Chitosan Adsorbent Derivatives for Pharmaceuticals Removal from Effluents: A Review
Abstract
1. Introduction
2. Synthetic Routes and Characterizations
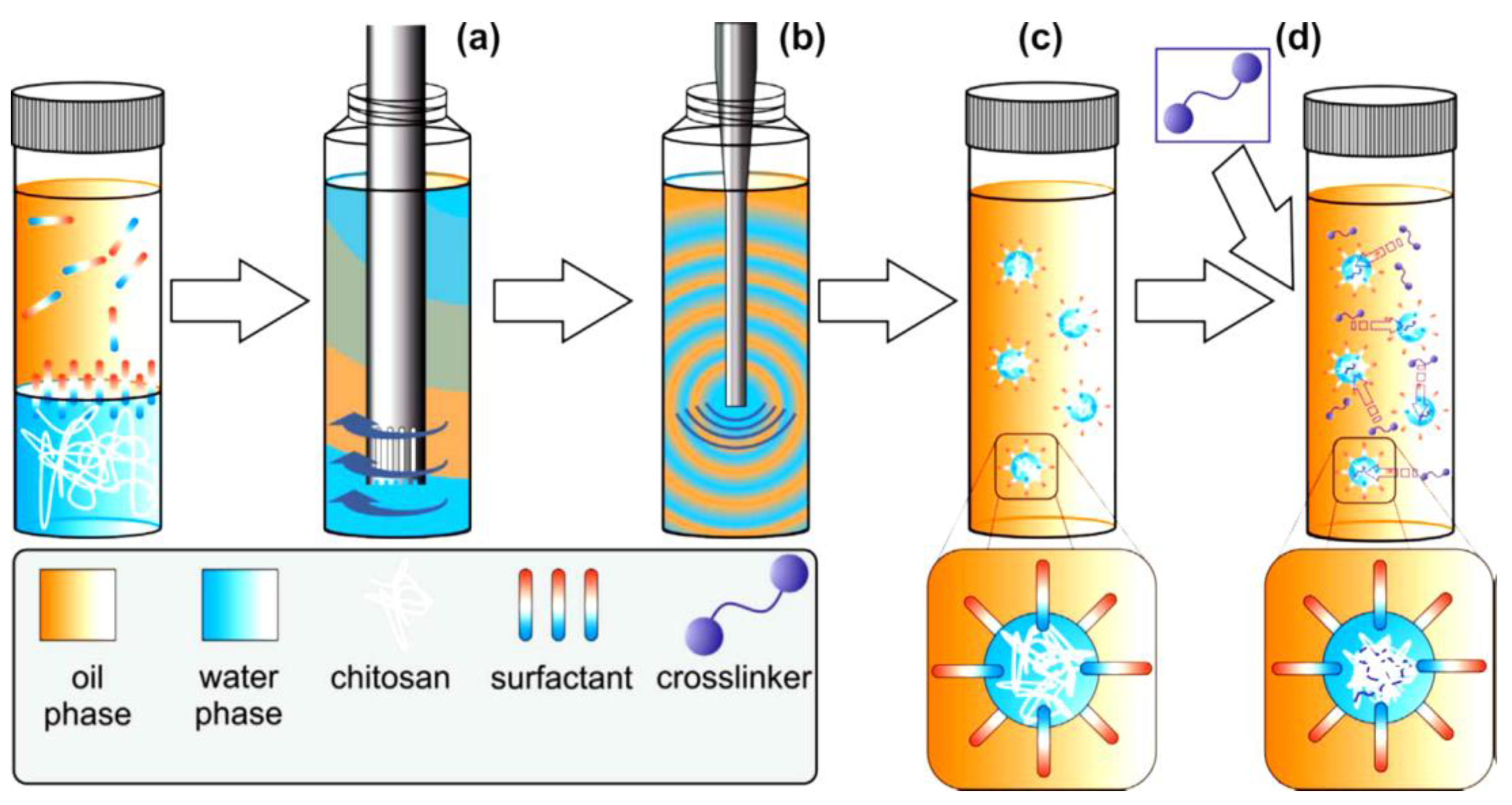
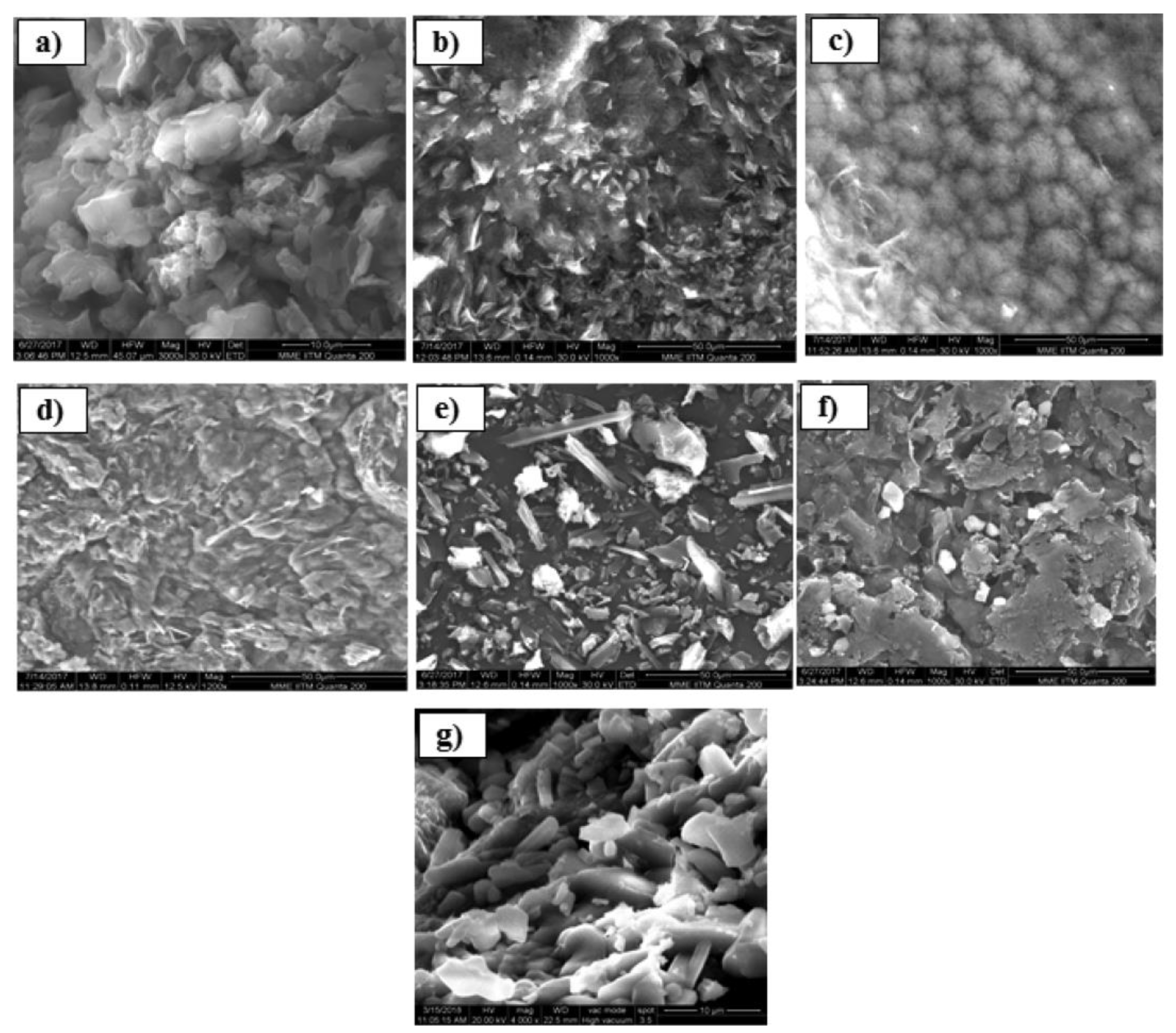
2.1. Chitosan/Modified Chitosan Beads
2.2. Chitosan Nanoparticles/Chitosan Film
2.3. Grafted Chitosan
2.4. Chitosan Composites with Magnetic Properties
2.5. Chitosan Combined with MOFs
2.6. Other Chitosan Composites
3. Adsorption Evaluation
3.1. Isotherm Models and Kinetic Equations
3.1.1. Isotherm Models
3.1.2. Kinetic Equations
3.2. Discussion
3.2.1. Pharmaceutical Compounds—Effect of pH
3.2.2. Pharmaceutical Compounds—Evaluation of Adsorption Isotherm Models
3.2.3. Pharmaceutical Compounds—Adsorption Kinetics
3.3. Personal Care Products
3.3.1. Personal Care Compounds—pH Effect
3.3.2. Personal Care Products—Evaluation of Adsorption Isotherm Models
3.3.3. Personal Care Products—Adsorption Kinetics
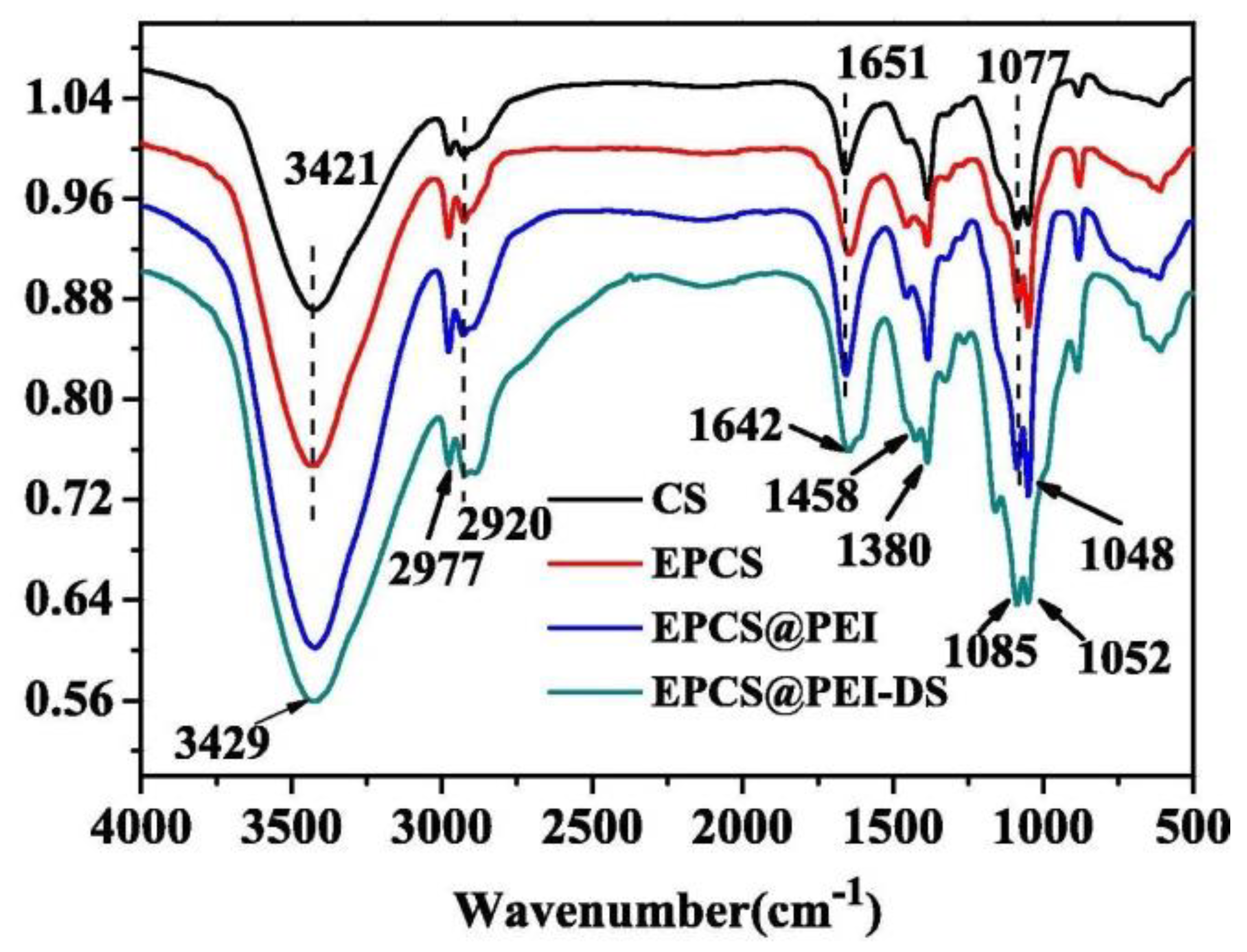
3.4. FTIR Analysis for Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Andrade, J.R.; Oliveira, M.F.; Da Silva, M.G.C.; Vieira, M.G.A. Adsorption of Pharmaceuticals from Water and Wastewater Using Nonconventional Low-Cost Materials: A Review. Ind. Eng. Chem. Res. 2018, 57, 3103–3127. [Google Scholar] [CrossRef]
- Tanhaei, B.; Ayati, A.; Iakovleva, E.; Sillanpää, M. Efficient carbon interlayed magnetic chitosan adsorbent for anionic dye removal: Synthesis, characterization and adsorption study. Int. J. Biol. Macromol. 2020, 164, 3621–3631. [Google Scholar] [CrossRef]
- Samadi, F.Y.; Mohammadi, Z.; Yousefi, M.; Majdejabbari, S. Synthesis of raloxifene-chitosan conjugate: A novel chitosan derivative as a potential targeting vehicle. Int. J. Biol. Macromol. 2016, 82, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Kyzas, G.Z.; Cai, Z.; Deliyanni, E.A.; Liu, W.; Zhao, D. Photocatalytic degradation of phenanthrene by graphite oxide-TiO2-Sr(OH)2/SrCO3 nanocomposite under solar irradiation: Effects of water quality parameters and predictive modeling. Chem. Eng. J. 2018, 335, 290–300. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Nanaki, S.G.; Koltsakidou, A.; Papageorgiou, M.; Kechagia, M.; Bikiaris, D.N.; Lambropoulou, D.A. Effectively designed molecularly imprinted polymers for selective isolation of the antidiabetic drug metformin and its transformation product guanylurea from aqueous media. Anal. Chim. Acta 2015, 866, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Bikiaris, D.N.; Mitropoulos, A.C. Chitosan adsorbents for dye removal: A review. Polym. Int. 2017, 66, 1800–1811. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Hosseini-Bandegharaei, A.; Fu, J.; Mitropoulos, A.C.; Kyzas, G.Z. Use of nanoparticles for dye adsorption: Review. J. Dispers. Sci. Technol. 2018, 39, 836–847. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Lazaridis, N.K.; Kostoglou, M. On the simultaneous adsorption of a reactive dye and hexavalent chromium from aqueous solutions onto grafted chitosan. J. Colloid Interface Sci. 2013, 407, 432–441. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Kyzas, G.Z. Composts as biosorbents for decontamination of various pollutants: A review. Waterairand Soil Pollut. 2015, 226. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Papageorgiou, M.; Kyzas, G.Z.; Bikiaris, D.N.; Lambropoulou, D.A. Preparation of molecularly imprinted solid-phase microextraction fiber for the selective removal and extraction of the antiviral drug abacavir in environmental and biological matrices. Anal. Chim. Acta 2016, 913, 63–75. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Ayati, A.; Davoodi, R.; Tanhaei, B.; Karimi, F.; Malekmohammadi, S.; Orooji, Y.; Fu, L.; Sillanpää, M. Recent advances in using of chitosan-based adsorbents for removal of pharmaceutical contaminants: A review. J. Clean. Prod. 2021, 291. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Z.; Ouyang, X.-k.; Ji, C.; Liu, Y.; Huang, F.; Yang, L.-Y. Fabrication of cross-linked chitosan beads grafted by polyethylenimine for efficient adsorption of diclofenac sodium from water. Int. J. Biol. Macromol. 2020, 145, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Ranjbari, S.; Tanhaei, B.; Ayati, A.; Khadempir, S.; Sillanpää, M. Efficient tetracycline adsorptive removal using tricaprylmethylammonium chloride conjugated chitosan hydrogel beads: Mechanism, kinetic, isotherms and thermodynamic study. Int. J. Biol. Macromol. 2020, 155, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Riegger, B.R.; Bäurer, B.; Mirzayeva, A.; Tovar, G.E.M.; Bach, M. A systematic approach of chitosan nanoparticle preparation via emulsion crosslinking as potential adsorbent in wastewater treatment. Carbohydr. Polym. 2018, 180, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, V.; Gubitosa, J.; Fini, P.; Romita, R.; Nuzzo, S.; Gabaldón, J.A.; Gorbe, M.I.F.; Gómez-Morte, T.; Cosma, P. Chitosan film as recyclable adsorbent membrane to remove/recover hazardous pharmaceutical pollutants from water: The case of the emerging pollutant Furosemide. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2020, 56, 145–156. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bikiaris, D.N.; Lambropoulou, D.A. Effect of humic acid on pharmaceuticals adsorption using sulfonic acid grafted chitosan. J. Mol. Liq. 2017, 230, 1–5. [Google Scholar] [CrossRef]
- Tzereme, A.; Christodoulou, E.; Kyzas, G.Z.; Kostoglou, M.; Bikiaris, D.N.; Lambropoulou, D.A. Chitosan Grafted Adsorbents for Diclofenac Pharmaceutical Compound Removal from Single-Component Aqueous Solutions and Mixtures. Polymers 2019, 11, 497. [Google Scholar] [CrossRef]
- Malesic-Eleftheriadou, N.; Evgenidou, E.; Lazaridou, M.; Bikiaris, D.N.; Yang, X.; Kyzas, G.Z.; Lambropoulou, D.A. Simultaneous removal of anti-inflammatory pharmaceutical compounds from an aqueous mixture with adsorption onto chitosan zwitterionic derivative. Colloids Surf. A Physicochem. Eng. Asp. 2021, 126498. [Google Scholar] [CrossRef]
- Ahamad, T.; Ruksana; Chaudhary, A.A.; Naushad, M.; Alshehri, S.M. Fabrication of MnFe2O4 nanoparticles embedded chitosan-diphenylureaformaldehyde resin for the removal of tetracycline from aqueous solution. Int. J. Biol. Macromol. 2019, 134, 180–188. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, Y.; Yang, Z.; Yang, W.; Wu, J.; Dong, C. Adsorption of pharmaceuticals on chitosan-based magnetic composite particles with core-brush topology. Chem. Eng. J. 2016, 304, 325–334. [Google Scholar] [CrossRef]
- Zhou, X.; Dong, C.; Yang, Z.; Tian, Z.; Lu, L.; Yang, W.; Wang, Y.; Zhang, L.; Li, A.; Chen, J. Enhanced adsorption of pharmaceuticals onto core-brush shaped aromatic rings-functionalized chitosan magnetic composite particles: Effects of structural characteristics of both pharmaceuticals and brushes. J. Clean. Prod. 2018, 172, 1025–1034. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, R.; Li, M.; Yu, F.; He, C. Removal of pharmaceuticals by novel magnetic genipin-crosslinked chitosan/graphene oxide-SO3H composite. Carbohydr. Polym. 2019, 220, 141–148. [Google Scholar] [CrossRef]
- Zhuo, N.; Lan, Y.; Yang, W.; Yang, Z.; Li, X.; Zhou, X.; Liu, Y.; Shen, J.; Zhang, X. Adsorption of three selected pharmaceuticals and personal care products (PPCPs) onto MIL-101(Cr)/natural polymer composite beads. Sep. Purif. Technol. 2017, 177, 272–280. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, B.; Chen, C.; Fu, X.; Huang, Q. Immobilization of chitosan grafted carboxylic Zr-MOF to porous starch for sulfanilamide adsorption. Carbohydr. Polym. 2021, 253, 117305. [Google Scholar] [CrossRef] [PubMed]
- Delhiraja, K.; Vellingiri, K.; Boukhvalov, D.W.; Philip, L. Development of Highly Water Stable Graphene Oxide-Based Composites for the Removal of Pharmaceuticals and Personal Care Products. Ind. Eng. Chem. Res. 2019, 58, 2899–2913. [Google Scholar] [CrossRef]
- Zhao, F.; Repo, E.; Yin, D.; Chen, L.; Kalliola, S.; Tang, J.; Iakovleva, E.; Tam, K.; Sillanpää, M. One-pot synthesis of trifunctional chitosan-EDTA-β-cyclodextrin polymer for simultaneous removal of metals and organic micropollutants. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Lessa, E.F.; Nunes, M.L.; Fajardo, A.R. Chitosan/waste coffee-grounds composite: An efficient and eco-friendly adsorbent for removal of pharmaceutical contaminants from water. Carbohydr. Polym. 2018, 189, 257–266. [Google Scholar] [CrossRef]
- Feng, Z.; Danjo, T.; Odelius, K.; Hakkarainen, M.; Iwata, T.; Albertsson, A.C. Recyclable Fully Biobased Chitosan Adsorbents Spray-Dried in One Pot to Microscopic Size and Enhanced Adsorption Capacity. Biomacromolecules 2019, 20, 1956–1964. [Google Scholar] [CrossRef]
- Yanyan, L.; Kurniawan, T.A.; Albadarin, A.B.; Walker, G. Enhanced removal of acetaminophen from synthetic wastewater using multi-walled carbon nanotubes (MWCNTs) chemically modified with NaOH, HNO3/H2SO4, ozone, and/or chitosan. J. Mol. Liq. 2018, 251, 369–377. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Matis, K.A. Nanoadsorbents for pollutants removal: A review. J. Mol. Liq. 2015, 203, 159–168. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bikiaris, D.N.; Seredych, M.; Bandosz, T.J.; Deliyanni, E.A. Removal of dorzolamide from biomedical wastewaters with adsorption onto graphite oxide/poly(acrylic acid) grafted chitosan nanocomposite. Bioresour. Technol. 2014, 152, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Kostoglou, M.; Lazaridis, N.K.; Lambropoulou, D.A.; Bikiaris, D.N. Environmental friendly technology for the removal of pharmaceutical contaminants from wastewaters using modified chitosan adsorbents. Chem. Eng. J. 2013, 222, 248–258. [Google Scholar] [CrossRef]







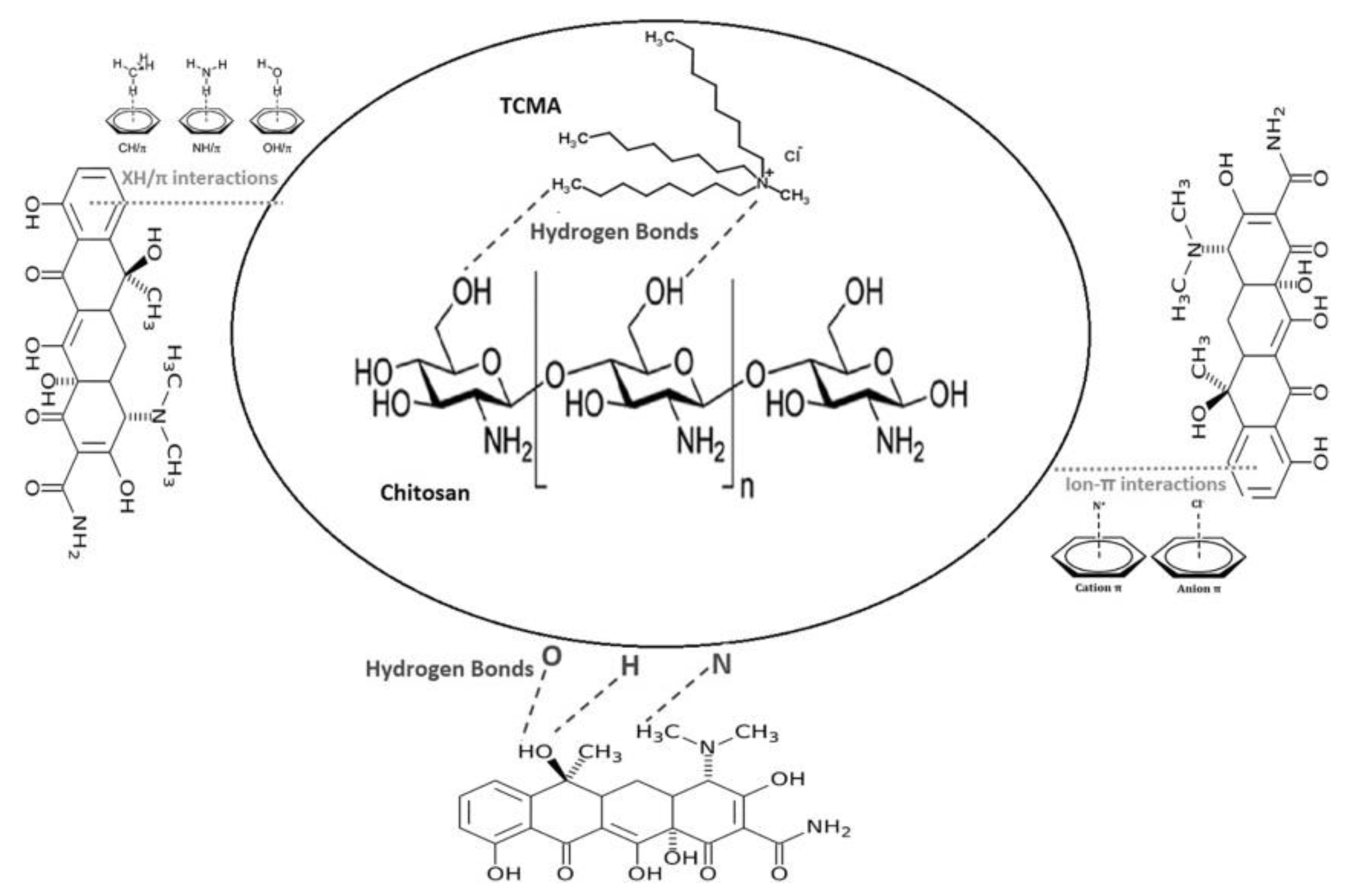
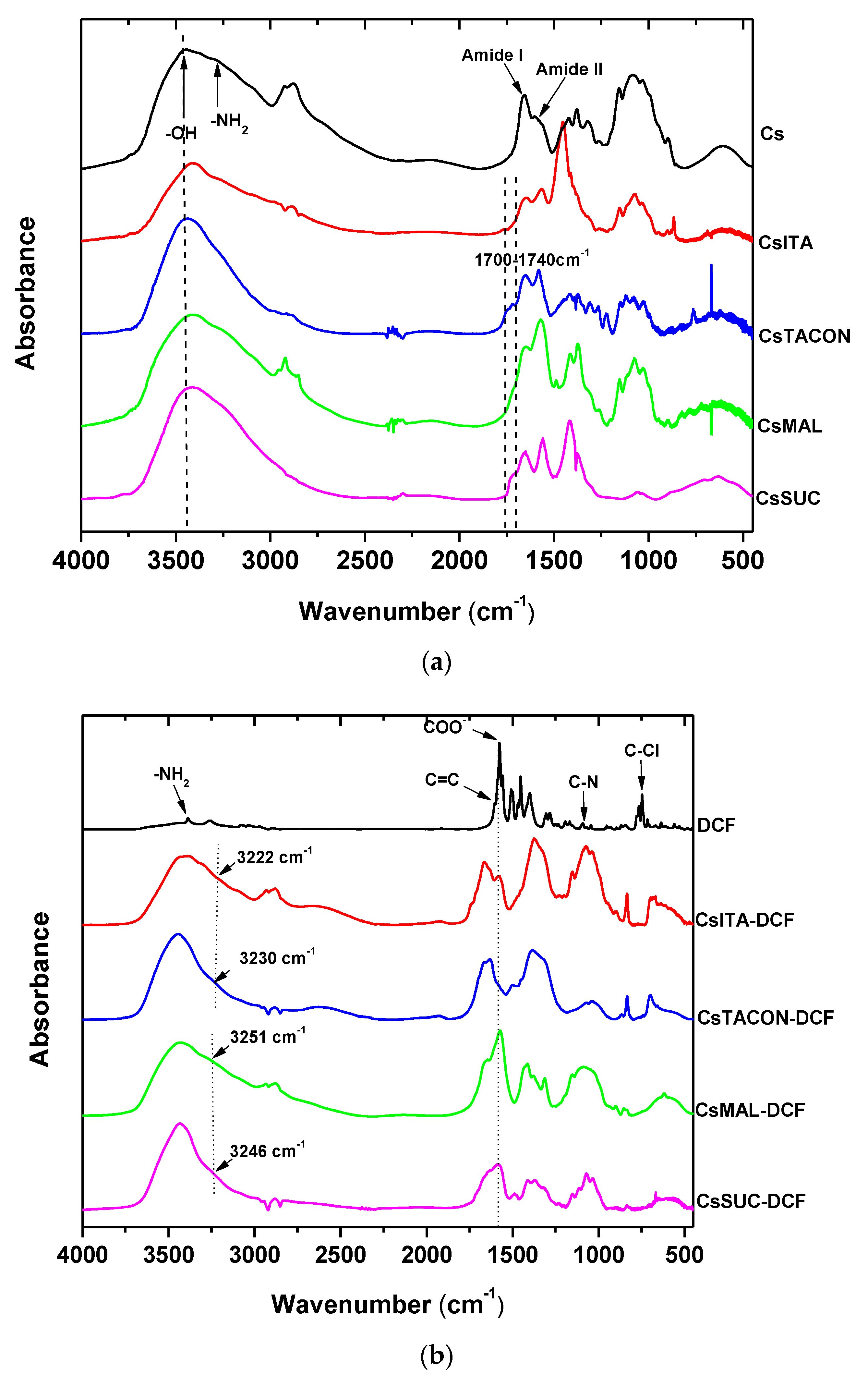
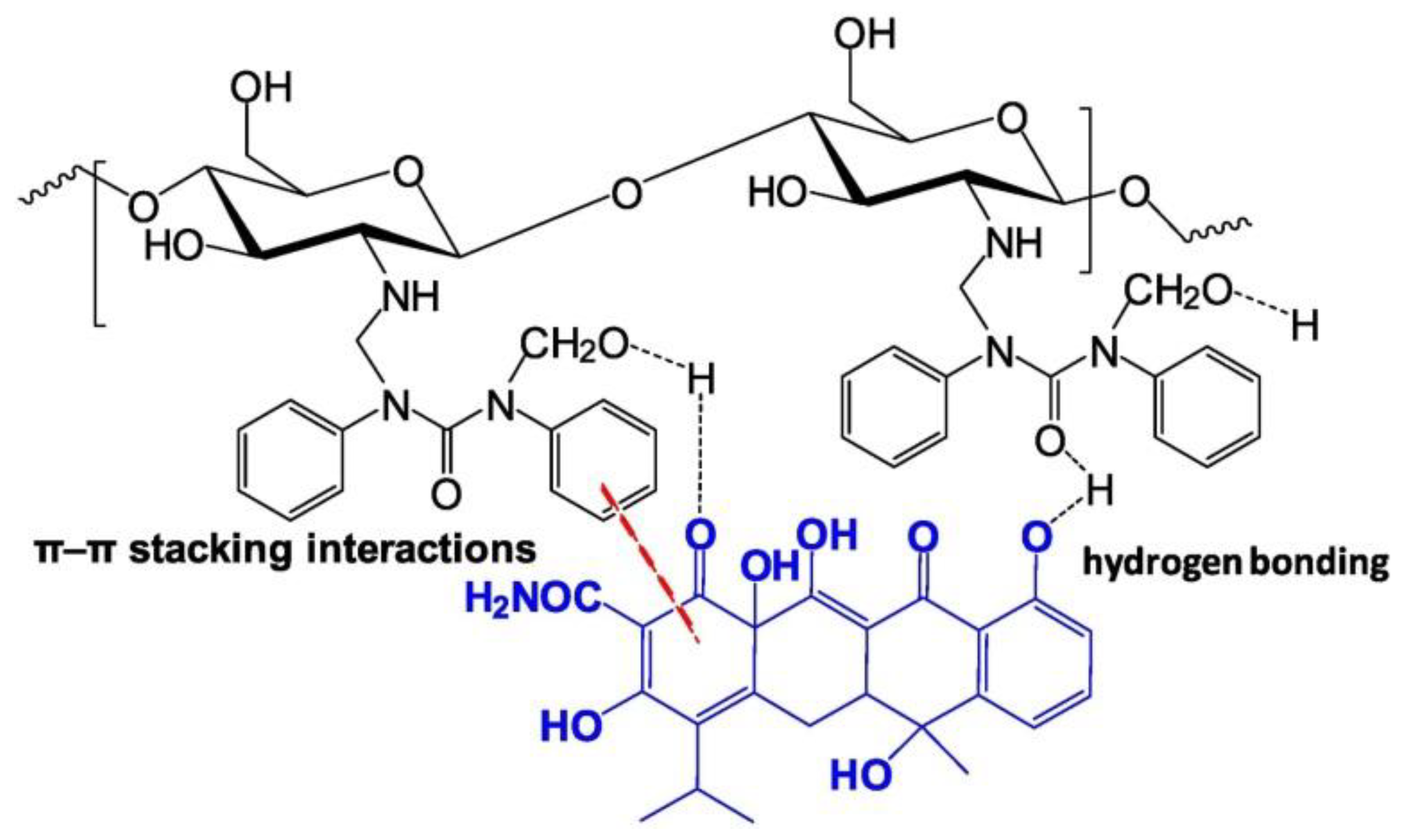
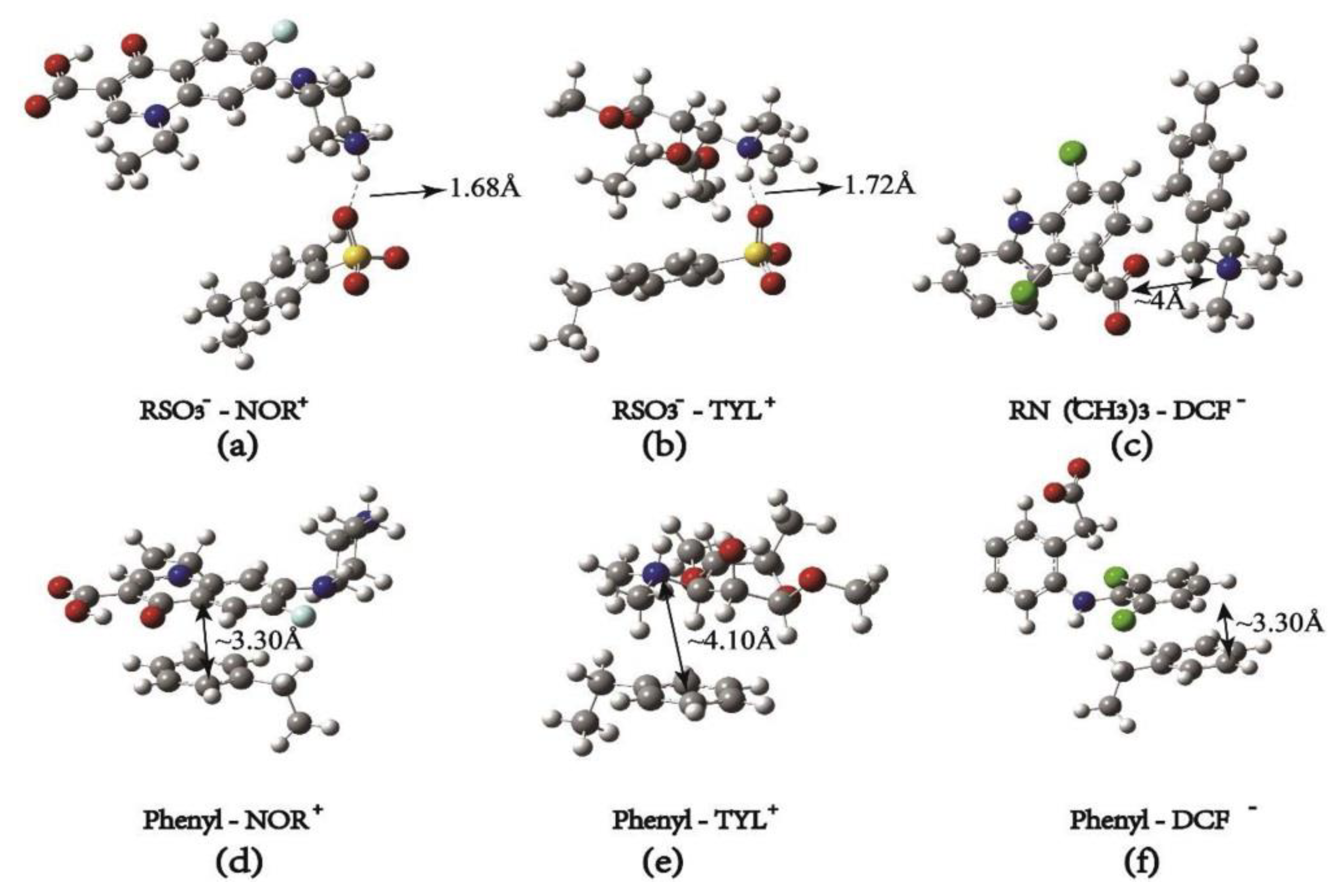

| Sorbent | pH | Pharmaceutical | Isotherms | Kinetics | Qmax (mg/g) | Ref. |
|---|---|---|---|---|---|---|
| MnFe2O4 nanoparticles embedded chitosan-diphenylureaformaldehyde Resin (CDF@MF) | 6 | Tetracycline | (L), F, T | (PFO), PSO, I-PD | 168.24 | [19] |
| Ionic liquid-impregnated chitosan hydrogel beads (CS-TCMA) | 7 | (L), F, S | (PFO), PSO, I-PD | 17.15 | [13] | |
| Chitosan/Fe3O4 composite particles (CD-MCP) | 10 | (L), F, D–R | PFO, (PSO), I-PD, ELV | 50.1 | [20] | |
| Genipin-crosslinked chitosan/graphene oxide-SO3H (GC/MGO-SO3H) | 7 | (L), F | PFO, (PSO) | 473.28 | [22] | |
| Chitosan/Fe3O4 composite particles (CD-MCP) | 6 | Diclofenac | (L), F, D–R | PFO, (PSO), I-PD, ELV | 196 | [20] |
| Magnetic composite particle (MCP) adsorbent, Core-brush shaped chitosan-based MCPs with core-brushes of polyanions (poly(sodium p-styrenesulfonate)) (PSA-MCP) | 6 | L, F, (T) | PFO, (PSO) | 151 | [21] | |
| Chitosan grafted with trans-aconitic acid (CsTACON) | 4 | L, F, (L–F) | PFO, (PSO) | 84.56 | [17] | |
| Epichlorohydrin-polyethylenimine adsorbent (EPCS@PEI) | 5 | (L), F | PFO, (PSO) | 253.32 | [12] | |
| Chitosan microspheres with nanographene oxide | --- | F | PFO, (PSO) | 20 | [28] | |
| Graphite oxide/poly(acrylic acid) grafted chitosan nanocomposite (GO/CSA) | 3 | Dorzolamide | L, (L–F) | PSO | 334 | [31] |
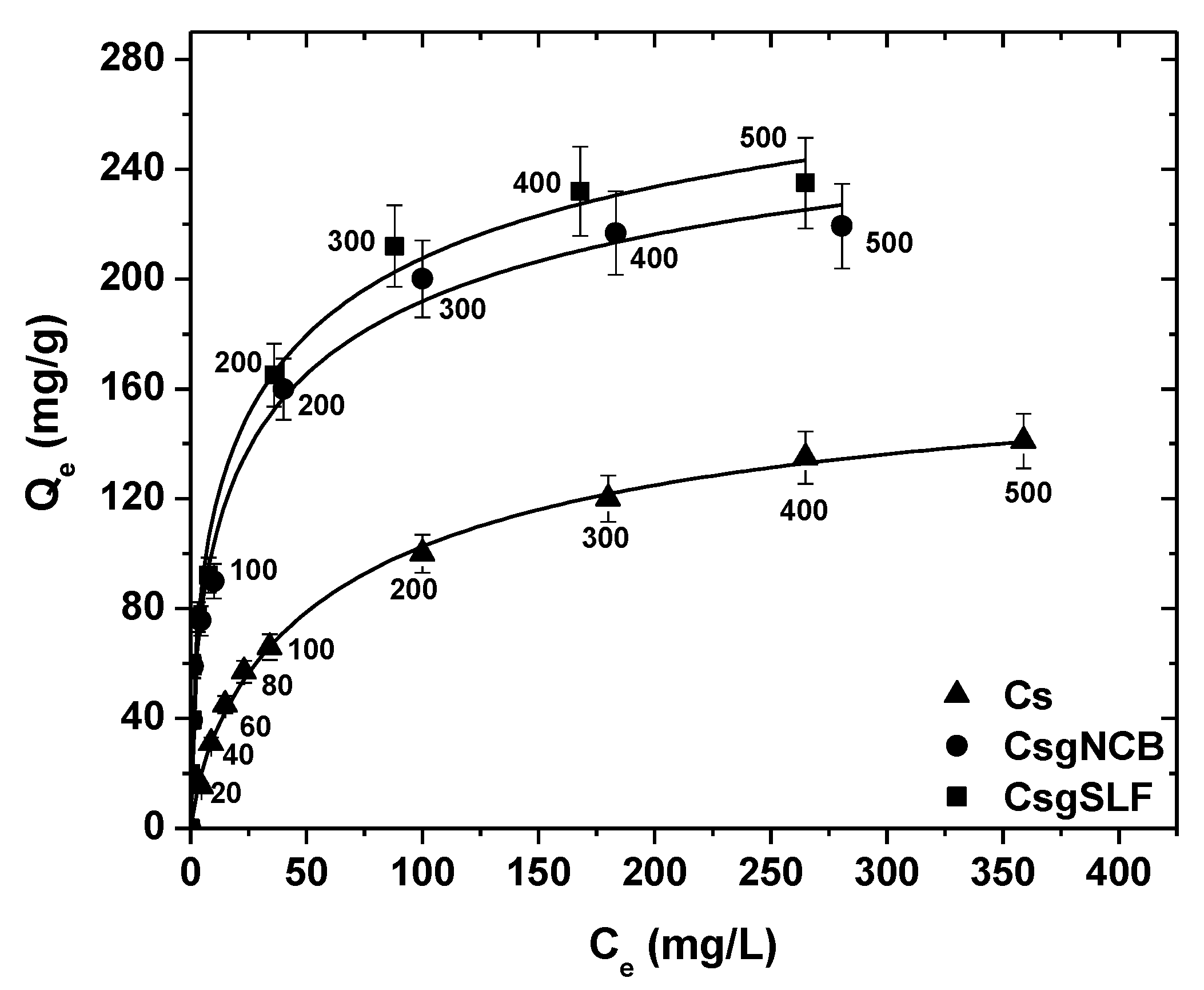
| Sulfonate-grafted chitosan adsorbent (CsSLF) | 10 | Pramipexole | L–F | PFO, (PSO), ELV | 337 | [32] |
| N-(2-carboxybenzyl)-grafted chitosan adsorbent (CsNCB) | 10 | L–F | PFO, (PSO), ELV | 181 | [32] | |
| Sulfonic acid-grafted chitosan adsorbent (CsSLA) | 10 | L, (L–F) | Not presented | 339 | [16] | |
| Magnetic composite Particle, Core-brush shaped chitosan-based MCPs with core-brushes of polyanions (poly(sodium p-styrenesulfonate)) | 3 | Norfloxacin | L, F, (T) | PFO, (PSO) | 165 | [21] |
| Magnetic composite Particle, Core-brush shaped chitosan-based MCPs with core-brushes of polyanions (poly(sodium p-styrenesulfonate)) | 4 | Tylosin | L, F, (T) | (PFO), PSO | 134 | [21] |
| Chitosan/waste coffee grounds | 6 | Metamizol | L, (F), T, D–R | PFO, (PSO), ELV, I-PD | 6.29 | [27] |
| Chitosan/waste coffee grounds | 6 | Caffeine | L, (F), T, D–R | PFO, (PSO), ELV, I-PD | 8.21 | [27] |
| Chitosan/waste coffee grounds | 6 | Acetaminophen | L, (F), T, D–R | PFO, (PSO), ELV, I-PD | 7.52 | [27] |
| Chitosan/waste coffee grounds | 6 | Acetylsalicylic acid | L, (F), T, D–R | PFO, (PSO), ELV, I-PD | 9.92 | [27] |
| Ozone-treated MWCNTs | 4 | L, (F) | PFO, (PSO) | 205 | [29] | |
| Graphene oxide-activated carbon-chitosan composite (GO-AC-CS) | 7 | (L), F | PFO, (PSO) | 13.7 | [25] | |
| Graphene oxide-activated carbon-chitosan composite (GO-AC-CS) | 4 | Carbamazepine | (L), F | PFO, (PSO) | 11.2 | [25] |
| Chitosan grafted carboxylic Zr-MOF to porous starch (PS-chitosan-UiO-66-COOH) | 3 | Sulfanilamide | (L), F | PFO, (PSO), I-PD | 70.20 | [24] |
| Chitosan film | 5–6 | Furosemide | (L), F, T, D–R | PFO, (PSO) | 3.5 | [15] |
| Sorbent | pH | Pollutant | Isotherms | Kinetics | Qmax (mg/g) | Ref. |
|---|---|---|---|---|---|---|
| Graphene oxide-activated carbon-chitosan composite (GO-AC-CS) | 4 | Bisphenol A | (L), F | PFO, (PSO) | 13.2 | [25] |
| 7 | Caffeine | (L), F | PFO, (PSO) | 14.8 | [25] | |
| 4 | Triclosan | (L), F | PFO, (PSO) | 14.5 | [25] | |
| Genipin-crosslinked chitosan/graphene oxide-SO3H (GC/MGO-SO3H) | 6 | Ibuprofen | (L), F | PFO, (PSO) | 113.27 | [22] |
| MIL-101(Cr)/chitosan composite beads | 4 | Ibuprofen | L, F, (R–P) | PFO, (PSO), I-PD | 103.2 | [23] |
| Benzoin acid | L, F, (R–P) | PFO, (PSO), I-PD | 66.5 | [23] | ||
| Ketoprofen | L, F, (R–P) | PFO, (PSO), I-PD | 156.5 | [23] | ||
| Bio-derived chitosan-EDTA-β-cyclodextrin(CS-ED-CD) | 4 | Bisphenol S | L, (L–F) | PSO | 44.3 | [26] |
| 4 | Ciprofloxacin | L, (L–F) | PSO | 47.1 | [26] | |
| 5 | Procaine | L, (L–F) | PSO | 48 | [26] | |
| 5 | Imipramine | L, (L–F) | PSO | 41.8 | [26] |
| Pharmaceutical | T | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
| K | KL | Qmax (mg/g) | R2 | KF | 1/n | R2 | |
| IBU | 298 | 2.017 | 138.62 | 0.9999 | 5.46 | 1.2530 | 0.9122 |
| 308 | 2.236 | 160.83 | 0.9992 | 6.35 | 1.1263 | 0.8247 | |
| 313 | 1.865 | 146.27 | 0.9978 | 5.68 | 1.2724 | 0.7893 | |
| Compound | Adsorbent | Capacity | PFO | PSO | ||||
|---|---|---|---|---|---|---|---|---|
| Qmax (mg/g) | k1 (h−1) | qe,cal (mg/g) | R2 | k2 (g/mg/h) | qe,cal (mg/g) | R2 | ||
| BPA | GO-AC-CS | 18.4 ± 0.55 | 0.527 | 14.2 | 0.976 | 0.04 | 30.3 | 0.999 |
| CAFF | GO-AC-CS | 19.8 ± 0.11 | 0.374 | 3.99 | 0.824 | 0.487 | 19.7 | 0.999 |
| TCS | GO-AC-CS | 19.5 ± 0.95 | 0.587 | 4.51 | 0.904 | 0.65 | 19.6 | 0.999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liakos, E.V.; Lazaridou, M.; Michailidou, G.; Koumentakou, I.; Lambropoulou, D.A.; Bikiaris, D.N.; Kyzas, G.Z. Chitosan Adsorbent Derivatives for Pharmaceuticals Removal from Effluents: A Review. Macromol 2021, 1, 130-154. https://doi.org/10.3390/macromol1020011
Liakos EV, Lazaridou M, Michailidou G, Koumentakou I, Lambropoulou DA, Bikiaris DN, Kyzas GZ. Chitosan Adsorbent Derivatives for Pharmaceuticals Removal from Effluents: A Review. Macromol. 2021; 1(2):130-154. https://doi.org/10.3390/macromol1020011
Chicago/Turabian StyleLiakos, Efstathios V., Maria Lazaridou, Georgia Michailidou, Ioanna Koumentakou, Dimitra A. Lambropoulou, Dimitrios N. Bikiaris, and George Z. Kyzas. 2021. "Chitosan Adsorbent Derivatives for Pharmaceuticals Removal from Effluents: A Review" Macromol 1, no. 2: 130-154. https://doi.org/10.3390/macromol1020011
APA StyleLiakos, E. V., Lazaridou, M., Michailidou, G., Koumentakou, I., Lambropoulou, D. A., Bikiaris, D. N., & Kyzas, G. Z. (2021). Chitosan Adsorbent Derivatives for Pharmaceuticals Removal from Effluents: A Review. Macromol, 1(2), 130-154. https://doi.org/10.3390/macromol1020011









