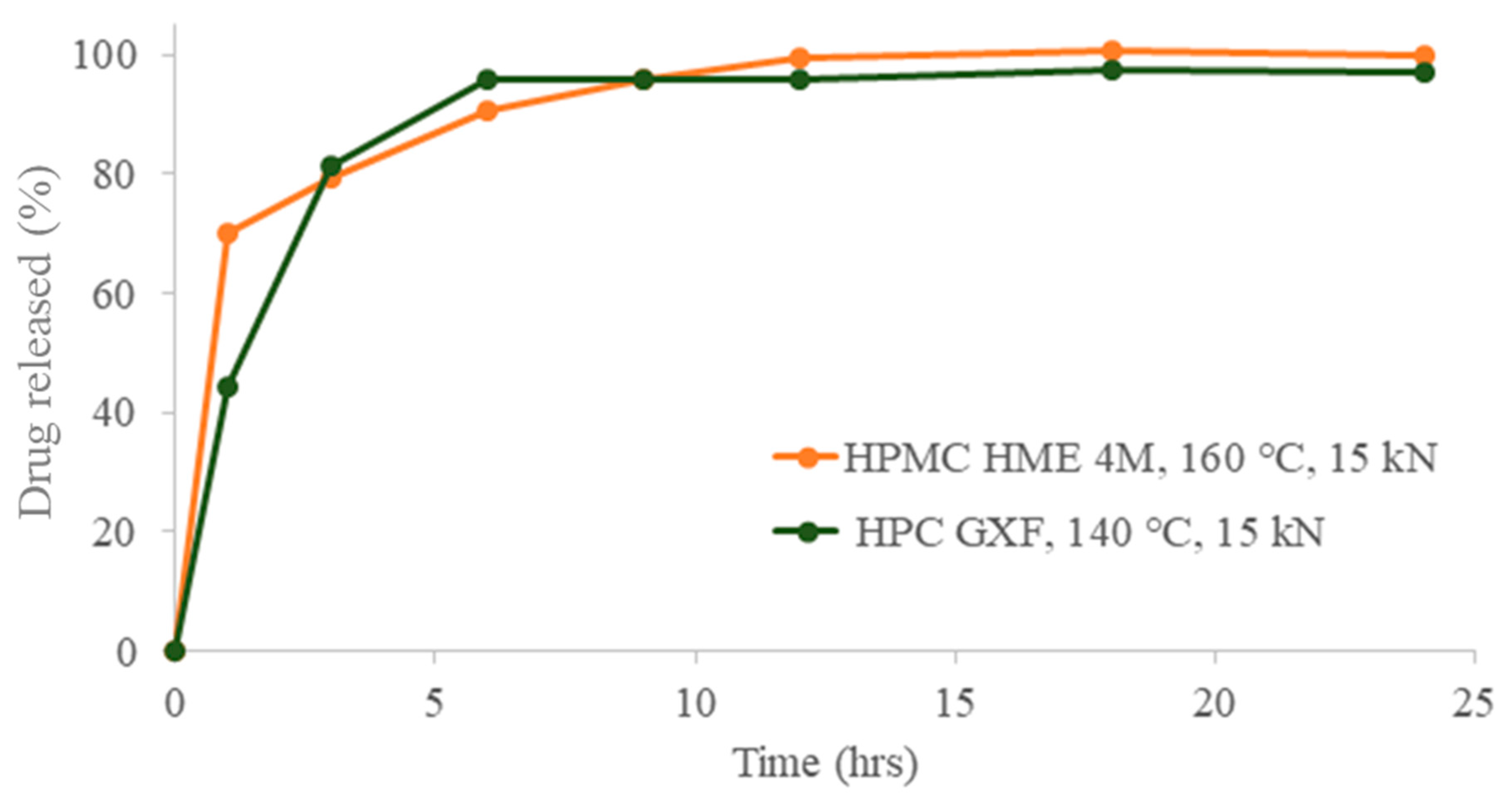
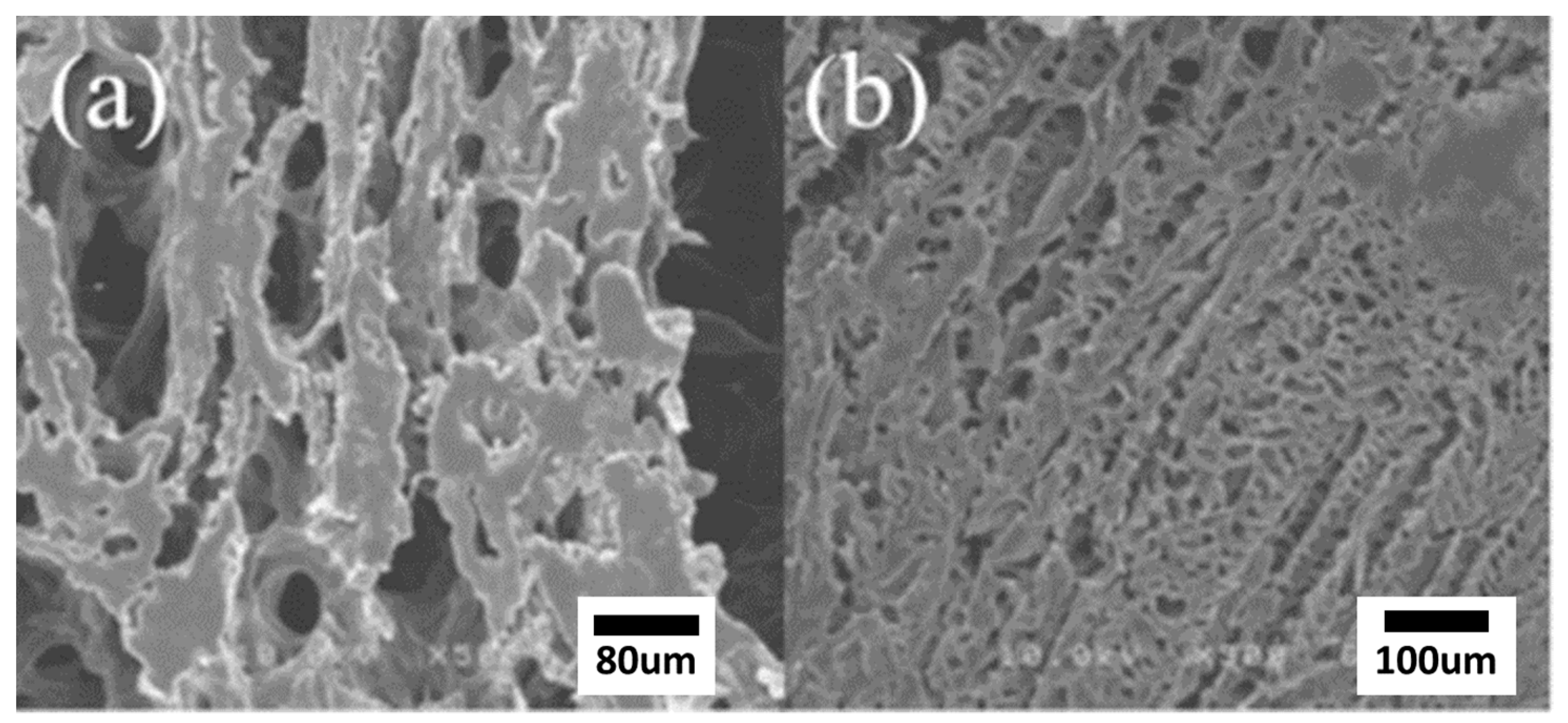
3.6. Dissolution and SEM
3.7. Melt Flow Index Using Extrusion Plastometer
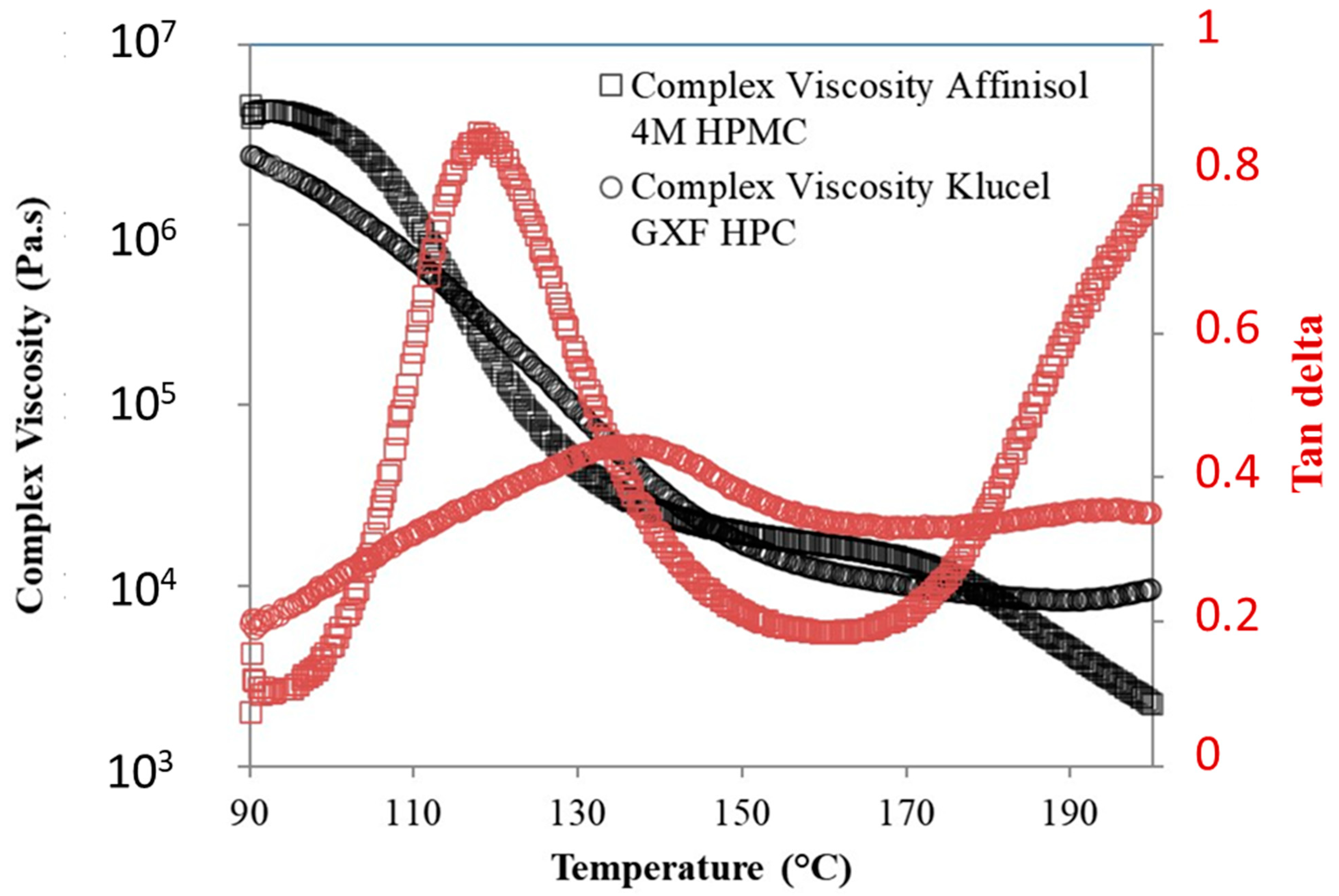
3.8. Steady-State Viscosity
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serajuddin, A.T. The future of tableting technology. J. Excip. Food Chem. 2016, 5, 1006. [Google Scholar]
- Bandari, S.; Nyavanandi, D.; Kallakunta, V.R.; Janga, K.Y.; Sarabu, S.; Butreddy, A.; Repka, M.A. Continuous twin screw granulation–An advanced alternative granulation technology for use in the pharmaceutical industry. Int. J. Pharm. 2020, 580, 119215. [Google Scholar] [CrossRef] [PubMed]
- Schaber, S.D.; Gerogiorgis, D.I.; Ramachandran, R.; Evans, J.M.B.; Barton, P.I.; Trout, B.L. Economic A nalysis of Integrated Continuous and Batch Pharmaceutical Manufacturing: A Case, Study. Ind. Eng. Chem. Res. 2011, 50, 10083–10092. [Google Scholar] [CrossRef] [Green Version]
- Grymonpre, W.; Verstraete, G.; Vanhoorne, V.; Remon, J.P.; De Beer, T.; Vervaet, C. Downstream processing from melt granulation towards tablets: In-depth analysis of a continuous twin-screw melt granulation process using polymeric binders. Eur. J. Pharm. Biopharm. 2018, 124, 43–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarabu, S.; Bandari, S.; Kallakunta, V.R.; Tiwari, R.; Patil, H.; Repka, M.A. An update on the contribution of hot-melt extrusion technology to novel drug delivery in the twenty-first century: Part II. Expert Opin. Drug Deliv. 2019, 16, 567–582. [Google Scholar] [CrossRef]
- Lakshman, J.P.; Kowalski, J.; Vasanthavada, M.; Tong, W.Q.; Joshi, Y.M.; Serajuddin, A.T. Application of melt granulation technology to enhance tabletting properties of poorly compactible high-dose drugs. J. Pharm. Sci. 2011, 100, 1553–1565. [Google Scholar] [CrossRef]
- Vasanthavada, M.; Wang, Y.; Haefele, T.; Lakshman, J.P.; Mone, M.; Tong, W.; Joshi, Y.M.; Serajuddin, A.T.M. Application of melt granulation technology using twin-screw extruder in development of high-dose modified-release tablet formulation. J. Pharm. Sci. 2011, 100, 1923–1934. [Google Scholar] [CrossRef]
- Vaingankar, P.; Amin, P. Continuous melt granulation to develop high drug loaded sustained release tablet of Metformin HCl. Asian J. Pharm. Sci. 2017, 12, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Vanhoorne, V.; Janssens, L.; Vercruysse, J.; De Beer, T.; Remon, J.P.; Vervaet, C. Continuous twin screw granulation of controlled release formulations with various HPMC grades. Int. J. Pharm. 2016, 511, 1048–1057. [Google Scholar] [CrossRef] [Green Version]
- Batra, A.; Desai, D.; Serajuddin, A.T.M. Investigating the Use of Polymeric Binders in Twin Screw Melt Granulation Process for Improving Compactibility of Drugs. J. Pharm. Sci. 2017, 106, 140–150. [Google Scholar] [CrossRef] [Green Version]
- Kowalski, J.; Kalb, O.; Serajuddin, A.T.M.; Joshi, Y. Treatment with Vildagliptin. European Patent 3087975A1, 2 November 2016. [Google Scholar]
- Ghosh, I.; Kowalski, J.; Snyder, J.; Tong, W.Q.; Vippagunta, S.; Novartis, A.G. Galenical Formulation Comprising Aliskiren and Process for Its Preparation by Melt Extrusion Granulation. U.S. Patent Application 13/120,182, 21 July 2009. [Google Scholar]
- Ghosh, I.; Li, S.; Tong, W.Q.; Vippagunta, S.; Wen, H.; Novartis, A.G. Galenical Formulations of a Fixed, Dose Combination of Valsartan and Aliskiren. U.S. Patent Application 13/256,454, 12 January 2012. [Google Scholar]
- Kowalski, J.; Lakshman, J.P.; Serajuddin, A.T.; Tong, W.Q.; Vasanthavada, M.; Novartis, A.G. Continuous Process for Making Pharmaceutical Compositions. U.S. Patent Application 12/990,151, 17 February 2009. [Google Scholar]
- Brady, J.; Dürig, T.; Lee, P.I.; Li, J.X. Polymer Properties and Characterization. In Developing Solid Oral Dosage Forms; Academic Press: Cambridge, MA, USA, 2017; pp. 181–223. [Google Scholar]
- Meena, A.; Parikh, T.; Gupta, S.S.; Serajuddin, A.T. Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion-II: Cellulosic polymers. J. Excip. Food Chem. 2016, 5, 1002. [Google Scholar]
- Gupta, S.S.; Meena, A.; Parikh, T.; Serajuddin, A.T. Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion-I: Polyvinylpyrrolidone and related polymers. J. Excip. Food Chem. 2016, 5, 1001. [Google Scholar]
- Parikh, T.; Gupta, S.S.; Meena, A.; Serajuddin, A.T. Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion-III: Polymethacrylates and polymethacrylic acid based polymers. J. Excip. Food Chem. 2016, 5, 1003. [Google Scholar]
- Ghosh, I.; Snyder, J.; Vippagunta, R.; Alvine, M.; Vakil, R.; Tong, W.-Q.; Vippagunta, S. Comparison of HPMC based polymers performance as carriers for manufacture of solid dispersions using the melt extruder. Int. J. Pharm. 2011, 419, 12–19. [Google Scholar] [CrossRef]
- Yi, S.; Wang, J.; Lu, Y.; Ma, R.; Gao, Q.; Liu, S.; Xiong, S. Novel Hot Melt Extruded Matrices of Hydroxypropyl, Cellulose and Amorphous Felodipine-Plasticized, Hydroxypropyl Methylcellulose as Controlled, Release Systems. AAPS PharmSciTech 2019, 20, 219. [Google Scholar] [CrossRef] [PubMed]
- Loreti, G.; Maroni, A.; Del Curto, M.D.; Melocchi, A.; Gazzaniga, A.; Zema, L. Evaluation of hot-melt extrusion technique in the preparation of HPC matrices for prolonged release. Eur. J. Pharm. Sci. 2014, 52, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.N.; Majumdar, S.; Singh, A.; Deng, W.; Murthy, N.S.; Pinto, E.; Tewari, D.; Durig, T.; Repka, M.A. Klucel™ EF and ELF polymers for immediate-release oral dosage forms prepared by melt extrusion technology. AAPS PharmSciTech 2012, 13, 1158–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallakunta, V.R.; Sarabu, S.; Bandari, S.; Batra, A.; Bi, V.; Durig, T.; Repka, M.A. Stable amorphous solid dispersions of fenofibrate using hot melt extrusion technology: Effect of formulation and process parameters for a low glass transition temperature drug. J. Drug Deliv. Sci. Technol. 2020, 58, 101395. [Google Scholar] [CrossRef] [PubMed]
- Sarabu, S.; Kallakunta, V.R.; Bandari, S.; Batra, A.; Bi, V.; Durig, T.; Zhang, F.; Repka, M.A. Hypromellose acetate succinate based amorphous solid dispersions via hot melt extrusion: Effect of drug physicochemical properties. Carbohydr. Polym. 2020, 233, 115828. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xie, X.; Zhao, Y.; Gao, Y.; Cai, C.; Zhang, Q.; Ding, Z.; Fan, Z.; Zhang, H.; Liu, M.; et al. Effect of plasticizers on manufacturing ritonavir/copovidone solid dispersions via hot-melt extrusion: Preformulation physicochemical characterization and pharmacokinetics in rats. Eur. J. Pharm. Sci. 2019, 127, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Su, Y.; Zhang, J.; DiNunzio, J.; Leone, A.; Huang, C.; Brown, C.D. Rheology Guided Rational Selection of Processing Temperature To Prepare Copovidone–Nifedipine Amorphous Solid Dispersions via Hot Melt Extrusion (HME). Mol. Pharm. 2016, 13, 3494–3505. [Google Scholar] [CrossRef]
- Batra, A.; Thongsukmak, A.; Desai, D.; Serajuddin, A.T.M. The Effect of Process Variables and Binder Concentration on Tabletability of Metformin Hydrochloride and Acetaminophen Granules Produced by Twin Screw Melt Granulation with Different Polymeric Binders. AAPS PharmSciTech 2021, 22, 154. [Google Scholar] [CrossRef] [PubMed]
- Maskova, E.; Kubova, K.; Raimi-Abraham, B.T.; Vllasaliu, D.; Vohlidalova, E.; Turanek, J.; Masek, J. Hypromellose-A traditional pharmaceutical excipient with modern applications in oral and oromucosal drug delivery. J. Control. Release 2020, 324, 695–727. [Google Scholar] [CrossRef] [PubMed]
- Affinisol™ HPMC HME. Available online: https://www.pharma.dupont.com/content/dam/dupont/amer/us/en/nutrition-health/general/pharmaceuticals/documents/Download_Affinisol%20HPMC%20HME%20Brochure.pdf (accessed on 5 November 2021).
- Huang, S.; O’Donnell, K.P.; Keen, J.M.; Rickard, M.A.; McGinity, J.W.; Williams, R.O., 3rd. A New Extrudable Form of Hypromellose: AFFINISOL HPMC HME. AAPS PharmSciTech 2016, 17, 106–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.S.; Solanki, N.; Serajuddin, A.T. Investigation of Thermal and Viscoelastic Properties of Polymers, Relevant to Hot Melt Extrusion, IV: Affinisol HPMC HME Polymers. AAPS PharmSciTech 2016, 17, 148–157. [Google Scholar] [CrossRef]
- Luebbert, C.; Stoyanov, E.; Sadowski, G. Phase behavior of ASDs based on hydroxypropyl cellulose. Int. J. Pharm. X 2021, 3, 100070. [Google Scholar] [CrossRef]
- Vippagunta, R.R.; LoBrutto, R.; Pan, C.; Lakshman, J.P. Investigation of Metformin HCl lot-to-lot variation on flowability differences exhibited during drug product processing. J. Pharm. Sci. 2010, 99, 5030–5039. [Google Scholar] [CrossRef] [PubMed]
- Metformin Hydrochloride Extended-Release Tablets. Available online: https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/revisions/metformin-hcl-ert-sept18-pending-nitr.pdf (accessed on 5 November 2021).
- Bird, R.B.; Armstrong, R.C.; Hassager, O. Fluid Mechanics. In Dynamics of Polymeric Liquids, 2nd ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 1987; Volume 1. [Google Scholar]
- Aminabhavi, T.M.; Nadagouda, M.N.; Joshi, S.D.; More, U.A. Guar gum as platform for the oral controlled release of therapeutics. Expert Opin. Drug Deliv. 2014, 11, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Dürig, T.; Fassihi, R. Guar-based monolithic matrix systems: Effect of ionizable and non-ionizable substances and excipients on gel dynamics and release kinetics. J. Control. Release 2002, 80, 45–56. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Jadaun, M.; Bajpai, M.; Jyotishi, P.; Shah, F.F.; Tiwari, S. Controlled release of Doxycycline from gum acacia/poly(sodium acrylate) microparticles for oral drug delivery. Int. J. Biol. Macromol. 2017, 104, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H.; Yoneyama, T.; Nakajima, K.; Kobayashi, M. Correlation between loose density and compactibility of granules prepared by various granulation methods. Int. J. Pharm. 2001, 216, 159–164. [Google Scholar] [CrossRef]
- Kittikunakorn, N.; Liu, T.; Zhang, F. Twin-screw melt granulation: Current progress and challenges. Int. J. Pharm. 2020, 588, 119670. [Google Scholar] [CrossRef] [PubMed]

| Chemical Name | Trade Name (Manufacturer) | Tg (°C) | Degradation Temperature (°C) |
|---|---|---|---|
| Hydroxypropyl cellulose (MW~370 kDa) | Klucel® GXF | 84 c | 227 a |
| Hydroxypropyl methylcellulose (MW~552 kDa) | Affinisol® 4M | 115 b | 220 b |
| Feed Rate (g/min) | Screw Speed (rpm) | Zone 1 (°C) | Zone 2 (°C) | Zone 3 (°C) | Zone 4 (°C) | Zone 5 (°C) | Zone 6 (°C) | Zone 7 (°C) | Zone 8 (°C) |
|---|---|---|---|---|---|---|---|---|---|
| 20 | 100 | 20 | 60 | 100 | 100 | 100 | 100 | 100 | 100 |
| 20 | 60 | 100 | 120 | 120 | 120 | 120 | 120 | ||
| 20 | 60 | 100 | 140 | 140 | 140 | 140 | 140 | ||
| 20 | 60 | 100 | 160 | 160 | 160 | 160 | 160 |
| Binder | Screw Configuration | Extrusion Temperature (°C) | <125 µm (%) | 125–300 µm (%) | 300–450 µm (%) | 450–600 µm (%) |
|---|---|---|---|---|---|---|
| HPC GXF | SC#1 | 140 | 83.2 | 16.0 | 0.7 | 0.1 |
| SC#1 | 160 | 80.5 | 18.2 | 1.1 | 0.2 | |
| HPMC HME 4M | SC#1 | 140 | 98.9 | 1.1 | - | - |
| SC#1 | 160 | 91.2 | 8.7 | 0.1 | - |
| Binder | Screw Configuration | Compaction Force (kN) | Processing Temperature (°C) | |||
|---|---|---|---|---|---|---|
| 160 | 140 | 120 | 100 | |||
| HPC GXF | SC#1 | 15 | + | + | + | + |
| 30 | + | + | + | + | ||
| SC#2 | 15 | + | + | + | + | |
| 30 | + | + | + | + | ||
| HPMC HME 4M | SC#1 | 15 | + | - | - | - |
| 30 | + | - | - | - | ||
| SC#2 | 15 | - | - | - | - | |
| 30 | - | - | - | - | ||
| Binder | Carreau-Yasuda Model (Tr = 140 °C) | ||||
|---|---|---|---|---|---|
| n | α | λ (s) | r2 | ||
| HPMC HME 4M | 1.5722 × 108 | 0.50855 | 28.6 | 404352 | 0.9837 |
| HPC GXF | 1.3485 × 108 | 0.24315 | 16.6 | 41061 | 0.9921 |
| Polymer | Initial Sample Weight (g) | Extrudate Collected (g) | Time (s) | MFI* (g/min) |
|---|---|---|---|---|
| HPC GXF | 4.50 | 3.70 | 5 | 44.39 |
| HPMC HME 4M | 4.50 | 3.68 | 3600 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batra, A.; Yang, F.; Kogan, M.; Sosnowik, A.; Usher, C.; Oldham, E.W.; Chen, N.; Lawal, K.; Bi, Y.; Dürig, T. Comparison of Hydroxypropylcellulose and Hot-Melt Extrudable Hypromellose in Twin-Screw Melt Granulation of Metformin Hydrochloride: Effect of Rheological Properties of Polymer on Melt Granulation and Granule Properties. Macromol 2022, 2, 1-19. https://doi.org/10.3390/macromol2010001
Batra A, Yang F, Kogan M, Sosnowik A, Usher C, Oldham EW, Chen N, Lawal K, Bi Y, Dürig T. Comparison of Hydroxypropylcellulose and Hot-Melt Extrudable Hypromellose in Twin-Screw Melt Granulation of Metformin Hydrochloride: Effect of Rheological Properties of Polymer on Melt Granulation and Granule Properties. Macromol. 2022; 2(1):1-19. https://doi.org/10.3390/macromol2010001
Chicago/Turabian StyleBatra, Amol, Fengyuan Yang, Michael Kogan, Anthony Sosnowik, Courtney Usher, Eugene W. Oldham, Ningyi Chen, Kamaru Lawal, Yunxia Bi, and Thomas Dürig. 2022. "Comparison of Hydroxypropylcellulose and Hot-Melt Extrudable Hypromellose in Twin-Screw Melt Granulation of Metformin Hydrochloride: Effect of Rheological Properties of Polymer on Melt Granulation and Granule Properties" Macromol 2, no. 1: 1-19. https://doi.org/10.3390/macromol2010001
APA StyleBatra, A., Yang, F., Kogan, M., Sosnowik, A., Usher, C., Oldham, E. W., Chen, N., Lawal, K., Bi, Y., & Dürig, T. (2022). Comparison of Hydroxypropylcellulose and Hot-Melt Extrudable Hypromellose in Twin-Screw Melt Granulation of Metformin Hydrochloride: Effect of Rheological Properties of Polymer on Melt Granulation and Granule Properties. Macromol, 2(1), 1-19. https://doi.org/10.3390/macromol2010001





