Current Research on Polyelectrolyte Nanostructures: From Molecular Interactions to Biomedical Applications
Abstract
1. Introduction
2. Polyelectrolyte Nanostructures
2.1. Universal and Intrinsic Properties
2.2. Nanoscopic Assemblies
2.3. Network and Film Nanostructures
2.4. Electrostatic Complexation
3. Interactions of Polyelectrolytes with Pharmaceutical and Biological Matter
3.1. Interactions with Molecular Drugs
3.2. Interactions Involving Proteins
3.3. Polyelectrolyte-Cell Interactions
4. Polyelectrolytes in Drug and Protein Delivery
4.1. Self-Assembled Nanoformulations
4.2. Macro- and Nano-Hydrogels from Polyelectrolytes
4.3. Polyelectrolyte Nano-Assemblies with Proteins as Building Blocks
4.4. Polyelectrolyte-Based Films for Sustained Drug Release
5. Polyelectrolytes in Tissue Regeneration
5.1. Wound Treatment
5.2. Bone and Cartilage Regeneration
5.3. Cardiac Muscle and Vitreous Humor Tissue Repair
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Varma, K.; Gopi, S. Chapter 7—Biopolymers and their role in medicinal and pharmaceutical applications. In Biopolymers and Their Industrial Applications; Thomas, S., Gopi, S., Amalraj, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 175–191. [Google Scholar]
- Luo, Y.; Wang, Q.; Zhang, Y. Biopolymer-Based Nanotechnology Approaches To Deliver Bioactive Compounds for Food Applications: A Perspective on the Past, Present, and Future. J. Agric. Food Chem. 2020, 68, 12993–13000. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, A.; Li, X.; Sun, L.; Guo, Y. An overview of classifications, properties of food polysaccharides and their links to applications in improving food textures. Trends Food Sci. Technol. 2020, 102, 1–15. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Amphiphilic block copolymers in drug delivery: Advances in formulation structure and performance. Expert Opin. Drug Deliv. 2018, 15, 1085–1104. [Google Scholar] [CrossRef]
- Liu, S.; Li, Z.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Recent advances on protein separation and purification methods. Adv. Colloid Interface Sci. 2020, 284, 102254. [Google Scholar] [CrossRef] [PubMed]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef] [PubMed]
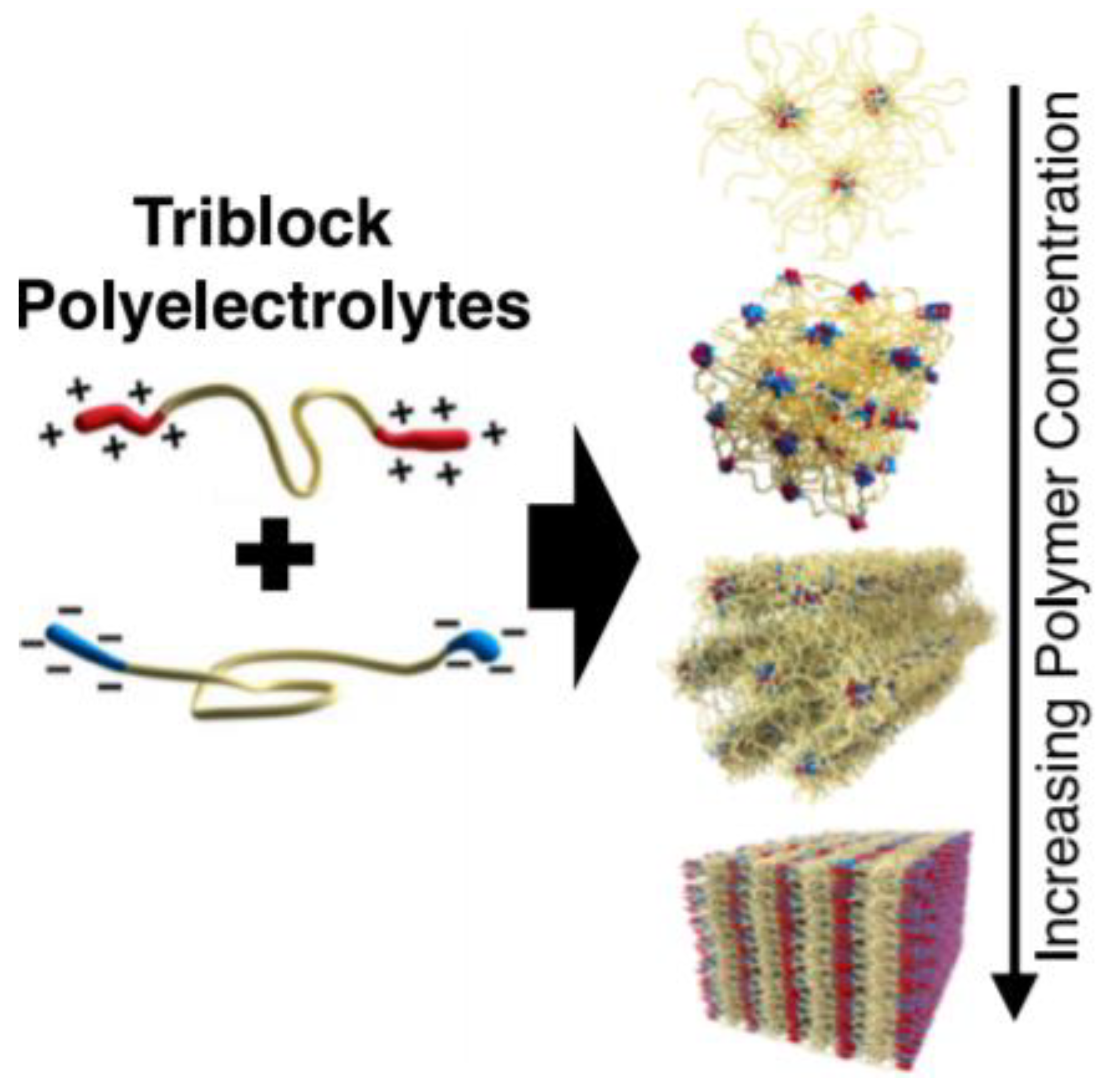
- Srivastava, S.; Levi, A.E.; Goldfeld, D.J.; Tirrell, M.V. Structure, Morphology, and Rheology of Polyelectrolyte Complex Hydrogels Formed by Self-Assembly of Oppositely Charged Triblock Polyelectrolytes. Macromolecules 2020, 53, 5763–5774. [Google Scholar] [CrossRef]
- Gao, S.; Holkar, A.; Srivastava, S. Protein–Polyelectrolyte Complexes and Micellar Assemblies. Polymers 2019, 11, 1097. [Google Scholar] [CrossRef] [PubMed]
- Papagiannopoulos, A.; Meristoudi, A.; Hong, K.; Pispas, S. Kinetics of temperature response of PEO-b-PNIPAM-b-PAA triblock terpolymer aggregates and of their complexes with lysozyme. Polymer 2016, 83, 111–115. [Google Scholar] [CrossRef]
- Fernandez-Alvarez, R.; Nová, L.; Uhlík, F.; Kereïche, S.; Uchman, M.; Košovan, P.; Matějíček, P. Interactions of star-like polyelectrolyte micelles with hydrophobic counterions. J. Colloid Interface Sci. 2019, 546, 371–380. [Google Scholar] [CrossRef]
- Samanta, R.; Ganesan, V. Influence of protein charge patches on the structure of protein–polyelectrolyte complexes. Soft Matter 2018, 14, 9475–9488. [Google Scholar] [CrossRef] [PubMed]
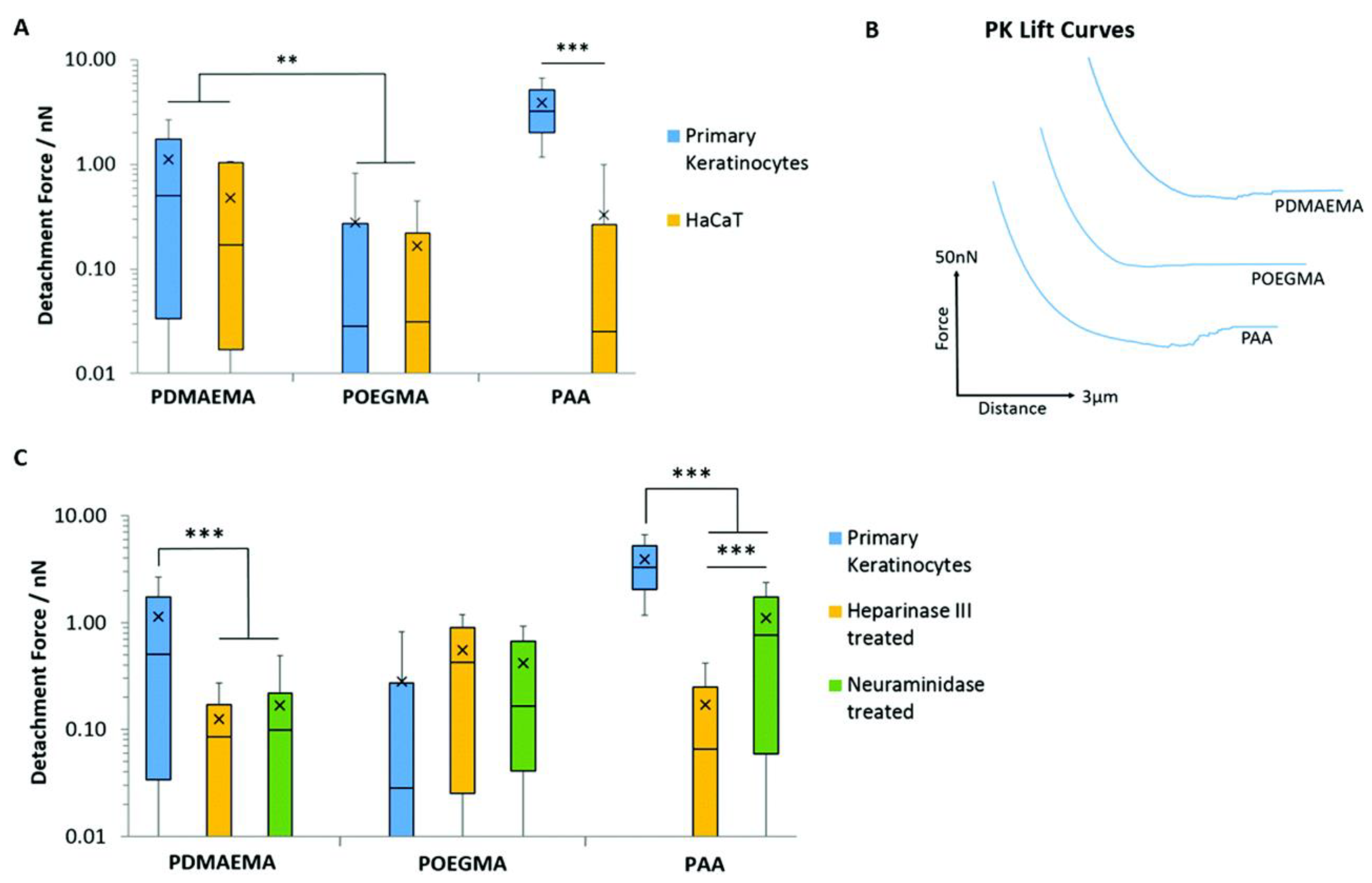
- Cozens, E.J.; Kong, D.; Roohpour, N.; Gautrot, J.E. The physico-chemistry of adhesions of protein resistant and weak polyelectrolyte brushes to cells and tissues. Soft Matter 2020, 16, 505–522. [Google Scholar] [CrossRef]
- Hsu, F.-M.; Hu, M.-H.; Jiang, Y.-S.; Lin, B.-Y.; Hu, J.-J.; Jan, J.-S. Antibacterial polypeptide/heparin composite hydrogels carrying growth factor for wound healing. Mater. Sci. Eng. C 2020, 112, 110923. [Google Scholar] [CrossRef]
- Yan, W.; Xu, X.; Xu, Q.; Sun, Z.; Jiang, Q.; Shi, D. Platelet-rich plasma combined with injectable hyaluronic acid hydrogel for porcine cartilage regeneration: A 6-month follow-up. Regen. Biomater. 2020, 7, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.C.; de Vries, R.; Lyklema, H. Chapter 2—Polyelectrolytes. In Fundamentals of Interface and Colloid Science; Lyklema, J., Ed.; Academic Press: Cambridge, MA, USA, 2005; pp. 2.1–2.84. [Google Scholar]
- Dobrynin, A.V.; Rubinstein, M. Theory of polyelectrolytes in solutions and at surfaces. Prog. Polym. Sci. 2005, 30, 1049–1118. [Google Scholar] [CrossRef]
- Dobrynin, A.V.; Colby, R.H.; Rubinstein, M. Scaling Theory of Polyelectrolyte Solutions. Macromolecules 1995, 28, 1859–1871. [Google Scholar] [CrossRef]
- Holm, C.; Joanny, J.F.; Kremer, K.; Netz, R.R.; Reineker, P.; Seidel, C.; Vilgis, T.A.; Winkler, R.G. Polyelectrolyte Theory. In Polyelectrolytes with Defined Molecular Architecture; Springer: Berlin, Germany, 2004. [Google Scholar]
- Hernández Cifre, J.G.; De La Torre, J.G. Ionic strength effect in polyelectrolyte dilute solutions within the Debye–Hückel approximation: Monte Carlo and Brownian dynamics simulations. Polym. Bull. 2014, 71, 2269–2285. [Google Scholar] [CrossRef]
- Blanco, P.M.; Madurga, S.; Narambuena, C.F.; Mas, F.; Garcés, J.L. Role of Charge Regulation and Fluctuations in the Conformational and Mechanical Properties of Weak Flexible Polyelectrolytes. Polymers 2019, 11, 1962. [Google Scholar] [CrossRef]
- Chremos, A.; Douglas, J.F. The Influence of Polymer and Ion Solvation on the Conformational Properties of Flexible Polyelectrolytes. Gels 2018, 4, 20. [Google Scholar] [CrossRef]
- Zhu, R.; Feng, Y.; Luo, P. Net Contribution of Hydrophobic Association to the Thickening Power of Hydrophobically Modified Polyelectrolytes Prepared by Micellar Polymerization. Macromolecules 2020, 53, 1326–1337. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Yang, Y.; Shu, X.; Yan, H.; Ran, Q. Effect of hydrophobic groups on the adsorption conformation of modified polycarboxylate superplasticizer investigated by molecular dynamics simulation. Appl. Surf. Sci. 2017, 407, 8–15. [Google Scholar] [CrossRef]
- Sadman, K.; Wang, Q.; Chen, Y.; Keshavarz, B.; Jiang, Z.; Shull, K.R. Influence of Hydrophobicity on Polyelectrolyte Complexation. Macromolecules 2017, 50, 9417–9426. [Google Scholar] [CrossRef]
- Lopez, C.G. Entanglement of semiflexible polyelectrolytes: Crossover concentrations and entanglement density of sodium carboxymethyl cellulose. J. Rheol. 2020, 64, 191–204. [Google Scholar] [CrossRef]
- Lopez, C.G.; Colby, R.H.; Graham, P.; Cabral, J.T. Viscosity and Scaling of Semiflexible Polyelectrolyte NaCMC in Aqueous Salt Solutions. Macromolecules 2016, 50, 332–338. [Google Scholar] [CrossRef]
- Midya, J.; Egorov, S.A.; Binder, K.; Nikoubashman, A. Phase behavior of flexible and semiflexible polymers in solvents of varying quality. J. Chem. Phys. 2019, 151, 034902. [Google Scholar] [CrossRef]
- Kierfeld, J.; Baczynski, K.; Gutjahr, P.; Lipowsky, R. Semiflexible Polymers and Filaments: From Variational Problems to Fluctuations. AIP Conf. Proc. 2008, 1002, 151–185. [Google Scholar] [CrossRef]
- Guilbaud, S.; Salomé, L.; Destainville, N.; Manghi, M.; Tardin, C. Dependence of DNA Persistence Length on Ionic Strength and Ion Type. Phys. Rev. Lett. 2019, 122, 028102. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, D.; Yan, J.; Matyjaszewski, K.; Tilton, R.D. Swelling of multi-responsive spherical polyelectrolyte brushes across a wide range of grafting densities. Colloid Polym. Sci. 2019, 298, 35–49. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Wang, Y.; Yang, Q.; Ye, Z.; Hua, C.; Tian, Y.; Von Klitzing, R.; Guo, X. Interaction among Spherical Polyelectrolyte Brushes in Concentrated Aqueous Solution. Langmuir 2020, 36, 3104–3110. [Google Scholar] [CrossRef] [PubMed]
- Laktionov, M.Y.; Zhulina, E.B.; Borisov, O.V. Proteins and Polyampholytes Interacting with Polyelectrolyte Brushes and Microgels: The Charge Reversal Concept Revised. Langmuir 2021, 37, 2865–2873. [Google Scholar] [CrossRef]
- Yigit, C.; Kanduč, M.; Ballauff, M.; Dzubiella, J. Interaction of Charged Patchy Protein Models with Like-Charged Polyelectrolyte Brushes. Langmuir 2016, 33, 417–427. [Google Scholar] [CrossRef]
- Papagiannopoulos, A.; Meristoudi, A.; Pispas, S.; Radulescu, A. Micelles from HOOC-PnBA-b-PAA-C12H15Diblock Amphiphilic Polyelectrolytes as Protein Nanocarriers. Biomacromolecules 2016, 17, 3816–3827. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.G.; Colby, R.H.; Cabral, J.T. Electrostatic and Hydrophobic Interactions in NaCMC Aqueous Solutions: Effect of Degree of Substitution. Macromolecules 2018, 51, 3165–3175. [Google Scholar] [CrossRef]
- Han, Y.; Tan, J.; Wang, D.; Xu, K.; An, H. Novel approach to promote the hydrophobic association: Introduction of short alkyl chains into hydrophobically associating polyelectrolytes. J. Appl. Polym. Sci. 2019, 136. [Google Scholar] [CrossRef]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive Polymers for Biomedical Applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef]
- Papagiannopoulos, A.; Meristoudi, A.; Pispas, S.; Keiderling, U. Thermoresponsive Behavior of Micellar Aggregates from end-functionalized PnBA-b-PNIPAM-COOH Block Copolymer and their Complexes with Lysozyme. Soft Matter 2016, 12, 6547–6556. [Google Scholar] [CrossRef]
- Papagiannopoulos, A.; Meristoudi, A.; Pispas, S.; Keiderling, U. Thermal response of self-organization in an amphiphilic triblock polyelectrolyte and the influence of the globular protein lysozyme. Eur. Polym. J. 2018, 99, 49–57. [Google Scholar] [CrossRef]
- Ullah, S.; Khalil, A.A.; Shaukat, F.; Song, Y. Sources, Extraction and Biomedical Properties of Polysaccharides. Foods 2019, 8, 304. [Google Scholar] [CrossRef]
- Lovegrove, A.; Edwards, C.H.; DE Noni, I.; Patel, H.; El, S.N.; Grassby, T.; Zielke, C.; Ulmius, M.; Nilsson, L.; Butterworth, P.J.; et al. Role of polysaccharides in food, digestion, and health. Crit. Rev. Food Sci. Nutr. 2017, 57, 237–253. [Google Scholar] [CrossRef]
- Huang, G.; Mei, X.; Xiao, F.; Chen, X.; Tang, Q.; Peng, D. Applications of Important Polysaccharides in Drug Delivery. Curr. Pharm. Des. 2015, 21, 3692–3696. [Google Scholar] [CrossRef]
- Li, M.; Ding, J.; Tao, Y.; Shi, B.; Chen, J.-H. Polysaccharides for Biomedical Applications. Int. J. Polym. Sci. 2019, 7841836. [Google Scholar] [CrossRef]
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic acid—Based wound dressings: A review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef]
- Sworn, G. Chapter 27—Xanthan gum. In Handbook of Hydrocolloids, 3rd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Cambridge, UK, 2021; pp. 833–853. [Google Scholar]
- Fittolani, G.; Seeberger, P.H.; DelBianco, M. Helical polysaccharides. Pept. Sci. 2020, 112, e24124. [Google Scholar] [CrossRef]
- Lv, B.; Bu, X.; Da, Y.; Duan, P.; Wang, H.; Ren, J.; Lyu, B.; Gao, D.; Ma, J. Gelatin/PAM double network hydrogels with super-compressibility. Polymer 2020, 210, 123021. [Google Scholar] [CrossRef]
- Chang, S.; Qin, D.; Yan, R.; Zhang, M.; Sui, B.; Xu, H.; Zheng, Z.; Hou, X.; Wang, Y.; Qi, C. Temperature and pH Dual Responsive Nanogels of Modified Sodium Alginate and NIPAM for Berberine Loading and Release. ACS Omega 2021, 6, 1119–1128. [Google Scholar] [CrossRef]
- Mirtič, J.; Ilaš, J.; Kristl, J. Influence of different classes of crosslinkers on alginate polyelectrolyte nanoparticle formation, thermodynamics and characteristics. Carbohydr. Polym. 2018, 181, 93–102. [Google Scholar] [CrossRef]
- Maity, S.; Sa, B. Development and Evaluation of Ca+ 2Ion Cross-Linked Carboxymethyl Xanthan Gum Tablet Prepared by Wet Granulation Technique. AAPS PharmSciTech 2014, 15, 920–927. [Google Scholar] [CrossRef][Green Version]
- Bui, V.T.N.T.; Nguyen, B.T.; Nicolai, T.; Renou, F. Mixed iota and kappa carrageenan gels in the presence of both calcium and potassium ions. Carbohydr. Polym. 2019, 223, 115107. [Google Scholar] [CrossRef] [PubMed]
- Brenner, T.; Tuvikene, R.; Parker, A.; Matsukawa, S.; Nishinari, K. Rheology and structure of mixed kappa-carrageenan/iota-carrageenan gels. Food Hydrocoll. 2014, 39, 272–279. [Google Scholar] [CrossRef]
- Meka, V.S.; Sing, M.K.G.; Pichika, M.R.; Nali, S.R.; Kolapalli, V.R.; Kesharwani, P. A comprehensive review on polyelectrolyte complexes. Drug Discov. Today 2017, 22, 1697–1706. [Google Scholar] [CrossRef]
- Raik, S.V.; Gasilova, E.R.; Dubashynskaya, N.V.; Dobrodumov, A.V.; Skorik, Y.A. Diethylaminoethyl chitosan–hyaluronic acid polyelectrolyte complexes. Int. J. Biol. Macromol. 2020, 146, 1161–1168. [Google Scholar] [CrossRef]
- Montero, N.; Alhajj, M.J.; Sierra, M.; Oñate-Garzon, J.; Yarce, C.J.; Salamanca, C.H. Development of Polyelectrolyte Complex Nanoparticles-PECNs Loaded with Ampicillin by Means of Polyelectrolyte Complexation and Ultra-High Pressure Homogenization (UHPH). Polymers 2020, 12, 1168. [Google Scholar] [CrossRef]
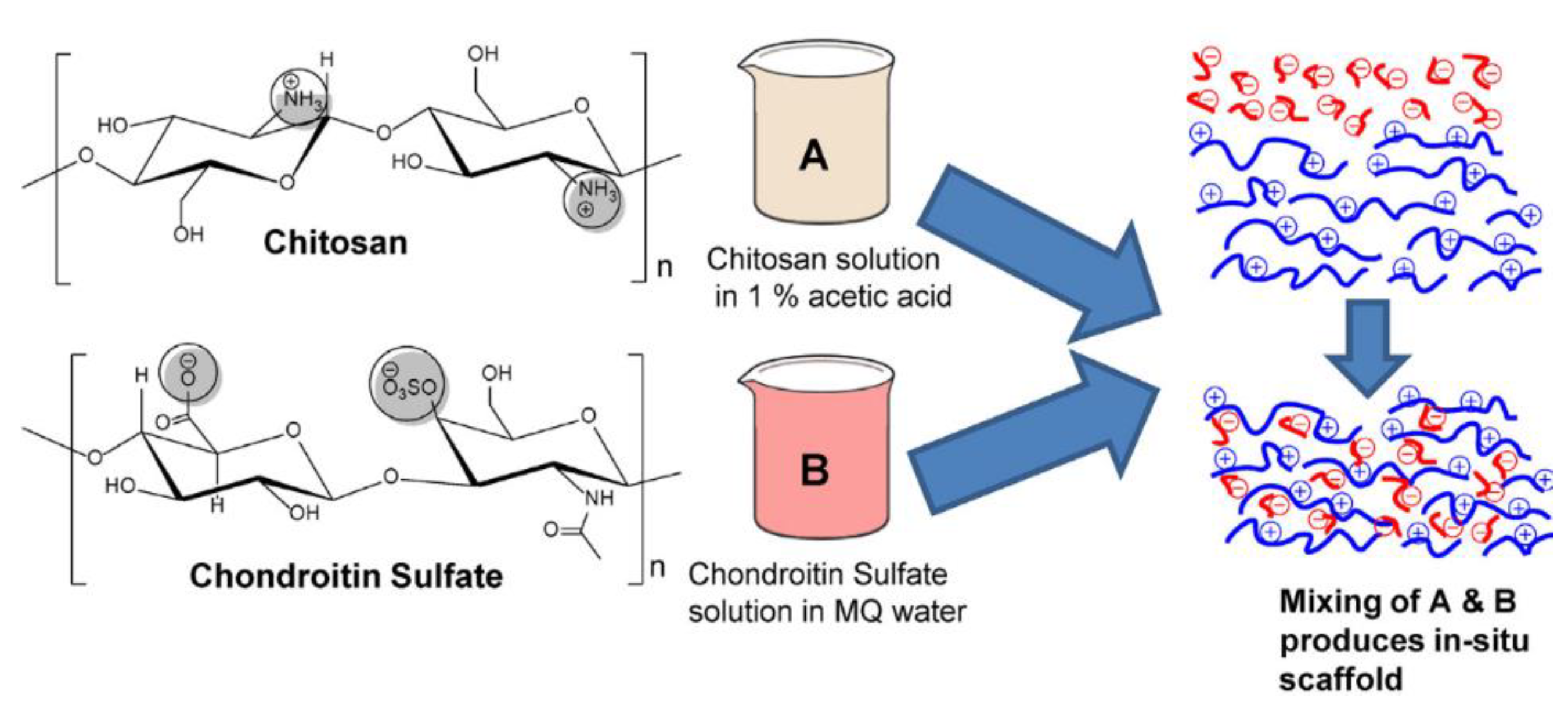
- Sharma, S.; Swetha, K.L.; Roy, A. Chitosan-Chondroitin sulfate based polyelectrolyte complex for effective management of chronic wounds. Int. J. Biol. Macromol. 2019, 132, 97–108. [Google Scholar] [CrossRef]
- Matsumoto, A.; Kurata, T.; Shiino, D.; Kataoka, K. Swelling and Shrinking Kinetics of Totally Synthetic, Glucose-Responsive Polymer Gel Bearing Phenylborate Derivative as a Glucose-Sensing Moiety. Macromolecules 2004, 37, 1502–1510. [Google Scholar] [CrossRef]
- Meunier, M.; Goupil, A.; Lienard, P. Predicting drug loading in PLA-PEG nanoparticles. Int. J. Pharm. 2017, 526, 157–166. [Google Scholar] [CrossRef]
- Otto, D.P.; De Villiers, M.M. Coarse-Grained Molecular Dynamics (CG-MD) Simulation of the Encapsulation of Dexamethasone in PSS/PDDA Layer-by-Layer Assembled Polyelectrolyte Nanocapsules. AAPS PharmSciTech 2020, 21, 292. [Google Scholar] [CrossRef]
- Gandhi, S.; Roy, I. Doxorubicin-loaded casein nanoparticles for drug delivery: Preparation, characterization and in vitro evaluation. Int. J. Biol. Macromol. 2019, 121, 6–12. [Google Scholar] [CrossRef]
- Sahu, A.; Kasoju, N.; Bora, U. Fluorescence Study of the Curcumin−Casein Micelle Complexation and Its Application as a Drug Nanocarrier to Cancer Cells. Biomacromolecules 2008, 9, 2905–2912. [Google Scholar] [CrossRef]
- Huang, J.; Wu, B.; Zhou, Z.; Hu, S.; Xu, H.; Piao, Y.; Zheng, H.; Tang, J.; Liu, X.; Shen, Y. Drug-binding albumins forming stabilized nanoparticles for efficient anticancer therapy. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102058. [Google Scholar] [CrossRef]
- Larsen, M.T.; Kuhlmann, M.; Hvam, M.L.; Howard, K.A. Albumin-based drug delivery: Harnessing nature to cure disease. Mol. Cell. Ther. 2016, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zheng, K.; Si, Y.; Guo, X.; Xu, Y. Protein–Polyelectrolyte Interaction: Thermodynamic Analysis Based on the Titration Method. Polymers 2019, 11, 82. [Google Scholar] [CrossRef]
- da Silva, F.L.B.; Joensson, B. Polyelectrolyte–protein complexation driven by charge regulation. Soft Matter 2009, 5, 2862–2868. [Google Scholar] [CrossRef]
- Kim, S.; Sureka, H.V.; Kayitmazer, A.B.; Wang, G.; Swan, J.W.; Olsen, B.D. Effect of Protein Surface Charge Distribution on Protein–Polyelectrolyte Complexation. Biomacromolecules 2020, 21, 3026–3037. [Google Scholar] [CrossRef] [PubMed]
- Henzler, K.; Haupt, B.; Lauterbach, K.; Wittemann, A.; Borisov, O.; Ballauff, M. Adsorption of β-Lactoglobulin on Spherical Polyelectrolyte Brushes: Direct Proof of Counterion Release by Isothermal Titration Calorimetry. J. Am. Chem. Soc. 2010, 132, 3159–3163. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sun, Y.; Davis, T.P.; Ke, P.C.; Wu, Y.; Ding, F. Understanding Effects of PAMAM Dendrimer Size and Surface Chemistry on Serum Protein Binding with Discrete Molecular Dynamics Simulations. ACS Sustain. Chem. Eng. 2018, 6, 11704–11715. [Google Scholar] [CrossRef]
- Guo, S.; Kwek, M.Y.; Toh, Z.Q.; Pranantyo, D.; Kang, E.-T.; Loh, X.J.; Zhu, X.; Jańczewski, D.; Neoh, K.G. Tailoring Polyelectrolyte Architecture To Promote Cell Growth and Inhibit Bacterial Adhesion. ACS Appl. Mater. Interfaces 2018, 10, 7882–7891. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Sekhar, V.C.; Nair, S.S. Polyelectrolyte complexes of carboxymethyl chitosan/alginate based drug carrier for targeted and controlled release of dual drug. J. Drug Deliv. Sci. Technol. 2019, 51, 569–582. [Google Scholar] [CrossRef]
- Chen, T.; Li, S.; Zhu, W.; Liang, Z.; Zeng, Q. Self-assembly pH-sensitive chitosan/alginate coated polyelectrolyte complexes for oral delivery of insulin. J. Microencapsul. 2019, 36, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Tabandeh, S.; Leon, L. Engineering Peptide-Based Polyelectrolyte Complexes with Increased Hydrophobicity. Molecules 2019, 24, 868. [Google Scholar] [CrossRef] [PubMed]
- Fatima, M.T.; Chanchal, A.; Yavvari, P.S.; Bhagat, S.D.; Gujrati, M.; Mishra, R.K.; Srivastava, A. Cell Permeating Nano-Complexes of Amphiphilic Polyelectrolytes Enhance Solubility, Stability, and Anti-Cancer Efficacy of Curcumin. Biomacromolecules 2016, 17, 2375–2383. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhu, Q.; Li, C.; Yuan, K.; Che, R.; Zhang, P.; Yang, C.; Lu, W.; Wu, W.; Jiang, X. Carbamoylmannose enhances the tumor targeting ability of supramolecular nanoparticles formed through host-guest complexation of a pair of homopolymers. J. Mater. Chem. B 2016, 5, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Sim, T.; Lim, C.; Hoang, N.H.; Kim, J.E.; Lee, E.S.; Youn, Y.S.; Oh, K.T. Synergistic photodynamic therapeutic effect of indole-3-acetic acid using a pH sensitive nano-carrier based on poly(aspartic acid-graft-imidazole)-poly(ethylene glycol). J. Mater. Chem. B 2017, 5, 8498–8505. [Google Scholar] [CrossRef]
- Folchman-Wagner, Z.; Zaro, J.; Shen, W.-C. Characterization of Polyelectrolyte Complex Formation Between Anionic and Cationic Poly(amino acids) and Their Potential Applications in pH-Dependent Drug Delivery. Molecules 2017, 22, 1089. [Google Scholar] [CrossRef]
- Ding, P.; Liu, W.; Guo, X.; Stuart, M.A.C.; Wang, J. Optimal synthesis of polyelectrolyte nanogels by electrostatic assembly directed polymerization for dye loading and release. Soft Matter 2021, 17, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Huang, J.; Wei, C.; Liu, W.; Zhou, W.; Wang, J.; Wang, M.; Guo, X.; Stuart, M.A.C.; Wang, J. Efficient and Generic Preparation of Diverse Polyelectrolyte Nanogels by Electrostatic Assembly Directed Polymerization. CCS Chem. 2020, 2, 1016–1025. [Google Scholar] [CrossRef]
- Mudassir, J.; Darwis, Y.; Yusof, S.R. Synthesis, characterization and toxicological evaluation of pH-sensitive polyelectrolyte Nanogels. J. Polym. Res. 2017, 24, 164. [Google Scholar] [CrossRef]
- Mudassir, J.; Darwis, Y.; Muhamad, S.; Khan, A.A. Self-assembled insulin and nanogels polyelectrolyte complex (Ins/NGs-PEC) for oral insulin delivery: Characterization, lyophilization and in-vivo evaluation. Int. J. Nanomed. 2019, 14, 4895–4909. [Google Scholar] [CrossRef]
- Rusu, A.G.; Chiriac, A.P.; Nita, L.E.; Rosca, I.; Pinteala, M.; Mititelu-Tartau, L. Chitosan Derivatives in Macromolecular Co-assembly Nanogels with Potential for Biomedical Applications. Biomacromolecules 2020, 21, 4231–4243. [Google Scholar] [CrossRef]
- Sana, S.S.; Arla, S.K.; Badineni, V.; Boya, V.K.N. Development of poly (acrylamide-co-diallyldimethylammoniumchloride) nanogels and study of their ability as drug delivery devices. SN Appl. Sci. 2019, 1, 1716. [Google Scholar] [CrossRef]
- Podgórna, K.; Szczepanowicz, K.; Piotrowski, M.; Gajdošová, M.; Štěpánek, F.; Warszynski, P. Gadolinium alginate nanogels for theranostic applications. Colloids Surf. B Biointerfaces 2017, 153, 183–189. [Google Scholar] [CrossRef]
- Gurav, D.D.; Kulkarni, A.S.; Khan, A.; Shinde, V.S. pH-responsive targeted and controlled doxorubicin delivery using hyaluronic acid nanocarriers. Colloids Surf. B Biointerfaces 2016, 143, 352–358. [Google Scholar] [CrossRef]
- Sarika, P.R.; Nirmala, R.J. Curcumin loaded gum arabic aldehyde-gelatin nanogels for breast cancer therapy. Mater. Sci. Eng. C 2016, 65, 331–337. [Google Scholar] [CrossRef]
- Nguyen, A.H.; McKinney, J.; Miller, T.; Bongiorno, T.; McDevitt, T.C. Gelatin methacrylate microspheres for controlled growth factor release. Acta Biomater. 2015, 13, 101–110. [Google Scholar] [CrossRef]
- Basu, S.; Pacelli, S.; Paul, A. Self-healing DNA-based injectable hydrogels with reversible covalent linkages for controlled drug delivery. Acta Biomater. 2020, 105, 159–169. [Google Scholar] [CrossRef]
- Sakr, O.S.; Jordan, O.; Borchard, G. Sustained protein release from hydrogel microparticles using layer-by-layer (LbL) technology. Drug Deliv. 2016, 23, 2747–2755. [Google Scholar] [CrossRef]
- Nolles, A.; Van Dongen, N.J.E.; Westphal, A.H.; Visser, A.J.W.G.; Kleijn, J.M.; Van Berkel, W.J.H.; Borst, J.W. Encapsulation into complex coacervate core micelles promotes EGFP dimerization. Phys. Chem. Chem. Phys. 2017, 19, 11380–11389. [Google Scholar] [CrossRef]
- Jiang, Y.; Fay, J.M.; Poon, C.; Vinod, N.; Zhao, Y.; Bullock, K.; Qin, S.; Manickam, D.S.; Yi, X.; Banks, W.A.; et al. Nanoformulation of Brain-Derived Neurotrophic Factor with Target Receptor-Triggered-Release in the Central Nervous System. Adv. Funct. Mater. 2018, 28, 1703982. [Google Scholar] [CrossRef] [PubMed]
- Papagiannopoulos, A.; Vlassi, E. Stimuli-responsive nanoparticles by thermal treatment of bovine serum albumin inside its complexes with chondroitin sulfate. Food Hydrocoll. 2019, 87, 602–610. [Google Scholar] [CrossRef]
- Vlassi, E.; Papagiannopoulos, A. Nanoformulation of fibrinogen by thermal stabilization of its electrostatic complexes with hyaluronic acid. Int. J. Biol. Macromol. 2020, 158, 251–257. [Google Scholar] [CrossRef]
- Papagiannopoulos, A.; Sklapani, A. Xanthan-based polysaccharide/protein nanoparticles: Preparation, characterization, encapsulation and stabilization of curcumin. Carbohydr. Polym. Technol. Appl. 2021, 2, 100075. [Google Scholar] [CrossRef]
- Tejada, G.; Barrera, M.G.; Piccirilli, G.N.; Sortino, M.; Frattini, A.; Salomón, C.J.; Lamas, M.C.; Leonardi, D. Development and Evaluation of Buccal Films Based on Chitosan for the Potential Treatment of Oral Candidiasis. AAPS PharmSciTech 2017, 18, 936–946. [Google Scholar] [CrossRef]
- Kilicarslan, M.; Ilhan, M.; Inal, O.; Orhan, K. Preparation and evaluation of clindamycin phosphate loaded chitosan/alginate polyelectrolyte complex film as mucoadhesive drug delivery system for periodontal therapy. Eur. J. Pharm. Sci. 2018, 123, 441–451. [Google Scholar] [CrossRef]
- Şen, F.; Uzunsoy, I.; Baştürk, E.; Kahraman, M.V. Antimicrobial agent-free hybrid cationic starch/sodium alginate polyelectrolyte films for food packaging materials. Carbohydr. Polym. 2017, 170, 264–270. [Google Scholar] [CrossRef]
- Tang, Q.; Lim, T.; Wei, X.-J.; Wang, Q.-Y.; Xu, J.-C.; Shen, L.-Y.; Zhu, Z.-Z.; Zhang, C.-Q. A free-standing multilayer film as a novel delivery carrier of platelet lysates for potential wound-dressing applications. Biomaterials 2020, 255, 120138. [Google Scholar] [CrossRef]
- Khanlari, A.; Schulteis, J.E.; Suekama, T.C.; Detamore, M.S.; Gehrke, S.H. Designing crosslinked hyaluronic acid hydrogels with tunable mechanical properties for biomedical applications. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Goh, M.; Hwang, Y.; Tae, G. Epidermal growth factor loaded heparin-based hydrogel sheet for skin wound healing. Carbohydr. Polym. 2016, 147, 251–260. [Google Scholar] [CrossRef]
- Pereira, R.F.; Barrias, C.C.; Bártolo, P.J.; Granja, P.L. Cell-instructive pectin hydrogels crosslinked via thiol-norbornene photo-click chemistry for skin tissue engineering. Acta Biomater. 2018, 66, 282–293. [Google Scholar] [CrossRef]
- Tavakoli, S.; Kharaziha, M.; Kermanpur, A.; Mokhtari, H. Sprayable and injectable visible-light Kappa-carrageenan hydrogel for in-situ soft tissue engineering. Int. J. Biol. Macromol. 2019, 138, 590–601. [Google Scholar] [CrossRef]
- Chimene, D.; Peak, C.W.; Gentry, J.L.; Carrow, J.K.; Cross, L.M.; Mondragon, E.; Cardoso, G.B.C.; Kaunas, R.; Gaharwar, A.K. Nanoengineered Ionic–Covalent Entanglement (NICE) Bioinks for 3D Bioprinting. ACS Appl. Mater. Interfaces 2018, 10, 9957–9968. [Google Scholar] [CrossRef]
- Lokhande, G.; Carrow, J.K.; Thakur, T.; Xavier, J.R.; Parani, M.; Bayless, K.J.; Gaharwar, A.K. Nanoengineered injectable hydrogels for wound healing application. Acta Biomater. 2018, 70, 35–47. [Google Scholar] [CrossRef]
- Gholizadeh, H.; Messerotti, E.; Pozzoli, M.; Cheng, S.; Traini, D.; Young, P.; Kourmatzis, A.; Caramella, C.; Ong, H.X. Application of a Thermosensitive In Situ Gel of Chitosan-Based Nasal Spray Loaded with Tranexamic Acid for Localised Treatment of Nasal Wounds. AAPS PharmSciTech 2019, 20, 299. [Google Scholar] [CrossRef] [PubMed]
- Borzacchiello, A.; Russo, L.; Malle, B.M.; Schwach-Abdellaoui, K.; Ambrosio, L. Hyaluronic Acid Based Hydrogels for Regenerative Medicine Applications. BioMed Res. Int. 2015, 2015, 871218. [Google Scholar] [CrossRef]
- Costantini, M.; Idaszek, J.; Szöke, K.; Jaroszewicz, J.; Dentini, M.; Barbetta, A.; Brinchmann, J.E.; Święszkowski, W. 3D bioprinting of BM-MSCs-loaded ECM biomimetic hydrogels for in vitro neocartilage formation. Biofabrication 2016, 8, 035002. [Google Scholar] [CrossRef]
- Kim, H.D.; Lee, E.A.; An, Y.-H.; Kim, S.L.; Lee, S.S.; Yu, S.J.; Jang, H.L.; Nam, K.T.; Im, S.G.; Hwang, N.S. Chondroitin Sulfate-Based Biomineralizing Surface Hydrogels for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2017, 9, 21639–21650. [Google Scholar] [CrossRef]
- Wang, T.; Lai, J.H.; Yang, F. Effects of Hydrogel Stiffness and Extracellular Compositions on Modulating Cartilage Regeneration by Mixed Populations of Stem Cells and Chondrocytes In Vivo. Tissue Eng. Part A 2016, 22, 1348–1356. [Google Scholar] [CrossRef]
- Hao, T.; Li, J.; Yao, F.; Dong, D.; Wang, Y.; Yang, B.; Wang, C. Injectable Fullerenol/Alginate Hydrogel for Suppression of Oxidative Stress Damage in Brown Adipose-Derived Stem Cells and Cardiac Repair. ACS Nano 2017, 11, 5474–5488. [Google Scholar] [CrossRef]
- Efraim, Y.; Sarig, H.; Cohen Anavy, N.; Sarig, U.; de Berardinis, E.; Chaw, S.-Y.; Krishnamoorthi, M.; Kalifa, J.; Bogireddi, H.; Duc, T.V.; et al. Biohybrid cardiac ECM-based hydrogels improve long term cardiac function post myocardial infarction. Acta Biomater. 2017, 50, 220–233. [Google Scholar] [CrossRef]
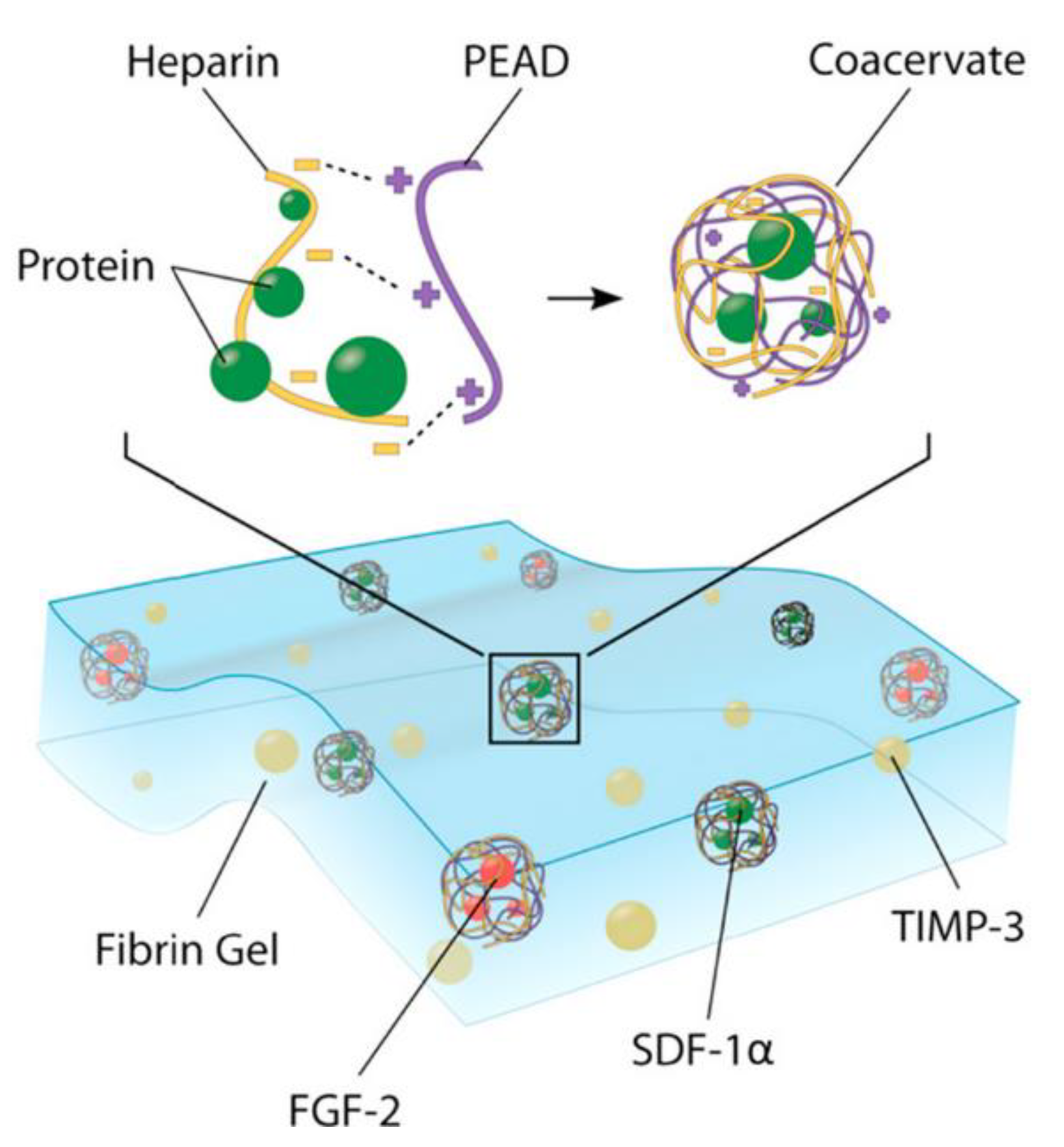
- Awada, H.K.; Long, D.W.; Wang, Z.; Hwang, M.; Kim, K.; Wang, Y. A single injection of protein-loaded coacervate-gel significantly improves cardiac function post infarction. Biomaterials 2017, 125, 65–80. [Google Scholar] [CrossRef]
- Noshadi, I.; Hong, S.; Sullivan, K.E.; Sani, E.S.; Lara, R.P.; Tamayol, A.; Shin, S.R.; Gao, A.E.; Stoppel, W.L.; Iii, L.D.B.; et al. In vitro and in vivo analysis of visible light crosslinkable gelatin methacryloyl (GelMA) hydrogels. Biomater. Sci. 2017, 5, 2093–2105. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Qin, A.; Lin, X.; Yang, L.; Wang, Q.; Wang, Z.; Shan, Z.; Li, S.; Wang, J.; Fan, S.; et al. Biodegradable and biocompatible high elastic chitosan scaffold is cell-friendly both in vitro and in vivo. Oncotarget 2017, 8, 35583–35591. [Google Scholar] [CrossRef] [PubMed]
- Raia, N.R.; Jia, D.; Ghezzi, C.E.; Muthukumar, M.; Kaplan, D.L. Characterization of silk-hyaluronic acid composite hydrogels towards vitreous humor substitutes. Biomaterials 2020, 233, 119729. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papagiannopoulos, A. Current Research on Polyelectrolyte Nanostructures: From Molecular Interactions to Biomedical Applications. Macromol 2021, 1, 155-172. https://doi.org/10.3390/macromol1020012
Papagiannopoulos A. Current Research on Polyelectrolyte Nanostructures: From Molecular Interactions to Biomedical Applications. Macromol. 2021; 1(2):155-172. https://doi.org/10.3390/macromol1020012
Chicago/Turabian StylePapagiannopoulos, Aristeidis. 2021. "Current Research on Polyelectrolyte Nanostructures: From Molecular Interactions to Biomedical Applications" Macromol 1, no. 2: 155-172. https://doi.org/10.3390/macromol1020012
APA StylePapagiannopoulos, A. (2021). Current Research on Polyelectrolyte Nanostructures: From Molecular Interactions to Biomedical Applications. Macromol, 1(2), 155-172. https://doi.org/10.3390/macromol1020012





